Abstract
BACKGROUND
The fungicide benzovindiflupyr belongs to the class of succinate dehydrogenase inhibitors (SDHIs). Certain SDHIs have shown plant physiological effects, so‐called secondary effects, that appeared to be related to the plant water status. Therefore, the effect of benzovindiflupyr on transpiration of leaves and whole wheat plants was studied under controlled conditions. Furthermore, wheat yield trials under controlled and natural drought stress in the field were conducted.
RESULTS
Transpiration of detached wheat leaves was reduced by benzovindiflupyr in a dose‐dependent manner. Similarly, whole‐plant transpiration decreased for several days following application of this fungicide. In 16 field trials under drought stress conditions that were classified as disease‐free, treatment of wheat plants at the flag leaf stage or at heading with benzovindiflupyr showed a grain yield increase (+5.2%; P ≤ 0.01) that was partially attributed to an increased thousand‐grain weight.
CONCLUSIONS
Water saving during pre‐anthesis as a result of benzovindiflupyr application may be associated with better seed setting and filling under dry field conditions in wheat. The results of this research provide new insights into secondary effects of SDHIs that lead directly to yield improvements. © 2017 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: drought stress, field trial, secondary effects, succinate dehydrogenase inhibitor, transpiration, Triticum
1. INTRODUCTION
The fungicide benzovindiflupyr is a broad‐spectrum pyrazole carboxamide originating from a novel chemical class. It is active on a broad spectrum of diseases in cereals (e.g. those caused by Zymoseptoria tritici, Puccinia spp. and Pyrenophora teres) and in other crops, in particular against soybean rust (Phakopsora pachyrhizi).1, 2, 3 The mode of action of benzovindiflupyr is based on the repression of fungal respiration through inhibition of succinate dehydrogenase (SDH).1
In plants, the activity of SDH not only plays a pivotal role in respiration, but also affects the oxidative status of plant cells and modulates photosynthesis.4 The contribution of mitochondrial function to photosynthesis is well documented.5 In particular, mitochondrial electron transport is involved in the synthesis and recycling of ascorbate, which is a key antioxidant protecting the photosynthetic machinery, especially under stress conditions. Moreover, parts of the photorespiratory pathway take place in the mitochondria. Recently, it was found that antisense inhibition of SDH affects the stomatal aperature and thus promotes photosynthesis.6 In view of the fact that the target of all succinate dehydrogenase inhibitors (SDHIs) is a part of the SDH, which is highly conserved across living organisms,7 it can be assumed that SDHIs also interact with the plant's SDH and, consequently, may have plant physiological effects.
Indeed, such secondary effects of SDHIs were detected in wheat under controlled conditions as well as in the field. The application of the SDHI bixafen, which is known to have high efficacy against many cereal pathogens,8 positively affected yield formation by delaying senescence.9 This delayed senescence was associated with a higher transpiration rate at late crop growth stages.10 In contrast, foliar application of fluxapyroxad, another SDHI which shows good efficacy against cereal pathogens, was found to reduce the transpiration rate, resulting in a higher water use efficiency of greenhouse and field‐grown wheat.11 It is an open question whether these effects occur only for particular SDHIs and whether the described secondary effects, i.e. the increased water use efficiency, would be beneficial under field conditions, in particular when water is limiting.
Drought stress may occur and affect crop performance at different crop development stages and results in substantial yield losses. Thus, the timing, severity and duration of the stress determine the extent of the yield loss.12 The principal reasons for these losses are reduced rates of net photosynthesis as a consequence of metabolic limitations resulting from oxidative damage to chloroplasts and stomatal closure, and poor grain set and development.13 Wheat is particularly sensitive to drought stress when water is limiting during reproductive growth.14 Consequently, the distribution of rainfall, and resultant water availability, during the crop cycle have a great impact on the final yield.15 Reduction of soil water uptake, which can be achieved by a reduction of transpiration via stomatal closure, before the critical phase of reproductive growth is a promising strategy for mitigating drought stress during reproductive growth.16 Therefore, a pre‐anthesis water‐saving phenotype, which preserves more soil water for the time of anthesis, can be a suitable approach for improving wheat production under limited water availability.17, 18 In maize, breeding for such a phenotype has resulted in varieties with improved yield under water‐limited conditions.19
Understanding the potential plant physiological effects of a fungicide can give the grower an additional tool to maximize the crop yield. In the current study, we addressed whether benzovindiflupyr affects plant physiology, in particular transpiration. We also addressed the question of whether it improves the crop performance in the field under a range of drought stress conditions, which would be beneficial for a soil water‐saving phenotype. Benzovindiflupyr's primary use is as a fungicide with a broad spectrum of control of foliar pathogens in wheat. Therefore, in order to differentiate fungicidal effects from potential physiological effects, we discuss only results from drought‐stressed field trials reported as disease‐free, meaning without symptoms of disease in foliar tissue.
2. MATERIALS AND METHODS
2.1. Detached leaf transpiration
Chemical effects on stomata were quantified by measuring the transpiration of detached leaves according to Munns and King.20 Winter wheat (Triticum aestivum L.) of the variety Arina was grown under optimum conditions in growth chambers until the second‐leaf stage (BBCH 12).21 The seedlings were dark‐adapted for 1 h; the second leaf was cut under water and immediately placed in a 1.5‐mL test‐tube filled with 1 mL of benzovindiflupyr solution. The solution was prepared by dissolving benzovindiflupyr in dimethyl sulfoxide (DMSO) and diluting in water to the final concentrations of 0, 3, 10, 50 and 100 ppm active ingredient (ai). After determining the initial weight of the leaf − tube system, the leaves were placed in growth chambers at 25 °C, 60% relative humidity and a light intensity of 150 μmol m‐2 s‐1 with a 14‐h photoperiod. Transpiration was quantified by measuring the weight of the leaf − tube system after 6 and 24 h. Tubes without leaves, filled only with water, were used to correct for evaporation.
The experimental design was a randomized complete block design with five replications for each treatment; the experimental unit was one leaf per test‐tube. Statistical analysis [analysis of variance (ANOVA)] was performed with agstat 1.6/acsap 4.29 (Syngenta, UK).
2.2. Whole‐plant transpiration analysis
For the whole‐plant transpiration analysis, spring wheat (T. aestivum) of the variety Taifun was grown under pest‐free conditions in 1.2‐L growth columns filled with a field soil (sandy loam). Plants were watered daily to a target soil water content of 0.25 g H2O g‐1 dry soil. Plant growth and transpiration analysis was carried out in growth chambers at 25/18 °C (day/night), 60% relative humidity and a light intensity of 600 μmol m‐2 s‐1 with a 14‐h photoperiod. Transpiration was measured by monitoring the weight of the plant − pot system on a gravimetric platform with precision irrigation (DroughtSpotter; Phenospex, Heerlen, the Netherlands). Application of benzovindiflupyr formulated as EC 100 at 75 g ai ha‐1 (375 ppm) was performed with a track sprayer at 200 L ha‐1 when plants were at tillering (BBCH 23). Control plants were sprayed with the same amount of water. Transpiration was monitored at optimal watering for 7 days after application, and then watering was stopped.
At the end of the experiment, shoots were harvested, oven‐dried for 3 days at 60 °C and weighed for shoot dry matter determination.
The experimental layout was a randomized complete block design with nine replications; the experimental unit was two plants per pot. The transpiration before treatment was used as a covariant for statistical analysis (ANOVA; agstat 1.6/acsap 4.29) (Syngenta, UK).
2.3. Field trials
Wheat field trials were designed as randomized complete blocks with six replicates. Sowing, fertilization and general crop maintenance were performed in accordance with locally accepted best practice. Plot quality and pest damage were visually observed several times per week in field trials with controlled drought stress conditions and on a monthly basis in field trials with natural drought stress conditions. Crop protection maintenance products were applied preventatively only if required across the whole trial area (check and experimental plots and boundary areas) and included locally registered seed treatment products, herbicides and insecticides. In the case of potential disease attack, demethylation inhibitors (DMIs) and/or contact fungicides were applied preventatively (see Supporting Information Table S1). Application of fungicides from the Qo inhibitor (strobilurine) and SDHI groups were not allowed to avoid possible interaction with benzovindiflupyr experimental treatments. Any products with plant growth regulator effects were also excluded. Pesticides were applied not less than 3 to 4 days before or after experimental applications of benzovindiflupyr.
The following assessments were performed: general phytotoxicity at 7 and 14 days after application, general pest damage ratings before harvest, grain yield adjusted to 10% standard moisture (calculation based on fresh grain weight and grain moisture at harvest), and thousand‐grain weight (TGW) adjusted to 10% standard moisture.
Entire plots or only the central treated areas were harvested manually using cutters with stationary threshers (for trials located in Argentina, Egypt and India) or with small‐plot combine‐harvesters (for trials located in France, Italy and the USA).
A single‐trial ANOVA was performed with agstat 1.6/acsap 4.29. Multi‐trial analysis was based on a paired t‐test with comparison of benzovindiflupyr experimental treatments with untreated checks and was performed with tibco® spotfire ® 7.0.1 (Tibco, Palo Alto, CA, USA).
2.3.1. Field trials with controlled drought stress conditions
To minimize the risk of environmental impacts from rain on drought stress period and level, all proposed locations were validated through an internal environment and crop modeling process. This modeling allowed the calculation of the potential drought stress intensity through estimation of climatic cumulative water deficit (sum of all daily water deficits during the reproductive stage of wheat development). Daily water deficit was calculated as the difference between daily precipitation and daily evapotranspiration by wheat plants.22 Twenty‐nine field trials were peformed at 15 locations within a time‐span of 3 years. Results are presented for the 11 disease‐free trials in seven locations across the 3 years: Egypt (Kaha), Italy (Foggia), India (Dongargaon, Mandleshwar and Sanwer) and the USA (Woodland, CA and Jerome, ID).
Each field trial had two adjacent blocks: a well‐watered (WW) block with untreated check plots used for stress level estimation (grain yield reference), and a water‐stressed (WS) block with experimental treatments including untreated check plots. Plot size varied between trials from 8.45 m2 (Woodland, USA) to 30 m2 (Kaha, Egypt) and harvested plot size varied from 8.45 m2 (Woodland, USA) to 20.7 m2 (India). Seven locally adapted drought‐sensitive commercial varieties of spring and winter bread wheat (T. aestivum) and durum wheat (Triticum durum Desf.) were tested: var. Massimo Meridio (Foggia, Italy), var. Saha 94 and Sods 12 (Kaha, Egypt), var. GW322 and GW366 (India), var. Alturas (Jerome, USA) and var. Summit 515 (Woodland, USA).
To manage drought stress, three types of water irrigation system were used: precise drip irrigation (Kaha, Egypt; Foggia, Italy; Woodland, USA), solid‐set sprinkler irrigation (Jerome, USA) and flood irrigation (all sites in India). Plants in WW blocks were well irrigated from sowing until harvest to compensate 100% of evapotranspiration demand, targeting the optimum yield achievable in the trial area. Plants in WS blocks were irrigated from planting until the beginning of the pre‐stress period exactly at the same level of water supply as in WW conditions. The pre‐stress period was initiated 1 to 4 weeks before BBCH 59 (end of heading) but not earlier than BBCH 39 (flag leaf stage) by water being withheld to deplete excess soil moisture with the objective of exposing plants to targeted drought stress conditions at BBCH 59. An example of the deficit irrigation management is shown in Figure 1. During the targeted transient drought stress period from BBCH 59 until harvest, only a deficient irrigation practice was used. If required, emergency irrigation cycles were maintained to prevent severe plant damage from drought. Soil moisture level was monitored with a solid‐state electrical resistance sensing device (Watermark 200SS; Irrometer Co., Riverside, CA, USA) at two soil horizons of 15 and 35 cm. The target drought stress level was between −20 and −40% grain yield reduction in WS untreated check plots relative to WW untreated check plots. The drought stress level varied from −1 to −59% (−0.5 to −42 dt ha‐1) with an average − 23% (−15 dt ha‐1) grain yield reduction in WS plants versus the WW crop across the 11 trials (Table S1).
Figure 1.
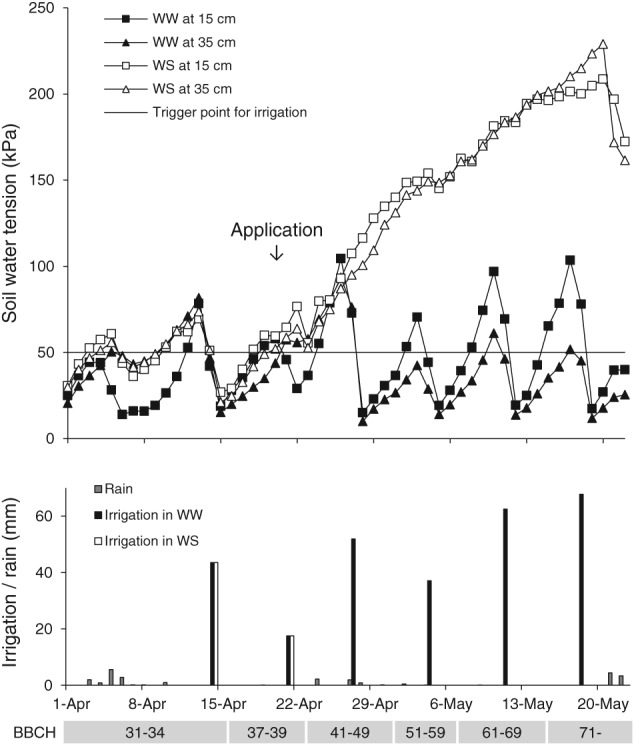
Deficit irrigation management for terminal drought stress in wheat. Examples are shown for well‐watered (WW) and drought stress (WS) management in the open field experiment in Foggia (Italy) in 2015 for the critical growth phases before and after application of benzovindiflupyr.
A single application of benzovindiflupyr formulated as EC 100 (SOLATENOL™; Syngenta Group Company, Basel, Switzerland) at 30 or 75 g ai ha‐1 was carried out with a hand‐held boom or motorized knapsack sprayers at 140‐300 L ha‐1 when plants were at BBCH 39 − 41 or 51 − 55. Control plants were untreated.
2.3.2. Field trials with natural (uncontrolled) drought stress conditions
During the 2014 and 2015 seasons, 15 open field trials were placed in typical commercial cereal‐growing areas of Argentina, France and Italy known for their high probability of suboptimal yields caused by natural drought stress occurring at reproductive stages. In the current study, we reviewed five trials in Argentina (Coronel Martinez de Hoz), France (Simandre) and Italy (Marsiliana and Borgo Santo Pietro) reported as disease‐free experiments with drought stress conditions occurring at the reproductive stage.
Harvested plot sizes were 5.6 m2 in Argentina, 9 m2 in Marsiliana (Italy), 12.5 m2 in Simandre (France) and 20 m2 in Borgo Santo Pietro (Italy). Five locally adapted drought‐sensitive commercial varieties of spring and winter bread wheat (T. aestivum) and durum wheat (T. durum) were tested: var. Simeto (Borgo Santo Pietro, Italy), var. SI100 and Baguette 601 (Argentina), var. Svevo (Marsiliana, Italy) and var. SY Moisson (Simandre, France).
Drought stress occurrence, timing and level were estimated by considering (a) the difference between actual precipitation and long‐term average precipitation for the crop‐growing period, and (b) the yield reduction compared with nonstressed plants of the same variety (in the case of irrigation) or versus optimal varietal characteristics known for favorable nonstressed conditions and/or year in the same geographical location.
A single application of benzovindiflupyr formulated as EC 100 at 75 g ai ha‐1 was performed with a hand‐held boom or motorized knapsack sprayers at 200‐300 L ha‐1 when plants were at BBCH 39 or 55. Control plants were untreated.
3. Results
3.1. Laboratory experiments
In order to study the effect of benzovindiflupyr on leaf transpiration, a detached leaf assay was conducted in which the dissolved compound was taken up at the cut leaf base and distributed within the leaf by the transpiration stream. Benzovindiflupyr significantly decreased leaf transpiration in a dose‐dependent manner (Fig. 2). This effect was clearly visible already within 6 h of treatment at 50 and 100 ppm ai (P ≤ 0.05); for the two lower concentrations (3 and 10 ppm ai), transpiration hardly decreased within the first 6 h but decreased significantly (P ≤ 0.05) during the remaining 18 h of the experiment (data not shown). Analysis of photosynthesis by measurement of chlorophyll fluorescence did not show a significant impact of the treatment with benzovindiflupyr on the operating quantum efficiency of photosystem II (data not shown).
Figure 2.
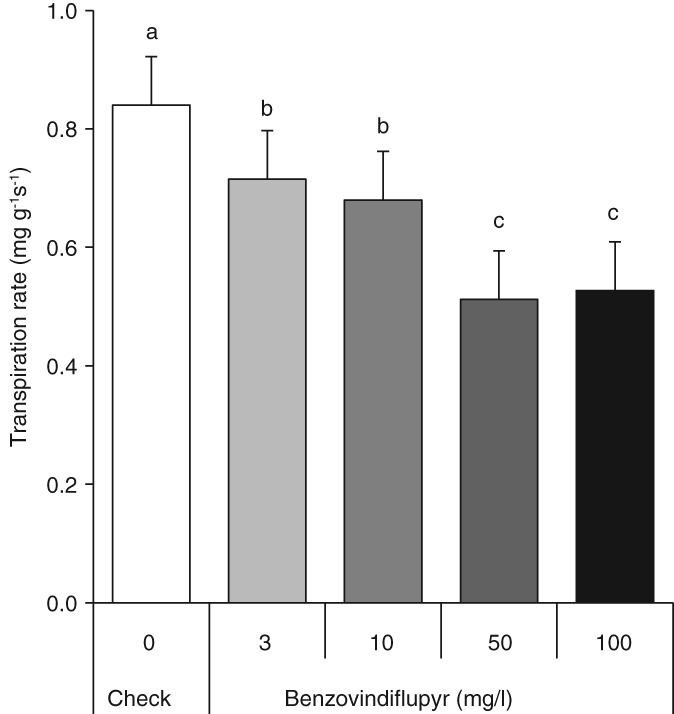
Benzovindiflupyr dose–response for the transpiration rate of detached wheat (Triticum aestivum var. Arina) leaves between 0 and 24 h after treatment. The compound was fed via the transpiration stream. Values are mean ± SD of five replications. Different letters indicate significant differences between treatments at P ≤ 0.05.
In order to confirm this observation at the whole‐plant level with benzovindiflupyr application comparable to that in the field, the transpiration of benzovindiflupyr‐treated wheat plants was studied on an automated weighing and irrigation system (Fig. 3a). Benzovindiflupyr was applied foliarly at the recommended field rate (75 g ai ha‐1) when plants were growing without water limitation. Within 1 day, transpiration had decreased by 5.7% (P ≤ 0.1) in benzovindiflupyr‐treated plants compared with untreated control plants (Fig. 3b). Three days after application, transpiration was 7.3% (P ≤ 0.05) lower in benzovindiflupyr‐treated plants compared with controls; this effect was consistent for at least a further 3 days. When irrigation was withheld 8 days after application, transpiration decreased strongly as a result of the decreasing soil water content (Fig. 3a). However, this transpiration decrease was delayed in benzovindiflupyr‐treated plants, resulting in a transiently higher transpiration rate compared with untreated control plants (Fig. 3c). The reduction of transpiration in benzovindiflupyr‐treated plants had no negative impact on biomass accumulation; at the end of the experiment, the shoot dry weight of benzovindiflupyr‐treated plants was 6.34 g plant‐1 and that of control plants was 6.31 g plant‐1 [standard deviation (SD) = 0.41; coefficient of variation (CV) = 6.6%].
Figure 3.
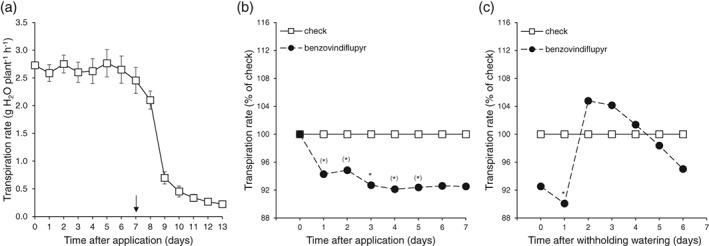
Time course of the transpiration rate of control wheat (Triticum aestivum var. Taifun) plants at constant soil water content and after withholding watering (arrow) (a) and the effect of foliar treatment with benzovindiflupyr on the transpiration rate of wheat plants under constant soil water content (b) and during soil drying (c). Soil drying was started 8 days after application by withholding watering. Values are means of nine replications. Statistically significant differences from check are indicated as follows: *P ≤ 0.05; (*) P ≤ 0.1. The transpiration rate 3 days before application was used as a covariate for statistical analysis.
3.2. Field trials
In order to study the effect of benzovindiflupyr on grain yield under drought stress at reproductive stages in wheat, 29 open field trials were conducted. Of these, 16 trials were reported to be disease‐free based on regular pest damage observation and final pest damage rating, meaning that the trials were without symptoms of diseases in the above‐ground parts of the plants.
The group of disease‐free trials included 26 variants (unique combination of location × year × variety × application rate × application time) of single treatments of benzovindiflupyr formulated as EC 100 at 30 or 75 g ai ha‐1 applied at BBCH 39 to 55 (Table S1). Benzovindiflupyr did not cause any phytotoxic effects on wheat plants in all trials at both 7 and 14 days after application.
The CV for grain yield adjusted to 10% standard moisture in single trials varied from 3.69 to 13.05% (average CV was 8.38%). The CV for TGW adjusted to 10% standard moisture varied from 2.24 to 10.34% (average CV was 5.41%). Single‐trial ANOVA did not detect any significant effect at P ≤ 0.05 of benzovindiflupyr on grain yield adjusted to 10% standard moisture and TGW compared with the untreated control.
Multitrial analysis (MTA) based on a paired t‐test performed for all variants identified a significant average grain yield increase (P ≤ 0.01) with benzovindiflupyr treatment compared to untreated check in disease‐free situations of +2.33 dt ha‐1 (+5.2%) with a 95% confidence interval (CI) of the mean between +1.45 and +3.21 dt ha‐1 (Fig. 4). This means that, based on our sample data, we are 95% confident that the “true” yield benefit a grower can expect is between +1.45 and +3.21 dt ha‐1. Considering only trials under controlled drought stress conditions, the yield increase was +2.86 dt ha‐1 (+6.0%; P ≤ 0.01) with a 95% CI of +1.69 to +4.03 dt ha‐1. Under these conditions, the average grain yield increase with 75 g benzovindiflupyr ha‐1 was +0.23 dt ha‐1 compared with the rate of 30 g ai ha‐1.
Figure 4.
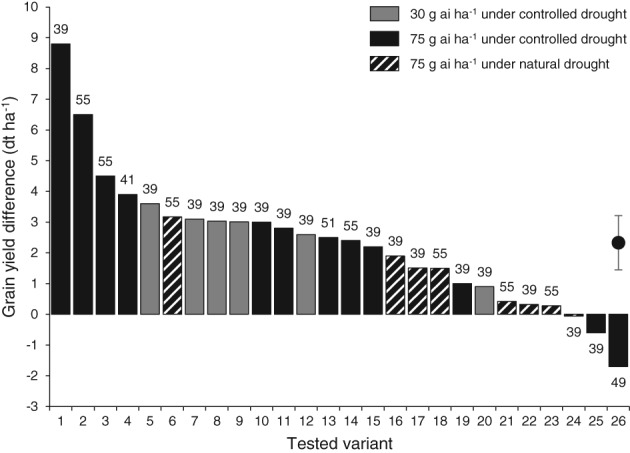
Grain yield difference in benzovindiflupyr treatments versus untreated check under controlled (filled bars) and natural (hatched bars) drought stress conditions during the reproductive period. The application rate of benzovindiflupyr was 30 (gray bars) or 75 g ai ha‐1 (filled or hatched black bars). The application timing (BBCH) is given at the top of the bars. Tested variants (in bold) were (location, country, year): Borgo Santo Pietro, Italy, 2015 (24); Dongargaone, India, 2015 (1, 2); Dongargaone, India, 2016 (12); Fogia, Italy, 2015 (15, 20); Jerome, USA, 2014 (10, 14); Jerome, USA, 2015 (5, 11); Kaha, Egypt, 2014 (4, 26); Kaha, Egypt, 2015 (3, 19); Kaha, Egypt, 2016 (7); Mandleshwar, India, 2016 (9); Marsiliana, Italy, 2015 (6); Matinez (var. Baguette 601), Argentina, 2014 (16, 18); Matinez (var. SI100), Argentina, 2014 (17, 21); Sanwer, India, 2016 (8); Simandre, France, 2015 (22, 23); Woodland, USA, 2014 (13, 25). Means with the upper and lower limits of the 95% confidence interval are shown on the right (paired t‐test with P ≤ 0.01; n = 26).
TGW adjusted to 10% standard moisture was reported for 13 disease‐free trials (20 variants). Under all drought‐stressed conditions (controlled and natural drought stress at the reproductive stage), the MTA confirmed a mean TGW increase with benzovindiflupyr versus untreated check of 0.68 g (1000 seeds)‐1 (+1.9%; P ≤ 0.01) with a 95% CI of +0.23 to +1.13 g (1000 seeds)‐1 (Fig. 5).
Figure 5.
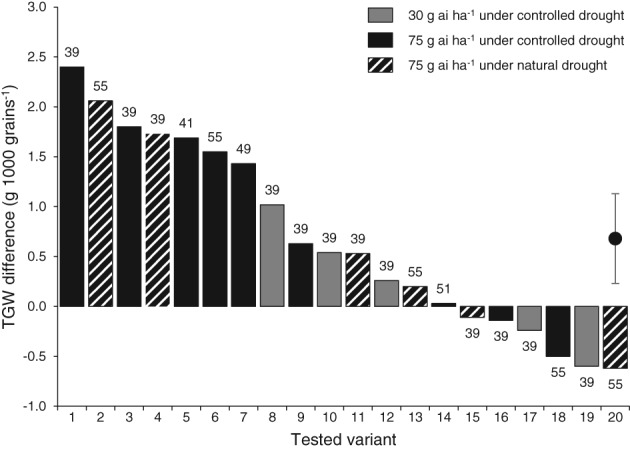
Thousand‐grain weight difference in benzovindiflupyr treatments versus untreated check under controlled (filled bars) and natural (hatched bars) drought stress conditions during the reproductive period. The application rate of benzovindiflupyr was 30 (gray bars) or 75 g ai ha‐1 (filled or hatched black bars). The application timing (BBCH) is given at the top of the bars. Tested variants (in bold) were (location, country, year): Borgo Santo Pietro, IT, 2015 (15); Dongargaone, India, 2015 (3, 18); Dongargaone, India, 2016 (17); Fogia, Italy, 2015 (1, 19); Kaha, Egypt, 2014 (5, 7); Kaha, Egypt, 2015 (6, 9); Kaha, Egypt, 2016 (8); Mandleshwar, India, 2016 (12); Marsiliana, Italy, 2015 (20); Matinez (var. Baguette 601), Argentina, 2014 (4, 13); Matinez (var. SI100), Argentina, 2014 (2, 11); Sanwer, India, 2016 (10); Woodland, USA, 2014 (14, 16). Mean with the upper and lower limits of the 95% confidence interval are shown on the right (paired t‐test with P ≤ 0.01; n = 20).
4. Discussion
Three years of extensive global validation of benzovindiflupyr in open field experiments under controlled and natural drought stress during generative growth and seed filling was conducted. The potential contribution of benzovindiflupyr treatment to the grain yield and TGW of wheat in disease‐free conditions was measured. A significant increase of grain yield after benzovindiflupyr treatment compared with the untreated check of +2.33 dt ha‐1 (+5.2%; P ≤ 0.01) with a positive response in 88% of the tested variants was observed. This grain yield increase was partially based on a positive effect of benzovindiflupyr on the individual grain weight. Indeed, TGW was observed to increase after benzovindiflupyr treatment in 67% of tested variants, with an overall increase of 1.9% compared with the untreated check (P ≤ 0.01). As the increase of TGW was smaller than the increase of grain yield, a considerable part of the grain yield increase must be attributable to higher grain number; grain number was estimated to increase by 3.2% as a result of the benzovindiflupyr treatment.
Under controlled field conditions, drought stress resulted in a reduction of grain yield (−17 %) which was mainly contributed by a reduction of TGW (−12 %) but also a reduction of grain number (−6 %). As drought stress before anthesis mainly affects grain number while terminal drought stress reduces the grain weight,14 the drought stress seemed to affect both developmental phases in the controlled field trials. With respect to the pesticide treatment, the beneficial effect of benzovindiflupyr on grain number (calculated as grain yield divided by TGW) and TGW indicates that benzovindiflupyr treatment increased seed setting (by reducing floret sterility or seed abortion) and, additionally, promoted to a certain extent seed filling. Consequently, benzovindiflupyr treatment appears to have a long‐lasting beneficial effect on drought tolerance from seed setting to seed filling. The stronger beneficial effect of benzovindiflupyr on seed number, however, indicates that it protects this very drought‐sensitive developmental phase. In view of the dependence of drought tolerance during these developmental phases on genetic factors,14 a certain genetic influence on the effect of benzovindiflupyr can be assumed. The present data do not provide evidence for a strong dependence on variety of the effect of benzovindiflupyr on grain yield. However, elucidating this interaction would require a larger field program that would include several varieties at each trial site.
Secondary effects of fungicides on wheat grain yield were observed in several studies.23, 24, 25 These effects were partially attributed to a delay of senescence. However, it has been suggested that such effects may be mainly attributable to the control of phylloplane microflora, which could explain delayed senescence.26, 27 At the trial sites where senescence was monitored by visual observation or by NDVI (normalized difference vegetation index) measurements, benzovindiflupyr did not affect this trait. This further supports the conclusion that the trials were largely disease‐free and that another mechanism seems to be responsible for the observed yield increase.
Indeed, the physiological experiments demonstrated that benzovindiflupyr was capable of reducing the transpiration rate in detached leaves as well as in whole plants. This effect was attributable neither to phytotoxicity nor to a reduced growth rate. In wheat, such a decrease of transpiration was also found after foliar application of the SDHI fluxapyroxad11 and might be a common characteristic of SDHIs. However, in wheat plants treated with the SDHI bixafen, higher transpiration rates were observed during the time course of senescence, which were probably caused by a delay of senescence and significantly extended green leaf area duration.9, 10 It has to be noted, as discussed above and also in Berdugo et al.,9 that a delay of senescence may be attributed to the control of saprophytic fungi on the plant surface and, therefore, the increased transpiration may have been caused by the disease control, as foliar pathogens can alter the plant water status by affecting transpiration.28
It is well known that the functioning of mitochondria plays a role in photosynthesis and consequently transpiration, especially under stress conditions.5 In particular, the malate:fumarate ratio, which is modulated by the activity of SDH, plays a role in regulating stomatal aperture.4, 6 The direct interaction of SDHIs such as benzovindiflupyr with plant SDH is likely, as all studied SDH structures (from prokaryotes to eukaryotes) show a high degree of homology of the functional quinone‐binding site,7 which is the fungal target of all SDHIs.29 Consequently, a modulation of transpiration by benzovindiflupyr through interaction with the plant's SDH could be one reason for the observed secondary effect.
In conclusion, a reduction of transpiration without a negative effect on photosynthetic efficiency and biomass accumulation, which can be explained by the nonlinear relationship between transpiration and photosynthesis,30 seems to be the physiological effect of benzovindiflupyr in wheat. Consequently, soil water saving, after application and before the critical growth stages that determine grain number and grain weight,14, 16, 18 seems to be an obvious crop physiological mode of action to improve wheat grain yield under dry conditions with benzovindiflupyr. As water limitation during anthesis, seed set and early seed filling phases is a yield‐reducing factor in many wheat‐growing areas,31 the application of benzovindiflupyr could provide an additional benefit under a large range of farming situations. Detailed crop physiological studies under field conditions will be required to confirm the hypothesized mode of action and to better understand the environmental and genotypic dependence of the secondary effects of benzovindiflupyr.
Supporting information
Table S1.
REFERENCES
- 1. Walter H, Tobler H, Gribkov D and Corsi C, Sedaxane, Isopyrazam and SOLATENOL™: novel broad‐spectrum fungicides inhibiting succinate dehydrogenase (SDH) – synthesis challenges and biological aspects. Chimia 69:425–434 (2015). [DOI] [PubMed] [Google Scholar]
- 2. Jeschke P, Progress of modern agricultural chemistry and future prospects. Pest Manag Sci 72:433–455 (2016). [DOI] [PubMed] [Google Scholar]
- 3. Jeschke P, Latest generation of halogen‐containing pesticides. Pest Manag Sci 73:1053–1066 (2016). [DOI] [PubMed] [Google Scholar]
- 4. Huang S and Millar AH, Succinate dehydrogenase: the complex roles of a simple enzyme. Curr Opin Plant Biol 16:344–349 (2013). [DOI] [PubMed] [Google Scholar]
- 5. Nunes‐Nesi A, Sulpice R, Gibon Y and Fernie AR, The enigmatic contribution of mitochondrial function in photosynthesis. J Exp Bot 59:1675–1684 (2008). [DOI] [PubMed] [Google Scholar]
- 6. Araújo WL, Nunes‐Nesi A, Osorio S, Usadel B, Fuentes D, Nagy R et al, Antisense inhibition of the iron‐sulphur subunit of succinate dehydrogenase enhances photosynthesis and growth in tomato via an organic acid‐mediated effect on stomatal aperture. Plant Cell 23:600–627 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maklashina E and Cecchini G, The quinone‐binding and catalytic site of complex II. Biochim Biophys Acta 1797:1877–1882 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suty‐Heinze A, Dunkel R, Krieg U and Rieck H, Bixafen – the new cereal fungicide with yield boosting effects, in Modern Fungicides and Antifungal Compounds VI, ed. by Dehme HW, Deising HB, Gisi U, Kuck KH, Russell PE, and Lyr H. DPG, Braunschweig, pp. 69–74 (2010). [Google Scholar]
- 9. Berdugo CA, Steiner U, Dehne H‐W and Oerke E‐C, Effect of bixafen on senescence and yield formation of wheat. Pestic Biochem Physiol 104:171–177 (2012). [Google Scholar]
- 10. Berdugo CA, Mahlein A‐K, Steiner U, Dehne H‐W and Oerke E‐C, Sensors and imaging techniques for the assessment of the delay of wheat senescence induced by fungicides. Funct Plant Biol 40:677–689 (2013). [DOI] [PubMed] [Google Scholar]
- 11. Smith J, Grimmer M, Waterhouse S and Paveley N, Quantifying the non‐fungicidal effects of foliar applications of fluxapyroxad (Xemium®) on stomatal conductance, water use efficiency and yield in winter wheat. Commun Agric Appl Biol Sci 78:523–535 (2013). [PubMed] [Google Scholar]
- 12. Farooq M, Hussain M, Wahid A and Siddique KHM, Drought stress in plants: an overview, in Plant Responses to Drought Stress, ed. by Aroca R, Springer, Berlin, pp. 1–33 (2012). [Google Scholar]
- 13. Farooq M, Hussain M and Siddique KHM, Drought stress in wheat during flowering and grain‐filling periods. Crit Rev Plant Sci 33:331–349 (2014). [Google Scholar]
- 14. Barnabás B, Jäger K and Fehér A, The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ 31:11–38 (2008). [DOI] [PubMed] [Google Scholar]
- 15. Fischer RA, Wheat physiology: a review of recent developments. Crop Pasture Sci 62:95–114 (2011). [Google Scholar]
- 16. Tardieu F, Any trait or trait‐related allele can confer drought tolerance: just design the right drought scenario. J Exp Bot 63:25–31 (2012). [DOI] [PubMed] [Google Scholar]
- 17. Deng X‐P, Shan L, Inanaga S and Inoue M, Water‐saving approaches for improving wheat production. J Sci Food Agric 85:1379–1388 (2005). [Google Scholar]
- 18. Vadez V, Kholova J, Medina S, Kakkera A and Anderberg H, Transpiration efficiency: new insights into an old story. J Exp Bot 65:6141–6153 (2014). [DOI] [PubMed] [Google Scholar]
- 19. Cooper M, Gho C, Leafgren R, Tang T and Messina C, Breeding drought‐tolerant maize hybrids for the US corn‐belt: discovery to product. J Exp Bot 65:6191–6204 (2014). [DOI] [PubMed] [Google Scholar]
- 20. Muuns R and King RW, Abscisic acid is not the only stomatal inhibitor in the transpiration stream of wheat plants. Plant Physiol 88:703–708 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lancashire PD, Bleiholder H, van den Boom T, Langelüddeke P, Stauss R, Weber E et al, A uniform decimal code for growth stages of crops and weeds. Ann appl Biol 119:561–601 (1991). [Google Scholar]
- 22. Lhomme J‐P and Katerji N, A simple modelling of crop water balance for agrometeorological applications. Ecol Model 57:11–25 (1991). [Google Scholar]
- 23. Fehrmann H, Reineck P and Weihofen U, Yield increase in winter wheat by unknown effects of MBC‐fungicides and Captafol. J Phytopathol 93:359–367 (1978). [Google Scholar]
- 24. Cook RJ, Effects of late‐season fungicide sprays on yield of winter wheat. Plant Pathol 29:21–27 (1980). [Google Scholar]
- 25. Priestley RH and Bayles RA, Effects of fungicide treatment on yield of winter wheat and spring barley cultivars. Plant Pathol 31:31–37 (1982). [Google Scholar]
- 26. Magan N and Lacey J, The phylloplane microflora of ripening wheat and effect of late fungicide applications. Ann Appl Biol 109:117–128 (1986). [Google Scholar]
- 27. Bertelsen JR, De Neergaard E and Smedegaard‐Petersen V, Fungicidal effects of azoxystrobin and epoxiconazole on phyllosphere fungi, senescence and yield of winter wheat. Plant Pathol 50:190–205 (2001). [Google Scholar]
- 28. Grimmer MK, Foulkes MJ and Paveley ND, Foliar pathogenesis and plant water relations: a review. J Exp Bot 63:4321–4331 (2012). [DOI] [PubMed] [Google Scholar]
- 29. Sierotzki H and Scalliet G, A review of current knowledge of resistance aspects for the next‐generation succinate dehydrogenase inhibitor fungicides. Phytopathology 103:880–887 (2013). [DOI] [PubMed] [Google Scholar]
- 30. Yoo CY, Pence HE, Hasegawa PM and Mickelbart MV, Regulation of transpiration to improve crop water use. Crit Rev Plant Sci 28:410–431 (2009). [Google Scholar]
- 31. Mueller ND, Gerber JS, Johnston M, Ray DK, Ramankutty N and Foley JA, Closing yield gaps through nutrient and water management. Nature 490:254–257 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.


