Abstract
This study tests the hypothesis that cardiovascular magnetic resonance–derived left ventricular ejection fraction and volumes would provide improved risk stratification in patients undergoing coronary artery bypass surgery for ischemic cardiomyopathy.
Ischemic cardiomyopathy accounts for approximately 50% of patients with heart failure and one-third of the patients undergoing coronary artery bypass surgery. Left ventricular (LV) ejection fraction (EF) and end-systolic volume index (ESVI) are predictors of mortality in these patients. Cardiovascular magnetic resonance (CMR) imaging can provide precise estimates of LV volumes and function. In the Surgical Treatment for Ischemic Heart Failure (STICH) Trial population, we tested the hypothesis that CMR-derived LVEF and volumes would provide improved risk stratification.
Methods
The rationale, patient enrollment, and inclusion and exclusion criteria for the surgical arm of the STICH Trial have been previously described. Briefly, this study included patients with LVEF of 35% or less, apical dyssynergy, coronary anatomy suitable for revascularization, age 18 years or older, and the ability of the patient to give informed consent. A baseline CMR study was performed, although it was not mandated by the protocol.
The protocol was developed and analyzed by the CMR Core Laboratory. Cardiac-gated CMR studies were performed on commercially available 1.5-T or 3-T platforms from an international group of hospitals. Short-axis cine imaging studies were used to determine LV volumes and EF using the Simpson method. Each of the participating institutions obtained approval from their respective institutional review boards. Written consent was obtained from all patients.
Results
Of the 1000 surgical ventricular reconstruction–eligible patients, 340 had baseline CMR studies and 660 did not; both groups were similar except for a slightly higher prevalence of renal insufficiency in those who did not have a CMR study (Table). The mean (SD) age of the CMR group was 60.9 (9.8) years, the mean (SD) LVEF was 28% (11%), the mean (SD) end-diastolic volume index was 108 (39) mL/m2, and the mean (SD) ESVI was 80 (36) mL/m2.
Table. Baseline Clinical and CMR Measurements.
| Variable | No. (%) | |||
|---|---|---|---|---|
| All Patients | CMR Patients | |||
| With No CMR (n = 660) |
With CMR (n = 340) |
With CABG Only (n = 177) |
With CABG Plus SVR (n = 163) |
|
| Age, mean (SD), y | 62.0 (9.6) | 60.9 (9.8) | 61.3 (9.9) | 60.4 (9.7) |
| Women | 100 (15.2) | 47 (13.8) | 24 (13.6) | 23 (14.1) |
| Hypertension | 384 (58.6) | 198 (58.2) | 101 (57.1) | 97 (59.5) |
| Diabetes | 230 (34.8) | 114 (33.5) | 57 (32.2) | 57 (35.0) |
| Stroke | 39 (5.9) | 17 (5.0) | 8 (4.5) | 9 (5.5) |
| Peripheral vascular disease | 106 (16.1) | 40 (11.8) | 26 (14.7) | 14 (8.6) |
| Renal insufficiency | 62 (9.4) | 23 (6.8) | 10 (5.6) | 13 (8.0) |
| Atrial fibrillation | 82 (12.4) | 35 (10.3) | 18 (10.2) | 17 (10.4) |
| Heart rate, mean (SD), bpm | 67 (12) | 70 (13) | 70 (14) | 70 (12) |
| Blood pressure, mean (SD), mm Hg | ||||
| Systolic | 121 (17) | 120 (18) | 121 (18) | 120 (18) |
| Diastolic | 73 (12) | 74 (11) | 74 (10) | 75 (11) |
| LV ejection fraction, mean (SD), % | NA | 28 (11) | 28 (11) | 27 (12) |
| LV, mean (SD), mL/m2 | ||||
| End-diastolic volume index | NA | 108 (39) | 106 (36) | 112 (42) |
| End-systolic volume index | NA | 80 (36) | 77 (33) | 83 (39) |
| Aspirin use | 488 (74) | 278 (82) | 143 (81) | 135 (84) |
| Angiotensin-converting enzyme inhibitors | 574 (87) | 302 (89) | 159 (90) | 143 (88) |
| Statin use | 502 (76) | 270 (79) | 140 (79) | 130 (80) |
| β-Blocker use | 548 (83) | 308 (91) | 159 (90) | 149 (91) |
| ICD use | ||||
| At baseline | 33 (5.0) | 1 (0.3) | 1 (0.6) | 0 (0) |
| During follow-up | 114 (17.3) | 72 (21.2) | 41 (23.2) | 31 (19.0) |
Abbreviations: bpm, beats per minute; CABG, coronary artery bypass grafting; CMR, cardiovascular magnetic resonance; ICD, implantable cardioverter defibrillator; LV, left ventricular; NA, not applicable; SVR, surgical ventricular reconstruction.
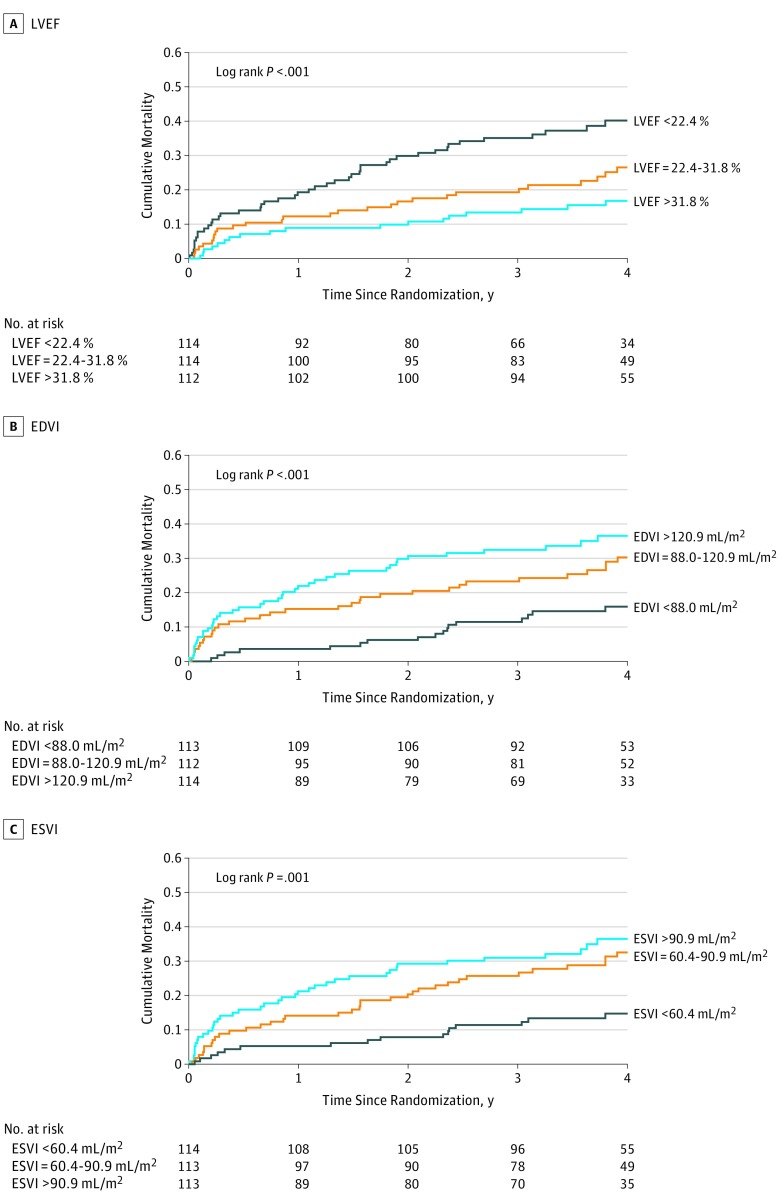
Based on Kaplan-Meier estimates, at 4 years, there were 102 deaths (28%). The Figure shows the survival curves of patients divided into tertiles of LVEF and volume indices. There was a graded increase in mortality in patients with lower EFs. Similarly, mortality was higher in patients with larger LV volume indices.
Figure. Kaplan-Meier Curves of Survival of Patients as a Function of Measures of Left Ventricular (LV) Ejection Fraction (EF), End-Diastolic Volume Index (EDVI), and End-Systolic Volume Index (ESVI) Separated by Tertiles.
We created multivariable models of survival outcomes adjusting for the effects of clinical and pharmacological variables and treatment assignment (age; sex; hypertension; diabetes; smoking; stroke; peripheral vascular disease; renal insufficiency; β-blocker, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, statin, and aspirin use; and assignment to surgical ventricular reconstruction or no surgical ventricular reconstruction groups). Adjusted for these variables, CMR EF was a strong predictor of survival (Wald χ2, 11.9; hazard ratio, 0.84 per 5%; 95% CI, 0.76-0.93; P < .001). Left ventricular end-diastolic volume index (hazard ratio, 1.11 per 10 mL/m2; 95% CI, 1.05-1.17; P < .001) and LVESVI (hazard ratio, 1.12 per 10 mL/m2; 95% CI, 1.05-1.19; P < .001) were incrementally predictive of outcomes when added individually to the model including CMR EF.
Mortality over 4 years of follow-up was 19% in those with CMR EFs greater than 30%, 34% in those with EFs of 20% to 30%, and 33% in those with an EF less than 20%. Adding CMR LVESVI to EF improved the discriminating power of CMR analysis. The 4-year mortality rates were 0% in those with an EF greater than 30% and ESVI less than 30 mL/m2, as well as 37% in those with an EF less than 20% and ESVI greater than 80 mL/m2.
Discussion
This study adds many insights into the diagnostic evaluation of patients with ischemic cardiomyopathy. Data quality is high in this prospectively studied population within the STICH clinical trial environment. This study demonstrates that the combination of CMR-determined LVEF with ESVI provides good annual mortality risk.
To our knowledge, the present study provides the largest population of patients with ischemic cardiomyopathy that has used CMR to predict surgical outcomes. In the Digitalis Investigation Group Trial, a diminishing EF was associated with an increase in mortality in a graded fashion. However, it has been suggested in other studies that there may be an inflection point at approximately 25% below which there is no apparent further reduction in mortality. Using CMR-determined LVEF in combination with LVESVI allows improvement in risk stratification in patients with severe LV dysfunction.
References
- 1.Hunt SA, Abraham WT, Chin MH, et al. ; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Rhythm Society . ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154-e235. [DOI] [PubMed] [Google Scholar]
- 2.Velazquez EJ, Lee KL, O’Connor CM, et al. ; STICH Investigators . The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) Trial. J Thorac Cardiovasc Surg. 2007;134(6):1540-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones RH, Velazquez EJ, Michler RE, et al. ; STICH Hypothesis 2 Investigators . Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360(17):1705-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velazquez EJ, Lee KL, Deja MA, et al. ; STICH Investigators . Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364(17):1607-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hallstrom A, Pratt CM, Greene HL, et al. Relations between heart failure, ejection fraction, arrhythmia suppression and mortality: analysis of the Cardiac Arrhythmia Suppression Trial. J Am Coll Cardiol. 1995;25(6):1250-1257. [DOI] [PubMed] [Google Scholar]
- 6.Hunt SA, Baker D, Chin MH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure); International Society for Heart and Lung Transplantation; Heart Failure Society of America . ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation. 2001;104:2996-3007. [DOI] [PubMed] [Google Scholar]