Summary
In the mantle cell lymphoma (MCL)‐002 study, lenalidomide demonstrated significantly improved median progression‐free survival (PFS) compared with investigator's choice (IC) in patients with relapsed/refractory MCL. Here we present the long‐term follow‐up data and results of preplanned subgroup exploratory analyses from MCL‐002 to evaluate the potential impact of demographic factors, baseline clinical characteristics and prior therapies on PFS. In MCL‐002, patients with relapsed/refractory MCL were randomized 2:1 to receive lenalidomide (25 mg/day orally on days 1–21; 28‐day cycles) or single‐agent IC therapy (rituximab, gemcitabine, fludarabine, chlorambucil or cytarabine). The intent‐to‐treat population comprised 254 patients (lenalidomide, n = 170; IC, n = 84). Subgroup analyses of PFS favoured lenalidomide over IC across most characteristics, including risk factors, such as high MCL International Prognostic Index score, age ≥65 years, high lactate dehydrogenase (LDH), stage III/IV disease, high tumour burden, and refractoriness to last prior therapy. By multivariate Cox regression analysis, factors associated with significantly longer PFS (other than lenalidomide treatment) included normal LDH levels (P < 0·001), nonbulky disease (P = 0·045), <3 prior antilymphoma treatments (P = 0·005), and ≥6 months since last prior treatment (P = 0·032). Overall, lenalidomide improved PFS versus single‐agent IC therapy in patients with relapsed/refractory MCL, irrespective of many demographic factors, disease characteristics and prior treatment history.
Keywords: lenalidomide, mantle cell lymphoma, non‐Hodgkin lymphoma
Mantle cell lymphoma (MCL) accounts for ~6% of all cases of non‐Hodgkin lymphoma (NHL) and typically presents as advanced stage disease in patients over 60 years of age (Avivi & Goy, 2015). First‐line dose‐intensive chemoimmunotherapy, with or without stem cell transplantation, leads improved progression‐free survival (PFS) in younger patients with MCL and an overall fit status (Dreyling et al, 2014). Older patients with multiple comorbidities are usually treated with less aggressive regimens. MCL typically relapses and becomes increasingly more challenging to manage over the course of the disease. With current therapies in the relapsed/refractory setting (bortezomib, temsirolimus, lenalidomide, ibrutinib), median overall survival (OS) following relapse is approximately 2 years (Avivi & Goy, 2015). While multiple treatment options are available, some with proven benefit in randomized trials (e.g., lenalidomide, ibrutinib), their role in the standard of care for relapsed/refractory disease and the best possible treatment sequence remains to be defined (Dreyling et al, 2014; Avivi & Goy, 2015).
Lenalidomide is an oral immunomodulatory drug (IMiD®) with direct and immune‐mediated mechanisms of action (Gribben et al, 2015) and has shown clinical activity and safety in multiple studies, including 2 single‐arm, phase II trials (NHL‐002 and NHL‐003) in heavily pretreated patients with relapsed/refractory aggressive NHL, including MCL (Habermann et al, 2009; Zinzani et al, 2013). Subsequently in the single‐arm, phase II MCL‐001 (EMERGE) study in 134 patients with MCL who had relapsed during treatment with, or developed disease refractory to, bortezomib, lenalidomide treatment resulted in an overall response rate (ORR) of 28%, with a median response duration of 16·6 months (Goy et al, 2013, 2015). More recently, in the randomized, open‐label, multicentre, phase II MCL‐002 (SPRINT) study, the lenalidomide arm showed a statistically significant and clinically meaningful improvement in the primary endpoint of PFS compared with investigator's choice (IC) of single‐agent therapy (rituximab, gemcitabine, fludarabine, chlorambucil or cytarabine), with a manageable safety profile (Trneny et al, 2016). This primary analysis of MCL‐002, which had a cut‐off date of 7 March 2014 and a median follow‐up of 15·9 months for the overall study population, found a median PFS of 8·7 months for lenalidomide versus 5·2 months for IC (hazard ratio [HR] 0·61, 95% confidence interval [CI] 0·44–0·84; P = 0·004). Per the study protocol, follow‐up to MCL‐002 continues until the death of 70% of patients, median follow‐up of responding patients is greater than 2 years, median duration of response has been reached, or 4 years have passed from last patient randomization, whichever comes later.
In the present report, we provide long‐term follow‐up data and results of preplanned subgroup exploratory analyses from the MCL‐002 study to evaluate the potential impact of demographic factors, baseline clinical characteristics, and prior therapies on PFS in patients with relapsed/refractory MCL randomized to receive lenalidomide versus IC.
Patients and methods
Study design
The methodology for MCL‐002 (ClinicalTrials.gov identifier, NCT00875667) has been previously described (Trneny et al, 2016). Key inclusion criteria were minimum age 18 years, Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2, histologically confirmed MCL with cyclin D1 overexpression by immunohistochemistry, measurable disease ≥2 cm in the longest diameter, refractory to prior therapy or ≤3 relapses and had documented progressive disease after ≥1 prior combination chemotherapy regimen with an alkylating agent and an anthracycline, cytarabine and/or fludarabine (with or without rituximab); and ineligibility for intensive chemotherapy or stem cell transplantation (SCT). Patients were stratified by time from diagnosis (<3 vs. ≥3 years), time from last antilymphoma therapy (<6 vs. ≥6 months), and prior autologous SCT and randomized 2:1 to lenalidomide or IC. Oral lenalidomide was initiated at 25 mg/day, days 1–21 of each 28‐day cycle until progressive disease (PD) or as tolerated. Rituximab and chlorambucil were administered until PD or unacceptable toxicity, whereas gemcitabine, fludarabine and cytarabine were given for ≤6 cycles. Patients randomized to IC were allowed to cross over to lenalidomide following documented PD.
All patients provided written informed consent prior to study initiation. The study protocol and its amendments were approved by an institutional review board or independent ethics committee, or centrally if required by national regulations, and were conducted in accordance with the ethical principles of the Declaration of Helsinki and in compliance with Good Clinical Practice.
Post hoc assessments
As prospectively outlined in the study protocol, planned analyses for longer follow‐up were performed by investigator assessment to evaluate PFS in the overall study population and for prespecified subgroups at baseline (i.e., the time of randomization unless otherwise stated). These subgroups are grouped in 3 categories based on their association with MCL International Prognostic Index (MIPI) score, other patient characteristics and treatment history. Specific parameters and cut‐off/comparison values within each subgroup are defined in Supplementary Table SI
We evaluated PFS in the intent‐to‐treat (ITT) population, which included all randomized patients irrespective of receipt of study treatment. Computed tomography (CT) scans (or magnetic resonance imaging if CT was contraindicated) were performed every 2 cycles (±7 days) for 6 months and then every 90 days (±15 days) until documented PD or death.
Statistical analyses
PFS was characterized by Kaplan–Meier estimates with P values per log‐rank test with determination of median values and 95% CIs. Univariate and multivariate Cox regression models evaluated whether baseline subgroup factors were predictive of the risk of progression or death. Variables with a P value <0·20 by univariate analysis were selected for multivariate analysis. Final variables were selected using a stepwise selection method with entry level P = 0·20 and stay level P = 0·15. ORR was defined according to Cheson et al (1999) and statistical significance determined by Wald Χ2 test (P < 0·05).
Results
Patient demographics and disposition
The ITT population comprised 254 patients (n = 170 lenalidomide; n = 84 IC) enrolled between April 2009 and March 2013. Three patients randomized to lenalidomide and 1 patient randomized to IC did not receive study treatment. Overall, patients had a median age of 68·5 years, 68% were 65 years or older, and 73% were male. Patients had received a median of 2 (range, 1–5) prior treatment regimens, of which 19% had received prior SCT. As previously reported, the treatment arms were balanced in baseline characteristics except for high‐risk MIPI score, high tumour burden, bulky disease, and high lactate dehydrogenase (LDH) concentration, which were more prevalent among patients randomized to lenalidomide versus IC (Trneny et al, 2016). Also, compared with the IC treatment arm, more patients in the lenalidomide arm had received a higher number of previous anti‐lymphoma treatments and had been refractory to their last previous therapy.
As of the data cut‐off of 7 March 2016, 163 of 250 patients (65%) overall who received treatment had died. While on study, only 17 (7%) patients had died during or within 30 days of their study treatment (lenalidomide or IC). Causes of death were similar in both treatment groups, primarily due to malignant lymphoma (46% lenalidomide vs. 45% IC), other/unknown causes (17% lenalidomide vs. 20% IC) and toxicity (1 lenalidomide patient vs. 2 IC patients). Sixteen patients were ongoing on initial lenalidomide treatment and 1 patient in the IC (rituximab) group. Additionally, 5 of 40 patients who crossed over from IC to lenalidomide were still receiving lenalidomide treatment.
Progression‐free survival
The median follow‐up for all surviving patients was 41·3 months, which was an additional 20 months from the initial assessment and published report (Trneny et al, 2016). Lenalidomide continued to show longer median PFS than IC (8·6 vs. 5·4 months, respectively; P = 0·006; Fig 1A). An improvement in PFS with lenalidomide over IC was evident across most baseline subgroups, particularly those with higher numbers of patients, and including patients aged ≥65 years (P = 0·001; Fig 1B); with advanced stage III/IV disease at diagnosis (P = 0·014; Fig 1C), high LDH (P = 0·016; Fig 1D), high tumour burden (P = 0·007; Fig 1E), bulky disease (P = 0·068; Fig 1F); and whose disease was refractory to their last therapy (P < 0·001; Fig 1G). In support of higher PFS in these same categories, lenalidomide treatment showed higher ORR compared with IC at the earliest efficacy assessment (Cycle 3) when treatment on all IC comparators was still ongoing (Supplementary Figure S1).
Figure 1.
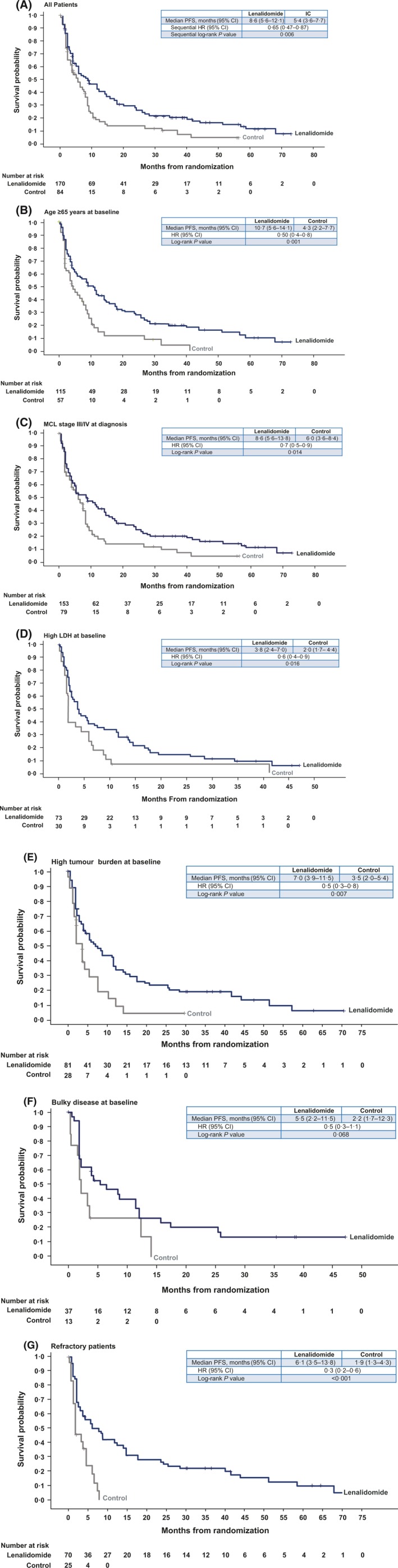
Kaplan–Meier curves of PFS in the lenalidomide versus IC treatment arms for all patients (A) and for patient subgroups with age ≥65 years (B), advanced MCL stage III/IV at diagnosis (C), high LDH at baseline (D), high tumour burden at baseline (E), bulky disease at baseline (F) and disease refractory to last treatment (G). 95% CI, 95% confidence interval; HR, hazard ratio; IC, investigator's choice; LDH, lactate dehydrogenase; MCL, mantle cell lymphoma; PFS, progression‐free survival.
Figure 2 lists the total number of patients per arm and subgroup depicted in the forest plots, along with their associated median PFS values and P value. Subgroup data were missing for some patients. Subgroups that had statistically significant improvements in PFS favouring lenalidomide over IC included patients with intermediate (P = 0·033) and high MIPI score at baseline (P = 0·037), age ≥65 years (P = 0·001), ECOG PS 0–1 (P = 0·025) or 2–4 (P = 0·019), normal (P = 0·049) or high LDH (P = 0·016), and <6·7 × 109/l white blood cell (WBC) counts (P = 0·011) (Fig 2A). The analysis of other patient and disease characteristics (Fig 2B) showed statistically significant improvements in PFS favouring lenalidomide in females (P = 0·035), stage III/IV disease at diagnosis (P = 0·014) irrespective of tumour burden (low P = 0·018; high P = 0·007), in patients without bulky disease (P = 0·004) or bone marrow involvement (P = 0·006) and in patients with both normal (P = 0·026) and moderate renal function (P = 0·019).
Figure 2.
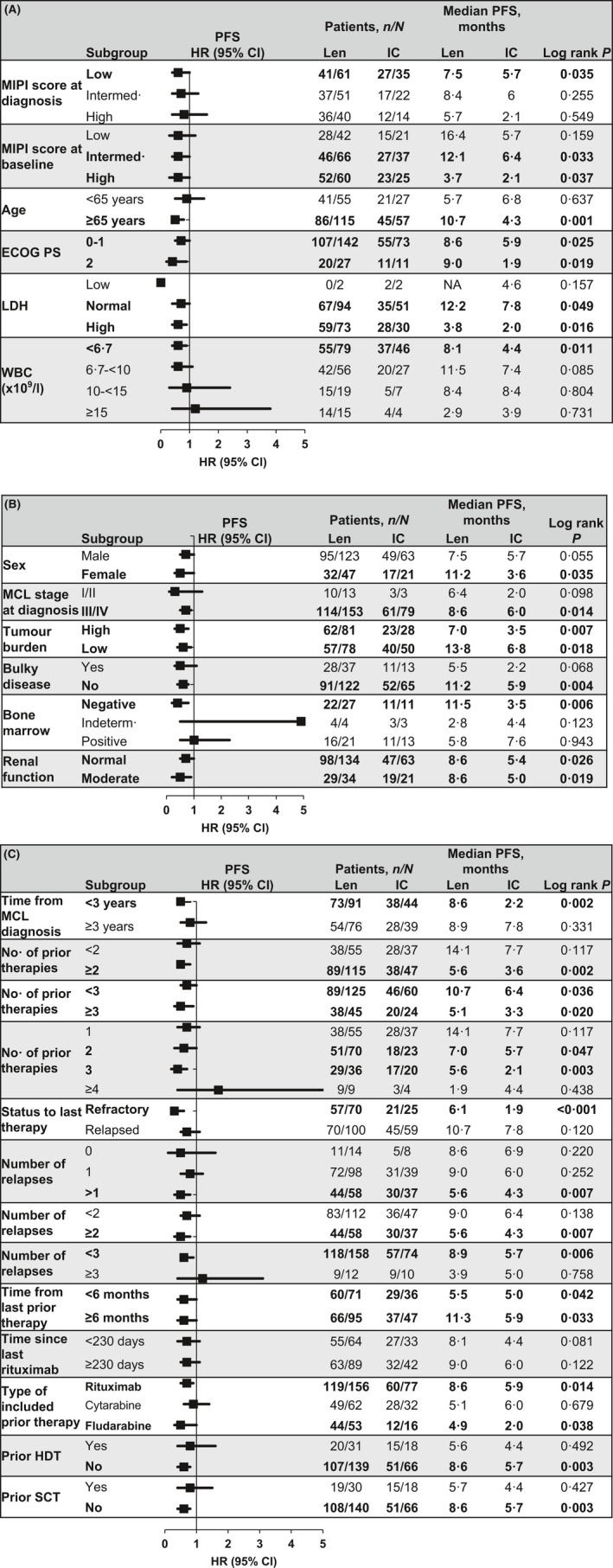
Forest plots of treatment effects on median PFS by subgroups according to MIPI‐based characteristics (A), other patient characteristics (B), and prior treatment history (C). Improved PFS to the left of the vertical line (i.e., at 1) favours lenalidomide and to the right of the line favours IC. Black squares represent the HR; horizontal lines lines represent 95% CI. Statistically significant (P ≤ 0·05) values and the specified factors are shown in bold. 95% CI, 95% confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; IC, investigator's choice; Intermed., intermediate; LDH, lactate dehydrogenase; Len, l lenalidomide; MCL, mantle cell lymphoma; MIPI, MCL International Prognostic Index; PFS, progression‐free survival; SCT, stem cell transplantation; WBC, white blood cell count.
We also evaluated subgroups to examine the potential impact of prior therapy on PFS outcomes. As shown in Fig 2C, lenalidomide significantly improved PFS compared with IC in patients who were <3 years from MCL diagnosis (P = 0·002); had more prior systemic antilymphoma therapies (P = 0·002 for ≥2; P = 0·020 for ≥3); were refractory to their last therapy (P < 0·001); had >1 prior relapses (P = 0·007 for >1, P = 0·007 for ≥2, P = 0·006 for <3); regardless of time from last prior therapy (P = 0·042 for <6 months, and P = 0·033 for ≥6 months); received prior rituximab‐ (P = 0·014) or fludarabine‐containing therapy (P = 0·038); and had not received prior high‐dose therapy (HDT; P = 0·003) or undergone prior SCT (P = 0·003). Despite the limitation of small patient numbers in some subgroups, these data suggest that lenalidomide may significantly improve PFS compared with IC treatment irrespective of ECOG status, high LDH and tumour burden.
Univariate and multivariate analyses for progression‐free survival
Further evaluation of subgroups by univariate Cox regression analysis showed that treatment group (lenalidomide favoured over IC) was the main effect associated with significantly improved PFS (HR = 0·65; P = 0·005), which was also highly significant by multivariate analysis (HR = 0·42; P < 0·001) (Table 1). Other subgroups with statistically significant improvements in PFS (P < 0·05) in the univariate analysis were low/intermediate MIPI score at diagnosis and baseline, normal LDH levels, <10 × 109/l WBC counts, normal renal function, <3 prior systemic antilymphoma therapies and ≥6 months since last prior therapy.
Table 1.
Univariate and multivariate analyses by Cox Regression on PFS by investigator assessment.a
| Baseline variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Treatment (lenalidomide versus IC) | 0·65 (0·48–0·87) | 0·005 | 0·42 (0·28–0·62) | <0·001 |
| MIPI‐based characteristics | ||||
| MIPI score at diagnosis (high versus low/intermediate)b | 1·57 (1·12–2·20) | 0·009 | — | — |
| MIPI score at baseline (high versus low/intermediate)b | 2·11 (1·57–2·83) | <0·001 | 1·51 (1·00–2·27) | 0·052 |
| Age, years (≥65 vs. <65) | 1·02 (0·75–1·38) | 0·919 | — | — |
| ECOG PS (2 vs. 0–1) | 1·46 (0·99–2·16) | 0·053 | — | — |
| LDH (high versus low/normal)c | 2·00 (1·49–2·67) | <0·001 | 2·02 (1·35–3·01) | <0·001 |
| WBC (≥10 × 109/l vs. <10 × 109/l) | 1·55 (1·08–2·21) | 0·017 | — | — |
| Other patient characteristics | ||||
| Sex (female versus male) | 0·86 (0·62–1·18) | 0·348 | — | — |
| MCL stage at diagnosis (III/IV versus I/II) | 0·81 (0·46–1·42) | 0·461 | — | — |
| Tumour burden (low versus high)d | 0·81 (0·60–1·08) | 0·155 | — | — |
| Bulky disease (yes versus no)e | 1·40 (0·98–2·01) | 0·063 | 1·57 (1·01–2·43) | 0·045 |
| Bone marrow assessment (negative versus indeterminate/positive)f | 0·72 (0·44–1·20) | 0·206 | — | — |
| Renal function (normal versus moderate/severe insufficiency)g | 0·60 (0·43–0·84) | 0·003 | — | — |
| Prior treatment history | ||||
| Time from MCL diagnosis to first dose (≥3 versus <3 years) | 0·85 (0·64–1·14) | 0·280 | — | — |
| Number of prior systemic antilymphoma therapies (≥3 versus <3) | 1·51 (1·11–2·06) | 0·009 | 1·75 (1·19–2·58) | 0·005 |
| Disease status to last prior therapy (relapsedh versus refractory) | 0·77 (0·58–1·03) | 0·075 | — | — |
| Time from last prior therapy to first dose (≥6 vs. <6 months) | 0·74 (0·55–0·98) | 0·034 | 0·68 (0·47–0·97) | 0·032 |
| Time since last rituximab to first dose (≥230 vs. <230 days) | 0·79 (0·59–1·07) | 0·127 | — | — |
| Prior HDT (yes versus no)i | 0·98 (0·68–1·42) | 0·930 | — | — |
| Prior SCT (yes versus no) | 0·96 (0·66–1·39) | 0·837 | — | — |
95% CI, 95% confidence interval; CR, complete response; CrCl, creatinine clearance; ECOG PS, Eastern Cooperative Oncology Group performance status; HDT, high‐dose therapy; HR, hazard ratio; LDH, lactate dehydrogenase; MCL, mantle cell lymphoma; MIPI, MCL International Prognostic Index; PFS, progression‐free survival; SCT, stem cell transplantation; WBC, white blood cell count.
Variables with P value <0·20 in the univariate analysis were selected for multivariate analysis. Final variables were selected using a stepwise selection method with entry level = 0·20 and stay level = 0·15. Multivariate survival analysis using Cox's regression model was estimated using 162 patients.
MIPI score = 0·03535 * age + 0·6978 * (if ECOG PS >1) + 1·367 * log10 (LDH/upper limit of normal) + 0·9393 * log10 (WBC per 10−6/l).
High LDH was >3·4 μkat/l for patients aged ≤60 years and >3·5 μkat/l for those aged >60 years; low LDH was <1·8 μkat/l; normal was defined per local laboratory criteria.
High tumour burden was defined by at least one lesion ≥5 cm in diameter or three lesions ≥3 cm in diameter by central radiology review.
Bulky disease was defined by at least one lesion ≥7 cm in the longest diameter by central radiology review.
For estimation of bone marrow involvement by local pathologist, negative was defined as having no aggregates or only a few well‐circumscribed lymphoid aggregates, indeterminate bone marrow was defined as having an increased number/size of lymphoid aggregates without overt malignancy, and positive was defined as an unequivocal malignancy.
Normal renal function was defined as CrCl of ≥60 ml/min; moderate insufficiency had CrCl ≥30 to <60 ml/min but not requiring dialysis; severe insufficiency had CrCl <30 ml/min. 2 patients had severe insufficiency in this study.
Relapse included patients with best response to last treatment of CR, unconfirmed CR, or partial response.
HDT was defined as SCT, hyper‐CVAD (hyper fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone plus methotrexate and cytarabine), or R‐hyper‐CVAD (rituximab + Hyper CVAD).
In the multivariate Cox regression analysis, normal LDH level was associated with highly significant improvement in PFS (P < 0·001) with lenalidomide treatment versus IC (Table 1). Other factors retaining significance in the multivariate model included no bulky disease (P = 0·045), <3 prior antilymphoma treatments (P = 0·005) and ≥6 months since last prior therapy (P = 0·032).
Univariate and multivariate analyses for overall survival
Median OS was 27·8 months (95% CI, 22·6–35·3) for lenalidomide versus 21·2 months (95% CI, 16·0–31·7) for IC (HR = 0·86; 95% CI, 0·62–1·18; Mantel‐Byar P = 0·34 [taking into account the effect of crossover]; Fig 3). We also performed univariate and multivariate analyses for OS as a way of identifying and/or confirming the role of potential independent factors on survival (Table 2). For OS, although the comparison between treatment groups (lenalidomide versus IC) did not achieve statistical significance, baseline factors that were statistically significant in the univariate analysis (P < 0·05) and led to improved OS were ECOG PS 0–1, normal LDH, low/intermediate MIPI score at diagnosis or baseline, <3 prior antilymphoma therapies, relapsed status to last therapy, ≥6 months from last prior therapy, low tumour burden and no bulky disease. Multivariate analysis of OS identified female sex as a signficiant independent prognostic factor (HR = 0·54; 95% CI, 0·33–0·89; P = 0·015).
Figure 3.
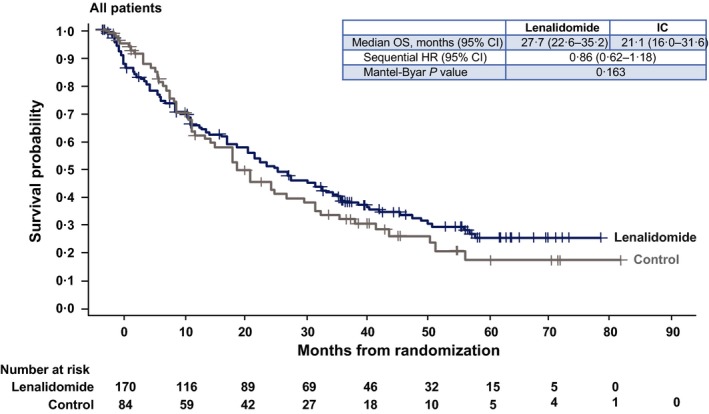
Kaplan–Meier curves of overall survival in the lenalidomide versus IC treatment arms for all patients. 95% CI, 95% confidence interval; HR, hazard ratio; IC, investigator's choice; OS, overall survival.
Table 2.
Univariate and multivariate analyses by Cox Regression on overall survival by investigator assessment.a
| Baseline variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Treatment (lenalidomide versus IC) | 0·86 (0·62–1·18) | 0·35 | — | — |
| MIPI‐based characteristics | ||||
| MIPI score at diagnosis (high versus low/intermediate)b | 1·80 (1·27–2·56) | 0·001 | — | — |
| MIPI score at baseline (high versus low/intermediate)b | 2·00 (1·47–2·74) | <0·001 | 1·49 (0·96–2·32) | 0·08 |
| Age, years (≥65 vs. <65) | 1·14 (0·82–1·60) | 0·44 | — | — |
| ECOG PS (2 vs. 0–1) | 1·62 (1·07–2·43) | 0·02 | — | — |
| LDH (high versus low/normal)c | 1·96 (1·44–2·68) | <0·001 | 1·50 (0·97–2·30) | 0·07 |
| WBC (≥10 × 109/l vs. <10 × 109/l) | 1·42 (0·96–2·08) | 0·08 | — | — |
| Other patient characteristics | ||||
| Sex (female versus male) | 0·77 (0·54–1·11) | 0·16 | 0·54 (0·33–0·89) | 0·02 |
| MCL stage at diagnosis (III/IV versus I/II) | 0·96 (0·50–1·82) | 0·89 | — | — |
| Tumour burden (low versus high)d | 0·68 (0·50–0·94) | 0·02 | — | — |
| Bulky disease (yes versus no)e | 1·55 (1·06–2·25) | 0·02 | 1·54 (0·97–2·44) | 0·07 |
| Bone marrow assessment (negative versus indeterminate/positive)f | 0·71 (0·42–1·22) | 0·22 | — | — |
| Renal function (normal versus moderate/severe insufficiency)g | 0·71 (0·50–1·01) | 0·06 | — | — |
| Prior treatment history | ||||
| Time from MCL diagnosis to first dose (≥3 vs. <3 years) | 0·82 (0·60–1·12) | 0·22 | — | — |
| Number of prior systemic antilymphoma therapies (≥3 vs. <3) | 1·59 (1·14–2·22) | 0·006 | 1·49 (0·98–2·25) | 0·06 |
| Disease status to last prior therapy (relapsedh versus refractory) | 0·70 (0·51–0·96) | 0·03 | — | — |
| Time from last prior therapy to first dose (≥6 vs. <6 months) | 0·60 (0·44–0·82) | 0·001 | 0·69 (0·47–1·04) | 0·08 |
| Time since last rituximab to first dose (≥230 vs. <230 days) | 0·74 (0·53–1·02) | 0·07 | — | — |
| Prior HDT (yes versus no)i | 1·13 (0·77–1·68) | 0·53 | — | — |
| Prior SCT (yes versus no) | 1·09 (0·74–1·62) | 0·66 | — | — |
95% CI, 95% confidence interval; CR, complete response; CrCl, creatinine clearance; ECOG PS, Eastern Cooperative Oncology Group performance status; HDT, high‐dose therapy; HR, hazard ratio; LDH, lactate dehydrogenase; MCL, mantle cell lymphoma; MIPI, MCL International Prognostic Index; PFS, progression‐free survival; SCT, stem cell transplantation; WBC, white blood cell count.
Variables with P value <0·20 in the univariate analysis were used to select for the multivariate. Final variables were selected using a stepwise selection method with entry level = 0·20 and stay level = 0·15. Multivariate survival analysis using Cox's regression model was estimated using 162 patients.
MIPI score = 0·03535 * age + 0·6978 * (if ECOG PS >1) + 1·367 * log10 (LDH/ULN) + 0·9393 * log10 (WBC per 10−6/l).
High LDH was >3·4 μkat/l for patients aged ≤60 years and >3·5 μkat/l for those aged >60 years; low LDH was <1·8 μkat/l; normal was defined per local laboratory criteria.
High tumour burden was defined by at least one lesion ≥5 cm in diameter or three lesions ≥3 cm in diameter by central radiology review.
Bulky disease was defined by at least one lesion ≥7 cm in the longest diameter by central radiology review.
For estimation of bone marrow involvement by local pathologist, negative was defined as having no aggregates or only a few well‐circumscribed lymphoid aggregates, indeterminate bone marrow was defined as having an increased number/size of lymphoid aggregates without overt malignancy, and positive was defined as an unequivocal malignancy.
Normal renal function was defined as CrCl of ≥60 ml/min; moderate insufficiency had CrCl ≥30 to <60 ml/min but not requiring dialysis; severe insufficiency had CrCl <30 ml/min. 2 patients had severe insufficiency in this study.
Relapse included patients with best response to last treatment of CR, unconfirmed CR, or partial response.
HDT was defined as SCT, hyper‐CVAD (hyper fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone plus methotrexate and cytarabine), or R‐hyper‐CVAD (rituximab + Hyper CVAD).
Discussion
The primary analysis of MCL‐002 demonstrated that lenalidomide significantly improved PFS compared with single‐agent IC therapy in patients with relapsed/refractory MCL, resulting in a significant risk reduction in PD or death (Trneny et al, 2016). The current exploratory subgroup and multivariate analyses extend these findings by uncovering an improved clinical benefit with lenalidomide compared with IC in patients with a wide range of demographic and baseline clinical characteristics. Moreover, the PFS benefit of lenalidomide over IC does not appear to be affected by the level of disease activity (measured by increased LDH), more advanced stage MCL or tumour burden. Additionally, lenalidomide treatment showed an early significant improvement in ORR compared with IC at cycle 3, supporting later differences in PFS. The PFS advantage of lenalidomide in patients with poor prognosis (high MIPI score at baseline) and the elderly, who represent the majority of patients with relapsed/refractory MCL, is of particular clinical relevance.
Previous subgroup analyses for lenalidomide were conducted in the MCL‐001 study, which evaluated lenalidomide in 134 MCL patients who had experienced relapse after bortezomib or whose disease was refractory to the drug (Goy et al, 2013). Because MCL‐001 did not have a control arm, the subgroup analyses evaluated the impact of baseline factors on ORR and duration of response (primary study endpoints). Lenalidomide treatment effects were consistent across subgroups in MCL‐001, with high LDH identified as the only significant factor for lower activity in the univariate and multivariate analyses (Goy et al, 2013).
The present MCL‐002 subgroup analyses confirm these findings in a randomized, controlled setting. High LDH is a known adverse prognostic factor in MCL (Hoster et al, 2014) and was identified in the current multivariate analysis as an independent factor for worse PFS. Notably, lenalidomide showed a significant improvement in PFS compared with IC in patients with high LDH. Similarly, lenalidomide exhibited a statistically significant PFS benefit in other high‐risk subgroups, including patients with high baseline MIPI score, older age (≥65 years), stage III/IV disease, high tumour burden and refractoriness to last prior therapy. Lenalidomide treatment was also associated with a non‐statistically significant trend toward longer median PFS in several other higher‐risk subgroups, including those with bulky disease (≥7 cm) and in those who received prior HDT and/or SCT.
The MCL‐002 study was prospectively conducted in a large number of patients across multiple centres to examine PFS and was the first randomized, controlled trial of lenalidomide in patients with relapsed/refractory MCL. The present subgroup analyses were prespecified for analysis per investigator assessment. One limitation of MCL‐002 is that temsirolimus, ibrutinib, and other newer agents that are now available for use in MCL were not considered standard treatment when recruitment in the MCL‐002 study began. Thus, although lenalidomide was favoured over IC in the univariate and multivariate analyses, the results may have been influenced by the treatment options available in the IC arm.
Several studies of temsirolimus and ibrutinib have reported similar efficacy by PFS or ORR across subgroups. Temsirolimus versus single‐agent IC (primarily, gemcitabine and fludarabine) showed consistently longer PFS across sex, performance status, disease stage at diagnosis, bone marrow involvement and number of prior regimens in exploratory subgroup analyses of a phase III trial (Hess et al, 2009) and in a recent retrospective analysis, across MIPI risk categories (Hess et al, 2015). Subgroup analyses of a single‐arm phase II trial of ibrutinib in 111 patients with relapsed/refractory MCL found similar ORRs, irrespective of multiple baseline factors, including tumour bulk (≥5 and ≥10 cm cut‐offs), ≥2 prior treatment regimens and refractory disease (less than partial response to last prior therapy) (Wang et al, 2015). More recently, an open‐label phase III study showed that ibrutinib was superior to temsirolimus with regard to improvements in PFS overall and when broken down by subgroups (Dreyling et al, 2016).
Another limitation of our analysis is that, despite the relatively large size of the study population, MCL‐002 was not powered to detect statistical differences in PFS between subgroups, and the subgroup analyses were prespecified to be exploratory in nature. Therefore, observed differences between lenalidomide and IC should not be overinterpreted. Similarly, the lack of statistical significance between lenalidomide and IC in some subgroups should be interpreted with caution. What makes lenalidomide unique and different from other treatments is the longevity of its responses.
It is interesting to consider the factors (i.e., normal LDH, no bulky disease, <3 prior antilymphoma therapies, ≥6 months since last prior therapy) identified by our multivariate analysis as having a significant positive impact on PFS, in addition to lenalidomide treatment. The MIPI has been validated and refined for previously untreated patients who received chemotherapy ± rituximab (Hoster et al, 2008, 2014). In our analysis, some but not all of the MIPI‐based factors were identified here as having a significant impact on PFS. How these factors might help risk‐stratify patients in the relapsed/refractory setting and with newer, more targeted agents remains to be defined in future larger analyses.
In conclusion, the prespecified subgroup and multivariate analyses for study MCL‐002 indicate that lenalidomide improves PFS compared with single‐agent IC therapy in patients with relapsed/refractory MCL, independent of most patient demographic and clinical characteristics, and prior treatment history.
Author contributions
All authors contributed equally to this work.
Disclosures
LA: received advisory board support from Bayer, Gilead, Roche, and Sandoz; consultancy for Celgene and Roche; research support from Gilead.
JW: reports consultancy and lecture honoraria from Roche, Mundipharma, Celgene, Takeda, Teva, Gilead, and Sanofi; consultancy agreements with Janssen‐Cilag, Teva, Boehringer Ingelheim, Karyopharm, Ariad, and Servier; and grant support from GSK/Novartis.
DB and JM: report grants and other remuneration from Celgene.
JR: reports advisory board support for Novartis and Cell Medica; advisory board, speaker's bureau, and research support from Takeda; and grant support from Celgene Ltd to Christie NHS Foundation Trust. JR reports holding of AstraZeneca and GlaxoSmithKline shares by his spouse.
WJ: reports research funding from Celgene, Gilead, Jansen, Novartis, Pfiser, Pharmacyclics, Roche, Sandoz, Spectrum, and Takeda and consulting/advisory boards for Mundipharma, Novartis, Spectrum, Takeda, and Teva.
FM: reports consultancy for Celgene, Genentech/Roche, Servier, and Gilead and honoraria for lectures for Celgene.
SR: reports research funding from Celgene, Janssen, and Roche; advisory boards for AstraZeneca, Celgene, Janssen, Kite, and Roche; and speaker fees from Janssen.
TB, MP, MLCB: are employees of Celgene International SARL and Celgene Corporation.
JC: reports contract fees from Celgene International SARL to the Institute, advisory board from MSD, and speakers’ bureau from Roche Pharma.
MT: reports grant support and other remuneration from Celgene and Roche and other remuneration from Janssen and Amgen.
TL and JA: declare no competing interests.
EC: reports honoraria for lectures from Celgene and Takeda, travel expenses and accommodation from Celgene, research grants, educational activity, and expert testimony from Gilead, and advisory board from Bayer and Gilead.
SAP: reports advisory board and speaker's bureau for Takeda.
Supporting information
Table SI. Prespecified baseline subgroups.
Fig S1. Subgroup analysis of ORR at cycle 3 in the intent‐to‐treat population for lenalidomide versus IC‐treated patients (investigator's assessment; March 7, 2016, data cut‐off). Statistical significance for P values of ORR comparisons was determined by Wald Χ2 test (P < 0·05). IC, investigator's choice; LDH, lactate dehydrogenase; MCL, mantle cell lymphoma; ORR, overall response rate.
Acknowledgements
This study was funded by Celgene Corporation. Editorial support for this manuscript was provided by Julie Kern, PhD, CMPP with Bio Connections LLC, which was funded by Celgene Corporation.
References
- Avivi, I. & Goy, A. (2015) Refining the mantle cell lymphoma paradigm: impact of novel therapies on current practice. Clinical Cancer Research, 21, 3853–3861. [DOI] [PubMed] [Google Scholar]
- Cheson, B.D. , Horning, S.J. , Coiffier, B. , Shipp, M.A. , Fisher, R.I. , Connors, J.M. , Lister, T.A. , Vose, J. , Grillo‐Lopez, A. , Hagenbeek, A. , Cabanillas, F. , Klippensten, D. , Hiddemann, W. , Castellino, R. , Harris, N.L. , Armitage, J.O. , Carter, W. , Hoppe, R. & Canellos, G.P. (1999) Report of an international workshop to standardize response criteria for non‐Hodgkin's lymphomas. NCI Sponsored International Working Group. Journal of Clinical Oncology, 17, 1244–1253. [DOI] [PubMed] [Google Scholar]
- Dreyling, M. , Geisler, C. , Hermine, O. , Kluin‐Nelemans, H.C. , Le Gouill, S. , Rule, S. , Shpilberg, O. , Walewski, J. & Ladetto, M. (2014) Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology, 25(Suppl. 3), iii83–iii92. [DOI] [PubMed] [Google Scholar]
- Dreyling, M. , Jurczak, W. , Jerkeman, M. , Silva, R.S. , Rusconi, C. , Trneny, M. , Offner, F. , Caballero, D. , Joao, C. , Witzens‐Harig, M. , Hess, G. , Bence‐Bruckler, I. , Cho, S.G. , Bothos, J. , Goldberg, J.D. , Enny, C. , Traina, S. , Balasubramanian, S. , Bandyopadhyay, N. , Sun, S. , Vermeulen, J. , Rizo, A. & Rule, S. (2016) Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle‐cell lymphoma: an international, randomised, open‐label, phase 3 study. Lancet, 387, 770–778. [DOI] [PubMed] [Google Scholar]
- Goy, A. , Sinha, R. , Williams, M.E. , Kalayoglu Besisik, S. , Drach, J. , Ramchandren, R. , Zhang, L. , Cicero, S. , Fu, T. & Witzig, T.E. (2013) Single‐agent lenalidomide in patients with mantle‐cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL‐001 (EMERGE) study. Journal of Clinical Oncology, 31, 3688–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goy, A. , Kalayoglu Besisik, S. , Drach, J. , Ramchandren, R. , Robertson, M.J. , Avivi, I. , Rowe, J.M. , Herbrecht, R. , Van Hoof, A. , Zhang, L. , Cicero, S. , Fu, T. & Witzig, T. (2015) Longer‐term follow‐up and outcome by tumour cell proliferation rate (Ki‐67) in patients with relapsed/refractory mantle cell lymphoma treated with lenalidomide on MCL‐001(EMERGE) pivotal trial. British Journal of Haematology, 170, 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribben, J.G. , Fowler, N. & Morschhauser, F. (2015) Mechanisms of action of lenalidomide in B‐cell non‐Hodgkin lymphoma. Journal of Clinical Oncology, 33, 2803–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann, T.M. , Lossos, I.S. , Justice, G. , Vose, J.M. , Wiernik, P.H. , McBride, K. , Wride, K. , Ervin‐Haynes, A. , Takeshita, K. , Pietronigro, D. , Zeldis, J.B. & Tuscano, J.M. (2009) Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. British Journal of Haematology, 145, 344–349. [DOI] [PubMed] [Google Scholar]
- Hess, G. , Herbrecht, R. , Romaguera, J. , Verhoef, G. , Crump, M. , Gisselbrecht, C. , Laurell, A. , Offner, F. , Strahs, A. , Berkenblit, A. , Hanushevsky, O. , Clancy, J. , Hewes, B. , Moore, L. & Coiffier, B. (2009) Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. Journal of Clinical Oncology, 27, 3822–3829. [DOI] [PubMed] [Google Scholar]
- Hess, G. , Coiffier, B. , Crump, M. , Gisselbrecht, C. , Offner, F. , Romaguera, J. , Kang, L. & Moran, P.J. (2015) Effect of prognostic classification on temsirolimus efficacy and safety in patients with relapsed or refractory mantle cell lymphoma: a retrospective analysis. Experimental Hematology & Oncology, 4, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoster, E. , Dreyling, M. , Klapper, W. , Gisselbrecht, C. , van Hoof, A. , Kluin‐Nelemans, H.C. , Pfreundschuh, M. , Reiser, M. , Metzner, B. , Einsele, H. , Peter, N. , Jung, W. , Wormann, B. , Ludwig, W.D. , Duhrsen, U. , Eimermacher, H. , Wandt, H. , Hasford, J. , Hiddemann, W. & Unterhalt, M. (2008) A new prognostic index (MIPI) for patients with advanced‐stage mantle cell lymphoma. Blood, 111, 558–565. [DOI] [PubMed] [Google Scholar]
- Hoster, E. , Klapper, W. , Hermine, O. , Kluin‐Nelemans, H.C. , Walewski, J. , van Hoof, A. , Trneny, M. , Geisler, C.H. , Di Raimondo, F. , Szymczyk, M. , Stilgenbauer, S. , Thieblemont, C. , Hallek, M. , Forstpointner, R. , Pott, C. , Ribrag, V. , Doorduijn, J. , Hiddemann, W. , Dreyling, M.H. & Unterhalt, M. (2014) Confirmation of the mantle‐cell lymphoma international prognostic index in randomized trials of the European mantle‐cell lymphoma network. Journal of Clinical Oncology, 32, 1338–1346. [DOI] [PubMed] [Google Scholar]
- Trneny, M. , Lamy, T. , Walewski, J. , Belada, D. , Mayer, J. , Radford, J. , Jurczak, W. , Morschhauser, F. , Alexeeva, J. , Rule, S. , Afanasyev, B. , Kaplanov, K. , Thyss, A. , Kuzmin, A. , Voloshin, S. , Kuliczkowski, K. , Giza, A. , Milpied, N. , Stelitano, C. , Marks, R. , Trumper, L. , Biyukov, T. , Patturajan, M. , Bravo, M.L. & Arcaini, L. (2016) Lenalidomide versus investigator's choice in relapsed or refractory mantle cell lymphoma (MCL‐002; SPRINT): a phase 2, randomised, multicentre trial. The Lancet. Oncology, 17, 319–331. [DOI] [PubMed] [Google Scholar]
- Wang, M.L. , Blum, K.A. , Martin, P. , Goy, A. , Auer, R. , Kahl, B.S. , Jurczak, W. , Advani, R.H. , Romaguera, J.E. , Williams, M.E. , Barrientos, J.C. , Chmielowska, E. , Radford, J. , Stilgenbauer, S. , Dreyling, M. , Jedrzejczak, W.W. , Johnson, P. , Spurgeon, S.E. , Zhang, L. , Baher, L. , Cheng, M. , Lee, D. , Beaupre, D.M. & Rule, S. (2015) Long‐term follow‐up of MCL patients treated with single‐agent ibrutinib: updated safety and efficacy results. Blood, 126, 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzani, P.L. , Vose, J.M. , Czuczman, M.S. , Reeder, C.B. , Haioun, C. , Polikoff, J. , Tilly, H. , Zhang, L. , Prandi, K. , Li, J. & Witzig, T.E. (2013) Long‐term follow‐up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL‐003 study. Annals of Oncology, 24, 2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Prespecified baseline subgroups.
Fig S1. Subgroup analysis of ORR at cycle 3 in the intent‐to‐treat population for lenalidomide versus IC‐treated patients (investigator's assessment; March 7, 2016, data cut‐off). Statistical significance for P values of ORR comparisons was determined by Wald Χ2 test (P < 0·05). IC, investigator's choice; LDH, lactate dehydrogenase; MCL, mantle cell lymphoma; ORR, overall response rate.


