This secondary analysis of a randomized clinical trial compares outcomes of evolocumab and placebo for subgroups of patients with stable atherosclerotic cardiovascular disease.
Key Points
Questions
Do patients with stable atherosclerotic cardiovascular disease treated with a statin who have a low-density lipoprotein cholesterol level of less than 70 mg/dL and those already receiving a maximal-potency statin benefit from the addition of evolocumab?
Findings
In this secondary analysis of a randomized clinical trial of 27 564 patients with stable disease, compared with placebo, evolocumab reduced cardiovascular events to a similar degreee in patients with a low-density lipoprotein cholesterol level of less than or at least 70 mg/dL and in those treated with a maximal-potency statin or a less potent statin regimen.
Meaning
In high-risk patients with stable atherosclerotic cardiovascular disease treated with a statin, patients who have a low level of low-density lipoprotein cholesterol and patients receiving a maximal-potency statin may experience further reduction of cardiovascular events with the addition of evolocumab.
Abstract
Importance
Current guidelines for atherosclerotic cardiovascular disease focus on high-intensity statins and targeting or using a threshold low-density lipoprotein cholesterol (LDL-C) level of less than 70 mg/dL for the highest-risk patients. Whether further reduction of LDL-C beyond these boundaries would be beneficial is unknown.
Objective
To compare outcomes of evolocumab vs placebo in patients with stable atherosclerotic cardiovascular disease and a baseline LDL-C of less than 70 mg/dL and in those receiving background treatment with a maximal-potency statin.
Design, Setting, and Participants
This secondary ad hoc analysis of the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial compared randomized treatments in 2 subgroups of patients with stable atherosclerotic cardiovascular disease currently receiving statin. Patients were classified by a baseline LDL-C of less than 70 or at least 70 mg/dL and by statin intensity (maximal: atorvastatin calcium, 80 mg/d, or rosuvastatin, 40 mg/d; submaximal: all other dosages). Patients with baseline LDL of less than 70 mg/dL either had a final screening LDL-C of at least 70 mg/dL or a final screening non–high-density lipoprotein cholesterol level of at least 100 mg/dL. Data were retrieved from 2013 to 2016 and analyzed in 2017 based on intention to treat.
Main Outcomes and Measures
The primary efficacy endpoint was the composite of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization. The secondary efficacy endpoint was the composite of cardiovascular death, myocardial infarction, or stroke. Safety outcomes included adverse events and events of interest identified in the FOURIER trial. Interaction testing was used to assess the consistency of results in patients who did vs did not satisfy the above criteria.
Results
A total of 27 564 patients (75.4% men and 24.6% women; mean [SD] age, 62.5 [9.0] years) were included in the analysis. Of 2034 patients (7.4%) who had a baseline LDL-C of less than 70 mg/dL, evolocumab reduced the risk for the primary endpoint (hazard ratio [HR], 0.80; 95% CI, 0.60-1.07) to a similar degree as in the 25 529 patients who had baseline LDL-C of at least 70 mg/dL (HR 0.86; 95% CI, 0.79-0.92; P = .65 for interaction; 1 patient was missing baseline LDL-C data). Of 7533 patients (27.3%) receiving maximal-potency statins, evolocumab significantly reduced the primary endpoint (HR, 0.86; 95% CI, 0.75-0.98) to a similar degree as in the 20 031 patients not receiving a maximal-potency statin (HR, 0.85; 95% CI, 0.78-0.93; P = .88 for interaction). The key secondary endpoint was reduced to a similar degree in both analyses. No major safety concerns were identified.
Conclusions and Relevance
Evolocumab was equally effective in reducing cardiovascular events in patients with stable atherosclerotic cardiovascular disease regardless of whether the baseline LDL-C was less than 70 or at least 70 mg/dL and whether the background statin was of maximal or submaximal potency.
Introduction
Several guidelines endorse a target low-density lipoprotein cholesterol (LDL-C) level of less than 70 mg/dL (to convert to millimoles per liter, multiply by 0.0259) or a threshold for treatment of at least 70 mg/dL in the highest-risk patients for secondary prevention of cardiovascular events. Likewise, high-intensity statin regimens (ie, atorvastatin calcium, ≥40 mg/d, or rosuvastatin, ≥20 mg/d) are recommended as foundational therapy. Whether more intensive lowering of LDL-C levels would benefit patients who already have an LDL-C level of less than 70 mg/dL or patients who are currently receiving maximal-potency statin therapy (highest doses possible) is, to our knowledge, unknown. We explored the efficacy and safety of evolocumab vs placebo in such patients in the Further Cardiovascular Outcomes Research With PCSK9 (proprotein convertase subtilisin/kexin type 9) Inhibition in Subjects With Elevated Risk (FOURIER) trial.
Methods
Study Design and Treatment
The design of the FOURIER trial has been reported elsewhere. In brief, 27 564 patients with prior myocardial infarction, nonhemorrhagic stroke, or symptomatic peripheral artery disease and additional characteristics that placed them at higher cardiovascular risk (including 1 major and 2 minor criteria) were randomized to receive the PCSK9 inhibitor evolocumab or placebo. Eligible patients had an LDL-C level of at least 70 mg/dL or a non–high-density lipoprotein cholesterol (non–HDL-C) level of at least 100 mg/dL at the end of screening while receiving moderate- or high-intensity statin therapy (defined as atorvastatin calcium, ≥20 mg/d, or the equivalent). In the FOURIER trial, LDL-C level was calculated on the basis of the Friedewald equation unless the calculated value was less than 40 mg/dL or the measured triglyceride level was greater than 400 mg/dL (to convert to millimoles per liter, multiply by 0.0113), in which case ultracentrifugation was performed. In the present ad hoc analysis, we compared outcomes of evolocumab treatment vs placebo in the following 2 subgroups: (1) patients with a baseline LDL-C level (the mean of the values obtained at the final screening visit and the day of randomization) of less than 70 (who either had a final screening LDL-C of at least 70 mg/dL or a final screening non-HDL-C of at least 100 mg/dL) vs at least 70 mg/dL and (2) patients receiving a maximal-potency background statin (ie, atorvastatin calcium, 80 mg/d, or rosuvastatin, 40 mg/d) vs submaximal statin at randomization. Ethics Committee approvals for the FOURIER trial were obtained from all relevant organizations locally or through a central institutional review board within the country (including 1242 centers from 49 countries), and each patient provided written informed consent.
Study Outcomes
Study data were retrieved from 2013 to 2016. The primary endpoint of the FOURIER trial was the composite of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization; the key secondary endpoint was the composite of cardiovascular death, myocardial infarction, or stroke. Safety endpoints included overall adverse events and adverse events of interest, including allergic and injection site reactions, and adverse events related to muscle symptoms, elevations in creatine kinase or transaminase levels, cataracts, new-onset diabetes, and neurocognitive events.
Statistical Analysis
Data were analyzed in 2017. We compared baseline categorical variables using χ2 or Fisher exact tests and continuous variables using the Wilcoxon rank sum test. Efficacy analyses were performed in the intention-to-treat population, including all patients who underwent randomization and provided written informed consent. Hazard ratios (HRs) and 95% CIs of the time to the first efficacy event were generated using a Cox proportional hazards model, and P values for time-to-event analyses were calculated using log-rank tests, with P < .05 indicating significance. Safety evaluations included all the patients who underwent randomization, who received at least 1 dose of a study agent, and for whom postdose data were available. Interaction testing was performed using Cox proportional hazards models for efficacy endpoints and logistic regression for safety endpoints.
Results
Patients With a Baseline LDL-C Level of Less Than 70 mg/dL
A total of 27 564 patients (75.4% men and 24.6% women; mean [SD] age, 62.5 [9.0] years) were included in the analysis. Baseline LDL-C level was unavailable for 1 patient. A total of 2034 patients (7.4%) had a baseline LDL-C level of less than 70 mg/dL. Compared with the 25 529 patients with LDL-C levels of at least 70 mg/dL at baseline, these patients tended to be younger (mean [SD] age, 62.1 [9.2] vs 62.5 [9.0] years) and have greater weight (mean [SD] weight, 88.2 [18.2] vs 85.0 [17.2] kg) and were more likely to be male (1632 [80.2%] vs 19 162 [75.1%]) and have had a prior stroke (430 [21.1%] vs 4907 [19.2%]), hypertension (1673 [82.3%] vs 20 410 [80.0%]), diabetes (987 [48.5%] vs 9093 [35.6%]), and metabolic syndrome (1481 [72.8%] vs 14 869 [58.2%]) (Table 1). The median baseline LDL-C level was 65.5 mg/dL (interquartile range [IQR], 61.0-68.0 mg/dL). In this subgroup, 1030 patients (51%) had a baseline non-HDL-C level of at least 100 mg/dL and 1004 patients (49%) had a non-HDL-C level less than 100 mg/dL.
Table 1. Patient Characteristics Stratified by Baseline LDL-C Level and Background Statin Intensitya.
| Characteristic | Baseline LDL-C Level, mg/dL (N = 27 563)b |
P Value | Baseline Statin Potency (N = 27 564) |
P Value | ||
|---|---|---|---|---|---|---|
| <70 (n = 2034)c |
≥70 (n = 25 529) |
Maximald (n = 7533) |
Submaximal (n = 20 031) |
|||
| Age, mean (SD), y | 62.1 (9.2) | 62.5 (9.0) | .051 | 61.1 (8.9) | 63.0 (9.0) | <.001 |
| Weight, mean (SD), kg | 88.2 (18.2) | 85.0 (17.2) | <.001 | 88.2 (17.6) | 84.2 (17.2) | <.001 |
| Male | 1632 (80.2) | 19 162 (75.1) | <.001 | 5722 (76.0) | 15 073 (75.2) | .22 |
| White racee | 1708 (84.0) | 21 749 (85.2) | .14 | 7027 (93.3) | 16 431 (82.0) | <.001 |
| Region | ||||||
| North America | 348 (17.1) | 4223 (16.5) | <.001 | 1877 (24.9) | 2694 (13.4) | <.001 |
| Europe | 1226 (60.3) | 16 108 (63.1) | 4862 (64.5) | 12 473 (62.3) | ||
| Latin America | 178 (8.8) | 1645 (6.4) | 180 (2.4) | 1643 (8.2) | ||
| Asia, Pacific, South Africa | 282 (13.9) | 3553 (13.9) | 614 (8.2) | 3221 (16.1) | ||
| Type of atherosclerosisf | ||||||
| Myocardial infarction | 1591 (78.2) | 20 759 (81.3) | <.001 | 6499 (86.3) | 15 852 (79.1) | <.001 |
| Nonhemorrhagic stroke | 430 (21.1) | 4907 (19.2) | .04 | 1182 (15.7) | 4155 (20.7) | <.001 |
| Peripheral artery disease | 276 (13.6) | 3366 (13.2) | .62 | 1012 (13.4) | 2630 (13.1) | .51 |
| Cardiovascular risk factors | ||||||
| Hypertension | 1673 (82.3) | 20 410 (80.0) | .01 | 6019 (79.9) | 16 065 (80.2) | .57 |
| Diabetes | 987 (48.5) | 9093 (35.6) | <.001 | 2536 (33.7) | 7545 (37.7) | <.001 |
| Metabolic syndrome | 1481 (72.8) | 14 869 (58.2) | <.001 | 4501 (59.8) | 11 850 (59.2) | .38 |
| Current cigarette use | 544 (26.7) | 7232 (28.3) | .13 | 2067 (27.4) | 5710 (28.5) | .08 |
| TIMI Risk Score for secondary prevention, mean (SD)g | 3.4 (1.2) | 3.3 (1.2) | <.001 | 3.3 (1.3) | 3.3 (1.2) | .002 |
| Statin intensity at baselineh | .02 | |||||
| High | 1365 (67.1) | 17 737 (69.5) | NA | 7533 (100) | 11 570 (57.8) | NA |
| Atorvastatin calcium, 80 mg/d, or rosuvastatin, 40 mg/d | 524 (25.8) | 7008 (27.5) | .10 | 7533 (100) | 0 | NA |
| Moderate | 667 (32.8) | 7725 (30.3) | NA | 0 | 8392 (41.9) | NA |
| Low, unknown, or no data | 2 (0.1) | 67 (0.3) | NA | 0 | 69 (0.3) | NA |
| Ezetimibe treatment | 83 (4.1) | 1357 (5.3) | .02 | 672 (8.9) | 768 (3.8) | <.001 |
| Other cardiovascular medicationsi | ||||||
| Aspirin and/or P2Y12 inhibitor | 1875 (92.2) | 23 556 (92.4) | .77 | 7122 (94.6) | 18 310 (91.5) | <.001 |
| β-Blocker | 1582 (77.8) | 19 232 (75.4) | .02 | 6056 (80.4) | 14 759 (73.8) | <.001 |
| Renin-angiotensin-aldosterone inhibitor | 1626 (79.9) | 19 906 (78.1) | .047 | 6016 (79.9) | 15 517 (77.5) | <.001 |
| Lipid levels, median (IQR), mg/dL | ||||||
| LDL-C | 65.5 (61.0-68.0) | 93.5 (82.0. 110.5) | NA | 93.0 (80.0-111.5) | 91.0 (79.5-107.5) | <.001 |
| Total cholesterol | 141.0 (132.0-152.0) | 170.0 (153.5-190.5) | NA | 168.0 (150.5-190.5) | 167.0 (151.0-187.5) | .004 |
| HDL-C | 38.5 (32.5. 47.0) | 44.0 (37.5-52.5) | NA | 43.0 (36.5-51.5) | 44.0 (37.0-53.0) | <.001 |
| Triglycerides | 181.0 (115.0-252.0) | 131.0 (99.5-177.0) | NA | 133.0 (98.5-181.0) | 133.0 (100.0-182.0) | .19 |
| LDL-C level <70 mg/dL at baseline | 2034 (100) | 0 | NA | 524 (7.0) | 1510 (7.5) | .10 |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; NA not applicable; TIMI, Thrombolysis in Myocardial Infarction.
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.0259; triglycerides to millomoles per liter, multiply by 0.0113.
Unless otherwise indicated, data are expressed as number (percentage) of patients. Percentages have been rounded and may not total 100. We found no nominally significant differences between the randomized treatments in either group stratified by baseline LDL-C level or stratified by baseline maximal statin potency except for baseline triglyceride in the submaximal statin intensity subgroup (P = .05).
Baseline LDL-C data were not available for 1 patient.
These patients either had a final screening LDL-C of at least 70 mg/dL or a final screening non–HDL-C level of at least 100 mg/dL.
Maximal statin potency indicates atorvastatin calcium, 80 mg/d, or rosuvastatin, 40 mg/d. All other statin regimens were considered to be submaximal.
Reported by the patients.
Patients could have more than 1 type of atherosclerosis.
As described by Bohula et al, scores range from 0 to 9, with higher scores indicating higher risk.
Categorized in accordance with the guidelines of the American College of Cardiology and American Heart Association.
Owing to missing patient data, denominators may be less than column headings.
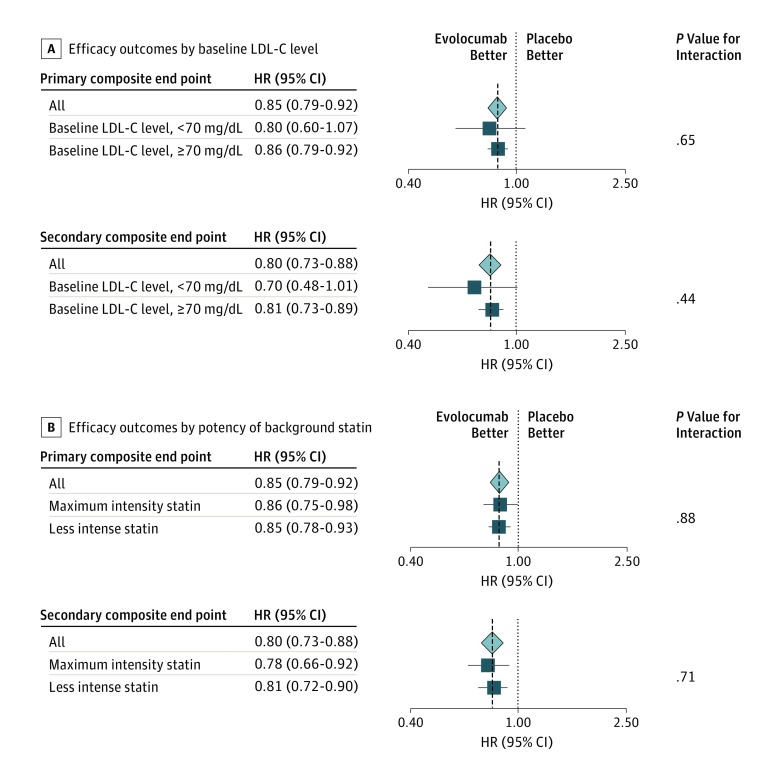
At 48 weeks, the least-squares mean percentage reduction in LDL-C level with evolocumab treatment, compared with placebo, was 66%, for a mean absolute reduction of 42 mg/dL and a median achieved concentration at 48 weeks of 21.0 mg/dL (IQR, 11.5-37.0 mg/dL). Evolocumab reduced the risk for the primary composite endpoint by 20% (HR, 0.80; 95% CI, 0.60-1.07) in patients with a baseline LDL-C level of less than 70 mg/dL and by 14% (HR, 0.86; 95% CI, 0.79-0.92) in patients with an LDL-C level of at least 70 mg/dL, with no evidence of treatment effect modification by baseline LDL-C (P = .65 for interaction) (Figure 1A). Likewise, evolocumab reduced the risk for the key secondary endpoint by 30% (HR, 0.70; 95% CI, 0.48-1.01) in patients with a baseline LDL-C level of less than 70 mg/dL (Figure 2A) and by 19% (HR, 0.81; 95% CI, 0.73-0.89) in patients with an LDL-C level of at least 70 mg/dL, with no evidence of treatment effect modification owing to baseline LDL-C level (P = .44 for interaction) (Figure 1A). We found no heterogeneity for any of the individual outcomes (eTable 1 in the Supplement). Likewise, we found no heterogeneity in the safety profile of evolocumab as a function of baseline LDL-C level (Table 2).
Figure 1. Efficacy Outcomes Stratified by Baseline Low-Density Lipoprotein Cholesterol (LDL-C) Levels and Intensity of Background Statin Treatment.
Hazard ratios (HRs) and 95% CIs are shown for the primary (composite of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, and coronary revascularization) and the key secondary (composite of cardiovascular death, myocardial infarction, and stroke) efficacy composite endpoints in the total population and (A) in patients with baseline LDL-C levels of less than 70 mg/dL vs those with LDL-C levels of at least 70 mg/dL and (B) in patients treated with maximal (atorvastatin calcium, 80 mg/d, or rosuvastatin, 40 mg/d) and submaximal background statin therapy.
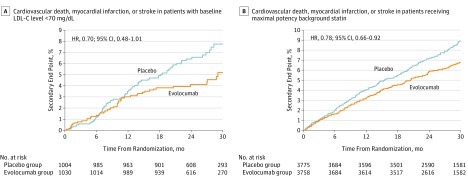
Figure 2. Cumulative Event Rate of the Key Secondary Endpoint With Evolocumab vs Placebo.
Cumulative event rate of the key secondary endpoint with evolocumab compared with placebo in (A) patients with a baseline LDL-C level of less than 70 mg/dL (evolocumab vs placebo, 5.2% vs 7.7%) and (B) in patients treated with a maximal-potency statin (evolocumab vs placebo, 6.8% vs 8.9%). To convert cholesterol levels to to millimoles per liter, multiply by 0.0259.
Table 2. Safety Outcomes of Evolocumab Treatment vs Placebo Stratified by Baseline LDL-C Levelsa.
| Outcome | Baseline LDL-C Level | |||
|---|---|---|---|---|
| <70 mg/dL (n = 2033)b |
≥70 mg/dL (n = 25 491) |
|||
| Evolocumab (n = 1030) |
Placebo (n = 1003) |
Evolocumab (n = 12 739) |
Placebo (n = 12 752) |
|
| Serious adverse event | 268 (26.0) | 274 (27.3) | 3142 (24.7) | 3130 (24.5) |
| Adverse event related to study drug and leading to therapy discontinuation | 19 (1.8) | 19 (1.9) | 207 (1.6) | 182 (1.4) |
| Injection site reaction | 30 (2.9)c | 16 (1.6) | 266 (2.1)c | 203 (1.6) |
| Muscle-related event | 49 (4.8) | 60 (6.0) | 633 (5.0) | 596 (4.7) |
| Cataract | 19 (1.8) | 16 (1.6) | 209 (1.6) | 226 (1.8) |
| New-onset diabetes (CEC adjudicated)d | 45/509 (8.8) | 53/475 (11.2) | 632/7828 (8.1) | 591/7864 (7.5) |
| Neurocognitive event | 17 (1.7) | 12 (1.2) | 200 (1.6) | 190 (1.5) |
| AST or ALT level >3 times normale | 27 (2.7) | 23 (2.3) | 213 (1.7) | 219 (1.7) |
| Creatine kinase level >5 times normal | 9 (0.9) | 9 (0.9) | 86 (0.7) | 90 (0.7) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CEC, Clinical End Point Committee; LDL-C, low-density lipoprotein cholesterol.
SI conversion factor: To convert LDL-C to millimoles per liter, multiply by 0.0259.
Baseline LDL-C level was not available for 1 patient, and 39 patients who did not receive the study drug were excluded. Unless otherwise indicated, data are expressed as number (percentage) of patients. No significant treatment × subgroup interaction was found.
These patients either had a final screening LDL-C of at least 70 mg/dL or a final screening non–high-density lipoprotein cholesterol level of at least 100 mg/dL.
Nominal P < .05 vs placebo.
Patients with prevalent diabetes were excluded.
Owing to missing patient data, denominators may be less than column headings.
Patients Receiving a Maximal-Potency Statin
A total of 7533 patients (27.3%) were receiving a maximal-intensity statin (baseline characteristics are shown in Table 1). The median baseline LDL-C level was 93.0 mg/dL (IQR, 80.0-111.5 mg/dL). At 48 weeks, the least-squares mean percentage reduction in LDL-C levels with evolocumab, compared with placebo, was 58%, for a mean absolute reduction of 57 mg/dL; the median achieved LDL-C concentration at 48 weeks was 32.0 mg/dL (IQR, 20.0-49.0 mg/dL). Evolocumab reduced the risk for the primary composite endpoint by 14% (HR, 0.86; 95% CI, 0.75-0.98) in patients receiving maximal-potency statin therapy and by 15% (HR, 0.85; 95% CI, 0.78-0.93) in patients treated with a submaximal statin, with no evidence of treatment effect modification owing to background statin intensity (P = .88 for interaction) (Figure 1B). Likewise, evolocumab reduced the risk for the key secondary endpoint by 22% (HR, 0.78; 95% CI, 0.66-0.92) in patients receiving maximal-potency statin therapy (Figure 2B) and by 19% (HR, 0.81; 95% CI, 0.72-0.90) in patients receiving less potent statin regimens, with no evidence of treatment effect modification owing to intensity of background statin therapy (P = .71 for interaction) (Figure 1B). We found no heterogeneity for any of the individual outcomes (eTable 2 in the Supplement). In addition, we found no heterogeneity in the safety profile of evolocumab as a function of intensity of background statin therapy (Table 3).
Table 3. Safety Outcomes of Evolocumab Treatment vs Placebo Stratified by Potency of a Background Statina .
| Outcome | Maximal Potency Background Statin (n = 7524) |
Submaximal Potency Background Statin (n = 20 001) |
||
|---|---|---|---|---|
| Evolocumab (n = 3754) |
Placebo (n = 3770) |
Evolocumab (n = 10 015) |
Placebo (n = 9986) |
|
| Serious adverse event | 979 (26.1) | 1010 (26.8) | 2431 (24.3) | 2394 (24.0) |
| Adverse event related to study drug and leading to drug discontinuation | 53 (1.4) | 53 (1.4) | 173 (1.7) | 148 (1.5) |
| Injection site reaction | 84 (2.2) | 68 (1.8) | 212 (2.1)b | 151 (1.5) |
| Muscle-related event | 207 (5.5) | 194 (5.1) | 475 (4.7) | 462 (4.6) |
| Cataract | 53 (1.4) | 64 (1.7) | 175 (1.7) | 178 (1.8) |
| New-onset diabetes (CEC adjudicated)c | 214/2385 (9.0)b | 176/2383 (7.4) | 463/5952 (7.8) | 468/5956 (7.9) |
| Neurocognitive event | 64 (1.7) | 63 (1.7) | 153 (1.5) | 139 (1.4) |
| AST or ALT level >3 times normald | 84 (2.3) | 81 (2.2) | 156 (1.6) | 161 (1.6) |
| Creatine kinase level >5 times normald | 28 (0.8) | 33 (0.9) | 67 (0.7) | 66 (0.7) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CEC, Clinical End Point Committee; LDL-C, low-density lipoprotein cholesterol.
Unless otherwise indicated, data are expressed as number (percentage) of patients in the safety cohort. No significant treatment × subgroup interaction was found.
Nominal P < .05 vs placebo.
Patients with prevalent diabetes were excluded.
Owing to missing patient data, denominators may be less than column headings.
Discussion
The principal findings of this analysis were that high-risk patients with stable atherosclerotic cardiovascular disease who were treated with statins derived similar clinical benefit with the addition of evolocumab during a median follow-up of 2.2 years regardless of whether the baseline LDL-C level was below 70 or at least 70 mg/dL and regardless of the intensity of background statin therapy (maximal vs submaximal). Patients enrolled with LDL-C levels of less than 70 mg/dL represented patients who either had a final screening LDL-C of at least 70 mg/dL or a final screening non–HDL-C level of at least 100 mg/dL; thus, these patients were more likely to have diabetes or metabolic syndrome and on average were younger and had more cardiovascular risk factors.
These findings extend prior observations reported with other therapies to lower lipid levels. For statins, the meta-analysis by the Cholesterol Treatment Trialists Collaboration noted consistent benefit in patients starting with an LDL-C level of less than 77 mg/dL, but because of the range of baseline LDL-C levels in these trials, few patients would have had an LDL-C level of less than 70 mg/dL. The Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) reported a consistent benefit of statin therapy in patients starting with an LDL-C level of no more than 60 mg/dL, but only 511 individuals were in that subgroup and the comparator was placebo. The Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) recently showed that the addition of ezetimibe to a background moderate-intensity statin (simvastatin, 40 mg/d) reduced cardiovascular events by 6.4% during a median of 6 years after acute coronary syndrome, with consistent benefit even among patients in the lowest quartile of baseline LDL-C level (<64 mg/dL); however, the achieved LDL-C level in that subgroup with the combination of ezetimibe and simvastatin was 45 mg/dL. More recently, the Heart Protection Study 3/Thrombolysis in Myocardial Infarction 55–Randomized Evaluation of the Effects of Anacetrapib through Lipid Modification (HPS3/TIMI55-REVEAL) Collaborative Group reported that patients with stable atherosclerotic disease and a baseline mean LDL-C level of 61 mg/dL who were randomized to the cholesterol ester transfer protein inhibitor anacetrapib had reduced mean LDL-C levels to 53 mg/dL and experienced an 9% reduction in major coronary events compared with those randomized to placebo. In the present analysis, we showed consistent benefit when starting with an LDL-C level of less than 70 mg/dL; the LDL-C levels were lowered by 66% to a median of 21.0 mg/dL, with 25% of patients having an LDL-C level of less than 11.5 mg/dL.
Strengths and Limitations
The consistent clinical benefit seen with randomized allocation to therapy that reduced LDL-C to a median concentration of 21 mg/dL supports and extends observational analyses that have shown that achievment of progressively lower LDL-C levels was associated with further reductions of major cardiovascular events. Before the FOURIER trial, no nonstatin therapy had shown clinical benefit when added to a background of maximal statin therapy. Last, the safety profile of evolocumab was consistent regardless of baseline LDL-C level or intensity of statin therapy. All patients in the FOURIER trial were at high risk, and a minority received ezetimibe; whether patients at lower risk or receiving ezetimibe and maximal statin would have similar benefit requires additional studies.
Conclusions
Evolocumab safely reduced cardiovascular events in patients with stable atherosclerotic cardiovascular disease to a similar degree whether the baseline LDL-C level was less than or at least 70 mg/dL and regardless of whether the background statin dosage was maximal or submaximal intensity. These findings support using evolocumab beyond what is recommended in current guidelines and, more broadly, the value of lowering LDL-C levels to approximately 20 mg/dL, even in high-risk patients starting at levels below current guideline targets or thresholds for treatment.
eTable 1. Efficacy Outcomes With Evolocumab vs Placebo Stratified by Baseline LDL-C Level
eTable 2. Efficacy Outcomes With Evolocumab vs Placebo Stratified by Potency of Background Statin Treatment
References
- 1.Catapano AL, Graham I, De Backer G, et al. ; Authors/Task Force Members; Additional Contributor . 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37(39):2999-3058. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. ; Writing Committee . 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2016;68(1):92-125. [DOI] [PubMed] [Google Scholar]
- 3.Orringer CE, Jacobson TA, Saseen JJ, et al. . Update on the use of PCSK9 inhibitors in adults: recommendations from an expert panel of the National Lipid Association. J Clin Lipidol. 2017;11(4):880-890. [DOI] [PubMed] [Google Scholar]
- 4.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S1-S45. [DOI] [PubMed] [Google Scholar]
- 5.Sabatine MS, Giugliano RP, Keech A, et al. . Rationale and design of the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk trial. Am Heart J. 2016;173:94-101. [DOI] [PubMed] [Google Scholar]
- 6.Sabatine MS, Giugliano RP, Keech AC, et al. ; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. [DOI] [PubMed] [Google Scholar]
- 7.Bohula EA, Bonaca MP, Braunwald E, et al. . Atherothrombotic risk stratification and the efficacy and safety of vorapaxar in patients with stable ischemic heart disease and previous myocardial infarction. Circulation. 2016;134(4):304-313. [DOI] [PubMed] [Google Scholar]
- 8.Baigent C, Blackwell L, Emberson J, et al. ; Cholesterol Treatment Trialists (CTT) Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsia J, MacFadyen JG, Monyak J, Ridker PM. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dL with rosuvastatin: the JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). J Am Coll Cardiol. 2011;57(16):1666-1675. [DOI] [PubMed] [Google Scholar]
- 10.Cannon CP, Blazing MA, Giugliano RP, et al. ; IMPROVE-IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. [DOI] [PubMed] [Google Scholar]
- 11.Giugliano RP, Cannon CP, Blazing MA, et al. . Baseline LDL-C and clinical outcomes with addition of ezetimibe to statin in 18,144 patients post ACS. J Am Coll Cardiol. 2015;65(10S):A4. [Google Scholar]
- 12.HPS3/TIMI55-REVEAL Collaborative Group Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;317:1217-1227. [DOI] [PubMed] [Google Scholar]
- 13.Boekholdt SM, Hovingh GK, Mora S, et al. . Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64(5):485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giugliano RP, Pedersen TR, Park JG, et al. ; FOURIER Investigators . Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial [published online August 25, 2017]. Lancet. doi: 10.1016/S0140-6736(17)32290-0 [DOI] [PubMed] [Google Scholar]
- 15.Giugliano RP, Wiviott SD, Blazing MA, et al. . Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT Trial. JAMA Cardiol. 2017;2(5):547-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabatine MS, Giugliano RP. Low-density lipoprotein cholesterol treatment in the proprotein convertase subtilisin/kexin type 9 inhibitor era: getting back on target. JAMA Cardiol. 2017;2(9):935-936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Efficacy Outcomes With Evolocumab vs Placebo Stratified by Baseline LDL-C Level
eTable 2. Efficacy Outcomes With Evolocumab vs Placebo Stratified by Potency of Background Statin Treatment