Abstract
Introduction
The aim of this study was to evaluate the value of absent fetal nasal bone in the prediction of fetal chromosomal abnormalities, according to whether it was associated with other soft markers or structural abnormalities in a prescreened population of Chinese pregnant women.
Material and methods
In this retrospective cohort study, women whose fetuses had absent nasal bone detected during the second trimester ultrasound scan were followed. Fetal karyotyping was performed and pregnancy outcomes were recorded. The association between absent fetal nasal bone with abnormal karyotype was evaluated according to whether soft markers or structural abnormalities were also observed.
Results
Fetal nasal bone was assessed in 56 707 singleton pregnancies. After exclusion of unqualified cases, 71 (71/56 707, 0.13%) fetuses were included in the final analyses, of which 16 (16/71, 22.54%) were detected to have chromosomal abnormalities, including 12 cases of trisomy‐21, three of trisomy‐18, and one of micro‐deletion (in 7q). Among the 42 cases with isolated absence of nasal bone, two had trisomy‐21 and one had a micro‐deletion. Absence of nasal bone in association with other structural abnormalities had a higher rate of abnormal karyotypes compared with isolated absence of nasal bone [83.33% (10/12) vs. 7.14% (3/42), Fisher's exact test χ2 = 25.620, p < 0.001].
Conclusion
Absent fetal nasal bone is a highly specific ultrasonographic soft marker that should be included in the routine second trimester ultrasound scan.
Keywords: Absence of nasal bone, karyotype abnormality, down syndrome, ultrasound, prenatal diagnosis
Abbreviations
- SNP
single nucleotide polymorphism
Key message.
Second trimester ultrasound scan of fetal nasal bone development has important clinical significance in detecting chromosomal abnormalities. Absent fetal nasal bone is a highly specific ultrasonographic marker in detecting chromosomal abnormalities in prescreened populations.
Introduction
It is well accepted that specific facial profile markers are associated with fetal chromosomal abnormalities 1, 2. Since Cicero et al. 3 first reported that absent nasal bone in fetuses was associated with Down syndrome, many studies have provided evidence supporting the notion that absence or hypoplasia of nasal bone is an ultrasound marker of fetal aneuploidy 4, 5, 6, 7. The positive likelihood ratio of nasal bone absence for Down syndrome screening has been reported to be around 29.00–66.75 4, 5, 8, 9. However, most of those studies were carried out in high‐risk populations, and only a few in samples representative of the general population. There are variations of fetal nasal bone length among different ethnic groups 10. So far, there are only a few studies investigating abnormal fetal nasal bone development in the Han Chinese population, with relatively small sample sizes 11, 12, 13, 14.
Nasal bone abnormalities, which include both nasal bone absence and hypoplasia, are commonly used as soft ultrasound markers to screen for Down syndrome during the first or second trimester 5, 8. A number of studies have shown that fetal nasal bone length grows with advancing gestational age 15, 16, 17. It is relatively difficult to detect fetal nasal bone during early pregnancy, but the detection becomes easier as the fetus grows. The nasal bone is clearly visible in the second trimester, so that it is more convenient to assess and take nasal bone measurements during this period of pregnancy. In most parts of China, second trimester prenatal screening for fetal anomalies has already become a routine. Therefore, it is possible to include nasal bone evaluation as a routine examination. Furthermore, with the gradual application of serological screening, ultrasound screening and non‐invasive DNA testing at the first or early second trimester, it is interesting to see whether fetal nasal bone is still a valuable ultrasound soft marker in detecting chromosomal abnormalities at the second trimester.
The current study mainly aimed to evaluate the clinical value of fetal nasal bone absence, alone or in combination with other soft markers or structural abnormalities, in detecting fetal chromosomal abnormalities in a prescreened population of Chinese pregnant women.
Material and methods
Study population
This retrospective cohort study was conducted at the Obstetrics and Gynecology Hospital of Fudan University in Shanghai, China. Pregnant women who underwent prenatal screening and also gave birth or induced labor at our hospital from January 2012 to December 2015 were included. There were 58 001 births during the study period at our hospital, including 1015 twins and other multiple births. In 279 cases, pregnancies were terminated mid‐to‐late gestation without prior invasive diagnostic procedures. Thus, 56 707 singleton pregnancies were included in the final analysis.
Ultrasonographic examinations
The second trimester ultrasound was performed using the GE Voluson E8 system (General Electric Medical Systems, Milwaukee, WI, USA), GE Voluson E6 (General Electric Medical Systems), or Philips iU 22 (Philips, USA) system equipped with 2–5 MHz transducers.
The procedure for prenatal examination was as follows: Down syndrome screening was performed at 15–18 gestational weeks of age, using a serum markers screening method which includes age and serological markers [AFP (alpha fetoprotein) + β‐hCG (human chorionic gonadotropin) + uE3]. Pregnant women with maternal age ≥35 years (without any prior risk assessment) or those determined to be at high‐risk by the screening test were counseled regarding invasive prenatal diagnostic procedures. A second trimester ultrasound scan was performed during routine screening at 20–23 weeks of gestation. The examination was carried out by certified physician sonologists following guidelines of the International Society of Ultrasound in Obstetrics & Gynecology 18. Sixteen images including observation of fetal head, face, neck, chest/heart, abdomen, skeleton, umbilical cord and placenta, were stored for each fetus, which were regularly checked and scored by senior sonologists to ensure quality. We applied the following commonly used ultrasound soft markers: absence or hypoplasia of nasal bone, increased nuchal fold thickness (≥6 mm), short femur or humerus, ventriculomegaly (≥10 mm), intestinal hyperechogenicity, choroid plexus cyst, echogenic intracardiac focus, mild pyelectasis (≥5 mm), aberrant right subclavian artery, absence of the middle phalanx of the fifth digit and short mandible.
Nasal bone length was assessed with two‐dimensional images of the fetal head in the sagittal plane enlarged to include nose, as well as lips, maxilla and mandibula, with an angle between the insonation beam and the nasal axis close to 45° or 135° to define the edges of the nasal bone more sharply. The nasal bone was considered absent only if it was not visualized on all appropriate views. Measurement of the exact length of fetal nasal bone was performed only when hypoplastic nasal bone was suspected. Cases with nasal bone length below the 2.5th percentile 11, including those with unilateral absence of nasal bone, were considered short. When fetal nasal bone abnormality was suspected on screening, further detailed ultrasonographic examination was performed by senior sonologists. If the diagnosis was confirmed, amniocentesis was performed for quantitative fluorescence polymerase chain reaction and karyotyping after counseling to exclude chromosomal abnormalities. After January 2015, cases with additional risk factors were tested by human whole genome single nucleotide polymorphism (SNP) genotyping. Copy number variation refers to chromosome fragment deletion greater than 1 Mb or repeat greater than 2 Mb. Suspected pathogenic areas are analyzed, using ISCA, DGV, Decipher, Ensemble, OMIM, UCSC or the PubMed database. A definite pathogenic fragment is given a positive diagnosis. As for the indefinite fragments, parents of the newborns also undergo SNP examinations. If the parents have the same copy number variation, and have no abnormal phenotype, this is considered a normal variation. If the newborn parents do not have the same copy number variation, an abnormal variation is suspected. For those who did not undergo prenatal invasive diagnostic procedures, general situations and clinical signs of the newborns such as neonatal facial features, muscle tonus, nervous system, cardiovascular system, digestive system and metabolic system were observed by neonatal pediatricians. At the same time, a family history of neonates was considered.
Statistical analyses
Data were transferred to a worksheet in EXCEL 2007 (Microsoft Corp., Redmond, WA, USA) and were analyzed by SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Median with range was calculated for continuous variables, and frequency or rate was calculated for discrete variables. Chi‐square test or Fisher's exact test was used to compare differences between groups when appropriate. Sensitivity, specificity, positive and negative predictive values for the absent fetal nasal bone marker in detecting Down syndrome were calculated. All significance tests were two‐sided; a p‐value < 0.05 was considered statistically significant.
Ethical approval
The study protocol was approved by the institutional review board of Obstetrics and Gynecology Hospital of Fudan University (Reference number: 2017‐20; date of approval: 5 May 2017). Oral informed consent was obtained from all participants.
Results
Of the 56 707 fetuses examined, 77 showed nasal bone absence at the second trimester ultrasound assessment. Of those 77 cases, two had termination of pregnancy because of multiple structural abnormalities and did not receive genetic amniocentesis, one underwent late‐term termination of pregnancy and was not tested for karyotype, one was one of the twins, and two were lost to follow‐up. Thus, 71 absent fetal nasal bone cases were included for the analyses. The prevalence rate of absent fetal nasal bone in our prescreened population was 0.13% (71/56 707).
The median maternal age of the 71 cases with absent fetal nasal bone was 29.6 (range 20–41) years. The median gestational weeks at the diagnosis was 22.7 (range 20.9–23.9) weeks. Of those 71 cases, 63 underwent genetic amniocentesis (88.73%, 63/71); another eight did not undergo amniocentesis but did not have any signs of genetic syndrome during postnatal examination and thus they were considered normal. A total of 16 cases (22.54%, 16/71) were diagnosed to have chromosomal abnormalities, including 12 cases of trisomy‐21 (75%, 12/16), three of trisomy‐18 (18.75%, 3/16), and one of micro‐deletion (in 7q) (6.25%, 1/16) (Table 1, Figure 1).
Table 1.
Summary of the cases identified with chromosomal abnormalities by genetic amniocentesis (n = 24)
| Case Number | Maternal age (Year) | Gestational age (week) | Ultrasound marker of nasal bone | Other ultrasound markers | Embryonic karyotype |
|---|---|---|---|---|---|
| 1 | 36 | 22.5 | Absence of nasal bone | Trisomy‐21 | |
| 2 | 37 | 23.1 | Absence of nasal bone | Trisomy‐21 | |
| 3 | 32 | 22.5 | Absence of nasal bone | Micro‐deletion [arr7q22.1(101,051,502‐102,053,414)×1] | |
| 4 | 32 | 22.1 | Absence of nasal bone | Short femur and humerus, increased nuchal fold thickness | Trisomy‐21 |
| 5 | 36 | 22.2 | Absence of nasal bone | Short femur and humerus, increased nuchal fold thickness | Trisomy‐18 |
| 6 | 32 | 23.5 | Absence of nasal bone | Round head, short mandible | Trisomy‐18 |
| 7 | 31 | 22 | Absence of nasal bone | Short femur, endocardial cushion defect | Trisomy‐21 |
| 8 | 25 | 22.3 | Absence of nasal bone | Bilateral ventricular dilatation, intestinal hyperechogenicity, ventricular septal defect, pulmonary stenosis | Trisomy‐21 |
| 9 | 28 | 22.4 | Absence of nasal bone | Endocardial cushion defect | Trisomy‐21 |
| 10 | 22 | 22.5 | Absence of nasal bone | Round head, increased nuchal fold thickness, small left heart | Trisomy‐21 |
| 11 | 29 | 23 | Absence of nasal bone | Short femur, endocardial cushion defect, bilateral absence of the middle phalanx of the fifth digit, intestinal hyperechogenicity | Trisomy‐21 |
| 12 | 29 | 23.1 | Absence of nasal bone | Short femur and short humerus, ventricular septal defect | Trisomy‐21 |
| 13 | 36 | 23.3 | Absence of nasal bone | Short femur and humerus, increased nuchal fold thickness, bilateral absence of the middle phalanx of the fifth digit, ventricular septal defect | Trisomy‐21 |
| 14 | 41 | 23.4 | Absence of nasal bone | Duodenal stenosis or atresia, increased nuchal fold thickness, endocardial cushion defect, double outlet right ventricle | Trisomy‐21 |
| 15 | 34 | 23.6 | Absence of nasal bone | Short femur, left lateral ventricular dilation, coarctation of aorta | Trisomy‐21 |
| 16 | 26 | 22.4 | Absence of nasal bone | Small head circumference, short femur, ventricular septal defect, bilateral choroid plexus cyst | Trisomy‐18 |
Figure 1.
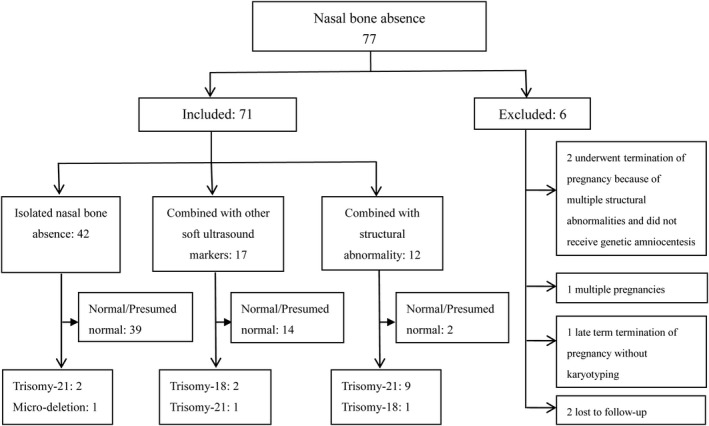
Characterization of 71 cases with nasal bone absence.
Among 71 cases with nasal bone absence, 42 had isolated nasal bone absence, 17 cases had additional soft ultrasound markers, and 12 cases had other structural abnormalities (Table 2). Among the 42 cases with isolated absence of nasal bone, two had trisomy‐21 and one had a micro‐deletion. A significantly higher rate of abnormal karyotype [83.33% (10/12) vs. 7.14% (3/42), Fisher's exact test χ2 = 25.620, p < 0.001] was observed among fetuses with absent nasal bone in association with structural abnormalities compared with those isolated absent nasal bone cases.
Table 2.
Number and rate of chromosomal abnormalities associated with absent nasal bone (aNB)
| NB absence (n = 71) | |||
|---|---|---|---|
| Isolated aNB (n = 42) | aNB + other marker (n = 17) | aNB + structural anomalies (n = 12) | |
| Type of chromosomal abnormalities | |||
| Trisomy‐21, n (%) | 2 (4.76%) | 1 (5.88%) | 9 (75.00%) |
| Trisomy‐18, n (%) | 0 (0%) | 2 (11.76%) | 1 (8.33%) |
| Micro‐deletion, n (%) | 1 (2.38%) | 0 (0%) | 0 (0%) |
| Total abnormalities, n (%) | 3 (7.14%) | 3 (17.65%) | 10 (83.33%) |
| Normal/Presumed normal, n (%) | 39 (92.86%) | 14 (82.35%) | 2 (16.67%) |
There were 38 cases of Down syndrome among the 56 707 singleton pregnancies in our hospital. The sensitivity and specificity of the absent fetal nasal bone marker in detecting Down syndrome were 31.58 and 99.90%, respectively, and the positive and negative predictive values were 16.90 and 99.95%, respectively.
We also observed 65 cases of nasal bone hypoplasia, including 34 cases with isolated nasal bone hypoplasia, 22 cases with other soft ultrasound markers, and nine cases with additional structural abnormalities. A total of eight fetuses with chromosomal abnormalities were detected from those 65 cases with nasal bone hypoplasia.
Discussion
Our study showed that the incidence of absent fetal nasal bone in the second trimester of pregnancy was 0.13% in a prescreened population of Chinese pregnant women, and it was significantly associated with fetal aneuploidy.
Absent nasal bone may be caused by nasal bone hypoplasia or delayed ossification; some cases will display nasal bone during late pregnancy. Nasal bone absence or hypoplasia in the second trimester can be physiological variations. Studies have also shown that the progress of fetal nasal bone development varies among different ethnic groups 10. Prefumo et al. 19 studied 3992 fetuses with normal karyotype at 11–14 weeks of gestation and reported that the rate of visualization of fetal nasal bone was associated with the ethnic origin of the mother. A meta‐analysis by Agathokleous et al. 5 reported a 0.35% incidence (27/7749) of fetal nasal bone loss among fetuses with normal karyotype at the second trimester 5. The current study was carried out in a prescreened population at the second trimester with significantly lower risk compared with the general or high‐risk populations. Our rate of absent fetal nasal bone was only 0.13%, lower than that of other studies (0.20–0.90%) 20, 21, 22. In our study, the sonologists who performed the nasal bone evaluation were trained and familiar with the anatomical features of fetal nasal bone. Pregnant women with abnormal fetal nasal bone were further evaluated by senior sonologists. Standard display and measurement sections were selected to ensure accuracy and comparability.
Ting et al. 12 studied 14 fetuses with absent or hypoplastic nasal bone at 17–22 gestational weeks; all six fetuses with isolated absent or hypoplastic nasal bone had normal karyotype (100%, 6/6), whereas six of the other eight fetuses with additional ultrasound markers had Down syndrome (75%, 6/8). Yang et al. 22 studied 126 cases with abnormal fetal nasal bone; found eight of the 63 fetuses with isolated absent or hypoplastic nasal bone had abnormal karyotpye (12.70%, 8/63), whereas 28 of the 63 fetuses with other ultrasound markers had abnormal karyotype (44.44%, 28/63). The current study also observed that fetuses with isolated absent nasal bone had a relatively low occurrence of abnormal karyotype (7.14%, 3/42), but if additional soft ultrasound markers were present, the detection rate increased to 17.65% (3/17). When the abnormal nasal bone was associated with structural abnormalities, the detection rate increased considerably to 83.33% (10/12). Therefore, it is necessary to perform a detailed fetal morphology scan when absent fetal nasal bone is detected at the second trimester ultrasound screening.
In our study, two cases of trisomy‐21 (both with advanced maternal age) and one case of micro‐deletion (in 7q) were detected among 42 isolated absent fetal nasal bone cases. Our study results showed that the sensitivity of absent fetal nasal bone marker in detecting Down syndrome is low (31.58%), whereas the specificity is almost 100% (99.90%). Abnormal fetal nasal bone development is currently the most effective soft marker in ultrasound scan 5, 12, 23. Markers with high specificity should be selected to reduce false‐positive results in low‐risk populations; therefore, fetal nasal bone should be included as a routine item in the second trimester ultrasound scan. Ting et al. 12 also reported that isolated fetal nasal bone absence or hypoplasia had limited value in clinical practice; they suggested a whole body check by experienced sonologists instead of invasive prenatal tests for those isolated abnormal fetal nasal bone cases.
The most frequent form of chromosomal defect in our study was Down syndrome (75%, 12/16). Different types of chromosomal abnormalities can be associated with abnormal fetal nasal bone development. Dukhovny et al. 23 reported three cases of micro‐deletion or duplication among 142 fetuses with abnormal nasal bone development (2.11%, 3/142). Similar to their result, our study observed one case of micro‐deletion (1.41%, 1/71), which was isolated nasal bone absence with chromosome karyotype arr7q22.1(101,051,502‐102,053,414)×1. There is the possibility of misdiagnoses before the routine application of human whole genome SNP genotyping with amniocentesis in our hospital a couple of years ago. It is highly likely we have underestimated the micro‐deletion rate in our series of fetuses with abnormal nasal bone development, since SNP‐array was applied hospital‐wide only after January 2015. One of our cases presented with fetal nasal bone absence, extreme flatness of the face, and vertebral stenosis. The CT scan of the new born discovered signs of chondrodysplasia punctata, and the newborn could not breathe due to collapse of the nose bridge and eventually died. However, karyotyping showed no abnormal results, so no further genetic testing was performed at the time. This case signifies that long‐term follow‐up is required when fetal nasal bone absence is present alone or along with other markers, although it is still debatable whether prenatal genetic sequencing is necessary.
This retrospective study did not measure nasal bone length of all fetuses, and it is very likely we underestimated the cases of hypoplastic nasal bone. In addition, karyotyping could not be performed on six fetuses with absent fetal nasal bone for various reasons, which this may also have affected our study results. Besides fetal nasal bone length, recent studies have reported that prenasal thickness and prenasal thickness‐to‐nasal bone length ratio can also be used as effective soft ultrasound markers to screen for Down syndrome 24, 25, 26, 27. However, there is also a study suggesting that prenasal thickness‐to‐nasal bone length ratio is not a very strong ultrasound marker in Chinese population 28. It would be of immense value to conduct a large prospective study in our region to establish gestational age‐specific normal reference values for fetal nasal bone length in our population, as well as follow‐up the prognoses of those cases with abnormal fetal nasal bone.
Conclusion
Absent fetal nasal bone is one of the strongest soft markers in the second trimester ultrasound screening. Detailed prenatal diagnosis is advisable to rule out abnormal karyotype when absent nasal bone is associated with other ultrasound markers of fetal aneuploidy or structural abnormality.
Du Y, Ren Y, Yan Y, Cao L. Absent fetal nasal bone in the second trimester and risk of abnormal karyotype in a prescreened population of Chinese women. Acta Obstet Gynecol Scand 2018; 97:180–186.
Conflicts of interest The authors have declared that they have no conflicts of interests in connection with this article.
References
- 1. Vos FI, de Jong‐Pleij EA, Bakker M, Tromp E, Kagan KO, Bilardo CM. Fetal facial profile markers of Down syndrome in the second and third trimesters of pregnancy. Ultrasound Obstet Gynecol. 2015;46:168–73. [DOI] [PubMed] [Google Scholar]
- 2. Kagan KO, Sonek J, Berg X, Berg C, Mallmann M, Abele H, et al. Facial markers in second‐ and third‐trimester fetuses with trisomy 18 or 13, triploidy or Turner syndrome. Ultrasound Obstet Gynecol. 2015;46:60–5. [DOI] [PubMed] [Google Scholar]
- 3. Cicero S, Curcio P, Papageorghiou A, Sonek J, Nicolaides K. Absent of nasal bone in fetuses with trisomy 21 at 11–14 weeks of gestation: an observational study. Lancet. 2001;358:1665–7. [DOI] [PubMed] [Google Scholar]
- 4. Moreno‐Cid M, Rubio‐Lorente A, Rodríguez MJ, Bueno‐Pacheco G, Tenías JM, Román‐Ortiz C, et al. Systematic review and meta‐analysis of performance of second‐trimester nasal bone assessment in detection of fetuses with Down syndrome. Ultrasound Obstet Gynecol. 2014;43:247–53. [DOI] [PubMed] [Google Scholar]
- 5. Agathokleous M, Chaveeva P, Poon LC, Kosinski P, Nicolaides KH. Meta‐analysis of second‐trimester markers for trisomy 21. Ultrasound Obstet Gynecol. 2013;41:247–61. [DOI] [PubMed] [Google Scholar]
- 6. Shanks A, Odibo A. Nasal bone in prenatal trisomy 21 screening. Obstet Gynecol Surv. 2010;65:46–52. [DOI] [PubMed] [Google Scholar]
- 7. Driscoll DA, Gross S. Clinical practice. Prenatal screening for aneuploidy. N Engl J Med. 2009;360:2556–62. [DOI] [PubMed] [Google Scholar]
- 8. Sonek JD, Cicero S, Neiger R, Nicolaides KH. Nasal bone assessment in prenatal screening for trisomy 21. Am J Obstet Gynecol. 2006;195:1219–30. [DOI] [PubMed] [Google Scholar]
- 9. Odibo AO, Sehdev HM, Gerkowicz S, Stamilio DM, Macones GA. Comparison of the efficiency of second‐trimester nasal bone hypoplasia and increased nuchal fold in Down syndrome screening. Am J Obstet Gynecol. 2008;199:281.e1–5. [DOI] [PubMed] [Google Scholar]
- 10. Papasozomenou P, Athanasiadis AP, Zafrakas M, Panteris E, Loufopoulos A, Assimakopoulos E, et al. Fetal nasal bone length in the second trimester: comparison between population groups from different ethnic origins. J Perinat Med. 2016;44:229–35. [DOI] [PubMed] [Google Scholar]
- 11. Hung JH, Fu CY, Chen CY, Chao KC, Hung J. Fetal nasal bone length and Down syndrome during the second trimester in a Chinese population. J Obstet Gynaecol Res. 2008;34:518–23. [DOI] [PubMed] [Google Scholar]
- 12. Ting YH, Lao TT, Lau TK, Chung MK, Leung TY. Isolated absent or hypoplastic nasal bone in the second trimester fetus: is amniocentesis necessary? J Matern Fetal Neonatal Med. 2011;24:555–8. [DOI] [PubMed] [Google Scholar]
- 13. Sahota DS, Leung TY, Chan LW, Law LW, Fung TY, Chan OK, et al. First‐trimester fetal nasal bone length in an ethnic Chinese population. Ultrasound Obstet Gynecol. 2009;34:33–7. [DOI] [PubMed] [Google Scholar]
- 14. Chen M, Lee CP, Leung KY, Hui PW, Tang MH. Pilot study on the midsecond trimester examination of fetal nasal bone in the Chinese population. Prenat Diagn. 2004;24:87–91. [DOI] [PubMed] [Google Scholar]
- 15. Sonek JD, McKenna D, Webb D, Croom C, Nicolaides K. Nasal bone length throughout gestation: normal ranges based on 3537 fetal ultrasound measurements. Ultrasound Obstet Gynecol. 2003;21:152–5. [DOI] [PubMed] [Google Scholar]
- 16. Kanagawa T, Fukuda H, Kinugasa Y, Son M, Shimoya K, Murata Y, et al. Mid‐second trimester measurement of fetal nasal bone length in the Japanese population. J Obstet Gynaecol Res. 2006;32:403–7. [DOI] [PubMed] [Google Scholar]
- 17. Tomai XH, Phan TH. Fetal nasal bone length at 19–26 weeks’ gestation in Vietnam. J Obstet Gynaecol Res. 2016;42:1245–9. [DOI] [PubMed] [Google Scholar]
- 18. International Society of Ultrasound in Obstetrics & Gynecology . Cardiac screening examination of the fetus: guidelines for performing the “basic” and “extended basic” cardiac scan. Ultrasound Obstet Gynecol. 2006;27:107–13. [DOI] [PubMed] [Google Scholar]
- 19. Prefumo F, Sairam S, Bhide A, Penna L, Hollis B, Thilaganathan B. Maternal ethnic origin and fetal nasal bones at 11‐14 weeks of gestation. BJOG. 2004;111:109–12. [DOI] [PubMed] [Google Scholar]
- 20. Gianferrari EA, Benn PA, Dries L, Brault K, Egan JF, Zelop CM. Absent or shortened nasal bone length and the detection of Down Syndrome in second‐trimester fetuses. Obstet Gynecol. 2007;109(2 Pt 1):371–5. [DOI] [PubMed] [Google Scholar]
- 21. Odibo AO, Sehdev HM, Sproat L, Parra C, Odibo L, Dunn L, et al. Evaluating the efficiency of using second‐trimester nasal bone hypoplasia as a single or a combined marker for fetal aneuploidy. J Ultrasound Med. 2006;25:437–41;quiz 443. [DOI] [PubMed] [Google Scholar]
- 22. Yang X, Han J, Zhen L, Pan M, Li D, Liao C. Relationship between absent or hypoplastic fetal nasal bone and chromosome abnormalities: analysis of 187 cases [In Chinese]. Chin J Perinat Med. 2015;18:339–42. [Google Scholar]
- 23. Dukhovny S, Wilkins‐Haug L, Shipp TD, Benson CB, Kaimal AJ, Reiss R. Absent fetal nasal bone: what does it mean for the euploid fetus? J Ultrasound Med. 2013;32:2131–4. [DOI] [PubMed] [Google Scholar]
- 24. Sonek J, Molina F, Hiett AK, Glover M, McKenna D, Nicolaides KH. Prefrontal space ratio: comparison between trisomy 21 and euploid fetuses in the second trimester. Ultrasound Obstet Gynecol. 2012;40:293–6. [DOI] [PubMed] [Google Scholar]
- 25. Persico N, Borenstein M, Molina F, Azumendi G, Nicolaides KH. Prenasal thickness in trisomy‐21 fetuses at 16–24 weeks of gestation. Ultrasound Obstet Gynecol. 2008;32:751–4. [DOI] [PubMed] [Google Scholar]
- 26. Tournemire A, Groussolles M, Ehlinger V, Lusque A, Morin M, Benevent JB, et al. Prenasal thickness to nasal bone length ratio: effectiveness as a second or third trimester marker for Down syndrome. Eur J Obstet Gynecol Reprod Biol. 2015;191:28–32. [DOI] [PubMed] [Google Scholar]
- 27. Vos FI, De Jong‐Pleij EA, Bakker M, Tromp E, Pajkrt E, Kagan KO, et al. Nasal bone length, prenasal thickness, prenasal thickness‐to‐nasal bone length ratio and prefrontal space ratio in second‐ and third‐trimester fetuses with Down syndrome. Ultrasound Obstet Gynecol. 2015;45:211–6. [DOI] [PubMed] [Google Scholar]
- 28. Yang X, Zhen L, Pan M, Han J, Li D, Liao C. PT/NBL ratio assessment at mid‐trimester in prenatal screening for Down syndrome in a Chinese population. J Matern Fetal Neonatal Med. 2014;27:1860–3. [DOI] [PubMed] [Google Scholar]


