Key Points
Question
How does treatment of skin disease in psoriasis associate with change in aortic vascular inflammation by 18fluorodeoxyglucose positron emission tomography/computed tomography at 1 year?
Findings
This cohort study found that improvement in skin disease severity is associated with an improvement in aortic vascular inflammation beyond cardiovascular risk factors, which was greater in patients with more improvement in skin disease severity. Furthermore, this association was stronger in those initiated with anti–tumor necrosis factor therapy.
Meaning
These findings suggest that alleviating inflammation at remote sites in the body (eg, skin) has beneficial effect on vascular inflammation at 1 year, a finding that may have important implications in other inflammatory disease states.
Abstract
Importance
Inflammation is critical in the development of atherosclerosis. Psoriasis is a chronic inflammatory skin disease that is associated with increased vascular inflammation by 18fluorodeoxyglucose positron emission tomography/computed tomography in vivo and future cardiovascular events. It provides a human model to understand the effect of treating inflammation in a target organ (eg, the skin) on vascular diseases.
Objective
To investigate the association between change in skin disease severity and change in vascular inflammation at 1 year and to characterize the impact of 1 year of anti–tumor necrosis factor therapy on vascular inflammation.
Design, Setting, and Participants
In this prospective cohort study, 220 participants from outpatient practices were recruited at the US National Institutes of Health. A total of 115 consecutively recruited patients with psoriasis were followed up at 1 year. The study was conducted from January 1, 2013, through October 31, 2016, with data analyzed in November 2016.
Exposure
Skin inflammation measured as Psoriasis Area and Severity Index (PASI) score.
Main Outcomes and Measures
Vascular inflammation assessed as target-to-background ratio by 18fluorodeoxyglucose positron emission tomography/computed tomography.
Results
Among the 115 patients, the mean (SD) age at 1-year follow-up was 50.8 (12.8) years and 68 were men (59%). The cohort had a low cardiovascular risk by Framingham risk score and mild-to-moderate psoriasis, with a median PASI score of 5.2 (interquartile range, 3.0-8.9). At follow-up, the total cohort had a median improvement in PASI score of 33%, with use of topical therapy (60%), biological therapy (66%, mostly anti–tumor necrosis factor) and phototherapy (15%) (P < .001). Moreover, improvement in PASI score was associated with improvement in target-to-background ratio of 6%, mainly driven by those with higher responses in PASI score (P < .001). This association persisted beyond traditional risk factors (β = 0.19; 95% CI, 0.012-0.375; P = .03) and was the strongest in those initiated with anti–tumor necrosis factor therapy (β = 0.79; 95% CI, 0.269-1.311; P = .03).
Conclusions and Relevance
Improvement in psoriasis skin disease severity was associated with improvement in aortic vascular inflammation by 18fluorodeoxyglucose positron emission tomography/computed tomography, with greater improvement in aortic vascular inflammation observed in those who had higher than 75% reduction in skin disease severity. These findings suggest that controlling remote target organ inflammation (eg, in the skin) may improve vascular diseases; however, randomized clinical trials are needed to confirm these findings.
This cohort study investigates the association between change in skin disease severity and change in vascular inflammation at 1 year and characterizes the impact of 1 year of anti–tumor necrosis factor therapy on vascular inflammation in patients with psoriasis.
Introduction
Psoriasis is a chronic inflammatory skin disease that affects an estimated 3% of the US adult population. Psoriasis, especially when it is severe, is an independent risk factor for myocardial infarction, stroke, and overall cardiovascular mortality. Moreover, vascular inflammation by 18fluorodeoxyglucose positron emission tomography/computed tomography (18FDG PET/CT) is associated with psoriasis skin disease severity, further suggesting shared mechanisms between remote inflammation in the skin and presence of vascular disease. Given the association with early cardiovascular events, psoriasis provides a reliable human model to study the longitudinal impact of modulating target organ inflammation (eg, skin) on vascular inflammation.
Vascular inflammation by 18FDG PET/CT is an important biomarker of cardiovascular risk. Aortic vascular inflammation is associated with inflammatory biomarkers in the serum, marks the distribution of atherosclerotic plaques with high-risk morphological features in carotid arteries and coronary arteries, and is predictive of future cardiovascular events. Additionally, vascular inflammation is highly sensitive to modulation of risk factors with preventive strategies such as statin therapy and therapeutic lifestyle changes, which are known to mitigate cardiovascular risk.
Thus, using vascular inflammation by 18FDG PET/CT as a primary outcome we hypothesized that (1) improvement in psoriasis severity would be associated with improvement in vascular inflammation at 1 year and (2) initiation of anti–tumor necrosis factor (TNF) therapy would lead to a reduction in vascular inflammation at 1 year.
Methods
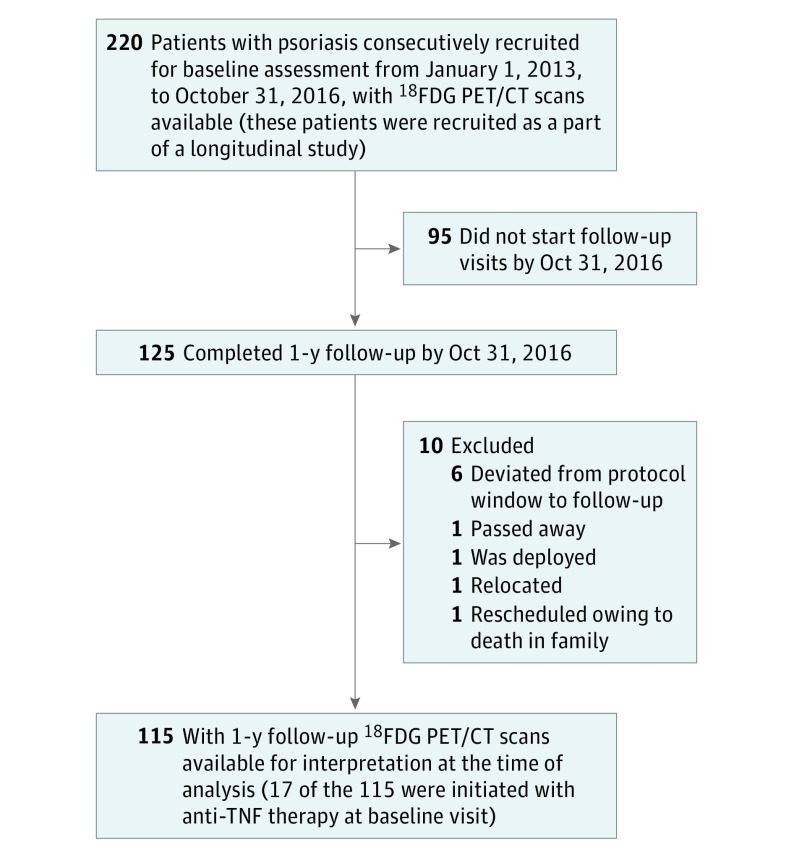
A total of 115 consecutive patients with psoriasis recruited from January 1, 2013, to October 31, 2016, participated in an ongoing case-cohort study to understand the association between psoriasis and cardiometabolic diseases (Figure 1). A study health care professional confirmed the onset and duration of psoriasis and assessed psoriasis severity using the Psoriasis Area and Severity Index (PASI) score, which combines the severity of lesions and the area affected into a single score, considering erythema, induration, and desquamation within each lesion. Previous psoriasis literature has established that the degree of PASI score response defined as greater than 50% improvement as well as greater than 75% improvement is clinically significant and denotes meaningful improvement. The institutional review board at the US National Institutes of Health approved the study protocol. All participants provided written informed consent and received financial compensation.
Figure 1. Recruitment Scheme for the Patients at the National Institutes of Health.
18FDG PET/CT indicates 18fluorodeoxyglucose positron emission tomography/computed tomography; TNF, tumor necrosis factor.
Patients were required to have a formal diagnosis of plaque psoriasis for study inclusion. Patients were excluded if they had any comorbid condition known to promote cardiovascular disease or systemic inflammation, such as clinically diagnosed cardiovascular disease, uncontrolled hypertension, internal malignancy within 5 years, human immunodeficiency virus, active infection within the past 72 hours of baseline, and major surgery within 3 months.
All patients underwent 18FDG PET/CT scans at baseline and at 1 year. All scans were read in a blinded fashion to patient characteristics and visit dates. Images were analyzed using a dedicated PET/CT analysis program (Extended Brilliance Workspace; Phillips Healthcare) to quantify vascular inflammation measured as target-to-background ratio. The primary outcome for our study was change in aortic vascular inflammation at 1 year (eAppendix in the Supplement).
Summary statistics are presented. Multivariable linear regression analyses were performed to evaluate the association between longitudinal changes in vascular inflammation and psoriasis severity. Statistical analyses were performed using STATA version 12 (StataCorp). We hypothesized that a 5-point change in PASI score would lead to a 0.1 change in target-to-background ratio, with an SD of 0.1, thus requiring a group of 50 patients to have 90% power to test the association of change in psoriasis severity with change in aortic vascular inflammation. Data were analyzed in November 2016.
Results
Characteristics of the Study Group Over 1-Year Follow-up
The cohort included 115 middle-aged patients with psoriasis (mean [SD] age, 49.7 [12.8] years), was male predominant (n = 68; 59%), and at low cardiovascular risk by Framingham score (median, 3; interquartile range [IQR], 1-6), with a PASI score of 5.2 (IQR, 3.0-8.9), indicating moderate skin disease with a broad range of psoriasis severity (0-40) at baseline.
At 1-year follow-up, the cohort had an improvement in high-density lipoprotein cholesterol level (mean [SD], 55.2 [18.3] mg/dL vs 58.5 [21.8] mg/dL; P = .003; to convert to millimoles per liter, multiply by 0.0259) without any change in the proportion of patients taking statins. Furthermore, high-sensitivity C-reactive protein level was reduced at 1 year (median, 1.8 mg/L; IQR, 0.7-3.8, vs 1.3 mg/L; IQR, 0.6-3.2; P = .02; to convert to nanomolers per liter, multiply by 9.524). Moreover, psoriasis severity improved significantly at follow-up by 33% (P < .001) with increased use of systemic or biologic anti-inflammatory therapies at 1 year (39% vs 60%; P < .001), which was accompanied by a 6% improvement in vascular inflammation (mean [SD] target-to-background ratio, 1.91 [0.29] vs 1.79 [0.22]; P < .001) (Table and Figure 2). No sex- or race-based interaction was found in vascular inflammation comparison over 1 year.
Table. Characteristics of Psoriasis Cohort at Baseline and 1 Year.
| Parameter | Baseline | 1-y Follow-up | P Valuea |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age, mean (SD), y | 49.7 (12.8) | 50.8 (12.8) | <.001 |
| Men, No. (%) | 68 (59) | 68 (59) | >.99 |
| Hypertension, No. (%) | 29 (25) | 25 (22) | .21 |
| Hyperlipidemia, No. (%) | 58 (50) | 56 (50) | .64 |
| Type 2 diabetes, No. (%) | 10 (9) | 9 (8) | .56 |
| Body mass index, mean (SD)b | 29.0 (5.5) | 29.0 (5.5) | .45 |
| Current smoker, No. (%) | 13 (11) | 11 (10) | .32 |
| Diabetes treatment, No. (%) | 8 (7) | 8 (7) | >.99 |
| Statins, No. (%) | 39 (34) | 38 (34) | .71 |
| Antihypertensive medication use, No. (%) | 23 (20) | 25 (22) | .48 |
| Exercise, No. (%)c | 95 (88) | 82 (79) | .06 |
| Clinical and laboratory values | |||
| Cholesterol, mean (SD), mg/dL | |||
| Total | 182.5 (37.6) | 183 (41.4) | .44 |
| HDL | 55.2 (18.3) | 58.5 (21.8) | .003 |
| LDL | 101.7 (32.2) | 99.2 (35.9) | .20 |
| Triglycerides, median (IQR), mg/dL | 101 (79-137) | 119 (79-162) | .10 |
| C-reactive protein, median (IQR), mg/L | 1.8 (0.7-3.8) | 1.3 (0.6-3.2) | .02 |
| Framingham risk score, median (IQR) | 3 (1-6) | 2 (1-5) | .06 |
| HOMA-IR, median (IQR) | 2.8 (1.7-4.7) | 3.0 (1.8-5.1) | .19 |
| Psoriasis characteristics | |||
| Psoriasis Area and Severity Index score, median (IQR) | 5.2 (3.0-8.9) | 3.6 (2.0-5.7) | <.001 |
| Total body surface area index, median (IQR) | 3.9 (2.4-10.0) | 2.7 (1.0-8.6) | .07 |
| Treatment, No. (%) | |||
| Topical | 77 (69) | 74 (66) | .73 |
| Light | 21 (19) | 17 (15) | .43 |
| Systemic or biologic treatment | 45 (39) | 68 (60) | <.001 |
| Vascular inflammation, mean (SD) | |||
| Target-to-background ratio | 1.91 (0.29) | 1.79 (0.22) | <.001 |
Abbreviations: HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; IQR, interquartile range; LDL, low-density lipoprotein.
SI conversion factors: to convert C-reactive protein to nanomolers per liter, multiply by 9.524; cholesterol to millimoles per liter, multiply by 0.0259; and triglycerides to millimoles per liter, multiply by 0.0113.
P values were calculated by using paired t test or Wilcoxon signed rank test for continuous variables and Pearson χ2 test for categorical variables. P < .05 was deemed significant.
Calculated as weight in kilograms divided by height in meters squared.
Exercise was defined as a categorical variable.
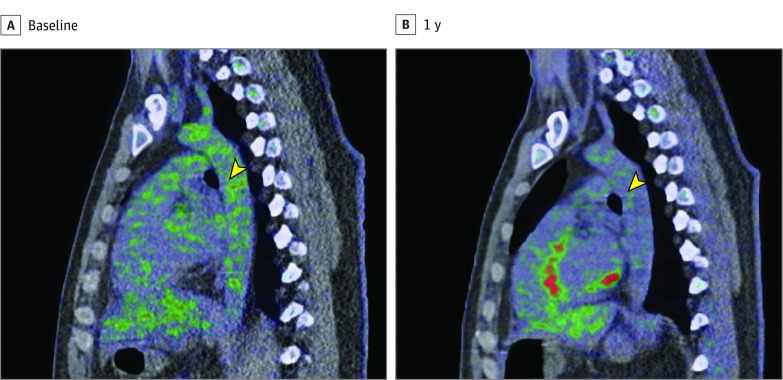
Figure 2. Patient With Improvement in Psoriasis Severity and Aortic Vascular Inflammation.
Representative images from a patient who experienced reduction in both Psoriasis Area and Severity Index score and aortic vascular inflammation. The images show a sagittal section at the level of the midaorta at baseline (A) and 1 year (B). Green represents 18fluorodeoxyglucose tracer uptake in the aorta, which is higher at baseline compared with 1 year of psoriasis treatment (yellow arrowheads).
Association of Psoriasis Skin Inflammation and Vascular Inflammation Beyond Traditional Risk Factors
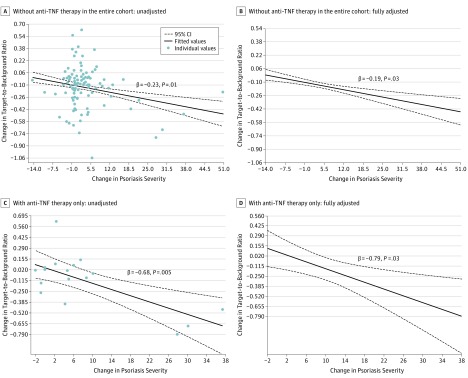
Psoriasis severity was associated with vascular inflammation at baseline (β = 0.20; 95% CI, 0.018-0.379; P = .03). At 1 year, improvement in psoriasis severity was associated with an improvement in vascular inflammation (β = 0.23; 95% CI, 0.051-0.417; P = .01) (Figure 3A), which remained significant beyond traditional cardiovascular risk factors, body mass index, high-sensitivity C-reactive protein, statin use, and use of systemic or biologic psoriasis therapy (β = 0.19; 95% CI, 0.012-0.375; P = .03) (Figure 3B and eTable 1 in the Supplement). Furthermore, sensitivity analyses after removing those taking any form of systemic or biologic therapy found consistent results (β = 0.22; 95% CI, 0.041-0.404; P = .03).
Figure 3. Association Between Change in Psoriasis Severity and Change in Aortic Vascular Inflammation.
Improvement in psoriasis skin disease severity is associated with improvement in vascular inflammation beyond traditional cardiovascular risk factors at 1 year: in all patients with psoriasis in the cohort (n = 115), skin disease improvement was associated with reduction in vascular inflammation in unadjusted (A) and adjusted (B) analyses. Improvement in psoriasis skin disease severity is associated with improvement in vascular inflammation beyond traditional cardiovascular risk factors at 1 year: in a subcohort of biologically naive patients with psoriasis, who were initiated with anti–tumor necrosis factor (TNF) therapy (n = 17), skin disease improvement was associated with reduction in vascular inflammation in unadjusted (C) and adjusted (D) analyses. Change in psoriasis severity = psoriasis severity at baseline − psoriasis severity at 1 year. Change in target-to-background ratio = target-to-background ratio at 1 year − target-to-background ratio at baseline.
Finally, there was a significant 11% reduction in vascular inflammation for those with greater than 75% reduction in psoriasis severity (mean [SD], 2.02 [0.36] to 1.79 [0.20]; P < .003), which was associated with improvement in psoriasis severity beyond adjustment for traditional risk factors (β = 0.57; 95% CI, 0.100-1.049; P = .04).
Anti-TNF Therapy, Psoriasis Severity, and Aortic Vascular Inflammation Over 1 Year in Severe Psoriasis
In patients with severe psoriasis who were eligible for biological therapy, anti-TNF therapy was initiated and patients were followed up for 1 year (n = 17). Patients were middle-aged (mean [SD] age, 48.6 [14.6] years), male predominant (59%), and at low cardiovascular risk by Framingham risk score (median, 3; IQR, 1-6), and they had characteristics similar to the entire sample (eTable 2 in the Supplement). At 1 year, the cohort had a reduction in psoriasis severity by 67% (median, 9; IQR, 6-15, to median, 3; IQR, 2-4; P = .002). This reduction in psoriasis severity was associated with a reduction in vascular inflammation of 6% (mean [SD], 1.90 [0.35] vs 1.78 [0.22]; P = .04). This association between psoriasis severity improvement and vascular inflammation improvement (β = 0.68; 95% CI, 0.323-1.056; P = .005) persisted beyond traditional risk factors (β = 0.79; 95% CI, 0.269-1.311; P = .03) (Figure 3C and D and eTable 3 in the Supplement).
Discussion
Using a longitudinal case-cohort study design, we demonstrated that (1) when psoriasis skin disease severity improved over 1 year, there was a reduction in vascular inflammation independent of cardiovascular risk factors and (2) initiation of TNF inhibitor therapy in patients with severe treatment-naive psoriasis led to successful clearance of severe skin disease associated with a significant improvement in vascular inflammation.
Atherosclerosis is an immune-mediated lipid-associated process involving multiple inflammatory cell lines. Multiple studies have demonstrated an increased risk for atherosclerotic cardiovascular disease in human psoriasis, which is a systemic inflammatory disease linked with cardiometabolic dysfunction at baseline including insulin resistance, impaired high-density lipoprotein function, and obesity. Psoriasis also has increased subclinical cardiovascular disease prevalence, as evidenced by higher coronary artery calcium, increased vascular inflammation by 18FDG PET/CT, and an elevated burden of coronary artery disease by coronary CT angiography.
Prior studies have shown that systemic or biologic treatment of psoriasis was associated with reduced vascular disease in psoriasis, demonstrating an important role for inflammation in cardiovascular disease. Furthermore, small studies using novel vascular imaging modalities showed that treatment of psoriasis with anti-TNF as well as anti–interleukin 12/23 therapies reduced vascular inflammation and reduced progression of coronary artery disease as assessed by coronary artery calcium score. Indeed, our findings—that reduction in skin disease severity, the target organ associated with inflammation in psoriasis, is associated with reduction in vascular inflammation—further support this concept that quelling remote target organ inflammation may improve vascular diseases.
Furthermore, our findings that initiation of biologic therapy with anti-TNF agents leads to pronounced improvement in vascular inflammation beyond traditional cardiovascular risk factors denote that modifying the magnitude of remote inflammation imparts benefits toward overall cardiovascular risk by direct as well as indirect effects. Statins are currently the most validated cardiovascular risk-modifying agents and have been shown to reduce vascular inflammation in dose-dependent fashion. In this study, we demonstrated that approximately 33% improvement in psoriasis severity was associated with a 6% decrease in vascular inflammation in the entire cohort. This reduction is, to a similar extent, as seen following low-dose statin therapy, which suggests that reducing remote skin disease severity may have short-term effects by reducing vascular inflammation comparable with low-dose statin therapy.
Limitations
Our study has certain limitations. This was an observational study; therefore, it is subject to potential for confounding including residual confounding secondary to adjustment for cohort-level changes. We did not have uniform therapy throughout the cohort. Moreover, the use of anti-TNF agents was open-label, nonrandomized, and in a small sample. Finally, we did not examine cardiovascular events but instead used vascular inflammation to understand modulation on cardiovascular disease risk. While these data strengthen the hypothesis of inflammation reduction leading to cardiovascular disease mitigation, they do not alone prove causality. Hence, future studies should attempt to characterize the shared mechanistic links involved in psoriasis and atherosclerosis, and they should try to assess how biologic therapies may modulate them.
To overcome our observational study design, an ongoing randomized clinical trial will examine the effect of anti-TNF treatment with adalimumab on both skin disease and vascular inflammation at 12 weeks and 52 weeks (the Vascular Inflammation in Psoriasis–Extension Trial, NCT01866592) compared with placebo.
Conclusions
We found that improvement in target organ inflammation (eg, the skin) modulated vascular inflammation. Furthermore, initiating anti-TNF treatment had favorable impact on both skin clearance and vascular inflammation. Collectively, these findings suggest that alleviating remote skin inflammation may have positive effects on vascular inflammation at 1 year, similar to the effect of low-dose statin therapy.
eAppendix. Methods.
eReferences
eTable 1. Relationship Between Change in Vascular Inflammation and Change in Psoriasis Severity at 1 Year.
eTable 2. Characteristics of Psoriasis Cohort at 1 Year Initiated on Anti-TNF Therapy.
eTable 3. Relationship Between Change in Vascular Inflammation and Change in Psoriasis Severity at 1 Year Initiated on Anti-TNF Therapy.
References
- 1.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735-1741. [DOI] [PubMed] [Google Scholar]
- 2.Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129(10):2411-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahlehoff O, Gislason GH, Lindhardsen J, et al. Prognosis following first-time myocardial infarction in patients with psoriasis: a Danish nationwide cohort study. J Intern Med. 2011;270(3):237-244. [DOI] [PubMed] [Google Scholar]
- 4.Naik HB, Natarajan B, Stansky E, et al. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol. 2015;35(12):2667-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figueroa AL, Abdelbaky A, Truong QA, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013;6(12):1250-1259. [DOI] [PubMed] [Google Scholar]
- 6.Duivenvoorden R, Mani V, Woodward M, et al. Relationship of serum inflammatory biomarkers with plaque inflammation assessed by FDG PET/CT: the dal-PLAQUE Study. JACC Cardiovasc Imaging. 2013;6(10):1087-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figueroa AL, Subramanian SS, Cury RC, et al. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ Cardiovasc Imaging. 2012;5(1):69-77. [DOI] [PubMed] [Google Scholar]
- 8.Tawakol A, Lo J, Zanni MV, et al. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Defic Syndr. 2014;66(2):164-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tawakol A, Fayad ZA, Mogg R, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62(10):909-917. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, On YK, Lee EJ, Choi JY, Kim BT, Lee KH. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med. 2008;49(8):1277-1282. [DOI] [PubMed] [Google Scholar]
- 11.Carlin CS, Feldman SR, Krueger JG, Menter A, Krueger GGA. A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol. 2004;50(6):859-866. [DOI] [PubMed] [Google Scholar]
- 12.Mehta NN, Li R, Krishnamoorthy P, et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis. 2012;224(1):218-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansouri B, Kivelevitch D, Natarajan B, et al. Comparison of coronary artery calcium scores between patients with psoriasis and type 2 diabetes. JAMA Dermatol. 2016;152(11):1244-1253. [DOI] [PubMed] [Google Scholar]
- 15.Hjuler KF, Bøttcher M, Vestergaard C, Bøtker HE, Iversen L, Kragballe K. Association between changes in coronary artery disease progression and treatment with biologic agents for severe psoriasis. JAMA Dermatol. 2016;152(10):1114-1121. [DOI] [PubMed] [Google Scholar]
- 16.Bissonnette R, Tardif JC, Harel F, Pressacco J, Bolduc C, Guertin MC. Effects of the tumor necrosis factor-α antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: results of a randomized controlled trial. Circ Cardiovasc Imaging. 2013;6(1):83-90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Methods.
eReferences
eTable 1. Relationship Between Change in Vascular Inflammation and Change in Psoriasis Severity at 1 Year.
eTable 2. Characteristics of Psoriasis Cohort at 1 Year Initiated on Anti-TNF Therapy.
eTable 3. Relationship Between Change in Vascular Inflammation and Change in Psoriasis Severity at 1 Year Initiated on Anti-TNF Therapy.