Abstract
Objective
The dopamine hypothesis is one of the most influential theories of the neurobiological background of schizophrenia (SCZ). However, direct evidence for abnormal dopamine-related subcortical-cortical circuitry disconnectivity is still lacking. The aim of this study was therefore to test dopamine-related substantia nigra (SN)-based striato-thalamo-cortical resting-state functional connectivity (FC) in SCZ.
Method
Based on our a priori hypothesis, we analyzed a large sample resting-state functional magnetic resonance imaging (fMRI) dataset from first-episode drug-naïve SCZ patients (n = 112) and healthy controls (n = 82) using the SN as the seed region for an investigation of striato-thalamo-cortical FC. This was done in the standard band of slow frequency oscillations and then in its subfrequency bands (Slow4 and Slow5). Results: The analysis showed in SCZ: (1) reciprocal functional hypo-connectivity between SN and striatum, with differential patterns for Slow5 and Slow4; (2) functional hypo-connectivity between striatum and thalamus, as well as functional hyper-connectivity between thalamus and sensorimotor cortical areas, specifically in Slow4; (3) correlation of thalamo-sensorimotor functional hyper-connectivity with psychopathological symptoms. Conclusions: We demonstrate abnormal dopamine-related SN-based striato-thalamo-cortical FC in slow frequency oscillations in first-episode drug-naive SCZ. This suggests that altered dopaminergic function in the SN leads to abnormal neuronal synchronization (as indexed by FC) within subcortical-cortical circuitry, complementing the dopamine hypothesis in SCZ on the regional level of resting-state activity.
Keywords: schizophrenia/substantia nigra/resting-state, fMRI/functional connectivity/slow frequency bands/neural synchronization
Introduction
Schizophrenia (SCZ) is a severe, chronic mental disorder that affects about 1% of the general population and is characterized by positive symptoms (eg, hallucinations, delusions and disorganized behavior), negative symptoms (eg, blunted affects, avolition and social withdrawal) and cognitive impairments.1,2 The investigation of biological underpinnings of SCZ is a crucial step for the rational development of effective treatments for this illness.3
Among many competing hypotheses,4 the dopamine hypothesis of SCZ is one of the most influential theories on the neurobiological background of the disorder.5,6 In the last decades, the hypothesis has evolved from a purely biochemical framework to a more complex anatomical-molecular pathophysiologic model.6–8 According to this model, the tonic activity of dopaminergic neurons of the ventral tegmental area and substantia nigra (SN) is decreased.6–8 This, in turn, leads to up-regulation with hyper-sensitization and increased stimulus-related phasic activity of dopaminergic receptors (especially D2 receptors) in subsequent subcortical and cortical regions and striato-thalamo-cortical pathways.6–8 In vivo molecular imaging studies give robust support for there being increased dopamine release and synthesis capacity in selected regions, such as the striatum, in SCZ.7,9,10 In contrast, the impact of dopaminergic changes on neural activity in dopamine-related subcortical-cortical circuitry from the SN, via the striatum and thalamus, to the cortex remains unclear.10
On a regional level, functional magnetic resonance imaging (fMRI) studies, including resting-state studies, have demonstrated that different subcortical and cortical brain regions are organized in functionally connected large-scale networks.11 The communication between these networks’ regions seems to be coordinated by low-frequency neural fluctuations, which are thought to be fundamental to information transfer and processing.11–14 Investigating resting-state fMRI functional connectivity (FC) allows the probing of long distance correlations in these fluctuations between different brain regions.12,14,15 A role of dopaminergic transmission in the modulation of intra-network FC between subcortical and cortical regions was demonstrated in fMRI studies in healthy subjects after administration of dopaminergic substances.16–19 In particular, pro-dopaminergic substances (eg, levodopa or amisulpiride) increased sensorimotor network FC between SN, putamen, midcingulate and sensorimotor cortical areas, as well as the FC between ventral striatum, prefrontal cortex and insula, while anti-dopaminergic challenge (eg, haloperidol) decreased the FC between the same subcortical-cortical regions.16–20 However, no investigation focusing a priori21 on how altered dopamine-related SN activity affects regional and network level activity has been reported so far (see22–26 for some indirect support though). More specifically, direct evidence for dopamine-related SN-based striato-thalamo-cortical resting-state disconnectivity in SCZ is still pending.
In this context, specific frequency bands can play a key role in activity synchronization within a particular network.11–14,27,28 Interestingly, within the standard frequency band (SFB) of low-frequency oscillations (0.01–0.08 Hz), Slow5 (0.01–0.027Hz) shows stronger power and longer cycle duration, while Slow4 (0.027–0.073Hz) is strongest throughout the basal ganglia, thalamus and sensorimotor cortical areas, potentially playing a role in subcortical-cortical communication loops.11–14,27–31 One would consequently expect differential effects in Slow5 and Slow4 in the SN-related subcortical-cortical FC abnormalities in SCZ. That remains to be tested though.
Finally, dopaminergic dysfunction, especially in the nigro-striatal pathway, has been strongly related to sensorimotor symptoms, further supporting its clinical relevance.32,33 Sensory processing alterations—eg, primary sensory dysfunction and integration—and psychomotor abnormalities—eg, psychomotor retardation and dyskinetic syndrome—ranging from subtle disturbances (neurological soft signs) to extreme psychomotor alterations (catatonic syndrome),34–38 have consistently been demonstrated in SCZ.33–35,39–41 These alterations are often present to some degree before clinical onset (as part of the basic symptoms and observed signs in high-risk populations) and clinically manifest in first-episode and drug-naïve patients; many of them then worsen during full-blown psychotic relapses and with antipsychotic treatment, and can persist in residual states.32,37,39,40,42 Furthermore, sensorimotor symptoms have been mainly related to negative symptoms and cognitive deficits,32,43 and may be considered as intermediaries between neurobiological dysfunction and typical psychotic symptomatology.41,44 Clinical correlations of dopamine-related SN-based striato-thalamo-cortical resting-state disconnectivity thus need to be further investigated.
Aims of the Study
The main aim of the study was to test the dopamine hypothesis of SCZ on a regional and network level; more specifically, SN-based abnormalities in striato-thalamo-cortical FC. For that purpose, we used the SN as a seed region for subsequent investigation of striato-thalamo-cortical FC in the SFB, and then in its subfrequency bands (Slow4 and Slow5) in order to better characterize these potential alterations, and explored clinical correlations. This was done in a large sample of first-episode, drug-naïve schizophrenic patients (which were compared with healthy subjects). Based on the dopamine hypothesis and previous literature (as above), we hypothesized that there would be subcortical-cortical alterations in FC from SN to striatum and thalamus, as well as from the latter to sensorimotor cortical areas, with differential patterns in Slow5 and Slow4.
Methods
The study population comprised of 112 treatment-naïve first-episode SCZ patients and 82 healthy controls (HC), part of a sample partly used in our previous work.45,46 A standard preprocessing pipeline was applied, including nonlinear alignment to the MNI ICBM152 template, motion scrubbing, and band-pass filtering within the SFB, Slow5 and Slow4 ranges.
For a detailed description of participants, acquisition parameters, and preprocessing steps, see supplementary methods and supplementary table 1.
Analysis Strategy
The aim of the study was to investigate how connectivity from the SN may be altered in SCZ and how this then affects striato-thalamo-cortical FC. We thus followed a seed-based FC approach where an a priori SN region-of-interest (ROI) was taken as a starting point, identifying subsequent ROIs within the striato-thalamo-cortical network based on the ongoing findings of the contrasts between SCZ and HC. This meant that we first calculated the FC from the SN and identified altered connectivity with the striatum. We took this alteration within the striatum as the seed point to then investigate altered connectivity with the thalamus, and the contrast result within the thalamus as the seed point for testing changes in connectivity between this region and the cortex. Bilateral ROIs were used for identifying these step-wise seed points, but we also calculated the connectivity for left and right hemisphere seeds separately in order to reveal any potential lateralization in connectivity changes in SCZ.47,48 Finally, correlations between clinical scores and connectivity alterations were done using the FC calculated from the bilateral ROIs.
Substantia Nigra FC
To investigate connectivity with the SN, a bilateral SN mask from the ATAG-Atlas (http://www.nitrc.org/projects/atag) was used as a ROI for a seed-based FC analysis.49 The seed reference time-series of the ROI was obtained by averaging the fMRI time-series of all voxels within. Pearson’s correlation coefficients were calculated between the seed region timecourses and the rest of the brain in a voxel-wise manner. The correlation coefficients were then transformed to z-values by means of the Fisher r-to-z transformation, in order to improve normality. This produced spatial maps in which the values of voxels represented the strength of the correlation with the ROI. This was done for bilateral and left or right hemisphere seeds separately.
To delineate the basic connectivity of the SN seed, we performed a 1-sample t test across only the HC whole-brain FC maps (bilateral seed). The FC maps from the SN were then contrasted between HC and SCZ patients to identify regions of altered FC, using a general linear model (GLM) and permutation-based nonparametric statistical thresholding (FSL’s randomize with threshold-free cluster enhancement; 5000 permutations; FWE corrected threshold, P < .05).50,51 Age, gender, and head motion were entered into this analysis as confound regressors to help ensure that any observed effect of group on FC was independent of age-, gender-, or motion-related changes. The FC analysis was performed in the SFB, and then in its subfrequency bands (ie, Slow4 and Slow5). SFB connectivity was used for identifying new seed regions.
Striatal and Thalamic FC
The clusters of voxels within the striatum that showed differences between groups (using a bilateral mask composed of the caudate, putamen, and globus pallidum from the Harvard-Oxford subcortical atlas) were used to create new seed regions. FC from the striatum was then calculated as described previously and contrasted between groups. The voxels with differences within the thalamus (using a bilateral thalamus mask from the Harvard-Oxford subcortical atlas) were then selected to create ROIs in that region. Again, connectivity was calculated and alterations in thalamo-cortical links were identified. In addition to the bilateral ROIs, this procedure was also done for left and right hemisphere seeds separately. Connectivity was calculated for the SFB, Slow4, and Slow5.
To identify the nature of the changes between groups, ROI-to-ROI FC values in SFB were extracted from each region and plotted. One-sample t tests against zero were conducted for each group separately to establish where significant connectivity was present or not.
Clinical Correlations
Finally, potential clinical correlations were investigated. ROI-to-ROI FC values (as calculated previously) were entered into partial correlation analyses with Positive and Negative Syndrome Scale (PANSS) total scores in the SCZ group, with age, gender and movement as covariates (Bonferroni corrected for multiple comparisons and bootstrapped effect size confidence intervals calculated). We then explored potential specific correlations for PANSS sub-scores for positive, negative and general symptomatology with the FC measures which were significantly correlated with PANSS total score (with age, gender and movement as covariates and Bonferroni corrected for multiple comparisons). Furthermore, the same ROI-to-ROI FC values were compared between SCZ patients with prevalent positive symptoms (SCZ PS) and patients with prevalent negative symptoms (SCZ NS). These were grouped according to the difference between positive and negative scores, yielding a composite scale that expresses the degree of predominance of positive (score > 0) or negative (score < 0) syndromes.52 Correlation analyses were performed in SPSS version19.
Results
Functional Connectivity of SN to Striatum
In a first step, we identified brain regions that were functionally connected with our SN seeds in the HC group alone. This analysis showed strong connectivity from the seed ROIs to other subcortical nuclei, including the basal ganglia, the thalamus, and the amygdala-hippocampal complex, as well as to the mid-cingulate cortex (supplementary figure 1).
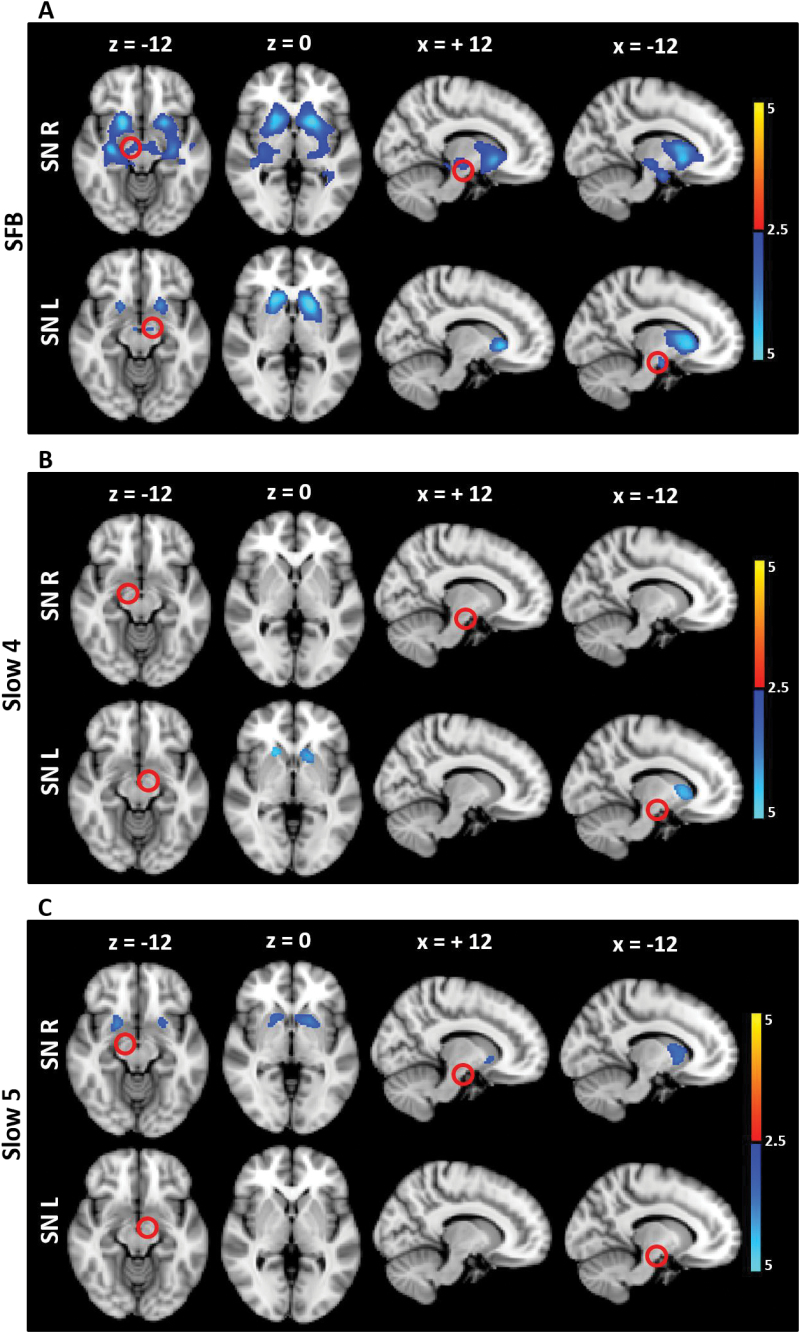
We then identified where FC from the SN was altered between HC and SCZ in each of the frequency bands. Taking separate left and right hemisphere SN seeds, the left and right SN were found to be hypo-connected with the bilateral striatum in SCZ in SFB compared to HC (figure 1 and supplementary table 2). The regions of the striatum that were found to be hypo-connected with SN were mainly composed of the caudate and putamen and, to a lesser extent, the pallidum (supplementary figure 2). When Slow4 and Slow5 were separately analyzed, the FC decrease was mainly observed in the right SN in Slow5, but in the left SN in Slow4 (figure 1 and supplementary table 2).
Fig. 1.
FC alteration in SCZ compared to HC in the different frequency bands (seed: SN). The t maps were thresholded at corrected P < .05. The color bar shows voxel-wise t values. Reduced FC is shown in blue, while increased FC is shown in red. In SCZ, the SN showed hypo-connectivity with the striatum in SFB (bilaterally), as well as in Slow5 (right SN) and Slow4 (left SN). Abbreviations: FC, functional connectivity; SN, Substantia Nigra; SCZ, schizophrenia; HC, healthy controls; SFB, Standard Frequency Band (0.01–0.08 Hz); Slow4 (0.027–0.073 Hz); Slow5 (0.01–0.027 Hz).
Functional Connectivity From Striatum to Thalamus and Cortical Regions
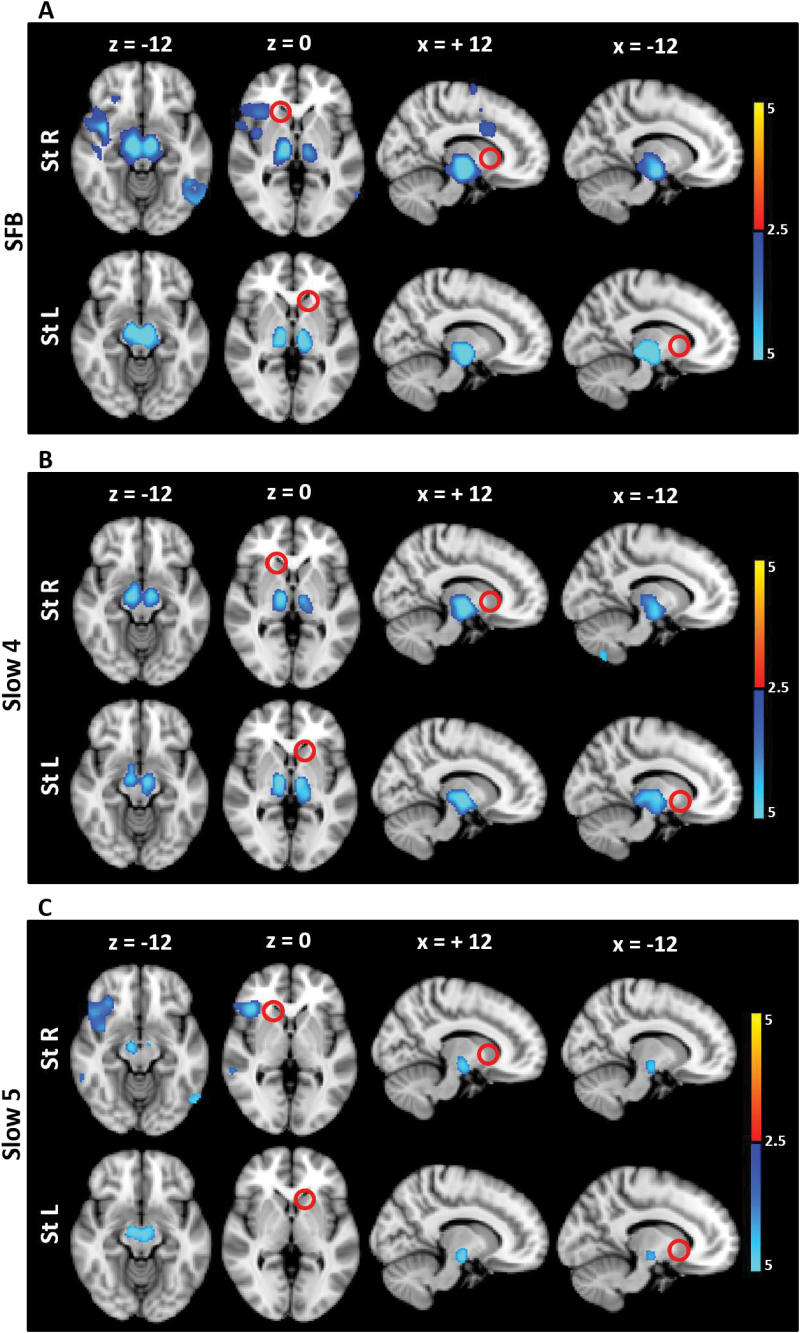
Having identified FC changes within the striatum, the significant voxels within this region were then used as a seed and the resulting connectivity contrasted between groups. In addition to the SN, the striatum was found to be hypo-connected with the thalamus in SFB in SCZ (figure 2 and supplementary table 3). The regions of the thalamus that were found to be hypo-connected with the striatum were mainly composed of ventral and lateral regions that are related to primary sensory-motor and premotor areas (and, to a lesser extent, dorsomedial regions, that are related to prefrontal areas) (supplementary figure 2). When Slow4 and Slow5 were analyzed separately, the FC decrease between the striatum and thalamus was observed in Slow4 specifically (figure 2 and supplementary table 3).
Fig. 2.
FC alteration in SCZ compared to HC in the different frequency bands (seed: St). The t maps were thresholded at corrected P < .05. The color bar shows voxel-wise t values. Reduced FC is shown in blue, while increased FC is shown in red. In SCZ, the striatum showed hypo-connectivity with the SN and thalamus in SFB, and mainly in Slow4 (SN and thalamus) rather than Slow5 (SN only). Abbreviations: FC, functional connectivity; St, Striatum; SCZ, schizophrenia; HC, healthy controls; SFB, Standard Frequency Band (0.01–0.08 Hz); Slow4 (0.027–0.073 Hz); Slow5 (0.01–0.027 Hz).
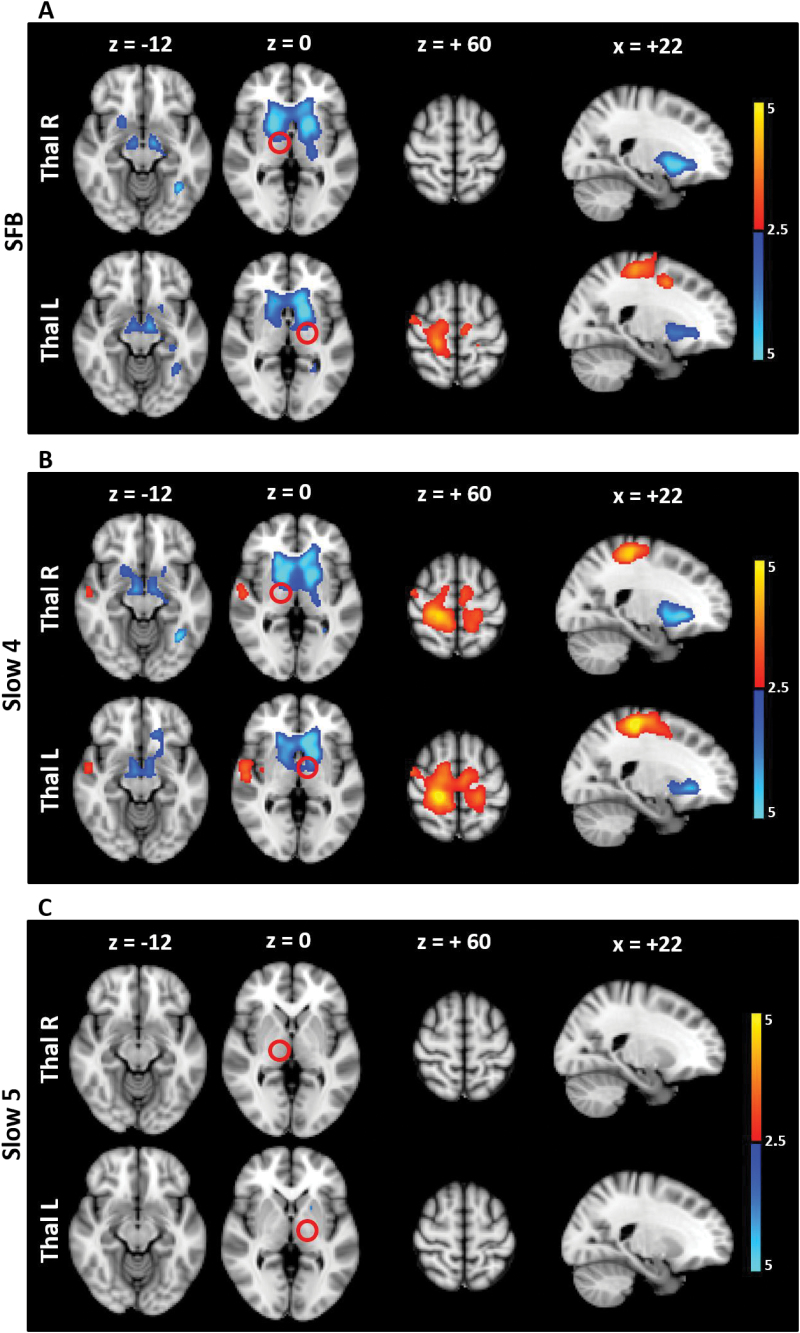
The left and right thalamic regions that were found to be hypo-connected with the bilateral striatum were then used as new seed ROIs. In SCZ, the thalamus showed downstream hypo-connectivity with subcortical regions (ie, bilateral striatum and SN), as well as upstream hyper-connectivity with sensorimotor cortical regions (primary sensory-motor and premotor areas) in SFB (figure 3 and supplementary table 4). When Slow4 and Slow5 were analyzed separately, the FC alterations were observed in Slow4 only (figure 3 and supplementary table 4). The abnormal coupling between the thalamus and sensorimotor cortical areas in SFB (and in Slow4 specifically) was further confirmed by taking the sensorimotor areas as seed ROI for FC analysis, which again revealed in SCZ an increase in FC with the thalamus in SFB and Slow4, and no results in Slow5 (supplementary figure 3 and supplementary table 5).
Fig. 3.
FC alteration in SCZ compared to HC in the different frequency bands (seed: Thal). The t maps were thresholded at corrected P < .05. The color bar shows voxel-wise t values. Reduced FC is shown in blue, while increased FC is shown in red. In SCZ, the thalamus showed hypo-connectivity with the striatum and SN as well as hyper-connectivity with the sensorimotor cortical areas in SFB, and Slow4, while no significant results were found in Slow5. Abbreviations: FC, functional connectivity; Thal, Thalamus; SCZ, schizophrenia; HC, healthy controls; SFB, Standard Frequency Band (0.01–0.08 Hz); Slow4 (0.027–0.073 Hz); Slow5 (0.01–0.027 Hz).
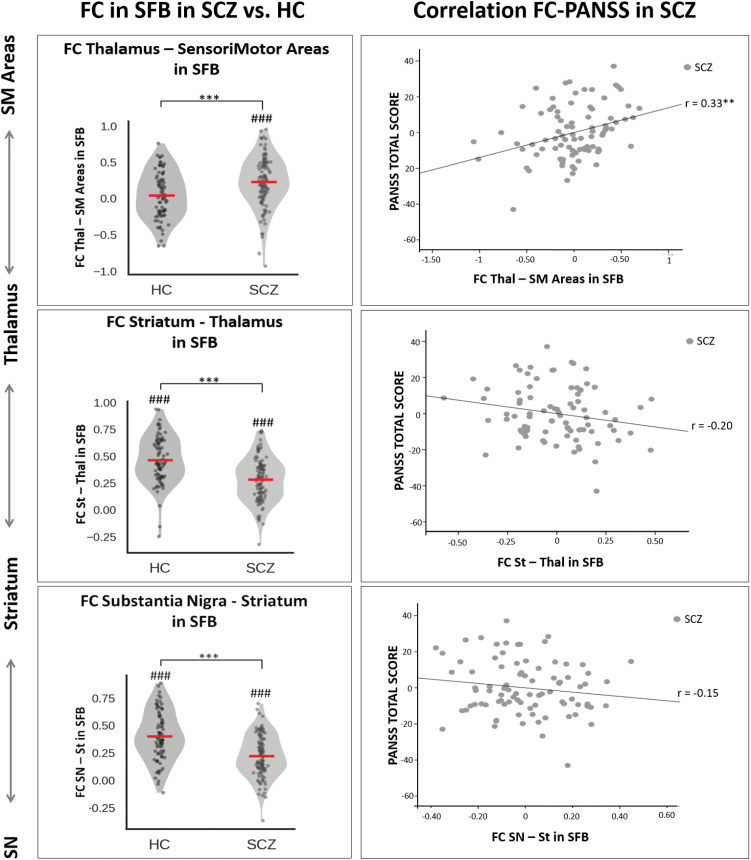
Finally, based on the voxel-wise FC results, the ROI-to-ROI FC was calculated between the relevant relay stations of the SN-related subcortical-cortical network in the SFB (ie, SN-striatum, striatum-thalamus and thalamus-sensorimotor cortical areas) and entered into 1-sample t tests, in order to show the individual group effects. HC showed positive FC between SN and striatum (t = 16.26; P = .000) as well as between striatum and thalamus (t = 19.05; P = .000), but no FC between thalamus and sensorimotor cortex (t = 1.17; P = .25). On the other hand, SCZ showed reduced positive connectivity between SN and striatum (t = 12.31; P = .000) as well as between striatum and thalamus (t = 14.73; P = .000), along with abnormal positive coupling between the thalamus and sensorimotor cortex (t = 6.95; P = .000).
Correlations With Psychopathological Symptoms
Potential clinical correlations of FC alterations were investigated. The ROI-to-ROI FC for the various relay stations of the SN-related striato-thalamo-cortical network in SFB were entered in partial correlation analyses with PANSS total scores in SCZ patients, with age, gender and movement as covariates (Bonferroni corrected P < .016). The PANSS total score correlated positively with the thalamus-sensorimotor cortex FC (r = .33; P = .002; CI: 0.14~0.50), while no significant correlations were detected with the SN-striatum FC (r = −.15; P = .19; CI: −0.34~0.06) or the striatum-thalamus FC (r = −.20; P = .07; CI: −0.38~−0.01; figure 4). Furthermore, in an exploratory partial correlation analysis on the PANSS subscales (with age, gender and movement as covariates, Bonferroni corrected P < .016), the thalamus-sensorimotor cortical areas FC was positively correlated with negative symptomatology (r = .27; P = .013; CI: 0.08~0.43) and general symptomatology (r = .30; P = .006; CI: 0.09~0.50), but not with positive symptomatology (r = .04; P = .73) (supplementary figure 4).
Fig. 4.
Functional disconnectivity in the SN-related striato-thalamo-cortical network and its clinical correlation in SCZ. Left side of the figure. The violin plots show the significant differences in mean FC in HC and SCZ separately, as well as the significant differences between the 2 groups, at the various relay stations of the SN-related striato-thalamo-cortical network in SFB. The SCZ group showed reduced positive FC between the SN and striatum as well as between the striatum and thalamus, when compared to highly positive FC in HC; by contrast, the SCZ group showed abnormal positive coupling between the thalamus and sensorimotor cortical areas, when compared to no FC in HC. ###P < .001 (1-sample t tests against zero); ***P < .001 (2-sample t tests). Right side of the figure. The scatter plots show in SCZ the partial correlation analysis (with age, gender and movement as covariates) between FC alterations at the various relay stations of the SN-related network in SFB and PANSS total score. A significant correlation between thalamo-sensorimotor FC alterations and psychopathological severity score was detected in SCZ. **P < .01 (correlation analysis). Abbreviations: FC, functional connectivity; SN, Substantia Nigra; St, striatum; Thal, Thalamus; SM Areas, SensoriMotor Areas; SCZ, schizophrenia; HC, healthy controls; SFB, Standard Frequency Band (0.01–0.08 Hz); PANSS, positive and negative syndrome scale.
Finally, the ROI-to-ROI FC of the SN-related subcortical-cortical network was analyzed and compared between SCZ PS (73.1% of SCZ sample) and SCZ NS (26.9% of SCZ sample) patients (Bonferroni corrected P < .016). This mainly showed that there was a greater level of abnormal coupling in the thalamo-sensorimotor cortical network specifically in patients with NS compared to those with PS (t = −3.02; P = .003) (supplementary figure 5).
Discussion
Main Findings
Comparisons of FC between HC and first-episode drug-naïve SCZ patients showed: (1) reciprocal functional hypo-connectivity between SN and striatum, with differential patterns for Slow5 and Slow4; (2) functional hypo-connectivity between striatum and thalamus, as well as functional hyper-connectivity between thalamus and sensorimotor cortical areas, specifically in Slow4; and (3) a correlation of thalamo-sensorimotor functional hyper-connectivity with psychopathological symptoms as measured with the PANSS (total score as well as negative and general psychopathology sub-scores). These findings may suggest a disruption of neural synchronization in slow frequencies between the different relay stations of the dopamine-related SN-based striato-thalamo-cortical resting-state network in drug-naïve SCZ.
Functional Disconnectivity Between SN and Striatum in Ultra-Slow Frequencies in SCZ
In our data, the SN was found to be connected with the amygdala-hippocampal complex, basal ganglia, thalamus, and mid-cingulate cortex. This is coherent with previous work, and is consistent with the SN’s neuroanatomical projections and with the role of dopamine in the modulation of brain FC.16–19,53–57
In line with our hypotheses, we found in SCZ robust resting-state functional hypo-connectivity of the SN with the striatum in the ultra-slow frequency bands (with differential patterns for Slow5 and Slow4), which was then related to functional disconnectivity in the striatum-thalamo-cortical loop (specifically in Slow4). These resting-state SN-based network FC alterations complement the dopamine hypothesis on the regional and network levels of neural activity.
Physiologically, resting-state FC measures the coherence of neuronal activity between different brain regions in the low-frequency range between 0.01 and 0.08Hz.58 Such low-frequency neuronal fluctuations are central to the tonic neural activity which provides the main substrate for the brain’s default activity in the resting-state.14,59,60 Convergent with that, dopamine in SN provides such tonic activity which, at least in part, may shape or synchronize the neuronal oscillations in related regions in the low-frequency range as measured with FC in fMRI (in accordance with evidence of intra-network FC modulation by dopaminergic substances16–19). One may therefore speculate that reduced activity in the low-frequency range, as indexed by decreased SN-based subcortical FC in our data, may thus reflect reduced tonic activity in dopaminergic subcortical circuitry. This remains to be tested experimentally in the future by combining both electrophysiological recording and dopaminergic manipulation of D2-receptors.
From this perspective, our findings of decreased SN-based subcortical FC, together with previous evidence of task-evoked SN hyper-activity,61,62 may be in accordance with the dopamine hypothesis of SCZ—for which a decrease in the tonic activity of dopaminergic neurons is associated with increased phasic activity in the dopaminergic system under stressors or stimulation.6–8
Functional Disconnectivity of SN-Related Striato-Thalamo-Cortical Sensorimotor Areas in Slow4 in SCZ
In our study, the regions of the striatum that were found to be hypo-connected with the SN were in turn hypo-connected with the thalamus, and, subsequently, the very same thalamic regions that were found to be downstream hypo-connected with the striatum showed an upstream hyper-connectivity with the sensorimotor cortical areas, specifically in Slow4, in SCZ. Anatomically, the striatal regions are mainly composed by GABAergic neurons which project and differentially inhibit the glutamatergic thalamo-cortical system.63 A deficit in striatum-thalamus coupling may therefore disinhibit excessive coupling of the thalamo-cortical system leading to thalamo-sensorimotor cortical functional hyper-connectivity, as observed in this study and in other prior work.22–24,64–73
More specifically, increased thalamo-sensorimotor cortical connectivity, in association with reduced thalamo-prefrontal cortical connectivity, represents a core fMRI abnormality in SCZ, both in chronic and early stage patients.22–24,64–74 Our finding of thalamo-sensorimotor cortical hyper-connectivity is consistent with the specific seed regions used for FC analysis: the a priori selected SN—which contains dopaminergic neurons mainly projecting to sensorimotor-related striatal regions75—and, subsequently, its disconnected thalamic areas—mainly ventral and lateral regions, which are connected to sensorimotor cortical regions.73 On the other hand, the use of these specific brainstem and thalamic ROIs may explain the absence of thalamo-prefrontal cortical hypo-connectivity in our findings (since the mediodorsal nucleus, which is mainly connected with the prefrontal cortex and not with subcortical structures,73 was poorly represented in our SN-related disconnected thalamic ROI).
Our findings therefore extend prior ones by: (1) showing that the increased thalamo-sensorimotor FC can be traced back to striatum and SN, thus reflecting functional disconnectivity in dopamine-related SN-mediated subcortical-cortical circuitry; (2) showing frequency-specific effects in Slow4 (rather than in Slow5), which is compatible with the predominance of Slow4 in basal ganglia, thalamus and sensorimotor regions in healthy subjects14; and (3) showing the presence of these specific SN-mediated subcortical-cortical Slow4 FC alterations at the illness onset and before antipsychotic use.
In turn, the functional disruption of an SN-related sensorimotor network as a core feature of SCZ is in line with previous evidence that showed both structural and functional MRI abnormalities in sensorimotor cortical regions of SCZ patients—such as reduced grey matter density (ie, in postcentral gyrus and superior temporal gyrus), reduced FC between cortical nodes, reduced temporal variability of neuronal activity, and decreased activation during motor tasks (ie, in sensorimotor and supplemental motor areas)23,76,77—as well as the central relevance of sensorimotor symptoms in SCZ.32–43
Clinical Correlations of SN-Related Thalamo-Cortical Disconnectivity in SCZ
The relevance of altered SN-related subcortical-cortical FC in slow frequencies may be further supported by our correlation findings. We observed a significant correlation of the thalamo-sensorimotor cortical disconnectivity (but not SN-striatal and striato-thalamic disconnectivity) with psychotic symptomatology in SCZ, and in particular with negative symptoms and general psychopathology (including several psychomotor alterations). The association between thalamo-cortical disconnectivity and negative symptomatology was also supported by the finding of a higher degree of FC impairment in the subgroup of patients with prevalent negative symptoms.
Previous findings in this regard are mixed73: some studies found significant positive correlations between thalamo-cortical hyper-connectivity and PANSS total score,65 PANSS negative syndrome sub-score24,74 and PANSS general psychopathology sub-score,24 while other studies did not.64,72 However, these clinical correlations, when detected, were small in magnitude, as also in our case (r around .3).73 This can suggest a more indirect relationship between thalamo-sensorimotor cortical disconnectivity and typical SCZ psychopathology. For instance, functional alterations of an SN-related sensorimotor network may more directly result in sensorimotor symptoms, as intermediaries between neurobiological dysfunction and typical psychotic symptomatology.41,44 This can be supported by the central relevance of functional abnormalities in the sensorimotor network and sensorimotor symptoms in SCZ, as well as their relationship with mainly negative symptoms,23,32–43,76,77 which is in line with our results. However, this needs to be tested in future studies that directly explore the relationship between functional abnormalities of the SN-related sensorimotor network, sensorimotor symptoms and SCZ psychopathology. Taken together, this evidence of associations between SN-related resting-state abnormalities and prevalent negative symptomatology (see above), as well as between SN-related task-evoked abnormalities and prevalent positive symptomatology,61,62 can be in accordance with the dopamine hypothesis of SCZ, for which altered dopaminergic tonic activity is associated with negative symptomatology and altered phasic activity with positive.6–8
How and why the dopamine-related SN-based striato-thalamo-sensorimotor cortical circuitry could be related to these clinical symptoms, which have been mainly associated with other cortical regions,78 remains unclear at this point in time though. We speculate that abnormal resting-state thalamo-sensorimotor cortical coupling impacts the spatiotemporal organization of subsequent sensory input and motor outputs (in a yet unclear way), subsequently generating the various psychopathological symptoms. Hence, considered in this way, our findings showing spatiotemporal alterations in subcortical-cortical circuitry support, albeit tentatively, the recently proposed idea of what has been described as “spatiotemporal psychopathology”.78,79
Limitations
There are a number of limitations of the present study. Our work is based purely on resting-state fMRI analysis, and no molecular imaging data was simultaneously acquired to detect any direct connection between FC and molecular changes. However, previous studies that combined resting-state FC with PET analysis have corroborated a role of dopamine in brain FC across subcortical-cortical pathways.17,80,81 Moreover, our findings are consistent with data from previous molecular imaging and PET-fMRI studies which showed changes in subcortical dopamine release and a direct relationship between dopaminergic receptor binding potential and altered connectivity patterns in subcortical-cortical regions in SCZ.9,82 Additionally, our resting-state findings complement previous fMRI studies that showed task-evoked hyper-activity of SN in association with striatal hypo-activity in SCZ patients.61,62 The resting-state FC techniques—which rely on detecting coherent patterns of spontaneous activity and eventually avoid potential confounds or limitations encountered in task-based approaches (eg, practice or differential performance levels)—appear to delineate entire functional networks which are typically observed in task activation-based studies in a more fragmentary manner.11,83–85 Thus, our resting-state findings of SN-related subcortical-cortical disconnectivity in a large sample of unmedicated and drug-naïve patients at their first psychotic episode, also avoiding medication or chronic disease-related imaging changes, support the role of alterations in a dopamine-related SN-based subcortical-cortical functional network in the pathophysiology of SCZ.
Conclusion
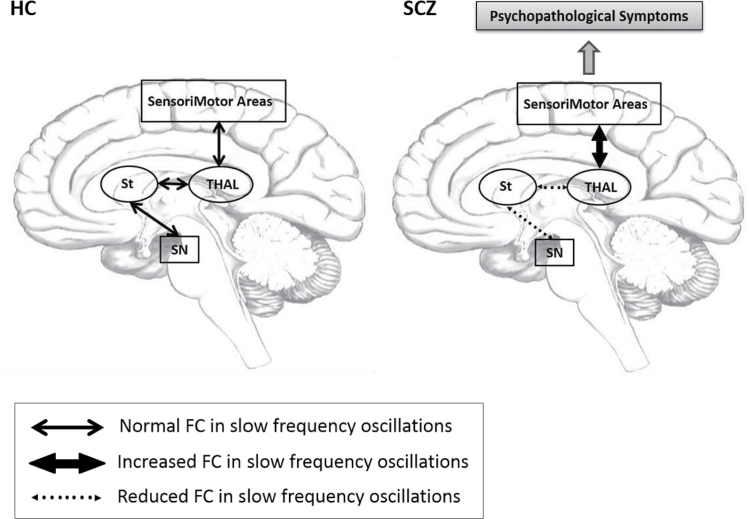
In summary, our findings show abnormal SN-based subcortical-cortical circuitry connectivity in low-frequency oscillations, with differential patterns for Slow5 and Slow4, in SCZ. FC measures the correlation of low-frequency fluctuations and is therefore thought to reflect cyclic modulation of long distance neuronal synchronization.12,14,15 Based on this, we hypothesize that dopamine-related SN dysfunction may desynchronize low-frequency oscillations and subsequently reduce tonic activity, decoupling the target subcortical striatal and thalamic regions, and resulting in the observed hypo-connectivity. This in turn may disinhibit neuronal synchronization and functional coherence between the thalamus and sensorimotor cortical areas, resulting in the observed hyper-connectivity and psychopathological symptoms (figure 5).
Fig. 5.
Schema. In this model of SCZ, the dopamine-related SN functional disconnectivity leads to desynchronization in the activity within striatum and between striatum and thalamus with subsequent abnormal coupling between thalamus and sensorimotor cortical areas, in the slow frequency oscillations. This, in turn, leads to abnormal input and output processing by baseline intrinsic neuronal activity, finally leading to psychopathological symptoms. Abbreviations: FC, functional connectivity; SN, Substantia Nigra; St, Striatum; Thal, Thalamus; SM Areas, SensoriMotor Areas; SCZ, schizophrenia; HC, healthy controls.
In conclusion, we demonstrated alterations in subcortical-cortical FC in slow frequency oscillations within the SN-based striatum-thalamic-sensorimotor cortex network, including its relation to psychopathologic symptomatology, in SCZ. As such the results might support a dopamine-related SN-based striato-thalamo-sensorimotor cortical network hypothesis in SCZ, complementing the dopamine hypothesis on the regional level of resting-state activity.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
This work was partly funded by National Nature Science Foundation of China Key Project 81630030 and 81130024 (to T.L.); National Key Research and Development Program of the Ministry of Science and Technology of China 2016YFC0904300 (to T.L.); 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZY2016203, ZY2016103); National Natural Science Foundation of China/Research Grants Council of Hong Kong Joint Research Scheme 81461168029 (to T.L.). G.N. received support from the Michael Smith Foundation (200809EJL- 194083-EJL-CECA-179644), the National Science Foundation of China (NSF No 31271195), and the Canadian Institutes of Health Research (201103MOP-244752- BSB-CECA-179644, 201103CCI- 248496-CCI-CECA- 179644). N.W.D. received support from the National Science Foundation of China (NSF31471072), the Taiwan Ministry of Science and Technology (105-2410-H-038-006-MY3, 105-2410-H-038-005-MY2), and from Taipei Medical University (104-6402-006-110).
Supplementary Material
Acknowledgment
The authors declare no conflicts of interest.
References
- 1. American Psychiatrich Association. Diagnostic and Statistical Manual for Mental Disorders. 5th ed. (DSM-5). Washington, DC: American Psychiatrich Association; 2013. [Google Scholar]
- 2. Abi-Dargham A. Schizophrenia: overview and dopamine dysfunction. J Clin Psychiatry. 2014;75:e31. [DOI] [PubMed] [Google Scholar]
- 3. Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006;12:1016–1022. [DOI] [PubMed] [Google Scholar]
- 4. Weinberger DR. The biological basis of schizophrenia: new directions. J Clin Psychiatry. 1997;58(suppl 10):22–27. [PubMed] [Google Scholar]
- 5. Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: a review. Schizophr Bull. 1976;2:19–76. [DOI] [PubMed] [Google Scholar]
- 6. Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. [DOI] [PubMed] [Google Scholar]
- 7. Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. [DOI] [PubMed] [Google Scholar]
- 8. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Howes OD, Kambeitz J, Kim E et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kambeitz J, Abi-Dargham A, Kapur S, Howes OD. Alterations in cortical and extrastriatal subcortical dopamine function in schizophrenia: systematic review and meta-analysis of imaging studies. Br J Psychiatry. 2014;204:420–429. [DOI] [PubMed] [Google Scholar]
- 11. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. [DOI] [PubMed] [Google Scholar]
- 12. Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. [DOI] [PubMed] [Google Scholar]
- 13. Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6:15–28. [DOI] [PubMed] [Google Scholar]
- 14. Zuo XN, Di Martino A, Kelly C et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49:1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balduzzi D, Riedner BA, Tononi G. A BOLD window into brain waves. Proc Natl Acad Sci U S A. 2008;105:15641–15642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cole DM, Beckmann CF, Oei NY, Both S, van Gerven JM, Rombouts SA. Differential and distributed effects of dopamine neuromodulations on resting-state network connectivity. Neuroimage. 2013;78:59–67. [DOI] [PubMed] [Google Scholar]
- 17. Kelly C, de Zubicaray G, Di Martino A et al. L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci. 2009;29:7364–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci. 2008;28:3697–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cole DM, Oei NY, Soeter RP et al. Dopamine-dependent architecture of cortico-subcortical network connectivity. Cereb Cortex. 2013;23:1509–1516. [DOI] [PubMed] [Google Scholar]
- 20. Metzger CD, Wiegers M, Walter M, Abler B, Graf H. Local and global resting state activity in the noradrenergic and dopaminergic pathway modulated by reboxetine and amisulpride in healthy subjects. Int J Neuropsychopharmacol 2015;19:1–9. doi:10.1093/ijnp/pyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weinberger DR, Radulescu E. Finding the elusive psychiatric “lesion” with 21st-century neuroanatomy: a note of caution. Am J Psychiatry. 2016;173:27–33. [DOI] [PubMed] [Google Scholar]
- 22. Pergola G, Selvaggi P, Trizio S, Bertolino A, Blasi G. The role of the thalamus in schizophrenia from a neuroimaging perspective. Neurosci Biobehav Rev. 2015;54:57–75. [DOI] [PubMed] [Google Scholar]
- 23. Kaufmann T, Skåtun KC, Alnæs D et al. Disintegration of sensorimotor brain networks in schizophrenia. Schizophr Bull. 2015;41:1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li T, Wang Q, Zhang J et al. Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr Bull. 2017;43:436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hadley JA, Nenert R, Kraguljac NV et al. Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2014;39:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. White TP, Wigton R, Joyce DW, Collier T, Fornito A, Shergill SS. Dysfunctional striatal systems in treatment-resistant schizophrenia. Neuropsychopharmacology. 2016;41:1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xue SW, Li D, Weng XC, Northoff G, Li DW. Different neural manifestations of two slow frequency bands in resting functional magnetic resonance imaging: a systemic survey at regional, interregional, and network levels. Brain Connect. 2014;4:242–255. [DOI] [PubMed] [Google Scholar]
- 28. Martino M, Magioncalda P, Huang Z et al. Contrasting variability patterns in the default mode and sensorimotor networks balance in bipolar depression and mania. Proc Natl Acad Sci U S A. 2016;113:4824–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buzsaki G. Rhythms of the brain. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 30. Huang Z, Zhang J, Longtin A et al. Is there a nonadditive interaction between spontaneous and evoked activity? Phase-dependence and its relation to the temporal structure of scale-free brain activity. Cereb Cortex. 2015;27:1037–1059. [DOI] [PubMed] [Google Scholar]
- 31. Penttonen M, Buszsaki G. Natural logarithmic relationship between brain oscillators. Thalamus Relat Syst. 2003;48:1–8. [Google Scholar]
- 32. Peralta V, Campos MS, De Jalón EG, Cuesta MJ. Motor behavior abnormalities in drug-naïve patients with schizophrenia spectrum disorders. Mov Disord. 2010;25:1068–1076. [DOI] [PubMed] [Google Scholar]
- 33. Javitt DC, Sweet RA. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat Rev Neurosci. 2015;16:535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Putzhammer A, Klein HE. Quantitative analysis of motor disturbances in schizophrenic patients. Dialogues Clin Neurosci. 2006;8:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bachmann S, Degen C, Geider FJ, Schröder J. Neurological soft signs in the clinical course of schizophrenia: results of a meta-analysis. Front Psychiatry. 2014;5:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dazzan P, Murray RM. Neurological soft signs in first-episode psychosis: a systematic review. Br J Psychiatry Suppl. 2002;43:s50–s57. [DOI] [PubMed] [Google Scholar]
- 37. Northoff G, Duncan NW. How do abnormalities in the brain’s spontaneous activity translate into symptoms in schizophrenia? From an overview of resting state activity findings to a proposed spatiotemporal psychopathology. Prog Neurobiol. 2016;145-146:26–45. [DOI] [PubMed] [Google Scholar]
- 38. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25:555–77; discussion 578. [DOI] [PubMed] [Google Scholar]
- 39. Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20:441–451. [DOI] [PubMed] [Google Scholar]
- 40. Walker E, Lewis N, Loewy R, Palyo S. Motor dysfunction and risk for schizophrenia. Dev Psychopathol. 1999;11:509–523. [DOI] [PubMed] [Google Scholar]
- 41. Javitt DC, Freedman R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry. 2015;172:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schultze-Lutter F. Subjective symptoms of schizophrenia in research and the clinic: the basic symptom concept. Schizophr Bull. 2009;35:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Docx L, Morrens M, Bervoets C et al. Parsing the components of the psychomotor syndrome in schizophrenia. Acta Psychiatr Scand. 2012;126:256–265. [DOI] [PubMed] [Google Scholar]
- 44. Javitt DC. Sensory processing in schizophrenia: neither simple nor intact. Schizophr Bull. 2009;35:1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. He Z, Deng W, Li M et al. Aberrant intrinsic brain activity and cognitive deficit in first-episode treatment-naive patients with schizophrenia. Psychol Med. 2013;43:769–780. [DOI] [PubMed] [Google Scholar]
- 46. Lei W, Li M, Deng W et al. Sex-specific patterns of aberrant brain function in first-episode treatment-naive patients with schizophrenia. Int J Mol Sci. 2015;16:16125–16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Molochnikov I, Cohen D. Hemispheric differences in the mesostriatal dopaminergic system. Front Syst Neurosci. 2014;8:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ribolsi M, Daskalakis ZJ, Siracusano A, Koch G. Abnormal asymmetry of brain connectivity in schizophrenia. Front Hum Neurosci. 2014;8:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Keuken MC, Bazin PL, Crown L et al. Quantifying inter-individual anatomical variability in the subcortex using 7 T structural MRI. Neuroimage. 2014;94:40–46. [DOI] [PubMed] [Google Scholar]
- 50. Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. [DOI] [PubMed] [Google Scholar]
- 52. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 53. Tomasi D, Volkow ND. Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cereb Cortex. 2014;24:935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Haber SN, Fudge JL. The primate substantia nigra and VTA: integrative circuitry and function. Crit Rev Neurobiol. 1997;11:323–342. [DOI] [PubMed] [Google Scholar]
- 55. Albin RL, Young AB, Penney JB. The functional anatomy of disorders of the basal ganglia. Trends Neurosci. 1995;18:63–64. [PubMed] [Google Scholar]
- 56. Amalric M, Koob GF. Functionally selective neurochemical afferents and efferents of the mesocorticolimbic and nigrostriatal dopamine system. Prog Brain Res. 1993;99:209–226. [DOI] [PubMed] [Google Scholar]
- 57. Freeman A, Ciliax B, Bakay R et al. Nigrostriatal collaterals to thalamus degenerate in parkinsonian animal models. Ann Neurol. 2001;50:321–329. [DOI] [PubMed] [Google Scholar]
- 58. He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc Natl Acad Sci U S A. 2008;105:16039–16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zou Q, Wu CW, Stein EA, Zang Y, Yang Y. Static and dynamic characteristics of cerebral blood flow during the resting state. Neuroimage. 2009;48:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Garrett DD, Samanez-Larkin GR, MacDonald SW, Lindenberger U, McIntosh AR, Grady CL. Moment-to-moment brain signal variability: a next frontier in human brain mapping?Neurosci Biobehav Rev. 2013;37:610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yoon JH, Minzenberg MJ, Raouf S, D’Esposito M, Carter CS. Impaired prefrontal-basal ganglia functional connectivity and substantia nigra hyperactivity in schizophrenia. Biol Psychiatry. 2013;74:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yoon JH, Westphal AJ, Minzenberg MJ et al. Task-evoked substantia nigra hyperactivity associated with prefrontal hypofunction, prefrontonigral disconnectivity and nigrostriatal connectivity predicting psychosis severity in medication naïve first episode schizophrenia. Schizophr Res. 2014;159:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wichmann T, DeLong M. The basal ganglia. In: Kandel E, Schwartz J, Jessel T, eds. Principles of Neural Sciences—Fifth edition. Ch 43. New York, NY: McGraw Hill; 2013:982–998. [Google Scholar]
- 64. Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Anticevic A, Cole MW, Repovs G et al. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24:3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Klingner CM, Langbein K, Dietzek M et al. Thalamocortical connectivity during resting state in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2014;264:111–119. [DOI] [PubMed] [Google Scholar]
- 68. Tu PC, Lee YC, Chen YS, Hsu JW, Li CT, Su TP. Network-specific cortico-thalamic dysconnection in schizophrenia revealed by intrinsic functional connectivity analyses. Schizophr Res. 2015;166:137–143. [DOI] [PubMed] [Google Scholar]
- 69. Lerman-Sinkoff DB, Barch DM. Network community structure alterations in adult schizophrenia: identification and localization of alterations. Neuroimage Clin. 2016;10:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Atluri G, Steinbach M, Lim KO, Kumar V, MacDonald A III. Connectivity cluster analysis for discovering discriminative subnetworks in schizophrenia. Hum Brain Mapp. 2015;36:756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang HL, Rau CL, Li YM, Chen YP, Yu R. Disrupted thalamic resting-state functional networks in schizophrenia. Front Behav Neurosci. 2015;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Woodward ND, Heckers S. Mapping thalamocortical functional connectivity in chronic and early stages of psychotic disorders. Biol Psychiatry. 2016;79:1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Giraldo-Chica M, Woodward ND. Review of thalamocortical resting-state fMRI studies in schizophrenia. Schizophr Res. 2017;180:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cheng W, Palaniyappan L, Li M et al. Voxel-based, brain-wide association study of aberrant functional connectivity in schizophrenia implicates thalamocortical circuitry. NPJ Schizophr. 2015;1:15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bentivoglio M, Morelli M. The organization and circuits of mesencephalic dopaminergic neurons and the distribution of dopamine receptors in the brain. In: Dunnett SB, ed. Handbook of Chemical Neuroanatomy. Vol. 21 Amsterdam, Netherlands: Elsevier; 2005:1–107. [Google Scholar]
- 76. Glahn DC, Laird AR, Ellison-Wright I et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schröder J, Wenz F, Schad LR, Baudendistel K, Knopp MV. Sensorimotor cortex and supplementary motor area changes in schizophrenia. A study with functional magnetic resonance imaging. Br J Psychiatry. 1995;167:197–201. [DOI] [PubMed] [Google Scholar]
- 78. Northoff G, Duncan NW. How do abnormalities in the brain’s spontaneous activity translate into symptoms in schizophrenia? From an overview of resting state activity findings to a proposed spatiotemporal psychopathology. Progress in Neurobiology In press 2016;145–146:26–45. [DOI] [PubMed] [Google Scholar]
- 79. Northoff G. Spatiotemporal Psychopathology II: how does a psychopathology of the brain’s resting state look like? Spatiotemporal approach and the history of psychopathology. J Affect Disord. 2016;190:867–879. [DOI] [PubMed] [Google Scholar]
- 80. Cole DM, Beckmann CF, Searle GE et al. Orbitofrontal connectivity with resting-state networks is associated with midbrain dopamine D3 receptor availability. Cereb Cortex. 2012;22:2784–2793. [DOI] [PubMed] [Google Scholar]
- 81. Rieckmann A, Karlsson S, Fischer H, Bäckman L. Caudate dopamine D1 receptor density is associated with individual differences in frontoparietal connectivity during working memory. J Neurosci. 2011;31:14284–14290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Horga G, Cassidy CM, Xu X et al. Dopamine-related disruption of functional topography of striatal connections in unmedicated patients with schizophrenia. JAMA Psychiatry. 2016;73:862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. [DOI] [PubMed] [Google Scholar]
- 84. Damoiseaux JS, Rombouts SA, Barkhof F et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dosenbach NU, Fair DA, Miezin FM et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.