Abstract
Autophagy is an evolutionarily conserved pathway in which cytoplasmic contents are degraded and recycled. This study found that submicromolar concentrations of urolithin A, a major polyphenol metabolite, induced autophagy in SW620 colorectal cancer (CRC) cells. Exposure to urolithin A also dose‐dependently decreased cell proliferation, delayed cell migration, and decreased matrix metalloproteinas‐9 (MMP‐9) activity. In addition, inhibition of autophagy by Atg5‐siRNA, caspases by Z‐VAD‐FMK suppressed urolithin A‐stimulated cell death and anti‐metastatic effects. Micromolar urolithin A concentrations induced both autophagy and apoptosis. Urolithin A suppressed cell cycle progression and inhibited DNA synthesis. These results suggest that dietary consumption of urolithin A could induce autophagy and inhibit human CRC cell metastasis. Urolithins may thus contribute to CRC treatment and offer an alternative or adjunct chemotherapeutic agent to combat this disease.
Keywords: autophagy, colorectal cancer, matrix metallo proteinases, metastasis, urolithin A
1. INTRODUCTION
Colorectal cancer (CRC) is the second most common gastrointestinal cancer worldwide,1 and preventing tumor cell metastasis is important for prolonging patient survival.2 In recent years, the relationship between cancer and autophagy has been extensively studied,3, 4, 5, 6, 7 and autophagy can promote or inhibit tumor metastasis under different circumstance. Autophagy may exert an inhibitory effect in early stage tumors, reducing invasion, and metastasis. During later stages, autophagy promotes metastasis by supporting cell survival following ECM detachment and preventing cell dormancy upon ECM reattachment.8, 9 Autophagy is already known to play an important role in CRC progression.10 Another key step in the process of metastasis is basement membrane and extracellular matrix degradation.11 Matrix metalloproteinase (MMPs) upregulation also promotes metastasis.12–14
Polyphenols provide a wide range of anti‐carcinogenic health benefits.15, 16, 17, 18, 19, 20, 21, 22 However, polyphenol absorption is reportedly very low. Urolithins are the major metabolites of polyphenols in the gut.23, 24, 25 We isolated and purified urolithins from the intestinal metabolites of pomegranate ellagitannins by high‐speed counter‐current chromatography.26 Urolithins can be absorbed by multiple tissue types and can suppress tumorigenesis,27 oxidation,28 inflammation,29, 30, 31 and microbial32 activity in vitro. Urolithins are reported to have antiproliferative effects on prostate,33, 34 colon,35, 36 bladder,37 breast,38 pancreatic, epidermal, ovarian, and neuroblastoma cancers39 at a range of five and 500 µM. In vitro studies conducted in colon cancer cells are of great relevance since it is in the GI tract where urolithins are produced and can reach bioactive concentrations. However, the exact mechanisms involved in urolithin activity are not yet fully elucidated. Of note, whether urolithins suppress cancer growth by regulating autophagy is still unknown.
In this study, we used SW620 CRC cells to test the hypothesis that urolithin A promotes autophagy. Our findings suggest that submicromolar urolithin A induces autophagy, thereby inhibiting SW620 cell survival and metastasis. Hence, urolithin A therapy may be efficacious against CRC in patients.
2. MATERIALS AND METHODS
2.1. Cell culture and treatment
Human SW620 cells were maintained in 75 cm2 Falcon flasks in L‐15 medium supplemented with 25 mM glucose, 10% heat‐inactivated (56°C) fetal bovine serum (FBS), 100 µg/mL streptomycin, 100 U/mL penicillin, and 1% non‐essential amino acids (Invitrogen Corp., France) in a humidified atmosphere at 37°C with 5% CO2. Cells were subcultured after trypsinization (0.5% trypsin/2.6 mM EDTA). For experiments, cells (1 × 106) were seeded in 10 cm culture dishes, with medium replacement every 48 h. At 24 h, SW620 cells were exposed to urolithin A dissolved in dimethylsulfoxide (DMSO, Sigma‐Aldrich, Germany). The final DMSO concentration in L‐15 culture medium did not exceed 0.1%.
2.2. Chamber migration assay
Transwell polycarbonate membrane inserts (8 µm poresize, 10 mm diameter; Corning Costar, MA) were utilized for chamber migration assays. SW620 cells with or without urolithin A were suspended in serum‐free medium (5 × 105 cells/mL) and added to the upper compartment, while L‐15 medium containing 10% FBS was added to the lower compartment. After 4 h, non‐invaded cells on the upper side of the Transwell polycarbonate membrane were removed with a cotton tip applicator. Invaded cells on the bottom surface of the membrane were fixed with methanol and stained with 0.5% crystal violet (Beyotime Institute of Biotechnology, Shanghai, China).
2.3. Measurement of MMP‐9 activation
MMP‐9 activation was determined by colorimetric assay (Sigma‐Aldrich for MMP‐9, abcam, ab100610) according to the manufacturer's instructions. Briefly, SW620 cells were washed with ice‐cold PBS and lysed; proteins were obtained and stored at −80°C. Twenty µL of cell lysate was added to buffer for 100 µL total reaction volume. Supernatants were incubated at room temperature. Released MMP concentrations were calculated from the absorbance values at 405 nm and compared to protein concentrations as determined using the Bradford method. MMP‐9 activity was shown as fold‐increase compared to untreated SW620 cells.
2.4. Western blot
SW620 cells were incubated with or without 1.5 µM urolithin A, harvested, washed with ice‐cold PBS and lysed for 30 min. Protein concentrations were determined using a BCA assay kit and 50 µg of protein per sample was used for Western blot analysis with the following antibodies: LC3‐I/II (1:250; Merck‐Millipore Transduction Laboratories, Billerica, MA) and anti‐mouse HRP‐conjugated IgG secondary antibody (1:2000). All samples were normalized to β‐actin.
2.5. Flow cytometry
SW620 cells were labeled with propidium iodide (PI) for cell cycle analysis. Cells were harvested by trypsinization after 24, 48, or 72 h treatment with urolithin A and were suspended in PBS (0.1 M, pH 7.4). Cells were fixed in 70% ethanol for at least 30 min at −20°C. Before analysis, cells were washed twice in cold PBS and re‐suspended in 200 µL PBS (0.25 mg/mL RNase A, 0.1 mg/mL PI). After incubation in darkness for 30 min at 37°C, samples were analyzed via flow cytometry and histograms were calculated by Cell Quest software (FACScan, BD Biosciences, San Jose, CA).
2.6. Detection of apoptosis
Apoptosis was evaluated using a flow cytometer with Annexin V‐FITC and PI staining. Briefly, SW620 cells were cultivated in six‐well plates. At the end of the incubation, cells were suspended in PBS three times, then resuspended in 195 µL binding buffer containing 5 µL Annexin V‐FITC and incubated for 10 min at room temperature. Cells were centrifuged at 1000 g for 5 min and resuspended in 190 µL of binding buffer containing 10 µL of PI working solution. After filtration, samples were analyzed on a flow cytometer FACSVantage flow cytometer. At least 10 000 events were analyzed.
2.7. Measurement of autophagy
Autophagy was monitored using the Cyto‐ID TM Autophagy Detection Kit (Enzo Life Sciences, France, ENZ‐51031‐K200) following the manufacturer's instructions. Briefly, for flow cytometric analysis, SW620 cells were harvested, washed with Assay Buffer, and stained with Cyto‐ID TM Green Detection Reagent at room temperature for 30 min in the dark. SW620 cell suspensions were then immediately analyzed by flow cytometry using Cell Quest software (FACScan, BD Biosciences).
2.8. Transmission electron microscopy
SW620 cells were first fixed at 4°C in solution containing 2% cacodylate‐buffered (0.1 M; pH 7.2) glutaraldehyde with 0.1 M sucrose, then fixed in 0.15 M cacodylate buffer containing 1% osmium tetroxide at room temperature, stained with 50% ethanol containing 2% uranyl acetate, dehydrated in a gradient of ethanol, and embedded in Epon. Thin sections were cut with a diamond knife, stained with lead citrate, and viewed with a 1200‐EX electron microscope.
2.9. Statistical analysis
All experiments were repeated at least three times. Data are reported as means ± SD. Statistical differences between groups were analyzed using the Student's t‐test or the Student‐Neuman‐Keuls multiple comparison test. Differences were considered statistically significant at P < 0.05.
3. RESULTS
3.1. Urolithin A inhibits SW620 cell proliferation and metastasis
To examine the effects of urolithin A on SW620 colon cancer cell proliferation, exponentially growing cells were exposed to various concentrations of urolithin A for 24 h, and proliferation was measured via MTT assay. A dose‐dependent proliferation decrease was observed in treated cells (Figure 1A, B). Exposure to high urolithin A concentrations (≥15 µM) produced the strongest anti‐proliferative effects, probably by promoting cell death.
Figure 1.

Effect of urolithin A on cell proliferation, metastasis, and MMP‐9 activity. SW620 cells were treated with 0 (a) 0.15 (b) 1.5 (c) or 15 (d) µM urolithin A for 24 h (A) and cell proliferation was measured using MTT assay (B). Photographs (×100 magnification) are representative of three independent experiments. Cell monolayers were wounded using 200 µL pipette tips, treated with 0 (a) 0.05 (b) µM urolithin A for 48 h and photographs were taken (×100 magnification) to measure cell migration (C). After treatment with 0 (a) 0.05 (b) µM urolithin A for 48 h, cells were seeded above transwell chamber membranes. After 4 h, membrane bottoms were stained with crystal violet and photographs were taken (×200 magnification) to assess cell invasion (D). MMP‐9 activity was determined by MMP assay in cells treated with 0.05 µM urolithin A for 48 h (E) *P < 0.05 represents significant difference from the control group
We next examined the effects of urolithin A on SW620 cell mobility via the chamber migration assay. The number of cells that migrated across the wound decreased by 35.2% (n = 3, P < 0.01) following treatment with 0.05 µM urolithin A for 48 h (Figure 1C), and cell migration was delayed up to 21.3% (n = 3, P < 0.05) (Figure 1D).
MMP‐9, which degrades the basement membrane and extracellular matrix, is enhanced in various malignant tumors and promotes invasion and metastasis. Treatment of SW620 cells with urolithin A (0.05 µM) strongly decreased MMP‐9 activity in these cells (reduced by 29.8%, n = 3, P < 0.01) (Figure 1E).
3.2. Submicromolar urolithin A triggers autophagy
Morphological features of autophagy in SW620 cells treated with 1.5‐15 µM urolithin A were examined by TEM. Submicromolar urolithin A concentrations resulted in the appearance of numerous vacuoles (Figure 2A) containing organelles and cellular fragments, indicating that they were autolysosomes (Figure 2Ab, c, e, f, h, i). Urolithin A caused the redistribution of GFP‐microtubule‐associated protein 1 light chain 3 (LC3) from a faint, diffused pattern to visible cytoplasmic puncta fluorescence, suggesting that urolithin A stimulates autophagic vacuolization (Figure 2B). These data demonstrate that dietary submicromolar urolithin A triggers autophagy in SW620 cells.
Figure 2.
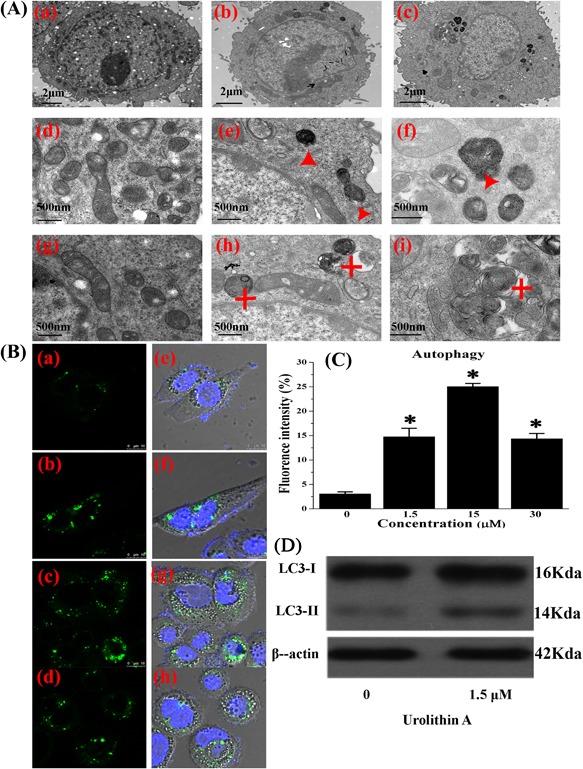
Autophagy in SW620 cells treated with urolithin A. Electron microscopy of cross sections of controls (a,d,g) and 1.5 µM (b, e, h) or 15 µM (c, f, i) urolithin A‐treated cells (A). Representative autophagic vesicles (red triangle) and late autophagic compartment containing electron dense cellular material (red star) are shown in treated cells. Immunocytochemical detection of LC3 in urolithin A‐treated cells via laser scanning confocal microscope (B). Representative photos of controls (a, e) and 1.5 µM (b, f), 15 µM (c, g), or 30 µM (d, h) urolithin A‐treated cells are shown (bar 500 µm). Results are representative of three independent experiments (C). SW620 cells were exposed to the indicated urolithin A concentrations for 24 h and then analyzed by Western blotting for LC3‐I and LC3‐II expression (D)
We then treated SW620 colon cancer cells with low (1.5 µM) and high (30 µM) urolithin A doses. We observed that urolithin A induced LC3 (GFP‐LC3) accumulation in cells 24 h after treatment (Figures 2Bb, c, f, g and 2C). Furthermore, submicromolar urolithin A treatment stimulated LC3 expression in SW620 cells (Figure 2D). Urolithin A‐induced autophagy is not dose‐dependent at micromolar concentrations; 15 µM urolithin A induces more autophagy than 1.5 µM, however, 30 µM urolithin A induces less autophagy than 15 µM (Figures 2Bd, h and 2C).
3.3. Micromolar urolithin A induces both autophagy and apoptosis
SW620 cells were stained with annexin V, an apoptosis marker. Incubation with 30 µM urolithin A increased both apoptosis (Annexin V‐fluorescein isothiocyanate [FITC] positive) and necrosis (PI positive) (Figures 3A and 3C). However, at concentrations ranging from 1.5 to 15 uM, urolithin did not induce apoptosis. These results suggest that micromolar urolithin A induces both autophagy and apoptosis approximately 24 h after treatment.
Figure 3.

Effect of urolithin A on apoptosis and cell cycle progression. SW620 cells were incubated with 0 (a), 1.5 (b), 15 (c), or 30 (d) µM urolithin A for 24 h, then stained with annexin‐V FITC and PI. Apoptosis was analyzed using flow cytometry (A). Results shown are representative of three independent experiments (C). *P < 0.05. Cells were treated with 0 (a), 1.5 (b), 15 (c) or 30 (d) µM urolithin A for 24 h, then harvested, fixed with ethanol, and stained with propidium iodide. DNA contents were determined by flow cytometry for assessment of the cell cycle distribution (B). Results shown are representative of three independent experiments (D). The time curve that 30 µM Urolithin A induces apoptosis after the treatment (E)
3.4. Urolithin A inhibits cell cycle progression in SW620 cells
Cell cycle distribution profiles for urolithin A‐exposed SW620 cells were assessed via flow cytometry. Urolithin A treatment resulted in the progressive accumulation of G2/M phase cells until 24 h (Figures 3B and 3D). Thus, urolithin A deregulates cell cycle progression and inhibits DNA synthesis in SW620 cells.
3.5. Inhibition of autophagy, caspases, or MMPs suppressed urolithin A‐activated cell death and anti‐metastasis activity
Inhibition of autophagy in SW620 cells by 3‐methyladenine (3‐MA), bafilomycin (BAF), chloroquine (CHL), and Atg5‐siRNA treatment suppressed submicromolar urolithin A‐activated cell death and MMP inhibition (Figure 4A–B). Inhibition of autophagy in SW620 cells by 3‐methyladenine (3‐MA) treatment suppressed submicromolar urolithin A‐activated metastasis inhibition (Figure 4C). However, the effects of micromolar urolithin A concentrations were unchanged by 3‐MA treatment. Thus, autophagy seems to play a critical role in the anticancer activity of urolithin A.
Figure 4.

Effect of autophagy, apoptosis inhibition on urolithin A‐mediated cell proliferation and anti‐metastasis activity. Cells were pretreated with pharmacological autophagy inhibitors (Atg5 siRNA, 3‐MA, bafilomycin, and chloroquine), then incubated with or without urolithin A for 24 h. Cell proliferation was measured using MTT assay (A) and MMP‐9 activity was determined by MMP assay (B). Cells were pretreated without (a) or with (b) 0.5 mM 3‐MA for 1 h, then incubated with 1.5 µM urolithin A for 48 h, cells were seeded above transwell chamber membranes. After 4 h, membrane bottoms were stained with crystal violet and photographs were taken (×200 magnification) to assess cell invasion (C). Cells were pretreated with 20 µM Z‐VAD‐FMK for 1 h, then incubated with or without urolithin A for 24 h. Cell proliferation was measured using MTT assay (D) and MMP‐9 activity was determined by MMP assay (E) and caspase‐3 activity was determined by caspase‐3 assay (F) **P < 0.05 represents significant difference from the control group. #P < 0.05 represents significant difference from the urolithin A treatment group. Data represent means ± S.D. for one experiment performed in triplicate
Similarly, inhibition of caspases by Z‐VAD‐FMK suppressed micromolar urolithin A‐activated cell death in SW620 cells (Figure 4D‐F), but did not affect submicromolar urolithin A activity. Metastasis inhibition by urolithin A was suppressed by Z‐VAD‐FMK treatment. Caspase‐3 inhibition by urolithin A was suppressed by Z‐VAD‐FMK treatment.
4. DISCUSSION
Epidemiological studies suggest that consumption of plant‐derived foods correlates with reduced cancer mortality.40, 41, 42 The anticancer effects of polyphenols are reportedly caused by apoptosis induction via cell survival pathways.43, 44 Several groups have shown that polyphenol‐induced apoptosis is correlated with increased caspase activity and decreased oxidative stress.45, 46, 47, 48 However, we found that submicromolar Urolithin A concentrations did not cause apoptosis, but induced autophagy in colon cancer SW620 cells. Higher concentrations (30 µM) did induce apoptosis in these cells. Other studies reported that urolithin A induced apoptosis in colon cancer HT‐29 cells (at 25, 50, and 100 µM) and prostate cancer LNCaP cells (at 40 µM).49, 50 Our studies suggested that the anticancer effects of urolithin A may result, at least in part, from autophagy induction at both lower and higher concentrations, and apoptosis at higher concentrations. Of note, the urolithin A concentrations that triggered autophagy were consistent with those found in the intestine, showing that dietary polyphenols play important roles in body functions.
Recently, studies showed that autophagy and MMP‐9 were correlated and autophagy may promote MMP‐9 induction.51, 52, 53 In the current study, we found that both autophagy and apoptosis were induced in response to urolithin A treatment, but MMP‐9 was reduced. Autophagy plays a complex role in tumorigenesis; at early stages of tumor formation, autophagy has been shown to be oncosuppressive. However, autophagy seems to be required for the progression of adenomas to carcinomas at later stages of disease. Autophagy may be activated in various cancers in response to treatment by polyphenols such as PGG and EGCG.[54,55] Here we demonstrated that dietary submicromolar urolithin A suppresses SW620 cell proliferation and metastasis by promoting autophagic cell death. Thus, autophagy could play an anti‐survival and potentially tumor suppressive role in colon cancer. Dietary polyphenol‐induced tumor cell autophagy might enhance mTOR inhibitor activity to accelerate tumor cell killing.
In summary, we showed for the first time that treatment of colon cancer SW620 cells with dietary submicromolar urolithin A can induce autophagy and inhibit CRC cell growth and metastasis. Our in vitro findings provide novel insights into understanding the anti‐tumor functions of dietary urolithin A in CRC.
CONFLICTS OF INTEREST
The authors have declared no conflict of interest.
ACKNOWLEDGMENTS
This research was supported by: funding from the National Science Foundation of China (21201124), the Importation and Development of High‐Caliber Talents Project funded by Beijing Municipal Institutions (CIT&TCD201304176), the Beijing Municipal Science & Technology Commission funded by the Chinese Government (Z141100002114049) and the Scientific Research Common Program of the Beijing Municipal Commission of Education funded by the Chinese Government (KM201310025007).
Zhao W, Shi F, Guo Z, Zhao J, Song X, Yang H. Metabolite of ellagitannins, urolithin A induces autophagy and inhibits metastasis in human sw620 colorectal cancer cells. Molecular Carcinogenesis. 2018;57: 193–200. https://doi.org/10.1002/mc.22746
REFERENCES
- 1. Zakraoui O, Marcinkiewicz C, Aloui Z, et al. Lebein, snake venom disintegrin, suppresses human colon cancer cells proliferation andtumor‐induced angiogenesis through cell cycle arrest, apoptosis induction and inhibition ofVEGF expression. Mol Carcinog. 2017; 56:18–35. [DOI] [PubMed] [Google Scholar]
- 2. O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004; 96:1420–1425. [DOI] [PubMed] [Google Scholar]
- 3. Ferraresi A, Phadngam S, Morani F, et al. Resveratrol inhibits IL‐6‐induced ovarian cancer cell migration through epigenetic up‐regulationof autophagy. Mol Carcinog. 2017; 56:1164–1181. [DOI] [PubMed] [Google Scholar]
- 4. Jane EP, Premkumar DR, Sutera PA, et al. Survivin inhibitor YM155 induces mitochondrial dysfunction, autophagy, DNA damage andapoptosis in Bcl‐xL silenced glioma cell lines. Mol Carcinog. 2017; 56:1251–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gewirtz DA. Cytoprotective and nonprotective autophagy in cancer therapy. Autophagy. 2013; 9:1263–1265. [DOI] [PubMed] [Google Scholar]
- 6. Thorburn A, Thamm DH, Gustafson DL. Autophagy and cancer therapy. Mol Pharmacol. 2014; 85:830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ágata RT, Alicia B, Cecilia M, et al. The pepper's natural ingredient capsaicin induces autophagy blockage in prostate cancer cells. Oncotarget. 2015; 27:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu XF, Shi WN, Zhang YH, et al. CXCL12/CXCR4 axis induced miR‐125b promotes invasion and confers 5‐fluorouracil resistance through enhancing autophagy in colorectal cancer. Sci Rep. 2017; 7:42226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Su Z, Yang Z, Xu Y, et al. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015; 14:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takeo Nitta YS, Ren X, Harada K, et al. Autophagy may promote carcinoma cell invasion and correlate with poor prognosis in cholangiocarcinoma. Int J Clin Exp Pathol. 2014; 7:4913–4921. [PMC free article] [PubMed] [Google Scholar]
- 11. Yang MP, Zhao H, Guo L, et al. Autophagy‐based survival prognosis in human colorectal carcinoma. Oncotarget. 2015; 9:7084–7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Babykutty S, S PP, J NR, et al. Nimbolide retards tumor cell migration, invasion, and angiogenesis by downregulating MMP‐2/9expression via inhibiting ERK1/2 and reducing DNA‐binding activity of NF‐κB in colon cancer cells. Mol Carcinog. 2012; 51:475–490. [DOI] [PubMed] [Google Scholar]
- 13. Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006; 25:9–34. [DOI] [PubMed] [Google Scholar]
- 14. Bendardaf R, Buhmeida A, Hilska M, et al. MMP‐9 (gelatinase B) expression is associated with disease‐free survival and disease‐specific survival in colorectal cancer patients. Cancer Invest. 2010; 28:38–43. [DOI] [PubMed] [Google Scholar]
- 15. Carlos MP, Carol W, Arran KT, et al. Antitumour activity of the novel flavonoid Oncamex in preclinical breast cancer models. Br J Cancer. 2016; 114:905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tian L, Song Z, Shao W, et al. Curcumin represses mouse 3T3‐L1 cell adipogenic differentiation via inhibiting miR‐17‐5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death Dis. 2017; 8:2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haraguch T, Kayashima T, Okazaki Y, et al. Cecal succinate elevated by some dietary polyphenols may inhibit colon cancer cell proliferation and angiogenesis. J Agr Food Chem. 2014; 62:5589–5594. [DOI] [PubMed] [Google Scholar]
- 18. Aichinger G, Beisl J, Marko D. Genistein and delphinidin antagonize the genotoxic effects of the mycotoxin alternariol in humancolon carcinoma cells. Mol Nutr Food Res. 2017; 61:1600462(1‐10). [DOI] [PubMed] [Google Scholar]
- 19. Khan N, Mukhtar H. Dietary agents for prevention and treatment of lung cancer. Cancer Lett. 2015; 359:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trung LQ, Espinoza JL, An DT, et al. Resveratrol selectively induces apoptosis in malignant cells with the JAK2V617F mutation byinhibiting the JAK2 pathway. Mol Nutr Food Res. 2015; 59:2143–2154. [DOI] [PubMed] [Google Scholar]
- 21. Tomás‐Barberán FA, González‐Sarrías A, García‐Villalba R, et al. Urolithins, the rescue of “old” metabolites to understand a “new” concept: metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol Nutr Food Res. 2017; 61:1500901(1‐35). [DOI] [PubMed] [Google Scholar]
- 22. Kasimsetty SG, Bialonska D, Reddy MK, et al. Colon cancer chemopreventive activities of pomegranate ellagitannins and urolithins. J Agr Food Chem. 2010; 58:2180–2187. [DOI] [PubMed] [Google Scholar]
- 23. Landete JM. Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Res Int. 2011; 44:1150–1160. [Google Scholar]
- 24. Zhao WH, Wang YJ, Hao WJ, et al. Preparative isolation and purification of urolithins from the intestinal metabolites of pomegranate ellagitannins by high‐speed counter‐current chromatography. J Chromatogr B. 2015; 990:111–117. [DOI] [PubMed] [Google Scholar]
- 25. Furlanetto V, Zagotto G, Pasquale R, et al. Ellagic acid and polyhydroxylated urolithins are potent catalytic inhibitors of human topoisomerase II: an in vitro study. J Agr Food Chem. 2012; 60:9162–9170. [DOI] [PubMed] [Google Scholar]
- 26. Bialonska D, Kasimsetty SG, Khan SI, et al. Urolithins, intestinal microbial metabolites of Pomegranate ellagitannins, exhibit potent antioxidant activity in a cell‐based assay. J Agr Food Chem. 2009; 57:10181–10186. [DOI] [PubMed] [Google Scholar]
- 27. Larrosa M, Gonzalez‐Sarrias A, Yanez‐Gascon MJ, et al. Anti‐inflammatory properties of a pomegranate extract and its metabolite urolithin‐A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2010; 21:717–725. [DOI] [PubMed] [Google Scholar]
- 28. Piwowarski JP, Kiss AK, Granica S, et al. Urolithins, gut microbiota‐derived metabolites of ellagitannins, inhibit LPS‐induced inflammation in RAW 264.7 murine macrophages. Mol Nutr Food Res. 2015; 59:2168–2177. [DOI] [PubMed] [Google Scholar]
- 29. Rodriguez J, Caille O, Ferreira D, et al. Pomegranate extract prevents skeletal muscle of mice against wasting induced by acute TNF‐α injection. Mol Nutr Food Res. 2017; 61: https://doi.org/10.1002/mnfr.201600169 [DOI] [PubMed] [Google Scholar]
- 30. Giménez‐Bastida JA, Truchado P, Larrosa M, et al. Urolithins, ellagitannin metabolites produced by colon microbiota, inhibit Quorum Sensing in Yersinia enterocolitica: phenotypic response and associated molecular changes. Food Chem. 2012; 32:1465–1474. [DOI] [PubMed] [Google Scholar]
- 31. Kasimsetty SG, Bialonska D, Reddy MK, et al. Effects of pomegranate chemical constituents/intestinal microbial metabolites on CYP1B1 in 22Rv1 prostate cancer cells. J Agr Food Chem. 2009; 57:10636–10644. [DOI] [PubMed] [Google Scholar]
- 32. Kang I, Kim Y, Tomás‐Barberán FA, et al. Urolithin A, C, and D, but not iso‐urolithin A and urolithin B, attenuate triglyceride accumulation in human cultures of adipocytes and hepatocytes. Mol Nutr Food Res. 2016; 60:1129–1138. [DOI] [PubMed] [Google Scholar]
- 33. Gonzalez‐Sarrias A, Espin JC, Tomas‐Barberan FA, et al. Gene expression, cell cycle arrest and MAPK signalling regulation in Caco‐2 cells exposed to ellagic acid and its metabolites, urolithins. Mol Nutr Food Res. 2009; 53:686–698. [DOI] [PubMed] [Google Scholar]
- 34. González‐Sarrías A, Núñez‐Sánchez MÁ, Tomé‐Carneiro J, et al. Comprehensive characterization of the effects of ellagic acid and urolithins on colorectal cancerand key‐associated molecular hallmarks: microRNA cell specific induction of CDKN1A (p21) as acommon mechanism involved. Mol Nutr Food Res. 2016; 60:701–716. [DOI] [PubMed] [Google Scholar]
- 35. Qiu Z, Zhou B, Jin L, et al. In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. Food Chem Toxicol. 2013; 59:428–437. [DOI] [PubMed] [Google Scholar]
- 36. Adams LS, Zhang Y, Seeram NP, et al. Pomegranate ellagitannin‐derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev Res. 2010; 3:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olennikov DN, Kashchenko NI, Chirikova NK. In vitro bioaccessibility, human gut microbiota metabolites and hepatoprotective potential of chebulic ellagitannins: a case of padma hepaten formulation. Nutrients. 2015; 13:8456–8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fantini M, Benvenuto M, Masuelli L, et al. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: perspectives on cancer treatment. Int J Mol Sci. 2015; 16:9236–9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lall RK, Syed DN, Adhami VM, et al. Dietary polyphenols in prevention and treatment of prostate cancer. Int J Mol Sci. 2015; 16:3350–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sancho M, Mach N. Effects of wine polyphenols on cancer prevention. Nutr Hosp. 2014; 31:535–551. [DOI] [PubMed] [Google Scholar]
- 41. Lee WS, Yun JW, Jung JH, et al. Polyphenols isolated from allium cepa L. induces apoptosis by induction of p53 and suppression of bcl‐2 through inhibiting PI3K/Akt signaling pathway in AGS human cancer cells. J Cancer Prev. 2014; 19:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mukherjee S, Ghosh S, Choudhury S, et al. Pomegranate reverses methotrexate‐induced oxidative stress and apoptosis in hepatocytes by modulating Nrf2‐NF‐kappaB pathways. J Nutr Biochem. 2013; 24:2040–2050. [DOI] [PubMed] [Google Scholar]
- 43. Zhao X, Pang L, Li J, et al. Apoptosis inducing effects of Kuding tea polyphenols in human buccal squamous cell carcinoma cell line BcaCD885. Nutrients. 2014; 6:3084–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Halder B, Bhattacharya U, Mukhopadhyay S, et al. Molecular mechanism of black tea polyphenols induced apoptosis in human skin cancer cells: involvement of Bax translocation and mitochondria mediated death cascade. Carcinogenesis. 2008; 29:129–138. [DOI] [PubMed] [Google Scholar]
- 45. Thakur VS, Gupta K, Gupta S. Green tea polyphenols causes cell cycle arrest and apoptosis in prostate cancer cells by suppressing class I histone deacetylases. Carcinogenesis. 2012; 33:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao WH, Wang YJ, Hao WJ, et al. In vitro inhibition of fatty acid synthase by 1,2,3,4,6‐penta‐O‐galloyl‐beta‐D‐glucose plays a vital role in anti‐tumour activity. BBRC. 2014; 445:346–351. [DOI] [PubMed] [Google Scholar]
- 47. Cho H, Jung H, Lee H, et al. Chemopreventive activity of ellagitannins and their derivatives from black raspberry seeds on HT‐29 colon cancer cells. Food Func. 2015; 6:1675–1683. [DOI] [PubMed] [Google Scholar]
- 48. Gonzalez‐Sarrias A, Tome‐Carneiro J, Bellesia A, et al. The ellagic acid‐derived gut microbiota metabolite, urolithin A, potentiates the anticancer effects of 5‐fluorouracil chemotherapy on human colon cancer cells. Food Funct. 2015; 6:1460–1469. [DOI] [PubMed] [Google Scholar]
- 49. Ito S, Koshikawa N, Mochizuki S, et al. 3‐Methyladenine suppresses cell migration and invasion of HT1080 fibrosarcoma cells through inhibiting phosphoinositide 3‐kinases independently of autophagy inhibition. Int J Oncol. 2007; 31:261–268. [PubMed] [Google Scholar]
- 50. Liao CC, Ho MY, Liang SM, et al. Recombinant protein rVP1 upregulates BECN1‐independent autophagy, MAPK1/3 phosphorylation and MMP9 activity via WIPI1/WIPI2 to promote macrophage migration. Autophagy. 2015; 9:5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tyagi N, Vacek JC, Givvimani S, et al. Cardiac specific deletion of N‐methyl‐d‐aspartate receptor 1 ameliorates mtMMP‐9 mediated autophagy/mitophagy in hyperhomocysteinemia. J Recept Signal Tr R. 2010; 30:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dong YH, Yin ST, Jiang C, et al. Involvement of autophagy induction in penta‐1,2,3,4,6‐O‐galloyl‐β‐D‐glucose‐induced senescence‐like growth arrest in human cancer cells. Autophagy. 2014; 10:296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Filomeni G, Desideri E, Cardaci S, et al. Carcinoma cells activate AMP‐activated protein kinase‐dependent autophagy as survival response to kaempferol‐mediated energetic impairment. Autophagy. 2014; 6:202–216. [DOI] [PubMed] [Google Scholar]


