Key Points
Question
How did physician prescribing behavior change after US academic medical centers implemented policies that limited pharmaceutical salesperson detailing?
Findings
Using a difference-in-differences design, the study compared changes in the prescribing behavior of 2126 physicians at 19 academic medical centers during the periods before and after enactment of detailing policies with changes in the prescribing behavior of 24 593 matched control physicians during the same period for 262 drugs in 8 drug classes. Policy enactment was associated with a 1.67 percentage point decrease in the market share of detailed drugs and a 0.84 percentage point increase in the market share of nondetailed drugs. Both estimates were statistically significant in the full sample, but were only statistically significant for 9 of the 19 individual academic medical centers.
Meaning
Academic medical center policies to regulate detailing were associated with modest but significant changes in prescribing behavior for some but not all of the centers that enacted policies.
Abstract
Importance
In an effort to regulate physician conflicts of interest, some US academic medical centers (AMCs) enacted policies restricting pharmaceutical representative sales visits to physicians (known as detailing) between 2006 and 2012. Little is known about the effect of these policies on physician prescribing.
Objective
To analyze the association between detailing policies enacted at AMCs and physician prescribing of actively detailed and not detailed drugs.
Design, Setting, and Participants
The study used a difference-in-differences multivariable regression analysis to compare changes in prescribing by physicians before and after implementation of detailing policies at AMCs in 5 states (California, Illinois, Massachusetts, Pennsylvania, and New York) that made up the intervention group with changes in prescribing by a matched control group of similar physicians not subject to a detailing policy.
Exposures
Academic medical center implementation of policies regulating pharmaceutical salesperson visits to attending physicians.
Main Outcomes and Measures
The monthly within-drug class market share of prescriptions written by an individual physician for detailed and nondetailed drugs in 8 drug classes (lipid-lowering drugs, gastroesophageal reflux disease drugs, diabetes drugs, antihypertensive drugs, hypnotic drugs approved for the treatment of insomnia [sleep aids], attention-deficit/hyperactivity disorder drugs, antidepressant drugs, and antipsychotic drugs) comparing the 10- to 36-month period before implementation of the detailing policies with the 12- to 36-month period after implementation, depending on data availability.
Results
The analysis included 16 121 483 prescriptions written between January 2006 and June 2012 by 2126 attending physicians at the 19 intervention group AMCs and by 24 593 matched control group physicians. The sample mean market share at the physician-drug-month level for detailed and nondetailed drugs prior to enactment of policies was 19.3% and 14.2%, respectively. Exposure to an AMC detailing policy was associated with a decrease in the market share of detailed drugs of 1.67 percentage points (95% CI, −2.18 to −1.18 percentage points; P < .001) and an increase in the market share of nondetailed drugs of 0.84 percentage points (95% CI, 0.54 to 1.14 percentage points; P < .001). Associations were statistically significant for 6 of 8 study drug classes for detailed drugs (lipid-lowering drugs, gastroesophageal reflux disease drugs, antihypertensive drugs, sleep aids, attention-deficit/hyperactivity disorder drugs, and antidepressant drugs) and for 9 of the 19 AMCs that implemented policies. Eleven of the 19 AMCs regulated salesperson gifts to physicians, restricted salesperson access to facilities, and incorporated explicit enforcement mechanisms. For 8 of these 11 AMCs, there was a significant change in prescribing. In contrast, there was a significant change at only 1 of 8 AMCs that did not enact policies in all 3 areas.
Conclusions and Relevance
Implementation of policies at AMCs that restricted pharmaceutical detailing between 2006 and 2012 was associated with modest but significant reductions in prescribing of detailed drugs across 6 of 8 major drug classes; however, changes were not seen in all of the AMCs that enacted policies.
This study compares changes in physician prescribing patterns before and after implementation of policies at academic medical centers restricting on-site activities of pharmaceutical salespeople.
Introduction
Between 2006 and 2012, a number of academic medical centers enacted policies restricting sales visits from pharmaceutical representatives to their practicing physicians (known as detailing), by far the most common form of interaction between physicians and the pharmaceutical industry. Between the same dates, the number of academic medical centers receiving A or B grades from the American Medical Student Association’s conflict of interest scorecard (which evaluates the strictness and comprehensiveness of academic medical center policies dealing with physician interactions with pharmaceutical and medical device companies and assigns each academic medical center a letter grade) increased from 22 to 91, and the number receiving an F grade or incomplete grade declined from 100 to 26.
Two studies have examined the relationship between academic medical center detailing policies and antipsychotic prescribing. In the first study, enactment of an academic medical center detailing policy was associated with a reduction in off-label prescribing of detailed drugs by pediatricians. In the second study, there was no association between academic medical center policies and prescribing of antipsychotics by psychiatrists. Both studies focused on a single physician specialty and a single drug class. In a third study, private practice physicians who did not routinely see pharmaceutical sales representatives were slower to change their prescribing after the release of new positive or negative information on 3 specific drugs; however, the study did not distinguish between physicians who chose not to receive sales visits and those who were not visited due to policies at their medical practice.
To our knowledge, no large-scale study has evaluated the association between academic medical center detailing policies and changes in prescribing, despite the large clinical and economic importance of the topic. The objective of this study was to examine the association between detailing policy enactment and prescribing behavior by (1) including prescribing data of physicians in all specialties (including generalists), (2) examining drugs in multiple drug classes containing a mix of actively detailed and generic drugs, and (3) estimating changes in prescribing after policy enactment across all academic medical centers in multiple states compared with a matched control group of physicians not subject to similar policies.
Methods
Study Design
The study received institutional review board approval with a waiver on informed consent from Carnegie Mellon University. The data contained no patient-identifying information. The study used a difference-in-differences multivariable regression design to compare changes in the prescribing behavior of physicians at academic medical centers that implemented new detailing policies between January 2006 and June 2012 (the intervention group) with changes in the prescribing behavior of a matched control group of physicians not subject to such policies. The analysis compared the change in prescribing behavior of intervention group physicians before and after their affiliated academic medical center’s policy went into effect with the change in prescribing behavior of matched control physicians at the same points in time. Difference-in-differences models are commonly used to examine large-scale policy changes when randomized clinical trials are infeasible.
Data
The analysis focused on US academic medical centers in the 5 states (California, Illinois, Massachusetts, Pennsylvania, and New York) with the largest number of academically affiliated physicians. These 5 states have 20.8% of all US academic medical centers and represented nearly 35% of all prescriptions written in the United States in 2015. The study period of January 2006 to June 2012 was selected because a large number of academic medical centers changed policies during this time frame.
Information about the content of each academic medical center’s policy was coded based on telephone and in-person interviews conducted from 2012 to 2013 with administrators at 29 of the 33 academic medical centers in the 5 states. In total, 9 of 29 interviews were conducted in person. For the 4 academic medical centers that did not respond to interview requests, coding was based on policy databases at the Institute on Medicine as a Profession and the American Medical Student Association.
The detailing policy of each academic medical center was compared with the code of conduct established in 2002 by the Pharmaceutical Research and Manufacturers of America (PhRMA), which placed some voluntary limits on detailing for all its corporate members. These limits banned all personal gifts except branded items such as pens and pads of paper carrying a drug name, company logo, or both. Meals given to physicians or staff as part of a sales call were also allowed. In 2009, an update to the code of conduct disallowed branded items but continued to allow meals and educational gifts. These policies applied nationwide to all sales visits, and therefore provided a baseline set of regulations for the study.
Of the 33 academic medical centers, 9 enacted policies stricter than the PhRMA code of conduct before the start of the study period and were excluded from the analysis because the study design required adequate data from both before and after the intervention. Of the remaining 24 academic medical centers, 20 implemented policies during the study period that were stricter than the PhRMA code of conduct. Specific regulations for these academic medical centers were coded in 3 areas: (1) salesperson gifts to physicians, (2) salesperson access to academic medical center facilities, and (3) mechanisms for enforcement of the policies.
The market research firm IMS provided data based on quarterly surveys of the physicians affiliated with each individual hospital within these 20 academic medical centers. The hospitals covered by each academic medical center policy are listed in eTable 1 in the Supplement. The study included all intervention group physicians who were full-time attending physicians at an academic medical center hospital for at least 1 quarter during the study period, and who wrote at least 50 prescriptions that were processed by CVS Caremark, a major pharmacy benefits manager. A physician who was reported by the IMS survey as having left the academic medical center was dropped from the intervention group beginning at that point in time.
Physicians who likely wrote prescriptions in environments outside the academic medical center, such as part-time attending physicians or other physicians who merely had admitting privileges at an academic medical center hospital, were excluded from the intervention group because the policies would not apply to them for prescriptions written in their main practice outside the academic medical center hospital. One academic medical center (Stony Brook University Medical Center) was excluded from the analysis because there were too few physicians (<25 full-time attending physicians during the study period according to the IMS data) to conduct reliable statistical testing, leaving the intervention group with 19 academic medical centers.
Data at the individual drug level regarding national sales force size for specific drugs were provided by the market research firm SDI Health. Drug classes were selected based on 2 criteria chosen by the researchers to ensure that the drug classes in the study had a mix of highly detailed drugs and at least 1 widely prescribed nondetailed drug. First, the overall drug class was required to have at least 2000 salespeople during the study period. Second, the detailed drugs within the drug class were required to have at least 25% but not more than 75% of the total market share during the study period.
The 8 drug classes meeting these criteria were lipid-lowering drugs, gastroesophageal reflux disease drugs, diabetes drugs, antihypertensive drugs, attention-deficit/hyperactivity disorder drugs, antidepressant drugs, antipsychotic drugs, and hypnotic drugs approved for the treatment of insomnia (referred to as sleep aids). All drugs within these 8 drug classes that had a within-drug class market share of at least 0.5% were included.
All 262 drugs included in the study appear in eTable 2 in the Supplement. The number of drugs in the database was not static during the study period because the US Food and Drug Administration (FDA) approved a number of new drugs between 2006 and 2012. Of the included drugs, 87 were detailed at some point during the study, although many of them were detailed for only part of the study period. Fifty drugs were detailed during the entire study period. Twenty-five generic drugs were approved during the study period in which the bioequivalent brand-name drug in question had never previously faced generic competition. Twelve brand-name drugs were newly approved by the FDA during the study period.
Among the 175 drugs never detailed during the study, 114 were generic drugs, meaning they did not have a brand name and were only referred to by their chemical name. The remaining 61 were brand-name drugs that were not detailed during the study period. Generic and bioequivalent brand-name drugs were treated as separate in the analysis.
The pharmacy benefits manager provided a database of potential control physicians whose prescriptions were filled in the 5 states in the study, and data on all prescriptions filled through its pharmacy benefits system. The resulting database contained more than 250 000 potential control physicians.
A strict matching protocol was used to create a control group of physicians who were similar to intervention group physicians in terms of background and prescribing habits. Intervention group physicians were matched to potential control physicians in the same metropolitan statistical area based on pharmacy location for the majority of each physician’s prescriptions. Control physicians also had to exactly match an intervention physician’s specialty and year of birth, which were identified using the American Medical Association Masterfile.
To ensure that matched physicians had similar prescribing habits, a control physician was required to be within 10% of the intervention group physician’s prescribing volume for all 8 of the drug classes within the first 6 months of the study period (before any of the academic medical centers had enacted policies). We included all physicians outside the 19 intervention academic medical centers who met these criteria as matched controls, which led to an overall ratio of control to intervention physicians of 11.7 to 1.
Statistical Analysis
The dependent variable in the statistical analysis was a physician’s market share during a given month for a given drug within a drug class. For example, if a physician prescribed drug A 5 times, drug B 10 times, drug C 15 times, drug D 20 times, and drug E 0 times within a month, the physician wrote 50 total prescriptions within that drug class and had a market share of 10% for drug A, 20% for drug B, 30% for drug C, 40% for drug D, and 0% for drug E. The sum of each physician’s market share within a drug class and within a month was 100%. Drugs that a physician never prescribed during the study period were not included in the data for that physician. Within a given drug class, a physician was only included if that physician prescribed drugs within that drug class at some point during the study period. Market share is frequently used as a dependent variable in studies on physician prescribing, especially when data on all drugs within a drug class are included.
The regression approach modeled the change in prescribing after enactment of an academic medical center policy by calculating a regression coefficient that estimated the postintervention between-group difference in prescribing compared with the preintervention between-group difference. This coefficient was calculated separately for drugs detailed and those not detailed because policies regulating detailing might affect these 2 drug categories in opposite ways, reducing the likelihood a physician would prescribe a detailed drug for a patient, and increasing the likelihood a physician would instead prescribe a nondetailed drug.
The study distinguished between these 2 categories by using 2 indicator variables. The first variable took a value of 1 if a drug was detailed within a given month, and the second variable took a value of 1 if the drug was not detailed within that month. For each drug within each month, exactly 1 of these indicators took a value of 1. These indicator variables were then each interacted with an indicator variable that designated whether the physician was in the intervention group, and an indicator variable that took a value of 1 for all periods after which a specific academic medical center had adopted its policy.
To focus on the changes in prescribing related to the intervention, the sample was limited to prescriptions written up to 3 years before and 3 years after each academic medical center’s policy intervention, with the preintervention data truncated for the centers that implemented their policy less than 3 years after the beginning of the study period and the postintervention period truncated for the centers that implemented their intervention less than 3 years before the end date of the study period.
Fixed effects were included in the regression analyses for individual physicians, months, and drugs. The fixed effects controlled for baseline differences in prescribing behavior across physicians, hospitals, geographies, drugs, and drug classes. The inclusion of physician fixed effects meant that any difference across physicians that did not vary over time did not affect the regression coefficients, even if these differences caused dissimilar prescribing behavior, because each individual physician was compared only with himself or herself before and after intervention. This is a conservative estimation strategy because observing an association between implementation of the policies and changes in prescribing required individual physicians to change their prescribing propensity after the policy was implemented. Similarly, the use of drug fixed effects limited the regression estimates to within-drug changes in market share following policy implementation. Month fixed effects accounted for time trends, and an interaction between each month fixed effect and the control group indicator variable allowed time trends to differ for the control and intervention groups.
Difference-in-differences models can produce spurious findings if an unobserved and unexpected event that affected only 1 group (eg, a news story about conflicts of interest at a particular academic medical center) occurred shortly before or after the intervention, or if the 2 groups being compared had different time trends before the intervention occurred. Two additional analyses were conducted to examine the exact timing of prescribing changes identified in the regression analyses to rule out these problems.
First, raw data plots were generated to show changes in the actual data across time. Because academic medical centers enacted policies at different points in time, the data for each academic medical center and its matched control group were centered on the date that the academic medical center implemented the policy. This meant that the raw data were analyzed not according to calendar months, but according to months before or months after the intervention, a variable termed months to intervention. For example, data from November 2007 for an academic medical center that enacted policies in December 2007 were paired with data from June 2007 for a different academic medical center that enacted policies in July 2007 because in both cases the data were from the month before intervention.
The market share data used in the raw data plots were calculated at the academic medical center level, not at the physician level, so that each academic medical center and control group pair had a single observation for each month before the intervention. This avoided the overweighting of data for any particular period so that time trends could be accurately assessed. One version of the raw data plot contained data on all drugs in the database. However, generic equivalents of 25 proprietary study drugs were introduced for the first time during the study period according to the FDA Orange Book data files.
The sudden introduction of generic drugs and the pharmacy benefit manager’s policy of mandated generic interchange caused a decline in the market share of detailed drugs at the time of such generic entry, which made plots of time trends more difficult to interpret. A second version of the raw data plot therefore excluded brand-name drugs that faced initial bioequivalent generic entry during the study period, and their corresponding generic equivalents. Specifically, if the first time a generic version of a proprietary drug as listed in the FDA Orange Book occurred during the study period, both the proprietary and generic equivalent drug were dropped for the plot’s second version. All of the detailed and nondetailed drugs included in this second plot, therefore, were actively prescribed throughout the study interval.
A second regression-based investigation of the timing of the changes in prescribing associated with the intervention was conducted. Instead of a single regression coefficient for the entire postintervention period, as in the main regression model, this regression model estimated a series of monthly regression coefficients (capturing the interactions between the months to intervention variables and the intervention group and postintervention variables) for each month within a 12-month window both before and after policy implementation. The regression provided a coefficient for each month before the intervention, representing the estimated between-group difference compared with the estimated difference in the month before intervention, which was the omitted month in the regression.
Ordinary least squares was used to estimate the regressions due to the ease of interpreting the coefficients. Observations were weighted by a physician’s total number of prescriptions within a given drug class within a given month. Standard errors were clustered at the physician level and were block bootstrapped with 1000 iterations.
Alternative versions of the regression model were estimated to assess the robustness of the model. These included (1) estimating the associations between the intervention and prescribing for generalists and specialists separately, (2) using the number of prescriptions rather than the market share as a dependent variable for a given physician, drug, and month, (3) limiting each intervention physician to his or her closest 5 control matches rather than all matches that met the matching criteria, (4) using a nonlinear maximum likelihood specification (the fractional logit) rather than ordinary least squares to estimate the regressions, and (5) conducting a series of 27 regressions in which all observations from a single academic medical center or drug class were omitted to ensure that no single academic medical center or drug class was driving the results. Testing was done at the 95% significance level using 2-sided tests. Stata version 14 (StataCorp) was used for all analyses.
Results
The analysis included 16 121 483 prescriptions written by 2126 attending physicians at the 19 intervention group academic medical centers between January 2006 and June 2012 and by the 24 593 matched control group physicians. Most intervention and control physicians only prescribed within a limited number of the study’s 8 drug classes.
The number of intervention physicians ranged from 34 to 285 across academic medical centers, and the number of control physicians they were matched to ranged from 241 to 4724 (Table 1). The policies enacted between October 2006 and May 2011 at the 19 intervention group academic medical centers differed in their focus, but all included limits or bans on pharmaceutical representatives providing meals, branded items, and educational gifts that went further than the PhRMA code of conduct. Seventeen academic medical centers placed limits on hospital access such as bans on salespeople in patient care areas or requiring salesperson registration and training. The policies for 11 academic medical centers contained penalties for salespeople, physicians, or both for noncompliance.
Table 1. Study Academic Medical Centers, Detailing Policies, and Distribution of Intervention and Control Physicians.
| Academic Medical Center (AMC) |
Date of Policy Change |
Initial Policya | AMC Physicians | Matched Control Non-AMC Physicians |
||||
|---|---|---|---|---|---|---|---|---|
| Included Gift Policyb |
Included Access Policyc |
Included Enforcement Policyd |
No. (%) (n = 2126) |
Amount of Time Included in Study Data, Mean (SD), moe |
No. (%) (n = 24 593) |
Amount of Time Included in Study Data, Mean (SD), moe |
||
| Stanford University School of Medicine | Oct 2006 | Yes | Yes | No | 108 (5.1) | 39.6 (24.2) | 545 (2.2) | 44.8 (22.5) |
| Northwestern University Feinberg School of Medicine | Jun 2007 | Yes | No | No | 87 (4.1) | 45.0 (24.9) | 1442 (5.9) | 48.9 (22.6) |
| University of California, Davis, School of Medicine | Jul 2007 | Yes | Yes | No | 50 (2.4) | 43.5 (23.1) | 308 (1.3) | 47.7 (21.5) |
| University of California, Los Angeles, School of Medicine | Jul 2007 | Yes | Yes | Yes | 260 (12.2) | 44.5 (17.9) | 4724 (19.2) | 50.4 (21.1) |
| University of California, San Francisco, School of Medicine | Jul 2007 | Yes | Yes | No | 127 (6.0) | 40.0 (22.3) | 730 (3.0) | 44.5 (21.6) |
| Boston University School of Medicine | Aug 2007 | Yes | Yes | Yes | 64 (3.0) | 43.9 (20.3) | 807 (3.3) | 51.9 (21.1) |
| University of Illinois, Chicago, College of Medicine | Dec 2007 | Yes | Yes | Yes | 78 (3.7) | 42.2 (22.6) | 1160 (4.7) | 50.2 (21.7) |
| Mount Sinai School of Medicine | Jan 2008 | Yes | Yes | No | 39 (1.8) | 38.7 (22.9) | 1053 (4.3) | 41.9 (22.2) |
| University of Southern California Keck School of Medicine | Jan 2008 | Yes | Yes | Yes | 123 (5.8) | 42.5 (21.9) | 1531 (6.2) | 49.4 (21.7) |
| University of Pittsburgh School of Medicine | Feb 2008 | Yes | Yes | Yes | 285 (13.4) | 49.0 (21.9) | 1668 (6.8) | 51.6 (22.6) |
| University of Rochester School of Medicine | May 2008 | Yes | Yes | Yes | 46 (2.2) | 41.5 (29.6) | 241 (1.0) | 49.9 (17.7) |
| University of California, San Diego, School of Medicine | Jul 2008 | Yes | Yes | No | 284 (13.4) | 46.3 (20.6) | 1902 (7.7) | 48.9 (21.2) |
| University of Massachusetts Medical School | Jul 2008 | Yes | Yes | Yes | 191 (9.0) | 51.6 (20.6) | 643 (2.6) | 56.0 (20.5) |
| Rush Medical College | Feb 2009 | Yes | Yes | Yes | 153 (7.2) | 45.6 (22.5) | 3698 (15.0) | 56.0 (21.1) |
| Temple University School of Medicine | Jun 2009 | Yes | Yes | No | 42 (2.0) | 32.2 (12.6) | 930 (3.8) | 36.1 (15.0) |
| New York Medical College | Jan 2010 | Yes | Yes | Yes | 18 (1.0) | 28.5 (8.5) | 843 (3.4) | 32.5 (9.8) |
| State University of New York, Downstate Medical School | Apr 2010 | Yes | Yes | Yes | 20 (1.0) | 32.6 (13.1) | 756 (3.1) | 39.9 (14.2) |
| Tufts University School of Medicine | Apr 2010 | Yes | Yes | Yes | 117 (5.5) | 36.7 (14.8) | 1168 (4.7) | 37.4 (17.1) |
| Thomas Jefferson University Medical College | May 2011 | Yes | No | No | 34 (1.6) | 32.3 (13.4) | 444 (1.8) | 36.2 (13.8) |
The policy was in place for all or nearly all of the data analyzed for each AMC. A number of AMCs enacted stricter policies at some point during the study period, usually several years after the initial policy.
Prohibitions on gifts that were stricter than the Pharmaceutical Research and Manufacturers of America code of conduct.
Access restrictions on salespeople.
Penalized salespeople, physicians, or both for noncompliance.
The maximum value of this variable is 72 months because the study was limited to 3 years before and 3 years after each AMC’s policy intervention. Because the data start in January 2006 and the end in June 2012, the effective maximum for most AMCs is less than 72 months.
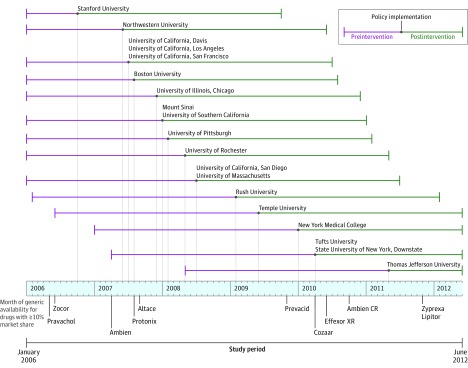
The introduction of academic medical center policies was not uniform across the study period (Figure 1). Specifically, 9 of 19 intervention academic medical centers, representing 53% of intervention physicians, introduced policies during the 9 months between June 2007 and February 2008. Several widely prescribed brand-name drugs faced initial generic competition during the months just around this period, most notably the brand-name version of the sleep aid zolpidem, whose generic equivalent was introduced in April 2007 (Figure 1).
Figure 1. Timing of Detailing Policy Implementations and Major Generic Drug Introductions.
At the physician level, the intervention group had a greater percentage of specialists than the control group (74.6% vs 54.0%, respectively) because intervention group specialists matched to many more control physicians in the pharmacy benefit manager’s database, according to the predetermined matching criteria (Table 2). The number of prescriptions written by control physicians was greater than those written by intervention physicians (mean prescriptions, 609.73 vs 530.36, respectively; median prescriptions, 401 vs 312). Within the combination unit of observation for physician, drug, and month used in the regression analyses, control physicians prescribed a larger number of drugs per month during the preintervention period and a higher proportion of detailed drugs.
Table 2. Summary Statistics for Intervention and Control Physiciansa.
| Intervention Physicians | Control Physicians | |
|---|---|---|
| Physician-Level Summary Statisticsb | ||
| No. of physicians | 2126 | 24 593 |
| Male sex, No. (%) | 1397 (65.7) | 16 083 (65.4) |
| Specialist, No. (%) | 1586 (74.6) | 13 287 (54.0) |
| Age, mean (SD), y | 45.4 (10.3) | 45.1 (9.5) |
| Total prescriptions in database | ||
| Mean (SD) [95% CI] | 530.36 (491.4) [53-1488] | 609.73 (987.5) [53-1911] |
| Median (IQR) | 312 (142-1208) | 401 (178-1617) |
| Preintervention Physician Drug-Level Summary Statisticsc | ||
| No. of prescriptionsd | ||
| Mean (SD) | 8.68 (7.21) | 12.85 (11.89) |
| Median (IQR) | 5.00 (3.91-12.21) | 7.00 (4.64-16.96) |
| Percentage of prescriptions for detailed drugs | ||
| Mean (SD) | 40 (32) | 43 (35) |
| Median (IQR) | 35 (15-56) | 42 (21-67) |
Abbreviation: IQR, interquartile range.
Between-group comparisons were significant except for percentage of male physicians and mean physician age. Calculated using a test of mean equivalency.
Data on sex, specialty, and age (as of 2006) were taken from the American Medical Association Masterfile.
Reflect monthly statistics for each physician and reflect the entire preintervention period.
Excludes drugs for which physician has no prescriptions.
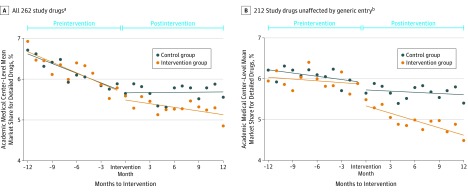
The analysis of the data that includes all 262 study drugs appears in Figure 2A. The levels of market share and time trends for detailed drugs were similar for the intervention and control groups during the preintervention period. After the intervention period, trends diverged between the 2 groups. The analysis of the data that excluded brand-name drugs that faced initial generic entry during the study period, and their generic equivalents, appears in Figure 2B. With these drugs removed, the preintervention trend against prescribing of detailed drugs is diminished for both groups. The preintervention trends are again similar in both the intervention and control groups, and there is a postintervention divergence in trends. The differences between the preintervention trends indicate that the initial generic entry affected the slopes of the time trends; however, both plots in Figure 2 show a postintervention divergence between the 2 groups in the prescribing of detailed drugs.
Figure 2. Mean Market Share of Detailed Drugs for Prescriptions Written by Academic Medical Center Intervention Physicians and Control Physicians by Time Relative to Each Center’s Intervention.
Months to intervention refers to the number of months relative to each academic medical center’s intervention. Data for each academic medical center is centered by the date of its intervention. Each data point represents the mean market share for detailed drugs across the 8 drug classes. For each academic medical center, the mean market share of detailed drugs in a given month to intervention was calculated separately for all 8 drug classes. This calculation yielded a total of 152 drug class mean market share values. Lines represent best linear fit, and both intercepts and slopes were allowed to vary before and after the intervention at each academic medical center. The market shares were calculated at the academic medical center level, not the individual physician level (which is the level of observation for the regressions). The mean market shares are not directly comparable with the sample means reported in Table 3. Eighteen academic medical centers are represented in every month to the intervention. A ± 12-month window was selected because pre- and postintervention data were available in that window for all but 1 academic medical center. One academic medical center, Stanford University, and its control group are not present in months to the intervention between -10 and -12 because this represented the last 3 months of 2005, which was before the study period.
aData are from all study drugs.
bData from 25 proprietary drugs that faced initial generic entry during the study period, and their corresponding generic equivalents, were removed from this analysis. The remaining 212 drugs were composed of 123 brand-name drugs that did not face initial generic entry during the study period, and 89 generic drugs that were introduced before the study period.
Implementation of academic medical center policies was associated with a mean decrease in market share of detailed drugs of 1.67 percentage points (95% CI, −2.18 to −1.18 percentage points; P < .001) and an increase in the market share of nondetailed drugs by 0.84 percentage points (95% CI, 0.54 to 1.14 percentage points; P < .001) (Table 3). Of the 19 academic medical centers, results were statistically significant for 9 academic medical centers for detailed drugs and for 8 academic medical centers for nondetailed drugs.
Table 3. Regression Analysis Results and Sample Mean Market Shares, 2006-2012.
| No. of Observationsa |
Detailed Drugs | Nondetailed Drugs | |||||
|---|---|---|---|---|---|---|---|
| Mean Market Share, 2006-2012, %b | Change in Market Share After Implementation of Detailing Policy, Percentage Points (95% CI)c |
P Value |
Mean Market Share, 2006-2012, %b | Change in Market Share After Implementation of Detailing Policy, Percentage Points (95% CI)c |
P Value |
||
| All Academic Medical Centers (AMCs) and All Drugs | 14 791 612 | 19.3 | −1.67 (−2.18 to −1.18) | <.001 | 14.2 | 0.84 (0.54 to 1.14) | <.001 |
| AMC-Specific Results Ordered by Date of Policy Intervention | |||||||
| Stanford University School of Medicine | 122 319 | 21.7 | −1.05 (−2.91 to 0.80) | .27 | 15.1 | −0.23 (−1.46 to 1.01) | .72 |
| Northwestern University Feinberg School of Medicine | 385 205 | 23.1 | −0.65 (−3.40 to 2.11) | .64 | 15.4 | −0.06 (−1.71 to 1.68) | .94 |
| University of California-Davis School of Medicine | 120 327 | 19.1 | −2.13 (−5.19 to 0.96) | .18 | 14.0 | 1.37 (−0.49 to 3.23) | .15 |
| University of California-Los Angeles School of Medicine | 2 539 672 | 18.3 | −2.72 (−4.39 to −1.04) | .02 | 13.4 | 1.60 (0.80 to 2.40) | <.001 |
| University of California-San Francisco School of Medicine | 232 137 | 21.9 | 0.54 (−1.54 to 2.67) | .62 | 16.3 | 0.21 (−0.21 to 0.64) | .33 |
| Boston University School of Medicine | 610 277 | 14.8 | −2.48 (−4.93 to −0.04) | .05 | 14.9 | 1.92 (−0.63 to 4.47) | .14 |
| University of Illinois-Chicago College of Medicine | 501 076 | 21.0 | −2.03 (−3.70 to −0.38) | .02 | 13.9 | 1.56 (0.15 to 2.96) | .03 |
| Mount Sinai School of Medicine | 605 651 | 21.2 | −2.29 (−5.09 to 0.51) | .11 | 12.6 | −0.14 (−3.23 to 2.97) | .93 |
| University of Southern California Keck School of Medicine | 869 500 | 19.4 | −1.87 (−3.69 to −0.02) | .04 | 13.7 | 0.90 (−0.21 to 2.00) | .11 |
| University of Pittsburgh School of Medicine | 1 058 972 | 19.9 | −1.96 (−2.87 to −0.82) | <.001 | 13.8 | 1.23 (0.52 to 1.95) | <.001 |
| University of Rochester School of Medicine | 116 538 | 21.9 | −1.46 (−5.30 to 2.61) | .48 | 18.6 | 1.03 (−1.82 to 3.87) | .48 |
| University of California-San Diego School of Medicine | 1 180 198 | 19.2 | −0.62 (−1.23 to −0.02) | .03 | 14.2 | 0.40 (0.01 to 0.62) | .03 |
| University of Massachusetts Medical School | 769 198 | 15.8 | −1.51 (−2.17 to −0.85) | <.001 | 13.8 | 1.47 (0.79 to 2.15) | <.001 |
| Rush Medical College | 4 246 339 | 17.7 | −0.91 (−2.23 to 0.41) | .18 | 11.8 | 0.62 (0.01 to 1.22) | .04 |
| Temple University School of Medicine | 233 190 | 22.3 | 0.32 (−7.29 to 7.34) | .94 | 16.7 | −0.51 (−12.88 to 11.89) | .93 |
| New York Medical College | 300 923 | 22.5 | −4.72 (−11.27 to 1.80) | .16 | 15.4 | 0.37 (−1.80 to 2.56) | .74 |
| State University of New York, Downstate Medical School | 325 786 | 21.9 | −1.59 (−2.87 to −0.31) | .01 | 14.0 | 1.58 (0.84 to 2.31) | <.001 |
| Tufts University School of Medicine | 434 177 | 17.6 | −1.02 (−2.02 to −0.01) | .04 | 17.6 | 0.70 (0.02 to 1.36) | .04 |
| Thomas Jefferson University Medical College | 140 109 | 21.5 | −1.23 (−4.36 to 1.89) | .44 | 14.6 | 0.51 (−0.37 to 1.40) | .25 |
| Drug Class–Specific Results Ordered by Size of Effect | |||||||
| Sleep aidsd | 375 753 | 51.4 | −10.5 (−18.87 to −2.16) | .01 | 50.9 | 4.67 (0.81 to 8.50) | .02 |
| Gastroesophageal reflux disease drugs | 1 166 894 | 37.0 | −6.89 (−9.10 to −4.70) | <.001 | 22.7 | 3.98 (2.63 to 5.31) | <.001 |
| Attention-deficit/hyperactivity disorder drugs | 149 703 | 29.0 | −5.03 (−9.92 to −0.05) | .04 | 40.6 | 2.51 (−1.02 to 6.05) | .16 |
| Antidepressant drugs | 2 453 111 | 22.5 | −2.32 (−3.26 to −1.37) | <.001 | 12.4 | 1.17 (0.68 to 1.66) | <.001 |
| Lipid-lowering drugs | 2 490 775 | 28.8 | −1.51 (−2.36 to −0.66) | .001 | 10.5 | 1.31 (0.69 to 1.92) | <.001 |
| Antihypertensive drugs | 5 496 003 | 9.2 | −0.84 (−1.34 to −0.35) | .002 | 9.1 | 0.67 (0.37 to 0.96) | <.001 |
| Antipsychotic drugs | 335 149 | 37.8 | −0.55 (−2.79 to 1.70) | .63 | 28.0 | 0.89 (−0.53 to 2.32) | .22 |
| Diabetes drugs | 2 311 089 | 10.9 | 0.60 (−0.26 to 1.47) | .24 | 16.6 | −0.05 (−1.08 to 0.98) | .92 |
Total number of physician-drug-month observations for the given sample.
Given as a reference to interpret the size of the reported change in market share associated with AMC policies restricting pharmaceutical detailing.
Associated with AMC policies restricting pharmaceutical detailing.
Hypnotic drugs approved for the treatment of insomnia.
Of the 11 academic medical centers with policies that limited salesperson gifts to physicians, regulated salesperson access to facilities, and provided enforcement mechanisms such as penalties for salespeople and physicians who violated the policy, there were significant results at 8 (University of California, Los Angeles; Boston University; University of Illinois, Chicago; University of Southern California; University of Pittsburgh; University of Massachusetts; State University of New York, Downstate; and Tufts University) and nonsignificant results at 3 (Rush Medical College, New York Medical Center, and University of Rochester). In contrast, there was a significant change at only 1 of 8 academic medical centers (University of California, San Diego) that failed to enact 1 or more of these policies and nonsignificant results at 7 (Stanford University; Northwestern University; University of California, Davis; University of California, San Francisco; Mount Sinai; Temple University; and Thomas Jefferson University).
At the drug class level, detailing policies were associated with statistically significant changes in market share for 6 of the 8 drug classes (Table 3). Sleep aid drugs, drugs for gastroesophageal reflux disease, and attention-deficit/hyperactivity disorder drugs all showed decreases of greater than 5 percentage points in market share for detailed drugs. Market share of detailed drugs for antidepressant agents, antihypertensive drugs, and lipid-lowering drugs decreased by 0.84 to 2.32 percentage points. All of these results were statistically significant. Coefficients for change in market share of nondetailed attention-deficit/hyperactivity disorder drugs, and detailed and nondetailed antipsychotic drugs and diabetes drugs, were not statistically significant.
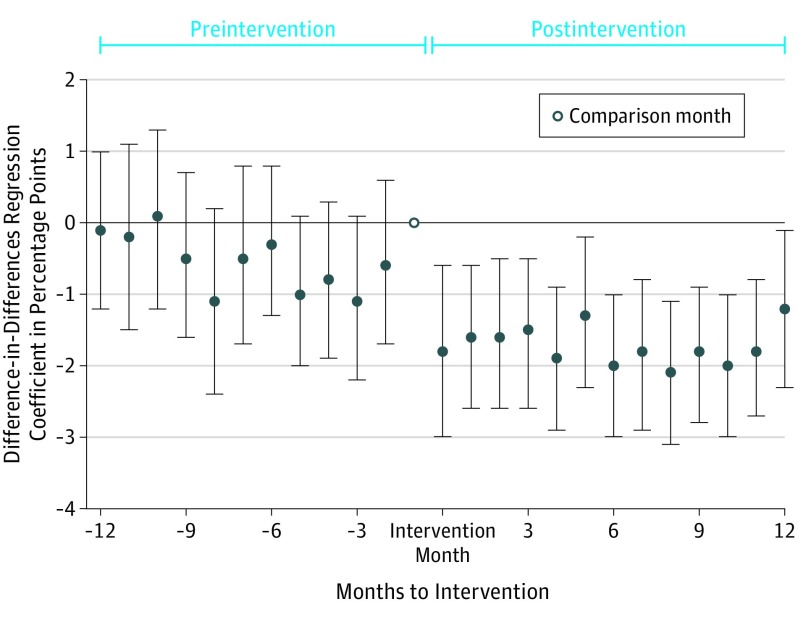
The results of the regression analysis that estimated a separate difference-in-differences coefficient for drug prescribing during the 12 pre- and postintervention months appear in Figure 3 and in eTable 3 in the Supplement. The estimated between-group difference was not statistically significant and close to 0 for the preintervention months, but was positive and statistically significant during all postintervention months.
Figure 3. Coefficient Estimates of Monthly Between-Group Differences in Market Share of Detailed Drugs.
Results of difference-in-differences regression estimating the difference in prescribing of detailed drugs between intervention and control physicians in a given month to intervention compared with the difference in the month before intervention (which was the omitted month to intervention in the regressions, and therefore represents the comparison month). Data markers represent the coefficient estimate and the error bars represent the 95% CIs. Negative values reflect lower prescribing of detailed drugs in the intervention group vs the control group during the given month to intervention compared with the same calculation during the month before intervention.
In each of the 5 classes of alternative models intended to test for the robustness of the results, the signs of coefficients of interest were the same as those for the main model, and the statistical significance of the results for both detailed and nondetailed drugs remained at P < .001. The results of these tests appear in eTable 4 in the Supplement.
Discussion
In this study comparing changes in the prescribing behavior of 2126 physicians at 19 academic medical centers with the prescribing behavior of 24 593 matched control physicians during the 10- to 36-month period before vs the 12- to 36-month period after implementation of detailing policies at academic medical centers, enactment of detailing restrictions at academic medical centers was associated with a decrease in the prescribing of detailed drugs of 1.67 percentage points of market share, and an increase in prescribing of nondetailed drugs of 0.84 percentage points. Evaluated at a sample mean market share of 19.3% for detailed drugs, the 1.67 percentage point reduction represented an 8.7% relative decrease in market share after the intervention. At the 14.2% sample mean market share for nondetailed drugs, the 0.84 percentage point increase represented a 5.6% relative increase in market share following the intervention.
Due to mandated generic interchange, whereby the pharmacy benefits manager automatically substituted generics for brand-name drugs when generic equivalents were available and when allowed by state law, more than 95% of nondetailed prescriptions in the data were for generic drugs. Therefore, across academic medical centers and drug classes, prescriptions shifted away from detailed drugs and toward generic drugs following the introduction of policies restricting pharmaceutical detailing.
Limitations
This study has several limitations. First, the observational design precludes proving causal relationships because other secular changes may have occurred that could have influenced the study results. For instance, some academic medical centers likely underwent other changes around the time that they enacted detailing policies, such as initiating a culture of evidence-based practice or providing education and training around industry interactions. However, the results in Figure 3, showing that the changes in prescribing started during the exact month of policy enactment, support the hypothesis that enactment of the policies was associated with changes in prescribing. Nevertheless, it is not possible to rule out that the prescribing changes observed in the study reflected other changes at academic medical centers that coincided with detailing policies.
Second, control group physicians were mainly in private practice, whereas intervention group physicians were associated with academic medical centers. Even though the use of fixed effects in the analysis controls for baseline physician differences in prescribing behavior, it is possible that control group physicians would have responded differently if exposed to the policies than did intervention group physicians. Third, the study used data sources for which accuracy has not been validated or for which there are known accuracy issues (ie, the American Medical Association Masterfile to code type of physician specialty, the affiliation data provided by IMS, and the detailing data provided by SDI Health). Fourth, researcher judgment was used to determine the 8 drug classes to include in the study. It is possible that examination of other drug classes may have produced different results. Fifth, the study period ended in 2012, and many academic medical centers have taken further measures since 2012 to limit conflicts of interest, so it is unknown whether the findings would translate to the current environment.
However, the study had several strengths that suggest that the most likely explanation for the associations identified in the results was that the policies influenced prescribing behavior. The difference-in-differences design used in the study is superior to the purely cross-sectional approaches used in several other studies addressing the same question. This design is commonly used to assess the effects of changes in policies when, as is true of the implementation of detailing policies, randomized clinical trials are impractical.
The study was also more comprehensive than previous studies examining drug detailing and conflicts of interest. The data set included all widely prescribed drugs within 8 major drug classes, prescribed by both generalist and specialist physicians. The academic medical centers in the study encompassed a large number of physicians who were exposed to the policies in the middle of an ongoing stream of prescribing, and an even larger control group of physicians matched on geography, age, specialty, and drug prescribing prior to policy enactment.
Academic medical centers in the study implemented policies at different points in time, which helps to reduce concern that unobserved events distinct from the policies can account for the results. Although implementation of the policies was not associated with significant changes in prescribing for all drug classes and academic medical centers, the aggregate results were highly significant, and this was true even when individual drug classes and academic medical centers were successively removed from the analysis.
Even though the overall results of the study demonstrated a significant association between implementation of detailing policies and change in prescribing of detailed drugs, the fact that only 9 of the 19 academic medical centers included in the study had statistically significant results may suggest that the results lack generalizability. However, as shown in Table 1, academic medical center detailing policies varied widely. Future research should examine the relationship between the content of academic medical center detailing policies and the prescribing patterns of physicians, especially because such policies are increasingly being adopted by private medical practices.
These results complement findings from other studies that have documented correlations between physician prescribing of detailed drugs and detailing strategies such as meals, as well as 2 studies that reported that physicians who had graduated from medical schools with strict conflict of interest policies prescribed fewer marketed (detailed) medications. The current study provides new evidence relevant to the evaluation of detailing policies by examining changes in the prescribing of detailed and nondetailed drugs by physicians who were and were not subject to detailing policies implemented at academic medical centers.
The reduction in the prescribing of detailed drugs and the increase in the prescribing of nondetailed drugs potentially represents a large reduction in costs. In 2010, pharmaceutical companies earned more than $60 billion in revenues for detailed drugs included in the study, and generic drugs are on average 80% to 85% less expensive than brand-name drugs. A 1-percentage point change in market share could represent approximately a 5% relative change in revenue for the average detailed drug, suggesting that the observed changes in prescribing could have important economic implications.
Conclusions
Implementation of policies at academic medical centers that restricted pharmaceutical detailing between 2006 and 2012 was associated with modest but significant reductions in prescribing of detailed drugs across 6 of 8 major drug classes; however, changes were not seen in all of the academic medical centers that enacted policies.
eTable 1. AMCs and hospitals included in study
eTable 2. Study drug classes and drugs
eTable 3. Results of regression estimating months to intervention differences between intervention and control groups
eTable 4. Results of alternative regression models
References
- 1.Steinbrook R. For sale: physicians’ prescribing data. N Engl J Med. 2006;354(26):2745-2747. [DOI] [PubMed] [Google Scholar]
- 2.American Medical Student Association American Medical Student Association scorecard. http://www.amsascorecard.org/. Accessed April 13, 2017.
- 3.Larkin I, Ang D, Avorn J, Kesselheim AS. Restrictions on pharmaceutical detailing reduced off-label prescribing of antidepressants and antipsychotics in children. Health Aff (Millwood). 2014;33(6):1014-1023. [DOI] [PubMed] [Google Scholar]
- 4.Anderson TS, Huskamp HA, Epstein AJ, et al. . Antipsychotic prescribing: do conflict of interest policies make a difference? Med Care. 2015;53(4):338-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chressanthis GA, Khedkar P, Jain N, Poddar P, Seiders MG. Can access limits on sales representatives to physicians affect clinical prescription decisions? a study of recent events with diabetes and lipid drugs. J Clin Hypertens (Greenwich). 2012;14(7):435-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wooldridge JM. Econometric Analysis of Cross Section and Panel Data. 2nd ed Cambridge, MA: MIT Press; 2010. [Google Scholar]
- 7.American Board of Medical Specialties Accredited medical schools (US and Canadian): official ABMS directory of board certified medical specialists. https://www.abmsdirectory.com/pdf/Resources_directory_MedSchools.pdf. Accessed April 13, 2017.
- 8.Henry J. Kaiser Family Foundation Total number of retail prescription drugs filled at pharmacies. http://kff.org/other/state-indicator/total-retail-rx-drugs/?activeTab=map¤tTimeframe=0&selectedDistributions=total-retail-rx-drugs&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed April 13, 2017.
- 9.Institute on Medicine as a Profession Conflict of interest policy database. http://imapny.org/conflicts-of-interest/conflicts-of-interest-2/. Accessed April 13, 2017.
- 10.PhRMA 2002. PhRMA code on interactions with healthcare professionals: UCSF continuing medical education. http://www.ucsfcme.com/physician/PhRMACode.pdf. Accessed April 13, 2017.
- 11.Spurling GK, Mansfield PR, Montgomery BD, et al. . Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7(10):e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volpp KG, Rosen AK, Rosenbaum PR, et al. . Mortality among patients in VA hospitals in the first 2 years following ACGME resident duty hour reform. JAMA. 2007;298(9):984-992. [DOI] [PubMed] [Google Scholar]
- 13.Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA. 2014;312(22):2401-2402. [DOI] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration Orange Book data files. https://www.fda.gov/Drugs/InformationOnDrugs/ucm129689.htm. Accessed April 13, 2017.
- 15.DeJong C, Aguilar T, Tseng CW, Lin GA, Boscardin WJ, Dudley RA. Pharmaceutical industry-sponsored meals and physician prescribing patterns for Medicare beneficiaries. JAMA Intern Med. 2016;176(8):1114-1122. [DOI] [PubMed] [Google Scholar]
- 16.Perlis RH, Perlis CS. Physician payments from industry are associated with greater Medicare part D prescribing costs. PLoS One. 2016;11(5):e0155474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeh JS, Franklin JM, Avorn J, Landon J, Kesselheim AS. Association of industry payments to physicians with the prescribing of brand-name statins in Massachusetts. JAMA Intern Med. 2016;176(6):763-768. [DOI] [PubMed] [Google Scholar]
- 18.King M, Essick C, Bearman P, Ross JS. Medical school gift restriction policies and physician prescribing of newly marketed psychotropic medications: difference-in-differences analysis. BMJ. 2013;346:f264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epstein AJ, Busch SH, Busch AB, Asch DA, Barry CL. Does exposure to conflict of interest policies in psychiatry residency affect antidepressant prescribing? Med Care. 2013;51(2):199-203. [DOI] [PubMed] [Google Scholar]
- 20.Drugs.com website Top 200 drugs for 2010. by sales. https://www.drugs.com/top200.html. Accessed April 13, 2017.
- 21.Generic Pharmaceutical Association An economic analysis of generic drug usage in the US, 2011. http://www.gphaonline.org/media/cms/AnnualReport_11.pdf. Accessed April 13, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. AMCs and hospitals included in study
eTable 2. Study drug classes and drugs
eTable 3. Results of regression estimating months to intervention differences between intervention and control groups
eTable 4. Results of alternative regression models