Key Points
Question
Does the addition of the mitogen-activated protein kinase kinase (MEK) inhibitor selumetinib to chemotherapy lead to improved outcomes in patients with advanced KRAS-mutant non–small cell lung cancer (NSCLC)?
Findings
In this randomized clinical trial of 510 patients (254 receiving selumetinib + docetaxel; 256 receiving placebo + docetaxel), there was no significant improvement in progression-free survival (median, 3.9 months for selumetinib + docetaxel vs 2.8 months for placebo + docetaxel).
Meaning
Among patients with previously treated advanced KRAS-mutant NSCLC, addition of selumetinib to docetaxel did not provide benefit over docetaxel alone.
Abstract
Importance
There are no specifically approved targeted therapies for the most common genomically defined subset of non–small cell lung cancer (NSCLC), KRAS-mutant lung cancer.
Objective
To compare efficacy of the mitogen-activated protein kinase kinase (MEK) inhibitor selumetinib + docetaxel with docetaxel alone as a second-line therapy for advanced KRAS-mutant NSCLC.
Design, Setting, and Participants
Multinational, randomized clinical trial conducted at 202 sites across 25 countries from October 2013 through January 2016. Of 3323 patients with advanced NSCLC and disease progression following first-line anticancer therapy tested for a KRAS mutation, 866 were enrolled and 510 randomized. Primary reason for exclusion was ineligibility. The data cutoff date for analysis was June 7, 2016.
Interventions
Patients were randomized 1:1; 254 to receive selumetinib + docetaxel and 256 to receive placebo + docetaxel.
Main Outcomes and Measures
Primary end point was investigator assessed progression-free survival. Secondary end points included overall survival, objective response rate, duration of response, effects on disease-related symptoms, safety, and tolerability.
Results
Of 510 randomized patients (mean age, 61.4 years [SD, 8.3]; women, 207 [41%]), 505 patients (99%) received treatment and completed the study (251 received selumetinib + docetaxel; 254 received placebo + docetaxel). At the time of data cutoff, 447 patients (88%) had experienced a progression event and 346 deaths (68%) had occurred. Median progression-free survival was 3.9 months (interquartile range [IQR], 1.5-5.9) with selumetinib + docetaxel and 2.8 months (IQR, 1.4-5.5) with placebo + docetaxel (difference, 1.1 months; hazard ratio [HR], 0.93 [95% CI, 0.77-1.12]; P = .44). Median overall survival was 8.7 months (IQR, 3.6-16.8) with selumetinib + docetaxel and 7.9 months (IQR, 3.8-20.1) with placebo + docetaxel (difference, 0.9 months; HR, 1.05 [95% CI, 0.85-1.30]; P = .64). Objective response rate was 20.1% with selumetinib + docetaxel and 13.7% with placebo + docetaxel (difference, 6.4%; odds ratio, 1.61 [95% CI, 1.00-2.62]; P = .05). Median duration of response was 2.9 months (IQR, 1.7-4.8; 95% CI, 2.7-4.1) with selumetinib + docetaxel and 4.5 months (IQR, 2.3-7.3; 95% CI, 2.8-5.6) with placebo + docetaxel. Adverse events of grade 3 or higher were more frequent with selumetinib + docetaxel (169 adverse events [67%] for selumetinib + docetaxel vs 115 adverse events [45%] for placebo + docetaxel; difference, 22%).
Conclusions and Relevance
Among patients with previously treated advanced KRAS-mutant non–small cell lung cancer, addition of selumetinib to docetaxel did not improve progression-free survival compared with docetaxel alone.
Trial Registration
clinicaltrials.gov: NCT01933932
This randomized clinical trial compares the effects of survival of the MEK inhibitor selumetinib plus docetaxel vs docetaxel alone as second-line therapy for advanced KRAS-mutant non–small cell lung cancer.
Introduction
Genotype-directed targeted therapy is the standard of care for patients with advanced non–small cell lung cancer (NSCLC). However, there are currently no targeted therapies specifically approved for patients with lung cancers related to a mutation in the v-Ki-ras2 Kirsten Rat Sarcoma Viral Oncogene Homologue (KRAS; OMIM:190070) gene, which are detected in approximately 25% of lung adenocarcinoma patients. Such patients have a worse prognosis and may derive less clinical benefit from chemotherapy than the overall population of patients with NSCLC.
KRAS mutations lead to tumor development and growth by activating downstream signaling pathways including the mitogen-activated protein kinase (MAPK) pathway involving MAPK kinase (MEK) and extracellular signal-regulated kinase (ERK). The development of pharmacological strategies to directly target KRAS has proven challenging; as such, therapeutic development has focused on inhibiting KRAS effector proteins downstream, including MEK. Selumetinib (also known as AZD6244 or ARRY-142886) is an oral, potent, and selective, allosteric MEK1 and MEK2 inhibitor with a short half-life. Studies of single-agent MEK inhibitors have demonstrated limited efficacy in KRAS-mutant NSCLC. In contrast, in a randomized phase 2 study (N = 87), selumetinib in combination with docetaxel, as a second-line treatment for patients with KRAS-mutant advanced NSCLC, significantly improved median progression-free survival and objective response rate, and numerically improved overall survival. Median progression-free survival was 5.3 months with selumetinib + docetaxel and 2.1 months with docetaxel alone (hazard ratio [HR] for progression with selumetinib, 0.58 [80% CI, 0.42-0.79]; 1-sided P = .01); objective response rate, 37% for selumetinib + docetaxel vs 0% for docetaxel alone (P < .001); and median overall survival was 9.4 months for selumetinib + docetaxel vs 5.2 months for docetaxel alone (HR for death, 0.80 [80% CI, 0.56-1.14]; 1-sided P = .21). These encouraging findings led to the development and initiation of the phase 3 Selumetinib Evaluation as Combination Therapy (SELECT-1; NCT01933932) trial, which assessed second-line selumetinib + docetaxel for patients with KRAS-mutant, locally advanced or metastatic NSCLC vs placebo + docetaxel.
Methods
Patients
This study included patients 18 years or older, with histologically or cytologically confirmed locally advanced or metastatic NSCLC (stage IIIB-IV). Patients had failure of 1 previous line of therapy for advanced disease, a centrally confirmed KRAS-mutant tumor (by cobas KRAS Mutation Test, Roche Molecular Systems; which detects codon 12 or 13, or 61 mutations) and had at least 1 lesion suitable for repeated measuring by Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Patients also had a World Health Organization (WHO) performance status of 0 or 1, and provided informed consent prior to any study-specific procedures. Exclusion criteria included mixed small cell and non–small cell lung cancer histology and presence of brain metastases or spinal cord compression (unless asymptomatic, treated, stable, and off steroids and anti-convulsants for ≥4 weeks prior to screening). Patients were also excluded if they had received more than 1 prior anticancer drug regimen for advanced or metastatic NSCLC, or prior treatment with an MEK inhibitor or any docetaxel-containing regimen. Patients could be prescreened if the investigator considered it appropriate for the patient to consent to central KRAS mutation status screening of archival tumor material prior to consenting to the main study. Patient race and ethnicity were recorded to gain a clear understanding of KRAS-mutant NSCLC across races and ethnicities. Patient-reported race and ethnicity were recorded by the investigator, based on categories including an option for “other”; race subgroup analyses included only categories for “white” and “other” and ethnicity subgroup analyses included only categories for “Hispanic” and “non-Hispanic.”
Study Oversight
The study was performed in accordance with the ethical principles of the Declaration of Helsinki and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice guidelines. The trial protocol (Supplement 1) was approved by an institutional review board or ethics committee at each participating site. All patients provided written informed consent prior to any study-specific procedures.
Study Design
Patients were randomly assigned to treatment in a 1:1 ratio based on a computer-generated random number, using an interactive voice or web response system. Patients were stratified by WHO performance status (0 or 1) and tumor histology (squamous or nonsquamous), and 1 randomization list was made for each of the 4 randomization strata. A blocked randomization was generated and all centers used the same list to minimize any imbalances in the number of patients assigned to each treatment group. Patients were randomized to receive either 75 mg of selumetinib (hydrogen sulfate) twice daily on a continuous oral administration schedule in combination with 75 mg/m2 of docetaxel intravenously on day 1 of every 21-day cycle or to receive matched placebo plus docetaxel (same schedule) (Figure 1). All patients received granulocyte colony-stimulating factor (G-CSF) or, where available, pegylated G-CSF (pegfilgrastim) starting within 24 hours following each docetaxel administration and not within 14 days before the next docetaxel dose. Patients received assigned study treatment until objective disease progression, intolerable toxicity, or withdrawal of study consent. Patients could continue to receive treatment following disease progression as long as the investigator considered them as continuing to derive clinical benefit in the absence of significant toxicity. Patients and investigators were blinded to randomized treatment.
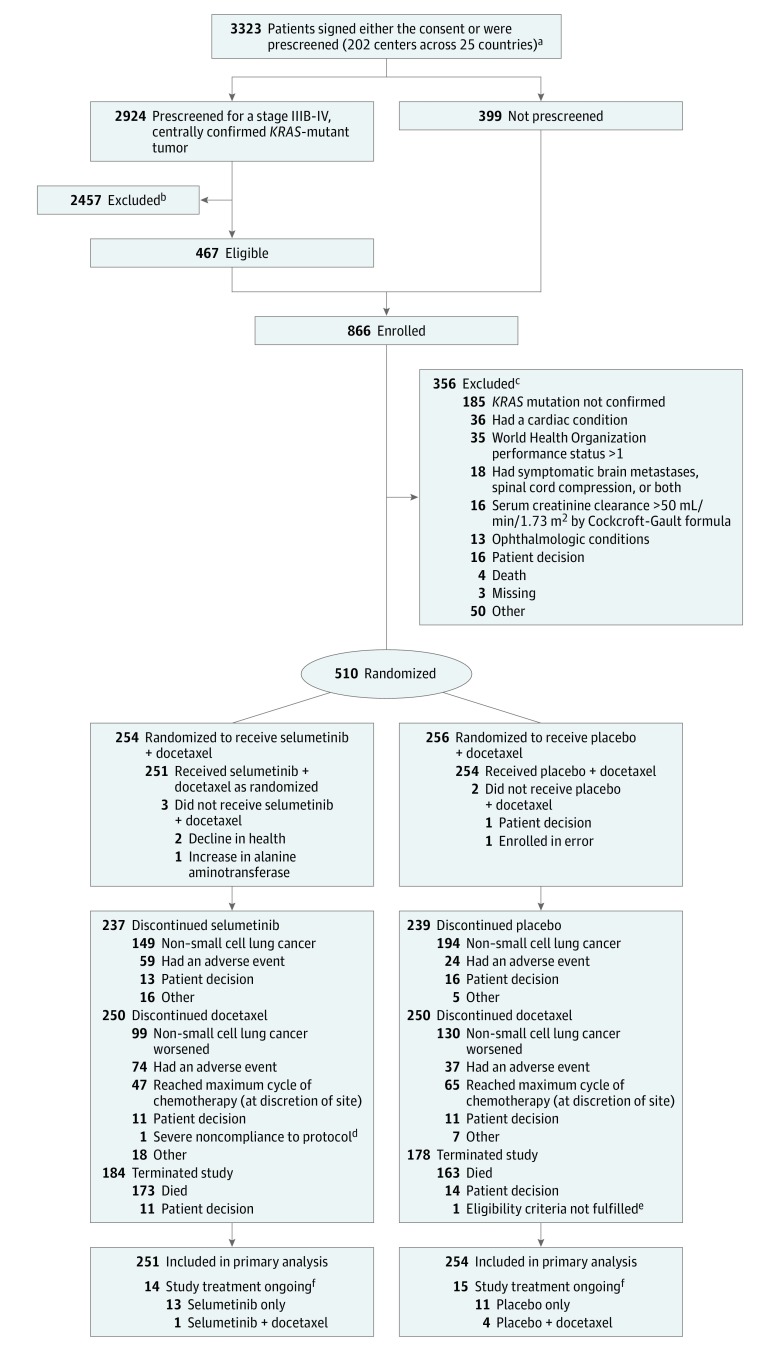
Figure 1. Flow of Patients Through Randomization and Treatment.
KRAS indicates v-Ki-ras2 Kirsten Rat Sarcoma Viral Oncogene Homologue. To convert creatinine clearance to mL/s/m2, multiply by 0.0167. The full analysis set included all randomized patients; the safety analysis set included all patients who received at least 1 dose of randomized investigational product (selumetinib or placebo).
aPatients could be prescreened if the investigator considered it appropriate for the patient to consent to central KRAS mutation status screening of archival tumor material prior to consenting to the main study.
bSpecific reasons for exclusion of 2457 prescreened patients are not available.
cAn individual patient could have had more than 1 reason for exclusion.
dSevere noncompliance was based on the Case Report Form categories. The choices were patient decision, adverse event, severe noncompliance to protocol, condition under investigation worsened, and other.
eIneligible due to cardiac conditions as specified in the protocol and World Health Organization performance status higher than 1.
fAt the time of data cutoff (June 7, 2016).
End Points and Study Assessments
The primary objective was investigator-assessed progression-free survival according to RECIST. Secondary objectives included overall survival, objective response rate (RECIST), duration of response (RECIST), effects of treatment on disease-related symptoms (time to symptom progression and symptom improvement rate; Average Symptom Burden Index [ASBI]), safety and tolerability of selumetinib in combination with docetaxel, and assessment of selumetinib and N-desmethyl selumetinib pharmacokinetics when administered in combination with docetaxel.
Exploratory objectives included evaluation of the influence of KRAS mutation subtypes (mutations in codons 12 or 13, or 61 of the KRAS gene using the cobas KRAS Mutation Test, Roche Molecular Systems); effects of programmed death-ligand 1 (PD-L1) expression using immunohistochemistry (PD-L1 IHC 28-8 pharmDx test, Dako) on treatment response; patient-reported outcomes; and the pharmacokinetics of selumetinib and its metabolite N-desmethyl selumetinib over time. Effects of specific KRAS mutation subtype status on treatment efficacy were also assessed, measured by progression-free survival and objective response rate. Next-generation sequencing was performed to determine the specific KRAS mutation subtype, and patients were assigned to 1 of 2 mutation groups: mutation group 1 included KRAS G12C or KRAS G12V; mutation group 2 included all other KRAS mutations.
Tumor evaluations were performed according to RECIST for all randomized patients using computerized tomography or magnetic resonance imaging scans at screening, week 6, and every 6 weeks thereafter relative to the date of randomization. A review of a random sample of scans from 220 evaluable patients was performed by blinded independent central review according to RECIST to assess concordance between investigator assessments and blinded independent central review. Patients were followed-up for survival status every 8 weeks after treatment discontinuation until withdrawal of consent, death, or the end of the study.
Effects of study treatment on disease-related symptoms and health-related quality of life were assessed as an exploratory objective using the Lung Cancer Symptom Scale and the 36-Item Short-Form Health Survey (version 2), prior to any study-related assessment and at specific time points during the study.
For patient-reported outcomes, the primary end point was the time to symptom progression based on the ASBI (loss of appetite, fatigue, cough, dyspnea, hemoptysis, and pain) from the Lung Cancer Symptom Scale. Items were scored 0 to 100 and a mean was calculated for overall score. Symptom progression was defined as an increase in the ASBI score of 10 points or more. Patients with a baseline score more than 90 were excluded from this analysis as they would not be able to show progression. Seven of the items have the anchors “none” or “not at all” and “as much as it could be” or “as bad as it could be.” The loss of appetite item ranges from “as good as it could be” to “as bad as it could be” and the global health-related quality of life item ranges from “very high” to “very low.”
Adverse events were recorded as Medical Dictionary for Regulatory Activities preferred terms and according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). Adverse events were collected from the time of informed consent until 30 days (±7) after the last dose of the last study treatment.
Blood samples were collected for pharmacokinetics assessments at baseline and at week 3 (predose, and 0.5-2 hours, 2.5-4.5 hours, and 6-8 hours after dose).
Sample Size and Statistical Analysis
The statistical analysis plan is available in Supplement 2. The primary end point was progression-free survival but the study was sized to be able to characterize the overall survival benefit of combining selumetinib with docetaxel. Approximately 500 patients with KRAS-mutant NSCLC who were randomized 1:1 were required to give the study more than 90% power to demonstrate a statistically significant difference for progression-free survival with a 1-sided significance level of 2.5% (2-sided 5%) if the true progression-free survival HR was 0.58. An increase in overall survival of approximately 2 months, based on the numerical difference observed between selumetinib + docetaxel and placebo + docetaxel in the phase 2 study (7.2 months for selumetinib + docetaxel vs 5.2 months for placebo + docetaxel), was considered clinically relevant and equates to an HR of 0.72. Randomizing 500 patients with KRAS-mutant NSCLC to obtain approximately 325 deaths (65% maturity) was estimated to give the study more than 80% power to demonstrate a statistically significant difference in overall survival, if the true overall survival HR was 0.72 and assuming a 2% 1-sided significance level. The final analysis was preplanned to occur when approximately 65% of patients had died (325 events). The significance levels given above are based on a multiple testing procedure with an α-exhaustive recycling strategy that strongly controlled the type I error at 2.5% 1-sided across primary (progression-free survival) and secondary (overall survival and objective response rate) end points. If the primary hypothesis of progression-free survival was rejected for superiority, the secondary end points would then be tested in the multiple testing procedure using a weighted proportion of α. The weighted proportion of α becomes available after each rejected hypothesis and is recycled to secondary end points not yet rejected. This testing procedure stops when the entire weighted proportion of α is allocated to nonrejected end points.
Data analyses were performed using the software program SAS (SAS Institute), version 9.2. Efficacy analyses were performed on all randomized patients on an intention-to-treat basis. Progression-free survival and overall survival were analyzed using a stratified log-rank test, with WHO performance status (0 or 1) as a stratification factor. For patients who had no postbaseline assessment or incomplete postbaseline assessments, these patients were classed as nonevaluable and included in the “nonresponders” category, so that all patients were included in the analyses. Procedures for censoring of patients are presented in the eMethods in Supplement 3. Tumor histology (nonsquamous or squamous) was also preplanned as a stratification factor in the analysis but due to the small number of events observed within the squamous strata it was not included in the final analyses. Results for progression-free survival and overall survival are presented in terms of the HR, associated 2-sided 95% CI, and P value. Kaplan-Meier plots of progression-free survival and overall survival are presented by treatment group. Objective response rate was analyzed using a logistic regression model including terms for treatment and WHO performance status. The analysis is presented in terms of an odds ratio together with its associated 95% CI and 2-sided P value. Median duration of response based on the investigator’s assessment of RECIST was summarized using the Kaplan-Meier method. Time to symptom progression was analyzed as described for progression-free survival and overall survival; safety analysis was summarized for all patients who received at least 1 dose of randomized treatment, and data were summarized based on treatment received.
Results
Patients and Treatment
Between October 2013 and January 2016, 3323 patients signed either the consent or were prescreened at 202 sites across 25 countries (Figure 1). Overall, 866 were enrolled and 356 were excluded with the primary reason being ineligibility. In total, 510 patients were randomized: 254 to the selumetinib + docetaxel group and 256 to the placebo + docetaxel group. Five hundred and five patients received treatment: 251 with selumetinib + docetaxel and 254 with placebo + docetaxel. The patient demographics and baseline disease characteristics were generally well balanced between treatment groups (Table 1). The mean age was 61.4 years, and 207 patients (41%) were women. All patients had previously received at least 1 prior anticancer drug regimen for advanced or metastatic NSCLC. The majority of patients (94%) had KRAS codon 12 or 13 mutations, and 93% of patients were former or current smokers and had adenocarcinoma histology (91%).
Table 1. Baseline Characteristics Among Patients With Advanced KRAS-Mutant Non–Small Cell Lung Cancer Randomized to Selumetinib Plus Docetaxel vs Placebo Plus Docetaxela.
| Characteristic | |||
|---|---|---|---|
| Selumetinib + Docetaxel, No. (%) (n = 254) |
Placebo + Docetaxel, No. (%) (n = 256) |
Total, No. (%) (n = 510) |
|
| Age, mean (SD), y | 61.9 (8.5) | 60.9 (8.1) | 61.4 (8.3) |
| Median (range) | 62 (36-85) | 61 (34-81) | 62 (34-85) |
| Sex | |||
| Women | 96 (38) | 111 (43) | 207 (41) |
| Men | 158 (62) | 145 (57) | 303 (59) |
| Race | |||
| White | 241 (95) | 243 (95) | 484 (95) |
| Other | 13 (5) | 13 (5) | 26 (5) |
| Ethnicity | |||
| Hispanic | 14 (6) | 15 (6) | 29 (6) |
| Non-Hispanic | 240 (95) | 241 (94) | 481 (94) |
| Smoking status | |||
| Never | 16 (6) | 21 (8) | 37 (7) |
| Current | 52 (21) | 62 (24) | 114 (22) |
| Former | 186 (73) | 173 (68) | 359 (70) |
| WHO performance statusb | |||
| 0 | 104 (41) | 104 (41) | 208 (41) |
| 1 | 150 (59) | 152 (59) | 302 (59) |
| Baseline ASBI scorec,d mean (SD) | 28.1 (15.8) | 29.3 (16.2) | 28.7 (16.0) |
| Median (range) | 25.3 (1-77) | 29.5 (0-68) | 27.3 (0-77) |
| Cancer histology | |||
| Squamous | 14 (6) | 14 (6) | 28 (6) |
| Nonsquamous | 240 (95) | 242 (95) | 482 (95) |
| Extent of disease | |||
| Locally advanced | 15 (6) | 10 (4) | 25 (5) |
| Metastatic | 239 (94) | 246 (96) | 485 (95) |
| KRAS mutation subtypee | |||
| Codon 12 or 13 | 237 (93) | 244 (95) | 481 (94) |
| Codon 61 | 16 (6) | 12 (5) | 28 (6) |
| PD-L1 marker statusf | |||
| <5% | 112 (44) | 112 (44) | 224 (44) |
| ≥5% | 79 (31) | 82 (32) | 161 (32) |
| Unknown | 63 (25) | 62 (24) | 125 (25) |
Abbreviations: ASBI, Average Symptom Burden Index; KRAS, v-Ki-ras2 Kirsten Rat Sarcoma Viral Oncogene Homologue; LCSS, Lung Cancer Symptom Scale; PD-L1, programmed death-ligand 1; WHO, World Health Organization.
Population: full analysis set (all randomized patients); data cutoff June 7, 2016.
WHO performance status: 0, asymptomatic; 1, symptomatic but ambulatory.
ASBI comprises 6 items from the LCSS (loss of appetite, fatigue, cough, dyspnea, hemoptysis, and pain) with scores ranging from 0 (best possible status) to 100 (worst possible status).
Baseline ASBI score was not available for all patients. Only patients with baseline ASBI scores are reported: selumetinib + docetaxel group (n = 234); placebo + docetaxel group (n = 247); total patients (n = 481).
Centrally confirmed using cobas KRAS Mutation Test (Roche); n = 509 (1 patient in the selumetinib + docetaxel group was tested locally and KRAS mutation subtype is unknown).
Centrally tested using the PD-L1 IHC 28-8 pharmDx test (Dako).
The median number of docetaxel cycles administered in the selumetinib + docetaxel group was similar (median, 4 cycles [range, 1-16]) to the placebo + docetaxel group (median, 4 cycles [range, 1-25]). Median duration of randomized treatment, excluding dose interruption time, with selumetinib or placebo was 74 days (range, 3-834) in the selumetinib + docetaxel group, and 85 days (range, 5-849 days) in the placebo + docetaxel group. The relative dose intensity of docetaxel was similar between the 2 groups over the first 6 cycles of therapy (86.7% in the selumetinib + docetaxel group and 90.3% in the placebo + docetaxel group). In both groups, 10% of patients (24 in the selumetinib group; 25 in the placebo group) received more than 6 cycles of docetaxel. In total, 87 patients (35%) in the selumetinib + docetaxel group and 96 patients (38%) in the placebo + docetaxel group received disease-related anticancer therapy after discontinuation, and rates of specific therapies were similar between groups.
Efficacy
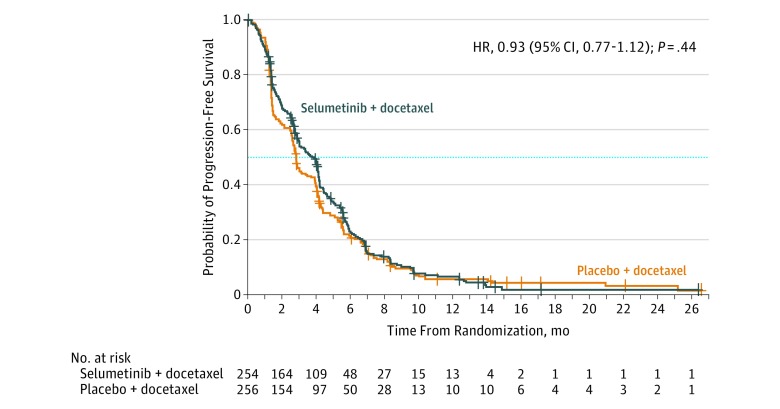
At the time of data cutoff for final analysis (June 7, 2016), 447 patients (88%) had experienced a progression event; 218 patients (86%) in the selumetinib + docetaxel group and 229 patients (89%) in the placebo + docetaxel group. At the time of primary analysis, 4 patients (2%) had censored RECIST progression and 8 patients (3%) had censored death in the selumetinib + docetaxel group; 6 patients (2%) had censored death in the placebo + docetaxel group. Median progression-free survival was 3.9 months (interquartile range [IQR], 1.5-5.9) in the selumetinib + docetaxel group compared with 2.8 months (IQR, 1.4-5.5) in the placebo + docetaxel group (difference, 1.1 months; Figure 2). The HR for progression-free survival was 0.93 (95% CI, 0.77-1.12) with a 2-sided P value of .44. Subgroup analyses of progression-free survival demonstrated no statistically significant interaction of treatment by subgroup (eFigure 1 in Supplement 3). A random selection of scans from 220 patients were also assessed by blinded independent central review, which agreed with investigative site review in terms of progression or no progression in more than 80% of cases, and analyses of ascertainment bias supported the consistency of the results based on investigative site review and blinded independent central review (HR ratio, 1.07 [90% upper confidence limit, 1.19]).
Figure 2. Estimated Progression-Free Survival in the Selumetinib Plus Docetaxel and Placebo Plus Docetaxel Groups.
HR indicates hazard ratio; IQR, interquartile range. The dotted line indicates median survival. In this full analysis set (performed using stratified log-rank test with factors for World Health Organization Performance Status), 88% patients had a progression event (447 of 510 events). Data cutoff was June 7, 2016. The crosses indicate censored observations. Median duration of follow-up for progression-free survival: selumetinib + docetaxel, 2.7 months (IQR, 0.6-5.6); placebo + docetaxel, 4.2 months (IQR, 0.03-11.1).
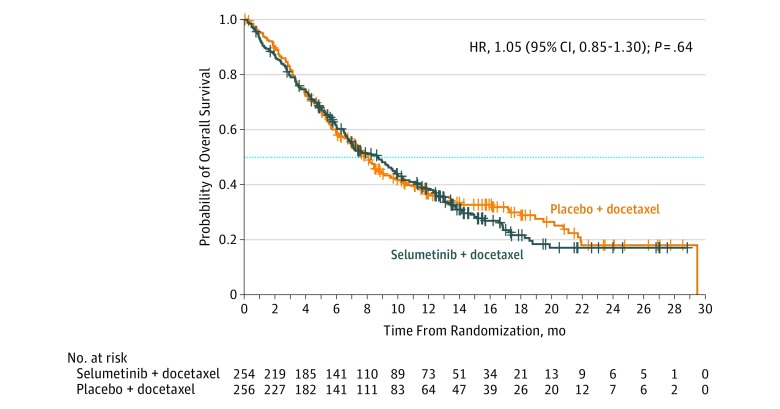
At the data cutoff, 346 death events (68%) had occurred. Median overall survival was 8.7 months (IQR, 3.6-16.8) in the selumetinib + docetaxel group (176 events) and 7.9 months (IQR, 3.8-20.1) in the placebo + docetaxel group (170 events)—a difference of 0.9 months, with an HR of 1.05 (95% CI, 0.85-1.30); 2-sided P = .64 (Figure 3). Subgroup analyses of overall survival demonstrated no statistically significant interaction of treatment by subgroup (eFigure 2 in Supplement 3). Objective response rate was 20.1% (2 complete response, 49 partial response) in the selumetinib + docetaxel group, and 13.7% (0 complete response, 35 partial response) in the placebo + docetaxel group (difference, 6.4%; odds ratio, 1.61 [95% CI, 1.00-2.62]; 2-sided P = .05) (eFigure 3 in Supplement 3). Median duration of response was 2.9 months (IQR, 1.7-4.8; 95% CI, 2.7-4.1) with selumetinib + docetaxel and 4.5 months (IQR, 2.3-7.3; 95% CI, 2.8-5.6) with placebo + docetaxel. One hundred and six patients (42%) in the selumetinib + docetaxel group, and 95 patients (37%) in the placebo + docetaxel group had stable disease for 6 weeks or more. At the time of data cutoff, 29 patients (6%) were receiving ongoing randomized study treatment (Figure 1).
Figure 3. Estimated Overall Survival in the Selumetinib Plus Docetaxel and Placebo Plus Docetaxel Groups.
HR indicates hazard ratio. The dotted line indicates median survival. In this full analysis set (performed using stratified log-rank test with factors for World Health Organization Performance Status), 68% of patients had a death event (346 of 510 events). Data cutoff was June 7, 2016. The crosses indicate censored observations. Median duration of follow-up for overall survival: selumetinib + docetaxel, 13.5 months (IQR, 7.4-17.3); placebo + docetaxel, 12.2 months (IQR, 8.1-16.8).
Adverse Events
The majority of patients experienced at least 1 adverse event (Table 2). The most commonly reported adverse events in the selumetinib + docetaxel group were diarrhea (154 events [61%]), nausea (94 events [38%]), rash (85 events [34%]) and peripheral edema (76 events [30%]). In the placebo + docetaxel group, the most frequent adverse events were diarrhea (89 events [35%]), fatigue (79 events [31%]), alopecia (64 events [25%]), and nausea (62 events [24%]). Grade 3 or higher adverse events were reported more frequently in the selumetinib + docetaxel group (169 events [67%]) than the placebo + docetaxel group (115 events [45%]; difference, 22%). Febrile neutropenia was reported in 4 patients (2%) in the selumetinib + docetaxel group and 2 patients (1%) in the placebo + docetaxel group. All were reported as grade 3 or higher, with the exception of 1 event in the placebo + docetaxel group. Adverse events causally related to randomized treatment are shown in eTable 1 in Supplement 3. More patients in the selumetinib + docetaxel group (49%) reported serious adverse events compared with the placebo + docetaxel group (32%). In total, 116 patients (46%) in the selumetinib + docetaxel and 76 patients (30%) in the placebo + docetaxel groups had adverse events leading to hospitalization. There were 346 deaths in this study; 313 were considered by the investigator to be related to underlying NSCLC, 10 due to adverse events, 17 related to both disease and an adverse event, and 6 related to other causes of death. The number and causes of death were balanced between treatment groups.
Table 2. Most Frequently Reported Adverse Events (All Causality) Among Patients With Advanced KRAS-Mutant Non–Small Cell Lung Cancer Receiving Selumetinib Plus Docetaxel vs Placebo Plus Docetaxela.
| Preferred Term, Participants With an Eventb |
Selumetinib + Docetaxel, No. (%) (n = 251) |
Placebo + Docetaxel, No. (%) (n = 254) |
||
|---|---|---|---|---|
| All Grades | CTCAE Grade ≥3 | All Grades | CTCAE Grade ≥3 | |
| Diarrhea | 154 (61) | 18 (7) | 89 (35) | 7 (3) |
| Nausea | 94 (38) | 3 (1) | 62 (24) | 1 (1) |
| Rash | 85 (34) | 9 (4) | 28 (11) | 1 (1) |
| Edema peripheral | 76 (30) | 6 (2) | 39 (15) | 0 |
| Fatigue | 70 (28) | 9 (4) | 79 (31) | 10 (4) |
| Asthenia | 67 (27) | 22 (9) | 47 (19) | 7 (3) |
| Vomiting | 67 (27) | 7 (3) | 32 (13) | 1 (1) |
| Stomatitis | 65 (26) | 9 (4) | 34 (13) | 1 (1) |
| Dyspnea | 61 (24) | 20 (8) | 44 (17) | 6 (2) |
| Decreased appetite | 56 (22) | 5 (2) | 60 (24) | 4 (2) |
| Pyrexia | 50 (20) | 4 (2) | 34 (13) | 2 (1) |
| Alopecia | 49 (20) | 2 (1) | 64 (25) | 0 |
| Anemia | 49 (20) | 12 (5) | 41 (16) | 11 (4) |
| Constipation | 41 (16) | 0 | 48 (19) | 1 (1) |
| Cough | 37 (15) | 0 | 35 (14) | 0 |
| Dermatitis acneiform | 30 (12) | 4 (2) | 2 (1) | 0 |
| Dry skin | 30 (12) | 0 | 14 (6) | 0 |
| Neutropenia | 26 (10) | 18 (7) | 15 (6) | 10 (4) |
| Abdominal pain | 25 (10) | 3 (1) | 21 (8) | 1 (1) |
Abbreviation: CTCAE, Common Toxicity Criteria for Adverse Events (score range: 1 [mild] to 5 [death]).
Population: safety analysis set.
All-causality adverse events reported during randomized treatment in 10% or more of patients in either treatment group, by frequency in the selumetinib + docetaxel group.
Dose reductions of selumetinib or placebo were required in 70 patients (28%) in the selumetinib + docetaxel group and 16 patients (6%) in the placebo + docetaxel group; interruptions of selumetinib or placebo were required in 103 patients (41%) in the selumetinib + docetaxel group and 53 patients (21%) in the placebo + docetaxel group. Dose reductions of docetaxel were required in 41 patients (16%) in the selumetinib + docetaxel group and 25 patients (10%) in the placebo + docetaxel group; docetaxel dose delays were required in 72 patients (29%) in the selumetinib + docetaxel group and 55 patients (22%) in the placebo + docetaxel group. Discontinuation of selumetinib or placebo due to adverse events occurred in 59 patients (23%) in the selumetinib + docetaxel group and 24 patients (9%) in the placebo + docetaxel group. Discontinuation of docetaxel due to adverse events was required in 74 patients (29%) in the selumetinib + docetaxel group and 37 patients (15%) in the placebo + docetaxel group.
Pharmacokinetics
Plasma concentrations of selumetinib and its N-desmethyl metabolite were analyzed using a population pharmacokinetics approach and were comparable with previous studies (eFigure 4 in Supplement 3).
Exploratory Analyses
Effects of PD-L1 on Treatment Response
PD-L1 status was successfully determined in 385 patients (75%). Of these 224 patients (58%) had staining in less than 5% of tumor cells whereas 161 patients (42%) had staining in 5% or more of cells (Table 1). The presence or absence of PD-L1 staining did not affect efficacy (progression-free survival or overall survival) of either selumetinib + docetaxel or placebo + docetaxel (eTable 2 in Supplement 3).
Influence of KRAS Mutation Subtypes
Of the 510 patients randomized, 13 samples were excluded from the KRAS mutation group analysis set because the subtype of the confirmed KRAS mutation could not be identified, and an additional patient had no tumor sample available for next-generation sequencing analysis. Overall, 496 patients were included in the KRAS mutation group analysis set (mutation group 1 [KRAS G12C or KRAS G12V], 301 patients; mutation group 2 [all other KRAS mutations], 195 patients); patients were similarly distributed between treatments in both mutation groups. Fifteen patients had multiple KRAS mutations and were excluded from the progression-free survival analysis (n = 481); no differences were observed in progression-free survival between treatments by mutation group or by individual KRAS mutation subtype (eFigure 6 in Supplement 3). In mutation group 1, objective response rate was greater in the selumetinib + docetaxel group compared with the placebo + docetaxel group, however this was not translated into a meaningful progression-free survival benefit (eTable 3 in Supplement 3). There were no differences in objective response rate between treatments in mutation group 2.
Patient-Reported Outcomes
There was no statistically significant effect of selumetinib on time to symptom progression (HR, 0.90 [95% CI, 0.73-1.11], P = .32; eFigure 5 in Supplement 3) or symptom improvement rates (odds ratio, 1.17 [95% CI, 0.74-1.86]).
Discussion
In the SELECT-1 trial, selumetinib + docetaxel did not improve progression-free survival or overall survival in patients with advanced KRAS-mutant NSCLC compared with placebo + docetaxel. Median progression-free survival was 3.9 months in the selumetinib + docetaxel group and 2.8 months in the placebo + docetaxel group, whereas median overall survival was 8.7 months in the selumetinib + docetaxel group vs 7.9 months in the placebo + docetaxel group. Grade 3 or higher adverse events were more frequent with selumetinib + docetaxel (67%) than placebo + docetaxel (45%).
The results of this study differ from those observed in a prior smaller randomized phase 2 trial with the same design. Efficacy in the control group was greater in the phase 3 trial compared with the phase 2 trial (objective response rate, 14% in the phase 3 trial vs 0% in the phase 2 trial; progression-free survival, 2.8 months in the phase 3 trial vs 2.1 months in the phase 2 trial). Conversely, the experimental group performed worse in the phase 3 trial compared with that of the phase 2 trial (objective response rate, 20% in the phase 3 trial vs 37% in the phase 2 trial; progression-free survival 3.9 months in the phase 3 trial vs 5.3 months in the phase 2 trial). The differences were unlikely to be influenced by the study design, which was the same in both cases; or by geography, because both were conducted in multiple regions around the world. The treatment regimen was better tolerated in the phase 3 trial given the mandatory use of G-CSF, which led to a lower incidence of neutropenia and febrile neutropenia in the current study (Table 2) compared with the prior phase 2 trial.
To our knowledge, SELECT-1 is the largest randomized trial for patients with KRAS-mutant NSCLC performed to date. However, not all KRAS mutations are functionally the same, and the genomic context in which KRAS mutations occur may also influence therapeutic efficacy. Approximately one-third of KRAS-mutant cancers harbor concurrent mutations in TP53 and another one-third have concurrent mutations in LKB1. A much smaller fraction harbor both concomitant TP53 and LKB1 mutations whereas the remaining portion harbor neither concomitant mutation. A co-clinical trial in mice demonstrated that although the addition of selumetinib to docetaxel significantly improved the response rate in KrasG12D and KrasG12D/p53−/− mice, this was not the case for KrasG12D/Lkb1−/− mice. The presence of either concomitant loss of p53 or Lkb1 also blunted the efficacy of docetaxel compared with mice with only KrasG12D. Increased sensitivity to MEK inhibition has also been demonstrated in KRAS-mutant NSCLC cell lines with concomitant LKB1 inactivation using the MEK inhibitor CI-1040.
KRAS mutations represent the largest genomically defined subset of lung cancer. There remains a great need to develop effective therapies for this subset of patients and the findings from the present study further highlight this. Patients with advanced KRAS-mutant NSCLC benefit from anti–programmed cell death protein 1 (PD-1) inhibitors including nivolumab, similarly to the general population of NSCLC patients. The benefit of anti–PD-1 therapy for patients with KRAS mutations may reflect the incidence of high PD-L1 expression in KRAS-mutant tumors. Although PD-L1 expression is used as a biomarker to select patients for anti–PD-1 therapy, it did not affect the efficacy observed in this study (eTable 2 in Supplement 3). Recent preclinical studies have identified inhibitors that can directly target KRAS. Based on in vitro studies, these inhibitors appear to have potential, and to date are selective for KRAS G12C, which represents the largest subset of KRAS-mutant NSCLC.
Limitations
There were several limitations in this study. Although the present trial was a randomized study, and hence should lead to a minimization of any imbalances, it is possible that differences in the distribution of concomitant genomic alterations, alongside KRAS mutations, contribute to the findings and to the differences observed between the phase 2 and phase 3 trials. Similarly, the particular codon 12 KRAS mutations may predict for differential sensitivity to MEK inhibitors. Preclinical studies suggest that cell lines harboring KRAS G12C or KRAS G12V mutations may have a greater dependence upon MAPK signaling and hence be more sensitive to MEK inhibitors. A subset analysis from the prior randomized phase 2 trial suggested a differential benefit for the selumetinib combination in patients with KRAS G12C or KRAS G12V mutations. However, we were unable to confirm this differential benefit in the present study. A further limitation is that the lack of efficacy of docetaxel in the phase 2 trial, from which results were used in the powering of the present study, may have led to an overestimation of the benefit of adding selumetinib to docetaxel. In a phase 2 randomized trial of trametinib compared with docetaxel, the response rate to docetaxel was 12% and the progression-free survival was 2.5 months, similar to the findings of the control group in the present study. Better performance of patients receiving placebo + docetaxel in the present study compared with the phase 2 trial may be explained by the administration of prophylactic G-CSF to all patients in the SELECT-1 trial, which is not part of routine clinical practice for docetaxel monotherapy administration in this patient population.
Conclusions
Among patients with previously treated advanced KRAS-mutant non–small cell lung cancer, addition of selumetinib to docetaxel did not improve progression-free survival compared with docetaxel alone.
Trial Protocol
Statistical Analysis
eMethods. Censoring of Patients
eTable 1. Most Frequently Reported Adverse Events Causally Related to Selumetinib/Placebo
eTable 2. PD-L1 Subgroup Analysis of Progression-Free Survival and Overall Survival Events
eTable 3. Objective Response Rate Analysis by KRAS Mutation Group Status (Next-Generation Sequencing Data)
eFigure 1. Prespecified Subgroup Analysis of Progression-Free Survival
eFigure 2. Prespecified Subgroup Analysis of Overall Survival
eFigure 3. Waterfall Plots of Best Percentage Change in Tumor Size for Target Lesions by Patient in (A) Selumetinib + Docetaxel, and (B) Placebo + Docetaxel Groups
eFigure 4. Selumetinib (A) and N-Desmethyl Selumetinib Metabolite (B) Plasma Concentration Over Time
eFigure 5. Kaplan-Meier Estimates of Time to Symptom Progression (ASBI)
eFigure 6. Progression-Free Survival Analysis by KRAS Mutation Subgroup (All Patients With Available Next-Generation Sequencing Data)
eReferences.
Abbreviation List
- ASBI
Average Symptom Burden Index
- G-CSF
granulocyte colony-stimulating factor
- MAPK
mitogen-activated protein kinase
- MEK
MAPK kinase
- NSCLC
non–small cell lung cancer
- PD-L1
programmed death-ligand 1
References
- 1.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72(10):2457-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2005;92(1):131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson ML, Sima CS, Chaft J, et al. Association of KRAS and EGFR mutations with survival in patients with advanced lung adenocarcinomas. Cancer. 2013;119(2):356-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlesi F, Mazieres J, Merlio JP, et al. ; Biomarkers France contributors . Routine molecular profiling of patients with advanced non–small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet. 2016;387(10026):1415-1426. [DOI] [PubMed] [Google Scholar]
- 6.Wood K, Hensing T, Malik R, Salgia R. Prognostic and predictive value in KRAS in non–small-cell lung cancer: a review. JAMA Oncol. 2016;2(6):805-812. [DOI] [PubMed] [Google Scholar]
- 7.Banerji U, Camidge DR, Verheul HM, et al. The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010;16(5):1613-1623. [DOI] [PubMed] [Google Scholar]
- 8.Denton CL, Gustafson DL. Pharmacokinetics and pharmacodynamics of AZD6244 (ARRY-142886) in tumor-bearing nude mice. Cancer Chemother Pharmacol. 2011;67(2):349-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh TC, Marsh V, Bernat BA, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13(5):1576-1583. [DOI] [PubMed] [Google Scholar]
- 10.Blumenschein GR Jr, Smit EF, Planchard D, et al. A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non–small-cell lung cancer (NSCLC). Ann Oncol. 2015;26(5):894-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter CA, Rajan A, Keen C, et al. Selumetinib with and without erlotinib in KRAS mutant and KRAS wild-type advanced non–small-cell lung cancer. Ann Oncol. 2016;27(4):693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jänne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non–small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14(1):38-47. [DOI] [PubMed] [Google Scholar]
- 13.Declaration of Helsinki. JAMA. 1966;197(11):32. doi: 10.1001/jama.1966.03110110016005 [DOI] [Google Scholar]
- 14.Jänne PA, Smith I, McWalter G, et al. Impact of KRAS codon subtypes from a randomised phase II trial of selumetinib plus docetaxel in KRAS mutant advanced non–small-cell lung cancer. Br J Cancer. 2015;113(2):199-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burman CF, Sonesson C, Guilbaud O. A recycling framework for the construction of Bonferroni-based multiple tests. Stat Med. 2009;28(5):739-761. [DOI] [PubMed] [Google Scholar]
- 16.Stone A, Macpherson E, Smith A, Jennison C. Model free audit methodology for bias evaluation of tumour progression in oncology. Pharm Stat. 2015;14(6):455-463. [DOI] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skoulidis F, Byers LA, Diao L, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5(8):860-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z, Cheng K, Walton Z, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 2012;483(7391):613-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahoney CL, Choudhury B, Davies H, et al. LKB1/KRAS mutant lung cancers constitute a genetic subset of NSCLC with increased sensitivity to MAPK and mTOR signalling inhibition. Br J Cancer. 2009;100(2):370-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab vs docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D, Zhu X, Li N, Li Y. Correlation of PD-L1 expression with EGFR, KRAS, or ALK alterations and with survival of non–small cell lung cancer (NSCLC) treated with EGFR-TKIs: a meta-analysis of published trials. J Clin Oncol. 2016;34(suppl; abstr e20576). [Google Scholar]
- 23.Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503(7477):548-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis
eMethods. Censoring of Patients
eTable 1. Most Frequently Reported Adverse Events Causally Related to Selumetinib/Placebo
eTable 2. PD-L1 Subgroup Analysis of Progression-Free Survival and Overall Survival Events
eTable 3. Objective Response Rate Analysis by KRAS Mutation Group Status (Next-Generation Sequencing Data)
eFigure 1. Prespecified Subgroup Analysis of Progression-Free Survival
eFigure 2. Prespecified Subgroup Analysis of Overall Survival
eFigure 3. Waterfall Plots of Best Percentage Change in Tumor Size for Target Lesions by Patient in (A) Selumetinib + Docetaxel, and (B) Placebo + Docetaxel Groups
eFigure 4. Selumetinib (A) and N-Desmethyl Selumetinib Metabolite (B) Plasma Concentration Over Time
eFigure 5. Kaplan-Meier Estimates of Time to Symptom Progression (ASBI)
eFigure 6. Progression-Free Survival Analysis by KRAS Mutation Subgroup (All Patients With Available Next-Generation Sequencing Data)
eReferences.