Key Points
Question
What are the effects of intra-articular injection of 40 mg of triamcinolone acetonide every 3 months on progression of cartilage loss and knee pain in patients with osteoarthritis?
Findings
In a randomized clinical trial of 140 patients with symptomatic knee osteoarthritis, the use of intra-articular triamcinolone compared with intra-articular saline resulted in greater cartilage volume loss. There was no significant difference on knee pain severity between treatment groups.
Meaning
Among patients with symptomatic knee osteoarthritis, intra-articular triamcinolone, compared with intra-articular saline, increased cartilage volume loss and had no effect on knee pain over 2 years.
Abstract
Importance
Synovitis is common and is associated with progression of structural characteristics of knee osteoarthritis. Intra-articular corticosteroids could reduce cartilage damage associated with synovitis but might have adverse effects on cartilage and periarticular bone.
Objective
To determine the effects of intra-articular injection of 40 mg of triamcinolone acetonide every 3 months on progression of cartilage loss and knee pain.
Design, Setting, and Participants
Two-year, randomized, placebo-controlled, double-blind trial of intra-articular triamcinolone vs saline for symptomatic knee osteoarthritis with ultrasonic features of synovitis in 140 patients. Mixed-effects regression models with a random intercept were used to analyze the longitudinal repeated outcome measures. Patients fulfilling the American College of Rheumatology criteria for symptomatic knee osteoarthritis, Kellgren-Lawrence grades 2 or 3, were enrolled at Tufts Medical Center beginning February 11, 2013; all patients completed the study by January 1, 2015.
Interventions
Intra-articular triamcinolone (n = 70) or saline (n = 70) every 12 weeks for 2 years.
Main Outcomes and Measures
Annual knee magnetic resonance imaging for quantitative evaluation of cartilage volume (minimal clinically important difference not yet defined), and Western Ontario and McMaster Universities Osteoarthritis index collected every 3 months (Likert pain subscale range, 0 [no pain] to 20 [extreme pain]; minimal clinically important improvement, 3.94).
Results
Among 140 randomized patients (mean age, 58 [SD, 8] years, 75 women [54%]), 119 (85%) completed the study. Intra-articular triamcinolone resulted in significantly greater cartilage volume loss than did saline for a mean change in index compartment cartilage thickness of −0.21 mm vs −0.10 mm (between-group difference, −0.11 mm; 95% CI, −0.20 to −0.03 mm); and no significant difference in pain (−1.2 vs −1.9; between-group difference, −0.6; 95% CI, −1.6 to 0.3). The saline group had 3 treatment-related adverse events compared with 5 in the triamcinolone group and had a small increase in hemoglobin A1c levels (between-group difference, −0.2%; 95% CI, −0.5% to −0.007%).
Conclusions and Relevance
Among patients with symptomatic knee osteoarthritis, 2 years of intra-articular triamcinolone, compared with intra-articular saline, resulted in significantly greater cartilage volume loss and no significant difference in knee pain. These findings do not support this treatment for patients with symptomatic knee osteoarthritis.
Trial Registration
ClinicalTrials.gov Identifier: NCT01230424
This randomized trial compares the effects of intra-articular triamcinolone vs saline injected every 3 months for 2 years on changes in cartilage volume and pain in older adult patients with knee osteoarthritis.
Introduction
Symptomatic knee osteoarthritis was estimated to affect more than 9 million individuals in the United States in 2005 and is a leading cause of disability and medical costs, most of which were attributable to arthroplasty. Treatments for osteoarthritis are primarily prescribed to reduce symptoms, with no interventions known to influence structural progression.
Evidence suggests that osteoarthritis is an inflammatory condition. Studies demonstrated the presence of synovitis in osteoarthritic joints accompanied by mononuclear cells and proinflammatory mediators with up-regulation of aggrecanases and collagenases. Clinical and epidemiological studies found that inflammation is common in the knee joints of people with knee osteoarthritis and associated with progression of cartilage damage. These observations suggest that suppression of inflammatory processes by corticosteroids (already in widespread clinical use for knee osteoarthritis) might reduce progression of knee osteoarthritis. This possibility is supported by interventional studies in animal models of osteoarthritis. However, associations of intra-articular corticosteroids with adverse joint outcomes in some observational studies involving people with osteoarthritis, together with their known antianabolic effects on healthy cartilage, have raised questions about their potential to damage joints. A 2-year clinical trial suggested that there were no adverse effects associated with intra-articular corticosteroids but was limited because radiography was used to evaluate osteoarthritis progression. Radiography is insensitive to osteoarthritis progression and does not directly image critical soft-tissue structures or bone marrow lesions. Therefore, a 2-year clinical trial of repeated intra-articular triamcinolone injections was performed to test the benefits and harms of intra-articular corticosteroids in the treatment of knee osteoarthritis, using magnetic resonance imaging (MRI) to evaluate articular structures.
Methods
Overview
This was a 2-year double-blind clinical trial of intra-articular triamcinolone administered every 3 months vs saline for symptomatic knee osteoarthritis with ultrasonic evidence of synovitis. Outcomes were cartilage loss, articular structural damage, pain, and physical function. The study was performed at Tufts Medical Center between June 2011 and January 2015. It was approved by the Institutional Review Board of Tufts Medical Center. (The study protocol is available in Supplement 1.) Adaptive interim monitoring was initially planned but this approach was removed in March 2014 with the approval of the data and safety monitoring board when it became apparent that its disadvantages outweighed any advantages.
Sample
Patients were recruited through clinics and local advertisements. Telephone-administered prescreening was conducted before scheduling an on-site visit that included knee radiographs and blood tests. All participants provided written informed consent. Self-reported race/ethnicity and sex were collected.
Eligibility criteria included age 45 years or older and presence of knee osteoarthritis defined by the American College of Rheumatology classification criteria. These criteria are based on a standardized question about knee pain, and tibiofemoral osteoarthritis evident on posteroanterior weight-bearing semi-flexed radiographs. Eligibility thresholds were placed for knee pain (score, ≥2 but ≤8 on the weight-bearing questions of the Western Ontario and McMaster Universities [WOMAC] pain subscale, range 0-12) and radiographic severity (Kellgren-Lawrence [KL] grade, 2 or 3). Potential participants had a clinical examination confirming pain from the knee joint and had to be willing to discontinue their analgesic medication for 48 hours before each pain assessment. Eligibility criteria included ultrasonographic evidence of effusion synovitis in the study knee, defined according to established protocols by a suprapatellar pouch depth larger than 2 mm. Ultrasonographic detection of effusion and synovitis is well-validated, and each is associated with prevalent and incident knee osteoarthritis. Exclusion criteria included other disorders affecting the study joint, such as systemic inflammatory joint disease, prior sepsis, osteonecrosis; chronic or recent use of oral corticosteroids, doxycycline, indomethacin, glucosamine, or chondroitin; recent (<3 months) intra-articular corticosteroids or hyaluronic acid; serious medical conditions (like uncontrolled diabetes, HIV infection, or hypertension) that could be contraindications to participation; and any contraindication to undergoing an MRI scan.
Randomization
Randomized treatment assignments were computer generated by the study statistician (M.L.) using SAS software and provided to the research pharmacy at Tufts Medical Center. The randomization was stratified by KL grade and sex, with 1:1 assignments permuted in blocks of 4. The investigative team and participants were blinded to group assignment.
Study Intervention
The active medication was 1 mL of triamcinolone (purchased from Bristol-Myers Squibb), 40 mg/mL, for injection. The comparator was 1 mL of 0.9% sodium chloride for injection (Hosperia Inc). Neither was mixed with local anesthetic. Both were administered every 12 weeks for 2 years. Synovial fluid (≤10 mL) was aspirated prior to the injection.
Toxicity Monitoring and Safety Procedures
At each visit, information on adverse effects was collected, vital signs obtained, including standard measurement of blood pressure, and blood was obtained for the hemoglobin A1c (HbA1c) assay. Knee MRI scans were screened for avascular necrosis or subchondral fracture. Oversight of treatment-specific results was provided by a National Institute of Arthritis and Musculoskeletal and Skin Disease–appointed data and safety monitoring board, which met in closed sessions.
Masking of Treatment Assignment
The research pharmacist prefilled the syringes and masked the contents using opacified labels and 3-way stopcock. Ultrasound guidance was used for the injection, but, after placement of the needle, the probe was removed to prevent visualization of medication. The clinician who performed the injections was not involved with outcome assessments in the study.
Concomitant Analgesic Use
Participants were asked to discontinue concomitant analgesics 2 days before each assessment to avoid masking symptoms of pain. Participants were advised to take acetaminophen only if needed.
Study Assessments
Following the screening visit, there were 9 visits scheduled at 3-month intervals over the 24-month period. Assessments included a knee examination; blood pressure measurement; WOMAC version 3.1 questionnaire (pain subscale range, 0 [no pain]-20 [extreme pain]; minimal clinically important improvement was a 3.94-difference in pain score; the stiffness subscale range, 0 [no stiffness]-8 [extreme stiffness]; function subscale range, 0 [no difficulty with daily activities]-68 [extreme difficulty], minimal clinically important improvement was a 6.66-difference in score); global knee pain assessment (range, 0 [no pain]-100 [extreme pain]), adverse event ascertainment; medication review; and serum HbA1c levels. Objective measures of functioning tests (timed 20-m walk and chair-stand test) every 6 months, and the 36-Item Short Form Health Survey (SF-36) were collected at baseline and at 12 and 24 months.
Standardized semiflexed posteroanterior knee radiographs and knee and hip dual x-ray absorptiometry (Lunar Prodigy Scanner, General Electric) were performed at baseline. Radiographs were used to classify the index compartment (knee compartment with greatest joint space narrowing), and malalignment, according to established methods. In cases that both compartments had equal joint space narrowing, the medial compartment was used. The readers were blinded to treatment assignment but not to sequence.
Knee Joint Ultrasonography
Knees were scanned according to a standardized protocol in the longitudinal plane with the joint in 30° flexion using a LOGIQe Ultrasound machine and a 13.0-MHz transducer (both by General Electric).
Magnetic Resonance Imaging
Participants underwent MRI scans at months 0, 12, and 24 (Achieva X-Series 3.0 Tesla scanner, Philips) operating sequences as follows: (1) for cartilage volume, 3-dimensional sagittal gradient echo with cartilage excitation, 3D_WATSc_SENSE: parallel imaging in right-left and anterior-posterior; recovery time, 20 ms; echo time, 7.6 ms; and field of view, 160 × 160 × 120 mm; matrix, 512 × 512; voxel size, 0.3 × 0.3 × 1.0 mm; flip angle, 12°; (2) for trabecular fast-field echo sequence, 3-dimensional T1 fast-field echo coronal recovery time, 20 ms; echo time, 4.92 ms; field of view, 120 mm; matrix, 51 × 512; voxel size, 0.2 × 0.2 × 1.0 mm; and flip angle, 50°; and (3) for the morphology sequence, proton density fat-suppressed in 3 planes, recovery time 3000 ms; echo time, 30 ms; field of view, 120 mm; matrix, 480 × 325; voxel size, 0.25 × 0.35 × 2.5 mm; flip angle, 90°.
MRI Quantitative Cartilage Analysis
Mean cartilage thickness was computed across prespecified MRI cross-sectional images segmented in each of the knee compartments according to previously developed methods. This had good reproducibility (intratester intraclass coefficient [ICC], 0.96-0.98; intertester ICC, 0.89-0.94). A validated volumetric cartilage damage index was also used to quantitate cartilage damage. This method was shown to have good reproducibility (intertester reliability, 0.86-0.95; intratester reliability, 0.94-0.99) and construct validity in relation to other measures of osteoarthritis severity (ie, KL grade, joint space narrowing, joint space width).
Bone Marrow Lesion and Effusion Volume Measurement
A validated semiautomated approach was used to measure bone marrow lesion volume using the sagittal proton density fat–suppressed images (intratester reliability, >0.90). Effusion volume was measured using thresholds with predefined perimeters based on anatomic landmarks on the sagittal proton density fat–suppressed knee images. Intratester reliability was good for effusion-synovitis volume (0.81).
Semiquantitative Assessment of Cartilage Damage
One reader (J.B.D.) evaluated MRIs for cartilaginous intrasubstance signal change, denudation, fissures, delamination, and superficial fibrillations, defined as fraying of the articular surface that appeared as a fine velvety surface or an indistinct articular margin. The intrareader agreement was good with a prevalence and bias-adjusted κ for progression of intrasubstance signal change of 0.80 and 0.55 for denudation. Fissures, delamination, and superficial fibrillations were uncommon. When present, these were reviewed with a musculoskeletal radiologist (R.J.W.) to reach consensus.
Analytic Plan
Coprimary outcomes were change in knee cartilage volume in the index compartment, assessed using cartilage thickness, and change in pain, assessed using the WOMAC pain subscale. All other outcomes were secondary and considered exploratory. Intention-to-treat analyses were used for all outcomes. Multiple imputation using the fully conditional specification method was performed to fill in missing values for the outcomes. For structural outcomes, KL grade, sex, age, self-reported race/ethnicity, and baseline and nonmissing values of the outcomes from other measurement points were used to impute missing values by treatment group. Pain and function outcomes were imputed similarly but with the addition of analgesia for breakthrough pain and without age. All analysis models were adjusted for randomization stratification factors of KL grade and sex. The pain and function outcomes were also adjusted for use of analgesia. Mixed-effects regression models were used with a random intercept for longitudinal repeated measures. Acetaminophen use was analyzed using logistic regression with the generalized estimating equations correction for repeated measures. All analyses were performed using SAS version 9.4 (SAS Institute Inc). All testing was 2-sided with P values <.05 considered significant. No adjustment was made for multiple comparisons.
Sample Size Calculation
Based on the original adaptive trial design and allowing for a 25% dropout rate, enrollment of 70 participants per group was selected to allow 80% power to detect a treatment difference of 90 mm3 in change cartilage volume over 2 years. There is no established minimally clinically important difference for cartilage volume loss. However, this corresponds to an effect size of 0.4 SDs using an anticipated SD of 224 mm3 as previously observed. This number also provided 80% power to detect a treatment difference of 2.3 units in WOMAC pain (range, 0-20) with an anticipated SD of 4.1.
Results
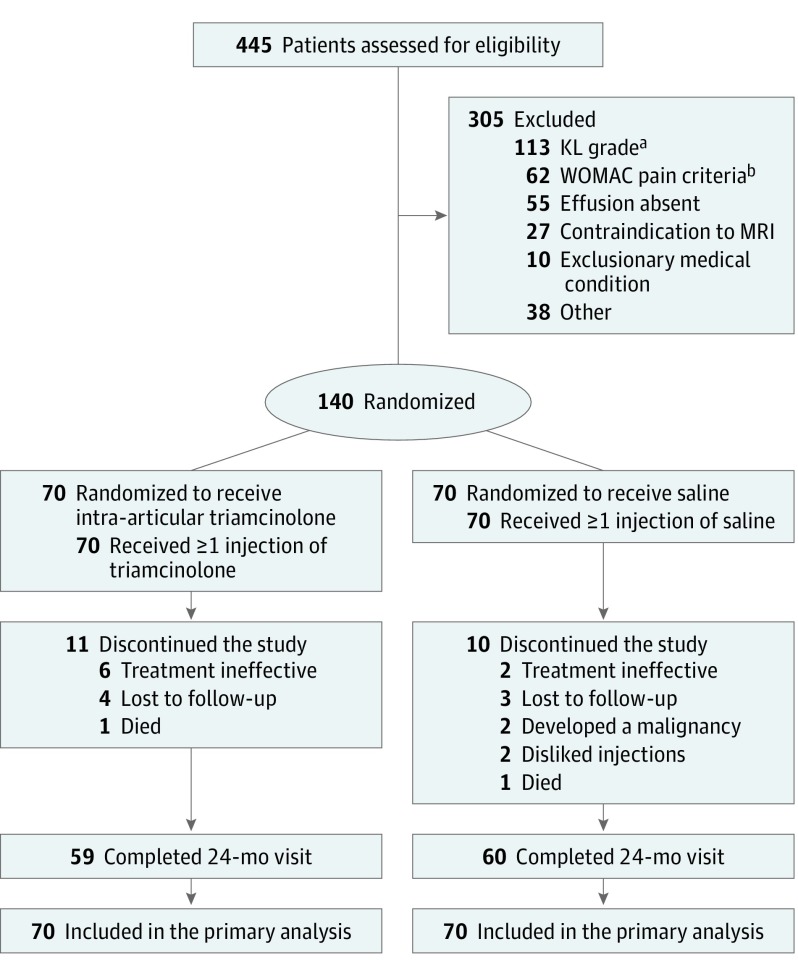
One hundred forty participants (70 to each group) were randomized from 445 in-person screening visits (Figure 1). The group assigned to receive triamcinolone injections was slightly older (Table 1) but otherwise comparable in demographic and clinical characteristics with the group randomized to receive saline injections (Table 1 and Table 2). Fifty-nine patients (84%) in the triamcinolone group and 60 (86%) in the saline group completed the final visit, with 990 of the possible 1120 intra-articular injections (88%) administered. In the mixed-model analyses, 408 imputations were made for structural outcomes (ie, 12% of 3360 possible data points). Adherence to washout protocol was 99%; use of medication for breakthrough pain was 7%.
Figure 1. Flow of Patients With Knee Osteoarthritis Through the Study.
Dropout is defined as not attending the 24-month visit.
aPatients who scored neither a Kellgren-Lawrence (KL) grade of 2 nor 3 were excluded.
bPatient scored 2 or higher on the weight-bearing question or 8 or less on the weight-bearing pain score according to the Western Ontario and McMaster Universities (WOMAC) index.
Table 1. Participant Characteristics at Baseline.
| Mean (SD) | ||
|---|---|---|
| Triamcinolone (n = 70) |
Saline (n = 70) |
|
| Age, y | 59.1 (8.3) | 57.2 (7.6) |
| Women, No. (%) | 37 (52.9) | 38 (54.3) |
| White, No. (%) | 47 (67.1) | 42 (60.0) |
| BMI | 30.8 (5.1) | 31.7 (6.6) |
| Varus or valgus malalignment, No. (%) | 53 (75.7) | 55 (78.6) |
| Synovial pouch depth, mm | 4.2 (1.9) | 4.5 (2.0) |
| KL score, No. (%) | ||
| 2 | 29 (41.4) | 29 (41.4) |
| 3 | 41 (58.6) | 41 (58.6) |
| Clinical | ||
| VAS pain scorea | 38.4 (22.2) | 42.6 (22.1) |
| WOMAC scoreb | ||
| Pain | 8.2 (3.0) | 8.4 (3.0) |
| Function | 28.3 (10.8) | 30.1 (9.5) |
| Stiffness | 3.7 (1.6) | 4.0 (1.4) |
| 20-m Walk, s | 19.8 (6.7) | 18.2 (3.8) |
| Chair stand, s | 18.3 (8.6) | 17.2 (6.5) |
| SF-36 scorec | ||
| Physical | 36.7 (9.1) | 35.4 (9.7) |
| Mental | 52.6 (10.2) | 52.2 (10.0) |
| Hemoglobin A1c, mean (SD), % | 6.0 (0.8) | 6.0 (0.6) |
| C-reactive protein, mean (SD), mg/L (log) | 0.6 (1.2) | 0.4 (1.1) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; KL, Kellgren-Lawrence; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities.
Values range from 0 to 100, for which 0 indicates no pain and 100, extreme pain.
Scores for WOMAC are defined in the Methods section.
The 36-Item Short Form Health Survey (SF-36) scores range from 0 to 100, with higher scores representing better health status.
Table 2. Treatment Effect on Structural Outcomes of Knees With Osteoarthritisa.
| Measurement | Mean (95% CI) | P Value | ||||
|---|---|---|---|---|---|---|
| Triamcinolone (n = 70) |
Saline (n = 70) |
Between-Group Difference in Change | ||||
| Baseline | 2-Year Change | Baseline | 2-Year Change | |||
| Cartilage thickness, mm | ||||||
| Index compartment | 2.43 (2.27 to 2.58) | −0.21 (−0.29 to −0.14) | 2.34 (2.19 to 2.50) | −0.10 (−0.16 to −0.04) | −0.11 (−0.20 to −0.03) | .01 |
| Total mean cartilage thickness | 5.58 (5.35 to 5.81) | −0.29 (−0.43 to −0.15) | 5.61 (5.38 to 5.84) | −0.13 (−0.23 to −0.03) | −0.16 (−0.31 to −0.01) | .04 |
| Cartilage damage index, mm3,b | ||||||
| Index compartmentc | 973.56 (855.78 to 1091.34) | −133.66 (−177.39 to −89.93) | 884.60 (767.49 to 1001.70) | −72.41 (−114.16 to −30.66) | −61.25 (−121.78 to −0.72) | .048 |
| Total | 2654.79 (2482.92 to 2826.67) | −177.63 (−257.20 to −98.06) | 2678.45 (2508.23 to 2848.67) | −82.01 (−145.42 to −18.60) | −95.62 (−194.93 to 3.68) | .06 |
| Area of denudation, mm2,d | ||||||
| Index compartment | 3.09 (2.37 to 3.81) | 0.41 (0.06 to 0.77) | 3.35 (2.61 to 4.06) | 0.41 (0.17 to 0.66) | 0.00 (−0.44 to 0.43) | .99 |
| Total | 4.40 (3.67 to 5.13) | 0.36 (−0.69 to 1.42) | 4.49 (3.77 to 5.20) | 0.32 (−0.11 to 0.76) | 0.04 (−1.11 to 1.20) | .93 |
| Semiquantitative measures, mm3 | ||||||
| Bone marrow lesion volume (log)d,e | 7.79 (6.47 to 9.11) | 0.89 (−0.29 to 2.08) | 6.80 (5.47 to 8.13) | 1.11 (−0.33 to 2.57) | −0.22 (−2.04 to 1.59) | .80 |
| Effusion volume (log)d,e | 10.70 (10.48 to 10.92) | −0.09 (−0.44 to 0.25) | 10.80 (10.57 to 11.02) | −0.32 (−0.56 to −0.09) | 0.23 (−0.11 to 0.57) | .17 |
Estimates and test for treatment by time interaction from repeated-measures, random intercept model, adjusted for KL and sex. Time used is months from baseline examination as a linear trend.
Mean thickness: lower baseline values indicate worse structural damage; high change values, worse damage.
Index compartment indicates compartment with greatest joint space narrowing.
Denudation, BML, effusion: Higher baseline values indicate worse structural damage; high change values indicate worse damage.
Higher natural log values for bone marrow lesions and effusion denote greater volumes affected by these findings. The natural log transformation was used for these measures due to pronounced skewness.
The rate of cartilage loss in the index compartment was greater in the triamcinolone group for cartilage thickness (−0.21 vs –0.10 mm; between-group difference, −0.11 mm; 95% CI, −0.20 to −0.03 mm), and for the secondary cartilage damage index (mean change, −133.66 vs −72.41 µm3; between-group difference, −61.25 µm3; 95% CI, −121.78 to −0.72 µm3; Table 2). There were no significant differences between the 2 groups in progression of cartilage denudation, bone marrow lesion, effusion volume (Table 2), or in trabecular morphology. There were no significant differences between the 2 groups in change in subchondral tibia or in hip and in bone mineral density. Results of a completers’ analysis are presented in the eTable in Supplement 2. Semiquantitative cartilage abnormalities were not significantly different, except for superficial fibrillation, which was more common in the saline group (34% vs 13%; between-group difference, 21%; 95% CI, 7%-35%).
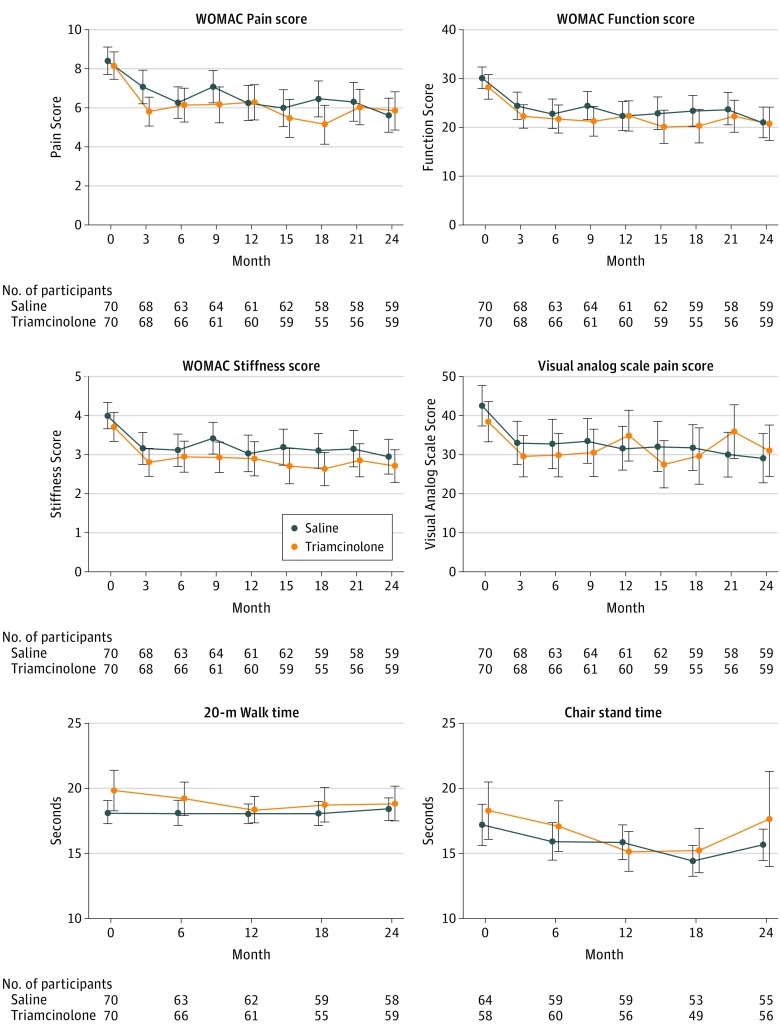
The decrease in knee pain did not significantly differ across treatment groups: −1.2 units in the triamcinolone vs −1.9 in the saline group; between-group mean difference, −0.64; 95% CI, −1.6 to 0.29). Also, there were no significant differences in any of the secondary patient-reported or objective clinical end points (Figure 2 and Table 3). Both groups exhibited a nonsignificant increase in high-sensitivity C-reactive protein (0.2 vs 0.1 mg/L; between-group mean difference, −0.1 mg/L; 95% CI, −0.4 to 0.2 mg/L). At the final visit, 45% of participants guessed their treatment assignment correctly.
Figure 2. Pain and Function Scores of Patients With Knee Osteoarthritis Treated With Triamcinolone vs Saline.
See the Methods section for range definitions for pain, stiffness, and function scores measure. Data markers indicate mean, error bars, 95% CIs. P values for treatment comparisons based on multiple imputation and adjusted for sex and Kellgren-Lawrence grade are in Table 3.
Table 3. Treatment Effect on Symptom and Function Outcomesa.
| Measurement | Mean (95% CI) | P Value | ||||
|---|---|---|---|---|---|---|
| Triamcinolone (n = 70) |
Saline (n = 70) |
Between-Group Difference in Change | ||||
| Baseline | 2-Year Change | Baseline | 2-Year Change | |||
| WOMACb | ||||||
| Pain | 7.50 (6.3 to 8.6) | −1.2 (−1.9 to −0.58) | 8.2 (7.0 to 9.3) | −1.9 (−2.52 to −1.23) | −0.64 (−1.6 to 0.29) | .17 |
| Function | 27.1 (23.1 to 31.0) | −4.1 (−7.4 to −0.83) | 29.2 (25.3 to 33.1) | −5.1 (−8.1 to −2.19) | −1.01 (−4.9 to 2.9) | .59 |
| Stiffness | 3.5 (3.0 to 4.1) | −0.59 (−1.1 to −0.06) | 3.8 (3.3 to 4.3) | −0.53 (−1.0 to −0.01) | −0.06 (−0.43 to 0.56) | .79 |
| VAS Pain scorec | 30.8 (22.9 to 38.7) | −2.7 (−11.9 to 6.6) | 35.4 (27.6 to 43.2) | −7.6 (−15.4 to 0.16) | −5.0 (−13.9 to 3.9) | .26 |
| Function tests, sd | ||||||
| 20-m Walk | 20.6 (19.0 to 22.2) | −0.29 (−1.03 to 0.44) | 19.2 (17.7 to 20.8) | 0.14 (−0.58 to 0.86) | 0.43 (−0.62 to 1.5) | .41 |
| Chair stand | 22.1 (19.0 to 25.2) | −1.1 (−3.5 to 1.2) | 21.2 (18.1 to 24.2) | −1.2 (−3.6 to 1.1) | −0.11 (−2.8 to 2.6) | .94 |
| Acetaminophen usee | .43 | |||||
| None | 5 | −2 | 9 | −6 | −4 | |
| % (95% CI) | 7.1 (1.1 to 13.1) | −2.8 (−10.5 to 4.9) | 12.9 (5.0 to 20.8) | −8.6 (−17.8 to 0.6) | −5.8 (−17.8 to 6.2) | |
Abbreviations: KL, Kellgren-Lawrence; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities.
Estimates and test for treatment by time interaction from repeated-measures, random intercept model, adjusted for KL, sex, and acetaminophen use. Time used is months from baseline exam as a linear trend.
See the Methods section for WOMAC scores for pain, stiffness, and function.
Values range from 0 to 100, for which 0 indicates no pain; 100, extreme pain.
Higher values for walk time and chair-stand indicate worse function.
Test for treatment by time interaction in a repeated-measures generalized estimating equation model, unadjusted for KL and sex.
There were more adverse events in the saline group (63 vs 52 participants, P = .02; 182 vs 131 events, P = .02). Eight were classified as treatment related, 3 in the saline group (1 cellulitis, 2 injection site pain) and 5 in the triamcinolone group (1 facial flushing, 4 injection site pain).
There were no significant differences in serious adverse events (P value = .06). One was classified as related (cellulitis, saline group). The incidence of new or worsening hypertension was not greater in the triamcinolone group (1 vs 2 events), and there were no instances of osteonecrosis or subchondral fracture. Hemoglobin A1c levels declined in the triamcinolone but increased in the saline group (−0.1% vs 0.2%; between-group difference, −0.2; 95% CI, −0.5 to −0.007, with adjustment for KL grade, sex, and body mass index).
Discussion
In this clinical trial investigating the benefits and risks of intra-articular corticosteroids, 40 mg of triamcinolone administered every 3 months over 2 years into knees with osteoarthritis and inflammation resulted in significantly greater cartilage volume loss and no significant difference in knee pain than did saline injections. These results contrast with a previous smaller trial that tested a similar regimen and found no difference in the rate of radiographic joint space loss and detected a benefit on knee pain in some secondary (but not primary) end points. The use of MRI in this study enabled direct quantitation of cartilage and soft-tissue structures and showed more cartilage loss in the triamcinolone group than in the saline group. Radiography does not image cartilage directly and is insensitive to change, so it may not have detected the small changes in cartilage loss measured on the MRIs in this study.
The 2-year change in the index compartment cartilage thickness was greater in the triamcinolone group with a between-group difference of −0.11 (95% CI, −0.20 to −0.03), which corresponds to a moderate effect size of 0.46 mm. A value for the amount of change in cartilage loss that would represent a minimally clinically important difference is not established; however, this change was smaller in magnitude than the cross-sectional differences between one KL grade measured in a prior natural history study (eg, 0.35 mm between grades 2 and 3). Increased progression was not detected in other osteoarthritis features, structurally or clinically. In fact, superficial fibrillation worsened more frequently in the saline group, although this may have been due to chance since secondary and semiquantitative structural measures showed no difference between groups. The effects that were detected on cartilage loss were statistically significant and consistent across different measurements. In vivo and clinical evidence show catabolic effects of corticosteroids. Although the cartilage loss was not associated with worsening of symptom outcomes, rates of cartilage loss have been associated with higher rates of arthroplasty, raising the possibility of potential for longer-term adverse consequences on the health of the joint. Cartilage structure should be evaluated in any future clinical studies of similar therapeutics.
The hypothesis that intra-articular corticosteroids might reduce the rate of cartilage loss and other structural manifestations of osteoarthritis was based on recognition of the role of inflammation in its pathogenesis, and reduced structural progression observed in vivo. Suppression of inflammation could attenuate catabolic effects of inflammation and reduce articular damage. However, these results showed greater progression of knee cartilage volume loss and no sustained effect on intra-articular inflammation as indicated by persistence of effusion. As a proof-of-concept study, the results raise questions about the role of inflammation in osteoarthritis progression.
It has been suggested that intra-articular saline injection might have a therapeutic effect in osteoarthritis, a hypothesis based on clinical trial results in which saline was used as a comparator with apparent symptomatic improvement. However, there is a strong placebo response to intra-articular injection, and no prior trials included a sham injection. Also, the rate of cartilage loss in this study was commensurate with that observed in prior natural history studies, so it is likely that the difference in cartilage loss rates between groups was due to an adverse effect of intra-articular corticosteroids on cartilage rather than a benefit from intra-articular saline.
There was a significant difference in HbA1c levels between groups observed by the end of the study, but this favored the triamcinolone group and may be due to chance.
Limitations
This study has several limitations. First, symptom ascertainment took place every 3 months with the goal of measuring long-term effects on these outcomes. Pain was not measured within the 4-week period after each injection, during which benefits are known to occur. Thus, any transient benefit on pain ending within the 3-month period between each injection could have been missed by these methods. Second, participants were permitted to continue their usual medications during the trial, which might have attenuated any between-group differences in symptom outcomes even though participants were asked to discontinue nonsteroidal anti-inflammatory drugs prior to each assessment, and the use of analgesics taken for breakthrough pain was also adjusted for in the multivariate models. Third, high expectations and large placebo responses could also have affected assessments of effects, although these appeared modest compared with typical osteoarthritis trials. Fourth, this study was targeted at osteoarthritic knees that had some degree of inflammation, determined using ultrasonography. It is possible that this imaging technique lacks specificity in identifying inflammation, or that pain from knees with osteoarthritis and features of inflammation are paradoxically less likely to respond to triamcinolone, as was found in a previous study. Alternatively, although the dose regimen tested was consistent with clinical practice guidelines, it is possible that the dose or frequency was insufficient to generate sufficient anti-inflammatory effect to reduce pain in the long term.
Conclusions
Among patients with symptomatic knee osteoarthritis, 2 years of intra-articular triamcinolone, compared with intra-articular saline, resulted in significantly greater cartilage volume loss and no significant difference in knee pain. These findings do not support this treatment for patients with symptomatic knee osteoarthritis.
Trial Protocol
eTable 1. Treatment Effect on Structural Outcomes, Completers’ Analysis
References
- 1.Lawrence RC, Felson DT, Helmick CG, et al. ; National Arthritis Data Workgroup . Estimates of the prevalence of arthritis and other rheumatic conditions in the United States, II. Arthritis Rheum. 2008;58(1):26-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mapel DW, Shainline M, Paez K, Gunter M. Hospital, pharmacy, and outpatient costs for osteoarthritis and chronic back pain. J Rheumatol. 2004;31(3):573-583. [PubMed] [Google Scholar]
- 3.Losina E, Paltiel AD, Weinstein AM, et al. . Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken). 2015;67(2):203-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21(1):16-21. [DOI] [PubMed] [Google Scholar]
- 5.Hill CL, Hunter DJ, Niu J, et al. . Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66(12):1599-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roemer FW, Guermazi A, Felson DT, et al. . Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70(10):1804-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13(5):361-367. [DOI] [PubMed] [Google Scholar]
- 8.Jüni P, Hari R, Rutjes AWS, et al. . Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;(10):CD005328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartmann K, Koenen M, Schauer S, et al. . Molecular actions of glucocorticoids in cartilage and bone during health, disease, and steroid therapy. Physiol Rev. 2016;96(2):409-447. [DOI] [PubMed] [Google Scholar]
- 10.Wada J, Koshino T, Morii T, Sugimoto K. Natural course of osteoarthritis of the knee treated with or without intraarticular corticosteroid injections. Bull Hosp Jt Dis. 1993;53(2):45-48. [PubMed] [Google Scholar]
- 11.Haddad IK. Temporomandibular joint osteoarthrosis: histopathological study of the effects of intra-articular injection of triamcinolone acetonide. Saudi Med J. 2000;21(7):675-679. [PubMed] [Google Scholar]
- 12.Wernecke C, Braun HJ, Dragoo JL. The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop J Sports Med. 2015;3(5):2325967115581163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raynauld JP, Buckland-Wright C, Ward R, et al. . Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370-377. [DOI] [PubMed] [Google Scholar]
- 14.Guermazi A, Roemer FW, Burstein D, Hayashi D. Why radiography should no longer be considered a surrogate outcome measure for longitudinal assessment of cartilage in knee osteoarthritis. Arthritis Res Ther. 2011;13(6):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altman R, Asch E, Bloch D, et al. ; Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association . Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29(8):1039-1049. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Woods R, Chaisson CE, et al. . Alcohol consumption as a trigger of recurrent gout attacks. Am J Med. 2006;119(9):800.e13-800.e18. [DOI] [PubMed] [Google Scholar]
- 17.Kellgren JH, Lawrence JS. The Epidemiology of Chronic Rheumatism: Atlas of Standard Radiographs. Vol 2 Oxford, UK: Blackwell Scientific; 1962. [Google Scholar]
- 18.Tarhan S, Unlu Z. Magnetic resonance imaging and ultrasonographic evaluation of the patients with knee osteoarthritis: a comparative study. Clin Rheumatol. 2003;22(3):181-188. [DOI] [PubMed] [Google Scholar]
- 19.Rubaltelli L, Fiocco U, Cozzi L, et al. . Prospective sonographic and arthroscopic evaluation of proliferative knee joint synovitis. J Ultrasound Med. 1994;13(11):855-862. [DOI] [PubMed] [Google Scholar]
- 20.Karim Z, Wakefield RJ, Quinn M, et al. . Validation and reproducibility of ultrasonography in the detection of synovitis in the knee: a comparison with arthroscopy and clinical examination. Arthritis Rheum. 2004;50(2):387-394. [DOI] [PubMed] [Google Scholar]
- 21.Sarmanova A, Hall M, Moses J, Doherty M, Zhang W. Synovial changes detected by ultrasound in people with knee osteoarthritis—a meta-analysis of observational studies. Osteoarthritis Cartilage. 2016;24(8):1376-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atukorala I, Kwoh CK, Guermazi A, et al. . Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis. 2016;75(2):390-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stratford PW, Kennedy DM, Woodhouse LJ, Spadoni GF. Measurement properties of the WOMAC LK 3.1 pain scale. Osteoarthritis Cartilage. 2007;15(3):266-272. [DOI] [PubMed] [Google Scholar]
- 24.Buckland-Wright JC, Wolfe F, Ward RJ, Flowers N, Hayne C. Substantial superiority of semiflexed (MTP) views in knee osteoarthritis: a comparative radiographic study, without fluoroscopy, of standing extended, semiflexed (MTP), and schuss views. J Rheumatol. 1999;26(12):2664-2674. [PubMed] [Google Scholar]
- 25.Kraus VB, Vail TP, Worrell T, McDaniel G. A comparative assessment of alignment angle of the knee by radiographic and physical examination methods. Arthritis Rheum. 2005;52(6):1730-1735. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Driban JB, Price LL, Lo GH, Miller E, McAlindon TE. Development of a rapid cartilage damage quantification method for the lateral tibiofemoral compartment using magnetic resonance images: data from the osteoarthritis initiative. Biomed Res Int. 2015;2015:634275. doi: 10.1155/2015/634275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Driban JB, Price LL, et al. . Development of a rapid knee cartilage damage quantification method using magnetic resonance images. BMC Musculoskelet Disord. 2014;15:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterfy CG, Guermazi A, Zaim S, et al. . Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177-190. [DOI] [PubMed] [Google Scholar]
- 29.Xu L, Hayashi D, Roemer FW, Felson DT, Guermazi A. Magnetic resonance imaging of subchondral bone marrow lesions in association with osteoarthritis. Semin Arthritis Rheum. 2012;42(2):105-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jungius KP, Schmid MR, Zanetti M, Hodler J, Koch P, Pfirrmann CW. Cartilaginous defects of the femorotibial joint: accuracy of coronal short inversion time inversion-recovery MR sequence. Radiology. 2006;240(2):482-488. [DOI] [PubMed] [Google Scholar]
- 31.McAlindon T, LaValley M, Schneider E, et al. . Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. JAMA. 2013;309(2):155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckstein F, Boudreau RM, Wang Z, et al. ; OAI investigators . Trajectory of cartilage loss within 4 years of knee replacement—a nested case-control study from the osteoarthritis initiative. Osteoarthritis Cartilage. 2014;22(10):1542-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsson E, Erlandsson Harris H, Larsson A, Månsson B, Saxne T, Klareskog L. Corticosteroid treatment of experimental arthritis retards cartilage destruction as determined by histology and serum COMP. Rheumatology (Oxford). 2004;43(4):428-434. [DOI] [PubMed] [Google Scholar]
- 34.Pelletier JP, DiBattista JA, Raynauld JP, Wilhelm S, Martel-Pelletier J. The in vivo effects of intraarticular corticosteroid injections on cartilage lesions, stromelysin, interleukin-1, and oncogene protein synthesis in experimental osteoarthritis. Lab Invest. 1995;72(5):578-586. [PubMed] [Google Scholar]
- 35.Houard X, Goldring MB, Berenbaum F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep. 2013;15(11):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altman RD, Devji T, Bhandari M, Fierlinger A, Niazi F, Christensen R. Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: a systematic review and meta-analysis of randomized trials. Semin Arthritis Rheum. 2016;46(2):151-159. [DOI] [PubMed] [Google Scholar]
- 37.Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328(7444):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bannuru RR, McAlindon TE, Sullivan MC, Wong JB, Kent DM, Schmid CH. Effectiveness and implications of alternative placebo treatments: a systematic review and network meta-analysis of osteoarthritis trials. Ann Intern Med. 2015;163(5):365-372. [DOI] [PubMed] [Google Scholar]
- 39.Chao J, Wu C, Sun B, et al. . Inflammatory characteristics on ultrasound predict poorer longterm response to intraarticular corticosteroid injections in knee osteoarthritis. J Rheumatol. 2010;37(3):650-655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Treatment Effect on Structural Outcomes, Completers’ Analysis