Key Points
Question
How does the survival for patients with borderline pulmonary hypertension compare with those with lower mean pulmonary arterial pressures?
Findings
In this cohort study of 4343 patients undergoing right heart catheterization at a large academic medical center, increased mortality was observed in patients with borderline pulmonary hypertension compared with reference patients after adjusting for clinically relevant covariates. Furthermore, patients with borderline pulmonary hypertension who underwent repeated catheterization often progressed to overt pulmonary hypertension.
Meaning
Patients with borderline pulmonary hypertension should be considered an at-risk subgroup with increased mortality compared with patients with lower mean pulmonary arterial pressures.
Abstract
Importance
Pulmonary hypertension (PH) is diagnosed by a mean pulmonary arterial pressure (mPAP) value of at least 25 mm Hg during right heart catheterization (RHC). While several studies have demonstrated increased mortality in patients with mPAP less than that threshold, little is known about the natural history of borderline PH.
Objective
To test the hypothesis that patients with borderline PH have decreased survival compared with patients with lower mPAP and frequently develop overt PH and to identify clinical correlates of borderline PH.
Design, Setting, and Participants
Retrospective cohort study from 1998 to 2014 at Vanderbilt University Medical Center, comprising all patients undergoing routine RHC for clinical indication. We extracted demographics, clinical data, invasive hemodynamics, echocardiography, and vital status for all patients. Patients with mPAP values of 18 mm Hg or less, 19 to 24 mm Hg, and at least 25 mm Hg were classified as reference, borderline PH, and PH, respectively.
Exposures
Mean pulmonary arterial pressure.
Main Outcome and Measures
Our primary outcome was all-cause mortality after adjusting for clinically relevant covariates in a Cox proportional hazards model. Our secondary outcome was the diagnosis of overt PH in patients initially diagnosed with borderline PH. Both outcomes were determined prior to data analysis.
Results
We identified 4343 patients (mean [SD] age, 59 [15] years, 51% women, and 86% white) among whom the prevalence of PH and borderline PH was 62% and 18%, respectively. Advanced age, features of the metabolic syndrome, and chronic heart and lung disease were independently associated with a higher likelihood of borderline PH compared with reference patients in a logistic regression model. After adjusting for 34 covariates in a Cox proportional hazards model, borderline PH was associated with increased mortality compared with reference patients (hazard ratio, 1.31; 95% CI, 1.04-1.65; P = .001). The hazard of death increased incrementally with higher mPAP, without an observed threshold. In the 70 patients with borderline PH who underwent a repeated RHC, 43 (61%) had developed overt PH, with a median increase in mPAP of 5 mm Hg (interquartile range, −1 to 11 mm Hg; P < .001).
Conclusions and Relevance
Borderline PH is common in patients undergoing RHC and is associated with significant comorbidities, progression to overt PH, and decreased survival. Small increases in mPAP, even at values currently considered normal, are independently associated with increased mortality. Prospective studies are warranted to determine whether early intervention or closer monitoring improves clinical outcomes in these patients.
This study tests the hypothesis that patients with borderline pulmonary hypertension have decreased survival compared with patients with lower mean pulmonary arterial pressure and frequently develop overt pulmonary hypertension.
Introduction
Pulmonary hypertension (PH) is a highly morbid disease that develops from a primary pulmonary vasculopathy or secondary to chronic pulmonary, cardiac, or systemic illnesses. Pulmonary hypertension is currently defined by a mean pulmonary arterial pressure (mPAP) value of at least 25 mm Hg on right heart catheterization (RHC), a definition “empirically and arbitrarily” established by a consensus gathering of PH experts in 1973. This mPAP threshold is not based on physiologic data; in fact, the mean (SD) mPAP in healthy patients is 14.0 (3.3) mm Hg, suggesting an upper limit of normal significantly less than 25 mm Hg.
Several investigators have evaluated the clinical characteristics and outcomes of patients with mPAP values less than the current diagnostic threshold of 25 mm Hg, referred to as borderline PH. In the largest report to our knowledge to date, Maron et al identified decreased adjusted survival in patients with borderline PH, defined as mPAP 19 to 24 mm Hg, in a large, multicentered Veterans Affairs cohort. The generalizability of these data to the broader population is not known. Importantly, data on longitudinal RHC measurements in patients with borderline PH have not been reported. Establishing the clinical profile, prognosis, and natural history of patients with borderline PH identified within a diverse patient population has important ramifications on characterizing the spectrum of clinical risk associated with pulmonary arterial pressures in clinical practice.
Methods
Study Population
The Vanderbilt University institutional review board approved this study. All patients were identified through Vanderbilt’s deidentified electronic health record, the Synthetic Derivative. The Vanderbilt Synthetic Derivative operates under a waiver of consent because all data are deidentified. Findings from this cohort have been previously reported, and additional details on patient exclusions and data acquisition are provided in the eMethods in the Supplement.
Patients were categorized by mPAP values available on the RHC report. In the event an individual had undergone multiple RHCs, data from the first procedure were used in this analysis. Pulmonary hypertension was defined as an mPAP value of at least 25 mm Hg. Although it does not have a consensus definition, borderline PH was defined as an mPAP value of 19 to 24 mm Hg for the purpose of this study according to published thresholds. Accordingly, the reference group of patients was defined as having an mPAP value of 18 mm Hg or less. Precapillary and postcapillary PH were defined as PH with a pulmonary arterial wedge pressure (PAWP) of 15 mm Hg or less and greater than 15 mm Hg, respectively. The medical records of all patients meeting the hemodynamic definition of pulmonary arterial hypertension (PAH) and who were receiving PAH-specific therapies were manually reviewed to confirm the treating physician’s diagnosis of PAH.
Statistical Analyses
Data are expressed as median and quartiles for continuous variables and counts and percentages for categorical variables unless stated otherwise. Differences between 2 groups were assessed using the Mann-Whitney U or χ2 test, as appropriate. Repeated measurements of categorical and continuous variables for an individual were analyzed using McNemar test and paired t test, respectively. Two-tailed P values less than .05 were deemed significant. To account for missing data, we imputed 5 data sets for all regression analyses using multiple imputation with additive regression, bootstrapping, and predictive mean. Additional imputation methods are found in the eMethods in the Supplement.
Univariate and multivariate logistic regression were used to identify clinical variables associated with borderline PH in all patients without PH (mPAP less than 25 mm Hg). Unadjusted and adjusted survival were analyzed using the Kaplan-Meier and Cox proportional hazard methods after adjusting for 34 clinically relevant covariates selected a priori, respectively. We included restricted cubic splines with 4 knots for all continuous variables in our model, with significant nonlinear effects. Results are reported as odds ratio (OR) or hazard ratio (HR) of all-cause mortality, as appropriate, with 95% confidence intervals and P values. All tests of significance were 2-tailed, with statistical significance deemed to be at an α level of less than .05. We also present the HR of all-cause mortality associated with mPAP values within the range of 11 to 60 mm Hg, with mPAP values of 10 mm Hg serving as the reference group for this analysis. To examine the prespecified effect modification by sex, race/ethnicity, heart failure type, and PH subtype, we performed analyses by including interaction terms between mPAP and these covariates in the Cox proportional hazards model for all patients. Additional analyses were performed after excluding patients with PAH and all patients with a pulmonary vascular resistance (PVR) greater than 3 Wood units. Statistical analysis was performed using R, version 3.3.1 (R Programming) and Stata for Macintosh, version 14.0 (StataCorp).
Results
Demographics and Clinical Characteristics
From 5797 unique patients referred for RHC (eFigure 1 in the Supplement), we identified 1454 patients who met the prespecified exclusion criteria. The remaining 4343 patients constituted the study cohort and were categorized further based on their mPAP values: 2684 (62%) with mPAP value of at least 25 mm Hg (PH), 783 (18%) with mPAP value 19 to 24 mm Hg (borderline PH), and 876 (20%) with mPAP value 18 mm Hg or less (reference group). Of the 2684 patients with PH, 1768 (66%) and 916 (37%) were classified as having postcapillary and precapillary PH, respectively. In total, 60% of the RHCs (n=2606) were performed on outpatients and two-thirds of RHCs were performed for coronary artery disease (25%; n = 1197), congestive heart failure (20%; n = 951), pulmonary hypertension (13%; n = 619), or aortic valve disease (9%; n =421; eTable 1 in the Supplement).
Patients with borderline PH were older with a higher prevalence of metabolic, vascular, cardiac, and pulmonary diseases compared with the reference group (Table 1). Diabetes, atrial fibrillation, obstructive sleep apnea, anemia, heart failure, and connective tissue disease were more prevalent in patients with PH. Brain natriuretic peptide, glomerular filtration rate, hemoglobin, and hemoglobin A1C values in borderline PH were intermediate between PH and reference patients. Patients with borderline PH were more likely to be treated with anticoagulant, lipid-lowering, diuretic, and antihypertensive medications than patients in the reference group. Anticoagulant and diuretic use were highest in the PH group.
Table 1. Demographic and Clinical Characteristics by mPAP Categorization.
| Characteristics | mPAP, No. (%) | ||
|---|---|---|---|
| ≥25 mm HG (n = 2684) |
19-24 mm HG (n = 783) |
≤18 mm HG (n = 876) |
|
| Age, median (IQR),y | 61 (50-69)a | 62 (52-71) | 59 (47-68)b |
| Female sex | 1348 (50)a | 360 (46) | 403 (46) |
| BMI, median (IQR) | 29 (25-35) | 29 (25-34) | 27 (23-31)b |
| Race/ethnicity | |||
| White | 2149 (80)a | 665 (85) | 784 (90)b |
| Black | 414 (15)a | 78 (10) | 63 (7)b |
| Other | 121 (5) | 40 (5) | 29 (3) |
| Comorbidities | |||
| Hypertension | 2131 (79) | 640 (82) | 641 (73)b |
| Diabetes mellitus | 1158 (43)a | 291 (37) | 219 (25)b |
| Obesity | 1001 (46) | 270 (43) | 198 (29)b |
| CAD | 1880 (70)a | 597 (76) | 628 (72)b |
| AF | 935 (35)a | 208 (27) | 178 (20)b |
| COPD | 386 (14) | 108 (14) | 45 (5)b |
| ILD | 188 (7) | 44 (6) | 41 (5) |
| OSA | 339 (13)a | 71 (10) | 48 (5)b |
| Anemia | 1333 (53)a | 327 (44) | 297 (36)b |
| Heart failure | 1563 (58)a | 309 (39) | 223 (25)b |
| Connective tissue disease | 129 (5)a | 19 (2) | 14 (2) |
| Medications | |||
| Anticoagulants | 970 (36)a | 217 (28) | 184 (21)b |
| Lipid lowering medications | 1246 (46)a | 435 (56) | 439 (50)b |
| Diuretics | 1831 (68)a | 365 (47) | 301 (34)b |
| Antihypertensives, any | 1972 (73) | 589 (75) | 582 (66)b |
| CCBs | 444 (17) | 137 (17) | 126 (14) |
| β-Blockers | 1114 (42) | 305 (39) | 262 (30)b |
| ACE inhibitors | 721 (27) | 207 (26) | 211 (24) |
| ARBs | 332 (12) | 90 (11) | 80 (9) |
| PAH-specific therapy, any | 242 (9)a | 9 (1) | 9 (1) |
| ERAs | 96 (4)a | 1 (<1) | 1 (<1) |
| PDE5 inhibitors | 117 (4)a | 8 (1) | 7 (<1) |
| Prostacyclins | 106 (4)a | 0 (0) | 1 (<1) |
| Laboratories, median (IQR) | |||
| BNP, pg/mLc | 453 (175-1052)a | 232 (103-579) | 156 (63-363)b |
| GFR, mL/min per 1.73 m2 | 62 (45-81)a | 69 (54-87) | 74 (58-90)b |
| Hemoglobin, g/dL | 12.6 (11-14.1)a | 13.1 (11.6-14.3) | 13.4 (12-14.5)b |
| Hemoglobin A1C, %d | 6.1 (5.6-7)a | 5.9 (5.5-6.6) | 5.7 (5.3-6.1)b |
Abbreviations: ACE, angiotensin converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BNP, brain natriuretic peptide; CAD, coronary artery disease; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; ERA, endothelin receptor antagonist; GFR, glomerular filtration rate; ILD, interstitial lung disease; IQR, interquartile range; mPAP, mean pulmonary arterial pressure; OSA, obstructive sleep apnea; PAH, pulmonary arterial hypertension; PDE5, phosphodiesterase type 5.
SI conversion factor: To convert BNP to nanograms per liter, multiply by 1; to convert hemoglobin to grams per liter, multiply by 10; to convert hemoglobin A1C to proportion of total hemoglobin, multiply by 0.01.
P < .05 for mean pulmonary arterial pressure ≥5 vs 19-24 mm Hg.
P < .05 for mean pulmonary arterial pressure ≤18 vs 19-24 mm Hg.
n = 2818.
n = 2602.
Hemodynamic and Echocardiographic Data
The hemodynamic characteristics of the patient groups are presented in Table 2. We observed a gradient in the severity of pulmonary hemodynamics across the reference, borderline PH, and overt PH groups including PVR, pulmonary arterial capacitance, and pulmonary arterial oxygen saturation. Cardiac function, as measured by stroke volume, cardiac output, and cardiac index, was similar between patients with borderline PH and reference patients, but all were lower compared with PH. Transthoracic echocardiography was performed in 4009 of 4343 patients (92%) within 6 months of RHC (median interval between RHC and echocardiography, 2 days; interquartile range [IQR], 19 days prior to 1 day prior). We observed increased left ventricle (LV) remodeling in patients with borderline PH compared with reference patients based on higher LV mass index and a higher prevalence of LV hypertrophy and left atrial enlargement (Table 2).
Table 2. Hemodynamic and Echocardiographic Characteristics by mPAP Categorization.
| Variable | No. | mPAP, median (IQR) | ||
|---|---|---|---|---|
| ≥25 mm Hg (n = 2684) |
19-24 mm Hg (n = 783) |
≤18 mm Hg (n = 876) |
||
| Invasive catheterization | ||||
| Heart rate, bpm | 2798 | 77 (66-88)a | 73 (62-85) | 70 (61-79)b |
| Blood pressure, mm Hg | ||||
| Systolic | 4343 | 123 (108-142) | 124 (106-142) | 117 (104-134)b |
| Diastolic | 4285 | 71 (62-81)a | 67 (60-76) | 65 (57-74)b |
| RAP, mm Hg | 4343 | 10 (6-15)a | 6 (4-8) | 3 (1-4)b |
| PA pressure, mm Hg | ||||
| Systolic | 4337 | 53 (43-68)a | 33 (30-35) | 23 (20-27)b |
| Diastolic | 4342 | 24 (19-30)a | 14 (12-16) | 7 (5-9)b |
| mPAP, mm Hg | 4343 | 36 (30-45)a | 21 (20-23) | 14 (12-17)b |
| PAWP, mm Hg | 4343 | 19 (14-24)a | 12 (9-15) | 6 (4-9)b |
| DPG, mm Hg | 4030 | 4 (-1-10)a | 1 (-2-4) | 1 (-1-3)b |
| TPG, mm Hg | 4029 | 15 (10-23)a | 9 (7-12) | 7 (6-9)b |
| PVR, Woods units | 4343 | 3.2 (2.1-5.3)a | 1.8 (1.2-2.4) | 1.4 (0.9-1.9)b |
| Cardiac output, L/min | 3997 | 4.95 (4.02-6.20)a | 5.88 (4.86-7.01) | 5.81 (4.84-7.01) |
| Cardiac index, L/min/m2 | 3996 | 2.5 (2.1-3.1)a | 3.0 (2.5-3.5) | 3.0 (2.6-3.7) |
| Stroke volume, mL | 2304 | 62 (47-82)a | 79 (62-101) | 83 (66-102) |
| Stroke volume index, mL/min/m2 | 2304 | 32 (24-41)a | 39 (32-51) | 44 (36-52)b |
| PA O2 saturation, % | 4113 | 65 (57-71)a | 71 (67-76) | 73 (69-77)b |
| PA capacitance, mL/mm Hg | 1957 | 2.0 (1.3-3.0)a | 4.3 (3.2-6.1) | 5.2 (3.9-7.0)b |
| Echocardiography | ||||
| LVEDD, mm | 3836 | 49 (41-58) | 48 (42-55) | 46 (41-53)b |
| LVESD, mm | 3744 | 33 (26-48) | 33 (27-43) | 30 (26-38)b |
| LV IVS thickness, mm | 3352 | 11 (10-13) | 12 (10-13) | 11 (10-13)b |
| Left atrial diameter, mm | 3719 | 44 (38-50)a | 41 (36-47) | 39 (33-43)b |
| LV ejection fraction, % | 3801 | 55 (28-55)a | 55 (35-55) | 55 (45-55)b |
| LV mass index, g/m2 | 3170 | 102 (76-138) | 101 (80-126) | 93 (73-118)b |
| LV hypertrophy, No. (%) | 4009 | 741 (32)a | 238 (38) | 188 (27)b |
| LAE, No. (%) | 4009 | 1489 (64)a | 348 (56) | 281 (40)b |
Abbreviations: DPG, diastolic pressure gradient; IQR, interquartile range; IVS, interventricular septum; LAE, left atrial enlargement; LV, left ventricular; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter; mPAP, mean pulmonary arterial pressure; PA, pulmonary arterial; PAWP, pulmonary arterial wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; TPG, transpulmonary gradient.
P < .05 for mean pulmonary arterial pressure ≥25 vs 19-24 mm Hg.
P < .05 for mean pulmonary arterial pressure ≤18 vs 19-24 mm Hg.
Correlates of Borderline PH in Patients With mPAP Less Than 25 mm Hg
We performed a univariate analysis of all patients with mPAP values less than 25 mm Hg to determine clinical and echocardiographic factors associated with borderline PH. On multivariate analysis adjusting for 34 demographic, clinical, laboratory, and echocardiographic indices, only older age (OR, 1.12 per 10 years; 95% CI, 1.03-1.21; P < .001), higher body mass index (calculated as weight in kilograms divided by height in meters squared, 1.05; 95% CI 1.03-1.08; P < .001), prevalent chronic obstructive pulmonary disease (OR, 3.22; 95% CI, 2.17-4.79; P < .001), higher hemoglobin A1C (OR, 1.12 per 1%; 95% CI, 1.01-1.25; P = .04), and increased left atrial diameter (OR, 1.21 per mm; 95% CI, 1.05-1.38; P = .01) were independently associated with a higher likelihood of borderline PH (eTable 2 in the Supplement).
Outcomes
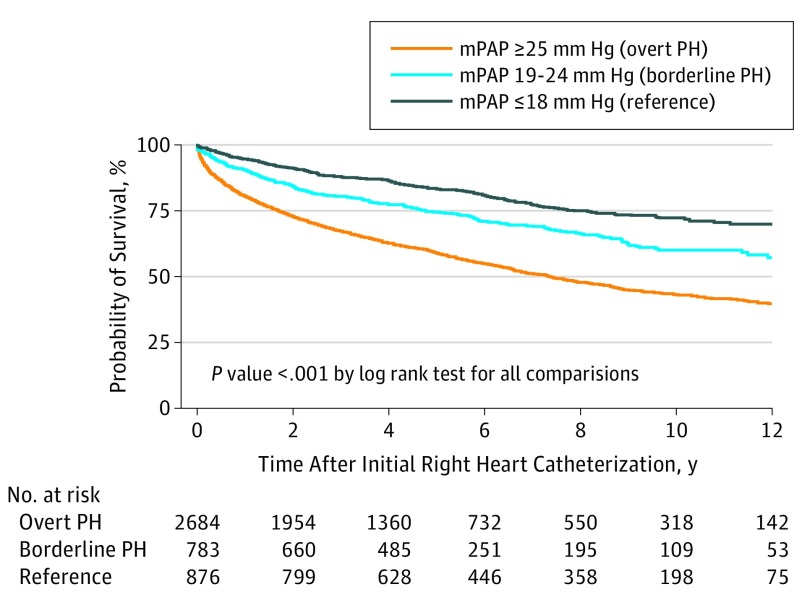
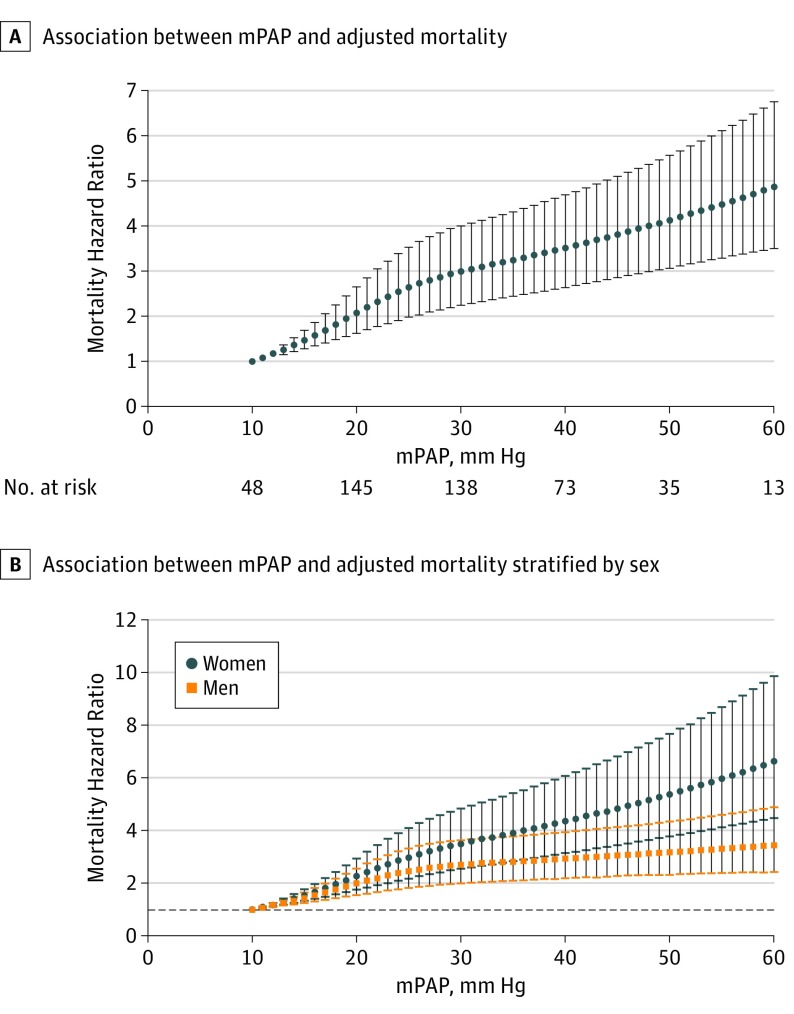
The unadjusted mortality was intermediate for borderline PH relative to overt PH and the reference group (Figure 1), with a 5-year survival of 83%, 75%, and 59% for reference patients, patients with borderline PH, and patients with overt PH, respectively. The difference in unadjusted 5-year survival for reference patients, patients with borderline PH, and patients with overt PH were similar after excluding patients treated with diuretics (90%, 80%, and 67%), treated with PAH-specific medications (83%, 74%, and 59%), or with a PVR of greater than 3 Wood units (84%, 76%, and 63%). When patients with borderline PH were stratified into tertiles of PAWP (≤9 mm Hg, 10-13 mm Hg, and ≥14 mm Hg) the unadjusted 5-year survival was 60%, 74%, and 69%, respectively. After adjusting for 34 relevant covariates in a Cox proportional hazards model, the risk of all-cause mortality was higher for patients with PH (HR, 1.70; 95% CI, 1.37-2.10; P < .001) and patients with borderline PH (HR, 1.31; 95% CI, 1.04-1.65; P = .001) when compared with reference patients (eTable 3 in the Supplement, including the list of adjusted covariates). When mPAP was modeled as a continuous, nonlinear variable adjusting for the same covariates, the HR for death compared with a reference group (mPAP value, 10 mm Hg) increased immediately and incrementally starting from mPAP values of 11 mm Hg (HR, 1.08; 95% CI, 1.05-1.11; P < .001) through 60 mm Hg (HR, 4.87; 95% CI, 3.51-6.74; P < .001) (Figure 2A). For patients with borderline PH, the relative HR of death ranged from 1.95 (95% CI, 1.56-2.44; P < .001) at 19 mm Hg to 2.54 (95% CI, 1.91-3.39; P < .001) at 24 mm Hg. A similar relationship was observed when patients with PAH were excluded, although the magnitude of risk was lower. There was an incremental increase in the hazard of death associated with a 1 mm Hg higher mPAP value in the entire range studied; however, the effect of a 1 mm Hg higher mPAP value was most pronounced in the reference and borderline PH range (eFigure 2 in the Supplement). In our model, sex significantly interacted with mPAP, such that women had a higher HR for death at a given mPAP compared with male patients (Figure 2B; β, −0.0131; P < .001). Because our population is enriched in patients with PAH who are predominantly women, we hypothesized that patients with PAH drove the sex interaction. However, when patients with were excluded from the analysis, the sex interaction remained (β, −0.0142; P = .001). No interaction was found between mPAP and our other prespecified analyses of heart failure subtype (preserved or reduced ejection fraction), race/ethnicity (white, black, or other), or PH subtype (precapillary or postcapillary PH). Receiver operator characteristic curve analyses for various mPAP cutoffs are reported in eFigure 3 in the Supplement, generally demonstrating that mPAP at any value is poorly discriminating in its ability to predict mortality.
Figure 1. Unadjusted Survival Analysis for Patients According to Mean Pulmonary Arterial Pressure (mPAP) Categorization.
Unadjusted mortality for patients with borderline pulmonary hypertension (PH) is intermediate to those with overt PH and mPAP values of 18 mm Hg or less.
Figure 2. Adjusted Mortality After Modeling Mean Pulmonary Arterial Pressure (mPAP) as a Continuous Variable and Sex Differences in the Relationship Between Mean Pulmonary Arterial Pressure and Mortality.
A, The hazard of death compared with the reference group (mean pulmonary arterial pressure 10 mm Hg) increased incrementally starting at an mPAP value of 11 mm Hg in an adjusted analysis after accounting for 34 relevant covariates in a Cox proportional hazards model. B, Relationship between mPAP and mortality in men and women.
Repeated Catheterization Data
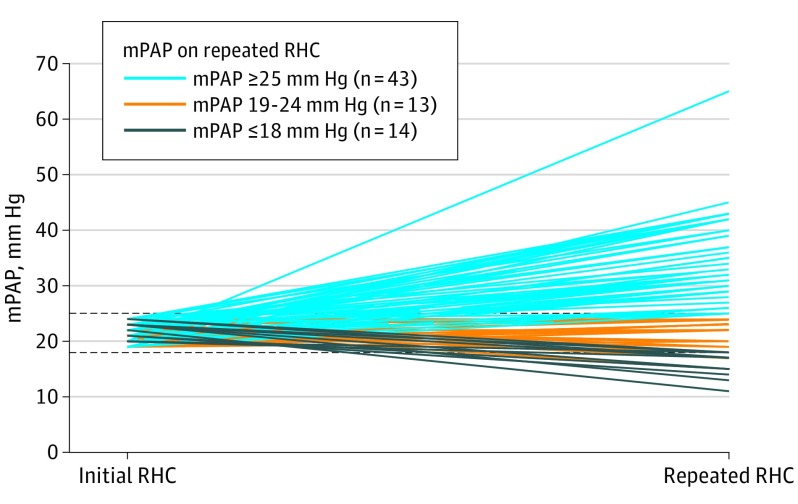
We identified 70 patients initially diagnosed with borderline PH who underwent a repeated RHC (median interval between RHC, 35 weeks; IQR, 12-124 weeks). Compared with the 713 patients with borderline PH who did not undergo a repeated RHC, these patients were younger, with more advanced and prevalent cardiac disease, although were otherwise similar (eTable 4 in the Supplement). From this subgroup, 43 of 70 patients (61%) were subsequently diagnosed as having PH, 18 (26%) remained having borderline PH, and 9 (13%) had an mPAP value of 18 mm Hg or less (Figure 3). The median mPAP and PAWP increased by 5 mm Hg (IQR, 21-26 mm Hg) and 3 mm Hg (IQR, 13-16 mm Hg), respectively, in these patients (both P < .001), with 13 patients (19%) developing an mPAP of at least 35 mm Hg. The PVR and transpulmonary gradient (difference between mPAP and PAWP) also increased in patients diagnosed with PH on repeated RHC (eTable 4 in the Supplement). Of the patients diagnosed as having PH on subsequent RHC, 34 (79%) and 9 (21%) developed postcapillary PH and precapillary PH, respectively. The demographic, clinical, echocardiographic, and baseline hemodynamic characteristics between the 43 patients with borderline PH with a follow-up mPAP level of at least 25 mm Hg and the 27 patients with a follow-up mPAP level of 25 mm Hg or less were identical, even after adjusting for age and sex (eTable 5 in the Supplement). Unadjusted 5-year survival in the patients who developed PH was 62%, compared with 85% in those who did not (P = .008 by log-rank test).
Figure 3. Repeated Mean Pulmonary Arterial Pressure (mPAP) in Patients Initially Diagnosed With Borderline Pulmonary Hypertension (PH).
Follow-up right heart catheterization (RHC) data in 70 patients initially diagnosed with borderline PH demonstrated most (61%) develop overt PH, with a median increase in mPAP from 21 to 26 mm Hg (P < .001 by paired t test).
Discussion
We aimed to determine the clinical correlates and prognostic effect of mPAP values less than the current diagnostic threshold for PH in a large and diverse referral population. We found that borderline PH is common in patients referred for RHC and associated with increased cardiopulmonary disease burden and mortality compared with lower mPAP values. The adjusted hazard of death increased incrementally and directly with a rise in mPAP, without an observed threshold. Finally, most patients (61%) with borderline PH who underwent a repeated RHC were subsequently diagnosed with overt PH, most commonly postcapillary PH. This study improves on prior work in this area by incorporating longitudinal mPAP measurements and statistical adjustments for LV function and medication exposures in a population enriched with women.
Based on historical assumptions of the normal pressures within the pulmonary vasculature, PH has traditionally been defined by an mPAP value of at least 25 mm Hg. Critical appraisal of this threshold has been limited by a lack of data, and consequently, international PH guidelines have deferred comment on the clinical importance of mPAP values below this threshold. While there is no universally accepted definition of borderline PH, several publications have challenged this diagnostic threshold by evaluating the clinical characteristics and outcomes of patients with mPAP values in the 17 to 24 mm Hg range. In smaller studies, Heresi et al and Kovacs et al found that patients with mPAP values between 17 and 24 mm Hg have increased mortality when compared with patients with lower mPAP values. In the largest study to our knowledge to date, the Veterans Affairs Clinical Assessment, Reporting, and Tracking (VA CART) investigators observed that many of the clinical characteristics and outcomes for borderline PH (mPAP values of 19-24 mm Hg) existed on a spectrum, with values of intermediate to overt PH and their reference group (mPAP values ≤18 mm Hg).
Two limitations of these earlier studies are a lack of generalizability and use of cross-sectional analyses. We sought to validate and expand on these previous studies in our electronic health record–based cohort that is enriched with female patients, echocardiographic data, medication exposure, and longitudinal hemodynamic measurements. Compared with the VA CART study, our cohort was younger (median age, 61 years; IQR, 50-70 years vs 65 years; IQR, 60-73 years) and included a more balanced sex distribution (49% vs 3% women). Of note, with the exception of age and sex, the distribution of comorbidities was similar between the VA CART and our study.
After adjusting for numerous clinical covariates, we found that chronic obstructive pulmonary disease, left atrial diameter, and features of the metabolic syndrome were independently associated with a higher likelihood of borderline PH. Chronic hypoxia and left heart disease are the 2 most common causes of PH, while higher hemoglobin A1C and body mass index in borderline PH provide further evidence of an association between insulin resistance and pulmonary vascular disease. Although PAH and other PH phenotypes have fundamentally different etiologies, several studies have demonstrated that borderline PH exists on a spectrum between normal pulmonary hemodynamics and PAH in patients with scleroderma. Our findings suggest that borderline PH is an early and intermediate pulmonary vascular phenotype in patients with exposure to numerous PH risk factors. The clinical profile of our cohort suggests most patients with borderline PH have existing or early left heart failure, rather than early PAH. Additional studies are needed to determine whether the mortality risk in patients with borderline PH can be modified, for example, by aggressive treatment of left heart disease and the attendant clinical risk factors.
We observed increased mortality in patients with borderline PH when compared with reference patients. When we modeled the adjusted survival of patients at each mPAP compared with a reference value of 10 mm Hg, we did not observe a risk threshold at 19 mm Hg, as previously reported by Maron et al. Rather, we saw an immediate and incremental increase in the hazard of death beginning at an mPAP of 11 mm Hg. There are several notable distinctions in our study population that may explain these different findings. The significantly higher proportion of patients who are younger, female, and have PAH in our cohort may have contributed to the difference, as suggested by a sex interaction with a higher HR among women at a given mPAP. Moreover, in contrast to the VA CART study, our model included echocardiographic variables, medication exposure, and accounted for continuous values in a nonlinear fashion.
Mortality in PH is generally attributed to right ventricular failure, and both our and the VA CART cohort demonstrated an increase in mortality at mPAP values currently considered normal. Despite our attempt to adjust for the complex medical illnesses in these patients, residual confounding may persist to explain in part the observed mortality differences. It remains unclear whether mild elevations of mPAP directly or indirectly contribute to the increased mortality in patients with borderline PH. Both experimental models of heart failure and human studies have demonstrated significant right ventricular–pulmonary arterial uncoupling and right ventricular dyssynchrony in the borderline PH range, suggesting right ventricular dysfunction occurs with only mild increases in mPAP. While patients with mPAP values of 11 to 19 mm Hg likely have worse survival secondary to increased prevalence of comorbid illnesses, it is possible that subclinical decline in right ventricular function may contribute to mortality in these patients. However, preserved cardiac output and stroke volume suggest right ventricular dysfunction was not highly prevalent in the borderline PH group.
The continuous relationship between mPAP and mortality parallels other continuous physiologic measurements in humans, such as systemic blood pressure and cholesterol, which are independently associated with an increased risk of vascular complications and death. These findings are potentially true for many other continuous variables that have somewhat arbitrary clinical thresholds. In the case of borderline PH, subtle elevations in mPAP reflect an increased burden of systemic disease. Mean pulmonary artery pressure is a surrogate that integrates the complex cardiopulmonary physiology of the patient. While mPAP is independently associated with mortality on a population level, it lacks sufficient discrimination to predict outcomes for an individual patient.
A novel observation from our study is that many patients with borderline PH are diagnosed with overt PH on subsequent RHC. It is important to note that there is selection bias in patients who were referred for repeated RHC in our retrospective cohort. However, this finding warrants further inquiry in prospective studies. In the 70 patients with borderline PH (9%) who underwent repeated RHC, the conversion rate to overt PH was 61%, most of whom fell into the postcapillary PH phenotype. The median increase in mPAP for patients who developed PH was 10 mm Hg, with nearly 20% of all patients achieving an mPAP value greater than 35 mm Hg. Furthermore, most of these patients were undergoing repeated RHC within 1 year, suggesting a relatively rapid transition to overt PH in these patients. While PAWP increased along with mPAP in these patients, the PVR and transpulmonary gradient significantly increased as well. This suggests that decompensated heart failure with worsening volume status is not solely responsible for the progression to overt PH in these patients. Pulmonary vascular physiology is dynamic, and these findings highlight the limitations of static measurements in the evaluation of pulmonary vascular disease. Most patients with borderline PH who progressed to PH developed postcapillary physiology with relatively low PVR, suggesting that early PAH-directed therapy would not improve outcomes in these patients. The role of closer monitoring or intensification of treatments directed at the underlying causes of pulmonary or LV disease warrants further investigation.
Limitations
Our study has several limitations that should be considered when interpreting our findings. As we have addressed in previous reports of this cohort, this is a retrospective and single-center sample of an academic medical center spanning 17 years. Despite this limitation, our findings are generalizable to other referral populations given the large and heterogeneous nature of our sample. Although we are unable to manually review RHC tracings or echocardiographic images, we have demonstrated excellent agreement between hemodynamic and echocardiographic values derived by manual review and from the procedural reports. Given the technical challenges of obtaining accurate RHC measurements, some patient misclassification may have occurred in our cohort; however, this is likely to be modest on a population level and unlikely to be biased in a single direction. Finally, as addressed previously, there is confounding by indication in the repeated catheterization cohort because decompensating patients may be more likely to undergo repeated invasive testing than stable patients.
Conclusions
Borderline PH is common in patients referred for RHC at an academic tertiary referral center. These patients have a high comorbidity burden with increased mortality compared with patients with lower mPAP. Most patients with borderline PH who underwent repeated RHC developed overt PH. Subtle elevations of mPAP within a range currently considered normal were independently associated with mortality. This work builds on an evolving body of literature that suggests borderline PH is clinically important and reconsideration of the diagnostic classification of PH may be warranted. Future studies may consider evaluating the efficacy of closer interval monitoring or early therapeutic interventions, particularly in patients with left heart disease.
eMethods. Further Details on the Cohort and Statistical Methods
eFigure 1. Flow Diagram for Patient Categorization.
eFigure 2. Incremental Increase In Hazard Of Death Per 1 mmHg Increase In Mean Pulmonary Arterial Pressure.
eFigure 3. Receiver Operator Characteristic Curve Analysis of Mean Pulmonary Artery Pressure and Mortality
eTable 1. Indications for Right Heart Catheterization.
eTable 2. Univariate and Multivariate Associations of Borderline Pulmonary Hypertension in Patients with a Mean Pulmonary Arterial Pressure < 25 mmHg.
eTable 3. Adjusted Mortality by Mean Pulmonary Arterial Pressure Categorization.
eTable 4. Complete Hemodynamics of Borderline Pulmonary Hypertension Patients Undergoing Repeat Right Heart Catheterization.
eTable 5. Univariate and Multivariate Associations of Developing Overt Pulmonary Hypertension in Patients Initially Diagnosed with Borderline Pulmonary Hypertension.
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, et al. . Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25)(suppl):D34-D41. [DOI] [PubMed] [Google Scholar]
- 2.Seeger W, Adir Y, Barberà JA, et al. . Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25)(suppl):D109-D116. [DOI] [PubMed] [Google Scholar]
- 3.Vachiéry JL, Adir Y, Barberà JA, et al. . Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25)(suppl):D100-D108. [DOI] [PubMed] [Google Scholar]
- 4.Hatano SST. Primary Pulmonary Hypertension: Report on a WHO Meeting. Geneva, Switzerland; World Health Organization; 1975. [Google Scholar]
- 5.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34(4):888-894. [DOI] [PubMed] [Google Scholar]
- 6.Heresi GA, Minai OA, Tonelli AR, et al. . Clinical characterization and survival of patients with borderline elevation in pulmonary artery pressure. Pulm Circ. 2013;3(4):916-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacs G, Avian A, Tscherner M, et al. . Characterization of patients with borderline pulmonary arterial pressure. Chest. 2014;146(6):1486-1493. [DOI] [PubMed] [Google Scholar]
- 8.Maron BA, Hess E, Maddox TM, et al. . Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133(13):1240-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulley J, Clayton E, Bernard GR, Roden DM, Masys DR. Principles of human subjects protections applied in an opt-out, de-identified biobank. Clin Transl Sci. 2010;3(1):42-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roden DM, Pulley JM, Basford MA, et al. . Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84(3):362-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assad TR, Brittain EL, Wells QS, et al. . Hemodynamic evidence of vascular remodeling in combined post- and precapillary pulmonary hypertension. Pulm Circ. 2016;6(3):313-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assad TR, Hemnes AR, Larkin EK, et al. . Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J Am Coll Cardiol. 2016;68(23):2525-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147-177. [PubMed] [Google Scholar]
- 14.Hoeper MM, Bogaard HJ, Condliffe R, et al. . Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25)(suppl):D42-D50. [DOI] [PubMed] [Google Scholar]
- 15.Badesch DB, Champion HC, Sanchez MA, et al. . Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1)(suppl):S55-S66. [DOI] [PubMed] [Google Scholar]
- 16.Galiè N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903-975. [DOI] [PubMed] [Google Scholar]
- 17.Pugh ME, Robbins IM, Rice TW, West J, Newman JH, Hemnes AR. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant. 2011;30(8):904-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benson L, Brittain EL, Pugh ME, et al. . Impact of diabetes on survival and right ventricular compensation in pulmonary arterial hypertension. Pulm Circ. 2014;4(2):311-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West J, Niswender KD, Johnson JA, et al. . A potential role for insulin resistance in experimental pulmonary hypertension. Eur Respir J. 2013;41(4):861-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valerio CJ, Schreiber BE, Handler CE, Denton CP, Coghlan JG. Borderline mean pulmonary artery pressure in patients with systemic sclerosis: transpulmonary gradient predicts risk of developing pulmonary hypertension. Arthritis Rheum. 2013;65(4):1074-1084. [DOI] [PubMed] [Google Scholar]
- 21.Visovatti SH, Distler O, Coghlan JG, et al. . Borderline pulmonary arterial pressure in systemic sclerosis patients: a post-hoc analysis of the DETECT study. Arthritis Res Ther. 2014;16(6):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovacs G, Maier R, Aberer E, et al. . Borderline pulmonary arterial pressure is associated with decreased exercise capacity in scleroderma. Am J Respir Crit Care Med. 2009;180(9):881-886. [DOI] [PubMed] [Google Scholar]
- 23.Pagnamenta A, Dewachter C, McEntee K, Fesler P, Brimioulle S, Naeije R. Early right ventriculo-arterial uncoupling in borderline pulmonary hypertension on experimental heart failure. J Appl Physiol (1985). 2010;109(4):1080-1085. [DOI] [PubMed] [Google Scholar]
- 24.Lamia B, Muir JF, Molano LC, et al. . Altered synchrony of right ventricular contraction in borderline pulmonary hypertension. Int J Cardiovasc Imaging. 2017;33(9):1331-1339. [DOI] [PubMed] [Google Scholar]
- 25.Rose G, Shipley M. Plasma cholesterol concentration and death from coronary heart disease: 10 year results of the Whitehall study. Br Med J (Clin Res Ed). 1986;293(6542):306-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ragland DR, Brand RJ. Coronary heart disease mortality in the Western Collaborative Group Study. Follow-up experience of 22 years. Am J Epidemiol. 1988;127(3):462-475. [DOI] [PubMed] [Google Scholar]
- 27.MacMahon S, Peto R, Cutler J, et al. . Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335(8692):765-774. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Peto R, Collins R, MacMahon S, Lu J, Li W. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. BMJ. 1991;303(6797):276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903-1913. [DOI] [PubMed] [Google Scholar]
- 30.Wells QS, Farber-Eger E, Crawford DC. Extraction of echocardiographic data from the electronic medical record is a rapid and efficient method for study of cardiac structure and function. J Clin Bioinforma. 2014;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Further Details on the Cohort and Statistical Methods
eFigure 1. Flow Diagram for Patient Categorization.
eFigure 2. Incremental Increase In Hazard Of Death Per 1 mmHg Increase In Mean Pulmonary Arterial Pressure.
eFigure 3. Receiver Operator Characteristic Curve Analysis of Mean Pulmonary Artery Pressure and Mortality
eTable 1. Indications for Right Heart Catheterization.
eTable 2. Univariate and Multivariate Associations of Borderline Pulmonary Hypertension in Patients with a Mean Pulmonary Arterial Pressure < 25 mmHg.
eTable 3. Adjusted Mortality by Mean Pulmonary Arterial Pressure Categorization.
eTable 4. Complete Hemodynamics of Borderline Pulmonary Hypertension Patients Undergoing Repeat Right Heart Catheterization.
eTable 5. Univariate and Multivariate Associations of Developing Overt Pulmonary Hypertension in Patients Initially Diagnosed with Borderline Pulmonary Hypertension.