Key Points
Questions
Do the lipid-lowering effects of evolocumab, a proprotein convertase subtilisin/kexin type 9 inhibitor, persist with long-term use?
Findings
In this open-label extension of a randomized clinical trial, evolocumab added to standard of care helped control low-density lipoprotein cholesterol (LDL-C) levels consistently over time, with a median LDL-C level reduction of 57% and achieved LDL-C level of 60 mg/dL for participants with 4 years of follow-up. No significant safety signals of concern and no neutralizing antibodies were observed with cumulative drug exposure.
Meaning
Hypercholesterolemia therapy with a proprotein convertase subtilisin/kexin type 9 inhibitor reduced LDL-C levels over an extended period, with no significant loss in efficacy or increasing annual rate of adverse effects from cumulative exposure.
Abstract
Importance
The Open-Label Study of Long-term Evaluation Against LDL-C (OSLER-1) evaluated the durability of long-term efficacy and safety during long-term therapy with evolocumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9 (PCSK9).
Objective
To determine whether LDL-C level reductions with evolocumab persist across different populations. Secondary objectives included assessment of adverse events, antidrug antibodies, and factors contributing to treatment discontinuation.
Design, Setting, and Participants
This ongoing, randomized open-label extension trial (OSLER-1) was conducted at 192 sites in 18 countries. A total of 1324 of 1666 patients randomized into 1 of 5 12-week double-blind phase 2 parent studies completed a parent study and chose to participate in OSLER-1; 1255 received 1 or more evolocumab doses. As of August 2016, 812 of 1324 (61%) had 208 weeks of follow-up. This current study was conducted from October 2011 to August 2016, with a data cutoff of August 26, 2016.
Interventions
During year 1, patients were randomized to evolocumab, 420 mg, plus standard of care (SOC) or SOC alone. After year 1, all patients continuing the study received evolocumab, 420 mg, plus SOC.
Main Outcomes and Measures
Lipids, safety, and tolerability every 12 weeks. A multivariate model identified factors associated with discontinuation of evolocumab.
Results
At parent study baseline, the mean (SD) age of the population was 57.1 (11.6) years, with 52.9% being women. The median LDL-C level was 133 mg/dL (to convert to millimoles per liter, multiply by 0.0259). After 52 weeks, evolocumab plus SOC was associated with a significant reduction in LDL-C level by 61% (95% CI, −63% to −60%) vs 2% (95% CI, −5% to −0.2%) with SOC alone (P < .001). At approximately 2, 3, and 4 years of study follow-up, the median LDL-C level was reduced by 59% (95% CI, −60% to −57%), 59% (95% CI, −61% to −58%), and 57% (95% CI, −59% to −55%), respectively, from parent study baseline. For patients receiving statin therapy unchanged from baseline, at week 208, the median LDL-C level reduction was 58%. No neutralizing antibodies to evolocumab were detected. The annualized incidence of new-onset diabetes was 4% in the SOC alone group and, adjusting for duration of evolocumab exposure, 2.8% in the evolocumab plus SOC group. Neurocognitive event rates were 0% (SOC alone) and 0.4% (evolocumab plus SOC). A total of 79% of patients persisted with evolocumab treatment, with a mean exposure duration of 44 months.
Conclusions and Relevance
In the longest clinical trial exposure to a PCSK9 inhibitor to date, evolocumab produced sustained reductions in LDL-C levels. The annual frequency of adverse events did not occur more frequently with cumulative exposure during open-label observation.
Trial Registration
clinicaltrials.gov Identifier: NCT01439880
This observational extension of the OSLER-1 randomized clinical trial determines whether low-density lipoprotein cholesterol level reductions with evolocumab persist across different patient populations.
Introduction
Lipid-lowering therapy has served as a cornerstone of the cardiovascular risk factor modification strategy that has led to improved outcomes over the past 3 decades. Although many studies have shown substantial benefits and an excellent overall safety profile of statins, the risk for cardiovascular complications in certain statin-treated populations remains high. Further, many patients do not use statins as recommended owing to adverse effects or the perception of poor tolerance of the drug class.
In recent years, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition has emerged as a promising approach for treating hypercholesterolemia. A novel strategy to inhibit PCSK9 involves therapeutic monoclonal antibodies that bind irreversibly to the molecule and prevent it from binding to the low-density lipoprotein (LDL) receptor/LDL cholesterol (C) complex. Two monoclonal antibodies targeting PCSK9, evolocumab and alirocumab, have received regulatory approval for treating hypercholesterolemia in patients with cardiovascular disease or heterozygous familial hypercholesterolemia (with evolocumab also approved for use in patients with homozygous familial hypercholesterolemia) with inadequate LDL-C level control on maximally tolerated statin therapy. Both drugs demonstrated significant reductions of LDL-C and other proatherosclerotic lipoprotein levels. A third anti-PCSK9 antibody, bococizumab, demonstrated significant reductions in LDL-C levels in phase 2 trials. However, a recent announcement of the decision to discontinue development of bococizumab cited “an unanticipated attenuation of LDL-C lowering over time, as well as a higher level of immunogenicity and higher rate of injection-site reactions.” To our knowledge, no studies to date have demonstrated the long-term efficacy of a PCSK9 inhibitor beyond 24 months.
The current analysis includes patients who completed double-blind phase 2 studies and entered into a multiyear, open-label extension conducted at 192 sites in 18 countries. We evaluated the long-term lipid-lowering efficacy, persistence, and safety of evolocumab in an ongoing clinical trial cohort of patients, many of whom have received treatment for up to 4 years.
Methods
Study Design and Patients
A previous report detailed the design and first-year safety and efficacy results of the Open-Label Study of Long-term Evaluation Against LDL-C (OSLER-1) from a cohort that included patients enrolled from 4 of the 5 phase 2 parent studies evaluated in the current analysis. In total, 192 sites in 18 countries enrolled eligible patients who completed 1 of the 5 phase 2 studies with evolocumab between October 2011 and December 2013. Patients could enroll in OSLER-1 provided that they did not discontinue treatment owing to a serious adverse event (SAE) during their phase 2 study duration or require unblinded lipid measurements and/or adjustment of background lipid therapy during the first 12 weeks of OSLER-1. The first patient consented for OSLER-1 in October 2011. The last patient enrolled in June 2013. The current analysis reports data from first enrollment to August 26, 2016, in this ongoing clinical trial. The mean (SD) age of the population was 57.1 (11.6) years.
Randomization of eligible patients occurred at or within 3 days of the phase 2 parent end-of-study visit. Patients were randomized 2:1 to 1 of 2 treatment groups, irrespective of their treatment assignments during the phase 2 parent study: evolocumab, 420 mg, subcutaneously monthly plus standard of care (SOC) (evolocumab plus SOC) or SOC alone. At randomization, and for 12 weeks thereafter, central laboratories lipid results were blinded to investigators. After 12 weeks, investigators received unblinded laboratory results and could adjust SOC therapies in either arm at their discretion. Local investigators determined SOC therapy.
Previous reports described the schedule of events for the first 52 weeks of the study. After the SOC-controlled period of OSLER-1, all patients who had been initially randomized to SOC alone and completed the first year could receive evolocumab. Thereafter, scheduled study visits occurred at 12-week intervals.
An independent ethics committee or institutional review board approved the protocol prior to study procedures at all sites. All patients provided written informed consent before enrollment in the extension study. Patients self-identified their race and ethnicity.
Efficacy and Safety End Points
The primary objective was to characterize the effects of long-term administration of evolocumab as assessed by changes in LDL-C level, non–high-density lipoprotein cholesterol (non–HDL-C) level, apolipoprotein B, total cholesterol to HDL-C ratio, lipoprotein(a) (Lp[a]), and apolipoprotein B to apolipoprotein A-1 ratio.
Safety end points included the incidence of AEs, SAEs, and AEs leading to discontinuation of evolocumab; the incidence of creatine kinase and liver function test result abnormalities; and the incidence of patients who developed anti-evolocumab antibodies (binding or neutralizing). Events of interest included new-onset diabetes, injection-site reactions, neurocognitive events, and adjudicated cardiovascular events, all analyzed by exposure-adjusted year event rate to evaluate a possible time-dependent relationship between evolocumab exposure and the event.
An independent clinical events committee adjudicated cardiovascular events for exploratory analysis. An independent data monitoring committee regularly reviewed data from all ongoing randomized evolocumab studies, prepared by an external biostatistical group. Amgen assumed safety monitoring for open-label evolocumab studies as of March 2015 at the request of the data monitoring committee.
Laboratory Methods
Details on laboratory methods have been previously described.
Statistical Analysis
For efficacy end points, data were summarized for patients by the randomized treatment group. Safety data analyses used descriptive statistics. Adverse events were coded using the Medical Dictionary for Regulatory Activities version 19.0. Adverse event rates were summarized for all AEs, SAEs, AEs leading to evolocumab discontinuation, and AEs of special interest. Adverse events were reported by preferred term and tabulated for the SOC alone group for the first year and by year of evolocumab exposure to evaluate a possible time-dependent relationship between evolocumab exposure and AE. Exposure-adjusted rates for AEs of special interest during evolocumab treatment were calculated using the total number of reported AEs divided by cumulative time of evolocumab exposure. Summary statistics for continuous variables included the number of patients, mean, median, SD or SE, 25th percentile (Q1), 75th percentile (Q3), minimum, and maximum. For categorical variables, the frequency and percentages were presented. All data analyses used observed values.
To best estimate the central tendency, we present mean values for normally distributed variables and median values for variables not normally distributed. The LDL-C values and percentage change from baseline at each visit were not normally distributed (P < .001 from the Shapiro-Wilk test), and a nonparametric analysis of Wilcoxon signed-rank test was used for the comparison of percentage change from baseline at week 52 between the evolocumab plus SOC group and SOC alone group.
Analysis of evolocumab discontinuation was performed by using Cox proportional hazards regression models. At the time of this analysis, of 1255 patients who received at least 1 dose of evolocumab, 268 patients (21%) discontinued evolocumab. Factors including age, sex, diabetes status, coronary artery disease, and high cardiovascular risk as defined by European Society of Cardiology and National Cholesterol Education Program scoring criteria, baseline statin intensity, and baseline lipid panel parameters including LDL-C, total cholesterol, triglycerides, and Lp(a) values, were tested separately in univariate Cox models and then in a multivariate stepwise selection procedure. All statistical tests were based on a 2-sided .05 significance level without multiplicity adjustment. All statistical analyses were performed using SAS version 9.3 (SAS Institute). Statisticians employed by the sponsor performed data analyses at the direction of the independent investigators who had access to the data analyses generated and could ask for queries of the database as desired.
Results
Patients
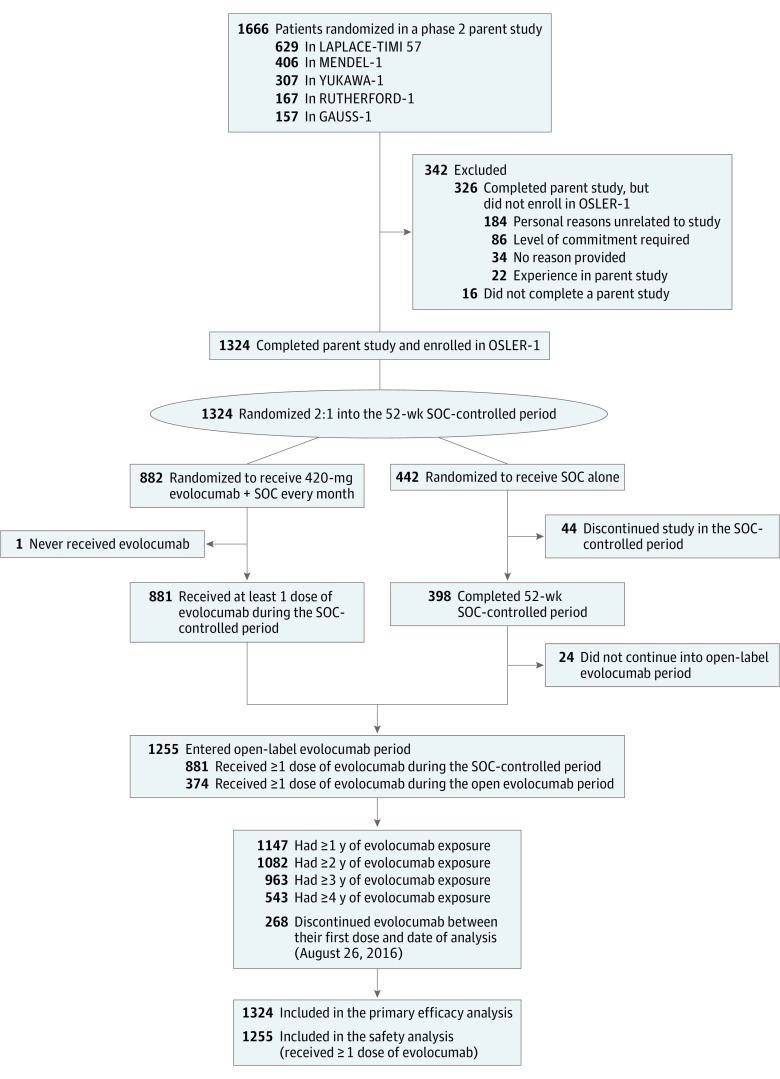
OSLER-1 enrolled 1324 of the 1650 eligible patients (80.2%) who completed a parent study without having experienced an SAE. Of those enrolled in OSLER-1, 882 patients were randomized to evolocumab plus SOC and 442 patients to SOC alone. Figure 1 shows a CONSORT diagram of the parent studies, enrollment into OSLER-1, and follow-up by year of evolocumab exposure. Table 1 provides summary demographic characteristics for the study population.
Figure 1. Patient Disposition in Open-Label Study of Long-term Evaluation Against LDL-C (OSLER-1).
Patients enrolled into OSLER-1 from 1 of 5 phase 2 parent studies. Patients were randomized 2:1 to receive evolocumab plus standard of care (SOC) or SOC alone for the first 52 weeks during the SOC-controlled period. Subsequently, all patients were eligible to receive 420 mg of evolocumab in addition to SOC every month. A total of 812 patients had 4 or more years of follow-up; the persistence rate was 79%. GAUSS-1 indicates Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects; LAPLACE-TIMI, LDL-C Assessment With Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined With Statin Therapy–Thrombolysis in Myocardial Infarction 57 Trial; MENDEL-1, Monoclonal Antibody Against PCSK9 to Reduce Elevated Low-Density Lipoprotein Cholesterol in Adults Currently Not Receiving Drug Therapy for Easing Lipid Levels; RUTHERFORD-1, Reduction of LDL-C With PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder; and YUKAWA-1, Study of LDL-Cholesterol Reduction Using a Monoclonal PCSK9 Antibody in Japanese Patients With Advanced Cardiovascular Risk.
Table 1. Baseline Patient Characteristics.
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| SOC
Patients at 1 y (n = 442) |
Length of Evolocumab Exposure, y | |||||
| First (n = 1255) |
Second (n = 1147) |
Third (n = 1082) |
Fourth (n = 963) |
>4 (n = 543) |
||
| Age, mean (SD), y | 57.6 (11.5) | 57.1 (11.5) | 57.2 (11.3) | 57.5 (10.9) | 57.4 (10.9) | 56.8 (11.0) |
| Female | 241 (54.5) | 662 (52.7) | 597 (52.0) | 556 (51.4) | 500 (51.9) | 293 (54.0) |
| Race/ethnicity | ||||||
| White | 325 (73.5) | 917 (73.1) | 838 (73.1) | 787 (72.7) | 745 (77.4) | 485 (89.3) |
| Asian | 87 (19.7) | 249 (19.8) | 234 (20.4) | 227 (21.0) | 158 (16.4) | 15 (2.8) |
| Black | 24 (5.4) | 74 (5.9) | 61 (5.3) | 55 (5.1) | 49 (5.1) | 35 (6.4) |
| Other | 6 (1.4) | 15 (1.2) | 14 (1.2) | 13 (1.2) | 11 (1.1) | 8 (1.5) |
| Coronary artery diseasea | 76 (17.2) | 249 (19.8) | 229 (20.0) | 221 (20.4) | 201 (20.9) | 127 (23.4) |
| Cardiovascular risk factors | ||||||
| Current cigarette use | 65 (14.7) | 224 (17.8) | 203 (17.7) | 189 (17.5) | 165 (17.1) | 90 (16.6) |
| Type 2 diabetes | 58 (13.1) | 182 (14.5) | 167 (14.6) | 161 (14.9) | 133 (13.8) | 50 (9.2) |
| Family history of coronary heart diseaseb | 107 (24.2) | 304 (24.2) | 280 (24.4) | 268 (24.8) | 252 (26.2) | 161 (29.7) |
| Metabolic syndromec | 153 (34.6) | 461 (36.7) | 422 (36.8) | 397 (36.7) | 360 (37.4) | 226 (41.6) |
| Statin therapy intensityd | ||||||
| High | 80 (18.1) | 253 (20.2) | 232 (20.2) | 222 (20.5) | 213 (22.1) | 148 (27.3) |
| Moderate | 130 (29.4) | 393 (31.3) | 356 (31.0) | 338 (31.2) | 304 (31.6) | 187 (34.4) |
| Low | 76 (17.2) | 223 (17.8) | 207 (18.0) | 198 (18.3) | 149 (15.5) | 33 (6.1) |
| No statin use | 156 (35.3) | 386 (30.8) | 352 (30.7) | 324 (29.9) | 297 (30.8) | 175 (32.2) |
| ESC/EAS risk category | ||||||
| Very high risk/high risk | 210 (47.5) | 625 (49.8) | 580 (50.6) | 557 (51.5) | 495 (51.4) | 278 (51.2) |
| Moderate risk/lower risk | 232 (52.5) | 630 (50.2) | 567 (49.4) | 525 (48.5) | 468 (48.6) | 265 (48.8) |
| NCEP risk category | ||||||
| High risk/moderately high risk | 181 (41.0) | 570 (45.4) | 530 (46.2) | 509 (47.0) | 441 (45.8) | 242 (44.6) |
| Moderate risk/lower risk | 261 (59.0) | 685 (54.6) | 617 (53.8) | 573 (53.0) | 522 (54.2) | 301 (55.4) |
| Lipid parameters at the parent study baseline, mean (SD), mg/dL | ||||||
| LDL-C by ultracentrifugation | 144.6 (37.5) | 141.4 (37.2) | 141.9 (37.2) | 142.4 (37.4) | 142.1 (38.2) | 141.2 (40.9) |
| LDL-C calculated | 143.2 (39.0) | 139.6 (38.1) | 140.1 (38.0) | 140.7 (38.2) | 140.2 (38.9) | 139.0 (41.5) |
| Total cholesterol | 224.8 (42.9) | 220.7 (43.1) | 221.1 (42.9) | 221.8 (42.8) | 221.3 (43.7) | 219.2 (46.4) |
| HDL-C | 54.1 (16.5) | 53.7 (16.2) | 53.5 (15.9) | 53.6 (16.0) | 53.6 (16.2) | 52.6 (15.7) |
| Non–HDL-C | 170.7 (43.0) | 167.0 (41.8) | 167.6 (41.7) | 168.2 (41.8) | 167.8 (42.6) | 166.6 (44.8) |
| Total cholesterol:HDL-C ratio | 4.5 (1.6) | 4.4 (1.5) | 4.4 (1.5) | 4.5 (1.5) | 4.5 (1.5) | 4.5 (1.5) |
| VLDL-C, median (IQR) | 22.5 (16.0-31.5) | 22.5 (16.5-31.0) | 22.5 (16.5-31.5) | 22.5 (16.5-31.5) | 22.5 (16.0-31.0) | 22.0 (16.0-30.5) |
| ApoB | 113.2 (25.3) | 111.4 (24.5) | 111.7 (24.5) | 112.1 (24.5) | 112.0 (24.9) | 111.7 (26.1) |
| ApoA1 | 154.8 (28.1) | 154.6 (28.1) | 154.5 (27.7) | 154.6 (27.6) | 154.5 (28.2) | 153.4 (27.9) |
| ApoB:ApoA1 ratio | 0.8 (0.2) | 0.7 (0.2) | 0.7 (0.2) | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) |
| Triglycerides, median (IQR) | 121.3 (90.5-167.5) | 121.5 (92.5-168.5) | 122.5 (93.0-169.5) | 122.0 (93.0-170.5) | 121.5 (92.0-171.0) | 123.5 (93.0-173.5) |
| Lp(a), median (IQR), nmol/L | 35.0 (12.0-107.0) | 37.0 (12.0-127.0) | 36.0 (12.0-124.0) | 36.0 (12.0-124.0) | 37.5 (12.0-128.0) | 39.0 (12.0-149.0) |
| Free PCSK9 | 417.0 (144.8) | 427.5 (141.1) | 428.4 (142.4) | 430.5 (142.9) | 432.2 (143.3) | 444.6 (141.2) |
Abbreviations: ApoA-1, apolipoprotein A-1; ApoB, apolipoprotein B; EAS, European Atherosclerosis Society; ESC, European Society of Cardiology; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); NCEP, National Cholesterol Education Program; PCSK9, proprotein convertase subtilisin/kexin 9; SOC, standard of care; VLDL-C, very low-density lipoprotein cholesterol.
SI conversion factors: to convert ApoA-1 to grams per liter, multiply by 0.01; ApoB to grams per liter, multiply by 0.01; cholesterol (HDL, LDL, non-HDL, total, and VLDL) to millimoles per liter, multiply by 0.0259; and triglycerides to millimoles per liter, multiply by 0.0113.
Based on the presence of angina, myocardial infarction, coronary artery bypass graft, or percutaneous coronary intervention.
Based on the presence of coronary heart disease in a first-degree male relative 55 years of age or younger or female 65 years of age or younger.
Defined as having 3 or more of the following factors: elevated waist circumference, triglyceride level of 150 mg/dL or greater, low HDL-C level (<40 mg/dL in men and <50 mg/dL in women), systolic blood pressure of 130 mm Hg or greater or diastolic blood pressure of 85 mm Hg or greater, or hyperglycemia (fasting blood glucose ≥100 mg/dL).
As defined by the American College of Cardiology/American Heart Association.
Of the 1255 patients who received at least 1 dose of evolocumab, 886 patients were taking statins at the time of receiving their first open-label dose of evolocumab. The 369 patients (29%) not taking background statin therapy reflect those phase 2 patients with statin intolerance or taking evolocumab monotherapy. Of those taking statins, 249 (28%) were taking high-intensity, 418 (47%) taking moderate-intensity, and 219 (25%) taking low-intensity statin treatment. During follow-up, of 1255 patients who received at least 1 dose of evolocumab, 157 patients (13%) decreased statin intensity, including 115 patients (9%) who stopped them; 48 patients (4%) either started (22 [2%]) or increased (26 [2%]) statin intensity. All patients taking ezetimibe at the beginning of the study (171 [14%]) continued taking ezetimibe through the end of their study participation or the data cutoff date. Nine patients (0.7%) added ezetimibe to their treatment during the course of the study (eTable 1 in the Supplement details background lipid therapy).
Lipid Efficacy Outcomes
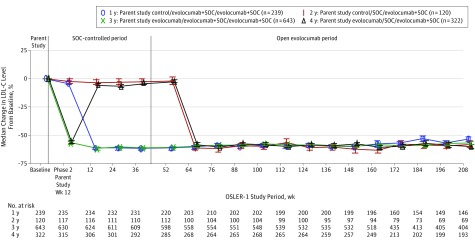
As of August 2016, lipid level measurements were available for 1215 patients (92%), 1122 (85%), 1057 (80%), and 812 (61%) at 1, 2, 3, and 4 years of follow-up, respectively. The median baseline LDL-C level at the time of parent study enrollment was 133.0 mg/dL (to convert to millimoles per liter, multiply by 0.0259). Following randomization, evolocumab lowered lipid levels significantly within 12 weeks for patients previously assigned to placebo in parent studies. In patients randomized to SOC alone in OSLER-1 who previously received evolocumab in parent studies, LDL-C levels returned to near baseline, without a rebound effect. Patients who did not change their evolocumab assignment during the transition from parent studies to OSLER-1 experienced no net changes in lipid levels over the first year of observation. Although baseline levels of LDL-C varied among patients enrolled from the 5 parent studies—lowest (124 mg/dL) in the LAPLACE-TIMI 57 (LDL-C Assessment With Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy–Thrombolysis in Myocardial Infarction 57 Trial) Study of high-risk patients treated with statins and highest (193 mg/dL) in the GAUSS (Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects) Study of patients with statin intolerance—percentage changes in LDL-C levels during OSLER related to evolocumab plus SOC treatment did not vary significantly by parent study (59% and 58% at week 208 for LAPLACE-TIMI 57 and GAUSS, respectively). Figure 2 shows the effects of evolocumab on LDL-C level over time.
Figure 2. Effects of Evolocumab on Low-Density Lipoprotein Cholesterol (LDL-C) Level Over 4 Years.
Calculated LDL-C percentage change from the phase 2 parent study baseline to week 208 of the Open-Label Study of Long-term Evaluation Against LDL-C (OSLER-1). The error bars represent SEs. Plot is based on observed data with no imputation for missing values. The median baseline LDL-C level was 133 mg/dL. The median week 208 on-treatment LDL-C level was 60 mg/dL. The key shows parent study assignment/year 1 assignment/long-term open-label assignment. To convert LDL-C to millimoles per liter, multiply by 0.0259. SOC indicates standard of care.
After 52 weeks of open-label therapy, median LDL-C levels in patients assigned to evolocumab plus SOC were reduced by 61% (95% CI, −63% to −60%) from baseline compared with 2% (95% CI, −5% to −0.2%) for those assigned to SOC alone. Median LDL-C levels in the evolocumab plus SOC vs SOC alone groups were 53 mg/dL vs 133 mg/dL (P < .001). Evolocumab similarly reduced lipids in this cohort of newly treated patients to levels nearly equal to those of patients previously dosed with evolocumab (median LDL-C levels were 58 mg/dL and 54 mg/dL at week 64, respectively). At weeks 100, 160, and 208 of OSLER-1 follow-up, the median absolute LDL-C level reductions across the cohort compared with baseline in the parent studies were 76.5, 78.5, and 75 mg/dL and median LDL-C levels were 57, 55, and 60 mg/dL, respectively. At weeks 64, 100, 160, and 208 of OSLER-1 follow-up, the median percentage reductions in LDL-C level across the cohort (including patients who were randomized to SOC alone and started evolocumab at week 52) compared with baseline in the parent studies were 60% (95% CI, −61% to −59%), 59% (95% CI, −60% to −57%), 59% (95% CI, −61% to −58%), and 57% (95% CI, −59% to −55%). For the 745 patients who remained receiving unchanged background statin therapy at week 208, the median LDL-C level reduction was 58%. At weeks 64, 100, 160, and 208 of OSLER-1 follow-up, the median percentage reductions in Lp(a) across the cohort compared with baseline in the parent studies were 32%, 29%, 30%, and 31% (eTable 2 in the Supplement details other lipid parameters).
Safety and Tolerability
Table 2 summarizes AEs that occurred by year of evolocumab exposure. During year 1 of evolocumab exposure, AEs occurred in 79.3% of patients taking evolocumab plus SOC compared with 74% of patients taking SOC alone during the 52-week SOC-controlled period. Serious AE rates were similar in the evolocumab plus SOC and SOC alone arms (6.9% vs 6.8%). The annualized AE rates in the evolocumab plus SOC group vs SOC alone were 2.8% vs 4.0% for new-onset diabetes, 0.4% vs 0% for neurocognitive events, and 4.7% vs 8.5% for muscle-related events. The rate of new reports of muscle-related AEs decreased in number as the years of drug exposure increased, a trend also noted for injection-site reactions and neurocognitive events. Mean body mass index and changes in fasting glucose between baseline and week 52 did not significantly differ in the SOC or evolocumab plus SOC arms.
Table 2. Summary of Adverse Eventsa.
| Adverse Event | No. (%) | |||||
|---|---|---|---|---|---|---|
| SOC
Exposure (0 to 1 y) (n = 442) |
Length of Evolocumab Exposure, y | |||||
| First (n = 1255) |
Second (n = 1147) |
Third (n = 1082) |
Fourth (n = 963) |
>4 (n = 543) |
||
| Any | 327 (74.0) | 995 (79.3) | 847 (73.8) | 728 (67.3) | 507 (52.6) | 204 (37.6) |
| Seriousb | 30 (6.8) | 87 (6.9) | 78 (6.8) | 84 (7.8) | 46 (4.8) | 19 (3.5) |
| Osteoarthritis | 1 (0.2) | 3 (0.2) | 3 (0.3) | 6 (0.6) | 2 (0.2) | 1 (0.2) |
| Angina | 2 (0.5) | 5 (0.4) | 3 (0.3) | 2 (0.2) | 3 (0.3) | 0 (0.0) |
| Chest pain | 1 (0.2) | 4 (0.3) | 3 (0.3) | 1 (0.1) | 1 (0.1) | 2 (0.4) |
| Noncardiac chest pain | 1 (0.2) | 4 (0.3) | 3 (0.3) | 3 (0.3) | 0 (0.0) | 1 (0.2) |
| Appendicitis | 1 (0.2) | 4 (0.3) | 1 (0.1) | 2 (0.2) | 2 (0.2) | 0 (0.0) |
| No. of patients discontinued evolocumab owing to adverse event | NA | 35 (2.8) | 7 (0.6) | 11 (1.0) | 5 (0.5) | 1 (0.2) |
| Most common adverse events | ||||||
| Nasopharyngitis | 64 (14.5) | 195 (15.5) | 166 (14.5) | 119 (11.0) | 56 (5.8) | 10 (1.8) |
| Upper respiratory tract infection | 29 (6.6) | 87 (6.9) | 68 (5.9) | 42 (3.9) | 44 (4.6) | 10 (1.8) |
| Back pain | 22 (5.0) | 77 (6.1) | 64 (5.6) | 48 (4.4) | 35 (3.6) | 10 (1.8) |
| Arthralgia | 18 (4.1) | 80 (6.4) | 59 (5.1) | 50 (4.6) | 31 (3.2) | 12 (2.2) |
| Hypertension | 20 (4.5) | 61 (4.9) | 48 (4.2) | 40 (3.7) | 13 (1.3) | 7 (1.3) |
| Bronchitis | 17 (3.8) | 59 (4.7) | 53 (4.6) | 47 (4.3) | 29 (3.0) | 11 (2.0) |
| Influenza | 24 (5.4) | 67 (5.3) | 50 (4.4) | 36 (3.3) | 22 (2.3) | 3 (0.6) |
| Cough | 19 (4.3) | 57 (4.5) | 46 (4.0) | 30 (2.8) | 18 (1.9) | 5 (0.9) |
| Pain in extremity | 14 (3.2) | 54 (4.3) | 42 (3.7) | 25 (2.3) | 15 (1.6) | 2 (0.4) |
| New-onset diabetesc | 19 (4.3) | 51 (4.1) | 22 (2.1) | 25 (2.6) | 26 (3.2) | 6 (1.4) |
| Potential hypersensitivityc | 19 (4.3) | 72 (5.7) | 43 (3.7) | 39 (3.6) | 20 (2.1) | 4 (0.7) |
| Potential injection-site reactionsc | NA | 52 (4.1) | 30 (2.6) | 23 (2.1) | 7 (0.7) | 0 (0.0) |
| Muscle relatedc | 41 (9.3) | 102 (8.1) | 68 (5.9) | 47 (4.3) | 30 (3.1) | 7 (1.3) |
| Neurocognitive relatedd | 0 (0.0) | 7 (0.6) | 4 (0.3) | 6 (0.6) | 2 (0.2) | 0 (0.0) |
| Antibody | ||||||
| Binding | NAe | 2 (0.16) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Neutralizing | NA | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Abbreviations: NA, not applicable; SOC, standard of care.
Observed incidence rates. Also, the length of evolocumab plus SOC exposure in OSLER-1 is defined as per patient-year.
The 5 most common serious adverse events are detailed.
Based on US Food and Drug Administration search terms.
Based on Medical Dictionary for Regulatory Activities search terms.
Two incidences of antidrug antibodies were observed during the SOC-controlled period in patients receiving SOC alone who had received evolocumab during the parent study. No neutralizing antibodies were reported.
Antidrug antibodies (ADAs) occurred infrequently. Four patients tested positive for binding ADAs during OSLER-1 follow-up: (1) at week 4 while taking SOC for a patient who received evolocumab, 70 mg, once every 2 weeks during the parent study, which resolved at week 12 and thereafter; (2) at week 4 while receiving SOC for a patient who received evolocumab, 420 mg, monthly during the parent study, which resolved at week 12 and thereafter; (3) at weeks 4, 12, and 48 while taking evolocumab during OSLER-1 for a patient who received placebo during the parent study, which resolved at week 52 and thereafter; and (4) at day 1 of OSLER-1, which resolved at week 4 and thereafter for a patient who received placebo during the parent study. No neutralizing antibodies were detected.
Table 3 shows the summary for the analysis of persistence with evolocumab therapy. Of predefined baseline factors, patients with higher cardiovascular risk defined by European Society of Cardiology/European Atherosclerosis Society risk categories taking baseline statins and with higher baseline LDL-C levels were more likely to continue to take evolocumab during open-label therapy. Patients who experienced AEs did not drop out at a higher rate than those who reported no AEs.
Table 3. Multivariate Analysis of Variables Contributing to Evolocumab Discontinuation.
| Characteristic | No. of Patients | All Patients With Evolocumab Exposure, No. (%) | Hazard Ratio of Drop
Out (95% CI)a |
P Valuea | |
|---|---|---|---|---|---|
| Discontinued
Evolocumab and/or Study (n = 268) |
Remained Taking Evolocumab in
Study (n = 987) |
||||
| ESC/EAS risk categories | |||||
| High/very high risk | 625 | 98 (15.7) | 527 (84.3) | High vs low: 0.707 (0.535-0.935) | .02 |
| Moderate/low risk | 630 | 170 (27.0) | 460 (73.0) | ||
| Baseline statin therapy intensity per ACC/AHA definition | |||||
| High | 253 | 39 (15.4) | 214 (84.6) | High vs no use: 0.522 (0.347-0.787) | .002 |
| Moderate | 393 | 78 (19.8) | 315 (80.2) | Moderate vs no use: 0.574 (0.415-0.794) | <.001 |
| Low | 223 | 32 (14.3) | 191 (85.7) | Low vs no use: 0.521 (0.346-0.784) | .002 |
| No statin use | 386 | 119 (30.8) | 267 (69.2) | NA | NA |
| Baseline LDL-C level, mg/dL | |||||
| Mean (SD) | NA | 135.5 (33.1) | 140.8 (39.2) | NA | .002 |
| Median (IQR) | NA | 131.5 (110.3-156.5) | 133.0 (115.0-156.0) | NA | |
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; EAS, European Atherosclerosis Society; ESC, European Society of Cardiology; IQR, interquartile range; LDL-C, low density lipoprotein cholesterol; NA, not applicable.
SI conversion factors: to convert LDL-C to millimoles per liter, multiply by 0.0259.
Hazard ratio and P values are from multivariate Cox regression model.
Adjudicated cardiovascular events occurred in 11 of 442 patients (2.5%) in the SOC arm during the SOC-controlled period as compared with 10 of 1255 (0.8%) during the first year of evolocumab exposure. Cardiovascular event rates during extended exposure to evolocumab remained low, with an incidence of cardiovascular events during years 2, 3, and 4 of evolocumab exposure of 1.2%, 1.2%, and 0.9%, respectively.
Discussion
OSLER-1, an open-label randomized trial followed by a long-term observational extension, provides valuable information about treating patients with hypercholesterolemia with a monoclonal antibody directed against PCSK9. In this analysis, we report data from 1255 patients exposed to evolocumab for 44 months on average. To our knowledge, these 4641 patient-years of follow-up represent the longest and most extensive experience reported to date for evolocumab or any other PCSK9 inhibitor. Among members of the cohort who reached 4 years, the efficacy of evolocumab persisted, with an overall median LDL-C level reduction of 57% to 60 mg/dL. In patients receiving statin coadministration at baseline and at 208 weeks, evolocumab lowered LDL-C level by 58% from baseline. The median Lp(a) level was persistently reduced by 31%. These data suggest that evolocumab can help many patients who need additional lipid lowering beyond their existing therapy.
Consensus opinion supports aggressive lipid-lowering therapy for patients at high risk for cardiovascular disease complications. Used with statins, PCSK9 antibodies reduce LDL-C to levels not previously achievable in routine clinical practice. As clinical outcomes results from large randomized blinded studies become available, the role of PCSK9 therapy as a part of the therapeutic armamentarium for hypercholesterolemia will emerge. OSLER-1 adds to this assessment by demonstrating, over a longer term, the persistence of atherogenic lipoprotein lowering and the absence of ADAs or a worsening AE profile.
Despite inherent limitations, long-term open-label trials can add important information relevant to clinical decision making for chronically administered therapies such as agents for hypercholesterolemia. Open-label extension trials usually do not provide comparative data between 2 or more treatment arms and can lead to biases related to knowledge of the therapy. Nonetheless, these trials can provide information on the long-term efficacy and tolerance of a therapy. In open-label trials, knowledge of the therapy received allows patients and their clinicians to mimic the decision-making patterns of clinical practice by choosing to continue a therapy, or not, based on their perception of whether the benefits of treatment outweigh any AEs or inconvenience at any given time. This dynamic differs from that of double-blind studies in which clinicians and many patients understand that the scientific validity of comparisons requires persistence on an unknown therapy. Further, long-term open-label trials that follow shorter-term randomized, double-blind studies provide an opportunity to analyze possible AEs that may derive from longer-term exposure to a medical therapy.
The evolocumab persistence rate of 79% during an average of 44 months of drug exposure in OSLER-1 compares favorably with published data for adherence to other therapies including statins. For mipomersen, an approved injectable treatment for severe forms of hypercholesterolemia, Santos and colleagues reported that 64 of 141 patients (45.4%) with familial hypercholesterolemia continued open-label therapy in a 2-year clinical trial. Monitoring clinical practice, Perreault et al reported an overall persistence rate for statin use in a middle-aged hypercholesterolemic population of 71% after 6 months and 45% after 3 years. Corresponding persistence rates for primary prevention patients in this cohort were 65% and 35%, respectively. Compared either with the investigational drug setting or clinical practice, the high persistence rates of OSLER-1 over a longer period indicate the tolerability of evolocumab. Further, lower dropout rates occurred among patients at higher cardiovascular risk, demonstrating a reassuring selection process toward patients who may benefit most from a novel lipid-lowering therapy.
Any parenterally administered protein-based therapeutic can elicit an immune response during chronic use. Because therapeutic antibodies for hypercholesterolemia require chronic administration, the risk for ADA development requires evaluation. The incidence of ADAs in OSLER-1 was low: only 4 transient events were observed during the course of the study and no neutralizing antibodies were detected. Of these events, 1 patient tested ADA positive, who received placebo during a parent study, on OSLER day 1. This finding distinguishes evolocumab, a fully human antibody, from bococizumab, an antibody with murine elements, previously shown to cause a 7% incidence of the development of ADAs over 24 weeks. Although the development of ADAs during treatment does not necessarily lead to losses of LDL-C level lowering efficacy, at least 1 bococizumab-treated patient demonstrated this effect over short-term drug exposure.
Limitations
The open-label design of OSLER-1 serves as an important limitation to the study’s findings. In particular, 16 patients who discontinued study participation owing to AEs in parent trials could not enroll in OSLER-1. This protocol design feature possibly introduces a selection bias. The ongoing placebo-controlled FOURIER study (NCT01764633) will provide a much more detailed and comprehensive analysis of the safety and risk reduction of evolocumab over a somewhat shorter time frame than OSLER-1. A prospective evaluation of neurocognitive function in a subset of the FOURIER population is being assessed in the EBBINGHAUS Study. Nonetheless, the finding of a lower adjudicated cardiovascular event rate in the evolocumab plus SOC arm of OSLER-1 compared with SOC alone in the first year with continued yearly event rates during evolocumab exposure of less than half of that of the SOC year provides reassurance. Further, subject to the limitations of observational studies, general AEs and AEs of special interest did not increase in incidence during continued exposure to evolocumab of up to 4 years.
Conclusions
OSLER-1 demonstrated the persistent effectiveness of evolocumab for lowering LDL-C levels over an average of 44 months of exposure in a diverse patient population with hypercholesterolemia. The incidences of AEs were comparable between patients randomized to SOC alone and evolocumab plus SOC during the first year of OSLER, and specific AEs did not increase with cumulative exposure to the drug. Rates of persistence on therapy were excellent, particularly among high-risk patients.
eTable 1. Statin Use From First Evolocumab Dose to Last Day of Reporting
eTable 2. Other Lipid Parameters
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics: 2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baigent C, Blackwell L, Emberson J, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stroes ES, Thompson PD, Corsini A, et al. ; European Atherosclerosis Society Consensus Panel . Statin-associated muscle symptoms: impact on statin therapy: European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients: the PRIMO Study. Cardiovasc Drugs Ther. 2005;19(6):403-414. [DOI] [PubMed] [Google Scholar]
- 5.McDonagh M, Peterson K, Holzhammer B, Fazio S. A systematic review of PCSK9 inhibitors alirocumab and evolocumab. J Manag Care Spec Pharm. 2016;22(6):641-653q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raal FJ, Stein EA, Dufour R, et al. ; RUTHERFORD-2 Investigators . PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):331-340. [DOI] [PubMed] [Google Scholar]
- 7.Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36(43):2996-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raal FJ, Honarpour N, Blom DJ, et al. ; TESLA Investigators . Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):341-350. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383(9911):60-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein EA, Giugliano RP, Koren MJ, et al. ; PROFICIO Investigators . Efficacy and safety of evolocumab (AMG 145), a fully human monoclonal antibody to PCSK9, in hyperlipidaemic patients on various background lipid therapies: pooled analysis of 1359 patients in four phase 2 trials. Eur Heart J. 2014;35(33):2249-2259. [DOI] [PubMed] [Google Scholar]
- 11.Gaudet D, Watts GF, Robinson JG, et al. Effect of alirocumab on lipoprotein(a) over ≥1.5 years (from the phase 3 ODYSSEY program). Am J Cardiol. 2017;119(1):40-46. [DOI] [PubMed] [Google Scholar]
- 12.Desai NR, Kohli P, Giugliano RP, et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C Assessment with Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy (LAPLACE)-Thrombolysis in Myocardial Infarction (TIMI) 57 Trial. Circulation. 2013;128(9):962-969. [DOI] [PubMed] [Google Scholar]
- 13.Ballantyne CM, Neutel J, Cropp A, et al. Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomized, placebo-controlled, dose-ranging study in statin-treated subjects with hypercholesterolemia. Am J Cardiol. 2015;115(9):1212-1221. [DOI] [PubMed] [Google Scholar]
- 14.Pfizer Pfizer discontinues global development of bococizumab, its investigational PCSK9 inhibitor [press release]. http://www.pfizer.com/news/press-release/press-release-detail/pfizer_discontinues_global_development_of_bococizumab_its_investigational_pcsk9_inhibitor. Published November 1, 2016. Accessed February 14, 2017.
- 15.Koren MJ, Giugliano RP, Raal FJ, et al. ; OSLER Investigators . Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial. Circulation. 2014;129(2):234-243. [DOI] [PubMed] [Google Scholar]
- 16.Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316(22):2373-2384. [DOI] [PubMed] [Google Scholar]
- 17.Giugliano RP, Desai NR, Kohli P, et al. ; LAPLACE-TIMI 57 Investigators . Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380(9858):2007-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirayama A, Honarpour N, Yoshida M, et al. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin-treated Japanese patients at high cardiovascular risk: primary results from the phase 2 YUKAWA Study. Circ J. 2014;78(5):1073-1082. [DOI] [PubMed] [Google Scholar]
- 19.Koren MJ, Scott R, Kim JB, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380(9858):1995-2006. [DOI] [PubMed] [Google Scholar]
- 20.Raal F, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126(20):2408-2417. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan D, Olsson AG, Scott R, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS Randomized Trial. JAMA. 2012;308(23):2497-2506. [DOI] [PubMed] [Google Scholar]
- 22.Reiner Z, Catapano AL, De Backer G, et al. ; European Association for Cardiovascular Prevention & Rehabilitation; ESC Committee for Practice Guidelines (CPG) 2008-2010 and 2010-2012 Committees . ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769-1818. [DOI] [PubMed] [Google Scholar]
- 23.Lipinski MJ, Benedetto U, Escarcega RO, et al. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J. 2016;37(6):536-545. [DOI] [PubMed] [Google Scholar]
- 24.Santos RD, Duell PB, East C, et al. Long-term efficacy and safety of mipomersen in patients with familial hypercholesterolaemia: 2-year interim results of an open-label extension. Eur Heart J. 2015;36(9):566-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perreault S, Blais L, Lamarre D, et al. Persistence and determinants of statin therapy among middle-aged patients for primary and secondary prevention. Br J Clin Pharmacol. 2005;59(5):564-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giugliano RP, Mach F, Zavitz K, et al. ; EBBINGHAUS Investigators . Design and rationale of the EBBINGHAUS trial: a phase 3, double-blind, placebo-controlled, multicenter study to assess the effect of evolocumab on cognitive function in patients with clinically evident cardiovascular disease and receiving statin background lipid-lowering therapy: a cognitive study of patients enrolled in the FOURIER Trial [published online February 16, 2017]. Clin Cardiol. doi: 10.1002/clc.22678 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Statin Use From First Evolocumab Dose to Last Day of Reporting
eTable 2. Other Lipid Parameters