Key Points
Question
What cost-effectiveness parameters for a statin plus proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor treatment strategy, compared with a statin alone strategy, can be elucidated from the results from the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial?
Findings
Per a Markov decision model, the incremental cost-effectiveness ratio was $337 729 per quality-adjusted life year at the current PCSK9 inhibitor treatment price of $14 300 per year, and PCSK9 inhibitors were associated with a negative return on investment of 86% for private payers. A price reduction to $5459 a year (a 62% discount) would permit PCSCK9 inhibitor drug therapy to reach the willingness-to-pay threshold of $100 000 per quality-adjusted life-year.
Meaning
Based on current pricing and the FOURIER trial results, the addition of a PCSK9 inhibitor to statin therapy is more than 3 times the commonly accepted willingness-to-pay threshold.
This study used a Markov decision model to determine the cost-effectiveness of a PCSK9 inhibitor and statin treatment strategy compared with a statin-alone strategy, using results of the FOURIER trial.
Abstract
Importance
Preliminary cost-effectiveness analyses of proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) were based on benefits estimated from reductions in low-density lipoprotein cholesterol that occurred in PCSK9i trials with variable results. The recent Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial provides better information about the effectiveness of the drug.
Objective
To use the trial results to determine the cost-effectiveness of a PCSK9i and statin treatment strategy compared with a statin alone strategy.
Design, Setting, and Participants
We derived observed rates of events, outcomes, cost of care, and health insurance from existing literature for a theoretical cohort of patients designed to resemble the FOURIER PCSK9i trial population and created a Markov model during the time horizon of a full lifetime.
Main Outcomes and Measures
We evaluated the incremental cost-effectiveness ratio from a health system perspective, and the return on investment from a private payer perspective. For both measures, we assumed an annual PCSK9i drug price of $14 300, with a lapse in US patent protection that would reduce the price by 43% in year 12. Costs were reported in 2016 US dollars.
Results
This study modeled 1000 hypothetical patients with attributes similar to those of the FOURIER trial cohort. At the current price, the incremental cost-effectiveness ratio of statin plus PCSK9i therapy was $337 729 per quality-adjusted life-year. Our probabilistic sensitivity analysis found that a statin plus PCSK9i strategy had a low probability (<1%) of being cost effective at the commonly accepted societal threshold of $100 000 per quality-adjusted life-year. Furthermore, PCSK9i produced a negative return on investment of 86% for private payers. In our threshold analysis, the price of PCSK9i would need to drop 62%, to $5459 per year, to reach $100 000 per quality-adjusted life year.
Conclusions and Relevance
At current prices, the addition of PCSK9i to statin therapy is estimated to provide an additional quality-adjusted life year for $337 729 . Significant discounts are necessary to meet conventional cost-effectiveness standards.
Introduction
In the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial, the addition of evolocumab, a proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) drug, to statin therapy was associated with a 59% greater reduction in low-density lipoprotein cholesterol and a 15% lower rate of major adverse cardiovascular events per year (cardiovascular deaths, myocardial infarctions, and stroke) over therapy with statins alone. Despite its effect on cardiovascular risk, there are substantial concerns about the high cost of evolocumab, which is $14 300 per year, and its value to the health care system. Recent studies assessing cost-effectiveness over a lifetime of treatment at current drug prices have shown that PCSK9i drugs were expensive from a health system perspective and were above the societal threshold of $100 000 per quality-adjusted life-year (QALY). Using the recent FOURIER trial results, we determined the health system perspective on the cost-effectiveness of adding PCSK9i to statin therapy. We also projected the return on investment (ROI) for the US private and multipayer insurance market, adding a private payer’s viewpoint, which has been lacking thus far, to our knowledge.
Methods
We used a Markov model to complete a cost-effectiveness analysis of 2 lipid-lowering strategies, statin plus PCSK9i vs statin alone, over a time horizon of a lifetime. Our research group have previously described the model in detail. Table 1 reports the parameters used in this cost-effectiveness analysis. The model used a hypothetical cohort of 1000 patients that resembled the FOURIER population as our baseline group.
Table 1. Parameters of the Markov Decision Model.
| Input Variable | Range of Probabilities, Statin Treatment Only vs Statin Plus PCSK9i Treatment | Uncertainty Range | Reference |
|---|---|---|---|
| Morbidity Rates, Annual, % | |||
| Incident MI | 2.17-8.62 vs 1.65-6.32 | Age-dependent | Sabatine et al |
| Incident stroke | 0.96-3.80 vs 0.80-3.05 | Age-dependent | Sabatine et al |
| Incident other CVD | 0.57-2.28 vs 0.65-2.50 | Age-dependent | Sabatine et al |
| Incident revascularization | 4.13-13.48 vs 3.36-11.21 | Age-dependent | Sabatine et al |
| Recurrent MI | 2.36-8.62 vs 1.82-6.32 | Age-dependent | D’Agostino et al |
| Recurrent stroke | 1.04-3.80 vs 0.88-3.05 | Age-dependent | D’Agostino et al |
| Recurrent other CVD | 0.62-2.28 vs 0.72-2.50 | Age-dependent | D’Agostino et al |
| Recurrent revascularization | 4.13-13.48 vs 3.36-11.21 | Age-dependent | D’Agostino et al |
| Mortality Rates, Annual, % | |||
| After incident MI | 6.28-100 | Age-dependent | CDC |
| After incident stroke | 7.63-100 | Age-dependent | CDC |
| After other CVD | 6.96-100 | Age-dependent | CDC |
| After revascularization | 7.45-100 | Age-dependent | CDC |
| After recurrent MI | 2.93-67.28 | Age-dependent | Brønnum-Hansen et al |
| After recurrent stroke | 4.19-60.91 | Age-dependent | Brønnum-Hansen et al |
| After recurrent other CVD | 3.56-64.09 | Age-dependent | Brønnum-Hansen et al |
| After recurrent revascularization | 3.56-64.09 | Age-dependent | Brønnum-Hansen et al |
| Non-CVD-related | 0.5-18.3 | Age-dependent | CDC |
| Costs, Annual, $ | |||
| Statin treatment | 48 | 36-60 | Erickson et al |
| Baseline treatment | 215 | 161-269 | Davis and Carper |
| MI, acute | 46 099 | 34 574-57 624 | Bonafede et al |
| Stroke, acute | 35 142 | 26 357-43 928 | Bonafede et al |
| Other CVD, acute | 16 442 | 12 332-20 553 | Bonafede et al |
| MI, chronic at 2 y | 3686 | 2765-4608 | Bonafede et al |
| Stroke, chronic at 2 y | 4997 | 4201-3150 | Bonafede et al |
| Other CVD, chronic at 2 y | 2272 | 1704-2840 | Bonafede et al |
| Fatal CVD event | 13 145 | 9859-16 431 | Bonafede et al |
| Statin/PCSK9i treatment | 0.79 | Sullivan and Ghushchyan | |
| MI, >5 y | 0.58-0.79 | 0.54-0.91 | Sullivan and Ghushchyan |
| Stroke, >5 y | 0.46-0.79 | 0.52-0.87 | Sullivan and Ghushchyan |
| Other CVD, >5 y | 0.63-0.79 | 0.57-0.96 | Sullivan and Ghushchyan |
| Premiums, $ | |||
| Age 58-64 y | 223-520 | Age-dependent | Arrieta et al |
| Age >65 y | 835 | 752-1044 | Arrieta et al |
| Age 58-64 y | 3882-3731 | Age-dependent | Arrieta et al |
| Age >65 y | 2158 | 1942-2698 | Arrieta et al |
| Tier 1, $a | |||
| Age 58-64 | 11 | 10-14 | Arrieta et al |
| Age >65 | 5 | 5-6 | Arrieta et al |
| Tier 2, $a | |||
| Age 58-64 | 31 | 28-39 | Arrieta et al |
| Age >65 | 11 | 10-14 | Arrieta et al |
| Turnover rate, % | 12.2 | 0.71 (SD) | Cutler and Gelber |
| Patent Protection | |||
| Time of patent protection, y | 12 | NA | |
| Reduction in price after patent protection period, % | 43 | NA | Conti and Berndt |
Abbreviations: CDC, Centers for Disease Control and Prevention; CVD, cardiovascular disease; MI, myocardial infarction; NA, not applicable; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor drug; SD, standard deviation.
Tier 1 and 2 refers to the groups that insurers use to determine medication copayments, with Tier 1 signifying generic medications and Tier 2 indicating preferred, brand-name medications.
Longitudinal cost parameters for myocardial infarction, stroke, unstable angina, and transient ischemic attack were obtained from the literature. We used observed cardiovascular risk reductions from the FOURIER study for up to 3 years. For all cardiovascular events, we assumed a 1-year probability of 3.7% for patients treated with statin only and a probability of 3.1% for those using statin plus PCSK9i (relative risk [RR], 0.84). We used a 2-year cardiovascular event probability of 6.8% and 5.5% (RR, 0.81) and a 3-year probability of 9.9% and 7.9% (RR, 0.80) for those using a statin only and those using a statin plus PCSK9i, respectively. We projected cardiovascular risk reductions beyond the duration of the trial by using the Framingham risk functions and imposing a constant RR restriction. The implicit assumption is that a ratio of the annual probability of a cardiovascular event is equivalent to 0.81 after the third year. Our model assumes no difference between treatment strategies for mortality risk after a cardiovascular event. Comparison between the hazard ratios of outcomes predicted by our model and those observed in FOURIER are presented in the Supplement.
We used the current drug price, which is $14 300 a year. We assumed that the future price of PCSK9i would be affected by the entry of generic drugs into the market and would therefore drop by 43% after 12 years of patent protection, the average reduction of specialty drug prices. Costs were reported in 2016 US dollars. All outcomes were discounted at a 3% discount rate.
Finally, we performed a sensitivity analysis that considered coronary revascularization as an additional cardiovascular event. Coronary revascularization is not an outcome, but rather a procedure; however, FOURIER incorporated it as a primary outcome. We initially followed previous studies that did not include coronary revascularization as a cardiovascular event, but we analyzed its economic impact in an alternative scenario. We also considered scenarios of permanent patent protection and a 10% increase in cardiovascular event costs in RR reduction and in QALYs.
Next, we examined the financial implications of PCSK9i from a private payer’s perspective by calculating the net present value (NPV) and ROI over a lifetime. In contrast with some national health systems, the United States has a multipayer insurance market that includes private payers. As a result, not all savings (eg, avoided costs) of PCSK9 inhibitor use will necessarily accrue to individual payers. Payers might therefore be reluctant to fund costly preventive treatment; because 12.2% of insurance plan members switch plans every year, insurers do not accrue all long-term benefits of their investment in these members. However, payers may obtain benefits from premium gains if patients live longer, which results in their contributing to the private payer’s premium revenue stream over a longer span of time. They can also obtain benefits by sharing treatment costs with patients via deductibles and copayments. We projected the NPV as the net of the present value of the payer’s premium income stream, including a patient’s cost sharing component, minus health expenditure costs. The ROI was calculated as the NPV divided by the treatment cost. An ROI or NPV of less than 0 indicates a net loss; an ROI or NPV of greater than 0 indicates a net monetary gain to the payer. Details of the cost projections and assumptions about insurance plans have been previously described.
To account for uncertainty in model parameters, we conducted probabilistic sensitivity analysis. Model parameters were assigned β and normal probability distributions. In addition, cost-effectiveness acceptability curves were constructed at different PCSK9i prices. Acceptability curves demonstrate the probability that PCSK9i would be cost effective at different thresholds of society’s willingness to pay.
Results
At the current price of $14 300 a year, the incremental cost-effectiveness ratio (ICER) of the addition of PCSK9i to statins per the FOURIER results was $337 729 per QALY (Table 2). From a health system perspective, our threshold analysis found that PCSK9i would be cost effective if the price dropped below $5459 a year, the point at which it reached the willingness-to-pay level of $100 000 per QALY. This is 62% lower than the current price.
Table 2. Cost-effectiveness Results and Sensitivity Analyses.
| Costs and Outcomes Per Member Per Lifetime | Health System | Private Payer |
|---|---|---|
| Baseline Casea | ||
| Treatment cost, $b | 136 101 | 41 846 |
| Avoided cost of cardiovascular events (savings), $c | –15 740 | –5423 |
| QALY gains, y | 0.36 | NA |
| Life year gains, y | 0.44 | NA |
| Premium revenue gains, $d | NA | 516 |
| ICER, $/QALYe | 337 729 | NA |
| NPV, $f | NA | 35 907 |
| ROI, %g | NA | –85.8 |
| Threshold PCSK9i priceh | 5459 | NA |
| Breakeven PCSK9i pricei | NA | 2381 |
| Alternative Cases (Sensitivity Analysis) | ||
| PCSK9i price remains at $14 300 with permanent patent protection | ||
| ICER, $/QALY | 496 936 | NA |
| ROI, % | NA | –91.6 |
| Including coronary revascularization as a cardiovascular eventj | ||
| ICER, $/QALY | 257 119 | NA |
| ROI, % | NA | –70.1 |
| Increasing cardiovascular event costs by 10% | ||
| ICER, $/QALY | 331 737 | NA |
| ROI, % | NA | –84.2 |
| Increasing cardiovascular risk reduction of PCSK9i by 10%k | ||
| ICER, $/QALY | 226 653 | NA |
| ROI, % | NA | –79.8 |
| Increasing QALY at baseline by 10%l | ||
| ICER, $/QALY | 325 509 | NA |
| ROI, % | NA | –85.8 |
Abbreviations: ICER, incremental cost-effectiveness ratio; NA, not applicable; NPV, net present value; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor drug; QALY, quality-adjusted life-years; ROI, return on investment.
The baseline case is defined as a PCSK9i price of $14 300, with 12 years of patent protection followed by a 43% price reduction.
Incremental cost of treating hyperlipidemia with PCSK9i plus statin compared with statin only. For the private payer, this cost excludes patient cost sharing (see insurance parameters in Table 1).
Incremental cost of CVD events.
Incremental gain in premium revenues that result from living longer and spending more time contributing to the private payer’s premium revenue stream.
ICER equals treatment costs minus savings (avoided costs).
NPV equals savings plus premium gains minus treatment cost.
ROI equals NPV divided by treatment cost.
The threshold price corresponds to the annual PCSK9i price that makes the ICER equal to $100 000 per QALY.
The break-even price is the annual PCSK9i price that makes ROI equal to 0.
Relative risk, costs, and utilities for this scenario are as reported in Table 1.
A scenario in which the risk reduction of cardiovascular events under PCSK9i is assumed to be 10% higher than in Table 1 because of higher PCSK9i efficacy.
A scenario assuming a higher health utility for the average person in the cohort replicating the FOURIER PCSK9i trial population.
The ROI analysis suggested that, at current prices, PCSK9i would produce a negative ROI of 86%, indicating that for every dollar invested, private insurers would lose $1.86. In dollar terms, putting 1 more plan patient on PCSK9i would produce an NPV loss of $35 907 to the payer. A price lower than $2381 would obtain a positive ROI and NPV.
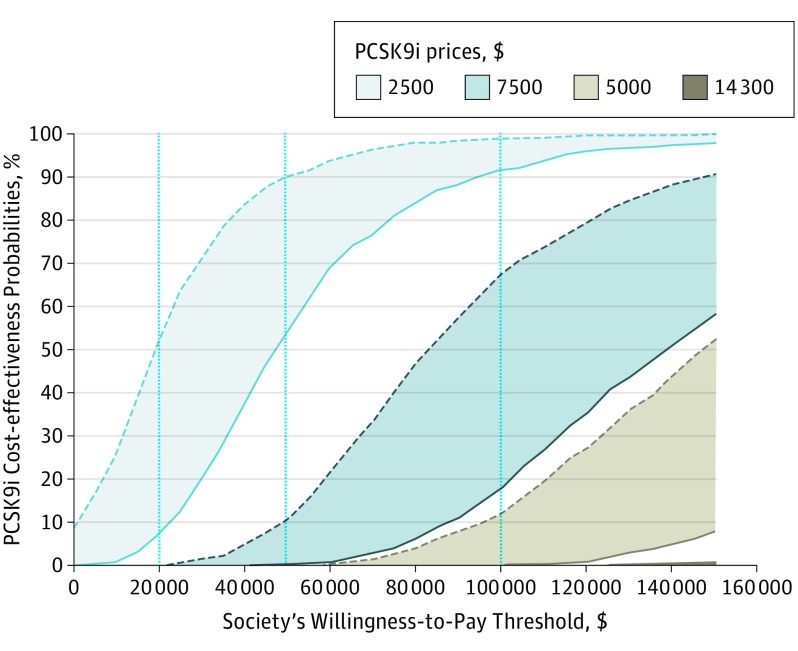
The uncertainty analysis suggests that, at the current price of $14 300, PCSK9i drugs have less than 1% probability of meeting the $100 000 per QALY threshold generally considered acceptable (Figure). If the price of PCSK9i were $5000, the probability of the drugs being cost effective would increase to 68%.
Figure. Cost-effectiveness Acceptability Curves at Different PCSK9i Prices With Permanent or Limited Patent Protection.
Dotted lines signify permanent patent protection of proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) drugs, while solid lines signify patent protection that lapses after 12 years, leading to a 43% reduction in price.
Finally, our sensitivity analysis showed that our results are highly sensitive to the patent protection assumption, the PCSK9i cardiovascular risk reduction assumption, and the coronary revascularization assumption. Specifically, ICER increased by 47% , or $496 936 per QALY, under permanent patent protection; decreased by 33%, or $226 653 per QALY, if cardiovascular risk reduction increased by 10%; and decreased by 24%, or $257 119 per QALY, if revascularization was included as a cardiovascular event.
Discussion
This cost-effectiveness analysis of PCSK9i was based on a lower-than-expected reduction in the major cardiovascular events from the FOURIER trial. We found that the addition of PCSK9i to statin therapy is currently priced at more than 3 times the accepted societal cost-effectiveness threshold. To be cost effective, the price of PCSK9i drugs would likely require a decrease of 62%, to $5459 per year, or more. Wider use of the drugs at current prices would create major financial losses. Treating 1 more plan patient with PCSK9i would produce a NPV loss of $35 907, which would be either assumed by the payer or transferred to plan patients via higher premiums. Only when the price dropped by 83%, to $2381 per year, would PCSK9i be financial viable for private payers.
Prior cost-effectiveness analyses found PCSK9i to exceed the acceptable societal threshold of $100 000 per QALY. However, none of these studies integrated discounts in drug prices with the expected expiration of patents and the possible introduction of generic versions of drugs. Our study, in contrast, was based on observed event rates in the intermediate term, as well as expected changes in drug costs over time. The findings are consistent with the more conservative preliminary assessments.
Furthermore, while previous studies did not include coronary revascularization as a cardiovascular event, our sensitivity analysis did include it. Adding revascularization into the model as an outcome reduced the ICER to $257 119 per QALY.
Finally, our study provides critical insights on the breakeven price for private payers, which is extremely relevant for the US private, multipayer, insurance market. Our findings suggest that even greater discounts will be necessary for PCSK9i to be a financially sound investment for insurers. Because private payers might face the majority of the up-front investment but only a fraction of the long-term benefits, extensive deliberations are needed to identify the right financial incentives to overcome access barriers to these emerging interventions.
Limitations
As is inherent to any decision analysis model, our methodology is limited by the simplifying assumptions about the underlying cohort that we used in analysis, which might not represent the US population accurately. This study is best thought of as applicable to a hypothetical baseline population that resembles the FOURIER trial participants. Additional factors that might have influenced the effect of PCSK9 inhibitors on total health expenditures include known factors, such as comorbidities and socioeconomic disparities, as well as use of high-intensity statins or ezetimibe, a second-line drug used as a substitute for or in addition to statins. The use of ezetimibe might lower the number of patients eligible for PCSK9i use. Finally, this study is not directive about who should be treated, but can only put in perspective costs and effectiveness parameters based on the recent FOURIER trial.
Conclusions
In conclusion, based on current PCSK9i prices and benefit estimates from the FOURIER trial, a price reduction of 62% to 83% is essential for PCSK9i drugs to meet the current societal thresholds of cost-effectiveness and become financially viable for private payers.
Technical appendix for reviewers.
References
- 1.Sabatine MS, Giugliano RP, Keech AC, et al. ; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. [DOI] [PubMed] [Google Scholar]
- 2.Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316(7):743-753. [DOI] [PubMed] [Google Scholar]
- 3.Arrieta A, Page TF, Veledar E, Nasir K. Economic evaluation of PCSK9 inhibitors in reducing cardiovascular risk from health system and private payer perspectives. PLoS One. 2017;12(1):e0169761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease [published online August 23, 2017]. JAMA Cardiol. doi: 10.1001/jamacardio.2017.2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kazi DS, Penko J, Coxson PG, et al. Updated cost-effectiveness analysis of PCSK9 inhibitors based on the results of the fourier trial. JAMA. 2017;318(8):748-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Health Data Interactive. 2017. https://www.cdc.gov/nchs/hdi/. Accessed May 27, 2017.
- 8.Brønnum-Hansen H, Jørgensen T, Davidsen M, et al. Survival and cause of death after myocardial infarction: the Danish MONICA study. J Clin Epidemiol. 2001;54(12):1244-1250. [DOI] [PubMed] [Google Scholar]
- 9.Brønnum-Hansen H, Davidsen M, Thorvaldsen P; Danish MONICA Study Group . Long-term survival and causes of death after stroke. Stroke. 2001;32(9):2131-2136. [DOI] [PubMed] [Google Scholar]
- 10.Erickson KF, Japa S, Owens DK, Chertow GM, Garber AM, Goldhaber-Fiebert JD. Cost-effectiveness of statins for primary cardiovascular prevention in chronic kidney disease. J Am Coll Cardiol. 2013;61(12):1250-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis K, Carper K Use and Expenses for Office-Based Physician Visits by Specialty, 2009: Estimates for the US Civilian Noninstitutionalized Population. Rockville, MD: Agency for Healthcare Research and Quality; August 2012. https://meps.ahrq.gov/data_files/publications/st381/stat381.pdf. Accessed September 13, 2017.
- 12.Bonafede MM, Johnson BH, Richhariya A, Gandra SR. Medical costs associated with cardiovascular events among high-risk patients with hyperlipidemia. Clinicoecon Outcomes Res. 2015;7:337-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutler DM, Gelber AM. Changes in the incidence and duration of periods without insurance. N Engl J Med. 2009;360(17):1740-1748. [DOI] [PubMed] [Google Scholar]
- 15.Conti RM, Berndt ER Specialty drug prices and utilization after loss of U.S. patent exclusivity, 2001-2007. National Bureau of Economic Research Working Paper Series. 2014;No. 20016. http://www.nber.org/papers/w20016. Accessed September 13, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technical appendix for reviewers.