Abstract
Background
A temporary ileostomy may reduce symptoms from anastomotic leakage after rectal cancer resection. Earlier results of the EASY trial showed that early closure of the temporary ileostomy was associated with significantly fewer postoperative complications. The aim of the present study was to compare health‐related quality of life (HRQOL) following early versus late closure of a temporary ileostomy.
Methods
Early closure of a temporary ileostomy (at 8–13 days) was compared with late closure (at more than 12 weeks) in a multicentre RCT (EASY) that included patients who underwent rectal resection for cancer. Inclusion of participants was made after index surgery. Exclusion criteria were signs of anastomotic leakage, diabetes mellitus, steroid treatment, and signs of postoperative complications at clinical evaluation 1–4 days after rectal resection. HRQOL was evaluated at 3, 6 and 12 months after resection using the European Organisation for Research and Treatment of Cancer (EORTC) questionnaires QLQ‐C30 and QLQ‐CR29 and Short Form 36 (SF‐36®).
Results
There were 112 patients available for analysis. Response rates of the questionnaires were 82–95 per cent, except for EORTC QLQ‐C30 at 12 months, to which only 54–55 per cent of the patients responded owing to an error in questionnaire distribution. There were no clinically significant differences in any questionnaire scores between the groups at 3, 6 or 12 months.
Conclusion
Although the randomized study found that early closure of the temporary ileostomy was associated with significantly fewer complications, this clinical advantage had no effect on the patients' HRQOL. Registration number: NCT01287637 (https://www.clinicaltrials.gov).
Short abstract
No different after early ileostomy closure
Introduction
Low anterior resection with total mesorectal excision is regarded as one of the optimal surgical treatments for potentially curable carcinoma of the rectum1, 2. Because of the low anastomosis close to the pelvic floor, patients often receive a temporary ileostomy at the time of the resection to reduce the risk of symptomatic anastomotic dehiscence3 and its clinical consequences3, 4, 5, 6. However, studies4, 7, 8, 9 have reported considerable morbidity related to the temporary ileostomy, with complication rates of up to 50 per cent. Most patients with a temporary ileostomy have the stoma for at least 3 months, and it is not unusual for it to be left in place much longer. For some patients the stoma becomes permanent10.
Data regarding quality of life (QOL) in patients receiving a diverting stoma as part of their rectal cancer treatment are limited11, 12, 13, 14. In prospective studies it has been suggested that patients with a stoma may suffer from impaired health‐related quality of life (HRQOL)15, which may improve at stoma closure2. Complications such as stoma leakage, parastomal skin irritation, dietary restrictions, retraction and prolapse of the stoma have been reported to have significant impact on the patient's daily life12.
The aim of the present study was to compare HRQOL at 3, 6 and 12 months after rectal resection for cancer in a multicentre RCT comparing early versus late closure of a temporary ileostomy (EASY trial)16, 17.
Methods
The EASY trial was designed as a randomized multicentre trial16 comparing early with late closure of a temporary ileostomy regarding risk of complications. Screening for and inclusion of participants was made after index surgery (total mesorectal excision for rectal cancer including creation of a temporary ileostomy). Exclusion criteria were diabetes mellitus, ongoing steroid treatment, signs of postoperative complications at clinical evaluation 1–4 days after rectal resection and inability to understand the Danish or Swedish language. Patients with no adverse signs were invited to participate and, after informed consent, underwent further investigation with contrast‐enhanced CT or a flexible endoscopy of the rectum, or both, performed 6–8 days after stoma creation to ensure that no patient with signs of anastomotic leakage was included. Patients were randomized to either the intervention group with early closure (day 8–13 after stoma creation) or the control group with late closure (more than 12 weeks after stoma creation) of the ileostomy.
The primary endpoint of the study was the mean number of complications after rectal resection and up to 12 months; these results have been published previously17. The present paper reports the secondary endpoints, HRQOL and QOL, at 3, 6 and 12 months after the index operation.
The study was approved in Denmark by the Science Ethics Committee for the Capital Region (H‐1‐2010‐113) and in Sweden by the Regional Ethics Approval Committee in Göteborg (Dnr 064‐2011). Before inclusion, patients were informed about the study and all participating patients returned a signed consent form.
The project was approved by the Data Protection Agency in Denmark, and by the Personal Data Representative at Sahlgrenska University Hospital, Göteborg, Sweden.
The protocol was registered at https://www.clinicaltrials.gov (NCT01287637) before patient inclusion.
Patients
Eight hospitals in Denmark and Sweden participated in the study, but three centres (with a total of 8 patients) were excluded as they failed to maintain a screening log. Consenting patients were asked to complete questionnaires at 3, 6 and 12 months after stoma creation (rectal resection). The questionnaires included the 36‐item Short Form 36 (SF‐36®; Rand Corporation, Santa Monica, California, USA) and the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ) CR29 and C30. Data regarding demographic details, tumour stage and height, chemoradiotherapy and all complications within 12 months of surgery were registered in case report forms.
Randomization
Consenting patients who fulfilled the inclusion criteria were randomized either to the intervention group with early closure of the ileostomy or to the control group with late closure. Randomization was executed in computer‐generated blocks of six. The randomization was performed on the surgical ward using sequentially numbered thick, opaque and sealed envelopes. Blinding of the intervention was not possible.
Health‐related quality‐of‐life instruments
Short Form 36
SF‐36® is a generic tool that evaluates patients' self‐reported quality of life18, 19. It consists of 36 items that measure eight dimensions of health on a multi‐item scale, including social and physical function. The scoring scale ranges from 0 to 100, with lower scores indicating worse health. The instrument has been validated, and for comparison in this study a Swedish reference population was used20.
EORTC QLQ‐C30 and QLQ‐CR29
The EORTC QLQ‐C30 is a questionnaire developed to assess QOL in patients with cancer, and consists of five functional scales (physical, role, cognitive, emotional and social), three symptom scales (fatigue, pain and nausea, and vomiting), one global health status and QOL scale, and six single‐item measures (dyspnoea, insomnia, appetite loss, constipation, diarrhoea and financial difficulties)21. A high score on the functional scale represents a high level of functioning, whereas a high score on the symptom scale represents a high level of symptoms.
The EORTC QLQ‐CR29 was designed for use in patients undergoing treatment for colorectal cancer. It was derived from the EORTC QLQ‐C38 questionnaire, as there was a need for an update in the colorectal module22. The questions assess disease symptoms, side‐effects of treatment, body image, future perspective, and sexual function and interest. Both questionnaires have been validated internationally and were available in Danish and Swedish versions22, 23.
All questionnaires were administered at 3, 6 and 12 months after stoma creation.
Statistical analysis
The study was part of a RCT with power calculated for the primary endpoint. Group sizes in the EASY trial were set to 72 patients per group to evaluate complication rates16, 17. In Krouse et al.24 a minimally important difference of 8 units was used for the different scales of SF‐36®. Physical and mental component scores had a standard deviation (s.d.) of up to 15 units, and the eight specific scales had s.d. values in the range 17–33. With group sizes of 72 or 55 and a s.d. of 17 or 15 respectively, there will be 80 per cent power to detect a true difference of 8 units.
The questionnaires SF‐36®25, EORTC QLQ‐C3026 and QLQ‐CR2923 were scored according to the methods recommended by the developers; missing data were handled as instructed in the scoring manuals. Before analysis, based on the literature and previous data, the authors chose to present the functional scales and global health status/QOL of the EORTC QLQ‐C30 questionnaire, and the functional scales (urinary frequency, stool frequency and body image) of the EORTC QLQ‐CR29 questionnaire. At 12‐month follow‐up, several patients (20 in the intervention group and 16 in the control group) were, by mistake, given an incomplete questionnaire in which questions 16–30 of the EORTC QLQ‐C30 were missing. Before analysis, the decision was made to include physical and role functioning, as these functional scales are scored using questions 1–15 in accordance with the EORTC manual. No other scales or items were analysed for these patients in the EORTC QLQ‐C30 at 12‐month follow‐up. However, EORTC QLQ‐CR29 and SF‐36® were analysed at 12‐month follow‐up. As the distribution of the incomplete questionnaires was independent of the observable characteristics of the patients who received them, interpreting the missing data as being completely random is reasonable and imputation was considered unnecessary.
Owing to the characteristics of the data, the different scales of SF‐36® and EORTC were summarized by median (i.q.r.) values, and group comparisons were made using the Wilcoxon rank sum test and the two‐sample Hodges–Lehmann estimator.
SF‐36® scores were compared with those in a general Swedish reference population20. For each of the eight scales, the individual levels were compared with age‐matched (17–34, 35–49, 50–64, and 65 or more years) mean levels from the reference population. The proportion of patients with values below the reference levels was compared between the intervention and control group using a χ2 test. The software packages SPSS® version 23 (IBM, Armonk, New York, USA), SAS® version 9.4 (SAS Institute, Cary, North Carolina, USA) and R version 3.2.327 were used for statistical analysis. Scores for SF‐36®, EORTC QLQ‐C30 and QLQ‐CR29 were derived and summarized by median (i.q.r.) values.
Results
The EASY trial assessed 418 patients for eligibility. After exclusion of 291 patients, 127 patients were randomized (Fig. 1). A further 15 patients were excluded, eight from three centres that were excluded from the study as they failed to maintain a screening log. In summary, 112 patients were included from February 2011, with the last follow‐up in November 2015. Some 55 patients in the intervention (early closure) group and 57 in the control (late closure) group were available for analysis. There were no violations of the randomization. Except for a larger proportion of women in the intervention group, baseline demographic characteristics and clinical data were similar in the two groups (Table 1).
Figure 1.
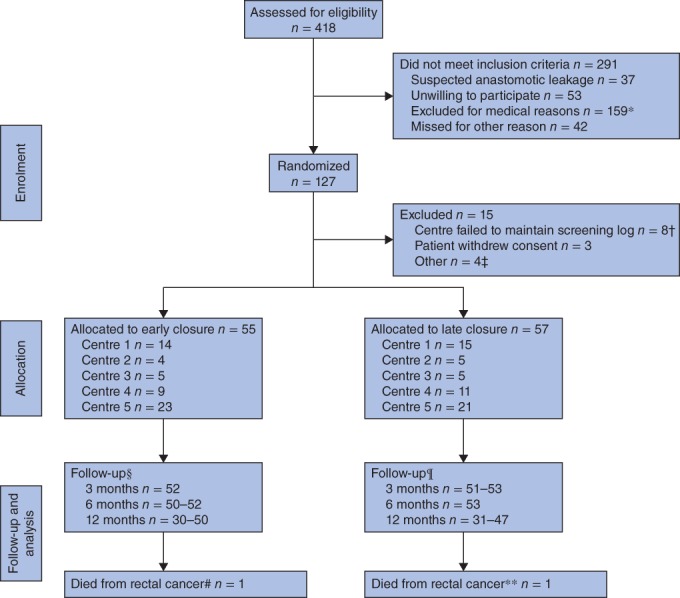
Participant flow diagram, as in the EASY trial17. *Paralytic ileus (24), Hartmann procedure with intersphincteric dissection (16), delayed postoperative recovery (15), perioperative complications (7), other infection (5), reoperation (7), high stoma output (5), pulmonary embolism (1), ulcerative colitis (1), extensive cancer disease (3), cardiovascular disease (2), language difficulty (5), diabetes (28), permanent or no stoma (29), steroid treatment (3), other (8). †Centre 6 (2), centre 7 (3), centre 8 (3). ‡Allocated to early closure, but not possible to perform surgery within 8–13 days (1); early closure outside the study (2); randomized, but no further information available (1). §3 months: Short Form 36 (SF‐36®) (52), European Organisation for Research and Treatment of Cancer (EORTC) QLQ‐C30 (52), EORTC QLQ‐CR29 (52); 6 months: 3 months: SF‐36® (52), EORTC QLQ‐C30 (50), EORTC QLQ‐CR29 (50); 12 months: 3 months: SF‐36® (50), EORTC QLQ‐C30 (30), EORTC QLQ‐CR29 (50). ¶3 months: SF‐36® (53), EORTC QLQ‐C30 (51), EORTC QLQ‐CR29 (52); 6 months: 3 months: SF‐36® (53), EORTC QLQ‐C30 (53), EORTC QLQ‐CR29 (53); 12 months: SF‐36® (47), EORTC QLQ‐C30 (31), EORTC QLQ‐CR29 (47). #Missing from follow‐up at 12 months. **Patient did not have closure; missing from follow‐up at 12 months
Table 1.
Baseline and preoperative characteristics of patients randomized to early or late closure
| Early closure (n = 55) | Late closure (n = 57) | |
|---|---|---|
| Age (years)* | 67 (36–82) | 67 (39–81) |
| Sex ratio (F : M) | 31 : 24 | 21 : 36 |
| BMI (kg/m2)* | 24 (17–32) | 23 (19–35) |
| Co‐morbidity | 23 (42) | 24 (42)† |
| Ischaemic heart disease | 5 | 8 |
| Hypertension | 17 | 13 |
| COPD | 2 | 2 |
| Renal disease | 0 | 0 |
| Other | 9‡ | 4§ |
| Radiotherapy | 16 (29) | 16 (28) |
| Long‐term | 5 (9) | 5 (9) |
| Adjuvant chemotherapy | 22 (40) | 23 (40) |
| Marital status | ||
| Single | 5 (9) | 9 (16) |
| Married | 42 (76) | 43 (75) |
| Widowed | 8 (15) | 5 (9) |
| Higher education | 34 (62) | 37 (65) |
| Employed | ||
| Yes | 25 (45) | 25 (44) |
| No | 29 (53)¶ | 32 (56) |
| Smoker | 6 (11) | 4 (7) |
| No. of pack years* | 30 (16–30)# | 26 (20–50)¶ |
| Alcohol intake > 60 g/day | 0 (0) | 0 (0)¶ |
| Lower border of tumour (cm from anal verge) | ||
| 5–9 | 27 (49) | 24 (42) |
| 10–15 | 27 (49) | 33 (58) |
| > 15 | 1 (2) | 0 (0) |
| UICC clinical stage** | ||
| I | 12 (22) | 19 (33) |
| II | 21 (38) | 13 (23) |
| III | 18 (33) | 20 (35) |
| IV | 3 (5) | 1 (2) |
| Method of evaluation of anastomosis before ileostomy closure | ||
| CT | 14 (25) | 19 (33) |
| Rectoscopy | 14 (25) | 10 (18) |
| CT + rectoscopy | 27 (49) | 28 (49) |
| Total length of hospital stay†† (days) * | 14 (11–42) | 14 (7–44) |
Values in parentheses are percentages unless indicated otherwise;
values are median (range).
Data missing for two patients.
Asthma (2), depression (1), idiopathic thrombocytopenic purpura (1), lymphoma (1), Waldenström's macroglobulinaemia (1), osteoporosis (1), Sjögren syndrome (1), thyrotoxicosis (1).
Depression (1), hyperlipidaemia (1), hypothyroidism (1), meningioblastoma (1).
Data missing for one patient.
Data missing for three patients.
Data missing for one patient in each group; in addition, three patients in the late closure group had T0 N0 M0 disease and were therefore not classified.
For both index surgery and loop ileostomy closure. COPD, chronic obstructive pulmonary disease; UICC, International Union Against Cancer. Table reproduced with permission from Wolters Kluwer Health Inc. Danielsen AK, Park J, Jansen JE, Bock D, Skullman S, Wedin A et al. Early closure of a temporary ileostomy in patients with rectal cancer: a multicenter randomized controlled trial. Ann Surg 2017; 265 (2): 284–290. https://journals.lww.com/annalsofsurgery/.
Questionnaire response rates were generally around 90 (range 82–95) per cent, excluding the EORTC QLQ‐C30 at 12‐month follow‐up, owing to missing questions as described in the Methods section (Fig. 1).
SF‐36® scores were similar between the two groups, with no differences in the physical component score or the mental component score, but significant differences in role physical, bodily pain and mental health at 3, 12 and 12 months respectively (Table 2). All dimensions in SF‐36® improved over time. At 3 months, a majority of patients in both groups scored values below mean levels in the reference population20, especially regarding role physical. At 12 months, 52–85 per cent of the patients scored higher than the reference group, with physical functioning scoring the highest among the dimensions.
Table 2.
SF‐36® scores at 3, 6 and 12 months after rectal resection
| 3 months | 6 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median (i.q.r.) | H–L* | P † | Median (i.q.r.) | H–L* | P † | Median (i.q.r.) | H–L* | P † | |
| Physical functioning | |||||||||
| Early | 90 (75–95) | 0 (−5, 5) | 0·646 | 90 (81·7–100) | 0 (−5, 5) | 0·630 | 95 (70–100) | 0 (0, 5) | 0·322 |
| Late | 90 (80–95) | 90 (80–95) | 95 (90–100) | ||||||
| Role physical | |||||||||
| Early | 75 (50–96·9) | 12·5 (0, 18·8) | 0·025 | 81·3 (50–100) | 6·3 (0, 18·8) | 0·140 | 81·3 (56·3–100) | 0 (−6·3, 6·3) | 0·718 |
| Late | 62·5 (43·8–75) | 75 (50–93·8) | 87·5 (75–100) | ||||||
| Bodily pain | |||||||||
| Early | 80 (52–100) | 0 (−10, 0) | 0·858 | 74 (62–100) | 0 (−16, 0) | 0·264 | 79 (51–100) | 0 (0, 20) | 0·035 |
| Late | 74 (62–100) | 84 (63–100) | 100 (74–100) | ||||||
| General health | |||||||||
| Early | 71·6 (52–88·5) | −5 (−15, 2) | 0·139 | 77 (56–87) | 0 (−10, 5) | 0·820 | 74·5 (45–92) | 5 (−5, 16·8) | 0·279 |
| Late | 77 (67–87) | 77 (65–87) | 82 (72–87) | ||||||
| Vitality | |||||||||
| Early | 62·5 (43·8–81·3) | 4·2 (−6·3, 12·5) | 0·441 | 68·8 (50–81·3) | 0 (−12·5, 6·3) | 0·796 | 68·8 (50–81·3) | 6·3 (0, 12·5) | 0·196 |
| Late | 68·8 (56·3–81·3) | 68·8 (56·3–81·3) | 75 (62·5–87·5) | ||||||
| Social functioning | |||||||||
| Early | 75 (62·5–100) | 0 (−12·5, 0) | 0·468 | 87·5 (62·5–100) | 0 (0, 0) | 0·976 | 87·5 (62·5–100) | 0 (0, 12·5) | 0·415 |
| Late | 87·5 (75–100) | 87·5 (62·5–100) | 100 (75–100) | ||||||
| Role emotional | |||||||||
| Early | 83·3 (58·3–100) | 0 (0, 8·3) | 0·345 | 87·5 (66·7–100) | 0 (0, 0) | 0·923 | 95·8 (66·7–100) | 0 (0, 0) | 0·697 |
| Late | 83·3 (50–100) | 83·3 (75–100) | 95·8 (75–100) | ||||||
| Mental health | |||||||||
| Early | 80 (55–90) | 5 (−5, 10) | 0·217 | 80 (60–90) | −5 (−10, 5) | 0·291 | 80 (60–90) | 10 (0, 15) | 0·020 |
| Late | 85 (65–90) | 85 (70–95) | 85 (75–95) | ||||||
| Mental component score | |||||||||
| Early | 52·5 (40·7–58·6) | 1 (−2·6, 5) | 0·588 | 54·4 (42·8–58·6) | 0·2 (−3, 3·9) | 0·939 | 54·1 (42·6–58·5) | 2·5 (−0·7, 6·3) | 0·105 |
| Late | 53 (44·8–57·8) | 54·6 (46·9–57·5) | 56·6 (52·9–59·2) | ||||||
| Physical component score | |||||||||
| Early | 51·8 (40·9–58·2) | −0·5(−3·8, 3·4) | 0·823 | 53·3 (43·3–57·1) | −0·2 (−3·6, 3) | 0·900 | 54·1 (44·5–59) | 1·6 (−1, 6·1) | 0·281 |
| Late | 51·2 (46·9–54·8) | 52·2 (45·8–57·9) | 56·8 (51–59·4) | ||||||
Two‐sample Hodges–Lehmann (H–L) estimator with 95 per cent asymptotic confidence limits in parentheses. SF‐36®, Short Form 36.
Wilcoxon rank sum test for difference between early and late closure.
EORTC QLQ‐C30 and QLQ‐CR29 scores were comparable between intervention and control groups. Emotional functioning was lower in the early closure group at 3 and 6 months, but similar to the late closure group at 12 months (Table 3). No statistically significant differences were seen in the dimensions of the QLQ‐CR29 questionnaire (Table 4).
Table 3.
EORTC QLQ‐C30 scores at 3, 6 and 12 months after rectal resection
| 3 months | 6 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median (i.q.r.) | H–L* | P † | Median (i.q.r.) | H–L* | P † | Median (i.q.r.) | H–L* | P † | |
| Global quality of life | |||||||||
| Early | 75 (50–83·3) | 0 (−8·3, 8·3) | 0·941 | 66·7 (50–83·3) | 0 (−8·3, 8·3) | 0·961 | 83·3 (50–91·7) | 0 (−16·7, 8·3) | 0·889 |
| Late | 66·7 (58·3–83·3) | 66·7 (66·7–83·3) | 83·3 (66·7–91·7) | ||||||
| Physical functioning | |||||||||
| Early | 93·3 (73·3–100) | 0 (0, 6·7) | 0·634 | 93·3 (80–100) | 0 (−6·7, 0) | 0·433 | 93·3 (73·3–100) | 0 (−13·3, 0) | 0·137 |
| Late | 93·3 (73·3–100) | 93·3 (80–100) | 100 (80–100) | ||||||
| Role functioning | |||||||||
| Early | 83·3 (66·7–100) | −16·7 (−16·7, 0) | 0·066 | 100 (66·7–100) | 0 (0, 0) | 0·503 | 100 (66·7–100) | 0 (0, 0) | 0·793 |
| Late | 66·7 (50–100) | 83·3 (66·7–100) | 100 (66·7–100) | ||||||
| Emotional functioning | |||||||||
| Early | 83·3 (66·7–100) | 8·3 (0, 16·7) | 0·023 | 83·3 (66·7–100) | −8·3 (−16·7, 0) | 0·031 | 91·7 (66·7–100) | 0 (−8·3, 0) | 0·409 |
| Late | 91·7 (83·3–100) | 91·7 (75–100) | 91·7 (83·3–100) | ||||||
| Cognitive functioning | |||||||||
| Early | 100 (83·3–100) | 0 (0, 0) | 0·447 | 83·3 (83·3–100) | 0 (−16·7, 0) | 0·131 | 100 (66·7–100) | 0 (0, 0) | 0·652 |
| Late | 100 (83·3–100) | 100 (83·3–100) | 100 (83·3–100) | ||||||
| Social functioning | |||||||||
| Early | 83·3 (66·7–100) | 0 (0, 0) | 0·583 | 83·3 (66·7–100) | 0 (0, 0) | 0·882 | 83·3 (66·7–100) | 0 (−16·7, 0) | 0·142 |
| Late | 83·3 (66·7–100) | 83·3 (66·7–100) | 100 (66·7–100) | ||||||
Two‐sample Hodges–Lehmann (H–L) estimator with 95 per cent asymptotic confidence limits in parentheses. EORTC, European Organisation for Research and Treatment of Cancer.
Wilcoxon rank sum test for difference between early and late.
Table 4.
EORTC QLQ‐CR29 scores for selected functions at 3, 6 and 12 months after rectal resection
| 3 months | 6 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median (i.q.r.) | H–L* | P † | Median (i.q.r.) | H–L* | P † | Median (i.q.r.) | H–L* | P † | |
| Urinary frequency | |||||||||
| Early | 16·7 (0–33·3) | 0 (0, 16·7) | 0·323 | 16·7 (0–50) | 0 (−16·7, 0) | 0·353 | 16·7 (0–33·3) | 0 (0, 16·7) | 0·268 |
| Late | 16·7 (0–50) | 16·7 (8·3–41·7) | 33·3 (0–50) | ||||||
| Stool frequency | |||||||||
| Early | 33·3 (16·7–50) | 33·3 (16·7, 33·3) | < 0·001 | 33·3 (16·7–50) | 16·7 (0, 33·3) | 0·068 | 33·3 (16·7–50) | 0 (0, 16·7) | 0·611 |
| Late | 0 (0–16·7) | 16·7 (0–66·7) | 33·3 (16·7–50) | ||||||
| Body image | |||||||||
| Early | 88·9 (66·7–100) | 0 (−11·1, 0) | 0·715 | 88·9 (77·8–100) | 0 (0, 11·1) | 0·364 | 94·4 (77·8–100) | 0 (0, 0) | 0·502 |
| Late | 77·8 (66·7–100) | 88·9 (66·7–100) | 100 (88·9–100) | ||||||
Two‐sample Hodges–Lehmann (H–L) estimator with 95 per cent asymptotic confidence limits in parentheses. EORTC, European Organisation for Research and Treatment of Cancer.
Wilcoxon rank sum test for difference between early and late closure.
Discussion
In this RCT no significant differences were observed in HRQOL within 12 months after rectal resection for cancer when early and late closure of temporary ileostomy were compared. Global QOL generally improved later in the follow‐up period (6–12 months), and at 12 months the results were comparable, not only between the two groups but also with respect to both age‐matched reference populations28, 29 and previous findings30. Although there was a tendency for improvement over time in global QOL and role functioning, no clinically significant changes were seen in QLQ‐C30 scores. The definition of a clinically significant change over time was based on the suggestion31 that a difference of 5–10 points be considered a ‘ little change’ and a difference of 10–20 points a ‘ moderate change’. When comparing the SF‐36® scores with Swedish reference data20, a general improvement was seen during the follow‐up interval.
No baseline data for preoperative assessment were available, owing to the fact that the patients were enrolled and randomized after the rectal resection. This is considered a weakness in the evaluation of HRQOL. Even though reference data were available, there was no opportunity to investigate the development and changes in HRQOL from preoperative to postoperative values. In addition, considering the length of time needed for recovery after rectal cancer surgery, follow‐up at 18 and 24 months might have been of value to observe any further development and potential improvement in HRQOL.
The lack of difference between the two groups differs somewhat from the findings of previous prospective studies2, 13, which have reported that patients with a temporary stoma may suffer from impaired HRQOL in comparison with patients who had undergone a similar operation but without a covering stoma, such as a high anterior resection. However, the results of previous studies are contradictory. A Danish study32 suggested that a temporary stoma left patients with feelings of uncertainty, that closure of the stoma was seen as a crucial event, and that knowing the date for closure was important. A prospective study11 found improved global QOL following stoma reversal after low anterior resection for rectal cancer, and physical function, as measured by both the QLQ‐C30 and SF‐36® questionnaires, was also significantly improved. However, a prospective interview study14 reported no change in QOL following closure of a temporary stoma after rectal cancer surgery, and the patient's personality rather than clinical variables has a strong and lasting influence on QOL33. As suggested in a review article34 from 2011, closure of the stoma and prompt redirection of intestinal contents to the rectum 3–6 months after rectal resection might be considered to be a cause of negative impact on patients' physical, social and psychological health for several months. Gastrointestinal symptoms, such as increased stool frequency, urgency, diarrhoea and persistent problems including low anterior resection syndrome, do not occur in the presence of a temporary stoma. Consequently, stoma reversal might result in the appearance of these symptoms. Although this was not observed in the present study, this could be because of the small size of the cohort. In the COLOR II trial35, patients with rectal cancer appeared to need a longer time to recover compared with those with colonic cancer. This could perhaps explain the lack of difference between the groups in the present study, as both involved extensive surgery for rectal cancer, where timing of the stoma closure is not reflected in the HRQOL follow‐up.
The EASY trial17 found that it was safe and advantageous to close the loop ileostomy early in patients with no clinical or radiological signs of anastomotic leakage. However, the present study did not find a link between this clinical advantage and patients' HRQOL.
Acknowledgements
The authors express their gratitude to the doctors, nurses and staff at the participating centres for their help and assistance during the course of this trial.
A.K.D. has received funding from the Research Council at Herlev and Gentofte Hospital, Denmark. S.S. has received funding from the Health and Medical Care Committee of the Regional Executive Board and Region Västra Götaland (numbers 311031 and 390991) and from Lions Cancerfond West, Sweden. E.A. has received funding from the Swedish Research Council (2012‐1786), Swedish Cancer Society (2013/500), Sahlgrenska University Hospital (agreement concerning research and education of doctors ALFGBG‐366481, ALFGBG‐526501 and ALFGBG‐493341), Swedish Society of Medicine (SLS‐247661 and SLS‐412151) and Lions Väst Cancer Foundation.
Disclosure: The authors declare no conflict of interest.
Presented to the annual meeting of the European Society of Coloproctology, Milan, Italy, September 2016
References
- 1. Heald RJ. Total mesorectal excision is optimal surgery for rectal cancer: a Scandinavian consensus. Br J Surg 1995; 82: 1297–1299. [DOI] [PubMed] [Google Scholar]
- 2.O'Leary DP, Fide CJ, Foy C, Lucarotti ME. Quality of life after low anterior resection with total mesorectal excision and temporary loop ileostomy for rectal carcinoma. Br J Surg 2001; 88: 1216–1220. [DOI] [PubMed] [Google Scholar]
- 3. Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg 2007; 246: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ihnat P, Gunkova P, Peteja M, Vavra P, Pelikan A, Zonca P. Diverting ileostomy in laparoscopic rectal cancer surgery: high price of protection. Surg Endosc 2016; 30: 4809–4816. [DOI] [PubMed] [Google Scholar]
- 5. Montedori A, Cirocchi R, Farinella E, Sciannameo F, Abraha I. Covering ileo‐ or colostomy in anterior resection for rectal carcinoma. Cochrane Database Syst Rev 2010; (5)CD006878. [DOI] [PubMed] [Google Scholar]
- 6. Hüser N, Michalski CW, Erkan M, Schuster T, Rosenberg R, Kleeff J et al Systematic review and meta‐analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg 2008; 248: 52–60. [DOI] [PubMed] [Google Scholar]
- 7. Gessler B, Haglind E, Angenete E. Loop ileostomies in colorectal cancer patients – morbidity and risk factors for nonreversal. J Surg Res 2012; 178: 708–714. [DOI] [PubMed] [Google Scholar]
- 8. Hallböök O, Matthiessen P, Leinsköld T, Nyström PO, Sjödahl R. Safety of the temporary loop ileostomy. Colorectal Dis 2002; 4: 361–364. [DOI] [PubMed] [Google Scholar]
- 9. Gessler B, Haglind E, Angenete E. A temporary loop ileostomy affects renal function. Int J Colorectal Dis 2014; 29: 1131–1135. [DOI] [PubMed] [Google Scholar]
- 10. den Dulk M, Smit M, Peeters KC, Kranenbarg EM, Rutten HJ, Wiggers T et al A multivariate analysis of limiting factors for stoma reversal in patients with rectal cancer entered into the total mesorectal excision (TME) trial: a retrospective study. Lancet Oncol 2007; 8: 297–303. [DOI] [PubMed] [Google Scholar]
- 11. Camilleri‐Brennan J, Steele RJ. Prospective analysis of quality of life after reversal of a defunctioning loop ileostomy. Colorectal Dis 2002; 4: 167–171. [DOI] [PubMed] [Google Scholar]
- 12. Gooszen AW, Geelkerken RH, Hermans J, Lagaay MB, Gooszen HG. Quality of life with a temporary stoma: ileostomy vs. colostomy. Dis Colon Rectum 2000; 43: 650–655. [DOI] [PubMed] [Google Scholar]
- 13. Tsunoda A, Tsunoda Y, Narita K, Watanabe M, Nakao K, Kusano M. Quality of life after low anterior resection and temporary loop ileostomy. Dis Colon Rectum 2008; 51: 218–222. [DOI] [PubMed] [Google Scholar]
- 14. Siassi M, Hohenberger W, Lösel F, Weiss M. Quality of life and patient's expectations after closure of a temporary stoma. Int J Colorectal Dis 2008; 23: 1207–1212. [DOI] [PubMed] [Google Scholar]
- 15. Anaraki F, Vafaie M, Behboo R, Maghsoodi N, Esmaeilpour S, Safaee A. Quality of life outcomes in patients living with stoma. Indian J Palliat Care 2012; 18: 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Danielsen AK, Correa‐Marinez A, Angenete E, Skullman S, Haglind E, Rosenberg J; SSORG (Scandinavian Outcomes Research Group) . Early closure of temporary ileostomy – the EASY trial: protocol for a randomised controlled trial. BMJ Open 2011; 1: e000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Danielsen AK, Park J, Jansen JE, Bock D, Skullman S, Wedin A et al Early closure of a temporary ileostomy in patients with rectal cancer: a multicenter randomized controlled trial. Ann Surg 2017; 265: 284–290. [DOI] [PubMed] [Google Scholar]
- 18. Anderson RT, Aaronson NK, Wilkin D. Critical review of the international assessments of health‐related quality of life. Qual Life Res 1993; 2: 369–395. [DOI] [PubMed] [Google Scholar]
- 19. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–483. [PubMed] [Google Scholar]
- 20. Taft C, Karlsson J, Sullivan M. Performance of the Swedish SF‐36 version 2.0. Qual Life Res 2004; 13: 251–256. [DOI] [PubMed] [Google Scholar]
- 21. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–376. [DOI] [PubMed] [Google Scholar]
- 22. Gujral S, Conroy T, Fleissner C, Sezer O, King PM, Avery KN et al; European Organisation for Research and Treatment of Cancer Quality of Life Group. Assessing quality of life in patients with colorectal cancer: an update of the EORTC quality of life questionnaire. Eur J Cancer 2007; 43: 1564–1573. [DOI] [PubMed] [Google Scholar]
- 23. Whistance RN, Conroy T, Chie W, Costantini A, Sezer O, Koller M et al; European Organisation for Research and Treatment of Cancer Quality of Life Group. Clinical and psychometric validation of the EORTC QLQ‐CR29 questionnaire module to assess health‐related quality of life in patients with colorectal cancer. Eur J Cancer 2009; 45: 3017–3026. [DOI] [PubMed] [Google Scholar]
- 24. Krouse RS, Herrinton LJ, Grant M, Wendel CS, Green SB, Mohler MJ et al Health‐related quality of life among long‐term rectal cancer survivors with an ostomy: manifestations by sex. J Clin Oncol 2009; 27: 4664–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ware JE, Kosinski M, Dewey JE. How to Score Version 2 of the SF‐36® Health Survey. QualityMetric: Lincoln, 2000. [Google Scholar]
- 26. Fayers PM , Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A; EORTC Quality of Life Group. The EORTC QLQ‐C30 Scoring Manual (3rd edn). European Organisation for Research and Treatment of Cancer: Brussels, 2001. [Google Scholar]
- 27. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, 2016. [Google Scholar]
- 28. Michelson H, Bolund C, Nilsson B, Brandberg Y. Health‐related quality of life measured by the EORTC QLQ‐C30 – reference values from a large sample of Swedish population. Acta Oncol 2000; 39: 477–484. [DOI] [PubMed] [Google Scholar]
- 29. Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ‐C30 in the general German population. Eur J Cancer 2001; 37: 1345–1351. [DOI] [PubMed] [Google Scholar]
- 30. Neuman HB, Patil S, Fuzesi S, Wong WD, Weiser MR, Guillem JG et al Impact of a temporary stoma on the quality of life of rectal cancer patients undergoing treatment. Ann Surg Oncol 2011; 18: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 31. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health‐related quality‐of‐life scores. J Clin Oncol 1998; 16: 139–144. [DOI] [PubMed] [Google Scholar]
- 32. Danielsen AK, Soerensen EE, Burcharth K, Rosenberg J. Impact of a temporary stoma on patients' everyday lives: feelings of uncertainty while waiting for closure of the stoma. J Clin Nurs 2013; 22: 1343–1352. [DOI] [PubMed] [Google Scholar]
- 33. Siassi M, Weiss M, Hohenberger W, Losel F, Matzel K. Personality rather than clinical variables determines quality of life after major colorectal surgery. Dis Colon Rectum 2009; 52: 662–668. [DOI] [PubMed] [Google Scholar]
- 34. Taylor C, Morgan L. Quality of life following reversal of temporary stoma after rectal cancer treatment. Eur J Oncol Nurs 2011; 15: 59–66. [DOI] [PubMed] [Google Scholar]
- 35. Andersson J, Angenete E, Gellerstedt M, Angeras U, Jess P, Rosenberg J et al Health‐related quality of life after laparoscopic and open surgery for rectal cancer in a randomized trial. Br J Surg 2013; 100: 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]


