Abstract
In the present article, we present a “Levels of Explanation” (LoE) approach to auditory verbal hallucinations (AVHs) in schizophrenia. Mental phenomena can be understood at different levels of explanation, including cultural, clinical, cognitive, brain imaging, cellular, and molecular levels. Current research on AVHs is characterized by accumulation of data at all levels, but with little or no interaction of findings between levels. A second advantage with a Levels of Explanation approach is that it fosters interdisciplinarity and collaboration across traditional borders, facilitating a real breakthrough in future research. We exemplify a Levels of Explanation approach with data from 3 levels where findings at 1 level provide predictions for another level. More specifically, we show how functional neuroimaging data at the brain level correspond with behavioral data at the cognitive level, and how data at these 2 levels correspond with recent findings of changes in neurotransmitter function at the cellular level. We further discuss implications for new therapeutic interventions, and the article is ended by suggestion how future research could incorporate genetic influences on AVHs at the molecular level of explanation by providing examples for animal work.
Keywords: auditory hallucinations, schizophrenia, cognition, neuroimaging, neurochemistry, dichotic listening, fMRI, glutamate, levels of explanation (LoE)
Introduction
One of the most perplexing phenomena of the human mind is the conviction of hearing and perceiving a “voice” in the absence of a corresponding auditory stimulus input. This is collectively called an auditory verbal hallucination (AVH), and is probably the most characteristic symptom of the most severe mental disorder that we know of, namely schizophrenia.1,2 AVHs also occur in other psychiatric disorders as well as in nonclinical individuals,3–5 which means that such experiences represent a fundamental property of the human mind. Understanding the phenomenology, cognitive, neuropsychological, and neurobiological underpinnings of AVHs would therefore not only provide new insights for explaining schizophrenia, but would also provide new insights into the complexities of the mind. Over the last decade, there has been an ever increasing number of systematic literature reviews and meta-analyses with a focus on neurobiological mechanisms, in particular, in relation to functional neuroimaging.6–12 As pointed out by Upthegrove et al8 there has been a large increase in publications over the last years, but integration with phenomenological and cognitive findings is necessary in order to avoid that models and theories become isolated from the very phenomenon they tend to explain. We, here, present a conceptual frame of reference for integrating data and findings which we have labeled a “Levels of Explanation” (LoE) approach.
Levels of Explanation
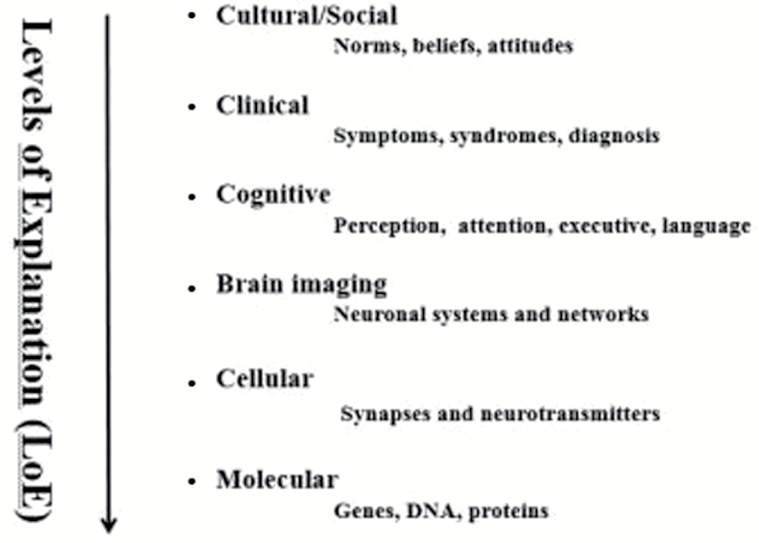
All mental phenomena can be described and studied at different “levels of explanation,” ranging from a cultural and social level to a molecular and genetic level.13Figure 1 shows a model with 6 levels: cultural, clinical, cognitive, brain imaging, cellular, and genetic levels.
Fig. 1.
Schematic illustration of a Levels of Explanation (LoE) approach to mental disorders. Adapted from text in Hugdahl.13
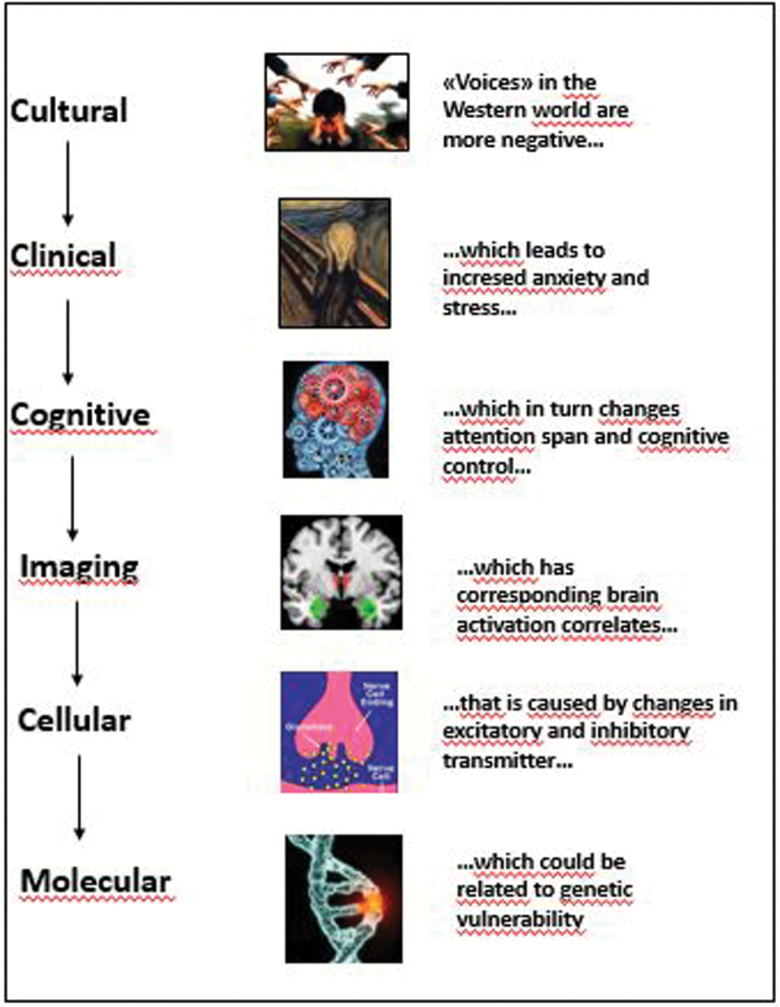
Figure 2 illustrates a thought case for auditory hallucinations across the different levels, starting with the fact that “voices” in the Western world have a more negative emotional content than “voices” in some African and Indian societies.14
Fig. 2.
A thought case illustration of a Levels of Explanation approach, from the cultural to the molecular level. The pictures used for illustrating the different levels are taken from the following websites: https://www.google.no/search?q=auditory+hallucinations&hl= no&dcr=0&source=lnms&tbm=isch&sa=X&ved=0ahUKEwi-ooSehIzWAhWCFZoKHeb5AZAQ_AUICigB&biw=1138&bih=457#imgrc=lKN643RO1fhO9M:&spf=1504545362788; https://www.google.no/search?q=munch+scream&hl=no&dcr=0&tbm=isch&imgil=-4j9OW6FXR40KM%253A%253B1iewB9Kj8MJFQM%253; https://www.google.fi/search?q=cognitive+control&hl=no&dcr=0&source=lnms&tbm=isch&sa=X&ved=0ahUKEwjgwK7dnP_VAhUCJJoKHVacC64Q_AUICigB&biw=1138&bih=456#imgrc=h-16UKGXwkqfYM:&spf=1504105261939; https://www.google.fi/search?q=glutamate&hl=no&dcr=0&source=lnms&tbm=isch&sa=X&ved=0ahUKEwjlvdWMnv_VAhXia5oKHX7OBc8Q_AUICigB#imgrc=tw3JA7pTBKPB_M:&spf=1504105628731 = GLUTAMAT.
In this article, we suggest that in order to advance the understanding of how auditory hallucinations are initiated and maintained both from a neuronal and phenomenological point of view, we need to integrate knowledge vertically from higher to lower levels, going from descriptions to explanations, and predictions. This may seem as a trivial task, but it is in stark contrast to the more common approach in today’s research, namely to stay at ones favorite level and expand horizontally with more and more advanced analysis methods. A recent review article in Schizophrenia Bulletin6 convincingly showed that no single feature of auditory hallucinations is unique for a diagnosis of schizophrenia. In a similar way, we would like to suggest that no single level sufficiently explains auditory hallucinations, whether part of a psychiatric diagnosis or occurring in nonclinical individuals. We will exemplify a Levels of Explanation approach by providing findings from 3 of the 6 levels where data from one level lead to new predictions and experiments at another level, either above or below the first level. Space does not allow us to elaborate on all levels, but studies have shown that cultural aspect plays a role for both the content and acceptance in society of hearing “voices.” For example, “voices” are more emotionally negative in the United States than in developing countries.14 People from cultures that provide accepted explanations for the phenomenon (ie, hearing arch angels, or Djinns) consistently more often experience AVH, without necessarily causing distress.15 At the clinical level, although AVHs are a core symptom of schizophrenia, they also appear in other psychiatric and neurological disorders, questioning the uniqueness of AVHs as a markers for psychoses.16,17 A Levels of Explanation approach shares terminology with the recently proposed Research Domain Criteria (RDoC),18,19 but differs in that it is not explicitly embedded in in a overarching classification system for mental disorders.
Brain Level—Neuroimaging Data
The Classic Finding
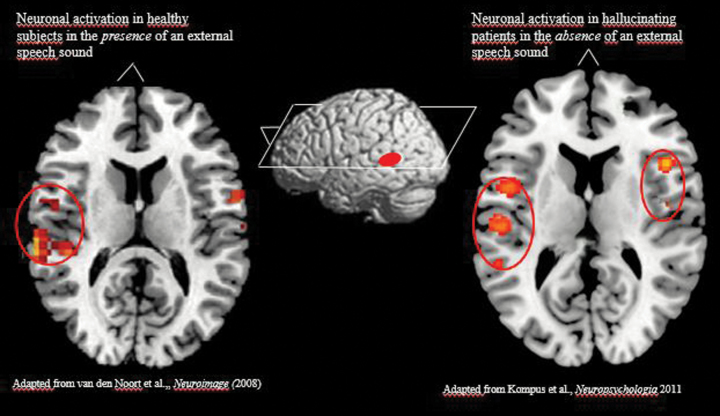
Functional neuroimaging with PET and fMRI has a long history in AVH research.7,9,20–26 Although findings have implicated several key regions in the brain being activated when patients are actively experiencing AVHs during scanning, the most robust and replicated findings are spontaneous activations in the upper, posterior part of the left temporal lobe, covering areas in the peri-Sylvian region. The right-hand panel of figure 3 shows activation in healthy subjects when listening to simple speech sounds, while the left-hand panel shows corresponding activation in hallucinating patients.
Fig. 3.
fMRI activations primarily in the speech perception areas in the upper posterior part of the temporal lobe and the right prefrontal cortex. Left-hand panel show activation in healthy subjects, and right-hand panel sow activation in hallucinating patients. Adapted from Kompus et al10 and Van den Noort et al.27
A second area prominently activated during AVH is the right inferior frontal area, covering the homologue of Broca’s area, frontal operculum and insula.11,25 At least 3 meta-analyses have confirmed spontaneous, state-driven, activations in these areas10–12 (see also Ćurčić-Blake et al20 for a recent and up-dated literature review). (We have used the more correct term “area” rather than the today more common term “network” when describing activations that are restricted to anatomically discrete regions.) Activations in the peri-Sylvia area would make sense from a perceptual view of auditory hallucinations, because this region of the brain contains the classic speech perception areas, including Heschl’s gyrus and the planum temporale; areas in the temporal plane of the superior temporal gyrus, collectively known as the Wernicke’s area. These state-effect findings in AVH patients, ie, activations in the absence of an external source to explain the activations, are remarkably similar to the activations found in healthy individuals when exposed to repeated presentations of simple consonant–vowel sounds, as seen in left-hand panel of figure 3 (adapted from Van den Noort et al27). Thus, the similarity in activations in healthy individuals when perceiving an external speech sound and in patients experiencing internal “voices” would support a perceptual basis for AVHs. The second area, located in the inferior frontal part, most likely corresponds to language production. It is of interest that this activation also involves the nondominant hemisphere. Right-sided speech production areas are typically capable of simple utterances, consisting of short sentences with little grammar and basic vocabulary elements. This type of utterances it often called “automatic speech” and may be the only source of speech in severely aphasic patients.25 The predominantly right-sided activation of speech production areas during AVH corresponds to the content of hallucinations which typically has few if any grammar and mainly contains simple words.28
The Paradoxical Finding
The finding of increased activations during AVH in the absence of a corresponding external stimulus to explain the activation could be predicted to be even stronger if these patients were also exposed to external speech sounds while in the scanner. Thus, one would predict that activations caused by the “outer voices” would relate to the activations caused by the “inner voices” in an additive way. This is, however, not what happens when AVH patients are compared with healthy individuals during presentation of external auditory stimuli (see meta-analysis by Kompus et al10, and review by Ćurčić-Blake et al20). Instead the 2 voice sources seem to subtract such that AVH patients show significantly reduced activations compared to healthy controls. Kompus et al10 called this phenomenon a “paradoxical finding” that the 2 types of stimulus input seems to compete for processing resources rather than strengthen processing capacity (see also refs.29–31 who have reported similar results using PET and EEG, respectively). There could be at least 3 possible explanations for the paradoxical effect reported by Kompus et al.10 First of all, the neurons could be set in a refractory state to external stimuli during AVHs, in which case it would be the perceptual system which would be shut down, or could be an attentional-bias effect toward the “inner voice” which prevents the recognition of an external stimulus, in which case it would be the cognitive system which is shut down. This was the explanation advanced by Kompus et al,10 or it could be a sensory-gating effect, at the brain-stem level, in which case it would be an early-stage processing deficit where the external signal never reaches the cortical processing areas.32 Future research should sort out these possibilities, which could have implications for development of new therapeutic interventions. A caveat that should be mentioned is that a shift in attention focus could also be induced when subjects are instructed to “press a button whenever you hear the voice” while in the scanner. As pointed out by van Lutterveld et al,33 this could cause an unwanted attention bias on when an episode will occur next, which in turn could confound the activations. Nevertheless, this paradoxical competition for brain resources encountered while patients with AVH listen to external speech provides an important anchor for treatment, as strong external auditory stimuli can be used to “steal” listening attention away from the AVH and thus help the patient to decrease hallucination intensity.34
Behavioral Level—Cognitive Data
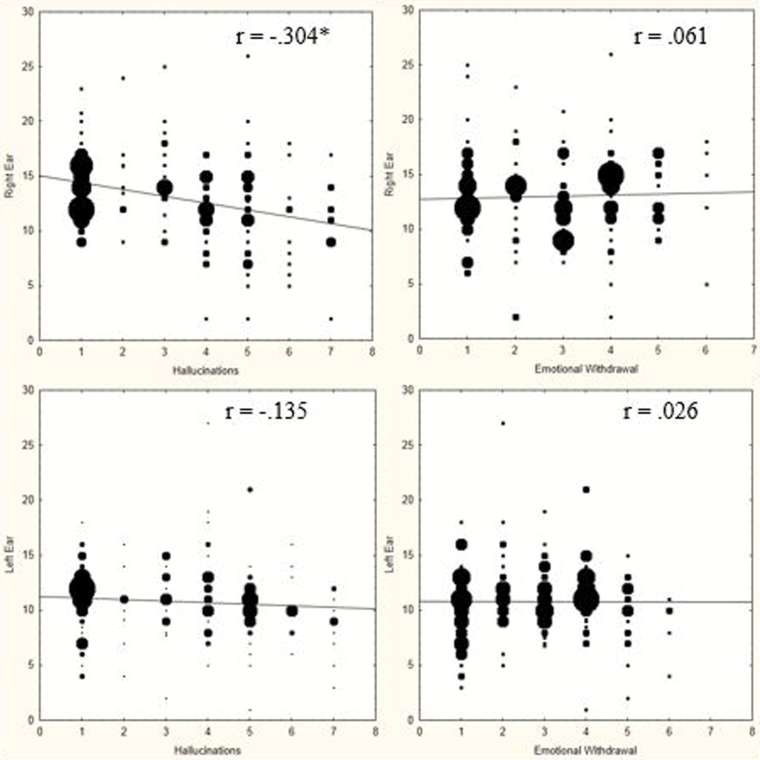
The interference caused by the “voices” at the brain level, should however have a corresponding effect at the cognitive level of explanation, for the simple reason that AVHs are about subjective experiences. A straightforward prediction at the behavioral and cognitive level that would follow from the findings at the brain level is a negative correlation between frequency and severity of AVHs and the accuracy of perception of an external speech sound. Frequency and severity of AVHs are typically quantified with the use of the Positive and Negative Syndrome Scale (PANSS),35 and particularly the P3-item. To test the prediction, Hugdahl et al36 used a dichotic listening task (see same reference for a description of the task) which identifies the hemisphere and temporal lobe, left or right, in which speech perception occurs, and also allows for the quantification of the strength of the lateralization to either hemisphere. The task consists of repeated presentations of simple consonant–vowels, with 2 different syllables presented simultaneously, one in the left and the other in the right ear on each trial. The task of the subject is simply to indicate which syllable he/she perceives on each trial, the one in the right or the one in the left ear. Healthy individuals with a left hemisphere speech perception dominance show a so called right ear advantage (REA) which means more correct responses for the right ear syllable. Hugdahl et al36 predicted that with increasing frequency and severity of AVHs, the REA will be attenuated, which would be a continuation at the cognitive level of the interference caused by AVHs at the brain level. The results are presented in the left-hand panels of figure 4, and show a significant negative correlation for the right ear syllables, with a nonsignificant effect for the left ear syllable, and consequently an attenuation of the REA with increasing frequency and severity, which thus supports the prediction and corresponds with the imaging data at the brain level of explanation.
Fig. 4.
Upper and lower left-hand panels: correlations between frequency and severity of AVHs, as measured with the PANSS P3 item (x-axis) and correct verbal responses from the right and left ear in the dichotic listening test (y-axis). Upper and lower right-hand panels: Correlations between frequency and severity of emotional withdrawal symptom, as measured with the PANSS N2 item (x-axis) and correct verbal responses from the right and left ear in the dichotic listening test (y-axis). Redrawn from Hugdahl et al36.
Interestingly, when a negative symptom was correlated with dichotic listening performance (right-hand panels of figure 3), the correlations were nonsignificant and close to zero for both ears, and consequently did not cause interference with regard to perception of an external speech sound. Taken together, these findings show an important correspondence with regard to the interference caused by AVHs at both the cognitive and the brain levels of explanation, and that this interference is unique for a positive symptom, and illustrate the utility of a LoE approach. Again, these experimental findings provide anchors for treatment, as the dichotic listening paradigm can also be used for focused attention toward correct ear. If a patient trains to improve his performance on this task, resources for AVH decrease and severity of hallucinations are reduced.
Cellular Level—Neurotransmitter Data
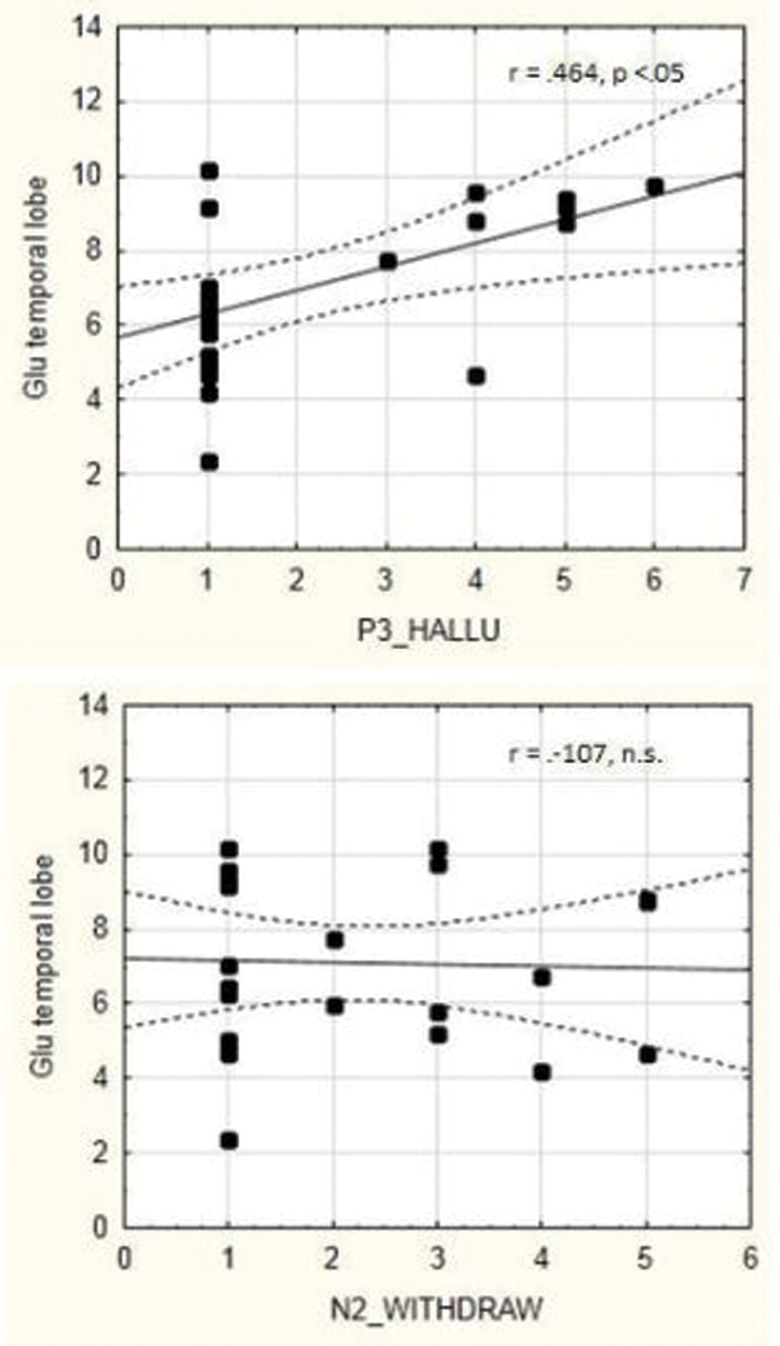
Every change in neuronal activation at the brain systems level, either spontaneously or stimulus-induced, must have a corresponding change at the receptor and neurotransmitter level that causes the neurons to alter their firing rate and metabolism. However, there is almost total absence of empirical studies of the underlying neurochemistry of AVHs (see Sanjuan et al37 for a discussion). A widely distributed excitatory transmitter in the cortex is glutamate which acts on 3 specific receptor types. Glutamate is a brain metabolite, ie, it plays a role in brain metabolism, in particular, in the conversion of glucose and oxygen to ATP. Knowing that the blood-oxygen-level-dependent (BOLD) response, which is the signal recorded in fMRI studies, is a measure of the cerebral metabolic rate of oxygen, and a marker of metabolic turnover in the neurons, further speaks to the validity of a glutamate hypothesis for the initiation of a hallucinatory episode, which in turn could explain the spontaneous activation increase during AVHs (see meta-analyses by Kompus et al10 and Jardri et al11). Hugdahl et al38 used MR spectroscopy to measure glutamate levels (measured as Glx which is the sum of glutamate and glutamine), and found increased Glx levels in frontal and temporal lobe areas (corresponding to the language network observed with fMRI), in AVH patients as compared to patients of similar clinical history but without AVH. This finding was recently replicated in a study from the University of Groningen, Netherlands39 who reported increased Glx levels in patients with a history of life-time AVHs as compared to patients without AVH. Hugdahl et al38 also found a significant positive correlation between frequency and severity of AVHs, as measured with PANSS, and Glx levels in the selected voxel in the temporal lobe, which further points to a role for glutamate in the initiation of a AVH episode, the correlations are seen in figure 5. The lower panel in figure 5 shows the corresponding correlation for the negative symptom of emotional withdrawal, which was nonsignificant.
Fig. 5.
Upper panel: correlation between frequency and severity of AVHs as measured with the PANSS P3 item (x-axis) and glutamate concentration in the temporal lobe, measured as Glx (y-axis). Lower panel: correlation between frequency and severity of emotional withdrawal symptom as measured with the PANSS N2 item (x-axis) and glutamate concentration in the temporal lobe, measured as Glx (y-axis). Redrawn from Hugdahl et al.38
This leads to a hypothesis of excess of glutamate levels and/or of glutamate receptors which causes a neuronal state characteristic of hyper-excitation in the language areas in the brain which in turn initiates a hallucinatory episode. Glutamate excitatory influences are normally balanced by GABA inhibitory influences in a fine-tuned interaction.40 We suggest that this balance is upset in the initiation of an AVH episode with glutamate excess, but that the balance is temporarily restored in the cessation of an episode, such that the glutamate-GABA excitatory-inhibitory balance is a key factor in understanding the oscillatory fluctuations seen in AVHs across time.41
Molecular Level—Genetic Data
Despite the fact that genetic studies have shown that heritability may be as high as 70% for schizophrenia,42 no studies exist that have implicated a specific link between heritability and AVHs. Similarly, of the 108 genetic loci that were identified in the gene-wide association study (GWAS) as associated with schizophrenia,43 none of the identified genes were specifically associated with AVHs. This could mean that AVHs are not uniquely linked to schizophrenia, or any other diagnostic category, which would be in line with recent suggestions that AVHs are not necessarily a marker of psychosis disorder, but could be a category of its own, and that characteristic features of AVH not necessarily can be used for diagnostic purposes (see overview by Waters and Fernyhough6). While large collaborate efforts have been made to collect GWAS data from large samples of patients with schizophrenia, such collaborative efforts are not yet made to investigate the genetic association with AVHs. This is unfortunate, since a diagnostic category carries much more heterogeneity and unexplained variance than a single symptom. We therefore encourage future studies of AVHs to also approach the genetic level of explanation. While GWAS information on association with AVH is scarce, some interesting work has been done in the field of copy number variation (CNVs). The 22q11 deletion syndrome (22q11 DS) provides a much higher risk for psychosis in general and for AVH specifically. Approximately one-third of children with 22q11 DS have increased plasma levels of the aminoacid proline, which influences glutamatergic neurotransmission.44 Two studies found evidence linking plasma proline levels to deviant brain function,45,46 suggesting that strategies to alter glutamatergic neurotransmission may be of particular relevance for this population. Another study by Chun et al47 identified a specific disruption of thalamocortical glutamatergic projections to the auditory cortex in a murine model of schizophrenia-associated 22q11 deletion syndrome (22q11DS). This deficiency could be alleviated by haloperidol, suggesting that the thalamocortical glutamatergic projections could be involved in humans suffering from AVH just the same.
Summary
We have suggested an LoE approach to the study of AVHs in schizophrenia that looks at integration of data and findings across different levels of explanation. With this, we mean that AVHs are manifest in different domains, what we call “levels” from a cultural to molecular and genetic domain, with clinical, cognitive, brain, and cellular levels in between. We have presented data from functional neuroimaging, using fMRI and behavior using dichotic listening which shows how findings from one level of explanation can be used to generate new hypotheses and predictions at another level. We finally show how these findings are linked to changes in glutamatergic neurotransmitter function, using magnetic resonance spectroscopy, MRS, which could yield new hypotheses regarding targets for drug developments.
Funding
The research presented in this article was funded by ERC Advanced Grant (693124), Research Council of Norway (RCN) (912945 and 223273 to K.H.), Helse-Vest, Norway (912045 to K.H.), and VIDI grant from the Netherlands Organization for Health Research and Development (ZonMW) (017106301 to I.E.S.).
Acknowledgment
The authors would like to thank all PhD students, postdocs, Research technicians, and other colleagues, and the patients who participated, which made the research possible. Conflict of interest: Kenneth Hugdahl owns shares in the company NordicNeuroLab Ltd., who produces equipment used for data acquisition in some of the MR imaging studies reviewed. He declares no conflict of interest.
References
- 1. Larøi F, Aleman A.. Hallucinations—A Guide to Treatment and Management. Oxford, UK: Oxford University Press; 2010. [Google Scholar]
- 2. Sommer IE, Slotema CW, Daskalakis ZJ, Derks EM, Blom JD, van der Gaag M. The treatment of hallucinations in schizophrenia spectrum disorders. Schizophr Bull. 2012;38:704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johns LC, Kompus K, Connell W et al. Auditory verbal hallucinations in persons with and without a need for care. Schizophr Bull. 2014suppl 4:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kompus K, Falkenberg LE, Bless JJ et al. The role of the primary auditory cortex in the neural mechanism of auditory verbal hallucinations. Front Hum Neurosci. 2013;7:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daalman K, van Zandvoort M, Bootsman F, Boks M, Kahn R, Sommer I. Auditory verbal hallucinations and cognitive functioning in healthy individuals. Schizophr Res. 2011;132:203–207. [DOI] [PubMed] [Google Scholar]
- 6. Waters F, Fernyhough C. Hallucinations: a systematic review of points of similarity and difference across diagnostic classes. Schizophr Bull. 2017;43:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bohlken MM, Hugdahl K, Sommer IE. Auditory verbal hallucinations: neuroimaging and treatment. Psychol Med. 2017;47:199–208. [DOI] [PubMed] [Google Scholar]
- 8. Upthegrove R, Broome MR, Caldwell K, Ives J, Oyebode F, Wood SJ. Understanding auditory verbal hallucinations: a systematic review of current evidence. Acta Psychiatr Scand. 2016;133:352–367. [DOI] [PubMed] [Google Scholar]
- 9. Aleman A, Vercammen A. Functional neuroimaging of hallucinations. In: Blom JD, Sommer IEC, eds. Hallucinations—Research and Practice. New York: Springer; 2012:267–282. [Google Scholar]
- 10. Kompus K, Westerhausen R, Hugdahl K. The “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: a meta-analysis of functional neuroimaging studies. Neuropsychologia. 2011;49: 3361–3369. [DOI] [PubMed] [Google Scholar]
- 11. Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168:73–81. [DOI] [PubMed] [Google Scholar]
- 12. Kühn S, Gallinat J. Quantitative meta-analysis on state and trait aspects of auditory verbal hallucinations in schizophrenia. Schizophr Bull. 2012;38:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hugdahl K. Auditory hallucinations: a review of the ERC “VOICE” project. World J Psychiatry. 2015;5:193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luhrmann TM, Padmavati R, Tharoor H, Osei A. Differences in voice-hearing experiences of people with psychosis in the U.S.A., India and Ghana: interview-based study. Br J Psychiatry. 2015;206:41–44. [DOI] [PubMed] [Google Scholar]
- 15. Blom JD, Eker H, Basalan H, Aouaj Y, Hoek HW. Hallucinations attributed to djinns. Ned Tijdschr Geneeskd. 2010;154:A973. [PubMed] [Google Scholar]
- 16. Waters F, Blom JD, Jardri R, Hugdahl K, Sommer IEC. Auditory hallucinations, not necessarily a hallmark of psychotic disorder. Psychol Med. 2017. doi: 10.1017/S0033291717002203. [DOI] [PubMed] [Google Scholar]
- 17. Sommer IE, Kahn RS. Psychosis susceptibility syndrome: an alternative name for schizophrenia. Lancet Psychiatry. 2014;1:111. [DOI] [PubMed] [Google Scholar]
- 18. Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Badcock JC, Hugdahl K. A synthesis of evidence on inhibitory control and auditory hallucinations based on the Research Domain Criteria (RDoC) framework. Front Hum Neurosci. 2014;8:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ćurčić-Blake B, Ford JM, Hubl D et al. Interaction of language, auditory and memory brain networks in auditory verbal hallucinations. Prog Neurobiol. 2017;148:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allen P, Larøi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev. 2008;32:175–191. [DOI] [PubMed] [Google Scholar]
- 22. Allen P, Modinos G, Hubl D et al. Neuroimaging auditory hallucinations in schizophrenia: from neuroanatomy to neurochemistry and beyond. Schizophr Bull. 2012;38:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Silbersweig DA, Stern E, Frith C et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. [DOI] [PubMed] [Google Scholar]
- 24. Bentaleb LA, Beauregard M, Liddle P, Stip E. Cerebral activity associated with auditory verbal hallucinations: a functional magnetic resonance imaging case study. J Psychiatry Neurosci. 2002;27:110–115. [PMC free article] [PubMed] [Google Scholar]
- 25. Sommer IE, Diederen KM, Blom JD et al. Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain. 2008;131:3169–3177. [DOI] [PubMed] [Google Scholar]
- 26. Ford JM, Dierks T, Fisher DJ et al. Neurophysiological studies of auditory verbal hallucinations. Schizophr Bull. 2012;38:715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van den Noort M, Specht K, Rimol LM, Ersland L, Hugdahl K. A new verbal reports fMRI dichotic listening paradigm for studies of hemispheric asymmetry. Neuroimage. 2008;40:902–911. [DOI] [PubMed] [Google Scholar]
- 28. de Boer JN, Heringa SM, van Dellen E, Wijnen FN, Sommer IE. A linguistic comparison between auditory verbal hallucinations in patients with a psychotic disorder and in nonpsychotic individuals: not just what the voices say, but how they say it. Brain Lang. 2016;162:10–18. [DOI] [PubMed] [Google Scholar]
- 29. Woodruff PW, Wright IC, Bullmore ET et al. Auditory hallucinations and the temporal cortical response to speech in schizophrenia: a functional magnetic resonance imaging study. Am J Psychiatry. 1997;154:1676–1682. [DOI] [PubMed] [Google Scholar]
- 30. Hubl D, Koenig T, Strik WK, Garcia LM, Dierks T. Competition for neuronal resources: how hallucinations make themselves heard. Br J Psychiatry. 2007;190:57–62. [DOI] [PubMed] [Google Scholar]
- 31. Ford JM, Roach BJ, Jorgensen KW et al. ; FBIRN Tuning in to the voices: a multisite FMRI study of auditory hallucinations. Schizophr Bull. 2009;35:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thoma R, Meier A, Houck B et al. Diminished auditory sensory gating during active auditory hallucinations in schizophrenia. Schizophr Res. 2017. doi: 10.1016/j.schres.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Lutterveld R, Diederen K, Schutte M, Zabdbelt B, Sommer IE. Brain correlates of auditory hallucinations: stimulus detection is a potential confounder. Schizophr Res. 2009;150:319–320. [DOI] [PubMed] [Google Scholar]
- 34. Farhall J, Greenwood KM, Jackson HJ. Coping with hallucinated voices in schizophrenia: a review of self-initiated strategies and therapeutic interventions. Clin Psychol Rev. 2007;27:476–493. [DOI] [PubMed] [Google Scholar]
- 35. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 36. Hugdahl K, Løberg EM, Falkenberg LE et al. Auditory verbal hallucinations in schizophrenia as aberrant lateralized speech perception: evidence from dichotic listening. Schizophr Res. 2012;140:59–64. [DOI] [PubMed] [Google Scholar]
- 37. Sanjuan J, Aguilar EJ, Artigas F. Pharmacological treatment of hallucinations. In: Larøi F, Aleman A, eds. Hallucinations—A Guide to Treatment and Management. New York: Oxford University Press; 2010:9–28. [Google Scholar]
- 38. Hugdahl K, Craven AR, Nygård M et al. Glutamate as a mediating transmitter for auditory hallucinations in schizophrenia: a (1)H MRS study. Schizophr Res. 2015;161:252–260. [DOI] [PubMed] [Google Scholar]
- 39. Ćurčić-Blake B, Bais L, Sibeijn-Kuiper A et al. Glutamate in dorsolateral prefrontal cortex and auditory verbal hallucinations in patients with schizophrenia: a (1)H MRS study. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:132–139. [DOI] [PubMed] [Google Scholar]
- 40. Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. [DOI] [PubMed] [Google Scholar]
- 41. Jardri R, Hugdahl K, Hughes M et al. Are hallucinations due to an imbalance between excitatory and inhibitory influences on the brain?Schizophr Bull. 2016;42:1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gandal MJ, Leppa V, Won H, Parikshak NN, Geschwind DH. The road to precision psychiatry: translating genetics into disease mechanisms. Nat Neurosci. 2016;19:1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Witt SH; Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vorstman JA, Turetsky BI, Sijmens-Morcus ME et al. Proline affects brain function in 22q11DS children with the low activity COMT 158 allele. Neuropsychopharmacology. 2009;34:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goodman BK, Rutberg J, Lin WW, Pulver AE, Thomas GH. Hyperprolinaemia in patients with deletion (22)(q11.2) syndrome. J Inherit Metab Dis. 2000;23:847–848. [DOI] [PubMed] [Google Scholar]
- 46. Raux G, Bumsel E, Hecketsweiler B et al. Involvement of hyperprolinemia in cognitive and psychiatric features of the 22q11 deletion syndrome. Hum Mol Genet. 2007;16: 83–91. [DOI] [PubMed] [Google Scholar]
- 47. Chun S, Westmoreland JJ, Bayazitov IT et al. Specific disruption of thalamic inputs to the auditory cortex in schizophrenia models. Science. 2014;344:1178–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]