This meta-analysis investigates the association of primary prevention implantable cardioverter defibrillators with all-cause mortality in patients with nonischemic cardiomyopathy.
Key Points
Question
Does use of primary prevention implantable cardioverter defibrillators improve survival among patients with nonischemic cardiomyopathy?
Findings
In this meta-analysis of 4 randomized clinical trials that included 1874 patients with nonischemic cardiomyopathy, use of primary prevention implantable cardioverter defibrillators reduced all-cause mortality by 25%.
Meaning
These findings support professional guidelines that recommend implantable cardioverter defibrillators in patients with nonischemic cardiomyopathy.
Abstract
Importance
Conflicting data have emerged on the efficacy of implantable cardioverter defibrillators (ICDs) for primary prevention of sudden cardiac death (primary prevention ICDs) in patients with nonischemic cardiomyopathy.
Objective
To investigate the association of primary prevention ICDs with all-cause mortality in patients with nonischemic cardiomyopathy.
Data Sources
PubMed was searched from January 1, 2000, through October 31, 2016, for the terms implantable defibrillator OR implantable cardioverter defibrillator AND non-ischemic cardiomyopathy. Additional references were identified from bibliographies of pertinent articles and queries to experts in this field.
Study Selection
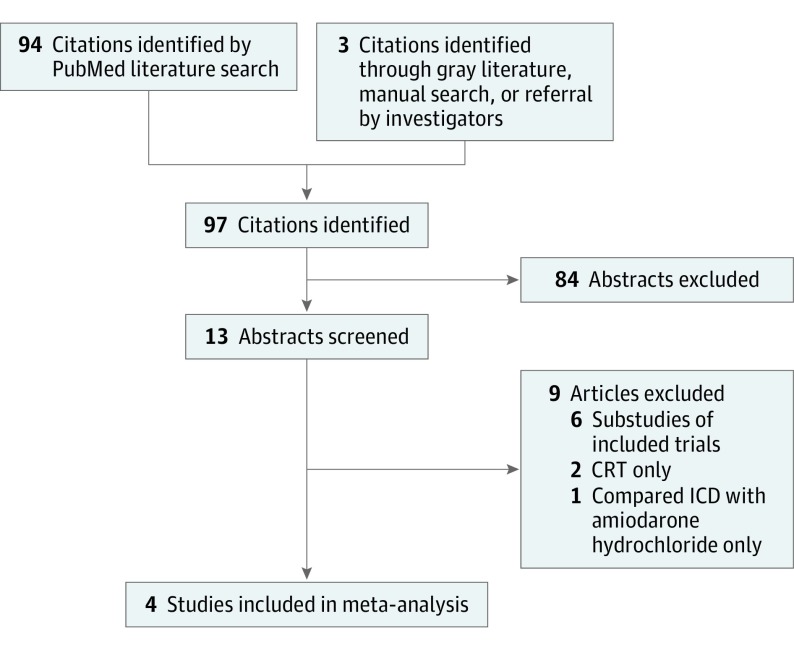
Inclusion criteria consisted of a randomized clinical trial design and comparison of the ICD with medical therapy (control) in at least 100 patients with nonischemic cardiomyopathy. In addition, studies had to report on all-cause mortality during a follow-up period of at least 12 months and be published in English. The search yielded 10 studies, of which only 1 met the inclusion criteria. A search of bibliographies of pertinent articles and queries of experts in this field led to 3 additional studies.
Data Extraction and Synthesis
The PRISMA guidelines were used to abstract data and assess data quality and validity. Data were pooled using fixed- and random-effects models.
Main Outcomes and Measures
The primary end point was all-cause mortality. Before data collection started, primary prevention ICDs were hypothesized to reduce all-cause mortality among patients with nonischemic cardiomyopathy.
Results
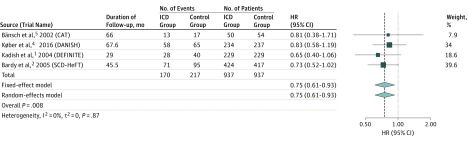
Four randomized clinical trials met the selection criteria and included 1874 unique patients; 937 were in the ICD group and 937 in the control group. Pooling data from these trials showed a significant reduction in all-cause mortality with an ICD (hazard ratio, 0.75; 95% CI, 0.61-0.93; P = .008; P = .87 for heterogeneity).
Conclusions and Relevance
Primary prevention ICDs are efficacious at reducing all-cause mortality among patients with nonischemic cardiomyopathy. These findings support professional guidelines that recommend the use of ICDs in such patients.
Introduction
Patients with heart failure due to nonischemic cardiomyopathy are at an increased risk for sudden cardiac death (SCD). In the mid-2000s, 2 randomized clinical trials provided helpful data on the benefit of implantable cardioverter defibrillators (ICDs) for primary prevention of SCD (hereafter referred to as primary prevention ICDs) in these patients. The Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial showed a significant reduction in the risk for SCD with use of primary prevention ICDs in patients with a left ventricular ejection fraction of 35% or less owing to nonischemic cardiomyopathy and premature ventricular complexes or nonsustained ventricular tachycardia. A trend toward reduced all-cause mortality was seen with use of an ICD. The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) showed a significant improvement in the survival of patients with New York Heart Association (NYHA) class II or III heart failure symptoms due to ischemic or nonischemic cardiomyopathy with use of a primary prevention ICD. Data from these trials led to the designation of the primary prevention ICD as a class I therapy in patients with NYHA class II or III heart failure symptoms due to nonischemic cardiomyopathy.
The recently published Danish Study to Assess the Efficacy of ICDs in Patients With Nonischemic Systolic Heart Failure on Mortality (DANISH trial) enrolled patients with NYHA class II or III heart failure symptoms (or NYHA class IV symptoms if cardiac resynchronization therapy [CRT] was planned) with a left ventricular ejection fraction of 35% or less owing to nonischemic cardiomyopathy and an increased level of N-terminal pro–brain natriuretic peptide (NT-proBNP) despite guideline-directed medical therapy. The DANISH trial showed a reduction in SCD but not in all-cause mortality with use of an ICD. To better understand the effect of primary prevention ICDs on survival among patients with nonischemic cardiomyopathy, we conducted a meta-analysis of randomized clinical trials on this topic.
Methods
Selection criteria included a randomized clinical trial design with ICD vs medical therapy as the comparators and a follow-up of at least 12 months. Studies had to have at least 100 patients and to have been published in English from January 1, 2000, through October 31, 2016. We excluded trials of CRT and those that compared the ICD with an antiarrhythmic medication, including amiodarone. Studies that enrolled patients with ischemic and nonischemic cardiomyopathy were included if data on patients with nonischemic cardiomyopathy were reported separately or could be obtained directly from the study investigators. Studies that included an ICD arm, a medical therapy arm, and other comparators were included if data on the ICD and medical therapy arms were reported separately. We searched PubMed using the following search terms: implantable defibrillator OR implantable cardioverter defibrillator AND non-ischemic cardiomyopathy. This search yielded 10 studies, of which only 1 (the DEFINITE trial) met our criteria (2 studies included CRT only, 1 compared ICD with amiodarone only, and 6 were substudies of the DEFINITE trial). A search of bibliographies of pertinent articles and queries of experts in this field resulted in 3 additional studies: the Cardiomyopathy Trial (CAT), the SCD-HeFT (ICD and medical therapy groups of patients with nonischemic cardiomyopathy), and the DANISH trial (patients with an ICD and medical therapy without CRT) (Figure 1). We used the Cochran Q test to examine heterogeneity among the included trials. P < .05 indicated statistical significance.
Figure 1. PRISMA Diagram of the Search for Pertinent Trials.
CRT indicates cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator.
Results
The 4 randomized clinical trials that met our selection criteria included 1874 unique patients with 937 in the ICD group and 937 in the medical therapy (control) group. Mortality rates are provided in the Table. Pooling data with fixed- and random-effects models from these 4 studies showed a significant reduction in all-cause mortality with use of an ICD (hazard ratio, 0.75; 95% CI, 0.61-0.93; P = .008; P = .87 for heterogeneity) (Figure 2). We performed 2 sensitivity analyses. One excluded the CAT, which was smaller and had a shorter follow-up than the other 3 trials, and the other excluded the DANISH trial, given the time difference between the completion of that trial relative to the other 3 trials. The results did not change appreciably. To further address the issue of the time difference among the trials, we conducted a meta-regression that accounted for each trial’s year of publication in the meta-analysis. We found no evidence that time was a modifier of the relationship between use of an ICD and all-cause mortality (P = .53).
Table. All-Cause Mortality in the Individual Trials.
| Source (Trial Name) | Follow-up, moa | All-Cause Mortality, No. With End Point/Total No. | |
|---|---|---|---|
| ICD Group | Control Group | ||
| Bänsch et al, 2002 (CAT) | 66.0 (26.4) | 13/50 | 17/54 |
| Kadish et al, 2004 (DEFINITE) | 29.0 (14.4) | 28/229 | 40/229 |
| Bardy et al, 2005 (SCD-HeFT) | 45.5 (34.8-55.2) | 71/424 | 95/417 |
| Køber et al, 2016 (DANISH) | 67.6 (49.0-85.0) | 58/234 | 65/237 |
| All | NA | 170/937 | 217/937 |
Abbreviations: CAT, Cardiomyopathy Trial; DANISH, Danish Study to Assess the Efficacy of ICDs in Patients With Nonischemic Systolic Heart Failure on Mortality; DEFINITE, Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation; ICD, implantable cardioverter defibrillator; NA, not applicable; SCD-HeFT, Sudden Cardiac Death in Heart Failure Trial.
Presented as mean (SD) in the CAT and DEFINITE trials and median (interquartile range) in the SCD-HeFT and DANISH trials.
Figure 2. Results of Meta-analysis.
Findings include data from 4 randomized clinical trials of implantable cardioverter defibrillators (ICDs) in patients with nonischemic cardiomyopathy using fixed- and random-effects models. CAT indicates Cardiomyopathy Trial; DANISH, Danish Study to Assess the Efficacy of ICDs in Patients With Nonischemic Systolic Heart Failure on Mortality; DEFINITE, Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation; HR, hazard ratio; and SCD-HeFT, Sudden Cardiac Death in Heart Failure Trial.
Discussion
We showed a significant survival benefit of use of an ICD in patients with nonischemic cardiomyopathy. Our findings are consistent with the results of the DEFINITE and SCD-HeFT trials. Compared with these 2 trials, the DANISH trial differed in a number of ways. First, 58% of patients in the ICD and the control groups in the DANISH trial received a CRT device. Therefore, data from the overall DANISH trial should not be extrapolated to patients with nonischemic cardiomyopathy who are eligible for an ICD but not for CRT. The frequent use of CRT likely contributed to the lower than projected mortality rate in both groups in the DANISH trial. Second, medical therapy in both groups in the DANISH trial was superior to that used in clinical practice. Third, the DANISH trial is the only trial to date to require an elevated NT pro-BNP level for a patient to qualify for enrollment, and the median level of NT pro-BNP of enrolled patients was 1244 pg/mL in the ICD group and 1110 pg/mL in the medical therapy group (to convert NT pro-BNP to nanograms per liter, multiply by 1.0). This finding, along with the older age of patients in the DANISH trial (mean age, 64 years compared with 52-60 years in the other 3 trials), may have led to the inclusion of patients who are more likely to die of non-SCD causes. More granular data on the mode of death from the DANISH trial would be important. Finally, the high use of CRT and guideline-directed medical therapy in the DANISH trial likely reduced their statistical power for showing a significant difference in the primary outcome, despite extending the follow-up period to more than 67 months.
Strengths and Limitations
Another meta-analysis of primary prevention ICDs in patients with nonischemic cardiomyopathy was recently published. Compared with that analysis, our approach was more robust at reducing heterogeneity because we included only trials that compared the ICD with optimal medical therapy and excluded trials of CRT and antiarrhythmic medications. Furthermore, through our access to patient-level data in the DEFINITE and SCD-HeFT trials, we were able to provide actual mortality rates from all the trials (Table). However, a limitation of our meta-analysis is noteworthy. Although we used patient-level data from the SCD-HeFT and the DEFINITE trials, we had no access to patient-level data in the CAT or the DANISH trial.
Conclusions
Based on the totality of evidence, our findings support the 2012 guidelines of the American Heart Association, American College of Cardiology Foundation, and Heart Rhythm Society for ICDs. The guidelines recommend the use of ICDs in eligible patients with nonischemic cardiomyopathy and a depressed left ventricular ejection fraction.
References
- 1.Kadish A, Dyer A, Daubert JP, et al. ; Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators . Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151-2158. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, et al. ; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators . Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225-237. [DOI] [PubMed] [Google Scholar]
- 3.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society . 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61(3):e6-e75. [DOI] [PubMed] [Google Scholar]
- 4.Køber L, Thune JJ, Nielsen JC, et al. ; DANISH Investigators . Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375(13):1221-1230. [DOI] [PubMed] [Google Scholar]
- 5.Bänsch D, Antz M, Boczor S, et al. . Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT). Circulation. 2002;105(12):1453-1458. [DOI] [PubMed] [Google Scholar]
- 6.Chin KL, Skiba M, Tonkin A, et al. . The treatment gap in patients with chronic systolic heart failure: a systematic review of evidence-based prescribing in practice. Heart Fail Rev. 2016;21(6):675-697. [DOI] [PubMed] [Google Scholar]
- 7.Golwala H, Bajaj NS, Arora G, Arora P. Implantable cardioverter-defibrillator for non ischemic cardiomyopathy: an updated meta-analysis. Circulation. 2017;135(2):201-203. [DOI] [PMC free article] [PubMed] [Google Scholar]