Key Points
Question
Is there an association between baseline levels of proprotein convertase subtilisin kexin type 9 (PCSK9) and the low-density lipoprotein cholesterol (LDL-C) level lowering achieved with evolocumab?
Findings
In this analysis of 3016 patients from 4 phase 3 randomized trials, evolocumab, 140 mg every 2 weeks and 420 mg every month, suppressed circulating PCSK9 levels by 90% to 100% within 1 week and were associated with significant and consistent reductions in LDL-C levels between 64% and 71% across quartiles of baseline PCSK9 levels.
Meaning
Regardless of baseline PCSK9 levels, the doses of evolocumab being studied in a large cardiovascular outcomes trial suppress PCSK9 levels and consistently and substantially reduce LDL-C levels.
Abstract
Importance
Levels of proprotein convertase subtilisin kexin type 9 (PCSK9) vary markedly across the population and are influenced by genetic and nongenetic factors. Evolocumab is a fully human, monoclonal antibody against PCSK9 that reduces low-density lipoprotein cholesterol (LDL-C) levels by 55% to 75%. Whether the efficacy of evolocumab varies based on an individual’s baseline PCSK9 level remains unknown.
Objective
To characterize variability in PCSK9 levels and determine whether the LDL-C level reduction achieved with evolocumab differs based on PCSK9 levels.
Design, Setting, and Participants
This study included pooled data from 3016 patients from 4 phase 3 randomized clinical trials of evolocumab as part of the Program to Reduce LDL-C and Cardiovascular Outcomes Following Inhibition of PCSK9 in Different Populations. Circulating PCSK9 levels were measured at baseline using quantitative enzyme-linked immunosorbent assays and used to stratify patients into quartiles, and LDL-C level was measured at baseline and weeks 10 and 12. In an additional 138 patients enrolled in a pharmacokinetic and pharmacodynamic substudy from 4 phase 2 trials, circulating PCSK9 levels were measured at baseline and then weekly at weeks 8 through 12.
Main Outcomes and Measures
Placebo-controlled percentage change in LDL-C level with evolocumab, 140 mg every 2 weeks and 420 mg once monthly, across quartiles of baseline PCSK9 levels.
Results
Of the 3016 patients, 1492 (49.5%) were female and 2758 (91.4%) were white. The median baseline circulating PCSK9 level was 323 ng/mL (interquartile range, 258-406 ng/mL). Patients with higher levels of PCSK9 were more likely to be receiving intensive statin therapy (56%, 36%, 25%, and 13% in the fourth through first quartiles; P < .001) and had significantly lower baseline LDL-C level (123 mg/dL, 124 mg/dL, 128 mg/dL, and 137 mg/dL in the fourth through first quartiles; P < .001). After stratifying by statin use, there was no correlation between PCSK9 levels and LDL-C levels (ρ = 0.03 [95% CI, −0.04 to 0.10] for nonstatin users, P = .39, and ρ = 0.03 [95% CI, −0.01 to 0.08] for statin users, P = .12). Across all quartiles of baseline PCSK9 levels, both evolocumab 140 mg every 2 weeks and 420 mg once monthly suppressed circulating PCSK9 levels by 90% to 100% within 1 week of administration. Both evolocumab 140 mg every 2 weeks and 420 mg once monthly were associated with significant reductions in LDL-C levels between 64% and 71% (P < .001), regardless of PCSK9 levels (P for interaction = .76 and .21, respectively).
Conclusions and Relevance
Regardless of baseline PCSK9 levels, the doses of evolocumab being studied in a large cardiovascular outcomes trial suppress PCSK9 levels and consistently and substantially reduce LDL-C levels.
This study of pooled data from patients in 4 randomized clinical trials characterizes variability in proprotein convertase subtilisin kexin type 9 (PCSK9) levels and determines whether the reduction in low-density lipoprotein cholesterol level achieved with evolocumab differs based on PCSK9 levels.
Introduction
Proprotein convertase subtilisin kexin type 9 (PCSK9) is synthesized and secreted by hepatocytes, binds to low-density lipoprotein cholesterol (LDL-C) receptor, and targets it for lysosomal degradation. Levels of PCSK9 vary markedly across the population and are influenced by such factors as genetics, clinical comorbidities, and statin therapy. Evolocumab is a fully human, monoclonal antibody against PCSK9 that reduces LDL-C level between 55% to 75% in patients with hypercholesterolemia. It was approved by the US Food and Drug Administration in 2015 based on its LDL-C level lowering, while a cardiovascular outcomes trial is under way. Whether the efficacy of evolocumab varies based on an individual’s baseline PCSK9 level, and hence whether assessment of these levels could identify individuals who may derive greater benefit, remains unknown. Accordingly, we sought to characterize variability in PCSK9 levels and then determine whether the LDL-C level reduction observed with evolocumab differs based on PCSK9 levels.
Methods
We pooled data from 3016 patients from 4 phase 3 randomized clinical trials of evolocumab as part of the Program to Reduce LDL-C and Cardiovascular Outcomes Following Inhibition of PCSK9 in Different Populations. The protocols of each trial were approved by the relevant institutional review board for each participating site and patients participated in the trials after providing informed consent as detailed in the primary publications of those trials. As part of the protocol for those trials, circulating PCSK9 levels were measured at baseline using quantitative enzyme-linked immunosorbent assays and used to stratify patients into quartiles. In brief, the lower limit of quantification of the assay is 15.0 ng/mL and the upper limit of quantification is 1200 ng/mL. The coefficient of variation is less than 20%. Baseline demographic, clinical, and background statin therapy was compared across groups using the Kruskal-Wallis tests for continuous variables and χ2 tests for categorical variables. The LDL-C level was measured at baseline and weeks 10 and 12 using the Friedewald equation unless the LDL-C level was 40 mg/dL or less (to convert to millimoles per liter, multiply by 0.0259) or triglyceride level was 400 mg/dL or greater (to convert to millimoles per liter, multiply by 0.0113), in which case it was measured by ultracentrifugation. The primary end point for this analysis, the placebo-controlled percentage change in LDL-C level, was determined for evolocumab, 140 mg every 2 weeks and 420 mg once monthly, the 2 doses approved for use, across quartiles of baseline PCSK9 level. Spearman correlation coefficients were calculated for PCSK9 and LDL-C levels at baseline as well as for the change in PCSK9 level and the change in LDL-C level at week 10. In an additional 138 patients enrolled in pharmacokinetic and pharmacodynamic substudies that were part of 4 phase 2 trials, circulating PCSK9 levels were measured at baseline and then weekly at weeks 8 through 12 for evolocumab, 140 mg every 2 weeks and 420 mg once monthly. Both 95% CIs and P values were 2-sided and a significance level of less than .05 was adopted.
Results
The median baseline circulating PCSK9 level was 323 ng/mL (minimum, 0; 25th percentile, 258; 75th percentile, 406; and maximum, 964 ng/mL) (eFigure 1 in the Supplement). Baseline characteristics across quartiles of PCSK9 level are shown in the Table. As expected, patients with higher levels of PCSK9 were more likely to be receiving intensive statin therapy (56%, 36%, 25%, and 13% in the fourth through first quartiles; P < .001) and had significantly lower baseline LDL-C levels (123 mg/dL, 124 mg/dL, 128 mg/dL, and 137 mg/dL in the fourth through first quartiles; P < .001). However, after stratifying by statin use, there was no correlation between PCSK9 and LDL-C levels (ρ = 0.03 [95% CI, −0.04 to 0.10] for nonstatin users, P = .39, and ρ = 0.03 [95% CI, −0.01 to 0.08] for statin users; P = .12) (eFigure 2 in the Supplement).
Table. Baseline Characteristics of Patients Across Quartiles of Baseline PCSK9 Level.
| Characteristic | Baseline PCSK9 Quartile, No. (%) | P Value for Trend | |||
|---|---|---|---|---|---|
| First ≤258 ng/mL (n = 759) |
Second >258-≤323 ng/mL (n = 758) |
Third >323-≤406 ng/mL (n = 748) |
Fourth >406 ng/mL (n = 751) |
||
| Age, median (quartile 1-quartile 3), y | 59 (49-67) | 60 (51-66) | 59 (52-66) | 59 (51-65) | .79 |
| Female | 345 (46) | 393 (52) | 344 (46) | 410 (55) | <.001 |
| White | 677 (89) | 690 (91) | 692 (92) | 699 (93) | .03 |
| Hypertension | 344 (45) | 396 (52) | 384 (51) | 362 (48) | .03 |
| Current smoker | 103 (14) | 98 (13) | 98 (13) | 124 (16) | .15 |
| Type 2 diabetes | 83 (11) | 87 (12) | 108 (14) | 85 (11) | .13 |
| Statin | 356 (47) | 523 (69) | 626 (84) | 686 (91) | <.001 |
| Intensive statin | 96 (13) | 187 (25) | 272 (36) | 420 (56) | <.001 |
| Cholesterol, mean (SD), mg/dL | |||||
| LDL-C | 137 (45) | 128 (48) | 124 (49) | 123 (50) | <.001 |
| HDL-C | 55 (17) | 54 (16) | 53 (16) | 54 (17) | .59 |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin kexin type.
SI conversion factor: to convert high- and low-density lipoprotein cholesterol to millimoles per liter, multiply by 0.0259.
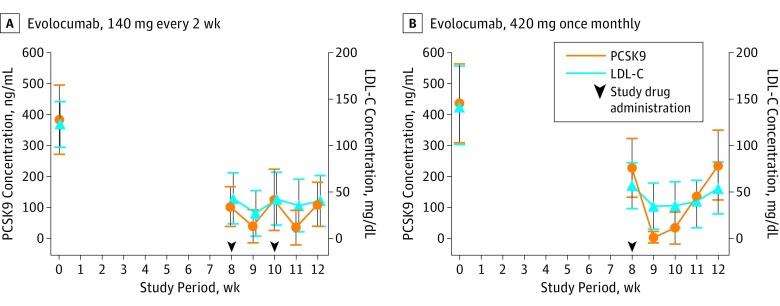
In patients who participated in the pharmacokinetic and pharmacodynamics substudies, evolocumab, 140 mg every 2 weeks, decreased PCSK9 levels by more than 90% to approximately 50 ng/mL 1 week after dosing (Figure 1A). Evolocumab, 420 mg once monthly, rendered PCSK9 levels undetectable 1 week after dosing and levels remained reduced by more than 90% at 2 weeks (Figure 1B). The PCSK9 levels started to return to baseline more rapidly after the 140-mg dose than with the 420-mg dose. Every 2-week dosing of the former kept peak PCSK9 levels at approximately one-quarter of baseline (approximately 100-125 ng/mL) (Figure 1A). For 420 mg once monthly, PCSK9 levels remained low (approximately 150 ng/mL) 3 weeks after dose and, by 4 weeks, were still reduced by approximately half (Figure 1B). The LDL-C level changes followed after PCSK9 level changes (Figure 1).
Figure 1. Levels of Circulating Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) and Low-Density Lipoprotein Cholesterol (LDL-C) Level.
Levels of circulating PCSK9 and LDL-C over time in 138 patients in the pharmacokinetic and pharmacodynamic substudy treated with evolocumab, 140 mg every 2 weeks (A) or 420 mg monthly (B). The error bars represent the 95% CIs. The arrowheads represent administration of evolocumab.
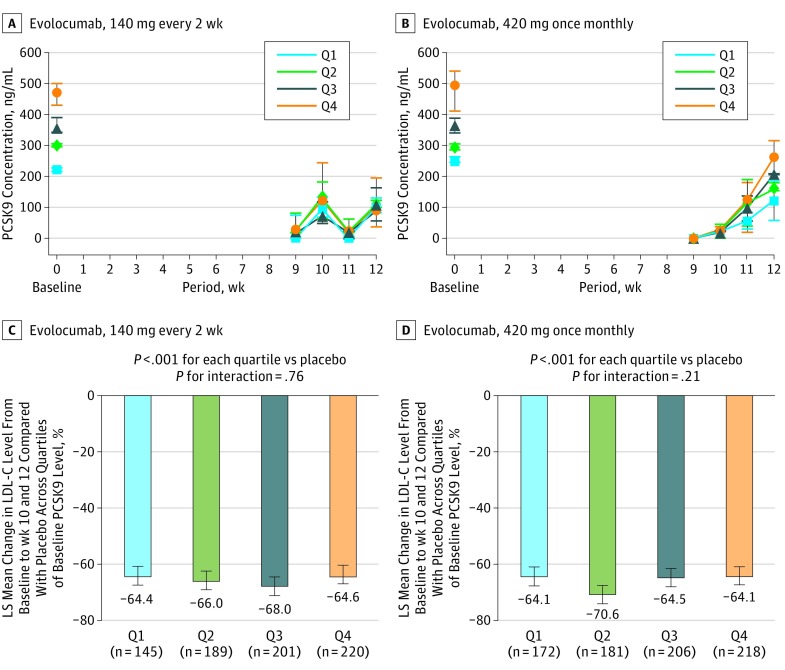
Across all quartiles of baseline PCSK9 levels, both evolocumab 140 mg every 2 weeks and 420 mg once monthly suppressed circulating PCSK9 levels within 1 week of administration by 90% to 100% (Figure 2A and B). Both evolocumab 140 mg every 2 weeks and 420 mg once monthly were associated with significant reductions in LDL-C levels between 64% and 71% (P < .001), regardless of baseline PCSK9 levels (P for interaction = .76 and .21, respectively) (Figure 2C and D). There was no meaningful correlation between change in PCSK9 level and percentage change in LDL-C level (ρ = 0.27; 95% CI, 0.23-0.32).
Figure 2. Levels of Circulating Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) and Placebo-Controlled Percentage Reductions in Low-Density Lipoprotein Cholesterol (LDL-C) Stratified by Baseline PCSK9 Level.
Levels of circulating PCSK9 over time in 138 patients in the pharmacokinetic and pharmacodynamic substudy treated with evolocumab 140 mg every 2 weeks (A) or 420 mg monthly (B), stratified by quartile of baseline PCSK9 level. Placebo-controlled, percentage reduction in LDL-C in 3016 patients treated with evolocumab 140 mg every 2 weeks (C) or 420 mg monthly (D), stratified by quartile of baseline PCSK9 level. The error lines represent 95% CIs. LS indicates least squares.
Discussion
Our analysis of baseline PCSK9 levels and LDL-C level reductions with evolocumab demonstrates that despite marked variation in baseline PCSK9 levels, evolocumab 140 mg every 2 weeks and 420 mg once monthly suppressed PCSK9 levels by at least 90% within 1 week of administration and significantly and consistently reduced LDL-C levels by approximately 65%.
We observed substantial variation in baseline PCSK9 levels consistent with prior studies noting that PCSK9 expression is affected by genetics, demographics, and statin therapy. While we found that patients with higher levels of baseline PCSK9 tended to be receiving high-intensity statin therapy and have lower levels of baseline LDL-C, after stratifying by statin use, there was no correlation between PCSK9 and LDL-C levels at baseline. There was no meaningful correlation between change in PCSK9 level and percentage change in LDL-C level. The relationship between PCSK9 level and LDL-C level reduction is likely tied to the steady-state expression of the LDL receptor and how much LDL receptor reserve is present to be brought to the hepatocyte surface when PCSK9 is fully suppressed.
In prior studies of patients with familial and nonfamilial hypercholesterolemia, as well as patients intolerant to statin therapy, evolocumab has been shown to significantly reduce LDL-C levels. In post hoc analyses of patients who successfully completed a phase 2 or 3 clinical trial and were enrolled in a long-term extension study, evolocumab, as compared with placebo, was associated with a 53% reduction in the rate of major adverse cardiovascular events. Large cardiovascular outcomes trials to definitively assess the efficacy and safety of PCSK9 inhibition are under way for evolocumab and for alirocumab.,
Given the mechanism of action of evolocumab and the variation in PCSK9 levels in the population, we tested whether there was heterogeneity in LDL-C reduction with evolocumab across quartiles of baseline PCSK9 level. Such a finding would have supported “biomarker-guided” therapy, tailoring therapy based on PCSK9 levels. Our results, however, demonstrate that evolocumab 140 mg every 2 weeks and 420 mg once monthly, the 2 doses being studied in the ongoing large cardiovascular outcomes trial, suppress PCSK9 levels over the interdose interval and lead to substantial and consistent reductions in LDL-C levels regardless of baseline PCSK9 levels. These data, taken together with previous data demonstrating similar LDL-C level reduction regardless of background lipid-lowering therapy and across clinical and sociodemographic subgroups, suggest that both doses of evolocumab will be associated with consistent LDL-C percentage reduction. Thus, instead of looking to biomarkers or clinical subgroups as a mechanism to selectively apply PCSK9 inhibition, clinicians may best apply therapy cognizant of the fact that the absolute reduction in LDL-C level is associated with relative reductions in cardiovascular events and absolute reductions in cardiovascular events are contingent on the underlying risk.
eFigure 1. Distribution of Baseline PCSK9 Levels.
eFigure 2. Correlation of Baseline PCSK9 Levels and Baseline LDL-C.
References
- 1.Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. 2009;94(7):2537-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almontashiri NA, Vilmundarson RO, Ghasemzadeh N, et al. Plasma PCSK9 levels are elevated with acute myocardial infarction in two independent retrospective angiographic studies. PLoS One. 2014;9(9):e106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awan Z, Seidah NG, MacFadyen JG, et al. Rosuvastatin, proprotein convertase subtilisin/kexin type 9 concentrations, and LDL cholesterol response: the JUPITER Trial. Clin Chem. 2012;58(1):183-189. [DOI] [PubMed] [Google Scholar]
- 4.Giugliano RP, Desai NR, Kohli P, et al. ; LAPLACE-TIMI 57 Investigators . Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380(9858):2007-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blom DJ, Hala T, Bolognese M, et al. ; DESCARTES Investigators . A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370(19):1809-1819. [DOI] [PubMed] [Google Scholar]
- 6.Koren MJ, Lundqvist P, Bolognese M, et al. ; MENDEL-2 Investigators . Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2531-2540. [DOI] [PubMed] [Google Scholar]
- 7.Robinson JG, Nedergaard BS, Rogers WJ, et al. ; LAPLACE-2 Investigators . Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 2014;311(18):1870-1882. [DOI] [PubMed] [Google Scholar]
- 8.Stroes E, Colquhoun D, Sullivan D, et al. ; GAUSS-2 Investigators . Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. [DOI] [PubMed] [Google Scholar]
- 9.Raal FJ, Stein EA, Dufour R, et al. ; RUTHERFORD-2 Investigators . PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):331-340. [DOI] [PubMed] [Google Scholar]
- 10.Colbert A, Umble-Romero A, Prokop S, Xu R, Gibbs J, Pederson S. Characterization of a quantitative method to measure free proprotein convertase subtilisin/kexin type 9 in human serum. MAbs. 2014;6(4):1103-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koren MJ, Scott R, Kim JB, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380(9858):1995-2006. [DOI] [PubMed] [Google Scholar]
- 12.Raal F, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126(20):2408-2417. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan D, Olsson AG, Scott R, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308(23):2497-2506. [DOI] [PubMed] [Google Scholar]
- 14.Sabatine MS, Giugliano RP, Wiviott SD, et al. ; Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500-1509. [DOI] [PubMed] [Google Scholar]
- 15.Sabatine MS, Giugliano RP, Keech A, et al. Rationale and design of the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk Trial. Am Heart J. 2016;173:94-101. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GG, Bessac L, Berdan LG, et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168(5):682-689. [DOI] [PubMed] [Google Scholar]
- 17.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289-1297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Distribution of Baseline PCSK9 Levels.
eFigure 2. Correlation of Baseline PCSK9 Levels and Baseline LDL-C.