Abstract
The C−H amination of benzene derivatives was achieved using DDQ as photocatalyst and BocNH2 as the amine source under aerobic conditions and visible light irradiation. Electron‐deficient and electron‐rich benzenes react as substrates with moderate to good product yields. The amine scope of the reaction comprises Boc‐amine, carbamates, pyrazoles, sulfonimides and urea. Preliminary mechanistic investigations indicate arene oxidation by the triplet of DDQ to radical cations with different electrophilicity and a charge transfer complex between the amine and DDQ as intermediate of the reaction.
Keywords: C−H amination, charge-transfer complexes, electron-deficient arenes, heterocycles, photocatalysis
The transformation of aromatic C−H into C−N bonds is important in synthetic organic chemistry as many biologically active target compounds1 or fine chemicals2 contain amine functional groups. A well‐established arsenal of C−N bond forming reactions by cross‐coupling of aryl halides and amines in the presence of a transition‐metal catalyst have been developed.3 Transition‐metal‐catalyzed direct C−H activation4 allow the functionalization of complex molecules without the need of C−X bonds.5 However, typically a directing group is required.6
Visible‐light‐mediated photocatalysis has emerged as a mild and useful tool for the functionalization of organic molecules.7 The visible‐light mediated direct C−H amination of aromatic compounds was reported by Nicewicz in 2015.8 Excellent product yields and good regioselectivity were achieved using an acridinium salt as catalyst (cat. A in Scheme 1) and TEMPO (2,2,6,6‐tetramethylpiperidinyloxyl) as a co‐catalyst under aerobic conditions. However, the impressive C−H amination protocol requires electron‐rich aromatic compounds.9 Electron‐deficient aromatic rings or simple benzene (1 a) are not within the substrate scope, due to the high oxidation potential of benzene (2.48 V vs. SCE)10 exceeding the estimated excited state oxidation power of the acridinium salt A (≈2.20 V vs. SCE). A complementary elegant approach of photochemical C−N bond formation by C−H arylation uses nitrogen radicals.11
Scheme 1.
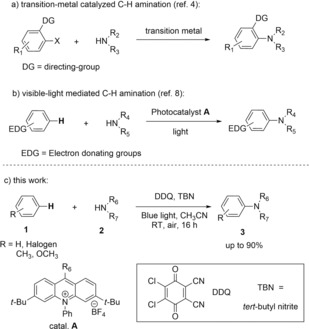
Reported procedures for C−H amination of arenes; (a) transition‐metal catalyzed directed C−H amination, (b) visible‐light‐mediated C−H amination. (c) This work.
Fukuzumi and co‐workers have shown that 2,3‐dichloro‐5,6‐dicyano‐p‐benzoquinone (DDQ) converts benzene into phenol under visible‐light irradiation.12, 13 Lei et al. reported the photocatalytic oxidative C−N coupling of thiophene with pyrazole using DDQ and tert‐butyl nitrite (TBN).14 Very recently, Murakami, Itami and co‐workers reported the C−N functionalization of naphthalene with sulfonimides promoted by DDQ and blue light.15 Wu, Tung and co‐workers used the combination of a quinolinum photocatalyst and a hydrogen‐evolving catalyst under UV‐A irradiation and a Lewis acid additive for the conversion of benzene into aniline and phenol.16 Despite the impressive progress, the substrate scope remains narrow. Herein, we present a general protocol for direct C−H amination of arenes using DDQ as a photocatalyst under aerobic conditions.
We started our investigation using a stoichiometric amount of DDQ as oxidizing agent and t‐BocNH2 as the amine source. Irradiation of a 1:1 mixture of benzene and amine in acetonitrile with blue light (λ=455±10 nm) in air resulted in 78 % of product 3 aa (entry 1, Table 1) after 16 h. Excess of benzene increased the yield slightly (entries 2–3). Next, DDQ was regenerated in the reaction under aerobic conditions following literature procedures using tert‐butyl nitrite under oxygen or in the presence of air.17, 18
Table 1.
Reaction optimization for the C−H amination of benzene 1 a using tert‐butyl carbamate 2 a (BocNH2) as amine source under visible light.

| ||||
|---|---|---|---|---|
| Entry[a] | Cat. loading [mol %] | Additive (mol %) | Benzene 1 a [Equiv.] | Yield of 3 aa [%][b] |
| 1 | 100 | – | 1.0 | 78 |
| 2 | 100 | – | 1.5 | 84 |
| 3 | 100 | – | 2.0 | 87 |
| 4 | 20 | t‐BuONO (20) | 1.5 | 94 |
| 5 | 20 | – | 1.5 | 26 |
| 6[c] | 20 | t‐BuONO (20) | 1.5 | 33 |
| 7 | 10 | t‐BuONO (10) | 1.5 | 77 |
| 8 | 30 | t‐BuONO (20) | 1.5 | 96 |
| 9[d] | 30 | t‐BuONO (20) | 1.5 | 86 |
| 10 | 30 | RFTA (10) | 1.5 | 13 |
| 11 | – | 1.5 | 0 | |
| 12[e] | 100 | 1.5 | 0 | |
[a] Reactions were carried out using 0.1 mmol of BocNH2 in 1 mL of CH3CN under air for 16 h. [b] The yield was determined by gas chromatography (GC) using 1,2‐dimethoxybenzene as internal standard. [c] Green light was used. [d] An oxygen balloon was used instead of air. [e] No light.
To our delight, we obtained a 94 % product yield (from GC) using 1:1 DDQ/TBN (20 mol %) under air (entry 4). Using only air was not sufficient to regenerate the DDQ catalyst (entry 5). Changing the light source from blue to green light (λ=530±10 nm) under otherwise similar conditions decreased the product yield to 33 % (entry 6). With lower catalyst loading of 10 mol % the yield dropped to 77 % (entry 7), whereas an increase to 30 mol % did not affect the yield (entry 8). Using an O2 balloon instead of air, reduced the yield slightly (entry 9). Other catalysts, such as riboflavin tetraacetate (RFTA) were tried, but failed to regenerate the DDQ catalyst (entry 10). Control experiments without catalyst or without light did not give any conversion to the product (entries 11 and 12).
With the optimized conditions in hand (20 mol % DDQ/TBN), we next focused on the arene scope with t‐BocNH2 as amine nucleophile. A general trend in reactivity was observed for different arenes used under the optimized reaction conditions. Although electron‐deficient arenes gave satisfactory yields of the C−H amidation product, electron‐rich ones, for example, anisole, did not yield the corresponding product 3 na (Scheme 2).
Scheme 2.
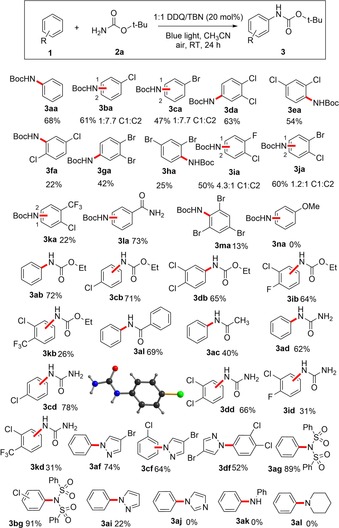
Substrate scope for the DDQ‐catalyzed direct C−H amination of electron‐deficient arenes under visible light irradiation. Unless otherwise stated, the general reaction conditions include 1.5–2.0 equiv arene, 1.0 equiv amine and 20 mol % DDQ/TBN under blue light irradiation in air.
Regioselectivity was observed for substituted arenes. In case of chlorobenzene, the p‐disubstituted product 3 ba was obtained as the major isomer. The compound is an intermediate in the synthesis of Efavirenz, an HIV‐1 reverse transcriptase inhibitor.19 For dihalobenzenes, 1,2‐derivatives gave the best product yields followed by 1,3‐dihalobenzenes; for example, 3 da was obtained in 63 % yield as compared to 3 ea, which was obtained in 54 % isolated yield. Chloro‐derivatives gave better yields than the corresponding bromoarenes (product 3 da vs. product 3 ga). 1,4‐Dihalobenzenes were found to be the least reactive of the dihalobenzene isomers: 1,4‐dichlorobenzene gave only 22 % of product 3 fa,20 while 1,4‐dibromobenzene yielded no product. Dihalobenzenes bearing different halogen atoms and benzamide showed C−H amidation with lower regioselectivity (3 ia and 3 ja, Scheme 2). Other electron deficient arenes, such as 1‐chloro‐2‐trifluoromethyl toluene react, but give only low product (3 ka) yield. We next turned our attention to the amine scope. Ethyl‐ and tert‐butyl carbamates gave high product yields with good regioselectivity. Carbamates are common structural motifs in pharmaceuticals.21 For example, the herbicide Swep22 3 db can be accessed directly in one step from dichlorobenzene and ethyl carbamate in a 65 % isolated yield. Other amides, such as benzamide and acetamide also gave satisfactory yields of the corresponding N‐arylated products. Remarkably, urea can be used as amine source and the product N‐phenyl urea (3 ad) was obtained in 62 % isolated yield. To the best of our knowledge, this is the first report of direct C−H amination of benzene using urea.23 Aryl urea derivatives find many applications in medicinal chemistry and biology.24 Traditionally they are synthesized from the corresponding aryl amines reacting with isocyanates. Our method provides access to urea derivatives starting from easily available arenes and urea. Pyrazole, bromo‐pyrazole and sulfonimide were found to be suitable reaction partners under our catalytic condition. In general, less nucleophilic amines and amides were found to be suitable substrates, while more nucleophilic ones did not yield C−H amidation products under our reaction condition. Imidazole being more nucleophilic than pyrazole is the limit of the substrate scope and gave no product due to catalyst deactivation.
Next, we turned our attention to electron‐rich arenes, for example, anisole as it was not giving any product when reacted with BocNH2 (Scheme 2). After screening different amines and conditions, it turned out that using 5 equivalents of anisole and 1.0 equivalent of pyrazole, the N‐arylated product 5 ni could be isolated in 72 % yield (Scheme 3). Other anisole derivatives were successfully employed under the optimized condition and moderate to good product yields were obtained. Moving from anisole to other electron‐rich arenes such as naphthalene, phenanthrene, toluene derivatives and 2‐methyl furan, N‐arylated products were obtained with good to excellent isolated yields with varying regioselectivities. For more nucleophilic arenes, such as 1‐methyl indole, the reaction failed to give any product (5 si) due to catalyst deactivation (vide supra). We evaluated the amine scope, and other pyrazole derivatives such 4‐bromopyrazole, 4‐chloropyrazole and benzopyrazole gave satisfactory product yields under our reaction conditions. Other azole derivatives, such as triazole and benzotriazole, react in moderate to good product yields with 2‐methylfuran.
Scheme 3.
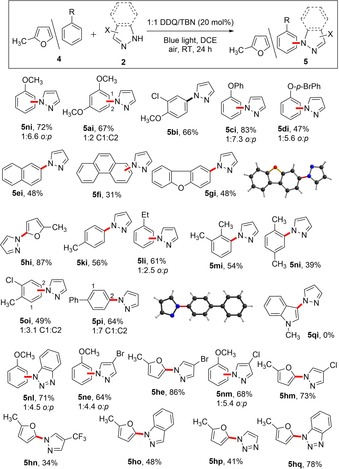
Substrate scope for the DDQ‐catalyzed direct C−H amination of electron‐rich arenes under visible light irradiation. Unless otherwise stated, general reaction condition include 5.0 equiv arene, 1.0 equiv amine and 20 mol % DDQ/TBN under blue light irradiation in air.
To rationalize the observed reactivity for electron‐rich arenes (e.g. anisole) and electron‐deficient arenes (e.g. chlorobenzene) with different amines, we investigated their interaction with DDQ by UV/Vis spectroscopy. At first, different amines were mixed at identical concentration with DDQ in CH3CN and the spectra were recorded. A colour change from yellow to deep brown, characteristic for the DDQ radical anion, with two new maxima at 588 and 545 nm (Figure 1 a)25 was observed for every amine added to the DDQ solution. The DDQ radical anion formation was confirmed by ESR (electron spin resonance) measurements (see Supporting Information). For more nucleophilic amines (e.g. imidazole, aniline), the absorption spectra changed completely indicating the formation of a new species different from the charge‐transfer complex between DDQ and an amine. Next, we investigated the interaction between different arenes and DDQ. Similar spectral changes were only observed for electron‐rich arenes (e.g. anisole and naphthalene), but not for the electron‐deficient ones (e.g. benzene or chlorobenzene). Again, the color change was assigned to the formation of a charge‐transfer complex between the electron‐rich arenes and DDQ (Figure 1 b). For more nucleophilic arenes, such as 1‐methyl indole, the absorption spectra changed completely, indicating the formation of a reaction product. In fact, we could isolate product 6 when 1‐methyl indole was used as substrate under the reaction conditions (Figure 2). This explains the catalyst deactivation and their inactivity as arene substrate under our C−H amination condition.26
Figure 1.
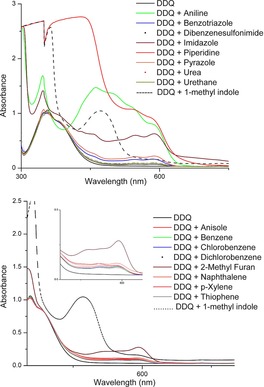
UV/Vis spectra observed from mixtures of different amines and arenes added to CH3CN/DDQ solutions. UV/Vis spectra obtained with amines (0.005 m) and DDQ (0.001 m) (top). UV/Vis spectra obtained with arenes (0.05 m) and DDQ (0.001 m) (bottom).
Figure 2.
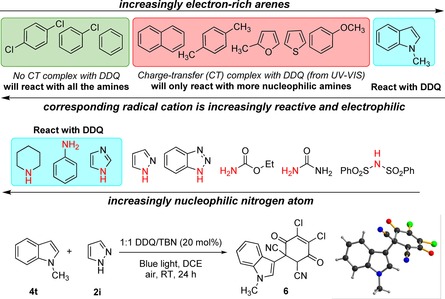
Prediction of arene and amine reactivities in DDQ photocatalysis from UV/Vis measurements. Nucleophilicity is a relative estimation, as no measured values are available for the depicted amines. Bottom: Covalent addition product of DDQ and 1‐methyl indole obtained under the reaction condition; structure of reaction product in the crystal.
The outcome of the C−H amination reaction is rationally predicted from UV/Vis measurements (Figure 2.) Arenes, which do not form a charge‐transfer complex with DDQ (e.g. benzene, chlorobenzene; green box) react with all amines, which are not reacting with DDQ in the ground state and thereby destroy the catalyst. Electron‐rich arenes, which form a charge‐transfer complex with DDQ (red box) will react with more nucleophilic amines, such as pyrazole. Amines that are more nucleophilic than pyrazole (e.g. imidazole; blue box) react with DDQ and inactivate the photocatalyst.
Based on our observations and the literature evidence,12, 27 we propose two possible mechanistic pathways shown in Figure 3. Photoexcitation yields DDQ in its triplet state, which is a very strong oxidant (≈+3.18 V vs. SCE) and converts all arenes to their corresponding radical cations (Figure 3, mechanism I). The radical cation is nucleophilic attacked by the amine and oxidation of the resulting species yields the arene C−N substitution product. The different reactivity of electron‐deficient and electron‐rich arenes towards the amines is explained by the different electrophilicity of the aromatic radical cation.12 Thus, the radical cation generated from benzene or chlorobenzene will react with all amine nucleophiles, while the radical cation of anisole is less reactive and yields products only with strong nucleophiles, such as pyrazole and other azoles.
Figure 3.
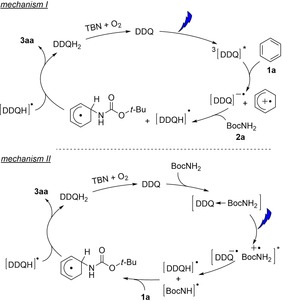
Proposed mechanism for DDQ‐photocatalyzed C−H amination of different arenes under visible light irradiation.
The second mechanistic proposal (mechanism II in Figure 3) is based on our observation of the charge‐transfer complex formation between amines and DDQ in the ground state. Excitation by visible light initiates hydrogen atom transfer to DDQ generating the amine radical and [DDQH].. The amine radical attacks the arene and another H‐atom abstraction yields the product and DDQH2. DDQ is regenerated by TBN. At the current state of investigations, we cannot exclude N‐centred radicals as intermediates, but two observations favor mechanism I: The DDQ charge‐transfer band spans from 450 nm to almost 600 nm, but green light excitation yields significantly less product (entry 6, Table 1) suggesting that DDQ excitation is the more productive pathway. Electron‐deficient arenes with very high oxidation potentials (e.g. ethyl benzoate, benzonitrile)13c are beyond the substrate scope. The electron transfer rate to triplet DDQ is known to be small for these substrates, which makes the nucleophilic trapping inefficient.
In conclusion, we have developed a method for the predictable direct photocatalytic C−H amination of benzene and other arenes with Boc‐amine, amides, pyrazole, sulfonimide and urea. DDQ was used as photocatalyst under aerobic conditions and blue light irradiation at ambient temperature. The simplicity of the method recommends the procedure for C−H aminations of arenes in synthesis.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (GRK 1626) and the European Research Council (ERCadv grant Pharos) for financial support. P.N. thanks the Alexander von Humboldt (AvH) foundation for support for a renewed research stay in Germany.
S. Das, P. Natarajan, B. König, Chem. Eur. J. 2017, 23, 18161.
References
- 1.
- 1a. Roughley S. D., Jordan A. M., J. Med. Chem. 2011, 54, 3451–3479. [DOI] [PubMed] [Google Scholar]
- 1b.In fact, 84 % out of the total number of unique drugs contain at least one nitrogen atom:
- 1c. Vitaku E., Smith D. T., Njardarson J. T., J. Med. Chem. 2014, 57, 10257–10274. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Mann A., in Amino Group Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, (Ed. A. Ricci) 2008, pp. 207–256; [Google Scholar]
- 2b. Amines: Synthesis Properties and Applications, Cambridge University Press, (Ed. S. A. Lawrence), 2004, pp. 265–305. [Google Scholar]
- 3.
- 3a. Ruiz-Castillo P., Buchwald S. L., Chem. Rev. 2016, 116, 12564–12649; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3b. Bariwal J., Van der Eycken E., Chem. Soc. Rev. 2013, 42, 9283–9303; [DOI] [PubMed] [Google Scholar]
- 3c. Hartwig J. F., Acc. Chem. Res. 1998, 31, 852–860; [Google Scholar]
- 3d. Wolfe J. P., Wagaw S., Marcoux J.-F., Buchwald S. L., Acc. Chem. Res. 1998, 31, 805–818. [Google Scholar]
- 4.
- 4a. Chen X., Engle K. M., Wang D.-H., Yu J.-Q., Angew. Chem. Int. Ed. 2009, 48, 5094–5115; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 5196–5217; [Google Scholar]
- 4b. Davies H. M. L., Morton D., J. Org. Chem. 2016, 81, 343–350; [DOI] [PubMed] [Google Scholar]
- 4c. Gensch T., Hopkinson M. N., Glorius F., Wencel-Delord J., Chem. Soc. Rev. 2016, 45, 2900–2936; [DOI] [PubMed] [Google Scholar]
- 4d. Tzouras N. V., Stamatopoulos I. K., Papastavrou A. T., Liori A. A., Vougioukalakis G. C., Coord. Chem. Rev. 2017, 343, 25–138. [Google Scholar]
- 5.
- 5a. Cernak T., Dykstra K. D., Tyagarajan S., Vachal P., Krska S. W., Chem. Soc. Rev. 2016, 45, 546–576; [DOI] [PubMed] [Google Scholar]
- 5b. Sharma A., Hartwig J. F., Nature 2015, 517, 600–604; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5c. Larsen M. A., Hartwig J. F., J. Am. Chem. Soc. 2014, 136, 4287–4299; [DOI] [PubMed] [Google Scholar]
- 5d. Wencel-Delord J., Glorius F., Nat. Chem. 2013, 5, 369–375. [DOI] [PubMed] [Google Scholar]
- 6.Only a small number of aromatic C−H aminations or amidation are reported that do not need a directing group in the molecule:
- 6a. Liu Y.-J., Xu H., Kong W.-J., Shang M., Dai H.-X., Yu J.-Q., Nature 2014, 515, 389–393; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6b. Zhang F.-L., Hong K., Li T.-J., Park H., Yu J.-Q., Science 2016, 351, 252–256; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6c. Park Y., Kim Y., Chang S., Chem. Rev. 2017, 117, 9247–9301. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a. König B., Eur. J. Org. Chem. 2017, 1979–1981; [Google Scholar]
- 7b. Romero N. A., Nicewicz D. A., Chem. Rev. 2016, 116, 10075–10166; [DOI] [PubMed] [Google Scholar]
- 7c. Lang X., Zhao J., Chen X., Chem. Soc. Rev. 2016, 45, 3026–3038; [DOI] [PubMed] [Google Scholar]
- 7d. Angnes R. A., Li Z., Correia C. R. D., Hammond G. B., Org. Biomol. Chem. 2015, 13, 9152–9167; [DOI] [PubMed] [Google Scholar]
- 7e. Prier C. K., Rankic D. A., MacMillan D. W. C., Chem. Rev. 2013, 113, 5322–5363; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7f. Shaw M. H., Twilton J., MacMillan D. W. C., J. Org. Chem. 2016, 81, 6898–6926; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7g. Narayanam J. M. R., Stephenson C. R. J., Chem. Soc. Rev. 2011, 40, 102–113. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Romero N. A., Margrey K. A., Tay N. E., Nicewicz D. A., Science 2015, 349, 1326–1330. Recent report on ipso-substitution: [DOI] [PubMed] [Google Scholar]
- 8b. Tay N. E. S., Nicewicz D. A., J. Am. Chem. Soc. 2017, 139, 16100–16104. [DOI] [PubMed] [Google Scholar]
- 9.
- 9a. Kawakami T., Murakami K., Itami K., J. Am. Chem. Soc. 2015, 137, 2460–2463; [DOI] [PubMed] [Google Scholar]
- 9b. Brachet E., Ghosh T., Ghosh I., Konig B., Chem. Sci. 2015, 6, 987–992; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9c. Qin Q., Yu S., Org. Lett. 2014, 16, 3504–3507; [DOI] [PubMed] [Google Scholar]
- 9d. Foo K., Sella E., Thomé I., Eastgate M. D., Baran P. S., J. Am. Chem. Soc. 2014, 136, 5279–5282; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9e. Allen L. J., Cabrera P. J., Lee M., Sanford M. S., J. Am. Chem. Soc. 2014, 136, 5607–5610; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9f. Tong K., Liu X., Zhang Y., Yu S., Chem. Eur. J. 2016, 22, 15669. [DOI] [PubMed] [Google Scholar]
- 10. Merkel P. B., Luo P., Dinnocenzo J. P., Farid S., J. Org. Chem. 2009, 74, 5163–5173. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a. Davies J., Booth S. G., Essafi S., Dryfe R. A. W., Leonori D., Angew. Chem. Int. Ed. 2015, 54, 14017–14021; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 14223–14227; [Google Scholar]
- 11b. Davies J., Svejstrup T. D., Reina D. F., Sheikh N. S., Leonori D., J. Am. Chem. Soc. 2016, 138, 8092–8095; [DOI] [PubMed] [Google Scholar]
- 11c. Margrey K. A., Levens A., Nicewicz D. A., Angew. Chem. Int. Ed. 2017, 56, 15644–15648; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 15850–15854. [Google Scholar]
- 12. Ohkubo K., Fujimoto A., Fukuzumi S., J. Am. Chem. Soc. 2013, 135, 5368–5371. [DOI] [PubMed] [Google Scholar]
- 13.
- 13a. Ohkubo K., Hirose K., Fukuzumi S., Chem. Asian J. 2016, 11, 2255–2259; [DOI] [PubMed] [Google Scholar]
- 13b. Hirose K., Ohkubo K., Fukuzumi S., Chem. Eur. J. 2016, 22, 12904–12909; [DOI] [PubMed] [Google Scholar]
- 13c. Ohkubo K., Hirose K., Fukuzumi S., Chem. Eur. J. 2015, 21, 2855–2861. [DOI] [PubMed] [Google Scholar]
- 14. Song C., Yi H., Dou B., Li Y., Singh A. K., Lei A., Chem. Commun. 2017, 53, 3689–3692. [DOI] [PubMed] [Google Scholar]
- 15. Sakakibara Y., Ito E., Kawakami T., Yamada S., Murakami K., Itami K., Chem. Lett. 2017, 46, 1014–1016. [Google Scholar]
- 16. Zheng Y.-W., Chen B., Ye P., Feng K., Wang W., Meng Q.-Y., Wu L.-Z., Tung C.-H., J. Am. Chem. Soc. 2016, 138, 10080–10083. [DOI] [PubMed] [Google Scholar]
- 17.
- 17a. Rusch F., Schober J.-C., Brasholz M., ChemCatChem 2016, 8, 2881–2884; [Google Scholar]
- 17b. Shen Z., Dai J., Xiong J., He X., Mo W., Hu B., Sun N., Hu X., Adv. Synth. Catal. 2011, 353, 3031–3038. [Google Scholar]
- 18.
- 18a. Chandrasekhar S., Sumithra G., Yadav J. S., Tetrahedron Lett. 1996, 37, 1645–1646; [Google Scholar]
- 18b. Sharma G. V. M., Lavanya B., Mahalingam A. K., Krishna P. R., Tetrahedron Lett. 2000, 41, 10323–10326; [Google Scholar]
- 18c. Liu L., Floreancig P. E., Org. Lett. 2010, 12, 4686–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pierce M. E., R. L. Parsons, Jr. , Radesca L. A., Lo Y. S., Silverman S., Moore J. R., Islam Q., Choudhury A., Fortunak J. M. D., Nguyen D., Luo C., Morgan S. J., Davis W. P., Confalone P. N., J. Org. Chem. 1998, 63, 8536–8543. [Google Scholar]
- 20.A 1:3 mixture of product and trichlorinated product (confirmed from HR-MS and NMR) were obtained; the reason is yet unclear.
- 21. Ghosh A. K., Brindisi M., J. Med. Chem. 2015, 58, 2895–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Braverman S., Cherkinsky M., Kedrova L., Reiselman A., Tetrahedron Lett. 1999, 40, 3235–3238. [Google Scholar]
- 23. Bandara H. M. D., Sosin M. H., McKeogh B. J., Emmert M. H., GSTF J. Chem. Sci. 2013, 1, 17–39. [Google Scholar]
- 24.
- 24a. Gallou I., Org. Prep. Proced. Int. 2007, 39, 355–383; [Google Scholar]
- 24b. Sikka P., Sahu J. K., Mishra A. K., Hashim S.R., Med. Chem. 2015, 5, 479–483. [Google Scholar]
- 25.
- 25a. Ottenberg A., Brandon R. L., Browne M. E., Nature 1964, 201, 1119–1120; [Google Scholar]
- 25b. Miller J. S., Krusic P. J., Dixon D. A., Reiff W. M., Zhang J. H., Anderson E. C., Epstein A. J., J. Am. Chem. Soc. 1986, 108, 4459–4466; [Google Scholar]
- 25c. Fukuzumi S., Ohkubo K., Tokuda Y., Suenobu T., J. Am. Chem. Soc. 2000, 122, 4286–4294; [Google Scholar]
- 25d. Al-Ahmary K. M., El-Kholy M. M., Al-Solmy I. A., Habeeb M. M., Spectrochim. Acta Mol. Biomol. Spectrosc. 2013, 110, 343–350. [DOI] [PubMed] [Google Scholar]
- 26. Guo X., Mayr H., J. Am. Chem. Soc. 2013, 135, 12377–12387. [DOI] [PubMed] [Google Scholar]
- 27. Chang X.-P., Cui G., Fang W.-H., Thiel W., ChemPhysChem 2015, 16, 933–937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


