Abstract
Inhibition of apoptosis signal–regulating kinase 1, a serine/threonine kinase, leads to improvement in inflammation and fibrosis in animal models of nonalcoholic steatohepatitis. We evaluated the safety and efficacy of selonsertib, a selective inhibitor of apoptosis signal–regulating kinase 1, alone or in combination with simtuzumab, in patients with nonalcoholic steatohepatitis and stage 2 or 3 liver fibrosis. In this multicenter phase 2 trial, 72 patients were randomized to receive 24 weeks of open‐label treatment with either 6 or 18 mg of selonsertib orally once daily with or without once‐weekly injections of 125 mg of simtuzumab or simtuzumab alone. The effect of treatment was assessed by paired pretreatment and posttreatment liver biopsies, magnetic resonance elastography, magnetic resonance imaging–estimated proton density fat fraction, quantitative collagen content, and noninvasive markers of liver injury. Due to the lack of effect of simtuzumab on histology or selonsertib pharmacokinetics, selonsertib groups with and without simtuzumab were pooled. After 24 weeks of treatment, the proportion of patients with a one or more stage reduction in fibrosis in the 18‐mg selonsertib group was 13 of 30 (43%; 95% confidence interval, 26‐63); in the 6‐mg selonsertib group, 8 of 27 (30%; 95% confidence interval, 14‐50); and in the simtuzumab‐alone group, 2 of 10 (20%; 95% confidence interval, 3‐56). Improvement in fibrosis was associated with reductions in liver stiffness on magnetic resonance elastography, collagen content and lobular inflammation on liver biopsy, as well as improvements in serum biomarkers of apoptosis and necrosis. There were no significant differences in adverse events between the treatment groups. Conclusion: These findings suggest that selonsertib may reduce liver fibrosis in patients with nonalcoholic steatohepatitis and stage 2‐3 fibrosis. (Hepatology 2018;67:549‐559).
Abbreviations
- ALT
alanine aminotransferase
- ASK1
apoptosis signal–regulating kinase 1
- AST
aspartate aminotransferase
- CI
confidence interval
- CRN
Clinical Research Network
- JNK
c‐Jun N‐terminal kinase
- MRE
magnetic resonance elastography
- MRI‐PDFF
magnetic resonance imaging–estimated proton density fat fraction
- NAS
nonalcoholic fatty liver disease activity score
- NASH
nonalcoholic steatohepatitis
- α‐SMA
α‐smooth muscle actin
Nonalcoholic steatohepatitis (NASH) is a progressive liver disease characterized by hepatic steatosis, lobular inflammation, ballooning, and perisinusoidal fibrosis in individuals who consume little or no alcohol and who do not have a secondary cause of steatosis.1, 2 NASH is estimated to affect up to 5% of the population in the United States.3, 4, 5 Between 30% and 50% of patients with NASH develop progressive fibrosis, which increases the risk for cirrhosis, liver decompensation, and hepatocellular carcinoma.6, 7, 8, 9 In recent years, NASH has become a leading indication for liver transplantation.10 Emerging data suggest that fibrosis is the most important predictor of all‐cause and liver‐related mortality in NASH; the risk of liver‐related mortality increases with increase in the stage of fibrosis.11, 12, 13, 14 An effective and safe pharmacologic treatment to halt or reverse fibrosis in NASH would address an important unmet medical need.15, 16, 17, 18, 19
Ballooned hepatocytes, representing the activation of apoptosis‐related pathways, are a hallmark of NASH and fibrosis progression.3, 20 In the setting of oxidative stress, activation of apoptosis signal–regulating kinase 1 (ASK1), a serine/threonine signaling kinase, can lead to phosphorylation of p38 mitogen‐activated kinase and c‐Jun N‐terminal kinase (JNK), leading in turn to activation of stress response pathways that worsen hepatic inflammation, apoptosis, and fibrosis.21, 22, 23 Inhibition of ASK1 has therefore been proposed as a target for the treatment of NASH. In a murine model of NASH, selonsertib (formerly GS‐4997), a selective inhibitor of ASK1, significantly improved not only metabolic parameters associated with NASH but also reduced hepatic steatosis, inflammation, and fibrosis.21 Simtuzumab is a humanized monoclonal antibody directed against lysyl oxidase‐like molecule 2, an enzyme that catalyzes the crosslinkage of collagen and elastin, leading to remodeling of the extracellular matrix.24 When the current study was designed, it was hypothesized that inhibition of lysyl oxidase‐like molecule 2 with simtuzumab could have an antifibrotic effect in NASH. Moreover, preclinical data from a murine model of advanced fibrosis suggested that simtuzumab has an additive effect when combined with an ASK1 inhibitor.25 However, results from phase 2 studies of simtuzumab monotherapy—one in patients with F3 fibrosis (NCT01672866) and another in patients with cirrhosis (NCT01672879)—which became available after the start of the current trial, indicated that simtuzumab was ineffective at reducing hepatic fibrosis.26
Currently, the diagnosis of NASH requires a liver biopsy demonstrating the histologic features of steatosis, lobular inflammation, ballooning, and variable degrees of fibrosis.1 Recent studies have demonstrated that the presence of fibrosis is the only independent factor associated with mortality and liver‐related clinical complications in patients with NASH.13 Although liver biopsy is the current gold standard for NASH diagnosis, its limitations include sampling error due to variable distribution of fibrosis and the invasive nature of the procedure. Noninvasive approaches, including imaging and serum tests, are being explored.27, 28 Estimations of liver stiffness by magnetic resonance elastography (MRE) and of the quantity of hepatic fat by magnetic resonance imaging‐estimated proton density fat fraction (MRI‐PDFF) have shown strong correlations with histology and low interobserver variability, suggesting that these noninvasive methods may be reliable, accurate, and precise tools for clinical assessment of the extent of liver fibrosis.29, 30, 31, 32, 33, 34
We conducted a phase 2, randomized, open‐label study to assess the safety and efficacy of selonsertib with and without simtuzumab or simtuzumab alone in patients with moderate to severe fibrosis resulting from NASH. This trial included longitudinal paired assessment with advanced MRI‐derived parameters including MRI‐PDFF and MRE along with liver biopsy, all assessed centrally by blinded reviewers in a multicenter setting.
Patients and Methods
PATIENTS
Patients 18‐70 years of age were enrolled at 23 sites in the United States and Canada from June 8, 2015, to March 31, 2016. All patients provided written informed consent, and the protocol was approved by individual institutional review boards.
The patients included in the trial must have had a liver biopsy within 3 months of screening consistent with a diagnosis of NASH, with presence of either stage 2 or stage 3 fibrosis based upon the NASH Clinical Research Network (CRN) Histologic Scoring System and a nonalcoholic fatty liver disease activity score (NAS) of 5 or higher, with a score of at least 1 for each of the three components (steatosis, hepatocellular ballooning, and lobular inflammation).1 Patients were also required to have at least three of the following features of the metabolic syndrome: abdominal obesity, hypertension, elevated fasting glucose, elevated levels of serum triglycerides, or low levels of high‐density lipoprotein cholesterol (see Supporting Information for ranges). Eligible patients had levels of alanine aminotransferase (ALT) no more than 5 times the upper limit of normal and a liver stiffness by transient elastography (FibroScan; Echosens, Paris, France) of at least 7 kPa. The FibroScan requirement did not apply to patients who qualified on the basis of a historical biopsy.
Key exclusion criteria were presence of cirrhosis or history of decompensated liver disease, as well as positive tests for hepatitis B surface antigen or human immunodeficiency virus RNA. Patients with other causes of chronic liver disease or secondary causes of hepatic steatosis were also excluded. Alcohol consumption in excess of 21 ounces per week for men or 14 ounces per week for women was excluded.
STUDY DESIGN
In this multicenter, open‐label trial, patients were randomly assigned in a 2:2:1:1:1 ratio to receive 24 weeks of treatment with 6 mg or 18 mg of selonsertib alone, 6 mg or 18 mg of selonsertib with 125 mg of simtuzumab, or 125 mg of simtuzumab alone. Selonsertib was administered orally once daily with or without food. Simtuzumab was self‐administered by subcutaneous injection once a week. Randomization and treatment assignment were managed with an Interactive Web Response System using sequentially numbered containers (Bracket, San Francisco, CA) and a block size of 7. Randomization was stratified by presence or absence of diabetes mellitus. Screening assessments included medical history and physical examination, as well as standard laboratory and clinical testing.
ASSESSMENTS AND OUTCOMES
Histology
The presence of NASH was assessed in a blinded fashion by a central pathologist (Z.D.G.) with extensive experience in assessing treatment response in NASH clinical trials. Biopsy specimens were graded according to the NAS, a standardized grading system for steatosis (on a scale of 0‐3), lobular inflammation (on a scale of 0‐3), and hepatocellular ballooning (on a scale of 0‐2), with higher scores indicating increasing disease activity. Improvement in liver fibrosis was defined as a reduction of one or more stage using the NASH CRN Histologic Scoring System. Additional histologic outcomes included progression to cirrhosis and improvement in the NAS and its individual components. Computer‐assisted morphometry was also used to quantify hepatic collagen and fat content using picrosirius red–stained liver sections, as well as α‐smooth muscle actin (α‐SMA) expression.35
Imaging Assessments
In this trial, longitudinal changes in liver fat and liver stiffness from baseline to week 12 and end of study were assessed using MRI‐PDFF and two‐dimensional MRE (60 Hz), respectively. Assessments were performed by an experienced central reader (University of California San Diego Radiology Reading Center) blinded to treatment assignments and clinical and histologic data.35, 37 The methodology and assessments of changes in these parameters in colocalized regions of interest were performed as described.29, 30, 31, 32, 33, 34, 37, 38 All sites underwent a quality‐assessment process prior to study initiation, and all images were approved by the central reader. We determined the proportion of patients in each treatment group who had at least a 30% decrease between baseline and week 24 in MRI‐PDFF40 and at least a 15% reduction in MRE.41 Liver stiffness was also measured by FibroScan.
Serum Biomarkers
Changes from baseline in noninvasive markers of fibrosis, including the Enhanced Liver Fibrosis test (Siemens, Tarrytown, NY) and FibroSure/FibroTest (LabCorp, Burlington, NC), were assessed, as well as changes in markers of liver injury and function, including serum ALT, aspartate aminotransferase (AST), bilirubin, gamma‐glutamyltransferase, and alkaline phosphatase. Changes from baseline in serum levels of cytokeratin‐18 M30 and M65 fractions were measured as indicators of hepatocellular apoptosis and necrosis, respectively, using the M30 Apoptosense and M65 EpiDeath enzyme‐linked immunosorbent assays (Diapharma, West Chester, OH).
Safety
Safety was assessed by the incidence of treatment‐emergent adverse events, including serious adverse events, clinical laboratory tests, and vital signs collected at every visit from first dose to 30 days after the last dose of study drug. The number and percentage of patients who prematurely discontinued the study drug or the study owing to adverse events were calculated.
STATISTICAL ANALYSIS
The sample size assessment was based on clinical experience with other proof‐of‐concept studies to assess directionality across multiple outcomes rather than statistical significance. Descriptive statistics were calculated for continuous exploratory parameters by treatment group. For categorical variables, descriptive statistics with count, percentage of patients in each category, and 95% confidence intervals (CIs) for the percentages within each treatment group based on Clopper‐Pearson were calculated.
Safety was assessed in all patients who received at least one dose of study drug. The effect of treatment on histologic endpoints was assessed in all patients with evaluable liver biopsy results at baseline and week 24. Given the demonstrated lack of efficacy of simtuzumab,26 we conducted an analysis pooling the results from selonsertib groups with and without simtuzumab. Results by the original unpooled dose groups are provided in the Supporting Information.
We also conducted exploratory logistic regression analyses to assess associations between fibrosis improvement and changes from baseline at week 24 in select factors (after adjustment for baseline values).
STUDY OVERSIGHT
This study was approved by the institutional review board or independent ethics committees at all participating sites and conducted in compliance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local regulatory requirements. The study was designed and conducted according to protocol by the sponsor (Gilead) in collaboration with the principal investigators. The sponsor collected the data, monitored study conduct, and performed the statistical analyses. An independent data‐monitoring committee reviewed the progress and provided oversight of the study. The investigators, participating institutions, and sponsor agreed to maintain confidentiality of the data. All authors had access to the data and assumed responsibility for the integrity and completeness of the reported data. A professional writer employed by Gilead Sciences helped prepare the manuscript with input from the authors, and all authors approved the final manuscript. The study protocol is available with this article.
Results
STUDY PATIENTS
Of the 242 patients who were screened, 72 with NASH and stage 2 or 3 fibrosis on biopsy were randomized into this multicenter trial (reasons for screen failure are provided in Supporting Table S1). The majority (65%) of patients had stage 3 fibrosis and a NAS ≥6 (71%) at baseline. Overall, 86% had severe hepatocellular ballooning. Detailed characteristics of the study cohort are provided in Table 1. Approximately two thirds (69%) of patients were female, the median age was 56 years, and 71% of patients had diabetes mellitus. The overall median body mass index was 33 kg/m2; the median body mass index in the simtuzumab‐alone group was nonsignificantly higher at 37 kg/m2. Otherwise, there were no substantial differences between the study cohorts across the treatment groups at baseline.
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic |
Selonsertib 18 mg ± Simtuzumab (n = 32) |
Selonsertib 6 mg ± Simtuzumab (n = 30) |
Simtuzumab (n = 10) |
|---|---|---|---|
| Demographic factors | |||
| Age, years | 55 (49‐61) | 54 (46‐62) | 57 (56‐59) |
| Female sex | 22 (69) | 22 (73) | 6 (60) |
| White race | 28 (88) | 27 (90) | 10 (100) |
| Diabetes mellitus | 21 (66) | 22 (73) | 8 (80) |
| Serum biochemical levels | |||
| ALT, U/L | 69 (52‐93) | 56 (46‐91) | 61 (46‐83) |
| AST, U/L | 48 (37‐71) | 50 (36‐82) | 56 (37‐76) |
| Gamma‐glutamyl transferase, U/L | 52 (38‐83) | 62 (40‐78) | 48 (35‐86) |
| Alkaline phosphatase, U/L | 86 (73‐100) | 88 (64‐101) | 66 (53‐97) |
| Total bilirubin, mg/dL | 0.5 (0.4‐0.6) | 0.4 (0.3‐0.5) | 0.5 (0.4‐0.6) |
| Metabolic factors | |||
| Body mass index, kg/m2 | 33 (30‐37) | 33 (29‐41) | 37 (31‐37) |
| Lipids | |||
| Triglycerides, mg/dL | 181 (138‐271) | 176 (125‐232) | 178 (111‐238) |
| Total cholesterol, mg/dL | 187 (151‐211) | 201 (170‐213) | 183 (168‐196) |
| High‐density lipoprotein, mg/dL | 40 (33‐49) | 43 (36‐52) | 42 (36‐44) |
| Low‐density lipoprotein, mg/dL | 111 (80‐135) | 122 (109‐143) | 110 (100‐123) |
| Noninvasive measures | |||
| MRI‐PDFF, % | 18 (11‐24) | 17 (11‐22) | 16 (9‐20) |
| MRE, kPa | 3.7 (2.9‐4.8) | 3.7 (3.1‐4.5) | 3.4 (3.0‐4.3) |
| FibroSure/FibroTest | 0.35 (0.16‐0.49) | 0.29 (0.20‐0.46) | 0.38 (0.28‐0.61) |
| FibroScan, kPa | 10.4 (8.5‐14.9) | 11.0 (8.3‐11.3) | 13.5 (9.4‐15.0) |
| Liver histologic findings | |||
| NASH CRN fibrosis stage 3 | 21 (66) | 20 (67) | 6 (60) |
| NAS 6‐8 | 22 (69) | 24 (80) | 5 (50) |
| Steatosis grade 2‐3 | 13 (41) | 9 (30) | 2 (20) |
| Lobular inflammation grade 3 | 21 (66) | 23 (77) | 5 (50) |
| Hepatocyte ballooning grade 2 | 26 (81) | 27 (90) | 9 (90) |
| Hepatic collagen content, % | 3.8 (2.6‐4.8) | 4.3 (2.8‐5.6) | 3.5 (2.4‐5.8) |
| α‐SMA, % | 3.2 (2.0‐6.4) | 3.8 (2.3‐7.2) | 4.9 (2.3‐9.9) |
Values are either n (%) or median (interquartile range).
EFFICACY
Histologic Outcomes
The proportion of patients with at least a one‐stage reduction in fibrosis after 24 weeks of treatment in the 18‐mg selonsertib group was 13 of 30 (43%; 95% CI, 26‐63); in the 6‐mg selonsertib group, 8 of 27 (30%; 95% CI, 14‐50); and in the simtuzumab‐alone group, 2 of 10 (20%; 95% CI, 3‐56) (Fig. 1). The proportion with progression to cirrhosis at week 24 in the 18‐mg selonsertib group was 1 of 30 (3%; 95% CI, 0‐17); in the 6‐mg selonsertib group, 2 of 27 (7%; 95% CI, 1‐24); and in the simtuzumab‐alone group, 2 of 10 (20%; 95% CI, 3‐56). Fibrosis responses according to the original five unpooled treatment groups are provided in Supporting Fig. S2.
Figure 1.
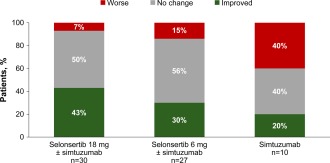
Proportions of patients with improved, unchanged, or worse fibrosis from baseline to week 24. Fibrosis improvement is defined as a decrease of at least one stage in fibrosis according to the NASH CRN Histologic Scoring System. Data are shown for the 67 patients with liver biopsies evaluable for fibrosis at baseline and week 24.
Median percent change from baseline at week 24 in morphometric collagen content for patients receiving 18 and 6 mg of selonsertib was –8.7% and –8.2%, respectively, compared with an increase of 2.1% in those receiving simtuzumab alone. Changes in fibrosis stage correlated with changes in hepatic collagen content by morphometry (r = 0.54, P < 0.001).
The proportion of patients with improvement of ≥1 point in the NAS in the 18‐mg selonsertib group was 16 of 31 patients (52%); in the 6‐mg selonsertib group, 11 of 27 patients (41%); and in the simtuzumab group, 6 of 10 (60%). Improvements were noted in all three components of the NAS—steatosis, lobular inflammation, and hepatocellular ballooning (Table 2)—but did not differ substantially by treatment group.
Table 2.
Change in Histology and Imaging From Baseline to Week 24
|
Selonsertib 18 mg ± Simtuzumab (n = 32) |
Selonsertib 6 mg ± Simtuzumab (n = 30) |
Simtuzumab (n = 10) | |
|---|---|---|---|
| Histology | |||
| Patients with improvement in fibrosis (CRN) | 13/30 (43) | 8/27 (30) | 2/10 (20) |
| Patients with progression to cirrhosis | 1/30 (3) | 2/27 (7) | 2/10 (20) |
| Hepatic collagen content (% change from baseline) | −8.7 (−48.7 to 29.6) | −8.2 (−48.1 to 40.0) | 2.1 (−27.3 to 37.9) |
| Patients with ≥1 point reduction in NAS | 16/31 (52) | 11/27 (41) | 6/10 (60) |
| Patients with ≥2 point reduction in NAS | 7/31 (23) | 5/27 (19) | 2/10 (20) |
| Steatosis ≥1 point reduction | 10/31 (32) | 8/27 (30) | 2/10 (20) |
| Lobular inflammation ≥1 point reduction | 10/31 (32) | 6/27 (22) | 2/10 (20) |
| Ballooning ≥1 point reduction | 5/31 (16) | 9/27 (33) | 3/10 (30) |
| Imaging | |||
| MRI‐PDFF, % change from baseline | −4.55 (−32.72 to 20.87) | −6.67 (−21.66 to 15.25) | −12.72 (−24.62 to −6.53) |
| Patients with ≥30% reduction | 8/31 (26) | 3/24 (13) | 1/10 (10) |
| MRE, % change from baseline | 1.79 (−10.96 to 11.19) | −0.09 (−19.00 to 8.10) | 2.06 (−8.65 to 22.33) |
| Patients with ≥15% reduction | 4/26 (15) | 7/22 (32) | 0 |
| FibroScan, kPa | 0.20 (−3.50 to 1.40) | −0.80 (−1.90 to 2.30) | −0.50 (−3.80 to 3.40) |
Values are either n/N (%) or median (interquartile range).
Factors Associated With Fibrosis Improvement
To identify factors associated with improvement in fibrosis at week 24, we performed exploratory logistic regression analyses of changes from baseline to week 24 in several factors (see Fig. 2). Changes in body weight were not associated with changes in fibrosis. After adjustment for baseline values, an improvement in the NAS, as well as relative reductions in hepatic collagen content, α‐SMA expression, liver stiffness by FibroScan, gamma‐glutamyltransferase, and the M30 and M65 fractions of cytokeratin‐18 were significantly associated with fibrosis improvement (Fig. 2). Fibrosis responders also had greater reductions in liver stiffness by MRE and were more likely to have improvement or no change in the lobular inflammation component of the NAS compared with worsening (odds ratio, 4.80; 95% CI, 0.68‐33.80; P = 0.12), but these differences were not statistically significant.
Figure 2.
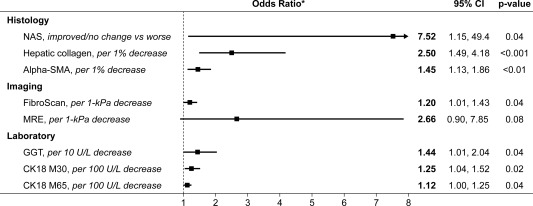
Factors associated with fibrosis improvement. Changes in body weight, MRI‐PDFF, and grades of steatosis and ballooning were not associated with fibrosis improvement. *All odds ratios were adjusted for baseline values. Abbreviations: CK18, cytokeratin‐18; GGT, gamma‐glutamyltransferase.
Imaging Outcomes
The proportion of patients who achieved ≥15% relative reductions in liver stiffness by MRE from baseline to week 24 in the 18‐mg selonsertib group was 4 of 26 (15%); in the 6‐mg selonsertib group, 7 of 22 (32%); and in the simtuzumab‐alone group, 0 of 10. Compared with fibrosis nonresponders, fibrosis responders had nonsignificantly greater reductions in liver stiffness by MRE (median –2.3% versus 3.0%; P = 0.17; Fig. 3A). Changes in liver stiffness by FibroScan were not associated with selonsertib treatment (Table 3) or with fibrosis improvement. The proportion of patients who achieved ≥30% relative reductions in steatosis by MRI‐PDFF from baseline to week 24 in the 18‐mg selonsertib group was 8 of 31 (26%); in the 6‐mg selonsertib group, 3 of 24 (13%); and in the simtuzumab‐alone group, 1 of 10 (10%). Patients with improved steatosis on biopsy had greater reductions in MRI‐PDFF compared to those without steatosis improvement (median –20.2% versus –0.8%; P = 0.01; Fig. 3B).
Figure 3.
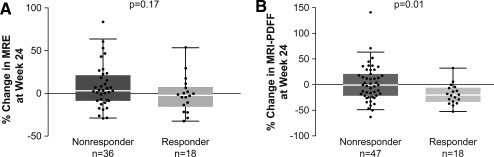
Change from baseline in MRE and MRI‐PDFF from baseline to week 24. Central line represents median value, box represents interquartile range, and whiskers show range not including outliers, which are represented by dots. (A) Change in MRE stiffness from baseline to week 24 in fibrosis responders and nonresponders. Fibrosis response was defined as a reduction of one or more stage in fibrosis. (B) Change in MRI‐PDFF from baseline to week 24 in steatosis responders and nonresponders. Steatosis response was defined as a reduction of one or more grade in steatosis.
Table 3.
Change in Serum Biomarkers and Metabolic Factors From Baseline to Week 24
|
Selonsertib 18 mg ± Simtuzumab (n = 32) |
Selonsertib 6 mg ± Simtuzumab (n = 30) |
Simtuzumab (n = 10) | |
|---|---|---|---|
| Enhanced Liver Fibrosis test | 0.02 (–0.34 to 0.52) | –0.07 (–0.46 to 0.36) | –0.13 (–0.35 to 0.05) |
| FibroSure/FibroTest | –0.01 (–0.03 to 0.03) | 0.02 (–0.03 to 0.08) | 0.01 (–0.04 to 0.05) |
| ALT, U/L | –8 (–24 to 23) | –6 (–29 to 7) | –3 (–16 to –1) |
| AST, U/L | –5 (–13 to 13) | –4 (–25 to 17) | –3 (–28 to –1) |
| Gamma‐glutamyltransferase, U/L | –7 (–19 to 5) | –2 (–15 to 11) | –2 (–9 to 2) |
| Triglycerides, mg/dL | –21 (–41 to 29) | 12 (–6 to 32) | –30 (–9 to 28) |
| Total cholesterol, mg/dL | –10 (–33 to 8) | –5 (–24 to 14) | –13 (–36 to 2) |
| High‐density lipoprotein, mg/dL | –2 (–3 to 1) | 1 (–5 to 5) | 2 (–4 to 5) |
| Low‐density lipoprotein, mg/dL | –10 (–24 to 6) | –5 (–19 to 9) | –25 (–31 to 0) |
| HOMA‐IR | 0.98 (–2.4 to 7.63) | 2.17 (0.16‐4.77) | –0.22 (–1.90 to 0.12) |
| Hemoglobin A1c, % | –0.2 (–0.5 to 0.2) | 0.2 (0.0‐0.5) | –0.2 (–1.1 to 0.6) |
| CK‐18 fractions | |||
| M30, U/L | –110 (–338 to 124) | –34 (–445 to 241) | –89 (–378 to 146) |
| M65, U/L | –222 (–811 to 238) | –162 (–820 to 341) | –185 (–820 to 251) |
Values are median (interquartile range)
Abbreviations: CK, cytokeratin; HOMA‐IR, homeostatic model assessment for insulin resistance.
Percentage change from baseline in MRE and MRI‐PDFF, as well as proportions of patients achieving a ≥15% reduction in MRE and a ≥30% reduction in MRI‐PDFF by week 12 of treatment, is shown in Supporting Table S3.
LIVER BIOCHEMISTRY
Across all groups, decreases from baseline in median ALT and gamma‐glutamyltransferase were observed at week 24 (Table 3). Greater median relative decreases in cytokeratin‐18 M30 and M65 fractions were observed in patients receiving 18 mg of selonsertib (−31% and −44%, respectively) and in those receiving 6 mg of selonsertib (−6% and −35%, respectively) than among patients receiving simtuzumab alone (22% and −4%, respectively). Changes from baseline in Enhanced Liver Fibrosis scores were minimal in all treatment groups (Table 3).
SAFETY
Table 4 provides a summary of safety results. The majority of patients in all three treatment groups experienced at least one adverse event, most of which were mild to moderate in severity. Higher proportions of patients in the selonsertib groups experienced headache, nausea, sinusitis, nasopharyngitis, upper abdominal pain, back pain, and fatigue. Three selonsertib‐treated patients discontinued treatment owing to adverse events (worsening schizophrenia, numbness of face and upper extremities, and elevated liver enzymes). Five patients experienced serious adverse events (see Table 4). No single serious adverse event was experienced by more than 1 patient.
Table 4.
Safety
|
Selonsertib 18 mg ± Simtuzumab (n = 32) |
Selonsertib 6 mg ± Simtuzumab (n = 30) |
Simtuzumab (n = 10) | |
|---|---|---|---|
| Patients with adverse events | 24 (75) | 26 (87) | 7 (70) |
| Patients with grade 3‐4 adverse events | 3 (9) | 1 (3) | 1 (10) |
| Patients with serious adverse events | 3 (9)a | 2 (7) † | 0 |
| Patients who discontinued study treatment due to adverse event | 2 (6) | 1 (3) | 0 |
| Most common adverse events | |||
| Headache | 9 (28) | 4 (13) | 0 |
| Nausea | 6 (19) | 4 (13) | 0 |
| Sinusitis | 4 (13) | 3 (10) | 1 (10) |
| Nasopharyngitis | 3 (9) | 4 (13) | 0 |
| Upper abdominal pain | 5 (16) | 1 (3) | 0 |
| Fatigue | 5 (16) | 1 (3) | 0 |
| Grade 3‐4 laboratory abnormalities ‡ | 9 (28) | 5 (17) | 4 (40) |
| Lymphocytes <500 mm3 | 1 (10) | 0 | 0 |
| Hypocalcemia <7.0 mg/dL | 0 | 0 | 1 (10) |
| Alkaline phosphatase >5 × ULN | 1 (3) | 0 | 0 |
| ALT >5 × ULN | 2 (6) | 0 | 0 |
| AST >5 × ULN | 2 (6) | 0 | 0 |
| Gamma‐glutamyl transferase >5 × ULN | 2 (6) | 1 (3) | 0 |
| Phosphate <1.5 mg/dL | 1 (3) | 0 | 0 |
| Serum glucose >250 mg/dL | 2 (6) | 4 (13) | 2 (20) |
| Glomerular filtration rate <30 mL/minute/1.73 m2 | 0 | 0 | 1 (10) |
| INR >2.5 × ULN | 1 (3) | 0 | 0 |
| Triglycerides >500 mg/dL | 2 (6) | 0 | 1 (10) |
A 59‐year‐old woman had a transient ischemic attack, a 54‐year‐old woman had hypoesthesia, and a 52‐year‐old woman had two serious adverse events: abdominal pain and influenza.
A 32‐year‐old woman experienced a serious adverse event of rectal bleeding, and a 57‐year‐old man experienced seven serious adverse events: chest pain, bronchitis, congestive cardiac failure, pneumonia, sepsis, and two events of dyspnea.
Values that were increased at least one toxicity grade from baseline at any time postbaseline, up to and including the last dosing date plus 30 days.
Abbreviations: INR, international normalized ratio; ULN, upper limit of normal.
Treatment‐emergent grade 3 and 4 laboratory abnormalities are also provided in Table 4. Four patients developed a transient increase of ALT or AST of at least 2 × baseline and at least 3 × upper limit of normal. Three of these 4 patients had grade 2 elevations at baseline. Only 1 patient (mentioned above) discontinued study treatment owing to elevated liver enzymes.
Discussion
In this multicenter, phase 2 trial using paired longitudinal assessment of treatment response with liver biopsy as well as advanced MRI methods including MRE and MRI‐PDFF, selonsertib‐treated patients had numerically higher rates of fibrosis improvement and lower rates of fibrosis progression than patients treated with simtuzumab alone over a 24‐week treatment period. These findings suggest that selonsertib may reduce liver fibrosis in patients with NASH and moderate to severe fibrosis. The novelty of this proof‐of‐concept trial includes its use of standardized assessments of treatment response using MRI‐PDFF and MRE with central reading of the images in a colocalized manner. Moreover, this study evaluated a combination of drugs with distinct mechanisms of action in patients with NASH.
Selonsertib is a selective inhibitor of ASK1, a ubiquitously expressed serine/threonine kinase which is activated by oxidative stress to promote hepatocellular apoptosis, inflammation, and fibrosis.21, 41 ASK1 is normally bound and repressed by thiol‐containing antioxidant proteins, including thioredoxin 1.42 Pathological conditions that increase oxidative stress result in ASK1 autoactivation, resulting in downstream phosphorylation of p38 and JNK, which mediate diverse cellular responses by phosphorylating cytosolic substrates and nuclear transcription factors including activating transcription factor 2 and c‐Jun.43 Both p38 and JNK have well‐characterized roles in hepatocytes, macrophages, and myofibroblasts to promote lipotoxicity, inflammation, and fibrosis. In preclinical models of NASH, genetic deletion22, 23 or pharmacological inhibition of ASK121 reduces p38 and JNK phosphorylation, resulting in reduced hepatic steatosis, inflammation, and fibrosis. Based on the complementary mechanisms of action of selonsertib and simtuzumab, the heterogeneity of patients with NASH, and preclinical data suggesting a benefit of dual therapy over the single agents in a murine model of advanced fibrosis,25 we hypothesized that a combination of agents would improve efficacy. However, the addition of simtuzumab to selonsertib had no discernible benefit.
Given that fibrosis stage is the most important determinant of outcome in patients with NASH,11, 12, 13 the possible antifibrotic effect of selonsertib may be clinically significant. Patients who responded to selonsertib, i.e., those who had an improvement of one or more stage from baseline to week 24 according to the NASH CRN Histologic Scoring System, had consistent reductions in other markers of fibrosis, including hepatic collagen content, liver stiffness by MRE, and α‐SMA expression. The consistency and directionality of these responses suggest that these findings are not likely to be due to sampling error of liver biopsy. The decreased expression of α‐SMA suggests that decreased collagen formation through diminished stellate activation may have contributed to the observed attenuation of hepatic fibrosis in the selonsertib‐containing treatment groups. Moreover, compared with nonresponders, fibrosis responders demonstrated improvements in liver biochemistry, serum markers of apoptosis and necrosis (cytokeratin‐18 M30 and M65), and improvements in lobular inflammation and hepatic steatosis by morphometry. Although changes in liver stiffness by FibroScan were not significant, this may reflect the reduced precision and accuracy of FibroScan in obese patients.44
This randomized trial assessed changes in liver fat content by MRI‐PDFF and liver stiffness by MRE, along with liver biopsy assessments before and after treatment, with results assessed by central reviewers blinded to treatment assignments. Liver fibrosis on histology was assessed using a comprehensive set of parameters including NASH CRN fibrosis stage, quantitative digital morphometry, and α‐SMA immunostaining before and after treatment. The study population included patients with more active NASH and had a higher proportion of patients with advanced fibrosis than in previous phase 2 trials. It likely underscores a phenomenon that inclusion of patients with greater disease severity may help shorten the duration of phase 2 studies because the likelihood of improvement due to a type 2 error is low.
Generalization of the results of this study is limited by its small size and exploratory nature. The pooling of the selonsertib groups with and without simtuzumab was also not prespecified. The open‐label design of this trial is unlikely to have affected the interpretation of the histology and imaging assessments because the central readers were blinded to group assignment. Although the trial did not include a placebo group, the simtuzumab‐alone group served as a de facto placebo group due to lack of additive efficacy to selonsertib in this study and as monotherapy in two large phase 2b studies in patients with NASH and advanced fibrosis.26
In this phase 2 exploratory trial, selonsertib appeared to improve liver fibrosis in a substantial proportion of patients with NASH and stage 2 or 3 fibrosis, suggesting that it has the potential to help address an important unmet medical need for an effective antifibrotic therapy for patients with NASH and advanced fibrosis. Because fibrosis is a key predictor of liver mortality in nonalcoholic fatty liver disease, further studies are needed to assess the benefits of selonsertib in improving long‐term outcomes associated with NASH‐related fibrosis in a larger, randomized controlled trial. Phase 3 studies of selonsertib in patients with NASH and bridging fibrosis (STELLAR‐3; NCT03053050) and compensated cirrhosis (STELLAR‐4; NCT03053063) are currently under way.
Author names in bold designate shared co‐first authorship.
Supporting information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.29514/suppinfo.
Supporting Information
Acknowledgment
We thank the patients and their families, as well as the investigators and site personnel. Editorial and writing assistance was provided by David McNeel of Gilead Sciences.
Potential conflict of interest: Dr. Caldwell received grants from Gilead, Genfit, Galmed, NGM, Conatus, Immuron, VitalTherapy, and Intercept. Dr. Lawitz received grants from Bristol‐Myers Squibb, Conatus, Exalenz, Galectin, Galmed, Genfit, Gilead, Intercept, Madrigal, Novartis, Octeta, and Zydus. Dr. Mantry consults, advises, is on the speakers' bureau, and received grants from AbbVie, Gilead, Bristol‐Myers Squibb, Genfit, Intercept, Salix, Tobira, Merck, and Conatus. Dr. Jayakumar is on the speakers' bureau for Gilead and Astellas. She received grants from Bristol‐Myers Squibb. Dr. Loomba consults and received grants from Gilead. Dr. Charlton consults and received grants from Gilead, Intercept, NGM, Genfit, and Novartis. He received grants from Conatus. Dr. Diehl consults, advises, and received grants from Cellgene and Immuron. She consults and advises Taiwan Pharmaceuticals and Novartis. She received grants from Metabolomics, Gilead, Galmed, NGM, Bristol‐Myers Squibb, Madrigal, and Galactin.
Supported by Gilead Sciences.
Presented in part at the annual meeting of the American Association for the Study of Liver Diseases held in Boston on November 11‐15, 2016.
REFERENCES
- 1. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver‐related mortality. Hepatology 2011;53:1874‐1882. [DOI] [PubMed] [Google Scholar]
- 3. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005‐2023. [DOI] [PubMed] [Google Scholar]
- 4. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274‐285. [DOI] [PubMed] [Google Scholar]
- 5. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 6. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) . EASL‐EASD‐EASO clinical practice guidelines for the management of non‐alcoholic fatty liver disease. Diabetologia 2016;59:1121‐1140. [DOI] [PubMed] [Google Scholar]
- 7. Ratziu V, Goodman Z, Sanyal A. Current efforts and trends in the treatment of NASH. J Hepatol 2015;62(1 Suppl.):S65‐S75. [DOI] [PubMed] [Google Scholar]
- 8. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686‐690. [DOI] [PubMed] [Google Scholar]
- 9. Torres DM, Harrison SA. Nonalcoholic fatty liver disease: fibrosis portends a worse prognosis. Hepatology 2015;61:1462‐1464. [DOI] [PubMed] [Google Scholar]
- 10. Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011;141:1249‐1253. [DOI] [PubMed] [Google Scholar]
- 11. Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in non‐alcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology 2017;65:1557‐1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology 2015;61:1547‐1554. [DOI] [PubMed] [Google Scholar]
- 13. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, et al. Decreased survival of subjects with elevated liver function tests during a 28‐year follow‐up. Hepatology 2010;51:595‐602. [DOI] [PubMed] [Google Scholar]
- 15. Bugianesi E, Marzocchi R, Villanova N, Marchesini G. Non‐alcoholic fatty liver disease/non‐alcoholic steatohepatitis (NAFLD/NASH): treatment. Best Pract Res Clin Gastroenterol 2004;18:1105‐1116. [DOI] [PubMed] [Google Scholar]
- 16. Neuschwander‐Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo‐controlled trial. Lancet 2015;385:956‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ratziu V. Novel pharmacotherapy options for NASH. Dig Dis Sci 2016;61:1398‐1405. [DOI] [PubMed] [Google Scholar]
- 18. Friedman SL, Bansal MB. Reversal of hepatic fibrosis—fact or fantasy? Hepatology 2006;43(2 Suppl. 1):S82‐S88. [DOI] [PubMed] [Google Scholar]
- 19. Perazzo H, Dufour JF. The therapeutic landscape of non‐alcoholic steatohepatitis. Liver Int 2017;37:634‐647. [DOI] [PubMed] [Google Scholar]
- 20. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413‐1419. [DOI] [PubMed] [Google Scholar]
- 21. Budas G, Karnik S, Jonnson T, Shafizadeh T, Watkins S, Breckenridge D, et al. Reduction of liver steatosis and fibrosis with an ASK1 inhibitor in a murine model of NASH is accomplished by improvements in cholesterol, bile acid and lipid metabolism [Abstract]. J Hepatol 2016;64(Suppl.):S170. [Google Scholar]
- 22. Yamamoto E, Dong YF, Kataoka K, Yamashita T, Tokutomi Y, Matsuba S, et al. Olmesartan prevents cardiovascular injury and hepatic steatosis in obesity and diabetes, accompanied by apoptosis signal regulating kinase‐1 inhibition. Hypertension 2008;52:573‐580. [DOI] [PubMed] [Google Scholar]
- 23. Wang PX, Ji YX, Zhang XJ, Zhao LP, Yan ZZ, Zhang P, et al. Targeting CASP8 and FADD‐like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat Med 2017;23:439‐449. [DOI] [PubMed] [Google Scholar]
- 24. Talal AH, Feron‐Rigodon M, Madere J, Subramanian GM, Bornstein JD. Simtuzumab, an antifibrotic monoclonal antibody against lysyl oxidase‐like 2 (LOXL2) enzyme, appears safe and well tolerated in patients with liver disease of diverse etiology [Abstract]. J Hepatol 2013;58(Suppl. 1):S532. [Google Scholar]
- 25. Ikenaga N, Liu SB, Peng ZW, Greenstein AE, French D, Smith V, et al. Dual combination therapy directed against lysyl oxidase‐like 2 (LOXL2) and apoptosis signal–regulating kinase 1 (ASK1) potently inhibits fibrosis and portal hypertension in a new mouse model of PSC‐like liver disease [Abstract]. Hepatology 2015;62(Suppl.):881A. [Google Scholar]
- 26. Sanyal A, Abdelmalek MF, Diehl AM, Caldwell S, Shiffman ML, Ghalib R, et al. Efficacy and safety of simtuzumab for the treatment of NASH with bridging fibrosis or cirrhosis: results of two phase 2b, dose‐ranging, randomized, placebo‐controlled trials [Abstract]. J Hepatol 2017;66(Suppl):S54. [Google Scholar]
- 27. Karanjia RN, Crossey MM, Cox IJ, Fye HK, Njie R, Goldin RD, et al. Hepatic steatosis and fibrosis: non‐invasive assessment. World J Gastroenterol 2016;22:9880‐9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu K, Xu W, Wong VWS. Serum biomarkers for nonalcoholic fatty liver disease: are we there yet? Hepatology 2017;65:8‐11. [DOI] [PubMed] [Google Scholar]
- 29. Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015;61:1239‐1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 2012;56:922‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013;58:1930‐1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non‐alcoholic fatty liver disease—MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther 2012;36:22‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, et al. Sitagliptin vs. placebo for non‐alcoholic fatty liver disease: a randomized controlled trial. J Hepatol 2016;65:369‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non‐invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol 2016;65:1006‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fontana RJ, Goodman ZD, Dienstag JL, Bonkovsky HL, Naishadham D, Sterling RK, et al. Relationship of serum fibrosis markers with liver fibrosis stage and collagen content in patients with advanced chronic hepatitis C. Hepatology 2008;47:789‐798. [DOI] [PubMed] [Google Scholar]
- 36. Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging 2011;34:729‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bannas P, Kramer H, Hernando D. Quantitative magnetic resonance imaging of hepatic steatosis: validation in ex vivo human livers. Hepatology 2015;62:1444‐1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging 2013;37:544‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Asrani SK, Talwalkar JA, Kamath PS, Shah VH, Saracino G, Jennings L, et al. Role of magnetic resonance elastography in compensated and decompensated liver disease. J Hepatol 2014;60:934‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Middleton MS, Lawitz E, Jayakumar S, Mantry P, Caldwell S, Diehl AM, et al. Hepatic proton density fat fraction correlates with histologic measures of steatosis and is responsive to changes in those measures in a multicenter nonalcoholic steatohepatitis clinical trial [Abstract]. J Hepatol 2017;66(Suppl.):S668. [Google Scholar]
- 41. Loomba R, Lawitz E, Ghalib R, Elkhashab M, Caldwell S, Abdelmalek M, et al. Longitudinal changes in liver stiffness by magnetic resonance elastography, liver fibrosis, and serum markers of fibrosis in a multicenter clinical trial in nonalcoholic steatohepatitis [Abstract]. J Hepatol 2017;66(Suppl.):S671. [Google Scholar]
- 42. Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997;275:90‐94. [DOI] [PubMed] [Google Scholar]
- 43. Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal–regulating kinase (ASK) 1. EMBO J 1998;17:2596‐2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy‐proven nonalcoholic fatty liver disease. Gastroenterology 2017;152:598‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.29514/suppinfo.
Supporting Information


