Key Points
Question
Do procedure volumes accurately identify low-performing and high-performing aortic and mitral valve surgical centers in the United States?
Findings
In an all-payer data set consisting of 682 US hospitals performing aortic and mitral valve surgical procedures, volume was only modestly associated with in-hospital mortality. The use of volume-based tertiles to categorize hospitals for quality misclassified 305 of 682 valve hospitals as either low performing or high performing when assessing performance based on tertiles of in-hospital risk-standardized mortality rate.
Meaning
Hospital procedure volume alone frequently misclassifies hospital performance with regard to risk-standardized outcomes after aortic and mitral valve surgical procedures.
Abstract
Importance
Identifying high-performing surgical valve centers with the best surgical outcomes is challenging. Hospital surgical volume is a frequently used surrogate for outcomes. However, its ability to distinguish low-performing and high-performing hospitals remains unknown.
Objective
To examine the association of hospital procedure volume with hospital performance for aortic and mitral valve (MV) surgical procedures.
Design, Setting, and Participants
Within an all-payer nationally representative data set of inpatient hospitalizations, this study identified 682 unique hospitals performing surgical aortic valve replacement (SAVR) and MV replacement and repair with or without coronary artery bypass grafting (CABG) between 2007 and 2011. Procedural outcomes were further assessed for a 10-year period (2005-2014) to assess representativeness of study period.
Main Outcomes and Measures
In-hospital risk-standardized mortality rate (RSMR) calculated using hierarchical models and an empirical bayesian approach with volume-based shrinkage that allowed for reliability adjustment.
Results
At 682 US hospitals, 70 295 SAVR, 19 913 MV replacement, and 17 037 MV repair procedures were performed between 2007 and 2011, with a median annual volume of 43 (interquartile range [IQR], 23-76) SAVR, 13 (IQR, 6-22) MV replacement, and 9 (IQR, 4-19) MV repair procedures. Of 225 SAVR hospitals in the highest-volume tertile, 34.7% and 36.0% were in the highest-RSMR tertile for SAVR + CABG and isolated SAVR procedures, respectively, while 21.5% and 17.5% of the 228 SAVR hospitals in the lowest-volume tertile were in the lowest respective RSMR tertile. Similarly, 36.8% and 43.5% of hospitals in the highest tertile of volume for MV replacement and repair, respectively, were in the corresponding highest-RSMR tertile, and 17.4% and 11.2% of the low-volume hospitals were in the lowest-RSMR tertile for MV replacement and repair, respectively. There was limited correlation between outcomes for SAVR and MV procedures at an institution. If solely volume-based tertiles were used to categorize hospitals for quality, 44.7% of all valve hospitals would be misclassified (as either low performing or high performing) when assessing performance based on tertiles of RSMR.
Conclusions and Relevance
Hospital procedure volume alone frequently misclassifies hospital performance with regard to risk-standardized outcomes after aortic and MV surgical procedures. Valve surgery quality improvement endeavors should focus on a more comprehensive assessment that includes risk-adjusted outcomes rather than hospital volume alone.
This study uses the AHRQ NIS data set to examine the association of hospital procedure volume with hospital performance for aortic and mitral valve surgical procedures.
Introduction
Valvular heart disease affects 2.5% of the US adult population, with aortic and mitral valve (MV) disorders representing a large majority of this group. Surgical management remains a cornerstone of therapy and is associated with substantial improvements in patient morbidity and mortality for both aortic and MV disease. However, valvular procedures are complex and have substantial perioperative morbidity and mortality. With the recent advent and expansion of percutaneous options, such as transcatheter aortic valve replacement (TAVR) and MitraClip (Abbott Laboratories), there is renewed interest in regionalization of services for the care for complex valvular heart disease. An institution’s surgical experience will undoubtedly serve as a major determinant of its role in such a network. Even currently, the Centers for Medicare & Medicaid Services mandates that hospitals must exceed specific minimum annual surgical volumes to be reimbursed for TAVR and MitraClip, irrespective of their actual surgical outcomes.
After initial reports of an inverse association between hospital volume and surgical outcomes, payers and policy makers started endorsing volume minimums as a proxy for quality of care delivered. Volume as a metric appears to be rather intuitive and is fairly easy to collect, interpret, and monitor. However, there are several downsides to this approach. Most important, using solely volume as a surrogate may be less relevant when carefully collected and risk-adjusted outcomes are directly available.
Despite these advances in quality assessment, institutional volume remains an entrenched metric of surgical care quality. Prior investigations that examined hospital volume-outcome associations for valve surgery were either conducted more than a decade ago, were limited by the studied age groups or geographic regions, or did not account for uncertainty in outcome estimates at low-volume centers. Therefore, using a large nationally representative all-payer data set, as well as a bayesian approach accounting for uncertainty in outcome estimates at low-volume centers, we sought to elucidate the hospital volume-mortality association for aortic and MV surgical procedures and to further assess the validity of hospital valve surgery volume as a surrogate for quality of care.
Methods
Data Source
We used the Agency for Healthcare Research and Quality’s National Inpatient Sample (NIS), the largest all-payer database of hospitalized patients in the United States. We included data for 2007 through 2011, the most recent 5-year period with complete hospital-level data. We further used data from a 10-year period between 2005 and 2014 to assess temporal changes in volumes and outcomes of surgical aortic and MV procedures. Because the study used deidentified data, the University of Texas Southwestern Medical Center exempted it from institutional review board approval or the need to obtain informed consent.
The design of the NIS has been described previously. Briefly, the NIS is a random 20% sample of all inpatient hospitalizations in the United States each year. For 2005 to 2011, the NIS was constructed from a 20% random sample of all reporting hospitals, and 100% of discharges from sampled hospitals were included. Each hospital has the same probability of being selected in subsequent years of data. Beginning in 2012, the NIS was constructed as a 20% patient-level sample across hospitals without representative sampling at each hospital; hence, data from 2012 to 2014 were only included to assess procedure volumes and outcomes (and thus representativeness of hospital-level analyses to the contemporary valve surgery landscape), but not for the actual volume-outcome relationship analyses. In both performing and reporting this study, we followed statistical best practices for NIS investigations and include the NIS study checklist in eTable 1 in the Supplement.
Study Population and Variables
The NIS includes information on patient demographics (age, sex, and race), primary discharge diagnosis, up to 24 secondary diagnoses, and 15 procedures performed during the index hospitalization. Both diagnoses and procedures are available as International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes and their validated combinations into broad categories (Clinical Classifications Software [CCS] codes). We used a combination of ICD-9-CM and CCS codes to identify specific comorbidities and procedures.
We identified all hospitalizations in adults (≥18 years) who underwent a surgical aortic valve replacement (SAVR) (ICD-9-CM codes 35.21 and 35.22), MV replacement (ICD-9-CM codes 35.23 and 35.24), or MV repair (ICD-9-CM code 35.12) at each hospital for every year of participation during 2007 to 2011. We identified the valve procedures with concomitant coronary artery bypass grafting (CABG) using CCS code 44. Only hospitals performing both aortic and MV surgery were included. To capture the breadth of surgical experience at a hospital, all of the SAVR, MV replacement, and MV repair procedures were used to define hospital procedure volumes for SAVR and MV procedures. However, to allow a comparison of outcomes across more homogeneous patient groups across these hospitals, we identified the following 4 specific procedure subsets for assessment of the hospital volume-outcome relationship: (1) SAVR performed with CABG, (2) SAVR performed without CABG, (3) MV replacement with or without CABG, and (4) MV repair with or without CABG (eFigure 1 in the Supplement).
Statistical Analysis
We calculated the annual rate of all SAVR and MV replacement and repair procedures at participating hospitals by dividing the procedure volume at each hospital by the number of years a hospital was included in the study. To account for differences in patient characteristics across hospitals, we calculated each hospital’s risk-standardized mortality rate (RSMR) for SAVR + CABG, isolated SAVR, MV replacement, and MV repair. For this investigation, we constructed a multivariable hierarchical regression model with hospital-level random effects and patient-level fixed effects, accounting for clustering of patients within each hospital, which is similar to the approach our group has described previously. The patient-level factors included in the analysis were patient demographics (age, sex, and race) and the Elixhauser comorbidities, a set of comorbid conditions that are accurately captured from administrative records and have been shown to independently predict early mortality across a spectrum of medical and surgical conditions.
The conditions were selected using an algorithm that correlated the diagnosis related group for the hospitalization with the secondary diagnosis codes and are listed in eTable 2 in the Supplement. From our hierarchical model, the pooled random-effects intercept across all included hospitals determined the “expected” mortality at each hospital. The “predicted” mortality at individual hospitals was determined from the hospital-specific random-effects intercept from the above model. We calculated RSMR for each hospital as the ratio of its predicted to expected mortality multiplied by the unadjusted mortality rate. To account for uncertainty in mortality estimates at low-volume hospitals, we modeled our risk-standardization algorithm using an empirical bayesian estimator. Furthermore, to allow for shrinkage of estimates closer to hospitals with similar volumes, we included each hospital’s actual procedure volume as an additional hospital-level fixed effect in the model. We calculated hospital RSMRs for SAVR + CABG, isolated SAVR, MV replacement, and MV repair separately. For risk standardization in MV procedures, we also accounted for concomitant CABG.
Next, we examined the correlation between annual hospital volumes of all SAVR procedures and their corresponding unadjusted mortality and RSMR for both SAVR + CABG and isolated SAVR using Spearman rank correlation coefficients. Similarly, we examined the correlation between overall hospital MV replacement and repair volumes and the corresponding RSMRs for these procedures. Next, we categorized hospitals into tertiles based on their annual SAVR or MV procedures. We then compared differences in patient and hospital characteristics across these tertiles. We then obtained rates of misclassification of hospital performance of SAVR + CABG, isolated SAVR, MV replacement, and MV repair based on a volume-based criterion alone. This was defined as the proportion of hospitals in the highest-volume tertile in the highest tertile for RSMR and in the lowest-volume tertile in the lowest tertile for RSMR for all 4 procedures. To examine the association of volumes and outcomes at the extremes, we repeated these analyses after classification of hospitals into volume deciles for SAVR, MV replacement, and MV repair.
Finally, to examine the contemporary landscape of procedure volumes and outcomes for aortic and MV procedures, we used survey analysis tools to obtain weighted estimates for both the number of SAVR and MV replacement and repair procedures nationally, as well as rates of in-hospital mortality during a 10-year period between 2005 and 2014.
The level of statistical significance was set at P = .05. All analyses were performed using statistical software (SAS, version 9.4; SAS Institute).
Results
A total of 682 unique hospitals with at least one SAVR and at least one MV replacement or repair procedure each year were included in the study (eFigure 1 in the Supplement). At these hospitals, 70 295 SAVR, 19 913 MV replacement, and 17 037 MV repair procedures were performed between 2007 and 2011, with a median annual volume of 43 (interquartile range [IQR], 23-76) SAVR, 13 (IQR, 6-22) MV replacement, and 9 (IQR, 4-19) MV repair procedures. Based on the distribution, hospitals were categorized as low, medium, and high volume, respectively, for SAVR (<28, 28-62, and >62 per year), MV replacement (<8, 8-18, and >18 per year), and MV repair (<6, 6-15, and >15 per year).
Hospital characteristics, both overall and across volume tertiles for SAVR and MV replacement or repair, are listed in Table 1. Higher–surgical volume hospitals were more likely to have larger bed sizes, be teaching hospitals, and represent urban locations. Patient characteristics are listed in Table 2. Patients seen at higher–surgical volume hospitals were more likely to be older and male. There was a higher prevalence of certain comorbidities, such as chronic obstructive pulmonary disease and diabetes, at lower-volume hospitals, while many other comorbidities (eg, peripheral artery disease) were more prevalent among patients seen at high-volume hospitals.
Table 1. Hospital Characteristics Overall and in Annual Hospital Volume–Based Tertiles for Surgical Aortic Valve Replacement (SAVR) and Mitral Valve (MV) Replacement or Repair.
| Variable | Overall | SAVR | MV Replacement or Repair | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | P Valuea | Tertile 1 | Tertile 2 | Tertile 3 | P Valuea | ||
| No. of hospitals | 682 | 228 | 229 | 225 | NA | 232 | 222 | 228 | NA |
| Annual Volume, Median (IQR) | |||||||||
| All SAVR | 43 (23-76) | 17 (11-23) | 43 (36-52) | 97 (76-153) | <.001 | 19 (11-26) | 42 (31-57) | 94 (69-151) | <.001 |
| SAVR + CABG | 15 (8-27) | 6 (4-9) | 16 (12-20) | 34 (26-50) | <.001 | 7 (4-11) | 15 (11-22) | 32 (23-49) | <.001 |
| Isolated SAVR | 20 (10-35) | 8 (5-10) | 20 (16-24) | 44 (35-70) | <.001 | 8 (5-13) | 19 (14-25) | 41 (30-69) | <.001 |
| All MV replacement and repair | 21 (11-40) | 8 (5-12) | 22 (15-28) | 51 (36-81) | <.001 | 7 (5-11) | 21 (18-25) | 52 (40-82) | <.001 |
| MV replacement and repair + CABG | 5 (3-10) | 2 (1-4) | 6 (4-9) | 12 (8-18) | <.001 | 2 (1-4) | 5 (4-8) | 13 (9-19) | <.001 |
| Isolated MV replacement and repair | 10 (4-19) | 4 (2-6) | 10 (7-14) | 25 (16-42) | <.001 | 4 (2-5) | 10 (8-13) | 25 (19-42) | <.001 |
| Bed Size, %b | |||||||||
| Small | 8.7 | 12.6 | 6.7 | 6.7 | <.001 | 12.4 | 6.4 | 7.1 | <.001 |
| Medium | 21.8 | 29.7 | 21.0 | 14.8 | 29.3 | 23.9 | 12.4 | ||
| Large | 69.5 | 57.7 | 72.3 | 78.5 | 58.2 | 69.7 | 80.5 | ||
| Hospital Characteristics, % | |||||||||
| Teaching hospital | 50.2 | 35.6 | 46.0 | 69.1 | <.001 | 33.8 | 47.2 | 69.5 | <.001 |
| Urban location | 94.3 | 90.1 | 96.0 | 96.9 | .002 | 88.9 | 95.9 | 98.2 | <.001 |
| Regionc | |||||||||
| Northeast | 13.0 | 5.7 | 10.9 | 22.7 | <.001 | 6.0 | 14.4 | 18.9 | <.001 |
| Midwest | 27.4 | 35.1 | 26.2 | 20.9 | 33.6 | 25.7 | 22.8 | ||
| South | 37.7 | 41.2 | 38.0 | 33.8 | 41.4 | 35.1 | 36.4 | ||
| West | 21.9 | 18.0 | 24.9 | 22.7 | 19.0 | 24.8 | 21.9 | ||
Abbreviations: CABG, coronary artery bypass grafting; IQR, interquartile range; NA, not applicable.
P value for negative trend.
Bed size categorization using Agency for Healthcare Research and Quality methods (large bed size vs others).
Northeast vs other regions.
Table 2. Patient Characteristics Overall and in Annual Hospital Volume–Based Tertiles for Surgical Aortic Valve Replacement (SAVR) and Mitral Valve (MV) Replacement or Repair.
| Variable | SAVR | MV Replacement or Repair | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Tertile 1 | Tertile 2 | Tertile 3 | P Value | Overall | Tertile 1 | Tertile 2 | Tertile 3 | P Value | |
| No. of patients | 70295 | 6063 | 15908 | 48324 | NA | 36950 | 2730 | 7361 | 26859 | NA |
| Patient Characteristics | ||||||||||
| Age, mean (SD), y | 68.7 (13.1) |
68.0 (12.7) |
68.3 (12.9) |
68.9 (13.2) |
NA | 64.5 (13.4) |
64.7 (12.8) |
64.7 (13.1) |
64.4 (13.5) |
NA |
| Female, % | 37.3 | 37.9 | 37.8 | 37.0 | .06 | 47.9 | 50.2 | 48.7 | 47.4 | .002 |
| Age >65 y, % | 67.9 | 66.4 | 67.0 | 68.4 | <.001 | 54.5 | 54.5 | 54.9 | 54.3 | .55 |
| Race, % | ||||||||||
| White | 69.2 | 63.2 | 66.4 | 70.9 | <.001 | 61.9 | 57.3 | 64.5 | 61.7 | <.001 |
| Black | 4.3 | 4.9 | 4.7 | 4.1 | 7.6 | 7.7 | 7.1 | 7.7 | ||
| Other | 9.5 | 12.2 | 11.7 | 8.5 | 11.6 | 13.3 | 12.5 | 11.1 | ||
| Missing or unknown | 17.0 | 19.7 | 17.2 | 16.5 | 18.9 | 21.8 | 16.0 | 19.5 | ||
| Comorbid Conditions, % | ||||||||||
| Heart failure | 6.4 | 6.8 | 7.0 | 6.1 | <.001 | 9.3 | 10.3 | 9.7 | 9.1 | .01 |
| Peripheral artery disease | 17.5 | 14.4 | 15.6 | 18.6 | <.001 | 7.8 | 6.9 | 7.7 | 7.9 | .09 |
| COPD | 18.2 | 20.9 | 19.1 | 17.5 | <.001a | 17.8 | 19.9 | 20.1 | 17.0 | <.001a |
| Pulmonary circulation disease | 1.5 | 1.5 | 1.6 | 1.5 | .89 | 3.4 | 3.4 | 3.3 | 3.4 | .61 |
| Hypertension | 62.4 | 61.6 | 62.6 | 62.4 | .89 | 50.8 | 50.8 | 51.3 | 50.7 | .62 |
| Obesity | 11.5 | 11.3 | 11.7 | 11.5 | .07 | 7.5 | 7.2 | 7.9 | 7.4 | .65 |
| Weight loss | 3.9 | 5.1 | 4.2 | 3.7 | <.001 | 5.6 | 6.3 | 6.1 | 5.3 | .004 |
| Paralysis | 1.6 | 1.6 | 1.7 | 1.5 | .15 | 1.8 | 2.2 | 1.8 | 1.7 | .04 |
| Other neurological disorders | 3.5 | 3.6 | 3.5 | 3.5 | .96 | 3.3 | 2.6 | 3.4 | 3.4 | .13 |
| Diabetes without complications | 21.5 | 24.0 | 22.4 | 20.9 | <.001 | 14.8 | 16.8 | 15.9 | 14.3 | <.001a |
| Diabetes with chronic complications | 3.3 | 3.0 | 3.2 | 3.3 | .15 | 2.4 | 2.3 | 2.6 | 2.3 | .47 |
| Hypothyroidism | 9.5 | 8.9 | 9.0 | 9.7 | .004 | 8.9 | 8.6 | 8.9 | 8.9 | .67 |
| Renal failure | 12.8 | 12.6 | 12.3 | 13.0 | .08 | 12.6 | 12.9 | 12.4 | 12.6 | .96 |
| Fluid and electrolyte disorders | 26.5 | 23.7 | 24.4 | 27.4 | <.001 | 28.8 | 27.9 | 27.2 | 29.4 | .002 |
| Liver disease | 1.3 | 1.3 | 1.3 | 1.3 | .002a | 1.4 | 1.5 | 1.6 | 1.3 | .18 |
| Alcohol abuse | 1.9 | 2.0 | 2.2 | 1.8 | .02a | 1.7 | 2.2 | 2.2 | 1.5 | <.001a |
| Drug abuse | 0.9 | 1.4 | 1.0 | 0.8 | <.001a | 1.3 | 1.5 | 1.4 | 1.2 | .12 |
| Psychoses | 1.5 | 1.9 | 1.5 | 1.4 | .02a | 1.6 | 2.3 | 1.8 | 1.4 | .001a |
| Depression | 4.5 | 4.1 | 4.6 | 4.5 | .51 | 4.8 | 4.7 | 5.2 | 4.7 | .34 |
| Chronic blood loss anemia | 1.5 | 1.7 | 1.8 | 1.4 | .27 | 1.6 | 1.6 | 1.3 | 1.3 | .35 |
| Iron deficiency anemia | 16.5 | 19.2 | 17.9 | 15.8 | <.001a | 15.9 | 18.8 | 16.2 | 15.6 | <.001 |
| AIDS | 0.1 | 0.1 | 0.1 | 0.1 | .60 | 0.1 | 0.1 | 0.1 | 0.1 | .82 |
| Lymphoma | 0.7 | 0.6 | 0.6 | 0.7 | .13 | 0.6 | 0.7 | 0.5 | 0.6 | .85 |
| Metastatic cancer | 0.2 | 0.2 | 0.2 | 0.2 | .56 | 0.1 | 0.1 | 0.1 | 0.1 | .29 |
| Solid tumor without metastasis | 0.9 | 0.8 | 0.8 | 1.0 | .03 | 0.6 | 0.8 | 0.4 | 0.6 | .58 |
| Coagulopathy | 21.7 | 20.4 | 21.9 | 21.8 | .09 | 21.3 | 19.4 | 21.0 | 21.6 | .01 |
| Rheumatologic diseases | 2.4 | 2.2 | 2.4 | 2.4 | .25 | 2.3 | 1.6 | 2.1 | 2.4 | .004 |
| History | ||||||||||
| Prior MI | 5.7 | 5.1 | 5.6 | 5.8 | .02 | 6.0 | 5.0 | 6.0 | 6.2 | .03 |
| Prior PCI | 6.4 | 6.1 | 6.3 | 6.4 | .28 | 4.4 | 2.4 | 3.2 | 3.5 | .001 |
| Prior CABG | 4.7 | 3.7 | 4.5 | 4.9 | <.001 | 3.4 | 4.4 | 4.7 | 4.4 | .48 |
| Admission, % | ||||||||||
| Elective | 67.0 | 66.4 | 65.2 | 67.7 | <.001 | 62.9 | 58.0 | 62.4 | 63.5 | <.001 |
| Procedure, % | ||||||||||
| Intra-aortic balloon pump | 4.7 | 5.7 | 5.3 | 4.3 | <.001a | 9.0 | 12.4 | 11.1 | 8.1 | <.001a |
| Length of Stay, d | ||||||||||
| Mean (SD) | 11.1 (10.0) | 11.3 (9.9) | 11.1 (9.4) | 11.0 (10.2) | NA | 13.1 (12.4) | 13.3 (11.0) | 13.4 (12.2) | 13.0 (12.6) | NA |
Abbreviations: CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; NA, not applicable; PCI, percutaneous coronary intervention.
P value for negative trend.
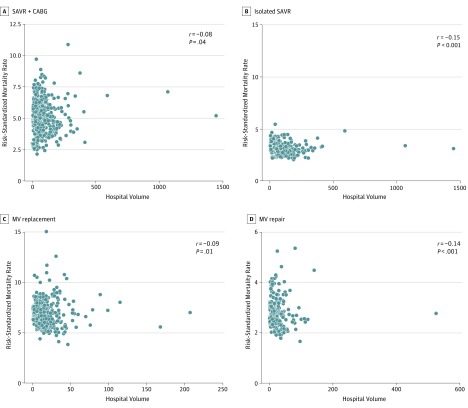
The median hospital RSMR was 4.8% (IQR, 4.5%-5.6%) for SAVR + CABG, 2.9% (IQR, 2.8%-3.2%) for isolated SAVR, 6.4% (IQR, 6.1%-7.2%) for MV replacement, and 2.7% (IQR, 2.6%-3.0%) for MV repair. There was a statistically significant but weak inverse correlation between annual hospital SAVR volume and RSMR for both SAVR + CABG (r = −0.08, P = .04) and isolated SAVR (r = −0.15, P < .001). Similarly, there was a statistically significant but weak inverse correlation between hospital volume of MV replacement and its corresponding RSMR (r = −0.09, P = .01). Furthermore, while the volume-outcome correlation for MV repair (r = −0.14, P < .001) procedures was numerically stronger, it was still fairly modest (Figure 1).
Figure 1. Spearman Rank Correlation Plots for Annual Hospital Volume.
A, Surgical aortic valve replacement (SAVR) procedures per year and risk-standardized in-hospital mortality rate for SAVR + coronary artery bypass grafting (CABG). B, SAVR procedures per year and risk-standardized in-hospital mortality for isolated SAVR. C, Mitral valve (MV) replacement procedures per year and risk-standardized in-hospital mortality rate for MV replacement. D, Mitral valve repair procedures per year and risk-standardized in-hospital mortality rate for MV repair.
The SAVR and MV procedure volumes had a high correlation with each other (r = 0.84, P < .001 for SAVR and MV replacement; r = 0.80, P < .001 for SAVR and MV repair). Despite this finding, the correlation between RSMRs for aortic and MV surgical procedures was low (r = 0.13, P = .001 for SAVR and MV replacement; r = 0.08, P = .046 for SAVR and MV repair; r = 0.16, P < .001 for SAVR + CABG and MV replacement; r = 0.20, P < .001 for SAVR + CABG and MV repair).
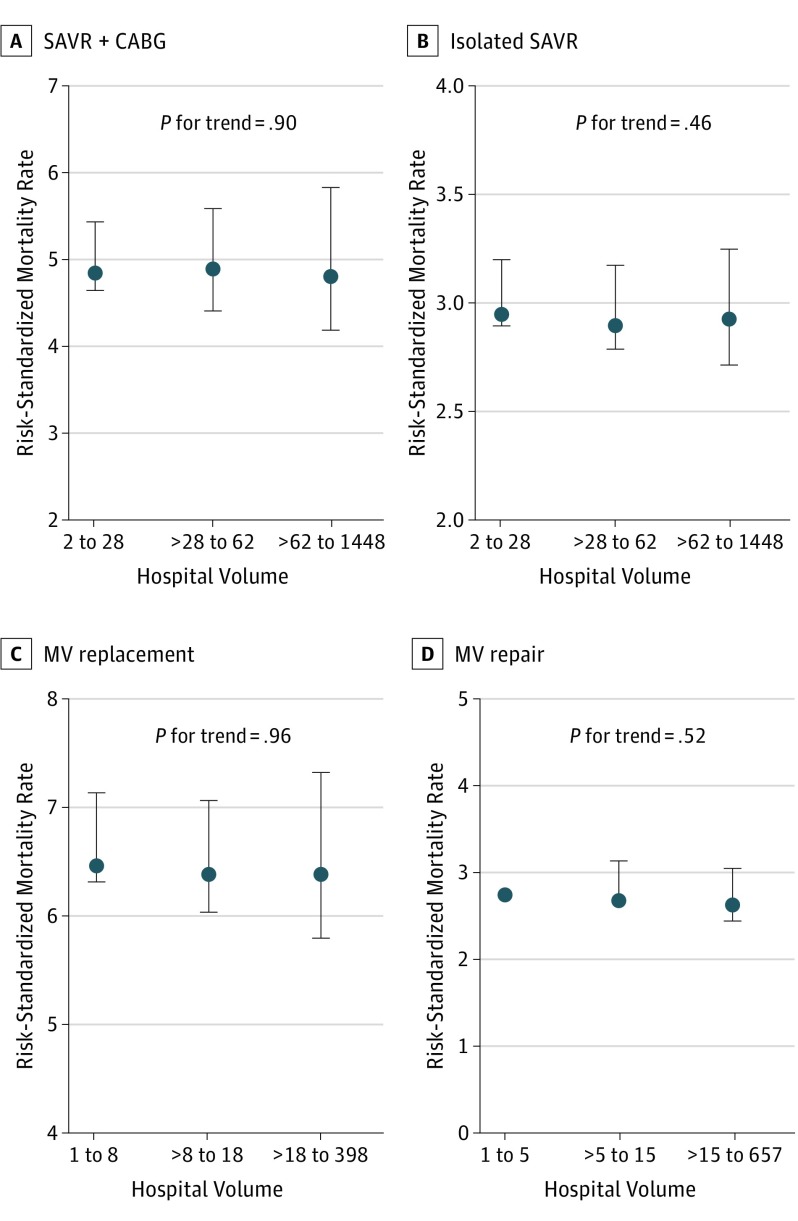
The raw observed outcomes at each hospital did not show any meaningful consistent association with outcomes (eTables 3, 4, 5, 6, and 7 and eFigure 2 in the Supplement). There was no consistent association between volume-based tertiles and RSMR for SAVR + CABG, isolated SAVR, MV replacement, and MV repair (Figure 2). The median (IQR) volumes for all procedures are listed in eTable 8 in the Supplement. Of 225 SAVR hospitals in the highest-volume tertile, 34.7% (78 of 225) and 36.0% (81 of 225) were also in the highest-RSMR tertile for SAVR + CABG and isolated SAVR procedures (RSMR>5.3% and >3.1%, respectively). Similarly, 21.5% (49 of 228) and 17.5% (40 of 228) of the 228 SAVR hospitals in the lowest-volume tertile had an RSMR for SAVR + CABG and isolated SAVR that was in the lowest-mortality tertile (RSMR<4.6 and <2.9, respectively). Similar findings were observed for MV replacement and repair procedures, where 36.8% (81 of 220) and 43.5% (91 of 209) of hospitals in the highest-volume tertile for replacement and repair procedures were in the highest corresponding RSMR tertiles (RSMR>6.9% and >2.7%, respectively), and 17.4% (38 of 218) and 11.2% (23 of 205) of hospitals in the lowest-volume tertile were in the lowest-RSMR tertile for MV replacement and repair procedures (RSMR<6.2% and <2.5%, respectively).
Figure 2. Median Risk-Standardized Mortality Rate Across Annual Hospital Volume–Based Tertiles.
A, Surgical aortic valve replacement (SAVR) + coronary artery bypass grafting (CABG) across hospital volume tertiles for SAVR. B, Isolated SAVR across hospital volume tertiles for SAVR. C, Mitral valve (MV) replacement across hospital volume tertiles for MV replacement. D, Mitral valve repair across hospital volume tertiles for MV repair. Error bars represent corresponding interquartile ranges.
Of the SAVR hospitals in the highest-volume tertile, 35.6% (80 of 225) and 39.3% (88 of 224) had an RSMR that was in the highest-RSMR tertile for MV replacement and MV repair, respectively. Similarly, of the high-volume MV replacement and MV repair hospitals, 35.4% and 33.0%, respectively, had an RSMR that was in the highest-mortality tertile for SAVR + CABG, and 35.0% and 36.8%, respectively, were in the highest-mortality tertile for isolated SAVR. If these volume-based (low, medium, and high) tertiles were used to categorize hospitals for quality, 44.7% (305 of 682) of all hospitals would be misclassified as low performing or high performing for their actual risk-standardized mortality for either SAVR or MV procedures (29.5% of SAVR-performing hospitals and 28.6% of MV replacement or repair–performing hospitals would be misclassified).
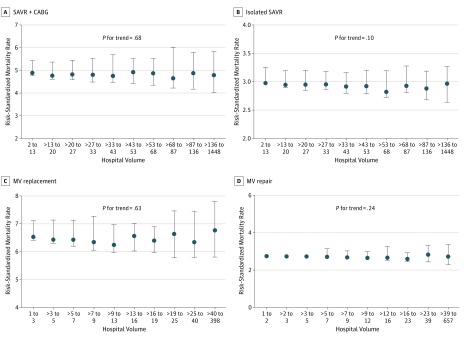
In further assessment of hospital volumes and outcomes at extremes of hospital volumes using data-derived volume deciles, we still found no evidence for lower risk-standardized mortality across the deciles of volume for each procedure. These results are shown in Figure 3.
Figure 3. Median Risk-Standardized Mortality Across Annual Hospital Volume–Based Deciles.
A, Surgical aortic valve replacement (SAVR) + coronary artery bypass grafting (CABG) across hospital volume–based deciles for SAVR. B, Isolated SAVR across hospital volume–based deciles for SAVR. C, Mitral valve (MV) replacement across hospital volume–based deciles for MV replacement. D, Mitral valve repair across hospital volume–based deciles for MV repair. Error bars represent corresponding interquartile ranges.
Finally, during the 10-year period from 2005 to 2014, the overall number of SAVR procedures increased gradually, while the number of MV procedures remained stable over time (eFigure 3 in the Supplement). Most important, compared with 2011, the characteristics of patients and in-hospital mortality for SAVR and MV replacement or repair did not change substantially during 2012 to 2014 (eFigure 4 in the Supplement).
Discussion
In a large national all-payer sample of 682 US hospitals performing 70 295 aortic and 36 950 MV surgical procedures between 2007 and 2011, we made the following important observations. First, the overall correlation between annual hospital volumes for valve surgical procedures and their corresponding in-hospital RSMR was poor. Second, although there is robust correlation between SAVR and MV procedure volumes at individual hospitals, there was little correlation between RSMRs for aortic and MV surgical procedures within the same institution. Third, using a pure volume-based criterion as a surrogate to define quality of care has the potential to misclassify a substantial number of the hospitals performing these surgical procedures in the United States.
Our findings have potentially important policy implications. Hospital variation in surgical outcomes has been central to advocating regionalization of high-risk procedures to well-performing centers. Volume-outcome associations for surgical procedures are being used as a surrogate to identify high-performing centers for both cardiovascular and noncardiovascular procedures. However, our findings suggest that an approach relying heavily on institutional surgical volume might not adequately categorize low-performing or high-performing institutions for aortic or MV procedures in the current era.
Compared with earlier analyses, what might explain the significantly attenuated volume-outcome relationship for valve surgical procedures noted in our analysis? Several studies have reported that a large proportion of hospital variation in outcomes is related to a surgeon’s experience, particularly for valve surgical procedures. Therefore, individual operator volumes may be more important than institutional volume for these procedures. It is also possible that the risk profile of patients included in the present analysis (which excluded repeated valve or multiple-valve surgical procedures) is lower and thus may not be influenced by the additional expertise potentially offered by higher-volume centers. Next, valve surgery is a fairly mature procedure in the contemporary era. Evidence shows that the volume-outcome relationship is stronger for procedures that are early in their evolution spectrum and declines as the technology matures. For example, there was a stronger volume-outcome relationship for percutaneous coronary intervention in the angioplasty era than exists in the contemporary drug-eluting stent era. Similarly, newer valve procedures (eg, TAVR) may have a more pronounced volume-outcome relationship.
From a methodological standpoint, we chose to study an all-inclusive all-payer national database. Because the observed mortality rates at low-volume centers can vary substantially by chance alone, we specifically used an empirical bayesian approach that accounts for uncertainty in outcomes at low-volume centers.Although this is an effective approach, it has also been criticized as shrinking outcomes for lower-volume hospitals toward the mean. Accordingly, we constructed all of our analyses by including hospital-level fixed effects, specifically each hospital’s procedure volumes as additional covariates in the risk-adjustment model to allow for shrinkage of estimates closer to hospitals with similar volumes. The associations for each 10-U increase in procedure volumes and mortality from this model are listed in eTable 9 in the Supplement. In analyses with and without such volume-based shrinkage, the results of the analyses were not substantially different. Some earlier analyses were either region or payer restricted or did not typically account for low-frequency events, as was done in the present analysis.
Another notable observation was that the mortality rates for aortic and MV surgery were not strongly correlated within the same institution, despite a strong correlation in procedure volumes. This result suggests a prominent procedure-specific rather than institution-specific mediator of surgical outcomes and may reflect increasingly specialized surgical expertise; indeed, many hospitals now differentiate aortic and MV surgeons given the differences in technique between the 2 procedures. This finding also argues that categorization of quality based on outcomes for one procedure type may not necessarily provide a suitable assessment for other valve procedures, suggesting that a systems-of-care approach for valve surgical procedures (eg, streamlining preoperative and postoperative processes of care and resources) may be less meaningful compared with other procedures. Again, the finding may be a reflection of the greater influence of operator-dependent variation in outcomes rather than an institution-dependent variation.
This is not to completely undermine the role of hospital procedure volumes. In fact, almost paradoxically, relying on RSMR alone may be problematic if hospitals do not have adequate caseloads for appropriate risk adjustment. Lower-volume hospitals may also have higher failure-to-rescue rates from a given complication than higher-volume hospitals, despite a similar overall complication rate.
Limitations
This study has certain limitations. Given our study’s administrative nature, variables defined in The Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Risk Calculator (http://riskcalc.sts.org/stswebriskcalc/#/calculate) were not directly available. We attempted to address this issue in multiple ways. Our risk-adjustment model uses Elixhauser comorbidities that have been validated to predict early mortality across a spectrum of medical and surgical conditions and had good discrimination for mortality (model C statistic, 0.753), comparable to the previously reported model discrimination for STS (C statistic rage, 0.71-0.76). Moreover, the mortality rates observed in our database are similar to those reported in the STS Adult Cardiac Surgery Database. For instance, the observed mortality for isolated SAVR of 2.9% in the present study is identical to that reported in the STS database. Furthermore, the algorithm used herein specifically identifies comorbidities from complications, the latter of which should not be included in risk-standardization algorithms. We also did not account for some potentially important information, such as ejection fraction, pulmonary artery pressures, and the number of diseased vessels. We report in-hospital rather than 30-day mortality, which is the STS standard. However, almost 80% of the surgical 30-day mortality occurs during hospitalization.
Next, given the administrative nature of the study, we could not conclusively identify procedural complications, such as stroke, reoperation, and readmissions, but rather have focused on hard clinical end points of mortality. Finally, for hospital-level analysis, our study does not use the most recent 2012-2014 NIS data but instead focuses on 2007-2011 data, in which all procedures at a hospital are available in the data set. We particularly chose this strategy for 2 reasons. First, the 2012-2014 NIS data have a different sampling strategy such that 20% of hospital discharges are sampled on average, and hospital-level estimates for low-volume procedures based on discharge weights are not appropriate. Second, these dates predate the introduction of TAVR and thus represent a cleaner relationship between volume and outcomes for SAVR in particular. Both patient-level characteristics and their outcomes for all studied procedures in 2012 to 2014 (eTable 10 and eTable 11 in the Supplement), representing the more recent years for which the NIS data are available, are unchanged from 2011; therefore, our findings are likely an accurate reflection of contemporary surgical practice.
Conclusions
Hospital procedure volume is an imperfect surrogate for risk-adjusted outcomes after aortic and MV surgical procedures. Furthermore, while the same hospitals were likely to be high-volume and low-volume centers for SAVR and MV procedures, outcomes for SAVR do not predict an institution’s MV surgery outcomes. From a health care policy and systems-of-care perspective, quality improvement endeavors should focus on a more comprehensive assessment that includes risk-adjusted mortality and morbidity metrics rather than on hospital volume alone.
eTable 1. National Inpatient Sample (NIS) Study Checklist
eTable 2. Elixhauser Comorbidities Used in the Risk-Adjustment Model in This Study
eTable 3. Correlation Between Procedure and Unadjusted In-Hospital Mortality
eTable 4. Trends in Unadjusted Median In-Hospital Mortality (and Interquartile Range) Across Deciles of Procedure Volume for Isolated Surgical Aortic Valve Replacement (SAVR)
eTable 5. Trends in Unadjusted Median In-Hospital Mortality (and Interquartile Range) Across Deciles of Procedure Volume for Surgical Aortic Valve Replacement (SAVR) + Coronary Artery Bypass Grafting (CABG)
eTable 6. Trends in Unadjusted Median In-Hospital Mortality (and Interquartile Range) Across Deciles of Procedure Volume for Mitral Valve (MV) Repair
eTable 7. Trends in Unadjusted Median In-Hospital Mortality (and Interquartile Range) Across Deciles of Procedure Volume for Mitral Valve (MV) Replacement
eTable 8. Risk-Standardized Mortality Rates (Median and Interquartile Range) for Operative Procedures in This Study
eTable 9. Association Between Volume and In-Hospital Mortality in the Hierarchical Risk-Adjustment Models for Valve Procedures, Reported for a 10-Unit Increase in Procedure Volumes
eTable 10. SAVR Patient Characteristics, 2011 to 2014
eTable 11. MV Repair and Replacement Patient Characteristics, 2011 to 2014
eFigure 1. Patient Selection Flowsheet
eFigure 2. Unadjusted In-Hospital Mortality by Hospital Volume
eFigure 3. Estimated National Procedural Volumes for (A) SAVR and (B) MVR Replacement and Repair
eFigure 4. Estimated In-Hospital Mortality Rates for (A) SAVR + CABG and Isolated CABG and (B) MV Replacement and Repair
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics: 2017 update: a report from the American Heart Association [published correction appears in Circulation. 2017;135(10):e646]. Circulation. 2017;135(10):e146-e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishimura RA, Otto CM, Bonow RO, et al. ; ACC/AHA Task Force Members . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(23):e650]. Circulation. 2014;129(23):2440-2492. [DOI] [PubMed] [Google Scholar]
- 3.Bonow RO, Adams DH. The time has come to define centers of excellence in mitral valve repair. J Am Coll Cardiol. 2016;67(5):499-501. [DOI] [PubMed] [Google Scholar]
- 4.Chambers J, Ray S, Prendergast B, et al. Standards for heart valve surgery in a “heart valve centre of excellence”. Open Heart. 2015;2(1):e000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? the empirical relation between surgical volume and mortality. N Engl J Med. 1979;301(25):1364-1369. [DOI] [PubMed] [Google Scholar]
- 6.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128-1137. [DOI] [PubMed] [Google Scholar]
- 7.Kumbhani DJ, Bittl JA. Much ado about nothing? the relationship of institutional percutaneous coronary intervention volume to mortality. Circ Cardiovasc Qual Outcomes. 2017;10(3):pii: e003610. [DOI] [PubMed] [Google Scholar]
- 8.Khuri SF. Quality, advocacy, healthcare policy, and the surgeon. Ann Thorac Surg. 2002;74(3):641-649. [DOI] [PubMed] [Google Scholar]
- 9.Shahian DM, He X, Jacobs JP, et al. The Society of Thoracic Surgeons isolated aortic valve replacement (AVR) composite score: a report of the STS Quality Measurement Task Force. Ann Thorac Surg. 2012;94(6):2166-2171. [DOI] [PubMed] [Google Scholar]
- 10.Badhwar V, Rankin JS, He X, et al. The Society of Thoracic Surgeons mitral repair/replacement composite score: a report of The Society of Thoracic Surgeons Quality Measurement Task Force. Ann Thorac Surg. 2016;101(6):2265-2271. [DOI] [PubMed] [Google Scholar]
- 11.Shahian DM, Edwards FH, Ferraris VA, et al. ; Society of Thoracic Surgeons Quality Measurement Task Force . Quality measurement in adult cardiac surgery, part 1: conceptual framework and measure selection. Ann Thorac Surg. 2007;83(4)(suppl):S3-S12. [DOI] [PubMed] [Google Scholar]
- 12.Kumbhani DJ, Cannon CP, Fonarow GC, et al. ; Get With the Guidelines Steering Committee and Investigators . Association of hospital primary angioplasty volume in ST-segment elevation myocardial infarction with quality and outcomes. JAMA. 2009;302(20):2207-2213. [DOI] [PubMed] [Google Scholar]
- 13.Kumbhani DJ, Nallamothu BK. PCI volume benchmarks: still adequate for quality assessment in 2017? J Am Coll Cardiol. 2017;69(24):2925-2928. [DOI] [PubMed] [Google Scholar]
- 14.Kidher E, Sepehripour A, Punjabi P, Athanasiou T. Do bigger hospitals or busier surgeons do better adult aortic or mitral valve operations? Interact Cardiovasc Thorac Surg. 2010;10(4):605-610. [DOI] [PubMed] [Google Scholar]
- 15.Grendar J, Shaheen AA, Myers RP, et al. Predicting in-hospital mortality in patients undergoing complex gastrointestinal surgery: determining the optimal risk adjustment method. Arch Surg. 2012;147(2):126-135. [DOI] [PubMed] [Google Scholar]
- 16.Khera R, Cram P, Lu X, et al. Trends in the use of percutaneous ventricular assist devices: analysis of National Inpatient Sample data, 2007 through 2012. JAMA Intern Med. 2015;175(6):941-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for Takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khera R, Pandey A, Kumar N, et al. Variation in hospital use and outcomes associated with pulmonary artery catheterization in heart failure in the United States. Circ Heart Fail. 2016;9(11):e003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houchens RL, Ross DN, Elixhauser A, Jiang J Nationwide Inpatient Sample redesign final report. https://www.hcup-us.ahrq.gov/db/nation/nis/reports/NISRedesignFinalReport040914.pdf. Published April 4, 2014. Accessed July 20, 2014.
- 20.Healthcare Cost and Utilization Project 2012 NIS redesign report available on HCUP website (May 2014). https://www.hcup-us.ahrq.gov/news/announcements/nisredesign_2012.jsp. Release date May 2014. Accessed July 20, 2014.
- 21.Khera R, Krumholz HM. With great power comes great responsibility: big data research from the National Inpatient Sample. Circ Cardiovasc Qual Outcomes. 2017;10(7):e003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elixhauser A, Steiner C, Palmer L. Clinical Classifications Software (CCS). Rockville, MD: US Agency for Healthcare Research and Quality; 2011.
- 23.Khera R, Chan PS, Donnino M, Girotra S; American Heart Association’s Get With The Guidelines–Resuscitation Investigators. Hospital variation in time to epinephrine for nonshockable in-hospital cardiac arrest. Circulation. 2016;134(25):2105-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. [DOI] [PubMed] [Google Scholar]
- 25.Li B, Evans D, Faris P, Dean S, Quan H. Risk adjustment performance of Charlson and Elixhauser comorbidities in ICD-9 and ICD-10 administrative databases. BMC Health Serv Res. 2008;8(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimick JB, Staiger DO, Birkmeyer JD. Ranking hospitals on surgical mortality: the importance of reliability adjustment. Health Serv Res. 2010;45(6, pt 1):1614-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quail JM, Lix LM, Osman BA, Teare GF. Comparing comorbidity measures for predicting mortality and hospitalization in three population-based cohorts. BMC Health Serv Res. 2011;11(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42(4):355-360. [DOI] [PubMed] [Google Scholar]
- 29.Juurlink D, Preyra C, Croxford R, et al. Canadian Institute for Health Information Discharge Abstract Database: A Validation Study. Ontario, Canada: Institute for Clinical Evaluation Sciences; 2006. [Google Scholar]
- 30.Iezzoni LI. Risk Adjustment for Measuring Health Care Outcomes. Chicago, IL: Health Administration Press; 2003. [Google Scholar]
- 31.Christiansen CL, Morris CN. Improving the statistical approach to health care provider profiling. Ann Intern Med. 1997;127(8, pt 2):764-768. [DOI] [PubMed] [Google Scholar]
- 32.Normand S-LT, Glickman ME, Gatsonis CA. Statistical methods for profiling providers of medical care: issues and applications. J Am Stat Assoc. 1997;92(439):803-814. [Google Scholar]
- 33.Staiger DO, Dimick JB, Baser O, Fan Z, Birkmeyer JD. Empirically derived composite measures of surgical performance. Med Care. 2009;47(2):226-233. [DOI] [PubMed] [Google Scholar]
- 34.Dimick JB, Staiger DO, Baser O, Birkmeyer JD. Composite measures for predicting surgical mortality in the hospital. Health Aff (Millwood). 2009;28(4):1189-1198. [DOI] [PubMed] [Google Scholar]
- 35.Dimick JB, Welch HG, Birkmeyer JD. Surgical mortality as an indicator of hospital quality: the problem with small sample size. JAMA. 2004;292(7):847-851. [DOI] [PubMed] [Google Scholar]
- 36.Hannan EL, O’Donnell JF, Kilburn H Jr, Bernard HR, Yazici A. Investigation of the relationship between volume and mortality for surgical procedures performed in New York State hospitals. JAMA. 1989;262(4):503-510. [PubMed] [Google Scholar]
- 37.Gonzalez AA, Dimick JB, Birkmeyer JD, Ghaferi AA. Understanding the volume-outcome effect in cardiovascular surgery: the role of failure to rescue. JAMA Surg. 2014;149(2):119-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel HJ, Herbert MA, Drake DH, et al. Aortic valve replacement: using a statewide cardiac surgical database identifies a procedural volume hinge point. Ann Thorac Surg. 2013;96(5):1560-1565. [DOI] [PubMed] [Google Scholar]
- 39.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117-2127. [DOI] [PubMed] [Google Scholar]
- 40.Kilic A, Shah AS, Conte JV, Baumgartner WA, Yuh DD. Operative outcomes in mitral valve surgery: combined effect of surgeon and hospital volume in a population-based analysis. J Thorac Cardiovasc Surg. 2013;146(3):638-646. [DOI] [PubMed] [Google Scholar]
- 41.Chikwe J, Toyoda N, Anyanwu AC, et al. Relation of mitral valve surgery volume to repair rate, durability, and survival [published online April 24, 2017]. J Am Coll Cardiol. 2017;S0735-1097(17)30677-0. doi: 10.1016/j.jacc.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 42.Dewey TM, Herbert MA, Ryan WH, et al. Influence of surgeon volume on outcomes with aortic valve replacement. Ann Thorac Surg. 2012;93(4):1107-1112. [DOI] [PubMed] [Google Scholar]
- 43.Shahian DM, Normand SL. The volume-outcome relationship: from Luft to Leapfrog. Ann Thorac Surg. 2003;75(3):1048-1058. [DOI] [PubMed] [Google Scholar]
- 44.O’Neill D, Nicholas O, Gale CP, et al. Total center percutaneous coronary intervention volume and 30-day mortality: a contemporary national cohort study of 427 467 elective, urgent, and emergency cases. Circ Cardiovasc Qual Outcomes. 2017;10(3):e003186. [DOI] [PubMed] [Google Scholar]
- 45.Khera S, Kolte D, Gupta T, et al. Association between hospital volume and 30-day readmissions following transcatheter aortic valve replacement. JAMA Cardiol. 2017;2(7):732-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edwards FH, Peterson ED, Coombs LP, et al. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol. 2001;37(3):885-892. [DOI] [PubMed] [Google Scholar]
- 47.Rankin JS, He X, O’Brien SM, et al. The Society of Thoracic Surgeons risk model for operative mortality after multiple valve surgery. Ann Thorac Surg. 2013;95(4):1484-1490. [DOI] [PubMed] [Google Scholar]
- 48.Overman DM, Jacobs JP, Prager RL, et al. Report from The Society of Thoracic Surgeons National Database Workforce: clarifying the definition of operative mortality. World J Pediatr Congenit Heart Surg. 2013;4(1):10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adult Cardiac Surgery Database: executive summary: 10 Years: STS period ending 3/31/2016. STS Adult Cardiac Surgery Database: period ending 3/31/2016 executive summary contents. https://www.sts.org/sites/default/files/documents/ACSD_2017Harvest2_ExecutiveSummary.pdf. Accessed March 18, 2017.
- 50.Thourani VH, Suri RM, Gunter RL, et al. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. Ann Thorac Surg. 2015;99(1):55-61. [DOI] [PubMed] [Google Scholar]
- 51.Hannan EL, Racz M, Culliford AT, et al. Risk score for predicting in-hospital/30-day mortality for patients undergoing valve and valve/coronary artery bypass graft surgery. Ann Thorac Surg. 2013;95(4):1282-1290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. National Inpatient Sample (NIS) Study Checklist
eTable 2. Elixhauser Comorbidities Used in the Risk-Adjustment Model in This Study
eTable 3. Correlation Between Procedure and Unadjusted In-Hospital Mortality
eTable 4. Trends in Unadjusted Median In-Hospital Mortality (and Interquartile Range) Across Deciles of Procedure Volume for Isolated Surgical Aortic Valve Replacement (SAVR)
eTable 5. Trends in Unadjusted Median In-Hospital Mortality (and Interquartile Range) Across Deciles of Procedure Volume for Surgical Aortic Valve Replacement (SAVR) + Coronary Artery Bypass Grafting (CABG)
eTable 6. Trends in Unadjusted Median In-Hospital Mortality (and Interquartile Range) Across Deciles of Procedure Volume for Mitral Valve (MV) Repair
eTable 7. Trends in Unadjusted Median In-Hospital Mortality (and Interquartile Range) Across Deciles of Procedure Volume for Mitral Valve (MV) Replacement
eTable 8. Risk-Standardized Mortality Rates (Median and Interquartile Range) for Operative Procedures in This Study
eTable 9. Association Between Volume and In-Hospital Mortality in the Hierarchical Risk-Adjustment Models for Valve Procedures, Reported for a 10-Unit Increase in Procedure Volumes
eTable 10. SAVR Patient Characteristics, 2011 to 2014
eTable 11. MV Repair and Replacement Patient Characteristics, 2011 to 2014
eFigure 1. Patient Selection Flowsheet
eFigure 2. Unadjusted In-Hospital Mortality by Hospital Volume
eFigure 3. Estimated National Procedural Volumes for (A) SAVR and (B) MVR Replacement and Repair
eFigure 4. Estimated In-Hospital Mortality Rates for (A) SAVR + CABG and Isolated CABG and (B) MV Replacement and Repair