Key Points
Question
Are characteristics of novel therapeutics known at the time of US Food and Drug Administration (FDA) approval associated with postmarket safety events, including withdrawal, boxed warnings, and safety communications?
Findings
Among 222 novel therapeutics approved by the FDA from 2001 through 2010, 71 (32.0%) were affected by a postmarket safety event. Postmarket safety events were more frequent among biologics, therapeutics indicated for the treatment of psychiatric disease, those receiving accelerated approval, and those with near–regulatory deadline approval.
Meaning
Postmarket safety events are common after FDA approval, highlighting the importance of continuous monitoring of the safety of novel therapeutics throughout their life cycle.
This study describes withdrawals, boxed warnings, and safety communications affecting pharmaceuticals and biologics approved by the US Food and Drug Administration between 2001 and 2010, and associations between characteristics known at the time of approval and those events.
Abstract
Importance
Postmarket safety events of novel pharmaceuticals and biologics occur when new safety risks are identified after initial regulatory approval of these therapeutics. These safety events can change how novel therapeutics are used in clinical practice and inform patient and clinician decision making.
Objectives
To characterize the frequency of postmarket safety events among novel therapeutics approved by the US Food and Drug Administration (FDA), and to examine whether any novel therapeutic characteristics known at the time of FDA approval were associated with increased risk.
Design and Setting
Cohort study of all novel therapeutics approved by the FDA between January 1, 2001, and December 31, 2010, followed up through February 28, 2017.
Exposures
Novel therapeutic characteristics known at the time of FDA approval, including drug class, therapeutic area, priority review, accelerated approval, orphan status, near–regulatory deadline approval, and regulatory review time.
Main Outcomes and Measures
A composite of (1) withdrawals due to safety concerns, (2) FDA issuance of incremental boxed warnings added in the postmarket period, and (3) FDA issuance of safety communications.
Results
From 2001 through 2010, the FDA approved 222 novel therapeutics (183 pharmaceuticals and 39 biologics). There were 123 new postmarket safety events (3 withdrawals, 61 boxed warnings, and 59 safety communications) during a median follow-up period of 11.7 years (interquartile range [IQR], 8.7-13.8 years), affecting 71 (32.0%) of the novel therapeutics. The median time from approval to first postmarket safety event was 4.2 years (IQR, 2.5-6.0 years), and the proportion of novel therapeutics affected by a postmarket safety event at 10 years was 30.8% (95% CI, 25.1%-37.5%). In multivariable analysis, postmarket safety events were statistically significantly more frequent among biologics (incidence rate ratio [IRR] = 1.93; 95% CI, 1.06-3.52; P = .03), therapeutics indicated for the treatment of psychiatric disease (IRR = 3.78; 95% CI, 1.77-8.06; P < .001), those receiving accelerated approval (IRR = 2.20; 95% CI, 1.15-4.21; P = .02), and those with near–regulatory deadline approval (IRR = 1.90; 95% CI, 1.19-3.05; P = .008); events were statistically significantly less frequent among those with regulatory review times less than 200 days (IRR = 0.46; 95% CI, 0.24-0.87; P = .02).
Conclusions and Relevance
Among 222 novel therapeutics approved by the FDA from 2001 through 2010, 32% were affected by a postmarket safety event. Biologics, psychiatric therapeutics, and accelerated and near–regulatory deadline approval were statistically significantly associated with higher rates of events, highlighting the need for continuous monitoring of the safety of novel therapeutics throughout their life cycle.
Introduction
The US Food and Drug Administration (FDA) is responsible for ensuring that novel therapeutics are safe and effective. When making approval decisions, the FDA weighs the risks and benefits of novel therapeutics using data gathered in premarket drug testing and clinical trials. The majority of pivotal trials that form the basis for FDA approval enroll fewer than 1000 patients with follow-up of 6 months or less, which may make it challenging to identify uncommon or long-term serious safety risks. These risks may only become evident when novel therapeutics are used in much larger patient populations and for longer durations in the postmarket period.
Given the inherent limitations of premarket clinical evaluation for drug safety, there may be opportunities to enhance patient safety if factors associated with postmarket safety events could be identified at the time of FDA approval. Additionally, monitoring efforts could focus on at-risk drugs to facilitate earlier detection of safety problems and prevent patients from unnecessary harm. It has been suggested that the deadlines for the FDA’s regulatory review process that were established by the Prescription Drug User Fee Act (PDUFA) may have implications for postmarket safety. One analysis of novel therapeutics approved by the FDA between 1993 and 2004 found that therapeutics approved in the 60 days prior to the statutory decision deadline, hereafter referred to as “near–regulatory deadline approvals,” were associated with more frequent postmarket safety events. Whether other regulatory factors may be associated with postmarket safety events remains unknown, such as the use of special accelerated approval and priority review pathways, which are intended to expedite clinical evaluation and premarket regulatory review.
This analysis characterizes the frequency of postmarket safety events among all novel therapeutics approved by the FDA from 2001 through 2010 and assesses the associations between 7 characteristics, which describe the novel therapeutics and the regulatory approval process, and postmarket safety events.
Methods
Sample Construction
We used the Drugs@FDA database to identify all novel therapeutics (ie, new pharmaceuticals and biologics) approved by the FDA between January 1, 2001, and December 31, 2010, excluding diagnostic agents, sunscreens, drug adjuvants, and drugs that were not intended for use in the United States (eFigure and eTable 1 in the Supplement).
Class and Therapeutic Areas
All therapeutics were classified as either pharmaceuticals (ie, small molecules) or biologics by the FDA. The indications for which each therapeutic was initially approved for use were abstracted from approval letters linked to the Drugs@FDA database and were classified into 1 of 8 therapeutic areas: autoimmune, musculoskeletal, and dermatology; cancer and hematology; cardiovascular disease, diabetes, and hyperlipidemia; genitourinary and renal; infectious disease; neurology; psychiatry; and other.
Special Regulatory Pathways
Promising therapeutics may be eligible to receive priority review status from the FDA, which requires that the agency complete its initial regulatory review within 6 months instead of the usual 10 months. Novel therapeutics approved through the priority review pathway were identified using a field in the Drugs@FDA database. Therapeutics that address a serious unmet medical need may be eligible for accelerated approval status, which allows the FDA to approve drugs on the basis of clinical trials using surrogate markers of disease for trial end points. Those therapeutics that received accelerated approval were identified by reviewing FDA approval letters linked in the Drugs@FDA database. The Fast Track designation, which provides enhanced access to the FDA during the development process, and the Breakthrough Therapy designation, which was not implemented until 2014, were not assessed in this study.
Orphan Product Designation
Drug makers producing a therapeutic that treats a rare disease can seek orphan status from the FDA, which confers a longer period of market exclusivity to offset the small size of the patient population. The Orphan Drug Product designation database was used to determine whether novel therapeutics had received designation as orphan products for the indication for which they were initially approved.
Regulatory Review Times
Key dates in the regulatory review process were collected from FDA approval letters. Review times were defined as the number of days between the submission date and the date on which the FDA made its regulatory decision (ie, approval or complete response). The number of review cycles required to secure approval was determined using FDA approval letters. Total review time was defined as the sum of the durations of all review cycles required for approval and was classified into 3 prespecified categories (<200, 200-399, and ≥400 days) based on FDA review process timing and patterns (priority review, 180 days [<200 days]; average first cycle review duration, 300 days [200-399 days]; or multiple review cycles [≥400 days]).
Near–Regulatory Deadline Approvals
Novel therapeutics were classified according to which iteration of PDUFA was in effect at the time their application was submitted to the FDA for review (eTable 2 in the Supplement). Near–regulatory deadline approvals were identified as those therapeutics approved within 60 days of the PDUFA deadline based on the submission and goal review dates. Therapeutics approved at least 60 days before the deadline, any time after the deadline, or after multiple review cycles were not considered to represent near–regulatory deadline approvals.
Postmarket Safety Events
Clinicians are informed about postmarket safety events in a variety of ways. Withdrawals occur when new safety information profoundly changes the balance of risks and benefits associated with a novel therapeutic, prompting the drug maker or the FDA to withdraw the product from the market. Boxed warnings, which highlight important safety risks at the top of a drug’s label, are issued by the FDA when a major, often life-threatening safety risk is detected, yet the overall risk-benefit profile favors continued market availability. The FDA releases safety communications often accompanied by labeling revisions to alert clinicians to pertinent but non-life-threatening safety risks.
Our primary outcome measure was a composite of the 3 principal types of postmarket safety events: (1) withdrawals due to safety concerns; (2) FDA issuance of incremental boxed warnings (ie, those added by the FDA in the postmarket period, after approval); and (3) FDA issuance of safety communications.
To identify safety-related withdrawals, a publicly available index of the FDA’s postmarket announcements was searched for all novel therapeutics using both the generic and brand names. When an announcement describing the withdrawal of a therapeutic was found, 2 reviewers (N.S.D. and J.S.R.) independently classified whether or not each withdrawal was safety related (when safety information that became apparent in the postmarket period made a significant contribution to the withdrawal) or other. There were no disagreements.
Incremental boxed warnings were identified by comparing the most recent version of the label of each therapeutic vs the initial approval label (eBox 1 in the Supplement). Safety communications were identified by searching the index of postmarket announcements (eBox 2 in the Supplement). Prior to 2010, the FDA used Information for Healthcare Professional Sheets, Public Health Advisories, and News Releases to communicate safety risks to clinicians; the agency also issued Early Communications when new but unsubstantiated safety information became available in the postmarket period. In 2010, the FDA adopted the Drug Safety Communication as its single means of communicating postmarket safety events to clinicians. To determine whether a safety communication occurred, all Drug Safety Communications, Information for Healthcare Professional Sheets, Public Health Advisories, and News Releases identified by our search of the FDA’s index of postmarket announcements were reviewed. Only those discussing a safety risk that emerged in the postmarket period were counted; communications notifying clinicians of the initiation of a safety review, updates to ongoing safety reviews, and non-safety-related labeling changes were not counted. Safety communications that were issued contemporaneously with a more serious postmarket safety event, namely a boxed warning or safety-related withdrawal, were excluded to avoid double counting (eTable 3 in the Supplement). Additionally, therapeutics accruing multiple postmarket safety events were reviewed to ensure that each safety event was distinct (eTable 4 in the Supplement). We searched for postmarket safety events through February 28, 2017.
Statistical Analysis
We used descriptive statistics to characterize the novel therapeutics. We produced Kaplan-Meier estimates to plot occurrence of a postmarket safety event as a function of time and report 2-sided log-rank tests to assess for differences in events over time according to 7 prespecified features of characteristics of novel therapeutics: (1) class (pharmaceutical, biologic), (2) therapeutic area, (3) priority review, (4) accelerated approval, (5) orphan product, (6) near–regulatory deadline approval, and (7) total review time (<200, 200-399, and ≥400 days). The proportion of novel therapeutics affected by a postmarket safety event at 10 years was derived from the Kaplan-Meier analysis, and we calculated the differences within these proportions for 7 prespecified features, using the Z test to determine statistical significance. Next, we performed a multivariable regression analysis using a Poisson model, and we report the resulting incidence rate ratios (IRRs) with 95% confidence intervals; all characteristics of novel therapeutics studied were included in the model given the limited utility of bivariate analysis in variable selection. To address the possibility that the experience of novel therapeutics approved prior to our study period contributed to postmarket safety events noted in our study, we performed a sensitivity analysis focused on novel therapeutics with a first-in-class approval in 2001 or later, a designation based on prior FDA research. All statistical tests were 2-tailed and used a P value of .05 as a threshold for significance. We used Stata version 14 (StataCorp LP) and JMP version 11.2 (SAS Institute Inc) statistical software to conduct all analyses.
Results
Characteristics of Novel Therapeutics
The study sample included 222 novel therapeutics approved by the FDA from 2001 through 2010 (Table 1), 183 (82.4%) of which were pharmaceuticals and 39 (17.6%) of which were biologics. Therapeutics indicated for the treatment of cancer and hematology were most common (47 [21.2%]), followed by those for infectious disease (37 [16.7%]) and those for cardiovascular disease, diabetes, and hyperlipidemia (26 [11.7%]). Special regulatory designations were common: 77 novel therapeutics (34.7%) received priority reviews and 28 (12.6%) received accelerated approval. Approximately one-quarter of novel therapeutics (62 [27.9%]) were designated as orphan products. The median total review time was 311 days (interquartile range [IQR], 203-485 days). The total review time was less than 200 days for 54 novel therapeutics (24.3%), between 200 and 399 days for 90 (40.5%), 400 days or longer for 76 (34.2%), and not available for 2 (0.9%). Approximately one-quarter of therapeutics (52 [23.6%]) were near–regulatory deadline approvals.
Table 1. Characteristics of 222 Novel Therapeutics Approved by the US Food and Drug Administration From 2001 Through 2010.
| Novel Therapeutic Characteristic | No. (%) |
|---|---|
| Class | |
| Pharmaceutical | 183 (82.4) |
| Biologic | 39 (17.6) |
| Therapeutic area | |
| Cancer and hematology | 47 (21.2) |
| Infectious disease | 37 (16.7) |
| Cardiovascular, diabetes, and hyperlipidemia | 26 (11.7) |
| Autoimmune, musculoskeletal, and dermatology | 17 (7.7) |
| Neurology | 17 (7.7) |
| Genitourinary and renal | 17 (7.7) |
| Psychiatry | 15 (6.8) |
| Other | 46 (20.7) |
| Priority review | |
| Yes | 77 (34.7) |
| No | 145 (65.3) |
| Accelerated approval | |
| Yes | 28 (12.6) |
| No | 194 (87.4) |
| Orphan drug | |
| Yes | 62 (27.9) |
| No | 160 (72.1) |
| Near–regulatory deadline approvala | |
| Yes | 52 (23.6) |
| No | 168 (76.4) |
| Regulatory review time, da | |
| Total review time, median (IQR) | 311 (203-485) |
| <200 | 54 (24.3) |
| 200-399 | 90 (40.5) |
| ≥400 | 76 (34.2) |
| Not available | 2 (0.9) |
| Follow-up, median (IQR), y | 11.7 (8.7-13.8) |
Abbreviation: IQR, interquartile range.
Key regulatory dates were not available for 2 therapeutics; near–regulatory deadline determination could not be made and total regulatory review time could not be calculated for these.
Postmarket Safety Events From 2001 Through 2015
Among all approved therapeutics, the median duration of market availability was 11.7 years (IQR, 8.7-13.8 years). During this period, there were 123 postmarket safety events, affecting 71 (32.0%) of the 222 novel therapeutics (Figure 1). There were 3 withdrawals. Valdecoxib, an anti-inflammatory, and tegaserod, a drug used for the treatment of irritable bowel syndrome, were withdrawn in 2005 and 2007, respectively, because of concerns about adverse cardiovascular events. Efalizumab, a drug used to treat psoriasis, was withdrawn in 2009 because of the risk of progressive multifocal leukoencephalopathy, 1 month after a boxed warning citing this risk was added to the drug label.
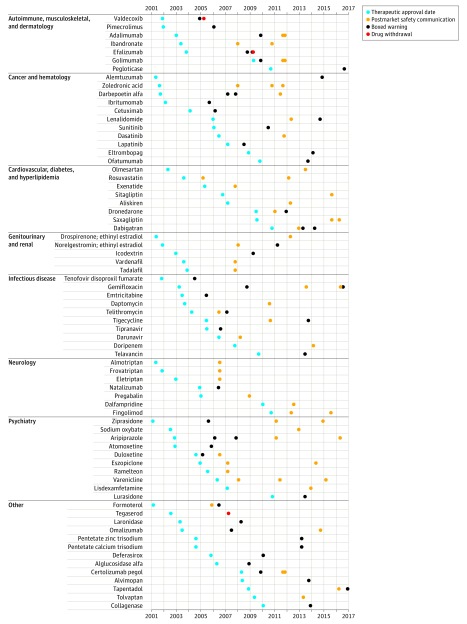
Figure 1. Timeline of Novel Therapeutics Approved by the US FDA, 2001-2010, That Experienced Postmarket Safety Events, Grouped by Therapeutic Area.
There were 61 incremental boxed warnings affecting 43 novel therapeutics, including classwide warnings for antipsychotics (ziprasidone, aripiprazole) and selective serotonin reuptake inhibitors (duloxetine, atomoxetine) in 2005 and 2006, and monoclonal antibodies acting against tumor necrosis factor α (adalimumab, certolizumab, golimumab) in 2009. Eight boxed warnings were preceded by safety communications; however, only 4 of these safety communications described the safety risk that triggered the subsequent boxed warning.
There were 59 safety communications affecting 44 novel therapeutics. Several of these safety communications affected entire drug classes: warnings were issued about triptans (almotriptan, frovatriptan, eletriptan) in 2006, phosphodiesterase 5 inhibitors (vardenafil, tadalafil) in 2007, bisphosphonates (zolendronic acid, ibandronate) in 2008, and dipeptidyl peptidase 4 inhibitors (saxagliptin, sitagliptin) in 2015.
Timing of Postmarket Safety Events
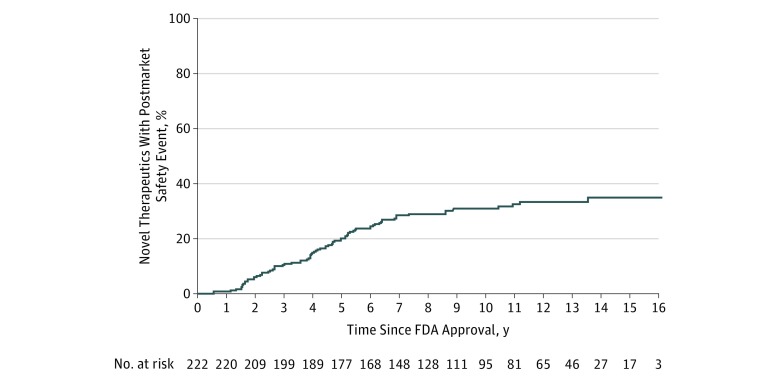
Among novel therapeutics experiencing a postmarket safety event, the median time from approval to the first event was 4.2 years (IQR, 2.5-6.0 years) (Figure 2). For the 3 withdrawn therapeutics, the time from approval to withdrawal was 3.4 years for valdecoxib, 4.7 years for tegaserod, and 5.4 years for efalizumab. For therapeutics receiving an incremental boxed warning, the median time from approval to boxed warning was 4.0 years (IQR, 2.6-5.5 years). For therapeutics receiving a safety communication, the median time from approval to the issuance of the safety communication was 4.9 years (IQR, 2.4-7.2 years). At 10 years, 30.8% (95% CI, 25.1%-37.5%) of the therapeutics had 1 or more safety events.
Figure 2. Proportion of Novel Therapeutics Approved by the US Food and Drug Administration (FDA) From 2001 Through 2010 Affected by Any Postmarket Safety Event as of February 2017.
The median time from approval to the first event was 4.2 years (interquartile range, 2.5-6.0 years).
Factors Associated With Postmarket Safety Events
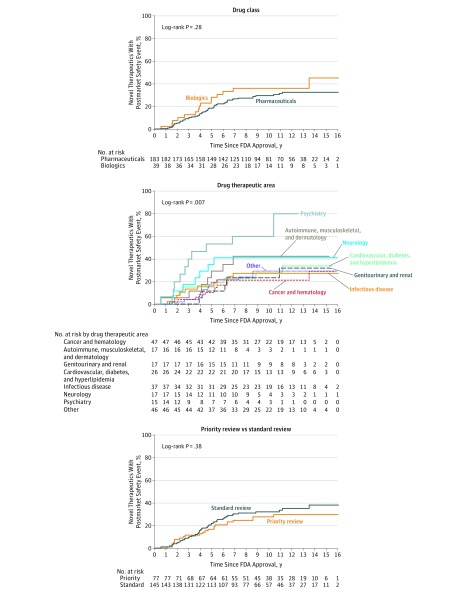
In bivariate analyses (Figure 3 and Figure 4), there was a statistically significant difference in the proportion of novel therapeutics affected by postmarket safety events according to therapeutic area (P = .007). Postmarket safety events were most common for therapeutics indicated for the treatment of psychiatric disease and least common for therapeutics for the treatment of cancer and hematologic disease (proportion at 10 years, 60.0% vs 21.4%; absolute difference in proportions, 38.6%; 95% CI, 11.2%-66.1%; P = .006) (Table 2). There were no statistically significant differences in the proportion of postmarket safety events for cancer and hematologic therapeutics vs other therapeutic classes or for any of the other bivariate comparisons.
Figure 3. Proportion of Novel Therapeutics Approved by the US Food and Drug Administration (FDA) From 2001 Through 2010 Affected by Any Postmarket Safety Event, According to Drug Class, Therapeutic Area, and Priority vs Standard Review.
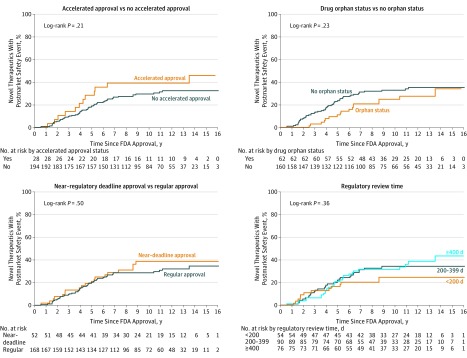
Figure 4. Proportion of Novel Therapeutics Approved by the US Food and Drug Administration (FDA) Between 2001 and 2010 Affected by Any Postmarket Safety Event, According to Accelerated Approval, Orphan Status, Near–Regulatory Deadline Approval, and Regulatory Review Time.
Table 2. Postmarket Safety Events at 10 Years and Associations Between These Events and Characteristics of Novel Therapeutics Approved by the US Food and Drug Administration Between 2001 and 2010.
| Characteristic | Proportion Affected by Postmarket Safety Event at 10 y, % (95% CI) | Bivariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|
| Difference in Proportion Affected by Postmarket Safety Event at 10 y, % (95% CI) | P Valuea | Incidence Rate Ratio (95% CI)b | P Value | ||
| Drug class | |||||
| Pharmaceutical | 29.7 (23.5 to 37.0) | 0 [Reference] | 1 [Reference] | ||
| Biologic | 36.1 (23.2 to 53.3) | 6.4 (−10.1 to 23.0) | .45 | 1.93 (1.06 to 3.52) | .03 |
| Therapeutic area | |||||
| Cancer and hematology | 21.4 (12.1 to 36.1) | 0 [Reference] | 1 [Reference] | ||
| Autoimmune, musculoskeletal, and dermatology | 42.5 (22.8 to 69.4) | 21.1 (−5.8 to 48.0) | .12 | 1.93 (0.87 to 4.28) | .11 |
| Cardiovascular, diabetes, and hyperlipidemia | 27.9 (14.3 to 50.0) | 6.5 (−14.8 to 27.8) | .55 | 1.46 (0.66 to 3.21) | .35 |
| Genitourinary and renal | 23.5 (9.6 to 51.2) | 2.1 (−21.2 to 25.5) | .86 | 1.23 (0.47 to 3.28) | .67 |
| Infectious disease | 27.4 (15.8 to 45.0) | 6.0 (−12.6 to 24.7) | .53 | 1.17 (0.57 to 2.41) | .67 |
| Neurology | 41.2 (22.2 to 67.5) | 19.8 (−6.4 to 46.0) | .14 | 1.53 (0.61 to 3.82) | .36 |
| Psychiatry | 60.0 (37.2 to 83.5) | 38.6 (11.2 to 66.1) | .006 | 3.78 (1.77 to 8.06) | <.001 |
| Other | 29.4 (18.2 to 45.4) | 8.0 (−9.9 to 26.0) | .38 | 1.75 (0.91 to 3.37) | .10 |
| Priority vs standard review | |||||
| Standard review | 32.3 (25.2 to 40.7) | 0 [Reference] | 1 [Reference] | ||
| Priority review | 27.8 (19.1 to 39.5) | 4.4 (−8.3 to 17.2) | .50 | 1.52 (0.87 to 2.65) | .14 |
| Accelerated vs not accelerated approval | |||||
| Not accelerated | 29.7 (23.7 to 36.8) | 0 [Reference] | 1 [Reference] | ||
| Accelerated | 39.3 (24.0 to 59.6) | 9.6 (−9.6 to 28.9) | .33 | 2.20 (1.15 to 4.21) | .02 |
| Orphan vs not orphan status | |||||
| Not orphan | 33.0 (26.2 to 41.0) | 0 [Reference] | 1 [Reference] | ||
| Orphan | 25.0 (15.9 to 38.2) | 8.0 (−5.3 to 21.3) | .24 | 0.60 (0.35 to 1.02) | .06 |
| Near–regulatory deadline vs regular approval | |||||
| Regular | 28.7 (22.5 to 36.2) | 0 [Reference] | 1 [Reference] | ||
| Near–regulatory deadline | 38.8 (26.6 to 54.2) | 10.1 (−5.4 to 25.6) | .20 | 1.90 (1.19 to 3.05) | .008 |
| Regulatory review time, d | |||||
| <200 | 24.7 (15.1 to 38.8) | 9.7 (−5.7 to 25.2) | .22 | 0.46 (0.24 to 0.87) | .02 |
| 200-399 | 34.4 (25.4 to 45.5) | 0 [Reference] | 1 [Reference] | ||
| ≥400 | 31.7 (22.5 to 43.4) | 2.7 (−11.8 to 17.3) | .71 | 1.27 (0.81 to 1.99) | .30 |
P values calculated using a Z test for differences in proportions. See text for full details.
Incidence rate ratios were derived from Poisson regression analysis using a composite of safety events as the dependent variable and 7 categories of novel therapeutic characteristics as independent variables. See text for full details.
In multivariable analysis, postmarket safety events were more frequent among biologics compared with pharmaceuticals (IRR = 1.93; 95% CI, 1.06-3.52; P = .03) and among psychiatric compared with cancer and hematologic therapeutics (IRR = 3.78; 95% CI, 1.77-8.06; P < .001). Priority review (IRR = 1.52; 95% CI, 0.87-2.65; P = .14) and orphan status (IRR = 0.60; 95% CI, 0.35-1.02; P = .06) were not associated with postmarket safety events, although confidence intervals were wide, while postmarket safety events were more frequent among therapeutics receiving accelerated approval (IRR = 2.20; 95% CI, 1.15-4.21; P = .02) and near–regulatory deadline approval (IRR = 1.90; 95% CI, 1.19-3.05; P = .008). When compared with therapeutics with regulatory review times of 200 to 399 days, postmarket safety events were less frequent for those with regulatory review times less than 200 days (IRR = 0.46; 95% CI, 0.24-0.87; P = .02) and no different for therapeutics approved at 400 days or later (IRR = 1.27; 95% CI, 0.81-1.99; P = .30).
Sensitivity Analysis
Among the 103 therapeutics with a first-in-class approval in 2001 or later, we identified 42 postmarket safety events (2 withdrawals, 20 boxed warnings, and 20 safety communications) affecting 29 therapeutics (28.2%), compared with 42 of 119 therapeutics (35.3%) with a first-in-class approval prior to 2001, with no difference in the median time from market availability to the first postmarket safety event (4.0 years [IQR, 2.5-5.3 years] vs 4.2 years [IQR, 2.2-6.4 years], respectively; P = .72). In sensitivity analyses, postmarket safety events were statistically significantly more frequent among neurologic therapeutics (difference in proportion affected by a postmarket safety event at 10 years, 44.4%; 95% CI, −0.37% to 89.1%) when compared with cancer and hematology therapeutics (IRR = 4.2; 95% CI, 1.1 to 16.2; P = .04), whereas near–regulatory deadline approval was no longer statistically significant (IRR = 2.0; 95% CI, 0.9 to 4.2; P = .08). All other results were qualitatively unchanged (eTable 5 in the Supplement).
Discussion
In this analysis of 222 novel therapeutics approved by the FDA from 2001 through 2010, 32% had a postmarket safety event during a median 11.7 years (IQR, 8.7-13.8 years) after approval. Although market withdrawals were rare (n = 3), new boxed warnings, indicating that potentially life-threatening or preventable safety events had been observed in the postmarket period, and safety communications, which describe serious but non-life-threatening postmarket safety events, each occurred for approximately one-fifth of the novel therapeutics (43 and 44 novel therapeutics, respectively). In multivariable analysis, postmarket safety events were significantly more frequent among biologics, therapeutics indicated for the treatment of psychiatric disease, those receiving accelerated approval, and those with near–regulatory deadline approval. In contrast, postmarket safety events were significantly less frequent among therapeutics approved after the shortest periods of regulatory review. These findings should be interpreted cautiously but can be used to inform ongoing surveillance efforts.
To date, evidence about whether preapproval drug characteristics are associated with or can predict postmarket safety events is mixed. Our finding that near–regulatory deadline approvals had higher rates of postmarket safety events is consistent with an earlier study of drugs approved between 1993 and 2004 (although the effect we observed was smaller in magnitude) and with prior research demonstrating that safety concerns are a frequent cause of delayed approval. Our finding that therapeutics approved after the shortest regulatory review times were associated with a lower frequency of postmarket safety events conversely raises the possibility that some approval packages provide clearer evidence of safety, allowing for more rapid regulatory approval. An analysis of regulatory review documents from the European Medicines Agency indicated that safety risks that would ultimately prompt a postmarket safety event were not always evident in the premarket period, suggesting that additional premarket review might only delay approval without identifying therapeutics that pose a future safety concern. Further research is needed to better understand the dynamics between more rapid and near–regulatory deadline approval and drug safety.
Collaboration between the FDA and other stakeholders is necessary to develop and maintain an effective system for detecting postmarket safety events, a suggestion echoed by the US Government Accountability Office in its recent review of FDA expedited regulatory pathways. The FDA’s Sentinel Initiative, which combines both administrative claims and clinical data, is an important first step because the integration of multiple data sources that include observations among large and diverse patient populations can facilitate the detection of postmarket safety events. The sharing of premarket clinical trial data may also bolster such efforts to more promptly identify postmarket safety events by providing patient-level data to independent researchers for further exploration and analysis. However, even with the most careful regulatory review and sensitive postmarket surveillance mechanisms, it may be impossible to detect other less common events until several years after approval, once the therapeutics are in broad use.
Limitations
This study has limitations. We focused on withdrawals and regulatory actions that the FDA has explicitly described as part of its postmarket enforcement apparatus, but there may be other signals of postmarket safety, such as labeling changes and dosage form discontinuations, that we did not assess. While the median length of follow-up in our analysis exceeded a decade, postmarket safety events affecting these novel therapeutics may yet occur. Because the study sample was necessarily limited by the number of novel therapeutics approved by the FDA, our analyses relied on limited sample sizes and had wide confidence intervals, so there is residual uncertainty even in our statistically significant findings.
Conclusions
Among 222 novel therapeutics approved by the FDA from 2001 through 2010, 32% were affected by a postmarket safety event. Biologics, psychiatric therapeutics, and accelerated and near–regulatory deadline approval were statistically significantly associated with higher rates of events. The high frequency of postmarket safety events highlights the need for continuous monitoring of the safety of novel therapeutics throughout their life cycle.
eTable 1. Data Sources
eTable 2. Iterations of the Prescription Drug User Fee Act (PDUFA) and Associated Goal Review Times That Were Used to Identify Near–Regulatory Deadline Approvals
eTable 3. Exclusion of Nonunique Safety Events
eTable 4. Therapeutics With Multiple Unique Postmarket Safety Events
eTable 5. Postmarket Safety Events at 10 Years and Associations Between Events and Characteristics of Novel Therapeutics Approved by the US Food and Drug Administration Between 2001 and 2010, Limited to Therapeutics With a First-in-Class Approval in 2001 or Later
eFigure. Flow Diagram Showing Approach to Identifying Novel Therapeutics Approved by the US Food and Drug Administration Between 2001 and 2010
eBox 1. Protocol for Identification of Incremental Boxed Warnings
eBox 2. Protocol for Identification of Safety Communications
References
- 1.Institute of Medicine The Future of Drug Safety: Promoting and Protecting the Health of the Public. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 2.Downing NS, Aminawung JA, Shah ND, Krumholz HM, Ross JS. Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005-2012. JAMA. 2014;311(4):368-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank C, Himmelstein DU, Woolhandler S, et al. . Era of faster FDA drug approval has also seen increased black-box warnings and market withdrawals. Health Aff (Millwood). 2014;33(8):1453-1459. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter D, Zucker EJ, Avorn J. Drug-review deadlines and safety problems. N Engl J Med. 2008;358(13):1354-1361. [DOI] [PubMed] [Google Scholar]
- 5.US Government Accountability Office Drug Safety: FDA expedites many applications, but data for postapproval oversight need improvement. http://www.gao.gov/products/GAO-16-192. January 14, 2016. Accessed March 10, 2017.
- 6.US Food and Drug Administration Priority review. https://www.fda.gov/ForPatients/Approvals/Fast/ucm405405.htm. Updated September 15, 2014. Accessed March 10, 2017.
- 7.US Food and Drug Administration Accelerated approval. https://www.fda.gov/ForPatients/Approvals/Fast/ucm405447.htm. Updated September 15, 2014. Accessed March 10, 2017.
- 8.US Food and Drug Administration Developing products for rare diseases and conditions. https://www.fda.gov/ForIndustry/DevelopingProductsforRareDiseasesConditions/ucm2005525.htm. Updated March 3, 2017. Accessed March 10, 2017.
- 9.US Food and Drug Administration Search orphan drug designations and approvals. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/. Accessed March 10, 2017.
- 10.Downing NS, Aminawung JA, Shah ND, Braunstein JB, Krumholz HM, Ross JS. Regulatory review of novel therapeutics—comparison of three regulatory agencies. N Engl J Med. 2012;366(24):2284-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration Guidance for industry: warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products—content and format. https://www.fda.gov/downloads/Drugs/.../Guidances/ucm075096.pdf. October 2011. Accessed March 10, 2017.
- 12.US Food and Drug Administration Guidance: drug safety information—FDA’s communication to the public. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm072281.pdf. March 2007. Accessed March 10, 2017.
- 13.US Food and Drug Administration Postmarket drug safety information for patients and providers: index to drug-specific information. https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm111085.htm. Updated November 1, 2016. Accessed March 10, 2017.
- 14.Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol. 1996;49(8):907-916. [DOI] [PubMed] [Google Scholar]
- 15.Lanthier M, Miller KL, Nardinelli C, Woodcock J. An improved approach to measuring drug innovation finds steady rates of first-in-class pharmaceuticals, 1987-2011. Health Aff (Millwood). 2013;32(8):1433-1439. [DOI] [PubMed] [Google Scholar]
- 16.Ross JS, Dzara K, Downing NS. Efficacy and safety concerns are important reasons why the FDA requires multiple reviews before approval of new drugs. Health Aff (Millwood). 2015;34(4):681-688. [DOI] [PubMed] [Google Scholar]
- 17.Zeitoun JD, Lefèvre JH, Downing NS, Bergeron H, Ross JS. Regulatory anticipation of postmarket safety problems for novel medicines approved by the EMA between 2001 and 2010: a cross-sectional study. Pharmacoepidemiol Drug Saf. 2016;25(6):687-694. [DOI] [PubMed] [Google Scholar]
- 18.Institute of Medicine Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risks. Washington, DC: National Academies Press; 2015. [PubMed] [Google Scholar]
- 19.Mott K, Graham DJ, Toh S, et al. . Uptake of new drugs in the early post-approval period in the Mini-Sentinel distributed database. Pharmacoepidemiol Drug Saf. 2016;25(9):1023-1032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Data Sources
eTable 2. Iterations of the Prescription Drug User Fee Act (PDUFA) and Associated Goal Review Times That Were Used to Identify Near–Regulatory Deadline Approvals
eTable 3. Exclusion of Nonunique Safety Events
eTable 4. Therapeutics With Multiple Unique Postmarket Safety Events
eTable 5. Postmarket Safety Events at 10 Years and Associations Between Events and Characteristics of Novel Therapeutics Approved by the US Food and Drug Administration Between 2001 and 2010, Limited to Therapeutics With a First-in-Class Approval in 2001 or Later
eFigure. Flow Diagram Showing Approach to Identifying Novel Therapeutics Approved by the US Food and Drug Administration Between 2001 and 2010
eBox 1. Protocol for Identification of Incremental Boxed Warnings
eBox 2. Protocol for Identification of Safety Communications