Key Points
Question
What is the prognosis of ischemic and bleeding events occurring beyond 1 year after coronary stenting?
Findings
Among 11 648 individuals who underwent randomization in the Dual Antiplatelet Therapy (DAPT) Study, ischemic events occurred in 4%, 11% died, and the cumulative incidence of death after an ischemic event was 0.5%. Bleeding events occurred in 2%, 18% died, and the cumulative incidence of death after a bleeding event was 0.3%.
Meaning
Among patients surviving 1 year after coronary stenting, ischemic events were more frequent than bleeding events, and both events were associated with high risk of mortality.
Abstract
Importance
Early cardiovascular and bleeding events after coronary stenting are associated with high risk of morbidity and mortality.
Objective
To assess the prognosis of cardiovascular and bleeding events occurring beyond 1 year after coronary stenting.
Design, Setting, and Participants
This secondary analysis is derived from data from the Dual Antiplatelet Therapy (DAPT) Study, a multicenter trial involving 220 US and international clinical sites from 11 countries. The study dates were August 2009 to May 2014. Individuals who underwent coronary stenting and completed 12 months of thienopyridine plus aspirin therapy without ischemic or bleeding events remained on an aspirin regimen and were randomized to continued thienopyridine therapy vs placebo for 18 additional months. Individuals were then followed up for 3 additional months while receiving aspirin therapy alone. The analysis began in August 2015.
Exposures
Ischemic events (myocardial infarction not related to stent thrombosis, stent thrombosis, and ischemic stroke) and bleeding events (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries [GUSTO] classification moderate or severe bleeding).
Main Outcomes and Measures
Ischemic events (myocardial infarction not related to stent thrombosis, stent thrombosis, and ischemic stroke) and bleeding events (GUSTO classification moderate or severe bleeding). Death at 21 months after randomization (33 months after coronary stenting).
Results
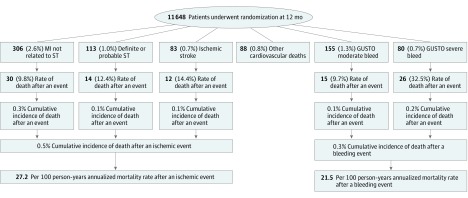
In total, 25 682 individuals older than 18 years with an indication for coronary stenting were enrolled, and 11 648 (mean age, 61.3 years; 25.1% female) were randomized. After randomization, 478 individuals (4.1%) had 502 ischemic events (306 with myocardial infarction, 113 with stent thrombosis, and 83 with ischemic stroke), and 232 individuals (2.0%) had 235 bleeding events (155 with moderate and 80 with severe bleeding). Among individuals with ischemic events, 52 (10.9%) died. The annualized mortality rate after an ischemic event was 27.2 (95% CI, 20.3-35.7) per 100 person-years. The cumulative incidence of death after an ischemic event among the total randomized study population was 0.5% (0.3% with myocardial infarction, 0.1% with stent thrombosis, and 0.1% with ischemic stroke). Among individuals with bleeding events, 41 (17.7%) died. The annualized mortality rate after a bleeding event was 21.5 (95% CI, 15.4-29.1) per 100 person-years. The cumulative incidence of death after a bleeding event among the total randomized study population was 0.3% (0.1% with moderate and 0.2% with severe bleeding).
Conclusions and Relevance
In patients treated with dual antiplatelet therapy for at least 1 year after coronary stenting, ischemic events were more frequent than bleeding events, and both events were associated with high risk of mortality.
Trial Registration
clinicaltrials.gov Identifier: NCT00977938
This randomized clinical trial assesses the prognosis of cardiovascular and bleeding events occurring beyond 1 year after coronary stenting.
Introduction
Advances in the management of patients with coronary artery disease undergoing percutaneous coronary intervention (PCI) have reduced cardiovascular events around the time of the procedure and within the first year after PCI. However, the risk of recurrent ischemia related to atherosclerosis progression and thrombosis in stented and nonstented coronary arteries persists indefinitely, despite treatment and risk factor modification. Antiplatelet medications, including aspirin and P2Y12 inhibitors, have become the standard of care as they decrease the risk of myocardial infarction (MI) and stent thrombosis (ST) within the first year after PCI. While recent randomized trials have shown the benefits of treatment beyond 1 year, these findings have been counterbalanced by risks of bleeding, which are of particular concern with long-term therapy.
Although the prognosis following early ischemic and bleeding events has been well described, data for events occurring beyond 1 year after PCI are limited. This scarcity may result from smaller size and duration of PCI trials, while larger observational studies often lack systematic follow-up or independent event adjudication.
We sought to assess the cumulative incidence of death after ischemic and bleeding events occurring among patients in the Dual Antiplatelet Therapy (DAPT) Study beyond 1 year after coronary stenting.
Methods
Study Population
The DAPT Study (described previously) was a randomized, double-blind trial comparing 12 vs 30 months of dual antiplatelet therapy in individuals undergoing PCI with a drug-eluting stent or a bare metal stent. The study involved 220 US and international clinical sites from 11 countries. After coronary stenting, 25 682 enrolled patients older than 18 years were prescribed thienopyridine plus aspirin therapy for 12 months. At 12 months, treatment-adherent patients who had no major adverse cardiovascular or cerebrovascular events, recurrent revascularization, or moderate or severe bleeding continued taking aspirin and were randomized to continued thienopyridine therapy vs placebo for 18 additional months. Randomized treatment was then discontinued, and patients were followed up for 3 additional months while receiving aspirin therapy alone. During the 21-month postrandomization period, all potential cardiovascular and bleeding events were adjudicated by a clinical events committee masked to treatment assignment. This study was conducted in accordance with the Declaration of Helsinki. The institutional review board at each institution approved the study, and all participating patients provided written informed consent. For the present secondary analysis, we examined all 11 648 randomized individuals.
Outcomes and Exposures
The primary outcome was death at 21 months after randomization (33 months after coronary stenting). The primary exposures were ischemic and bleeding events occurring 12 to 33 months after coronary stenting. Ischemic events included MI not related to ST, definite or probable ST as defined by the Academic Research Consortium, and ischemic stroke. While cardiovascular death as adjudicated in the DAPT Study included deaths that were associated with a concurrent ischemic event and deaths that could not be attributed to a noncardiovascular cause, only cardiovascular deaths related to MI, ST, or ischemic stroke were considered ischemic events in the present study. Bleeding events included moderate or severe bleeding as defined by the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries (GUSTO) classification. As a secondary bleeding end point, bleeding events were classified based on the Bleeding Academic Research Consortium (BARC) 2, 3, or 5 definitions. Intracranial hemorrhage (ICH) was classified as a BARC 3c bleeding event or hemorrhagic stroke as defined previously.
Statistical Analysis
Categorical variables were reported as counts and percentages, and continuous variables were reported as means (SDs). Between-group differences were assessed using Fisher exact test or χ2 test for categorical variables and t test or Wilcoxon rank sum test for continuous variables.
Among the randomized study population, we examined (1) the rate of ischemic and bleeding events after randomization, (2) the mortality risk among patients with each of these events, and (3) the cumulative incidence of mortality after each event. Patients experiencing multiple ischemic and bleeding events contributed data to both the ischemic events group and the bleeding events group; however, for patients with more than one of the same type of event, only the earliest event was included. We used Kaplan-Meier methods to estimate the cumulative incidence of death after an ischemic or bleeding event among those experiencing these events. Follow-up started at the time of the event and continued until death or study completion. Patients who died on the same day as the event were included in the analysis. To quantify the total population at risk of death after an event, we used Kaplan-Meier methods to estimate the cumulative incidence of death after the event among all randomized patients, with follow-up starting at the time of randomization.
Cox proportional hazards regression models with time-dependent covariates for exposure status were created for each exposure to evaluate the independent association of ischemic events or bleeding events with mortality. The time-updated model allowed for patients to contribute both unexposed (ie, before event) and exposed (ie, after event) person-time. A propensity score was calculated for each ischemic and bleeding event using all collected covariates, including the randomization arm, and each Cox proportional hazards regression model was stratified by propensity score quintile to control for confounding.
A two-sided P < .05 was considered statistically significant. Statistical analyses were performed using a software program (SAS, version 9.3; SAS Institute Inc).
Results
Incidence and Mortality Risk of Ischemic Events
Among 11 648 randomized patients (mean age, 61.3 years; 25.1% female), 478 (4.1%) had 502 ischemic events by 21 months. Of these 502 events, 306 (61.0%) were MI not related to ST, 113 (22.5%) were ST, and 83 (16.5%) were ischemic stroke. In addition, 88 patients had cardiovascular deaths that were not due to MI, ST, or ischemic stroke. Of these cardiovascular deaths, 63 (71.6%) had identifiable causes, including sudden cardiac death (n = 40), heart failure (n = 9), ventricular arrhythmia (n = 4), and other events (n = 10).
Patients with ischemic events had a higher prevalence of cardiovascular risk factors and prior cardiovascular disease (Table 1). They also more often initially presented with non–ST-segment elevation MI, had smaller minimum stent diameters, and received more paclitaxel-eluting stents (Table 2). Patients with ischemic events were more often randomized to placebo (283 patients [59.2%]) than to continued thienopyridine therapy (195 patients [40.8%]) (P < .01).
Table 1. Baseline Characteristics at Index Coronary Stenting by Ischemic and Bleeding Event Statusa.
| Variable | No./Total No. (%) | |||||
|---|---|---|---|---|---|---|
| By Ischemic Event Status | By Bleeding Event Status | |||||
| Ischemic Event (n = 478) |
No Ischemic Event (n = 11 170) |
P Value | Bleeding Event (n = 232) |
No Bleeding Event (n = 11 416) |
P Value | |
| Age, mean (SD), y | 62.2 (10.4) | 61.3 (10.3) | .05 | 66.6 (10.3) | 61.2 (10.3) | <.01 |
| Male | 345/478 (72.2) | 8378/11 170 (75.0) | .16 | 160/232 (69.0) | 8563/11 416 (75.0) | .04 |
| Race | ||||||
| White | 421/469 (89.8) | 10 024/10 961 (91.5) | .21 | 210/229 (91.7) | 10 235/11 201 (91.4) | >.99 |
| African American | 30/469 (6.4) | 545/10 961 (5.0) | .16 | 8/229 (3.5) | 567/11 201 (5.1) | .36 |
| Hispanic or Latino | 25/473 (5.3) | 381/10 959 (3.5) | .04 | 10/230 (4.4) | 396/11 202 (3.5) | .47 |
| BMI, mean (SD) | 30.1 (5.6) | 30.4 (5.7) | .19 | 29.6 (5.2) | 30.4 (5.7) | .02 |
| Diabetes mellitus | 191/475 (40.2) | 3200/11 126 (28.8) | <.01 | 74/231 (32.0) | 3317/11 370 (29.2) | .34 |
| Hypertension | 376/478 (78.7) | 8146/11 136 (73.2) | <.01 | 197/232 (84.9) | 8325/11 382 (73.1) | <.01 |
| Tobacco use | 153/469 (32.6) | 2989/11 009 (27.2) | .01 | 41/230 (17.8) | 3101/11 248 (27.6) | <.01 |
| Stroke or TIA | 27/475 (5.7) | 374/11 143 (3.4) | .01 | 18/227 (7.9) | 383/11 391 (3.4) | <.01 |
| Congestive heart failure | 47/475 (9.9) | 477/11 133 (4.3) | <.01 | 18/230 (7.8) | 506/11 378 (4.5) | .02 |
| LVEF<30% | 17/449 (3.8) | 190/10 266 (1.9) | <.01 | 6/213 (2.8) | 201/10 502 (1.9) | .31 |
| Renal disease | 35/472 (7.4) | 433/11 137 (3.9) | <.01 | 21/230 (9.1) | 447/11 379 (3.9) | <.01 |
| Peripheral arterial disease | 46/469 (9.8) | 603/10 995 (5.5) | <.01 | 31/227 (13.7) | 618/11 237 (5.5) | <.01 |
| Prior PCI | 201/477 (42.1) | 3167/11 126 (28.5) | <.01 | 88/232 (37.9) | 3280/11 371 (28.9) | <.01 |
| Prior CABG | 81/478 (17.0) | 1168/11 146 (10.5) | <.01 | 34/232 (14.7) | 1215/11 392 (10.7) | .07 |
| Prior myocardial infarction | 152/472 (32.2) | 2304/11 004 (20.9) | <.01 | 50/229 (21.8) | 2406/11 247 (21.4) | .87 |
| Atrial fibrillation | 17/477 (3.6) | 323/11 118 (2.9) | .40 | 12/231 (5.2) | 328/11 364 (2.9) | .05 |
| History of cancer | 47/472 (10.0) | 1023/11 085 (9.2) | .57 | 37/229 (16.2) | 1033/11 328 (9.2) | <.01 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass graft; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
For most variables, 0% to 2% of the patients had missing values; however, 7% to 8% of patients were missing data on LVEF.
Table 2. Procedural and Lesion Characteristics at Index Coronary Stenting by Ischemic and Bleeding Event Status.
| Variable | By Ischemic Event Status | By Bleeding Event Status | ||||
|---|---|---|---|---|---|---|
| Ischemic Event (n = 478) |
No Ischemic Event (n = 11 170) |
P Value | Bleeding Event (n = 232) |
No Bleeding Event (n = 11 416) |
P Value | |
| PCI indication, No. (%) | ||||||
| STEMI | 66 (13.8) | 1614 (14.4) | .74 | 22 (9.5) | 1658 (14.5) | .03 |
| NSTEMI | 103 (21.5) | 1793 (16.1) | <.01 | 31 (13.4) | 1865 (16.3) | .24 |
| Stable angina | 148 (31.0) | 4001 (35.8) | .03 | 82 (35.3) | 4067 (35.6) | >.99 |
| Unstable angina | 74 (15.5) | 1747 (15.6) | >.99 | 39 (16.8) | 1782 (15.6) | .59 |
| Other | 87 (18.2) | 2015 (18.0) | .90 | 58 (25.0) | 2044 (17.9) | <.01 |
| Randomization group, No. (%) | ||||||
| Placebo | 283 (59.2) | 5503 (49.3) | <.01 | 92 (39.7) | 5694 (49.9) | <.01 |
| Continued thienopyridine therapy | 195 (40.8) | 5667 (50.7) | 140 (60.3) | 5722 (50.1) | ||
| Type of stent, No. (%) | ||||||
| Nonpaclitaxel drug eluting | 249 (52.1) | 6836 (61.2) | <.01 | 150 (64.7) | 6935 (60.7) | .25 |
| Paclitaxel eluting | 155 (32.4) | 2511 (22.5) | <.01 | 50 (21.6) | 2616 (22.9) | .62 |
| Bare metal stent | 61 (12.8) | 1626 (14.6) | .31 | 24 (10.3) | 1663 (14.6) | .07 |
| >1 Drug-eluting stent type | 13 (2.7) | 197 (1.8) | .15 | 8 (3.4) | 202 (1.8) | .06 |
| No. of stents, mean (SD) | 1.5 (0.8) | 1.4 (0.7) | .05 | 1.4 (0.7) | 1.4 (0.7) | .40 |
| Minimum stent diameter, mm, No. (%) | ||||||
| <2.5 | 44 (9.2) | 715 (6.4) | .02 | 16 (6.9) | 743 (6.5) | .81 |
| 2.5-3 | 216 (45.2) | 4066 (36.4) | <.01 | 86 (37.1) | 4196 (36.8) | .92 |
| >3 | 218 (45.6) | 6389 (57.2) | <.01 | 130 (56.0) | 6477 (56.7) | .83 |
| Total stent length, mean (SD), mm | 28.5 (16.9) | 27.0 (16.4) | .04 | 25.9 (14.9) | 27.1 (16.5) | .25 |
| Treated vessel, No./total No. (%)a | ||||||
| Left main | 4/627 (0.6) | 107/14 332 (0.7) | >.99 | 2/300 (0.7) | 109/14 659 (0.7) | >.99 |
| Left anterior descending | 245/627 (39.1) | 5670/14 332 (39.6) | .84 | 121/300 (40.3) | 5794/14 659 (39.5) | .81 |
| Right | 192/627 (30.6) | 4907/14 332 (34.2) | .06 | 100/300 (33.3) | 4999/14 659 (34.1) | .81 |
| Circumflex | 151/627 (24.1) | 3241/14 332 (22.6) | .38 | 64/300 (21.3) | 3328/14 659 (22.7) | .63 |
| Venous graft | 31/627 (4.9) | 345/14 332 (2.4) | <.01 | 10/300 (3.3) | 366/14 659 (2.5) | .35 |
| Arterial graft | 4/627 (0.6) | 62/14 332 (0.4) | .36 | 3/300 (1.0) | 63/14 659 (0.4) | .15 |
| Modified ACC/AHA lesion class B2 or C, No./total No. (%) | 240/458 (52.4) |
5056/10 752 (47.0) | .03 | 108/229 (47.2) | 5188/10 981 (47.2) | .98 |
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
In total, 14 959 lesions were treated in the study population.
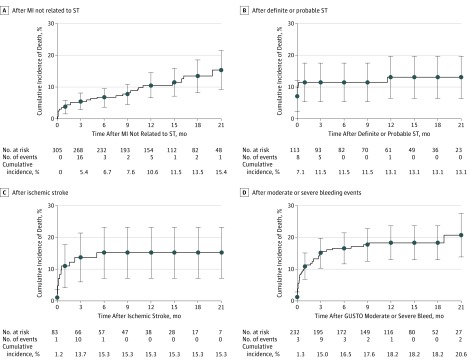
The median follow-up time after an ischemic event was 275.0 days (interquartile range [IQR], 90.0-456.0 days). Among the 478 patients with ischemic events, 52 (10.9%) died, and 41 deaths (78.8%) were attributable to cardiovascular causes. In comparison, of the 11 082 patients without a cardiovascular event, 82 (0.7%) died (P < .001). The cumulative incidence of death after an ischemic event among the total randomized study population was 0.5% (0.3% with MI, 0.1% with ST, and 0.1% with ischemic stroke) (Figure 1). The cumulative incidence of death for all cardiovascular events, including cardiovascular deaths that were not due to MI, ST, or ischemic stroke, was 1.3%. Death after ST or ischemic stroke primarily occurred within 1 month of presentation, whereas death after MI not related to ST occurred throughout the follow-up period (median survival time, 67.5 days; IQR, 4.0-281.0 days) (Figure 2). Nine ischemic events were immediately fatal, which occurred mainly among patients with ST (n = 8).
Figure 1. Frequency of Events and Death Among Randomized Patients in the Dual Antiplatelet Therapy (DAPT) Study.
Shown are cumulative rates of cardiovascular and bleeding events and subsequent risk of death. The number of each event represents the number of patients experiencing the event. Multiple types of events could be experienced by the same patient. For patients with more than one of the same type of event, only the earliest event contributed to the analysis. Other cardiovascular deaths were defined as any cardiovascular death not preceded by a myocardial infarction (MI), stent thrombosis (ST), or ischemic stroke. These events primarily consisted of sudden cardiac deaths (n = 40), heart failure–related deaths (n = 9), and fatal ventricular arrhythmias (n = 4). GUSTO indicates Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries.
Figure 2. Cumulative Incidence of Death After Ischemic and Bleeding Events.
Shown is the cumulative incidence of death based on Kaplan-Meier estimates. MI indicates myocardial infarction; ST, stent thrombosis.
The unadjusted annualized mortality rate after an ischemic event was 27.2 (95% CI, 20.3-35.7) per 100 person-years. Controlling for demographic characteristics, comorbid conditions, and procedural factors, the occurrence of an ischemic event was associated with a 12.6-fold (95% CI, 9.0-17.6) increased hazard of mortality compared with the absence of such event. Risks of death associated with ST (hazard ratio [HR], 14.6; 95% CI, 8.2-25.9) and ischemic stroke (HR, 13.1; 95% CI, 7.2-23.8) were greater in magnitude compared with MI not related to ST (HR, 9.1; 95% CI, 6.1-13.6) (Table 3). Risks of death within the first 30 days after an ischemic event and more than 30 days after the event are summarized in eTable 1 in the Supplement.
Table 3. Risk of Mortality After Ischemic and Bleeding Events During the 21-Month Postrandomization Period.
| Variable | Adjusted HR (95% CI) for Mortalitya |
|---|---|
| Ischemic events | |
| MI not related to ST | 9.1 (6.1-13.6) |
| Definite or probable ST | 14.6 (8.2-25.9) |
| Ischemic stroke | 13.1 (7.2-23.8) |
| Any MI, definite or probable ST, or ischemic stroke | 12.6 (9.0-17.6) |
| Bleeding events | |
| GUSTO moderate bleed | 8.0 (4.7-13.7) |
| GUSTO severe bleed | 36.3 (23.3-56.6) |
| GUSTO moderate or severe bleed | 18.1 (12.6-26.0) |
| BARC 2, 3, or 5 bleed | 9.3 (6.6-13.1) |
| BARC 2 or 3 bleed | 5.7 (3.8-8.4) |
| BARC 3 or 5 bleed | 16.2 (11.2-23.5) |
| BARC 2 bleed | 3.4 (1.9-6.1) |
| BARC 3 bleed | 8.6 (5.5-13.4) |
Abbreviations: BARC, Bleeding Academic Research Consortium; GUSTO, Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries; HR, hazard ratio; MI, myocardial infarction; ST, stent thrombosis.
Incidence and Mortality Risk of Bleeding Events
Among randomized patients, 232 (2.0%) had 235 bleeding events. Of all bleeding events, 155 (66.0%) were moderate, and 80 (34.0%) were severe. Intracranial hemorrhage occurred in 47 of 11 648 (0.4%), of whom 23 (0.4%) were randomized to continued thienopyridine therapy and 24 (0.4%) were randomized to placebo (P = .88).
Patients with bleeding events had lower body mass index, were older, and demonstrated a higher prevalence of cardiovascular risk factors and prior cardiovascular disease (Table 1). They also had higher rates of prior cancer, were less likely to have initially presented with non–ST-segment elevation MI at the time of PCI, and were more often randomized to continued thienopyridine therapy (140 [60.3%]) than to placebo (92 [39.7%]) (P < .01) (Table 2).
The median follow-up time after a bleeding event was 281.5 days (IQR, 98.5-445.0 days). Among 232 patients with a bleeding event, 41 deaths (17.7%) occurred, whereas among 11 416 patients without a bleeding event, 181 deaths (1.6%) occurred (P < .001). Of deaths after a bleeding event, 22 (53.7%) were attributable to cardiovascular causes (9 related to MI, ST, or ischemic stroke). The frequency of death after a moderate bleeding event was 9.7% and after a severe bleeding event was 32.5% (Figure 1). The cumulative incidence of death after a bleeding event was 0.3% (0.1% with moderate and 0.2% with severe bleeding). Results according to BARC definitions are summarized in eTable 2 in the Supplement. Of 47 patients with ICH, 14 (29.8%) died, with a cumulative incidence of death after an ICH of 0.1%. Death after moderate or severe bleeding occurred predominantly within 1 month of the event (Figure 2), with a median survival time of 16.0 days (IQR, 3.0-81.0 days). Three of the 235 bleeding events were immediately fatal.
The unadjusted annualized mortality rate after a moderate or severe bleeding event was 21.5 (95% CI, 15.4-29.1) per 100 person-years. Controlling for demographic characteristics, comorbid conditions, and procedural factors, a bleeding event was associated with an 18.1-fold (95% CI, 12.6-26.0) increased hazard for death compared with the absence of a bleeding event. There was a greater hazard of death after severe bleeding events (HR, 36.3; 95% CI, 23.3-56.6) compared with moderate (HR, 8.0; 95% CI, 4.7-13.7) bleeding events. Similar results were obtained when applying BARC bleeding definitions (Table 3). Risks of death within the first 30 days after a bleeding event and more than 30 days after the event are summarized in eTable 1 in the Supplement.
Incidence and Mortality Risk Among Patients With Multiple Ischemic or Bleeding Events
Among randomized patients, 101 (0.9%) had multiple events, whereas 558 (4.8%) had a single event. Of those with multiple events, 41 (0.4%) had more than 1 ischemic event, 9 (0.1%) had more than 1 bleeding event, and 51 (0.4%) experienced both ischemic and bleeding events. Among patients with both events, 24 (47.1%) had an ischemic event first, 14 (27.4%) had a bleeding event first, and 13 (25.5%) had both ischemic and bleeding events at the time of presentation. Patients with multiple events had a mortality rate of 23.8% compared with 9.3% among those with a single event (P < .01). Mortality among patients with more than 1 ischemic event was 12.2%, among those with more than 1 bleeding event was 22.2%, and among those with both ischemic and bleeding events was 33.3%.
Discussion
This post hoc analysis of randomized patients from the DAPT Study is the largest to date to examine the incidence and prognosis of very late ischemic and bleeding events after coronary stenting. From this analysis, we note the following observations. Among treatment-adherent patients who were event free for 1 year after coronary stenting, ischemic and bleeding events occurring between 12 and 33 months were associated with high risk of mortality. Ischemic events were more common than bleeding events, and death after either category of event accounted for an elevated mortality risk (27.2 deaths for ischemic events and 21.5 deaths for bleeding events per 100 person-years of follow-up, respectively). The risk of death after MI not related to ST was sustained throughout follow-up, whereas the mortality risk after bleeding events was greatest within 30 days of the event. A minority of randomized patients (0.9%) experienced more than 1 ischemic or bleeding event, and mortality was increased among patients with multiple events compared with a single event (23.8% vs 9.3%). The most common cause of death after an ischemic event or a bleeding event was cardiovascular. Overall, the cumulative incidence of death after an ischemic event was 0.5%, and the cumulative incidence of death after a bleeding event was 0.3%.
This study broadens our understanding of the clinical effect of ischemic and bleeding events by focusing on adjudicated events occurring beyond 1 year after coronary stenting. While a diverse group of patients was enrolled in the DAPT Study, including patients with or without MI at the time of the index procedure, randomization was limited to those patients who were treatment adherent and event free during the first year of follow-up. Despite this restriction, it is noteworthy that ischemic and bleeding events occurring beyond 1 year were associated with substantial mortality risk.
We observed a ratio of ischemic events to bleeding events of 2.1:1 and a ratio of MIs to bleeding events of 1.3:1. These findings are similar to the ratio of events that occurred among this study population in the first 12 months after enrollment in the DAPT Study. Other studies examining late events after MI or PCI have found comparable ratios of cardiovascular events to bleeding events, whereas analyses that included earlier events have demonstrated higher rates of bleeding. Prior analyses have also demonstrated that cardiovascular and bleeding events may differ depending on the timing of initiation of dual antiplatelet therapy.
In our analysis, very late ischemic events and very late bleeding events were associated with high hazards of mortality, even after adjusting for measured patient factors. Significant mortality risks have been similarly observed for events occurring earlier after PCI. Moderate bleeding, the most frequent bleeding event in the present study, was associated with a risk of mortality similar to that of MI as seen in prior analyses and had mortality risks comparable to those events occurring around the time of the procedure and within the first year after PCI. Conversely, severe (including fatal) bleeding occurred less commonly herein but was associated with a grave risk of death. Patients infrequently experienced ICH (0.4%), and rates of events occurred equally between randomized groups.
Of all ischemic events, MI not related to ST occurred most commonly and was associated with a sustained risk of death throughout the study period. This result differed from bleeding events, which were primarily followed by death within 1 month. These temporal relationships are the inverse of those seen in a prior analysis that involved earlier events after acute coronary syndromes. We hypothesize that these disparate findings are largely because of differing types of events. Bleeding events in the present analysis were mainly nonprocedure-related spontaneous bleeds, which have been associated with higher short-term mortality compared with procedure-related bleeding. Spontaneous MIs unrelated to the stented artery constituted most ischemic events in our study rather than periprocedural or ST-related MIs. Unlike spontaneous MIs, ST was associated with immediate mortality risk. The sustained risk associated with spontaneous MI may occur via the development of risk factors for future mortality, such as left ventricular dysfunction and arrhythmias, risks expected to persist beyond the time frame of the present study.
Few patients had both ischemic and bleeding events, which highlights the need to adapt dual antiplatelet therapy duration according to individual patient characteristics. A prediction rule for patient benefit and harm has been developed from the present study population and may be useful in optimizing care for individual patients.
Strengths and Limitations
This analysis has several strengths. It included patients with a range of comorbidities and procedural complexity. In addition, all events and outcomes were independently adjudicated. However, the results must also be interpreted in the context of the study design. Patients with a history of significant bleeding or treated with oral anticoagulation were excluded. While ischemic events were more common than bleeding events in the first year as reasons for exclusion from randomization, our analysis is only relevant to determining the prognosis after late events occurring among treatment-adherent patients who have survived event free in the first 12 months after coronary stenting. Fortunately, most patients undergoing PCI have excellent outcomes, without ischemic or bleeding events, recurrent procedures, or treatment nonadherence during the first year of follow-up. In addition, the incidences of ischemic and bleeding events depend on the definitions used. We found similar risks of mortality whether using GUSTO or BARC definitions for bleeding events in that more severe events were associated with higher mortality and less severe events were associated with less subsequent mortality. We excluded cardiovascular deaths not adjudicated to be preceded by MI, ST, or ischemic stroke and, in doing so, excluded 40 sudden cardiac deaths from classification as ischemic events in this study. While it is possible that many of these events were related to ischemia in the absence of documented MI or ST, their exclusion provides a conservative estimate of the proportion and number of ischemic events.
Conclusions
Among patients who survived to 12 months after coronary stenting event free, ischemic events were more frequent than bleeding events, yet both events were associated with high risk of mortality. The risk of death after very late MIs was sustained, whereas death after bleeding events primarily occurred within 30 days. These findings emphasize the need to adapt therapy to individual patient characteristics to avoid very late ischemic and bleeding events among patients after coronary stenting.
eTable 1. Risk of Mortality After Ischemic and Bleeding Events During the 21-Month Post-Randomization Period, Stratified by <30 or ≥30 Days From Time of Event
eTable 2. Frequency of Bleeding Events and Death by BARC Classification
References
- 1.Baber U, Mehran R, Sharma SK, et al. Impact of the everolimus-eluting stent on stent thrombosis: a meta-analysis of 13 randomized trials. J Am Coll Cardiol. 2011;58(15):1569-1577. [DOI] [PubMed] [Google Scholar]
- 2.Wiviott SD, Braunwald E, McCabe CH, et al. ; TRITON-TIMI 38 Investigators . Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015. [DOI] [PubMed] [Google Scholar]
- 3.Stone GW, Maehara A, Lansky AJ, et al. ; PROSPECT Investigators . A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226-235. [DOI] [PubMed] [Google Scholar]
- 4.Secemsky EA, Matteau A, Yeh RW, et al. ; PROTECT Trial Investigators . Comparison of short- and long-term cardiac mortality in early versus late stent thrombosis (from pooled PROTECT trials). Am J Cardiol. 2015;115(12):1678-1684. [DOI] [PubMed] [Google Scholar]
- 5.Levine GN, Bates ER, Blankenship JC, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Society for Cardiovascular Angiography and Interventions . 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58(24):e44-e122. [DOI] [PubMed] [Google Scholar]
- 6.Windecker S, Kolh P, Alfonso F, et al. ; Authors/Task Force Members . 2014 ESC/EACTS Guidelines on Myocardial Revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35(37):2541-2619. [DOI] [PubMed] [Google Scholar]
- 7.Bonaca MP, Bhatt DL, Cohen M, et al. ; PEGASUS-TIMI 54 Steering Committee and Investigators . Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791-1800. [DOI] [PubMed] [Google Scholar]
- 8.Mauri L, Kereiakes DJ, Yeh RW, et al. ; DAPT Study Investigators . Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371(23):2155-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kereiakes DJ, Yeh RW, Massaro JM, et al. ; Dual Antiplatelet Therapy (DAPT) Study Investigators . Antiplatelet therapy duration following bare metal or drug-eluting coronary stents: the Dual Antiplatelet Therapy randomized clinical trial [published correction appears in JAMA. 2015;313(21):2185]. JAMA. 2015;313(11):1113-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chhatriwalla AK, Amin AP, Kennedy KF, et al. ; National Cardiovascular Data Registry . Association between bleeding events and in-hospital mortality after percutaneous coronary intervention. JAMA. 2013;309(10):1022-1029. [DOI] [PubMed] [Google Scholar]
- 11.Kazi DS, Leong TK, Chang TI, Solomon MD, Hlatky MA, Go AS. Association of spontaneous bleeding and myocardial infarction with long-term mortality after percutaneous coronary intervention. J Am Coll Cardiol. 2015;65(14):1411-1420. [DOI] [PubMed] [Google Scholar]
- 12.Koskinas KC, Räber L, Zanchin T, et al. Clinical impact of gastrointestinal bleeding in patients undergoing percutaneous coronary interventions. Circ Cardiovasc Interv. 2015;8(5):e002053. [DOI] [PubMed] [Google Scholar]
- 13.Généreux P, Giustino G, Witzenbichler B, et al. Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol. 2015;66(9):1036-1045. [DOI] [PubMed] [Google Scholar]
- 14.Valgimigli M, Campo G, Monti M, et al. ; Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) Investigators . Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125(16):2015-2026. [DOI] [PubMed] [Google Scholar]
- 15.Park SJ, Park DW, Kim YH, et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med. 2010;362(15):1374-1382. [DOI] [PubMed] [Google Scholar]
- 16.Collet JP, Silvain J, Barthélémy O, et al. ; ARCTIC Investigators . Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet. 2014;384(9954):1577-1585. [DOI] [PubMed] [Google Scholar]
- 17.Mauri L, Kereiakes DJ, Normand SL, et al. Rationale and design of the dual antiplatelet therapy study, a prospective, multicenter, randomized, double-blind trial to assess the effectiveness and safety of 12 versus 30 months of dual antiplatelet therapy in subjects undergoing percutaneous coronary intervention with either drug-eluting stent or bare metal stent placement for the treatment of coronary artery lesions. Am Heart J. 2010;160(6):1035-1041, 1041.e1. [DOI] [PubMed] [Google Scholar]
- 18.Cutlip DE, Windecker S, Mehran R, et al. ; Academic Research Consortium . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344-2351. [DOI] [PubMed] [Google Scholar]
- 19.GUSTO Investigations. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329(10):673-682. [DOI] [PubMed] [Google Scholar]
- 20.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747. [DOI] [PubMed] [Google Scholar]
- 21.Yeh RW, Czarny MJ, Normand SL, et al. Evaluating the generalizability of a large streamlined cardiovascular trial: comparing hospitals and patients in the Dual Antiplatelet Therapy Study versus the National Cardiovascular Data Registry. Circ Cardiovasc Qual Outcomes. 2015;8(1):96-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeh RW, Kereiakes DJ, Steg PG, et al. ; DAPT Study Investigators . Benefits and risks of extended duration dual antiplatelet therapy after PCI in patients with and without acute myocardial infarction. J Am Coll Cardiol. 2015;65(20):2211-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonaca MP, Bhatt DL, Steg PG, et al. Ischaemic risk and efficacy of ticagrelor in relation to time from P2Y12 inhibitor withdrawal in patients with prior myocardial infarction: insights from PEGASUS-TIMI 54. Eur Heart J. 2016;37(14):1133-1142. [DOI] [PubMed] [Google Scholar]
- 24.Ducrocq G, Schulte PJ, Becker RC, et al. Association of spontaneous and procedure-related bleeds with short- and long-term mortality after acute coronary syndromes: an analysis from the PLATO trial. EuroIntervention. 2015;11(7):737-745. [DOI] [PubMed] [Google Scholar]
- 25.Matteau A, Yeh RW, Camenzind E, et al. Balancing long-term risks of ischemic and bleeding complications after percutaneous coronary intervention with drug-eluting stents. Am J Cardiol. 2015;116(5):686-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehran R, Pocock SJ, Stone GW, et al. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non–ST-elevation acute coronary syndromes: a risk model from the ACUITY trial. Eur Heart J. 2009;30(12):1457-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh RW, Secemsky EA, Kereiakes DJ, et al. ; DAPT Study Investigators . Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315(16):1735-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68(10):1082-1115. [DOI] [PubMed] [Google Scholar]
- 29.Mehran R, Baber U, Steg PG, et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet. 2013;382(9906):1714-1722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Risk of Mortality After Ischemic and Bleeding Events During the 21-Month Post-Randomization Period, Stratified by <30 or ≥30 Days From Time of Event
eTable 2. Frequency of Bleeding Events and Death by BARC Classification