Abstract
BACKGROUND
Outcomes for patients with relapsed or refractory acute myeloid leukemia (AML) are poor. Guadecitabine, a next‐generation hypomethylating agent, could be useful in treating such patients.
METHODS
In this multicenter, open‐label, phase 2 dose‐expansion study, AML patients from 10 North American medical centers were first randomized (1:1) to receive subcutaneous guadecitabine at 60 or 90 mg/m2 on 5 consecutive days in each 28‐day cycle (5‐day regimen). Subsequently, another cohort was treated for 10 days with 60 mg/m2 (10‐day regimen).
RESULTS
Between June 15, 2012, and August 19, 2013, 108 patients with previously treated AML consented to enroll in the study, and 103 of these patients were treated; 5 patients did not receive the study treatment. A total of 103 patients were included in the safety and efficacy analyses (24 and 26 patients who were randomly assigned to 60 and 90 mg/m2/d, respectively [5‐day regimen] and 53 patients who were assigned to 60 mg/m2/d [10‐day regimen]). The 90 mg/m2 dose showed no benefit in clinical outcomes in comparison with 60 mg/m2 in the randomized cohort. Composite complete response (CRc) and complete response (CR) rates were higher with the 10‐day regimen versus the 5‐day regimen (CRc, 30.2% vs 16.0%; P = .1061; CR, 18.9% vs 8%; P = .15). Adverse events (grade ≥ 3) were mainly hematologic, with a higher incidence on the 10‐day regimen. Early all‐cause mortality was low and similar between regimens. Twenty patients (8 on the 5‐day regimen and 12 on the 10‐day regimen) were bridged to hematopoietic cell transplantation.
CONCLUSIONS
Guadecitabine has promising clinical activity and an acceptable safety profile and thus warrants further development in this population. Cancer 2018;124:325‐34. © 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society. This is an open access article under the terms of the Creative Commons Attribution NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Keywords: acute myeloid leukemia (AML), guadecitabine, refractory, relapsed, SGI‐110
Short abstract
Outcomes for patients with relapsed or refractory acute myeloid leukemia are poor. Guadecitabine, a next‐generation hypomethylating agent, has promising clinical activity and an acceptable safety profile and warrants further development in this population.
See also pages 242‐4.
INTRODUCTION
Patients with acute myeloid leukemia (AML) who are refractory to or relapse after primary induction therapy have limited treatment options. Unless they achieve a second remission followed by allogeneic hematopoietic cell transplantation (HCT), their prognosis is poor.1, 2, 3
Hypomethylating agents (HMAs) show activity in patients with AML.4, 5, 6 However, the short plasma half‐life of S‐phase–dependent drugs such as decitabine and 5‐azacitidine limits their incorporation into the DNA of replicating leukemia cells. Guadecitabine (SGI‐110 or 2′‐deoxy‐5‐azacytidylyl‐(3′→5′)‐2′‐deoxyguanosine sodium salt), a next‐generation HMA that is resistant to cytidine deaminase, the main enzyme responsible for decitabine degradation, is designed to extend exposure to decitabine, its active metabolite.7, 8, 9 Guadecitabine is a dinucleotide of decitabine and deoxyguanosine linked by a phosphodiester bond. The gradual enzymatic cleavage of this bond results in the release of decitabine over an extended period, and this prolongs its in vivo exposure.
HMAs induce demethylation at low doses and cytotoxicity at high doses.10 We previously reported a phase 1 dose‐escalation study that determined a biologically effective dose (BED) for guadecitabine based on the hypomethylation effect of 60 mg/m2/d for 5 consecutive days in 28‐day cycles.11 A higher dose of 90 mg/m2/d was still well tolerated.11 The daily × 5 schedule is similar to what has been approved for decitabine.12 In addition, a 10‐day regimen for intravenous decitabine, which showed promising activity in single‐arm, single‐center studies,13 was explored with guadecitabine. The objective of this study was to explore the dose‐response relation beyond the BED for the 5‐day regimen and then to explore the safety and efficacy of the BED with the 10‐day regimen.
MATERIALS AND METHODS
This study included patients from the expansion phase of a phase 1/2, open‐label study of guadecitabine in adult patients with AML and myelodysplastic syndrome that was conducted at 10 North American academic medical centers. The trial is registered at ClinicalTrials.gov (NCT01261312). The human investigations were performed after the protocol was approved by the independent ethics committee at each study center. All patients gave written informed consent.
In this report, we present the experience for the phase 2 cohort of previously treated AML patients. Eligible patients were at least 18 years old with a confirmed diagnosis of AML (except for acute promyelocytic leukemia) that was refractory to or relapsed after standard treatment. There was no limit on the number or type of prior treatment regimens except that only 1 cycle of HMA was allowed. Patients whose disease was described as primary refractory had not achieved remission after 2 cycles of their primary induction regimen.
Patients were randomly assigned (1:1) to either 60 or 90 mg/m2/d (daily × 5), and the study was subsequently extended to treat a similar number of patients with 60 mg/m2/d in a 10‐day regimen as previously described for decitabine.13
Guadecitabine was administered subcutaneously on days 1 to 5 in the 5‐day regimen and on days 1 to 5 and on days 8 to 12 in the 10‐day regimen. Patients on the 10‐day regimen were allowed to change to the 5‐day treatment in subsequent cycles according to tolerability and clinical response. Treatment was given every 28 days (with allowances for dose delays or reductions based on tolerability) and was continued until progression or unacceptable toxicity.
Prior therapy (except hydroxyurea, which was permitted for the control of leukocytosis during cycle 1) must have concluded at least 2 weeks before randomization. Recipients of allogeneic HCT were eligible 8 or more weeks after transplantation with recovery from transplant‐related toxicities.
The primary endpoint was the composite complete response (CRc), which was composed of the complete response (CR), the CR without platelet recovery to 100,000/µL, and the CR without neutrophil recovery to 1000/µL (regardless of the platelet count), as defined by the International Working Group criteria in 2003.14 Secondary efficacy endpoints were the time to response, the duration of response (calculated from the day on which it was first observed to the day on which progression was noted), and the overall survival (calculated from the first day of treatment to the day of death or last contact). Complete blood counts and differentials were performed at least weekly for the blast percentage, absolute neutrophil count, platelet count, and hemoglobin. If a response was noted on blood counts, bone marrow aspiration was performed immediately to confirm the response, and it was repeated every 2 cycles until progression.
Whole blood samples for methylation assays were collected weekly during the first cycle of treatment. Guadecitabine effects on global DNA methylation were measured by a quantitative bisulfite pyrosequencing method for long interspersed nuclear element‐1 (LINE‐1) methylation analysis as previously reported.15 The primer sequences, computation, methodology, and assay conditions for LINE‐1 pyrosequencing were previously published.11
Patient‐reported and investigator‐observed adverse events (through physical examinations, clinical hematology and laboratory tests, and electrocardiograms) were collected and categorized according to the Common Terminology Criteria for Adverse Events (version 4.0) criteria.
All patients who received guadecitabine were included in the analyses on an as‐treated basis so that patients who did not receive any treatment with guadecitabine were excluded. Outcomes from patients randomly assigned to the 60 and 90 mg/m2 cohorts were compared. Outcomes from the combined 5‐day regimens were also compared with those achieved with the 10‐day regimen.
Data analyses in this exploratory phase 2 study are descriptive unless otherwise specified. The initial sample size of each phase 2 study cohort, 30 patients, was chosen so that if no responses were observed, it would be concluded with 95% confidence that the response rate was less than 10%. The safety review committee of the study allowed expansion to 50 patients per cohort for further assessment of efficacy. The 95% confidence intervals are based on binomial distributions. Ad hoc significance analyses using Fisher's exact test are reported for features exhibiting clinically meaningful differences between treatment groups. Mean maximum LINE‐1 demethylation was compared between patients categorized as responders and nonresponders with the Mann‐Whitney test. Survival data were displayed with Kaplan‐Meier estimates and were compared between the groups with a log‐rank test. We used SAS 9.3 for the statistical analysis.
RESULTS
Between June 15, 2012, and August 19, 2013, 108 patients with previously treated AML consented to enroll. Five patients did not receive treatment (1 patient died, 2 had rapid disease progression, 1 pursued alternative therapy, and 1 declined treatment). All 103 treated patients received at least 1 dose of guadecitabine and were included in the analysis (24 and 26 patients who were randomly assigned to 60 and 90 mg/m2/d [daily × 5], respectively, and 53 who were assigned to 60 mg/m2/d [10‐day regimen]). Ten patients were excluded from pharmacodynamic analyses because they did not have both baseline and posttreatment samples. At the time of the database lock on December 4, 2015, 16 patients (8 each on the 5‐ and 10‐day regimens) were alive, and 2 patients on the 5‐day regimen were continuing treatment. The median follow‐up was 29.2 months (95% confidence interval, 25‐32 months).
The baseline characteristics for the randomized 60 and 90 mg/m2 daily × 5 cohorts were generally well balanced (Table 1). The first‐line therapy was standard intensive induction chemotherapy (intermediate/high‐dose cytosine arabinoside and an anthracycline with or without a third drug) for most patients (86 patients or 83%). Eleven patients (11%) received prior HMA therapy; 4 received more than 1 cycle.
Table 1.
Demographic and Baseline Characteristics
| Characteristic | 5‐d Regimens | 10‐d Regimen at 60 mg/m2 (n = 53) | Total (n = 103) | ||
|---|---|---|---|---|---|
| 60 mg/m2 (n = 24) | 90 mg/m2 (n = 26) | Total (n = 50) | |||
| Age, median (range), y | 58 (22‐77) | 65 (30‐81) | 62 (22‐81) | 57 (29‐82) | 60 (22‐82) |
| Sex, No. (%) | |||||
| Male | 15 (63) | 20 (77) | 35 (70) | 27 (51) | 62 (60) |
| Female | 9 (38) | 6 (23) | 15 (30) | 26 (49) | 41 (40) |
| Race, No. (%) | |||||
| White | 18 (75) | 21 (81) | 39 (78) | 44 (83) | 83 (81) |
| Black or African American | 4 (17) | 0 | 4 (8) | 5 (9) | 9 (9) |
| Asian | 2 (8) | 4 (15) | 6 (12) | 2 (4) | 8 (8) |
| Pacific Islander | 0 | 0 | 0 | 1 (2) | 1 (<1) |
| Other/unknown | 0 | 1 (4) | 1 (2) | 1 (2) | 2 (2) |
| ECOG, No. (%) | |||||
| 0 | 2 (8) | 3 (12) | 5 (10) | 9 (17) | 14 (14) |
| 1 | 20 (83) | 20 (77) | 40 (80) | 35 (66) | 75 (73) |
| 2 | 2 (8) | 3 (12) | 5 (10) | 9 (17) | 14 (14) |
| No. of prior induction regimens, No. (%) | |||||
| Unknowna | 0 | 1 (2) | 0 | 2 (4) | 3 (3) |
| 1 | 3 (12) | 7 (27) | 10 (20) | 17 (32) | 27 (26) |
| 2 | 8 (33) | 6 (23) | 14 (28) | 17 (32) | 31 (30) |
| 3‐5 | 13 (54) | 9 (35) | 22 (44) | 17 (32) | 39 (38) |
| >5 | 0 | 2 (8) | 2 (4) | 0 | 2 (2) |
| No. of prior induction regimens | |||||
| Mean (SD) | 2.7 (1.1) | 2.9 (2.1) | 2.8 (1.7) | 2.3 (1.5) | 2.5 (1.6) |
| Median (range) | 2.5 (1‐5) | 2.0 (1‐10) | 2.0 (1‐10) | 2.0 (1‐7) | 2.0 (1‐10) |
| >1 cycle of prior HMA therapy, No. (%) | 0 | 1 (4) | 1 (2) | 3 (6) | 4 (4) |
| Disease status, No. (%) | |||||
| Primary refractory | 8 (33) | 12 (46) | 20 (40) | 28 (53) | 48 (47) |
| Relapse | 16 (66) | 14 (54) | 30 (60) | 25 (47) | 55 (53) |
| First relapse | 3 (13) | 2 (8) | 5 (10) | 9 (17) | 14 (14) |
| Later relapseb | 13 (54) | 12 (46) | 25 (50) | 16 (30) | 41 (40) |
| Length of 1st remission, No. (%) | |||||
| >1 y | 6 (25) | 7 (27) | 13 (26) | 9 (17) | 22 (21) |
| ≤1 y | 10 (42) | 7 (27) | 17 (34) | 16 (30) | 33 (32) |
| Prior HCT, No. (%) | 5 (21) | 5 (19) | 10 (20) | 9 (17) | 19 (18) |
| Cytogenetic risk, No. (%) | |||||
| Favorable | 0 | 0 | 0 | 0 | 0 |
| Intermediate | 12 (50) | 14 (54) | 26 (52) | 26 (49) | 52 (50) |
| Poor | 9 (38) | 11 (42) | 20 (40) | 22 (42) | 42 (42) |
| Unknown | 3 (13) | 1 (4) | 4 (8) | 5 (9) | 9 (9) |
| BM blast, % | |||||
| Mean (SD) | 40.3 (27.3) | 38.6 (29.0) | 39.4 (27.9) | 38.7 (25.1) | 39.1 (26.4) |
| Median (range) | 34.0 (9‐93) | 35.5 (2‐94) | 35.0 (2‐94) | 32.0 (4‐95) | 33.0 (2‐95) |
| PB blast, % | |||||
| Mean (SD) | 22.8 (30.9) | 25.1 (26.6) | 24.0 (28.5) | 23.0 (30.7) | 23.5 (29.5) |
| Median (range) | 5 (0‐95) | 14 (0‐81) | 10.0 (0‐95) | 2.5 (0‐99) | 6.0 (0‐99) |
| WBC, × 109/L | |||||
| Mean (SD) | 3 (3.9) | 3.9 (4.5) | 3.5 (4.2) | 5.8 (11.8) | 4.7 (9.0) |
| Median (range) | 1.7 (0.3‐18.7) | 2.1 (0.3‐18.6) | 1.7 (0.3‐18.7) | 2.1 (0.2‐75.5) | 1.8 (0.2‐75.5) |
Abbreviations: BM, bone marrow; d, day; ECOG, Eastern Cooperative Oncology Group; HCT, hematopoietic cell transplantation; HMA, hypomethylating agent; PB, peripheral blood; SD, standard deviation; WBC, white blood cell.
One patient in the 10‐day group with proliferative secondary acute myeloid leukemia received only a single dose of low‐dose cytosine arabinoside before enrollment.
This category includes patients refractory to 1 or more regimens during the first relapse before guadecitabine treatment.
The median number of guadecitabine cycles was 3 (range, 1‐29). Thirteen patients who had been assigned to the 10‐day regimen (25%) changed to the 5‐day treatment in subsequent cycles after a median of 2 cycles (range, 1‐6).
Discontinuations of study treatment were due to disease progression (54 patients or 50%), withdrawal to undergo HCT (17 patients or 16%), death (17 patients or 16%), the investigator's decision (6 patients or 6%), or an adverse event (4 patients or 4%). Eight patients on the 5‐day regimen received HCT after 1 to 8 cycles, and 12 on the 10‐day regimen received HCT after 1 to 4 cycles. Three patients resumed guadecitabine treatment after HCT. One patient from each dose group in the 5‐day regimen was still receiving treatment with guadecitabine at the time of the database lock.
Pharmacodynamics
Of the 103 treated patients, 93 (90%) provided sufficient samples for guadecitabine effects on LINE‐1 methylation to be assessed: 22 treated with 60 mg/m2 (daily × 5), 21 treated with 90 mg/m2 (daily × 5), and 50 treated with 60 mg/m2 on the 10‐day regimen. Figure 1A shows the kinetics of LINE‐1 demethylation. The extent and duration of LINE‐1 demethylation were similar between doses in the 5‐day regimen. The extent of the mean maximum LINE‐1 methylation percentage change from the baseline was better for the 10‐day regimen and was sustained until day 15. Overall, the mean percentage change from the baseline in maximum LINE‐1 methylation was significantly better in responders versus nonresponders (P = .0002; Fig. 1B).
Figure 1.
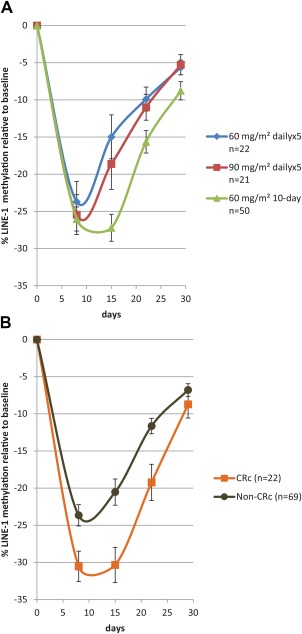
Mean LINE‐1 demethylation by (A) dose and schedule and (B) clinical response. The mean LINE‐1 demethylation is compared with the baseline during the first cycle of therapy for the 3 dose and schedule cohorts. The data are presented as means; the error bars are the standard errors. CRc indicates composite complete response; LINE‐1, long interspersed nuclear element‐1.
Clinical Response and Overall Survival
Of the 103 patients, 24 (23.3%; 95% confidence interval, 15.5%‐32.7%) responded to therapy (Table 2). Response rates were similar between the daily × 5 doses, so the cohorts were combined for comparison with the 10‐day cohort. Higher CRc and CR rates were observed with the 10‐day regimen versus the combined 5‐day regimen. The median time to the initial response was 82 days (range, 27‐295 days) for the 5‐day regimen and 42.5 days (range, 26‐143 days) for the 10‐day regimen (P = .102). Similarly, CRs were achieved significantly more rapidly on the 10‐day regimen (median, 77 days; range, 38‐172 days) than the 5‐day regimen (median, 236 days; range, 64‐987 days; P ≤ .04). The median duration of response was 444.5 days (range, 15‐880 days) for the 5‐day regimen and 233 days (range, 42‐898 days) for the 10‐day regimen. The difference in the duration of response was not statistically significant. The 10‐day regimen showed better activity in patients with poor‐risk features. Four of the 20 patients (20%) in the 5‐day group versus 11 of the 28 patients (39%) in the 10‐day group with primary refractory disease had a response. Among patients with poor cytogenetic risk, only 1 of 20 (5%) achieved a response in the 5‐day group, whereas 7 of 22 (32%) did in the 10‐day group (P = .0471). Among patients with prior HCT, 1 of 10 (10%) achieved a response in the 5‐day group, and 4 of 9 (44%) achieved a response in the 10‐day group (P = .1409). Six patients had mutations of TP53: 1 in the 5‐day regimen who did not respond to treatment and 5 in the 10‐day group, 2 of whom responded (CRc rate, 40%).
Table 2.
Best Responses
| Response Categorya | Response Rate | P c | ||||
|---|---|---|---|---|---|---|
| 5‐d Regimensb | 10‐d Regimen at 60 mg/m2 (n = 53) | Total (n = 103) | ||||
| 60 mg/m2 (n = 24) | 90 mg/m2 (n = 26) | Total (n = 50) | ||||
| CR, No. (%) | 2 (8.3) | 2 (7.7) | 4 (8.0) | 10 (18.9) | 14 (13.6) | .1515d |
| CRi, No. (%) | 1 (4.2) | 3 (11.5) | 4 (8.0) | 2 (3.8) | 6 (5.8) | NS |
| CRp, No. (%) | 0 | 0 | 0 | 4 (7.5) | 4 (3.9) | NS |
| CRc rate (CR + CRi + CRp) | ||||||
| No. (%) | 3 (12.5) | 5 (19.2) | 8 (16.0) | 16 (30.2) | 24 (23.3) | .1061d |
| 95% CI, % | 2.7‐32.4 | 6.6‐39.4 | 7.2‐29.1 | 18.3‐44.3 | 15.5‐32.7 | |
Abbreviations: CI, confidence interval; CR, complete response; CRc, composite complete response; CRi, complete response with incomplete blood count recovery; CRp, complete response with incomplete platelet recovery; d, day; NS, not significant.
Taken from the 2003 International Working Group acute myeloid leukemia response criteria.14
P for CR (60 vs 90 mg/m2), 1.00; P for CRc (60 vs 90 mg/m2), .704.
Comparing the 5‐day regimens (total) and the 10‐day regimen.
Fisher's exact 2‐sided Pr ≤ P.
HCT was performed in remission for 3 of the 8 patients undergoing transplantation in the 5‐day group and in 11 of the 12 patients undergoing transplantation in the 10‐day group. Patients who underwent HCT were not censored from the survival analysis.
The median overall survival ranged from 5.0 (5‐day regimen at the 90 mg/m2 dose) to 7.1 months (10‐day regimen). None of these survival differences were significant. Patients who achieved a response had significantly improved survival in comparison with those with other outcomes (median, not reached vs 5.6 months; P < .0001; Fig. 2C). Patients undergoing HCT after guadecitabine had improved survival (median, not reached) in comparison with those who did not (median, 5.6 mo; P < .0001; Fig. 3A). Patients who achieved a response after guadecitabine treatment had improved survival, regardless of HCT. No difference in survival was observed between responding patients who underwent HCT (median survival, not reached) and those who did not (median survival, not reached; P = .8768; Fig. 3B). Baseline cytogenetics were predictive of outcome with a median of 5.4 months for poor‐risk and 8.3 months for intermediate‐risk patients (P < .0018; Fig. 3C). The 1‐year survival rates were 14% and 39% for the poor‐risk and intermediate‐risk groups, respectively (P = .0064). The 10‐day regimen had higher rates of both response and transplantation but no significant improvement in overall survival. Notably, 7 of 16 responders in the 10‐day group (5 of whom underwent transplantation after a response) had poor cytogenetics; such patients are known to have poor overall outcomes even with HCT. Only 1 of the patients who responded in the 5‐day group had poor‐risk cytogenetics. The 1‐ and 2‐year survival rates of the entire study population were 28% and 19%, respectively.
Figure 2.
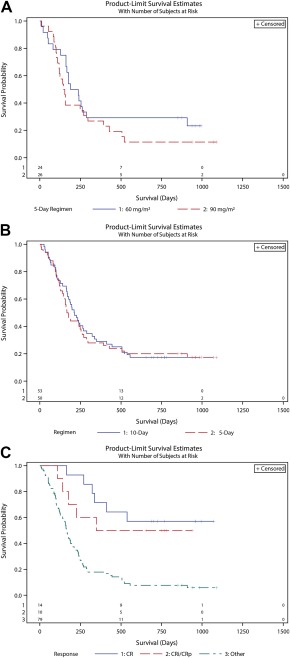
(A) Overall survival with the 60 and 90 mg/m2 5‐day regimens. Kaplan‐Meier estimates are presented for overall survival with the randomized 2 doses of the 5‐day schedule. Patients were not censored for hematopoietic cell transplantation. The median survival was 7.1 mo with 60 mg/m2 and 5.0 mo with 90 mg/m2 (P = .246). (B) Overall survival with the 5‐ and 10‐day regimens. Kaplan‐Meier estimates are presented for the overall survival of patients on the 5‐day regimens (combined arms) and the 10‐day regimen. Patients were not censored for hematopoietic cell transplantation. The median survival was 5.7 mo with the 5‐day regimens and 7.1 mo with the 10‐day regimen (P = .7783). (C) Overall survival by the response to guadecitabine. Kaplan‐Meier estimates are presented for all patients treated with guadecitabine according to their response: composite CR, CRi or CRp, or all other outcomes (P < .0001). CR indicates complete response; CRi, complete response with incomplete blood count recovery; CRp, complete response with incomplete platelet recovery.
Figure 3.
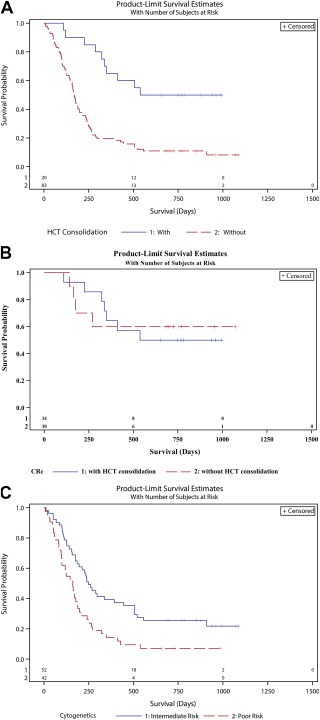
(A) Overall survival by HCT as subsequent therapy. Kaplan‐Meier estimates are presented for the overall survival of patients who were bridged to allogeneic HCT and those who were not. (P < .0001). (B) Overall survival of patients with a CRc by subsequent HCT. (C) Overall survival by baseline cytogenetic risk. Kaplan‐Meier estimates are presented for the overall survival of patients with intermediate‐risk cytogenetics and patients with poor cytogenetics (P < .0018). CRc indicates composite complete response; HCT, hematopoietic cell transplantation.
Adverse Events
The safety population consisted of all 103 treated patients: 50 in the 5‐day group and 53 in the 10‐day group. There was no difference in treatment exposure by the number of cycles between the 5‐day regimen (mean, 3.8 cycles; median, 3 cycles) and the 10‐day regimen (mean, 3.5 cycles; median, 3 cycles).
Myelosuppression, neutropenic fever, and infection are toxicities expected from guadecitabine, but they are also known complications of AML. Table 3 shows grade 3 or higher adverse events that occurred in ≥10% of the overall patient population, regardless of the relation to treatment. Table 4 shows guadecitabine‐related adverse events, regardless of grade, that occurred in ≥5% of the overall patient population. Injection‐site events were all grade 1 or 2. There was an increased incidence of grade 3 or higher adverse events of myelosuppression (febrile neutropenia, thrombocytopenia, and anemia) and infection (pneumonia and sepsis) with the 10‐day regimen versus the 5‐day regimen, Despite the increased incidence of grade 3 or higher adverse events with the 10‐day regimen, the rates of mortality and discontinuation for toxicity were similar to those with the 5‐day regimen, and only a few patients were changed from the 10‐day schedule to the 5‐day schedule. Four patients (2 in the 5‐day 90 mg/m2 group and 2 in the 10‐day group) experienced adverse events that were the primary reason for treatment discontinuation. All‐cause mortality at 30 and 60 days is reported in Table 5, and the results were similar with the 5‐ and 10‐day treatment regimens.
Table 3.
Grade 3 or Higher Adverse Events by Decreasing Incidence (at Least 10% of Total)
| No. of Subjects (%) | ||||||
|---|---|---|---|---|---|---|
| 5‐d Regimens | ||||||
| Adverse Event | 60 mg/m2 (n = 24) | 90 mg/m2 (n = 26) | Total (n = 50) | 10‐d Regimen at 60 mg/m2 (n = 53) | Total (n = 103) | P a |
| Any event | 20 (83) | 25 (96) | 45 (90) | 50 (94) | 95 (92) | |
| Febrile neutropenia | 10 (42) | 17 (65) | 27 (54) | 35 (66) | 62 (60) | .2296 |
| Pneumonia | 4 (17) | 9 (35) | 13 (26) | 24 (45) | 37 (36) | .0669 |
| Thrombocytopenia | 5 (21) | 5 (19) | 10 (20) | 27 (51) | 37 (36) | .0019 |
| Anemia | 5 (21) | 4 (15) | 9 (18) | 23 (43) | 32 (31) | .0123 |
| Neutropenia | 2 (8) | 3 (12) | 5 (10) | 15 (28) | 20 (19) | .0248 |
| Sepsis | 3 (13) | 2 (8) | 5 (10) | 11 (21) | 16 (16) | .176 |
| Hypokalemia | 5 (21) | 3 (12) | 8 (16) | 6 (11) | 14 (14) | >.5 |
| Bacteremia | 3 (13) | 3 (12) | 6 (12) | 6 (11) | 12 (12) | |
| Cellulitis | 4 (17) | 0 | 4 (8) | 6 (11) | 10 (10) | |
| Leukopenia | 3 (13) | 3 (12) | 6 (12) | 4 (8) | 10 (10) | |
Abbreviation: d, day.
Comparing the 5‐day regimens (total) and the 10‐day regimen.
Table 4.
Related Adverse Events of Any Grade by Decreasing Incidence (at Least 5% of Total)
| No. of Subjects (%) | ||||||
|---|---|---|---|---|---|---|
| 5‐d Regimens | ||||||
| Adverse Event | 60 mg/m2 (n = 24) | 90 mg/m2 (n = 26) | Total (n = 50) | 10‐d Regimen at 60 mg/m2 (n = 53) | Total (n = 103) | P a |
| Any related event | 16 (67) | 21 (81) | 37 (74) | 48 (91) | 85 (83) | |
| Injection‐site eventsb | 8 (33) | 10 (38) | 18 (36) | 28 (53) | 46 (45) | .113 |
| Fatigue | 3 (13) | 7 (27) | 10 (20) | 21 (40) | 31 (30) | .034 |
| Anemia | 3 (13) | 5 (19) | 8 (16) | 22 (42) | 30 (29) | .0051 |
| Thrombocytopenia | 5 (21) | 3 (12) | 8 (16) | 22 (42) | 30 (29) | .0051 |
| Diarrhea | 3 (13) | 5 (19) | 8 (16) | 20 (38) | 28 (27) | .009 |
| Nausea | 2 (8) | 3 (12) | 5 (10 | 19 (36) | 24 (23) | .0023 |
| Constipation | 1 (4) | 6 (23) | 7 (14) | 16 (30) | 23 (22) | .06 |
| Neutropenia | 2 (8) | 3 (12) | 5 (10) | 13 (25) | 18 (18) | .0698 |
| Decreased appetite | 1 (4) | 1 (4) | 2 (4) | 14 (26) | 16 (16) | .001 |
| Febrile neutropenia | 1 (4) | 3 (12) | 4 (8) | 11 (21) | 15 (15) | .094 |
| Vomiting | 0 | 0 | 0 | 13 (25) | 13 (13) | .00011 |
| Stomatitis | 0 | 0 | 0 | 12 (23) | 12 (12) | .00025 |
| Asthenia | 0 | 2 (8) | 2 (4) | 6 (11) | 8 (8) | |
| Epistaxis | 0 | 3 (12) | 3 (6) | 4 (8) | 7 (7) | |
| Leukopenia | 2 (8) | 4 (15) | 6 (12) | 1 (2) | 7 (7) | .055 |
| Headache | 1 (4) | 1 (4) | 2 (4) | 4 (8) | 6 (6) | |
| Hypomagnesemia | 0 | 2 (8) | 2 (4) | 4 (8) | 6 (6) | |
| Contusion | 0 | 1 (4) | 1 (2) | 4 (8) | 5 (5) | |
| Dysgeusia | 1 (4) | 0 | 1 (2) | 4 (8) | 5 (5) | |
| Dyspnea | 0 | 3 (12) | 3 (6) | 2 (4) | 5 (5) | |
| Weight decrease | 0 | 0 | 0 | 5 (9) | 5 (5) | |
Abbreviation: d, day.
Comparing the 5‐day regimens (total) and the 10‐day regimen.
Injection‐site events included the following: injection‐site erythema, hematoma, hemorrhage, infection, inflammation, nodule, pain, reaction, and swelling.
Table 5.
All‐Cause Early Mortality
| Event Rate, No. (%) | |||||
|---|---|---|---|---|---|
| 5‐d Regimens | |||||
| Early Mortality | 60 mg/m2 (n = 24) | 90 mg/m2 (n = 26) | Total (n = 50) | 10‐d Regimen at 60 mg/m2 (n = 53) | Total (n = 103) |
| 30 d | 2 (8.3) | 1 (3.8) | 3 (6) | 1 (1.9) | 4 (3.9) |
| 60 d | 4 (16.7) | 2 (7.7) | 6 (12) | 6 (11.3) | 12 (11.7) |
Abbreviation: d, day.
DISCUSSION
Trials in AML have failed to date to show a survival benefit in the salvage setting.3, 16, 17 We report here the first results of different doses and schedules of guadecitabine, a next‐generation HMA, in a multicenter study of 103 patients. The CRc rate of 30%, the median overall survival of 7.1 months, and the 30‐day mortality rate of 1.9% for patients treated with the 10‐day regimen compare favorably with published data. In a study by Roboz et al3 comparing elacytarabine with physician choice in a very similar patient population, the control group had a 21% CRc rate, a median overall survival of 3.3 months, and a 30‐day mortality rate of 14.8%. The increased efficacy observed with the 10‐day regimen (Table 2) is further supported by increased response rates in poor‐prognosis patients, such as those with poor cytogenetics, those with prior HCT, and those refractory to induction. Although the 10‐day regimen was associated with a higher incidence of grade 3 or higher adverse events, there was no increase in all‐cause mortality at 30 and 60 days. Overall, early mortality was lower than that observed in other studies of AML salvage therapy.3, 16
Initial intensive therapy using a 10‐day schedule of guadecitabine, followed by 5‐day cycles, appeared to be effective and slightly faster in inducing responses than the 5‐day schedule. This may have resulted, in part, from the deeper and more prolonged LINE‐1 demethylation observed with the 10‐day schedule (Fig. 1B). Similarly to our previous observations in the guadecitabine phase 1 study, responders had significantly more potent demethylation than nonresponders.11 Also, the mean maximum demethylation of approximately 25% or higher that was noted with guadecitabine in this study is more than what was observed in previously published LINE‐1 demethylation data for azacitidine and decitabine (approximately 7% and 18%, respectively).18, 19 Unlike cytotoxic chemotherapy, where treatment is usually stopped after a few cycles in patients with a response, treatment with HMAs can and should be continued until overt progression or unacceptable toxicity because responses occur late and disease relapse can be rapid upon discontinuation.20 Higher response rates allow more patients to successfully bridge to transplantation, which may further improve long‐term survival and cure rates for a subset of patients with heavily pretreated AML. Previous studies have shown high response rates in older patients with newly diagnosed AML with repeated cycles of 10‐day decitabine.13, 21, 22 In the study by Ritchie et al,22 relapsed/refractory patients were also studied, with a CR rate of 15.7% and a median survival of 5.9 months. There are no published studies comparing 5‐ and 10‐day schedules of decitabine.
In conclusion, the results of this phase 2 study support the initiation of the ongoing phase 3 trial (ASTRAL‐2), in which approximately 400 relapsed/refractory AML patients are being randomized to either guadecitabine administered for 10‐day cycles for up to 2 cycles followed by ongoing 5‐day cycles or the physician's choice of intensive salvage chemotherapy, low‐intensity therapy, or best supportive care (ClinicalTrials.gov number NCT02920008).
FUNDING SUPPORT
This study was sponsored by Astex Pharmaceuticals. Funding was also provided by Stand Up to Cancer.
CONFLICT OF INTEREST DISCLOSURES
Gail J. Roboz has served as a consultant for AbbVie, Agios Pharmaceuticals, Amgen, Amphivena Therapeutics, Astellas Pharma, Astex Pharmaceuticals, AstraZeneca, Array BioPharma, Celator Pharmaceuticals, Celgene, Clovis Oncology, CTI BioPharma, Genoptix, Immune Pharmaceuticals, Janssen Pharmaceutica, Jazz Pharmaceuticals, Orsenix, Juno Therapeutics, MEI Pharma, MedImmune, Novartis, Onconova Therapeutics, Pfizer, Roche/Genentech, and Sunesis Pharmaceuticals and has received research support from Cellectis. Hagop M. Kantarjian has received institutional research funds from Amgen, Ariad, Astex, Bristol‐Myers Squibb, Novartis, and Pfizer and honoraria from AbbVie, Actinium, Amgen, Ariad, Bristol‐Myers Squibb, ImmunoGen, Orsinex, and Pfizer. Karen W. L. Yee has participated in advisory board meetings for Celgene, Novartis, Hoffman–La Roche, Tolero Pharmaceuticals, Otsuka Pharmaceuticals, and Pfizer and has received research funding from OncoEthix, Merck, Agensys, Hoffmann–La Roche/Genentech, Astex Pharmaceuticals, and GlaxoSmithKline. Patricia L. Kropf has served as a consultant for Celgene and Takeda. Casey L. O'Connell has participated in a scientific advisory board for Astex Pharmaceuticals. Elizabeth A. Griffiths has participated in advisory board meetings for Celgene, Pfizer, Alexion Pharmaceuticals, and Novartis; has received honoraria from Alexion Pharmaceuticals and Celgene for lecturing; and has received research funding from Astex Pharmaceuticals and Celgene. Wendy Stock has served on advisory boards for Seattle Genetics, Pfizer, and Amgen. Ellen K. Ritchie reports participation in an advisory board and consulting for Novartis, participation in an advisory board and a speakers' bureau for Incyte, and participation in a speakers' bureau for Celgene. David Rizzieri is on a speakers' bureau supported by Celgene, Incyte, Gilead, and Seattle Genetics and is on advisory boards for Novartis, Kite, Gilead, Celgene, Seattle Genetics, Spectrum, Ariad, Astellas, Amgen, Pfizer, Agios, and Teva. Yong Hao, James N. Lowder, and Mohammad Azab are employees of Astex Pharmaceuticals. Jean‐Pierre J. Issa has received research funding from Astex Pharmaceuticals and is a consultant to Astex and Teva; he has served as an advisory board member for Janssen and GlaxoSmithKline.
AUTHOR CONTRIBUTIONS
Gail J. Roboz: Conceptualization, methodology, supervision, project administration, visualization, investigation, resources, writing–original draft, and writing–review and editing. Hagop M. Kantarjian: Conceptualization, methodology, supervision, project administration, visualization, investigation, resources, writing–original draft, and writing–review and editing. Karen W. L. Yee: Investigation, resources, and writing–review and editing. Patricia L. Kropf: Investigation, resources, and writing–review and editing. Casey L. O'Connell: Investigation, resources, and writing–review and editing. Elizabeth A. Griffiths: Investigation, resources, and writing–review and editing. Wendy Stock: Investigation, resources, and writing–review and editing. Naval G. Daver: Investigation, resources, and writing–review and editing. Elias Jabbour: Investigation, resources, and writing–review and editing. Ellen K. Ritchie: Investigation, resources, and writing–review and editing. Katherine J. Walsh: Investigation, resources, and writing–review and editing. David Rizzieri: Investigation, resources, and writing–review and editing. Scott D. Lunin: Investigation, resources, and writing–review and editing. Tania Curio: Resources and writing–review and editing. Woonbok Chung: Validation, formal analysis, data curation, resources, and writing–review and editing. Yong Hao: Formal analysis, visualization, data curation, resources, and writing–review and editing. James N. Lowder: Formal analysis, supervision, project administration, visualization, resources, writing–original draft, and writing–review and editing. Mohammad Azab: Conceptualization, methodology, funding acquisition, formal analysis, supervision, project administration, visualization, resources, and writing–review and editing. Jean‐Pierre J. Issa: Conceptualization, methodology, supervision, project administration, visualization, investigation, resources, and writing–review and editing.
See editorial on pages 242‐4, this issue.
The first 2 authors contributed equally to this article.
We thank Sherry Stinn, Renee Hansen, and Laurie Haynes for medical writing and administrative support; Sue Naim for overall management of the study; Xiang Yao Su for statistical support; and Pietro Taverna for assistance with the long interspersed nuclear element‐1 analysis. Sherry Stinn is a medical writer supported by funding from Astex Pharmaceuticals; Renee Hansen, Laurie Haynes, Sue Naim, and Xiang Yao Su are employees of Astex Pharmaceuticals; and Pietro Taverna is a former employee of Astex Pharmaceuticals.
REFERENCES
- 1. Mangan JK, Luger SM. Salvage therapy for relapsed or refractory acute myeloid leukemia. Ther Adv Hematol. 2011;2:73‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramos NR, Mo CC, Karp JE, Hourigan CS. Current approaches in the treatment of relapsed and refractory acute myeloid leukemia. J Clin Med. 2015;4:665‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roboz G, Rosenbalt T, Arellano M, et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol. 2014;32:1919‐1926. [DOI] [PubMed] [Google Scholar]
- 4. Issa JP, Garcia‐Manero G, Giles FJ, et al. Phase 1 study of low‐dose prolonged exposure schedules of the hypomethylating agent 5‐aza‐2′‐deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004;103:1635‐1640. [DOI] [PubMed] [Google Scholar]
- 5. Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, #open |label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low‐dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670‐2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fenaux P, Mufti GJ, Hellstrom‐Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562‐569. [DOI] [PubMed] [Google Scholar]
- 7. Griffiths EA, Choy G, Redkar S, Taverna P, Azab M, Karpf AR. SGI‐110: DNA methyltransferase inhibitor oncolytic. Drugs Future. 2013;38:535‐543. [PMC free article] [PubMed] [Google Scholar]
- 8. Chuang JC, Warner SL, Vollmer D, et al. S110, a 5‐aza‐2′‐deoxycytidine–containing dinucleotide, is an effective DNA methylation inhibitor in vivo and can reduce tumor growth. Mol Cancer Ther. 2010;9:1443‐1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lowder JN, Taverna P, Issa JPA. Will next generation agents deliver on the promise of epigenetic hypomethylation therapy? Epigenomics. 2015;7:1083‐1088. [DOI] [PubMed] [Google Scholar]
- 10. Kantarjian HM, Issa JP. Decitabine dosing schedules. Semin Hematol. 2005;42(3 suppl 2):S17‐S22. [DOI] [PubMed] [Google Scholar]
- 11. Issa JP, Roboz G, Rizzieri D, et al. Safety and tolerability of guadecitabine (SGI‐110) in patients with myelodysplastic syndrome and acute myeloid leukaemia: a multicentre, randomised, dose‐escalation phase 1 study. Lancet Oncol. 2015;16:1099‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steensma DP, Baer MR, Slack JL, et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the Alternative Dosing for Outpatient Treatment (ADOPT) trial. J Clin Oncol. 2009;27:3242‐3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blum W, Garzon R, Kisovic RB, et al. Clinical response and miR‐29b predictive significance in older AML patients treated with a 10‐day schedule of decitabine. Proc Natl Acad Sci U S A. 2010;107:7473‐7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, #Treatment |Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642‐4649. [DOI] [PubMed] [Google Scholar]
- 15. Yang AS, Estecio MRH, Doshi K, Kondo Y, Tahara EH, Issa JP. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Faderl S, Wetzler M, Rizzieri D, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I trial. J Clin Oncol. 2012;30:2492‐2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karanes C, Kopecky KJ, Head DR, et al. A phase III comparison of high dose ARA‐C (HIDAC) versus HIDAC plus mitoxantrone in the treatment of first relapsed or refractory acute myeloid leukemia Southwest Oncology Group Study. Leuk Res. 1999;23:787‐794. [DOI] [PubMed] [Google Scholar]
- 18. Bernstein I, Byun H, Mohrbacher A, et al. A phase 1 biological study of azacitidine (VidazaTM) to determine the optimal dose to inhibit DNA methylation. Epigenetics. 2010;5:750‐757. [DOI] [PubMed] [Google Scholar]
- 19. Kantarjian H, Oki Y, Garcia‐Manero G, et al. Results of a randomized study of 3 schedules of low dose decitabine in higher risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52‐57. [DOI] [PubMed] [Google Scholar]
- 20. Cabrero M, Jabbour E, Ravandi F, et al. Discontinuation of hypomethylating agent therapy in patients with myelodysplastic syndromes or acute myelogenous leukemia in complete remission or partial response: retrospective analysis of survival after long‐term follow‐up. Leuk Res. 2015;39:520‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhatnagar B, Duong VH, Gourdin TS, et al. Ten‐day decitabine as initial therapy for newly diagnosed patients with acute myeloid leukemia unfit for intensive chemotherapy. Leuk Lymphoma. 2014;55:1533‐1537. [DOI] [PubMed] [Google Scholar]
- 22. Ritchie EK, Feldman EJ, Christos PJ, et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk Lymphoma. 2013;54:2003‐2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


