Abstract
The identification of early biological changes associated with the psychotic disorder (PD) is important as it may provide clues to the underlying pathophysiological mechanisms. We undertook the first proteomic profiling of blood plasma samples of children who later develop a PD. Participants were recruited from the UK Avon Longitudinal Study of Parents and Children (ALSPAC) cohort who also participated in psychiatric assessment interviews at age 18. Protein expression levels at age 11 were compared between individuals who developed PD at age 18 (n = 37) with population-based age-matched controls (n = 38). Sixty out of 181 plasma proteins profiled were found to be differentially expressed (P < .05) in children with an outcome of the PD. Thirty-four of these proteins were found to be differentially expressed following correction for multiple comparisons. Pathway analysis implicated the complement and coagulation cascade. A second, targeted proteomic approach was used to verify these findings in age 11 plasma from subjects who reported psychotic experiences at age 18 (n = 40) in comparison to age-matched controls (n = 66). Our findings indicate that the complement and coagulation system is dysregulated in the blood during childhood before the development of the PD.
Keywords: ALSPAC, complement, coagulation, plasma, proteomics, psychotic disorder, schizophrenia
Introduction
There is firm evidence that the early identification and treatment of subjects with psychotic disorder (PD) significantly improve their clinical outcome.1 As clinical characteristics alone are of limited predictive value, there is a now a pressing need for further enhancement of predictive models to allow the earliest identification of subjects who may later develop psychosis.2,3
Blood-based precursors of the PD may offer the opportunity to enhance predictive models and may provide important clues for our understanding of pathophysiological cascades which may later be expressed as psychosis or PD. Our recent review of blood biomarkers in the PD schizophrenia points to a convergence of pathophysiological mechanisms that involve the acute-phase response, glucocorticoid receptor signalling, coagulation, and lipid and glucose metabolism.4 Furthermore, inflammatory cytokines, chemokines, and growth factors have been assessed in the blood during the perinatal periods and during childhood in subjects who subsequently developed schizophrenia, and in those with a first-episode psychosis.5–9 These studies have, in general, demonstrated a picture of enhanced inflammatory tone during and preceding psychosis. While the basis of these changes is not clear, numerous risk factors for schizophrenia such as genetic background but also exposures to abuse, maternal stress during pregnancy, prenatal famine, obstetric complications, and adolescent cannabis use have all been described and hypothesized to lead to raised inflammatory tone.10 Post-mortem brain studies, albeit possibly confounded by many variables, have furthermore underpinned the evidence for a role of inflammation, suggesting that this process is involved during early but also later stages of the disorder.11–13
One of the main challenges of the field is to identify differential profiles which can be measured prior to the clinical onset of the PD. Previous studies of the ALSPAC cohort, a prospective general population cohort based in Bristol area in South West England have characterized subgroups of subjects who developed PD and Psychotic Experiences (PEs)14 at age 18. These groups showed alterations in cortical white matter microstructure,15 working memory,16 and raised inflammatory markers in childhood5 in subjects later developing PD and PE. Supported by this evidence, we employed a discovery proteomic study on blood plasma samples at age 11 in order to yield insights into the underlying protein pathways dysregulated in children with PD outcomes at age 18. As expression of PEs can be considered as a liability phenotype for PDs and may involve the same etiopathogenesis,17 we next quantified candidate proteins in blood samples of subjects at age 11 in a separate subsample from the ALSPAC cohort, and compared subjects who reported PEs at age 18 with matched control subjects without PEs at age 18 as an verification study.
Methods
For extended materials and methods, please refer to online supplementary methods.
Participants
The ALSPAC cohort is a prospective general population cohort, and a rich resource of demographic, environmental, and clinical data on the individuals involved.18 Written informed consent was obtained prior to taking the plasma samples. Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. Please note that the study website contains details of all the data that is available through a fully searchable data dictionary (http://www.bristol.ac.uk/alspac/researchers/access).
Measures of PE and PD
PEs were identified at 11 and 18 years through the face-to-face, semi-structured Psychosis-Like Symptom (PLIKS) interview14 conducted by trained psychology graduates in assessment clinics, and were coded according to the definitions and rating rules for the Schedules for Clinical Assessment in Neuropsychiatry, Version 2.0 (Organisation 1994). Interviewers rated PEs as not present, suspected or definitely psychotic. Cases of PD were defined as individuals with definite PEs that were not attributable to the effects of sleep or fever and when the PE occurred at least once per month over the past 6 months and caused severe distress, had a very negative effect on social/occupational function, or led to help seeking from a professional source.14 All subjects categorized at the interview as having a PD met diagnostic criteria for PDs as defined in both DSM-IV and ICD-10, given that they had regular psychotic phenomena that were causing them severe distress or substantially impaired functioning.14 Control samples from age-matched individuals without suspected or definite PEs, or PD at age 18 were randomly selected to match cases (table 1). With regard to psychotropic drug use, 8 of the 37 subjects (21.6%) with PD and who provided plasma samples were on psychotropic medication at age 18. No subjects reported psychotropic drug use at age 11. Subjects categorized as having disorder in our study only included those whose PEs were not attributed to drug use.14
Table 1.
Descriptive Information for ALSPAC Subjects Included in the PD and PE Studies
| Psychotic Disorder (PD) Discovery Analysis |
Psychotic Experiences (PE) Verification Analysis |
|||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
| Number | 37 | 38 | 40 | 66 |
| Gender | 29F, 8M | 28F, 10M | 22F, 18M | 27F, 39M |
| BMI at age 11, Mean (SD) | 18.24 (3.39) | 18.066 (2.69) | 18.12 (2.72) | 17.75 (2.52) |
| Ethnicity | 28 W, 3 NW, 6 NA | 37 W, 1 NA | 37 W, 1 NW, 2 NA | 63 W, 0 NW, 3 NA |
| PLIKS at age 12 | 20 none, 8 suspected, 9 definite | 38 none | 40 none | 60 none, 6 NA |
| PLIKS at age 18 | 37 definite clinical | None | 29 = definite nonclinical, 11 = sleep/fever | 66 none |
Note: For gender: F, female; M, male. Body mass index (BMI) at age 12 is reported, where missing BMI variables were replaced with the mean according to gender. For ethnicity: W, white; NW, non-white; NA, missing. PLIKS at age 12 and age 18 are reported, however, in this analyses, we used PLIKS at age 18 as the main outcome measure for our proteomic analysis.
Study Design
We undertook a nested case-control study of the ALSPC cohort and chose to assess all available plasma samples from age 11 children with outcomes of PD or PE at age 18. Available plasma samples from controls of age-matched individuals were then randomly selected to arrive at the sample size for each analysis. The present study consisted of analyses of two datasets harboring mass-spectrometry-based proteomic profiles. (1) Discovery proteomic analysis was performed on n = 37 individuals with an outcome of PD at age 18 vs n = 38 age-matched controls without PD or PE (table 1). (2) Hypothesis-driven proteomic analysis to confirm the discovery protein findings was performed on n = 40 individuals with an outcome of PE at age 18 vs n = 67 controls without PD or PE (table 1).
Blood Collection
For all participants, blood samples from nonfasting individuals were collected at approximately 11 years of age. Blood was collected in 7.5 ml Plasma Lithium-Heparin S-Monovette tubes (Sarsted). Once collected, samples were stored on ice for a maximum of 90 min until processed. After centrifugation, the plasma was stored in aliquots at −80C. All samples underwent a single freeze-thaw cycle to allow aliquotting prior to the study. The standard quality of the plasma samples was ensured by assessing the overall MS protein profile to facilitate the identification of outlier protein expression profiles (see supplementary figure S1).
High-Abundance Protein Depletion of Plasma Samples
To improve the dynamic range for proteomic analysis, 40 µl of plasma from each case was immunodepleted of the 14 most abundant proteins (alpha-1-antitrypsin, A1-acid glycoprotein, serum albumin, alpha2-macroglobulin, apolipoprotein A-I, apolipoptrotein A-II, complement C3, fibrinogen alpha/beta/gamma, haptoglobin, IgG A, IgG G, IgG M, transthyretin, and serotransferrin) using the Agilent Hu14 Affinity Removal System (MARS) coupled to a high-performance liquid chromatography (HPLC) system19 (supplementary methods).
Sample Preparation for Mass Spectrometry
Protein digestion and peptide purification was performed as previously described,20 and are further detailed in supplementary methods.
Discovery Proteomic Analysis in PD
For the discovery proteomic analysis in PD (37 cases vs 38 controls; see table 1), 5 μl from each sample was injected on a Thermo Scientific Q-Exactive mass spectrometer, connected to a Dionex Ultimate 3000 (RSLCnano) chromatography system, and operated in data dependent analysis (DDA) mode, for label-free LC-MS/MS, which is standard proteomic profiling method for case/control clinical investigations.20–22 Please refer supplementary methods for further details.
Hypothesis-driven Proteomic Analysis in PE
For hypothesis-driven proteomic analysis of candidate proteins in a second subset of participants, we used targeted-data independent acquisition (DIA) analysis. Targeted-DIA is currently the gold standard for high-throughput quantification of protein targets.23–25 For targeted-DIA in the PE subset of participants (40 cases vs 66 controls; table 1), 5 μl of each sample was injected on the Thermo Scientific Q-Exactive, and data was acquired in DIA mode. The DIA isolation scheme and multiplexing strategy were based on that from Egertson et al., in which five separate 4-m/z isolation windows are analyzed per spectrum26,27 (supplementary methods).
Bioinformatics and Statistical Analysis
Discovery Proteomic Analysis in PD.
Label-free quantification for our discovery DDA files generated from the PD subjects was performed in Max Quant (v1.5.2.8),28,29 as described.20 Only proteins present in >80% of samples in at least one group were taken forward for quantification (supplementary table 1; n = 181 proteins), whereby the data were log2 transformed and normalized (supplementary figure S1).30 Data management and analyses were then performed in R version 3.0.1 (2013-05-16). Missing proteomic data was replaced with a random value from the normal distribution of each protein that was created separately for cases and controls.31 Linear regression analyses were performed on all proteins with the subject sample as a random effect, adjusting for gender and BMI at age 11. We adjusted for BMI as the prevalence of obesity in people with mental illness has been reported to higher than the general population,32,33 and recent evidence suggests childhood BMI between the ages 7 and 13 years is associated with risk of schizophrenia.34 A 5% false discovery rate (FDR) threshold was applied using the “fdrtool” package in R, as previously described.35,36 The demographic and clinical data were tested for differences between case and control group using the Fisher’s Exact test.
Hypothesis-Driven Proteomic Analysis in PE.
All DIA files from the PE analysis (see table 1 for groups) were analyzed in Skyline (V3.5.0; https://skyline.gs.washington.edu), as detailed by Egertson et al.26,27 We aimed to verify; (1) the specific proteins identified as differentially expressed following FDR from our “Discovery proteomic analysis in PD” and (2) the broader set and proteins from the complement and coagulation pathways which were generally implicated from the Discovery proteomic analysis in PD’. This was done in a separate ALSPAC subgroup of participants with a milder phenotype of PE (n = 40) but not PD at age 18, compared to controls (n = 66; table 1). For a full list of the fragments targeted and quantified please refer to supplementary table S2. All peptides and associated fragment ions were visually checked, and peak editing was undertaken where necessary (supplementary document 1). Preprocessing and statistical analysis of the fragment level data were undertaken in mapDIA37 (supplementary table S3), as detailed in supplementary methods.
Pathway Analysis
KEGG pathway analysis is traditionally performed on all proteins that are differentially expressed (P < .05) (http://www.genome.jp/kegg/pathway.html) in order to identify coordinated changes in pathways and cellular processes at the level of the proteome, and this was therefore undertaken on all proteins differentially expressed in the arising from the PD study. The STRING database (http://string-db.org/)38 was used to analyze the protein–protein interactions and pathway relationships within our dataset.
Results
Discovery Proteomic Analysis in PD
For the discovery proteomic analysis in PD (37 cases vs 38 controls; table 1), we identified 345 proteins (FDR < 0.01), of which 181 were present in >80% of samples and were taken forward for analysis (supplementary table 1). Sixty proteins out of 181 were significantly differentially expressed between PD cases and controls (P < .05), following adjustment for gender and BMI as covariates (see results, table 2). Thirty-four proteins (A2M, TNXB, IGHM, CLEC3B, BCHE, GSN, ECM1, IGF2, C4BPB, CTBS, SERPINA7, VCAM1, LUM, GPLD1, HABP2, IGHG3, SEPP1, CFH, SERPINF1, F5, F9, IGFBP3, F12, CFD, CFI, LAMP2, CRTAC1, PCOLCE, AFM, PLG, COL6A3, C7, APOA2, CD109) remained significant after FDR (table 2). The full list of proteins profiled, and those that were differentially expressed between groups is provided in supplementary table 1.
Table 2.
Differential Protein Expression in Psychotic Disorder
| Gene Names | Protein Names | Protein ID | P Value | FC in PD | FDR |
|---|---|---|---|---|---|
| A2M | Alpha-2-macroglobulin | P01023 | 2.35E-05 | −1.36 | 0.002452739084173 |
| TNXB | Tenascin-X | P22105 | 6.78E-05 | 1.60 | 0.003010022721392 |
| IGHM | Ig mu chain C region | P01871 | 8.93E-05 | −1.49 | 0.003100233074614 |
| CLEC3B | Tetranectin | P05452 | 0.000238536918096 | 1.21 | 0.005527149972427 |
| BCHE | Cholinesterase | P06276 | 0.00028867379359 | 1.22 | 0.006016201946107 |
| GSN | Gelsolin | P06396 | 0.001184331262904 | 1.13 | 0.013741065106703 |
| ECM1 | Extracellular matrix protein 1 | Q16610 | 0.00156813706243 | 1.23 | 0.015289744574204 |
| IGF2 | Insulin-like growth factor II | P01344 | 0.00185038627232 | 1.26 | 0.016146280605035 |
| C4BPB | C4b-binding protein beta chain | P20851 | 0.001968111624603 | −1.31 | 0.016452583474952 |
| CTBS | Di-N-acetylchitobiase | Q01459 | 0.002116574187167 | 1.23 | 0.016804037125592 |
| SERPINA7 | Thyroxine-binding globulin | P05543 | 0.002123057983554 | 1.17 | 0.016818582457348 |
| VCAM1 | Vascular cell adhesion protein 1 | P19320 | 0.002249659902707 | 1.34 | 0.01709034422445 |
| LUM | Lumican | P51884 | 0.002278966387226 | 1.19 | 0.017150104571853 |
| GPLD1 | Phosphatidylinositol-glycan- specific phospholipase D | P80108 | 0.00234614616507 | 1.42 | 0.017282940815611 |
| HABP2 | Hyaluronan-binding protein 2 | Q14520 | 0.002538350481953 | 1.17 | 0.017633778389896 |
| IGHG3 | Ig gamma-3 chain C region | P01860 | 0.002874794375478 | −1.27 | 0.018546283526309 |
| SEPP1 | Selenoprotein P | P49908 | 0.003146451379488 | 1.21 | 0.019193243065463 |
| CFH | Complement factor H | P08603 | 0.003414262231026 | 1.13 | 0.019765573474774 |
| SERPINF1 | Pigment epithelium-derived factor | P36955 | 0.003757243579115 | 1.13 | 0.02047865413338 |
| F5 | Coagulation factor V | Q28107 | 0.004067608434137 | 1.27 | 0.021055629451341 |
| F9 | Coagulation factor IX | P00740 | 0.004334224194447 | 1.13 | 0.021506865940923 |
| IGFBP3 | Insulin-like growth factor- binding protein 3 | P17936 | 0.007644758410206 | 1.17 | 0.032388279082007 |
| F12 | Coagulation factor XII | P00748 | 0.007824122898499 | 1.19 | 0.032887687197417 |
| CFD | Complement factor D | P00746 | 0.008444673418423 | 1.35 | 0.034556546176356 |
| CFI | Complement factor I | P05156 | 0.010003939954609 | 1.12 | 0.038384336560269 |
| LAMP2 | Lysosome-associated membrane glycoprotein 2 | P13473 | 0.011818700619699 | 1.19 | 0.042278849036246 |
| CRTAC1 | Cartilage acidic protein 1 | Q9NQ79 | 0.012282687547432 | 1.23 | 0.043191407027815 |
| PCOLCE | Procollagen C-endopeptidase enhancer 1 | Q15113 | 0.012858286614664 | 1.19 | 0.044281235146809 |
| AFM | Afamin | P43652 | 0.014449995361757 | 1.12 | 0.047073156606685 |
| PLG | Plasminogen | P00747 | 0.014808887658904 | 1.14 | 0.047661474616677 |
| COL6A3 | Collagen alpha-3(VI) chain | P12111 | 0.015123384190082 | 1.35 | 0.048165491709375 |
| C7 | Complement component C7 | P10643 | 0.015253718180595 | 1.14 | 0.048371295829491 |
| APOA2 | Apolipoprotein A-II | P02652 | 0.015768184543684 | −1.35 | 0.049166640144774 |
| CD109 | CD109 antigen | Q6YHK3 | 0.016302896370883 | 1.23 | 0.049965576376137 |
| ACTG1 | Actin, cytoplasmic 2 | P63261 | 0.01842844064171 | 1.23 | 0.052893098007721 |
| CD14 | Monocyte differentiation antigen CD14 | P08571 | 0.018648336393252 | 1.14 | 0.05317487598941 |
| KLKB1 | Plasma kallikrein | P03952 | 0.019913520723869 | 1.11 | 0.054727223568396 |
| KNG1 | Kininogen-1 | P01042 | 0.021034485331523 | 1.11 | 0.056011643691759 |
| F5 | Coagulation factor V | P12259 | 0.021375886441285 | 1.14 | 0.056387118262621 |
| SERPINF2 | Alpha-2-antiplasmin | P08697 | 0.024325248700793 | 1.09 | 0.059359684835652 |
| C1S | Complement C1s subcomponent | P09871 | 0.024366317843667 | 1.12 | 0.059397936011767 |
| F2 | Prothrombin | P00734 | 0.024740545362472 | 1.12 | 0.059742847153768 |
| APOA4 | Apolipoprotein A-IV | P06727 | 0.025696265563249 | 1.13 | 0.060594940495585 |
| IGKV2D-40 | Ig kappa chain V-II region TEW | A0A075B6R1 | 0.025902708420233 | −1.20 | 0.060773774727794 |
| LYVE1 | Lymphatic vessel endothelial hyaluronic acid receptor 1 | Q9Y5Y7 | 0.026443478521856 | 1.24 | 0.061233787247234 |
| F13A1 | Coagulation factor XIII A chain | P00488 | 0.027407334083503 | 1.14 | 0.062086047473999 |
| S100A8 | Protein S100-A8 | P05109 | 0.030888403899574 | 1.24 | 0.065695245585865 |
| ANPEP | Aminopeptidase N | P15144 | 0.030918599644144 | 1.22 | 0.065724623760281 |
| VTN | Vitronectin | P04004 | 0.031818887929209 | 1.10 | 0.066586456714209 |
| C4BPA | C4b-binding protein alpha chain | P04003 | 0.034467677540817 | −1.17 | 0.068973446582385 |
| APOH | Beta-2-glycoprotein 1 | P02749 | 0.035328247276582 | 1.13 | 0.06970463250612 |
| C6 | Complement component C6 | P13671 | 0.036921822018089 | 1.10 | 0.071005866471262 |
| C1R | Complement C1r subcomponent | P00736 | 0.03697367472293 | 1.13 | 0.07104710171526 |
| CLU | Clusterin | P10909 | 0.037047254149251 | 1.09 | 0.071105498589918 |
| COMP | Cartilage oligomeric matrix protein | P49747 | 0.038053606817258 | 1.27 | 0.071890746567978 |
| CPN2 | Carboxypeptidase N subunit 2 | P22792 | 0.039032541881775 | 1.11 | 0.07263130677403 |
| ADIPOQ | Adiponectin | Q15848 | 0.041918375605006 | 1.28 | 0.076309890815356 |
| ATRN | Attractin | O75882 | 0.045675287259397 | 1.09 | 0.080866376756604 |
| VASN | Vasorin | Q6EMK4 | 0.047805918283872 | 1.15 | 0.083340993340051 |
| RARRES2 | Retinoic acid receptor responder protein 2 | Q99969 | 0.048852426112363 | 1.15 | 0.084528878416831 |
Note: Label-free discovery proteomic was used to compare age 11 plasma samples from children with an outcome of PD at age 18 with age-matched healthy controls (n = 75). Sixty proteins were identified as significantly differentially expressed between cases and controls (ANCOVA, P < .05), following adjustment for gender and BMI as covariates (supplementary table 1). The gene name, protein name, accession, ANCOVA adjusted P value, fold change (FC) in disorder, and false discovery rate (FDR) values are listed for the 60 significant proteins which are sorted by FDR.
Pathway Analysis
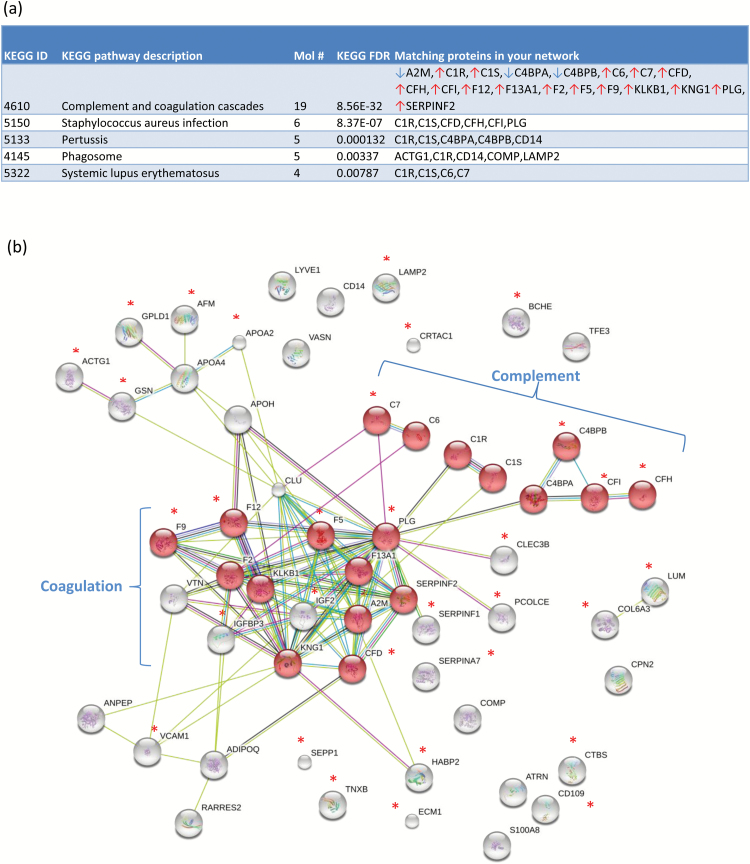
To identify potential protein pathways and processes whose expression is co-ordinately altered in PD, we uploaded the 60 significant proteins to KEGG for pathway analysis. The top pathway implicated was the “Complement and Coagulation Cascade” (figure 1a; Fisher’s exact test P = 8.56 × 10−32), followed by Staphylococcus aureus infection and Pertussis infection protein pathways (figure 1a). In addition, when the same proteins were analyzed using the STRING algorithm, the set of coagulation and complement proteins were found to cluster together as subnetworks (figure 1b), providing further evidence of coordinated perturbation of these pathways.
Fig. 1.
Differentially expressed proteins converge on complement and the coagulation cascade. (a) KEGG pathway analysis 60 proteins as differentially expressed between cases (n = 37) and controls (n = 38) identified the complement and coagulation cascade as the top process implicated (19 proteins; false discovery rate (FDR) 8.56e-32; (b) STRING network analysis provided strong evidence for co-regulation among coagulation and complement proteins, with the average interactions per protein being 3.65 (ie, average node degree) for 57 of the 60 proteins mapped. Proteins that were significant at the FDR level are marked with asterisks.
Hypothesis-Driven Proteomic Analysis in PE
These analyses aimed to verify and extend the findings of the discovery analysis of PD in a separate ALSPAC subgroup of participants with a milder phenotype of PE (n = 40) but not PD at age 18, compared to controls (n = 66; table 1). Two PE cases were excluded from the bioinformatics analysis due to poor chromatographic profiles.
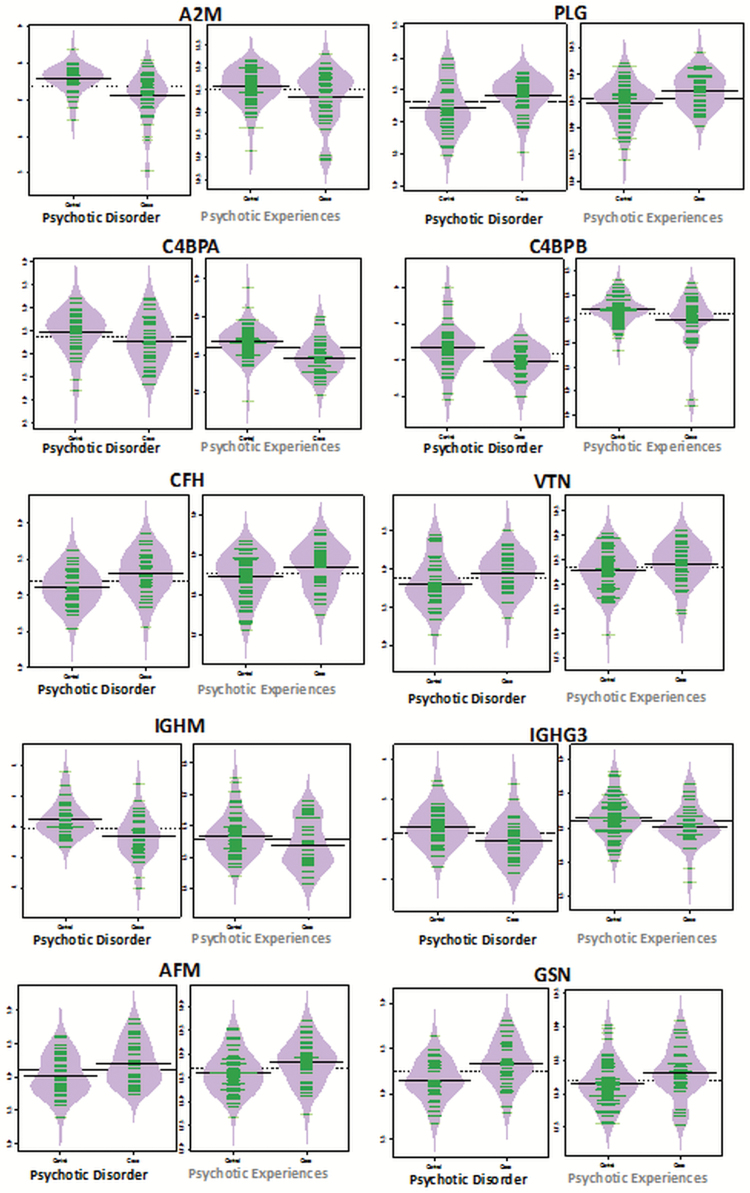
We used a targeted-DIA approach (see supplementary document 1) to quantify the levels of the 34 FDR significant proteins in the PE group. Twenty-seven of the 34 FDR candidates had peptides suitable for targeted-DIA analysis (supplementary table S3). We also used a targeted-DIA approach to target the proteins which mapped to the complement and coagulation cascade in PD but which were not in the list of the 34 FDR significant proteins (table 2). Thus of the total of 19 proteins which mapped to the complement and coagulation cascade (figure 1), we had already assessed 10 when undertaking our targeted-DIA assessment of the 34 FDR significant proteins, leaving 9 proteins (significant at P < .05) which mapped to this process (figure 1), and we assessed these 9. Therefore a total of 36 protein candidates (27 + 9) were quantified in the hypothesis-driven PE study. Out of the 36 targets, we confirmed the differential expression of 10 proteins using a Bayesian latent variable model at a 5% FDR threshold (C4BPA, A2M, PLG, AFM, CFH, IGHG3, VTN, GSN, C4BPB, and IGHM; supplementary table S3). The protein level DIA data illustrates that the direction of protein expression changes in the PD and PE phenotypes compared to controls was the same for all of these 10 proteins (figure 2).
Fig. 2.
Bean plots of proteins identified as altered in the same direction in both the psychotic disorder (PD) and psychotic experience (PE) cohorts. Bean plots represent the log-transformed intensities in protein expression (y-axis) between cases and controls (x-axis) for both the PD (n = 75) discovery proteomic analysis (left), and PE (n = 106) hypothesis-driven proteomic analysis (right). Please note the scale on the y-axis differs between the PD and PE cohorts because different quantification methods were used to assess protein expression in each cohort (ie, DDA vs DIA data). Nevertheless, bean-plots representing the expression changes in complement and coagulation proteins (A2M, PLG, C4BPA, C4BPB, CFH, and VTN), and others (IGHM, IGHG3, GSN, and AFM) are altered in the same direction in both cohorts. These protein candidates may represent persistent early hallmarks of psychotic outcomes at age 18 in the general population.
Discussion
An urgent challenge in the field of psychosis is to understand the pathophysiological basis of psychosis so that early prediction can be realized and earlier and more effective prevention and treatment options can be considered. We have undertaken the first discovery proteomic profiling study in early adolescence in order to identify differential plasma protein expression preceding PD. Using a unique prospective cohort, we investigated blood plasma samples obtained from children at age 11 who went on to develop PD at age 18. In the samples collected at age 11, we identified a set of 34 proteins significantly differentially expressed between children who developed PD vs age-matched population-based controls (table 2). Seven of these 34 proteins (A2M, APOA2, C4BPB, CLECB3, GPLD1, IGHM, IGFBP3) have previously been implicated in blood-based biomarker studies of patients diagnosed with the PD schizophrenia.4 These proteins, therefore, might have potential value in predicting the risk of and in contributing to future pathophysiological models for PD. However, this will require robust identification of markers, calibration of risk prediction models, and testing in independent cohorts.
The information that can be derived from the broader pathway- or network-based picture of differential protein expression (rather than the individual proteins) is relevant to understanding the biological basis of the PD. Pathway analysis identified the Complement and Coagulation Cascade as the most significant process altered (figure 1), with a total of 19 proteins involved in the process being differentially expressed between groups. These complement and coagulation proteins included 3 decreased proteins (A2M, C4BPA, C4BPB) and 16 increased proteins (C1R, C1S, C6, C7, CFD, CFH, CFI, F12, F13A1, F2, F5, F9, KLKB1, KNG1, PLG, SERPINF2) in children who developed PD. Due to significant overlap in proteins that mapped to the other top infection related processes in Staphylococcus and Pertussis infection, we focused our attention on complement and coagulation cascade. We followed the above discovery study with a hypothesis-driven proteomic study of children who went on to develop PEs (rather than PD) at age 18. In this group, we confirmed the differential expression of 10 proteins which were altered in the PD group, and all of these changes were in the same direction as in the PD group (figure 2). Six of these targeted proteins were implicated in complement and coagulation (A2M, C4BPA, C4BPB, CFH, PLG, VTN), two in the immune system (IGHM and IGHG3), and one each in Vitamin E binding (AFM), and synaptic elimination and myelination (GSN). As PEs is a milder phenotype that PD, with about 8% of individuals ultimately transitioning to PD,13 we consider these results to be supportive of our main findings.
The complement and coagulation cascade is part of the innate immune defense against infection, and the role of these processes in the pathophysiology of schizophrenia has gained attention in recent years. After decades of intensive research, collaborative GWAS efforts identified the strongest risk association for schizophrenia in the major histocompatibility complex (MHC)—best known for its role in immunity.39 Sekar et al13 have recently shown that within this MHC region the strongest risks are associated with the complement 4 (C4) gene, which reflects the heritable impairment in the complement system in schizophrenia. In this study, we found 9 complement proteins dysregulated in children preceding the onset of PD, including decreased C4BPA and C4BPB, and increased C1R, CFD, C1S, CFI, C6, C7, and CFH. The C4BPA and C4BPB proteins assemble together forming C4b-binding protein,which regulates the activation of the complement cascade, and also has a regulator role in the coagulation system. The coagulation system was also largely increased in expression in children preceding the onset of PD, including increased KNG1, PLG, KLKB1, F12, F9, F5, F13A1, SERPINF2, F2, and decreased A2M. In support, Li et al40 also reported the complement and coagulation cascade as the most significant pathway implicated in plasma samples of drug naïve schizophrenia patients, and again reported on the reduced expression of C4BPB. Other blood-based proteomic studies of first-episode psychosis support these findings.19,41,42 Dysfunction in coagulation has also been reported previously in schizophrenia4,43,44 and more recently by Hoirisch-Clapauch et al,45 who observed that schizophrenia patients on chronic warfarin therapy showed remission of psychotic symptoms. The complement system is linked functionally with inflammation,46 and within the brain has important functions in the regulation of synaptic plasticity.13,47–49 Our findings are thus in keeping with the literature implicating inflammation in major psychiatric disorders10,50 and with potential mechanisms involved in complement and altered synaptic plasticity.13,51–53 However, the functional relationship between complement activation within the blood and the complement activation within the brain has not been elucidated, and hence we cannot draw a direct inference between our findings and brain function. In the light of our findings, future studies need to address this issue.
Our study has several limitations which should be mentioned. Firstly, it was based on a unique characterized cohort, and we could not access an exactly similar age-matched sample in which we could perform a direct replication of the discovery analyses. However, we obtained supportive complementary data for our hypothesis-driven study using age 11 samples from a subject with PE outcomes, rather than PD outcomes, from the same cohort. Nevertheless, it has been argued that PEs form a measurable liability phenotype for PD and that PEs share (at least some of) the risk factors of PD, suggesting that their etiopathogenesis may involve similar biological cascades. Another potential weakness is that the subjects investigated in this study were age 18 at the time of last clinical assessment and therefore have not yet gone through the full period of risk for developing a PD and hence our sample is not representative of all individuals with the PD. While future work will be required to address this, it is notable that our findings find significant support from the plasma proteomic biomarker literature of schizophrenia which has implicated the complement and coagulation pathways very consistently.4,17,29,30,31 Finally, while various approaches for bioinformatics analyses of DIA datasets are still under development, the methods we used are in line with recent advances in the bioinformatic handling of DIA data by Teo et al.37 This tool considers the fragment level data as “repeated measurements” of the abundance of peptides and thus applies a sophisticated hierarchical Bayesian algorithm37 for the analysis of DIA data (supplementary table 3).
In conclusion, our study which is the first to undertake a proteomic analysis of plasma in childhood from subjects who develop PD in early adulthood has identified alterations in the complement and coagulation process. These changes are also present, but to a lesser extent, among subjects who go on to develop PE in early adulthood, suggesting that our findings may represent early changes associated with a vulnerability to psychosis. Further clarification of the roles of these proteins and processes will be obtained from future studies which will test how baseline levels of these proteins in subjects at ultra-high risk for PD relate to the varied clinical outcomes.54 Overall, our findings are indicative of the presence of a pathophysiological process long before the development of the disorder, and suggest that blood-based profiles may be tested in larger population for their value in predicting later PD.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
The UK Medical Research Council and Wellcome (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. This research was specifically funded by the Irish Health Research Board through a Clinician Scientist Award to D.R.C. This study is also supported by the NIHR Bristol BRC.
Supplementary Material
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. This publication is the work of the authors and will serve as guarantors for the contents of this paper. We also thank Prof Matthias Wilm and the Mass Spectrometry Core Facility at UCD Conway Institute, UCD, for support in the development of our proteomic workflows. In addition, we would like to thank everyone at the MacCoss Lab of Biological Mass Spectrometry, University of Washington, and everyone at the H. Choi Lab, National University of Singapore, for support and access to Skyline and MapDIA, respectively. The authors report no financial interests or potential conflicts of interest.
References
- 1. Larsen TK, Melle I, Auestad B et al. Early detection of psychosis: positive effects on 5-year outcome. Psychol Med. 2011;41:1461–1469. [DOI] [PubMed] [Google Scholar]
- 2. Addington J, Cornblatt BA, Cadenhead KS et al. At clinical high risk for psychosis: outcome for nonconverters. Am J Psychiatry. 2011;168:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cannon TD. Brain biomarkers of vulnerability and progression to psychosis. Schizophr Bull. 2016;42(suppl 1):S127–S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sabherwal S, English JA, Föcking M, Cagney G, Cotter DR. Blood biomarker discovery in drug-free schizophrenia: the contributionof proteomics and multiplex immunoassays. Expert Rev Proteomics. 2016;13:1141–1155. [DOI] [PubMed] [Google Scholar]
- 5. Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan MK, Krebs MO, Cox D et al. Development of a blood-based molecular biomarker test for identification of schizophrenia before disease onset. Transl Psychiatry. 2015;5:e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Beveren NJ, Schwarz E, Noll R et al. Evidence for disturbed insulin and growth hormone signaling as potential risk factors in the development of schizophrenia. Transl Psychiatry. 2014;4:e430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwarz E, van Beveren NJ, Ramsey J et al. Identification of subgroups of schizophrenia patients with changes in either immune or growth factor and hormonal pathways. Schizophr Bull. 2014;40:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perkins DO, Jeffries CD, Addington J et al. Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophr Bull. 2015;41:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cannon M, Clarke MC, Cotter DR. Priming the brain for psychosis: maternal inflammation during fetal development and the risk of later psychiatric disorder. Am J Psychiatry. 2014;171:901–905. [DOI] [PubMed] [Google Scholar]
- 11. Fillman SG, Weickert TW, Lenroot RK et al. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca’s area volume. Mol Psychiatry. 2016;21:1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trépanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry. 2016;21:1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sekar A, Bialas AR, de Rivera H et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zammit S, Kounali D, Cannon M et al. Psychotic experiences and psychotic disorders at age 18 in relation to psychotic experiences at age 12 in a longitudinal population-based cohort study. Am J Psychiatry. 2013;170:742–750. [DOI] [PubMed] [Google Scholar]
- 15. Drakesmith M, Dutt A, Fonville L et al. Mediation of developmental risk factors for psychosis by white matter microstructure in young adults with psychotic experiences. JAMA Psychiatry. 2016;73:396–406. [DOI] [PubMed] [Google Scholar]
- 16. Fonville L, Cohen Kadosh K, Drakesmith M et al. Psychotic experiences, working memory, and the developing brain: a multimodal neuroimaging study. Cereb Cortex. 2015;25:4828–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. [DOI] [PubMed] [Google Scholar]
- 18. Boyd A, Golding J, Macleod J et al. Cohort Profile: the ‘children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levin Y, Wang L, Schwarz E, Koethe D, Leweke FM, Bahn S. Global proteomic profiling reveals altered proteomic signature in schizophrenia serum. Mol Psychiatry. 2010;15:1088–1100. [DOI] [PubMed] [Google Scholar]
- 20. English JA, Fan Y, Föcking M et al. Reduced protein synthesis in schizophrenia patient-derived olfactory cells. Transl Psychiatry. 2015;5:e663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Föcking M, Dicker P, Lopez LM, et al. Proteomic analysis of the postsynaptic density implicates synaptic function and energy pathways in bipolar disorder. Transl Psychiatry. 2016;6:e959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Topol A, Zhu S, Hartley BJ et al. Dysregulation of miRNA-9 in a subset of schizophrenia patient-derived neural progenitor cells. Cell Rep. 2016;15:1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sajic T, Liu Y, Aebersold R. Using data-independent, high-resolution mass spectrometry in protein biomarker research: perspectives and clinical applications. Proteomics Clin Appl. 2015;9:307–321. [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Buil A, Collins BC et al. Quantitative variability of 342 plasma proteins in a human twin population. Mol Syst Biol. 2015;11:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aebersold R, Bensimon A, Collins BC, Ludwig C, Sabido E. Applications and developments in targeted proteomics: from SRM to DIA/SWATH. Proteomics. 2016;16:2065–2067. [DOI] [PubMed] [Google Scholar]
- 26. Egertson JD, Kuehn A, Merrihew GE et al. Multiplexed MS/MS for improved data-independent acquisition. Nat Methods. 2013;10:744–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Egertson JD, MacLean B, Johnson R, Xuan Y, MacCoss MJ. Multiplexed peptide analysis using data-independent acquisition and Skyline. Nat Protoc. 2015;10:887–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011;10:1794–1805. [DOI] [PubMed] [Google Scholar]
- 29. Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. [DOI] [PubMed] [Google Scholar]
- 30. Hubner NC, Bird AW, Cox J et al. Quantitative proteomics combined with BAC TransgeneOmics reveals in vivo protein interactions. J Cell Biol. 2010;189:739–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Webb-Robertson BJ, Wiberg HK, Matzke MM et al. Review, evaluation, and discussion of the challenges of missing value imputation for mass spectrometry-based label-free global proteomics. J Proteome Res. 2015;14:1993–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thorndike AN, Achtyes ED, Cather C et al. Weight gain and 10-year cardiovascular risk with sustained tobacco abstinence in smokers with serious mental illness: a subgroup analysis of a randomized trial. J Clin Psychiatry. 2016;77:e320–e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chouinard VA, Pingali SM, Chouinard G et al. Factors associated with overweight and obesity in schizophrenia, schizoaffective and bipolar disorders. Psychiatry Res. 2016;237:304–310. [DOI] [PubMed] [Google Scholar]
- 34. Sørensen HJ, Gamborg M, Sørensen TI, Baker JL, Mortensen EL. Childhood body mass index and risk of schizophrenia in relation to childhood age, sex and age of first contact with schizophrenia. Eur Psychiatry. 2016;34:64–69. [DOI] [PubMed] [Google Scholar]
- 35. Strimmer K. A unified approach to false discovery rate estimation. BMC Bioinform. 2008;9:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strimmer K. fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics. 2008;24:1461–1462. [DOI] [PubMed] [Google Scholar]
- 37. Teo G, Kim S, Tsou CC et al. mapDIA: preprocessing and statistical analysis of quantitative proteomics data from data independent acquisition mass spectrometry. J Proteomics. 2015;129:108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Szklarczyk D, Franceschini A, Wyder S et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schizophrenia Working Group of the Psychiatric Genomics Consortium, Ripke S, Neale BM, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li Y, Zhou K, Zhang Z et al. Label-free quantitative proteomic analysis reveals dysfunction of complement pathway in peripheral blood of schizophrenia patients: evidence for the immune hypothesis of schizophrenia. Mol Biosyst. 2012;8:2664–2671. [DOI] [PubMed] [Google Scholar]
- 41. Jaros JA, Martins-de-Souza D, Rahmoune H et al. Protein phosphorylation patterns in serum from schizophrenia patients and healthy controls. J Proteomics. 2012;76 Spec No:43–55. [DOI] [PubMed] [Google Scholar]
- 42. Yang Y, Wan C, Li H et al. Altered levels of acute phase proteins in the plasma of patients with schizophrenia. Anal Chem. 2006;78:3571–3576. [DOI] [PubMed] [Google Scholar]
- 43. Masopust J, Malý R, Andrýs C, Vališ M, Bažant J, Hosák L. Markers of thrombogenesis are activated in unmedicated patients with acute psychosis: a matched case control study. BMC Psychiatry. 2011;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang KC, Yang KC, Lin H, Tsao TT, Lee SA. Transcriptome alterations of mitochondrial and coagulation function in schizophrenia by cortical sequencing analysis. BMC Genomics. 2014;15(suppl 9):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoirisch-Clapauch S, Amaral OB, Mezzasalma MA, Panizzutti R, Nardi AE. Dysfunction in the coagulation system and schizophrenia. Transl Psychiatry. 2016;6:e704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Orsini F, De Blasio D, Zangari R, Zanier ER, De Simoni MG. Versatility of the complement system in neuroinflammation, neurodegeneration and brain homeostasis. Front Cell Neurosci. 2014;8:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kolev M, Le Friec G, Kemper C. Complement–tapping into new sites and effector systems. Nat Rev Immunol. 2014;14:811–820. [DOI] [PubMed] [Google Scholar]
- 49. Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. [DOI] [PubMed] [Google Scholar]
- 50. Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res. 2014;155:101–108. [DOI] [PubMed] [Google Scholar]
- 51. Curtis D. Schizophrenia genetics moves into the light. Br J Psychiatry. 2016;209:93–94. [DOI] [PubMed] [Google Scholar]
- 52. Ruzzo EK, Geschwind DH. Schizophrenia genetics complements its mechanistic understanding. Nat Neurosci. 2016;19:523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Whalley K. Psychiatric disorders: linking genetic risk to pruning. Nat Rev Neurosci. 2016;17:199. [DOI] [PubMed] [Google Scholar]
- 54. Rutigliano G, Valmaggia L, Landi P et al. Persistence or recurrence of non-psychotic comorbid mental disorders associated with 6-year poor functional outcomes in patients at ultra high risk for psychosis. J Affect Disord. 2016;203:101–110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.