Abstract
Pyruvate and acetyl-coenzyme A, located at the interface between glycolysis and TCA cycle, are important intermediates in yeast metabolism and key precursors for industrially relevant products. Rational engineering of their supply requires knowledge of compensatory reactions that replace predominant pathways when these are inactivated. This study investigates effects of individual and combined mutations that inactivate the mitochondrial pyruvate-dehydrogenase (PDH) complex, extramitochondrial citrate synthase (Cit2) and mitochondrial CoA-transferase (Ach1) in Saccharomyces cerevisiae. Additionally, strains with a constitutively expressed carnitine shuttle were constructed and analyzed. A predominant role of the PDH complex in linking glycolysis and TCA cycle in glucose-grown batch cultures could be functionally replaced by the combined activity of the cytosolic PDH bypass and Cit2. Strongly impaired growth and a high incidence of respiratory deficiency in pda1Δ ach1Δ strains showed that synthesis of intramitochondrial acetyl-CoA as a metabolic precursor requires activity of either the PDH complex or Ach1. Constitutive overexpression of AGP2, HNM1, YAT2, YAT1, CRC1 and CAT2 enabled the carnitine shuttle to efficiently link glycolysis and TCA cycle in l-carnitine-supplemented, glucose-grown batch cultures. Strains in which all known reactions at the glycolysis-TCA cycle interface were inactivated still grew slowly on glucose, indicating additional flexibility at this key metabolic junction.
Keywords: Saccharomyces cerevisiae, glycolysis-TCA cycle interface, PDH complex, Ach1, Cit2, carnitine shuttle
The glycolysis-TCA cycle interface in Saccharomyces cerevisiae was studied by analyzing the impact of genetic modifications affecting PDH complex, CoA-transferase (Ach1), extramitochondrial citrate synthase (Cit2) and carnitine shuttle.
INTRODUCTION
In many organisms, the Embden–Meyerhof variant of glycolysis catalyzes oxidation of glucose to pyruvate. The subsequent oxidative decarboxylation of pyruvate yields acetyl coenzyme A (acetyl-CoA), which can be fully oxidized to carbon dioxide in the tricarboxylic acid (TCA) cycle. In addition to their roles as dissimilatory pathways, glycolysis and TCA cycle provide key biosynthetic precursors. Both of these ‘textbook’ pathways have been intensively studied but, even in the intensively studied eukaryotic model organism Saccharomyces cerevisiae, their interface is less well understood.
Cytosolic pyruvate can be imported into the mitochondrial matrix via the transporters Mpc1, 2 and/or 3 (Bricker et al. 2012; Herzig et al. 2012). Direct oxidative decarboxylation by the mitochondrial pyruvate-dehydrogenase (PDH) complex yields acetyl-CoA in the mitochondrial matrix (pyruvate + NAD+ + CoA → acetyl-CoAmit + NADH + H+ + CO2). Null mutants in PDA1, which encodes the essential E1α subunit of the PDH complex, show a reduced growth rate in aerobic batch cultures on glucose synthetic media (Wenzel et al. 1992). Moreover, they exhibit an increased frequency of respiratory-deficient mutants and loss of mitochondrial DNA (Wenzel et al. 1992). Alternatively, acetyl-CoA can be formed in the cytosol of S. cerevisiae via a reaction sequence known as the PDH bypass (Holzer and Goedde 1957; Pronk, Steensma and Van Dijken 1996), consisting of pyruvate decarboxylase (pyruvate → acetaldehyde + CO2), acetaldehyde dehydrogenase (acetaldehyde + NAD+ + H2O → acetate + NADH + H+) and acetyl-CoA synthetase (acetate + ATP + CoA ⇌ acetyl-CoAcyt + AMP + PPi). Glucose-grown cultures of S. cerevisiae, which unlike many other eukaryotes does not contain ATP-citrate lyase (Boulton and Ratledge 1981), depend on this route for synthesis of acetyl-CoA in the nucleocytosolic compartment, where it acts as a precursor for synthesis of lipids, N-acetylglucosamine, sterols and lysine (Oura 1972; Flikweert et al. 1996, 1999) and as acetyl donor for protein acetylation (Takahashi et al. 2006; Henriksen et al. 2012). An observed 13% reduction of the biomass yield on glucose of pda1Δ mutants in aerobic, glucose-limited chemostat cultures was quantitatively consistent with rerouting of respiratory pyruvate metabolism via the cytosolic, ATP-consuming PDH bypass (Pronk et al. 1994).
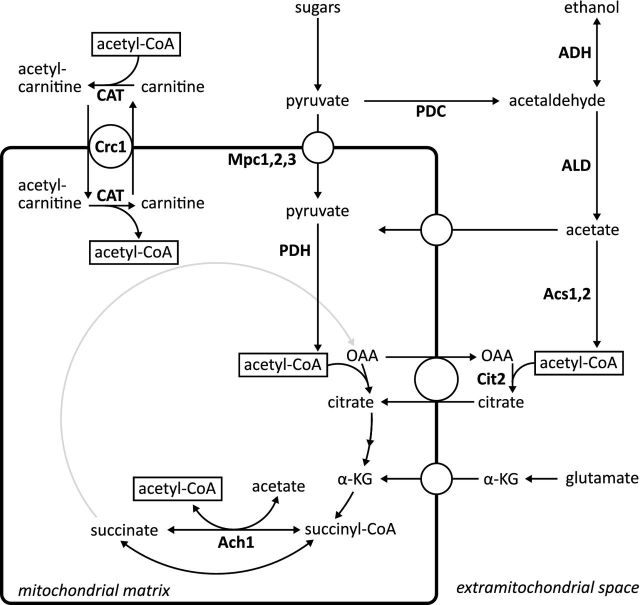
The nucleocytosolic localization of acetyl-CoA synthetase in S. cerevisiae (Van den Berg and Steensma 1995; Pronk, Steensma and Van Dijken 1996; De Jong-Gubbels et al. 1997; Takahashi et al. 2006) implies that the PDH bypass cannot directly generate intramitochondrial acetyl-CoA. Two mechanisms might link glycolysis and the TCA cycle in PDH-negative S. cerevisiae mutants (Fig. 1). First, cytosolic acetyl-CoA might be converted to citrate via the extramitochondrial citrate synthase isoenzyme Cit2 (Fig. 1; Kispal et al. 1989; Vélot et al. 1999), followed by uptake of citrate via the mitochondrial transporter Ctp1 (Kaplan et al. 1995). Alternatively, a transport or shuttle mechanism might catalyze translocation of cytosolic acetyl-CoA into the mitochondria.
Figure 1.
Mechanisms important for the provision of acetyl moieties in S. cerevisiae mitochondria. In glycolysis glucose is converted to pyruvate, which can be transported into the mitochondria via the mitochondrial pyruvate carriers Mpc1, Mpc2 and Mpc3, followed by its conversion to acetyl-CoA via the pyruvate dehydrogenase (PDH) complex. Alternatively, pyruvate can be converted to cytosolic acetyl-CoA via the pyruvate dehydrogenase bypass. Cytosolic acetyl-CoA can be condensed with oxaloacetate via Cit2 to form citrate, which can be exchanged with, for example, mitochondrial oxaloacetate, and hence fuel the TCA cycle. Acetate from the cytosol can also be activated to mitochondrial acetyl-CoA via Ach1 by transfer of the CoA group from succinyl-CoA to acetate. Only when cells are supplemented with l-carnitine, the carnitine shuttle can transport cytosolic acetyl units into the mitochondria. Abbreviations: α-KG, α-ketoglutarate; Acs1, Acs2, acetyl-CoA synthetase; Ach1, CoA transferase; ADH, alcohol dehydrogenase; ALD, acetaldehyde dehydrogenase; CAT, carnitine acetyltransferase; Cit2, citrate synthase; Crc1, acetyl-carnitine translocase; Mpc1, Mpc2, Mpc3, mitochondrial pyruvate carrier; OAA, oxaloacetate; PDC, pyruvate decarboxylase; PDH, pyruvate dehydrogenase complex.
In many eukaryotes, the carnitine shuttle is responsible for translocation of acetyl moieties across organellar membranes. The carnitine shuttle involves cytosolic and organellar acetyl-CoA:carnitine O-acetyltransferases (acetyl-CoA + l-carnitine ⇌ acetyl-l-carnitine + CoA) (Bieber 1988). In S. cerevisiae, acetyl-l-carnitine can be transported across the mitochondrial membrane by the mitochondrial acetyl-carnitine translocase Crc1 (Kohlhaw and Tan-Wilson 1977; Palmieri et al. 1999; Van Roermund et al. 1999; Franken et al. 2008). The three acetyl-carnitine transferases in S. cerevisiae have different subcellular localizations: Cat2 is peroxisomal and mitochondrial (Elgersma et al. 1995), Yat1 is localized to the outer mitochondrial membrane (Schmalix and Bandlow 1993) and Yat2 is reported to be cytosolic (Swiegers et al. 2001; Huh et al. 2003; Koh et al. 2015). Since S. cerevisiae cannot synthesize l-carnitine de novo, activity of the carnitine shuttle is strictly dependent on uptake of exogenous l-carnitine via the Hnm1 transporter, whose expression is regulated by Agp2 (Van Roermund et al. 1995, 1999; Swiegers et al. 2001; Aouida et al. 2013). Strong transcriptional repression of the carnitine shuttle structural genes by glucose (Kispal et al. 1991; Schmalix and Bandlow 1993; Elgersma et al. 1995) would seem to prevent an important contribution in glucose-grown batch cultures, even when l-carnitine is present.
At least two metabolic processes in S. cerevisiae are strictly dependent on intramitochondrial acetyl-CoA. Although the bulk of lipid synthesis in S. cerevisiae occurs in the cytosol, long-chain fatty acids needed for lipoic acid biosynthesis are exclusively synthesized in the mitochondrial matrix (Brody et al. 1997). Additionally, arginine biosynthesis requires catalytic amounts of intramitochondrial acetyl-CoA (Jauniaux, Urrestarazu and Wlame 1978). The ability of pda1Δ mutants to grow on glucose in synthetic media lacking l-carnitine indicates that, in addition to the PDH complex, S. cerevisiae must contain at least one other mechanism for mitochondrial acetyl-CoA provision. Ach1, a key candidate for this role, was first described as an acetyl-CoA hydrolase (Lee, Lin and Smith 1990; Buu, Chen and Lee 2003). Later, it was demonstrated to have in fact a much higher in vitro activity for the transfer of the CoA group from various acyl-CoA substrates to other organic acids (Fleck and Brock 2009). One such reaction involves the transfer of the CoA-group from succinyl-CoA to acetate, forming acetyl-CoA (succinyl-CoA + acetate ⇌ acetyl-CoA + succinate). This Ach1 catalyzed reaction could generate mitochondrial acetyl-CoA from acetate, derived from the PDH bypass, and mitochondrial succinyl-CoA, derived from the TCA cycle (via the α-ketoglutarate dehydrogenase complex) or via ATP-dependent activation of succinate by succinyl-CoA ligase (succinate + ATP + CoA ⇌ succinyl-CoA + ADP + Pi). Although a role of Ach1 in mitochondrial acetyl-CoA synthesis has been shown before (Fleck and Brock 2009; Orlandi, Casatta and Vai 2012; Eisenberg et al. 2014), its significance in glucose-grown batch cultures of wild-type and mutant S. cerevisiae strains remains to be elucidated.
The aim of the present study is to assess the relative importance of different alternative reactions active at the interface of glycolysis and TCA cycle in S. cerevisiae strains that lack a functional PDH complex. To this end, we reinvestigated growth of pda1Δ S. cerevisiae on glucose in synthetic medium and analyzed the effects of additional null mutations in ACH1 and CIT2 on growth rate, respiratory competence and metabolite formation. Moreover, we investigate whether constitutive expression of carnitine-shuttle genes enables the carnitine shuttle to function as an effective link between glycolysis and TCA cycle in glucose-grown batch cultures supplemented with l-carnitine.
MATERIALS AND METHODS
Growth media
Yeast-extract/peptone (YP) medium was prepared with demineralized water, 10 g ⋅ L−1 Bacto yeast extract (BD, Franklin Lakes, NJ, USA) and 20 g ⋅ L−1 Bacto peptone (BD). Synthetic medium with ammonium as nitrogen source (SM-ammonium) was prepared according to Verduyn et al. (1992). Synthetic medium with other nitrogen sources was prepared similarly, but with 38 mM K2SO4 instead of (NH4)2SO4 and with 38 mM urea (SM-urea), 76 mM l-glutamate (SM-glutamate). These modifications were made to maintain equivalent concentrations of nitrogen and sulfate relative to the original medium description. Media with these alternative nitrogen sources were sterilized with 0.2 μm bottle-top filters (Thermo Fisher Scientific, Waltham, MA, USA). Solid media were prepared by addition of 20 g ⋅ L−1 agar (BD) prior to heat sterilization of the medium for 20 min at 121°C.
Strains, growth conditions and storage
The S. cerevisiae strains used in this study (Table 1) share the CEN.PK genetic background (Entian and Kötter 2007; Nijkamp et al. 2012). Shake-flask cultures were grown at 30°C in 500 mL flasks containing 100 mL SM-ammonium with 20 g⋅L−1 glucose, in an Innova incubator shaker (New Brunswick Scientific, Edison, NJ, USA) set at 200 rpm. Stock cultures were grown in YP medium with 20 g⋅L−1 glucose. For strains IMK640, IMK641, IMX710 and IMX744, 2% (v/v) ethanol was used as carbon source to prevent loss of respiratory competence. Frozen stocks were prepared by adding 30% (v/v) glycerol to exponentially growing cultures and stored in 1 mL aliquots at −80°C. To induce sporulation, strains were pre-grown in YP medium with 10 g⋅L−1 potassium acetate, followed by incubation in sporulation medium (demineralized water, 20 g⋅L−1 potassium acetate, pH 7.0) (Fast 1973).
Table 1.
Saccharomyces cerevisiae strains used in this study and their relevant genotypes.
| Name | Relevant genotype | Parental strain(s) | Origin |
|---|---|---|---|
| CEN.PK113-7D | MATa | P. Kötter | |
| IMK439 | MATα ura3Δ::loxP-kanMX-loxP | CEN.PK113-1A | González-Ramos et al. (2013) |
| IMX585 | MATa can1Δ::cas9-natNT2 | CEN.PK113-7D | Mans et al. (2015) |
| CEN.PK541-1A | MATa pda1Δ::loxP-kanMX-loxP | This study | |
| CEN.PK542-1A | MATα cit2Δ::loxP-kanMX-loxP | This study | |
| CEN.PK544-4D | MATa pda1Δ::loxP-kanMX-loxP cit2Δ::loxP-kanMX-loxP | This study | |
| IMK627 | MATa ach1Δ::loxP-natNT2-loxP | CEN.PK113-7D | This study |
| IMK629 | MATα cit2Δ::loxP-kanMX-loxP ach1Δ::loxP-natNT2-loxP | CEN.PK542-1A | This study |
| IMK640 | MATa pda1Δ::loxP-kanMX-loxP ach1Δ::loxP-natNT2-loxP | CEN.PK541-1A | This study |
| IMK641 | MATa pda1Δ::loxP-kanMX-loxP cit2Δ::loxP-kanMX-loxP ach1Δ::loxP-natNT2-loxP | CEN.PK544-4D | This study |
| IMX710 | MATa can1Δ::cas9-natNT2 pda1Δ ach1Δ | IMX585 | This study |
| IMX744 | MATa can1Δ::cas9-natNT2 pda1Δ ach1Δ sga1Δ::{CARN}a | IMX710 | This study |
| IMD015 | MATα ura3Δ::loxP-kanMX-loxP × MATa URA3 can1Δ::cas9-natNT2 pda1Δ ach1Δ sga1Δ::{CARN} | IMK439 × IMX744 | This study |
| IMX868 | MATα can1Δ::cas9-natNT2 URA3 PDA1 ACH1 sga1Δ::{CARN} | IMD015 | This study |
a{CARN}, pTDH3-AGP2-tAGP2 pPGK1-HNM1-tHNM1 pADH1-YAT2-tYAT2 pPGI1-YAT1-tYAT1 pTPI1-CRC1-tCRC1 pTEF1-CAT2-tCAT2.
Strain and plasmid construction
Saccharomyces cerevisiae strains were transformed according to Gietz & Woods (2002). Mutants were selected on solid YP medium, supplemented with 20 g⋅L−1 glucose and the appropriate antibiotic, 200 mg⋅L−1 G418 (InvivoGen, San Diego, CA, USA) or 100 mg⋅L−1 nourseothricin (Jena Bioscience, Jena, Germany). SM containing 10 mM acetamide as the sole nitrogen source (SM-acetamide) was used for selection of the amdSYM marker (Solis-Escalante et al. 2013). Deletion cassettes for PDA1 and CIT2 were constructed with a PCR-based method using pUG6 as template (Güldener et al. 1996), using primer pairs 9087 & 9088 and 9089 & 9090, respectively. Thus amplified kanMX cassettes were used to replace the target genes in the prototrophic diploid strain CEN.PK122 (MATa/MATα). Transformants were verified for correct gene replacement by diagnostic PCR (Table S1, Supporting Information). After sporulation and tetrad dissection, the haploid deletion strains CEN.PK541-1A (MATa pda1Δ) and CEN.PK542-1A (MATα cit2Δ) were obtained. To obtain a strain with both CIT2 and PDA1 deleted, strains CEN.PK541-1A and CEN.PK542-1A were crossed. After tetrad dissection, spores showing the non-parental ditype for the kanMX marker were analyzed by diagnostic PCR to confirm correct deletion of both genes, resulting in strain CEN.PK544-4D (MATa pda1Δ cit2Δ). Deletion of ACH1 in strains CEN.PK113-7D, CEN.PK542-1A, CEN.PK541-1A and CEN.PK544-4D was achieved by integration of the natNT2 marker into its locus. The natNT2 cassette was PCR amplified from pUG-natNT2 (De Kok et al. 2012) with primer pair 3636 & 3637. Correct deletion was verified by colony PCR (Lõoke, Kristjuhan and Kristjuhan 2011) using the primers shown in Table S1 (Supporting Information), resulting in strains IMK627, IMK629, IMK640 and IMK641, respectively.
IMX710 was constructed by removing PDA1 and ACH1 in strain IMX585 using the CRISPR/Cas9 system by introduction of a plasmid containing two guideRNA (gRNA) cassettes, targeting PDA1 and ACH1. The plasmid was constructed as described before (Mans et al. 2015) with primers 5794 & 6159 to incorporate the appropriate target sites and with pROS13 as a backbone, resulting in plasmid pUDE340. Strain IMX585 was transformed with plasmid pUDE340 together with the repair fragments that were obtained by annealing oligonucleotides 6157 & 6158 (PDA1 deletion) and 6160 & 6161 (ACH1 deletion). After confirmation of the gene deletions using diagnostic PCR (Table S1, Supporting Information), plasmid pUDE340 was removed as described before (Mans et al. 2015), resulting in strain IMX710.
Strain IMX744 was obtained by placing the genes encoding the carnitine shuttle proteins (HNM1, AGP2, CRC1, YAT1, YAT2 and CAT2), under control of strong constitutive promoters and integrating them into the SGA1 locus. SGA1 encodes a glucoamylase that is not expressed during vegetative growth of S. cerevisiae (Yamashita and Fukui 1985). Its inactivation was therefore considered to be neutral under the conditions employed in this study. For this purpose, the constitutive promoters pTPI1, pTDH3, pADH1, pTEF1, pPGK1 and pPGI1 were amplified by PCR from plasmids pUD301–pUD306 (Kozak et al. 2014b) (Table S2 and S3, Supporting Information). The ORFs of the six genes involved in the carnitine shuttle, together with their terminator sequences, were amplified from the CEN.PK113-7D genome and fused to the constitutive promoters with fusion PCR (Yon and Fried 1989) (See Table S3, Supporting Information, for primers and templates). Gene cassettes were ligated in pJET1.2 (Life Technologies, Carlsbad, CA, USA) and the resulting plasmids (pUD366–pUD371; Table S2, Supporting Information) were verified by Sanger sequencing (BaseClear BV, Leiden, The Netherlands). The plasmid with the gRNA cassette targeting SGA1 (pUDR119) was constructed by Gibson Assembly of the pMEL11 backbone (Mans et al. 2015), obtained by PCR with pMEL11 as a template and using primers 5792 & 5980, and the gRNA cassette, obtained by PCR with primers 5979 & 7023 using the same template. Strain IMX710 was transformed with plasmid pUDR119 and the six gene cassettes, amplified from plasmids pUD366–pUDE371 with PCR using primers as indicated in Table S3 (Supporting Information). The six gene cassettes were concatenated via in vivo homologous recombination, mediated by 60 bp overlapping sequences, and after a Cas9-induced double-strand break, integrated into the SGA1 locus (see Data 1, Supporting Information for sequence in GenBank format). After confirmation of correct integration by diagnostic PCR (for primers see Table S1, Supporting Information), pUDR119 was removed as described before (Mans et al. 2015), resulting in strain IMX744 (MATa can1Δ::cas9-natNT2 pda1Δ ach1Δ sga1Δ::{pTDH3-AGP2-tAGP2 pPGK1-HNM1-tHNM1 pADH1-YAT2-tYAT2 pPGI1-YAT1-tYAT1 pTPI1-CRC1-tCRC1 pTEF1-CAT2-tCAT2}). The set of genes that are involved in the carnitine shuttle and introduced in the SGA1 locus are further referred to as {CARN}.
IMX868 was obtained by crossing, sporulation and spore dissection. Strain IMX744 (MATa) was crossed with IMK439 (MATα) by selecting for diploids on YP medium with 20 g·L−1 glucose, G418 and nourseothricin. The resulting diploid IMD015 was sporulated and the asci were dissected on YP with 20 g·L−1 glucose using a micromanipulator (Singer Instruments, Watchet, UK). One spore with the desired genotype (PDA1 ACH1 URA3 sga1Δ::{CARN}) was stocked as IMX868.
Molecular biology techniques
PCR amplification with Phusion® Hot Start II High Fidelity Polymerase (Thermo Fisher Scientific) was performed according to the manufacturer's instructions using HPLC- or PAGE-purified oligonucleotide primers (Sigma-Aldrich). Diagnostic PCR was done via colony PCR on randomly picked yeast colonies, using DreamTaq (Thermo Fisher Scientific) and desalted primers (Sigma-Aldrich). DNA fragments obtained by PCR were separated by gel electrophoresis on 1% (w/v) agarose gels (Thermo Fisher Scientific) in TAE buffer (Thermo Fisher Scientific) at 100 V for 30 min. Alternatively, fragments were purified using the GenElute PCR Clean-Up Kit (Sigma-Aldrich). Plasmids were isolated from Escherichia coli with Sigma GenElute Plasmid kit (Sigma-Aldrich) according to the supplier's manual. Yeast genomic DNA was isolated using a YeaStar Genomic DNA kit (Zymo Research) or using an SDS/LiAc-based lysis protocol (Lõoke, Kristjuhan and Kristjuhan 2011). E. coli DH5α (18258–012, Life Technologies) was used for chemical transformation or for electroporation. Chemical transformation was done according to Inoue, Nojima and Okayama (1990). Electrocompetent DH5α cells were prepared according to Bio-Rad's protocol, with the exception that during the preparation of competent cells, E. coli was grown in LB medium without NaCl. Electroporation was done in a 2 mm cuvette (165–2086, Bio-Rad, Hercules, CA, USA) using a Gene Pulser Xcell Electroporation System (Bio-Rad), following the manufacturer's protocol.
Growth studies on urea and l-glutamate as nitrogen source
Growth studies were conducted at 30°C in 500-mL flasks. To prevent nutrient carry over from stock cultures, strains were pre-grown in two sequential shake flasks in which biomass formation was not limited by the amount of carbon source but by the nitrogen source. To this end, 200-μL cell suspension from frozen stocks were inoculated in 100 mL SM-glutamate with a decreased initial l-glutamate concentration of 1.5 mM and with 20 g⋅L−1 glucose. After reaching stationary phase, biomass was centrifuged (4°C, 5 min at 3000 g). The pellet was washed twice with demineralized water, resuspended in demineralized water and used to inoculate a second shake flask with the same medium. After reaching stationary phase, the same wash procedure was performed and a final set of shake flasks, containing either 100 mL SM-urea or SM-glutamate and 20 g⋅L−1 glucose, was inoculated for growth rate determination,. Where indicated, l-carnitine (Sigma-Aldrich) was added at a final concentration of 0.4 g⋅L−1. Optical density at 660 nm was measured at regular time intervals with a Libra S11 spectrophotometer (Biochrom, Cambrige, UK).
Batch and chemostat cultures in bioreactors
Controlled batch and chemostat cultures were grown at 30°C in 2-L bioreactors (Applikon, Schiedam, The Netherlands) with working volumes of 1 L. Pre-cultures for batch cultivation were grown in shake flasks containing 100 mL SM-urea and 20 g⋅L−1 glucose. Pre-cultures for chemostat experiments were grown in shake flasks with 100 mL SM-glutamate and 20 g⋅L−1 glucose. Before inoculation of the bioreactors, pre-cultures were washed once with demineralized water. During the batch phase in bioreactors, cells were grown in SM-ammonium (Verduyn et al. 1992) with 20 g⋅L−1 glucose and 0.3 g⋅L−1 antifoam Pluronic PE 6100 (BASF, Ludwigshafen, Germany). When a rapid decrease in CO2 production indicated glucose depletion, continuous cultivation was initiated at a dilution rate of 0.05 h−1. During this chemostat phase, cells were grown on SM-ammonium with 7.5 g⋅L−1 glucose and 0.15 g⋅L−1 antifoam Pluronic PE 6100. Culture pH was maintained at 5.0 by automatic addition of 2 M KOH. Where indicated, 0.04 g⋅L−1l-carnitine was added to a sterilized bioreactor or medium vessel from a filter-sterilized stock of 40 g⋅L−1. To ensure fully aerobic conditions, the bioreactors were sparged with 500 mL⋅min−1 air and stirred at 800 rpm.
Analytical methods and calculations
Chemostat steady-state samples were taken between 7 and 12 volume changes after inoculation. Chemostats with CEN.PK113-7D were assumed to be in steady state when, after at least five volume changes, carbon dioxide production rates changed by less than by 4% over two volume changes. Due to selective pressure in the chemostats, IMK640 did not reach this requirement and was sampled after 7–12 volume changes. Dry weight measurements, HPLC analysis of supernatants and off-gas analysis were performed as described previously (De Kok et al. 2011). Biomass-specific production rates of ethanol were corrected for evaporation as described previously (Guadalupe Medina et al. 2010). Samples for analysis of extracellular metabolite concentrations (e.g. residual glucose) were taken with the stainless-steel-bead rapid-quenching method (Mashego et al. 2003). HPLC quantification of acetaldehyde measurements were performed as described previously (Bekers, Heijnen and Van Gulik 2015) with some modifications (Kozak et al. 2014a).
For aerobic batch cultures, maximum specific growth rates (μmax) in the glucose phase were calculated via linear regression of the natural logarithm of at least five OD660 measurements. Biomass yield on substrate (YX/S in g dry weight⋅(mmol glucose)−1) and product yields on substrate (Yi/S in mol⋅(mol glucose)−1) were calculated via linear regression on at least three experimental data points, with an interval of at least 2 h. Maximum biomass-specific glucose consumption rates (qSmax) were calculated by dividing μmax by YX/S and maximum biomass specific production rates were calculated by multiplying the ratio of produced compound over produced biomass by μmax, based on the assumption that growth stoichiometries remained constant during the exponential growth phase.
Determination of accumulation of respiratory deficient mutants
Shake flasks with YP and 2% (v/v) ethanol were inoculated from frozen stocks. Stationary-phase cultures were used to inoculate new 100-mL shake flasks, with 20 mL SM-glutamate and 20 g⋅L−1 glucose at an estimated initial OD660 of 0.001. After 12 generations (based on OD660 measurements), ∼100 cells per plate were applied on solid YP medium containing 20 g⋅L−1 glucose. Colonies were replica plated on YP with 2% ethanol and YP with 20 g⋅L−1 glucose. The number of colonies on each medium was counted and the fraction of respiratory-deficient mutants was estimated from the fraction of the colonies that grew on YP-glucose medium but not on YP-ethanol medium. Two independent plating experiments were performed for each strain, with 10 plates per experiment.
Carnitine acetyltransferase enzyme assay
Culture samples (corresponding to ca. 62.5 mg dry weight), harvested from exponentially growing shake flasks cultures on SM-ammonium with 20 g⋅L−1 glucose, were washed, stored and prepared as described previously (Postma et al. 1989). Cell extracts were prepared with a FastPrep-24 machine (M.P. Biomedicals, Irvine, CA, USA) using four bursts of 20 s at a speed of 6.0 m⋅s−1 with 30 s cooling intervals at 0°C as described before (De Kok et al. 2011). After removal of cells and debris by centrifugation (4°C, 20 min at 48 000 g), the supernatant was used for enzyme assays. Carnitine acetyltransferase activity was measured at 30°C on a Hitachi model U-3010 spectrophotometer (Sysmex, Norderstedt, Germany) by monitoring absorbance at 412 nm, which is proportional to the amount of free CoA (Fritz et al. 1963). The reaction mixture, with a final volume of 1 mL, contained 100 mM Tris-HCl (pH 8), 0.5 mM acetyl-CoA, 0.1 mM DTNB and cell extract. The reaction was started by adding 40 μL of 1 M l-carnitine solution, to a final concentration of 40 mM. Enzyme activities were calculated using Beer's law with an extinction coefficient (ɛ) for TNB2− of 14.15 mM−1⋅cm−1 (Riddles, Blakeley and Zerner 1983). To determine the quality of the cell extracts, and thereby to eliminate poor extract quality as the cause of the absence of carnitine acetyltransferase activity in some cultures, activity of glucose-6-phosphate dehydrogenase was determined as described previously (Postma et al. 1989). Reaction rates were proportional to the amounts of cell extract added. Enzyme activities were measured in cell extracts from two independently grown shake flask cultures. Protein concentrations in cell extracts were determined with the Lowry method (Lowry et al. 1951).
RESULTS
Determination of the specific growth rates of pda1Δ, cit2Δ and ach1Δ mutants
Studying the interface between glycolysis and the TCA cycle in S. cerevisiae is complicated by the different possible fates of mitochondrial acetyl-CoA: direct use for synthesis of arginine, leucine and lipoate; complete dissimilation via the TCA cycle; and generation of TCA-cycle intermediates as biosynthetic precursors. Only the first of these fates is strictly dependent on availability of intramitochondrial acetyl-CoA. To assess the relevance of the PDH complex, Cit2 and Ach1 (Fig. 1) for the three processes indicated above, specific growth rates of deletion mutants were determined in glucose synthetic medium with either urea or glutamate as the nitrogen source. Of these two nitrogen sources, only glutamate can yield α-ketoglutarate and, thereby, provide an alternative source of TCA-cycle intermediates as biosynthetic precursors.
In glucose-grown cultures with glutamate as the nitrogen source, only strains IMK640 (pda1Δ ach1Δ) and IMK641 (pda1Δ cit2Δ ach1Δ) showed substantially lower specific growth rates than the reference strain CEN.PK113-7D, while single deletions of PDA1, CIT2 or ACH1 did not affect growth (Table 2). In S. cerevisiae, activity of either the PDH complex or Ach1 therefore appears to be enough to provide sufficient intramitochondrial acetyl-CoA for synthesis of arginine, leucine and lipoate. Consistent with the hypothesis that mitochondrial acetyl-CoA availability is limiting growth, addition of l-carnitine, which enables supply of intramitochondrial acetyl-CoA via the carnitine shuttle, led to a 67%–94% increase of the specific growth rates of the pda1Δ ach1Δ and pda1Δ cit2Δ ach1Δ mutants (Table 2).
Table 2.
Effect of removing key reactions at the interface between glycolysis and TCA cycle interface on the specific growth rate on glucose. Strains were grown in shake flask cultures on synthetic medium with 20 g⋅L−1 glucose with either 38 mM urea or 76 mM glutamate as the sole nitrogen source. Where indicated, l-carnitine was added at a final concentration of 400 mg⋅L−1. The data represent averages of at least two independent experiments. In all cases the mean deviation was ≤ 0.01 h−1.
| Specific growth rate (h−1) | |||||||
|---|---|---|---|---|---|---|---|
| N-source | |||||||
| urea | glutamate | urea | glutamate | ||||
| Strain | Relevant genotype | w/o l-carnitine | w/o l-carnitine | w/ l-carnitine | w/ l-carnitine | ||
| CEN.PK113-7D | PDA1 | CIT2 | ACH1 | 0.35 | 0.37 | 0.35 | 0.38 |
| CEN.PK541-1A | pda1Δ | CIT2 | ACH1 | 0.19 | 0.34 | 0.19 | 0.33 |
| CEN.PK542-1A | PDA1 | cit2Δ | ACH1 | 0.34 | 0.36 | ||
| IMK627 | PDA1 | CIT2 | ach1Δ | 0.34 | 0.36 | ||
| IMK629 | PDA1 | cit2Δ | ach1Δ | 0.34 | 0.36 | ||
| IMK640 | pda1Δ | CIT2 | ach1Δ | 0.10 | 0.09 | 0.13 | 0.16 |
| CEN.PK544-4D | pda1Δ | cit2Δ | ACH1 | 0.05 | 0.33 | 0.09 | 0.33 |
| IMK641 | pda1Δ | cit2Δ | ach1Δ | 0.04 | 0.09 | 0.09 | 0.17 |
With urea as a nitrogen source, CEN.PK541-1A (pda1Δ) showed a 45% lower specific growth rate than the reference strain, while IMK627 (ach1Δ) showed near wild-type growth rates. This result is consistent with an earlier report, based on glucose-limited chemostat cultures, that the PDH complex is the predominant link between glycolysis and TCA cycle in S. cerevisiae (Pronk et al. 1994). Similar specific growth rates of the IMK640 (pda1Δ ach1Δ) strain on urea and glutamate media suggested that the observed reduction in those growth rates in comparison to the reference strain was not caused by a shortage of TCA-cycle intermediates. Additional disruption of CIT2 (strain IMK641, pda1Δ cit2Δ ach1Δ) led to even slower growth on urea, while the growth rate on glutamate medium was the same as that of the pda1Δ ach1Δ strain. This observation indicates that Cit2 is involved in funnelling TCA-cycle intermediates into the mitochondria in the pda1Δ ach1Δ strain during growth on urea medium. Additionally, strain CEN.PK544-4D (pda1Δ cit2Δ) showed strongly impaired growth on urea media (0.05 h−1), but near wild-type rates on glutamate (0.34 h−1). Together with the observation that with glutamate as a nitrogen source, CEN.PK544-4D (pda1Δ cit2Δ ACH1) shows much faster growth than IMK641 (pda1Δ cit2Δ ach1Δ), these growth experiments support the conclusion that Ach1 is sufficiently active to cover the major requirement for intramitochondrial acetyl-CoA as a precursor of arginine, leucine and lipoate biosynthesis. However, Ach1 cannot meet the entire demand of acetyl-CoA for dissimilation via the TCA cycle and for the generation of TCA-cycle intermediates and intramitochondrial acetyl-CoA as metabolic precursors. The residual growth of strain IMK641 (pda1Δ cit2Δ ach1Δ, 0.04 h−1 on urea medium) indicates that, in S. cerevisiae, at least one other mechanism can generate intramitochondrial acetyl-CoA.
Strains with decreased availability of mitochondrial acetyl-CoA show increased loss of respiration
An increased incidence of respiratory deficient mutants, often associated with loss of mitochondrial DNA (rho−petites) has been observed for several mutants in genes encoding components of the mitochondrial fatty-acid synthase system (Harington et al. 1993; Torkko et al. 2001; Hiltunen et al. 2005). Moreover, strains with reduced mitochondrial malonyl-CoA synthesis due to absence of the mitochondrial acetyl-CoA carboxylase Hfa1 exhibit a decreased lipoate content and loss of respiratory competence (Hoja et al. 2004). An increased incidence of rho0petites has also been observed in Pdh− strains (Wenzel et al. 1992). To investigate the impacts of deleting PDA1, CIT2 and/or ACH1 on respiratory competence, we analyzed loss of the ability to grow on the non-fermentable carbon source ethanol, after 12 generations of growth on synthetic medium with 20 g·L−1 glucose and glutamate. Most strains showed a low incidence of respiratory-deficient cells (Table 3). However, the two strains in which both routes of mitochondrial acetyl-CoA provision were disrupted, IMK640 (pda1Δ ach1Δ) and IMK641 (pda1Δ cit2Δ ach1Δ), displayed a spectacularly high incidence of respiratory deficient cells (94% and 92%, respectively). This result strengthened the conclusion that presence of either an active PDH complex or of Ach1 is essential for sufficient supply of intramitochondrial acetyl-CoA and that the role of Cit2 in pda1Δ mutants (Table 2) is limited to the provision of TCA-cycle intermediates.
Table 3.
Effect of removing key reactions at the glycolysis-TCA cycle interface on loss of respiratory competence in S. cerevisiae. After initial growth in YP with 2% (v/v) ethanol, cells were transferred to SM with 20 g⋅L−1 glucose and 76 mM glutamate as nitrogen source. After 12 generations, the cultures were plated on YP with 20 g⋅L−1 glucose. After colonies were observed, the plates were replica plated on YP with 2% (v/v) ethanol and YP with 20 g⋅L−1 glucose. Percentages of cells unable to grow on YP with 2% (v/v) ethanol are based on independent duplicate experiments, with 10 plates per strain per experiment and ∼100 cells per plate. Standard deviations are based on 20 plates per strain.
| Respiratory deficient | ||||
|---|---|---|---|---|
| Strain | Relevant genotype | cells (%) | ||
| CEN.PK113-7D | PDA1 | CIT2 | ACH1 | 0.00 ± 0.00 |
| CEN.PK541-1A | pda1Δ | CIT2 | ACH1 | 0.12 ± 0.31 |
| CEN.PK542-1A | PDA1 | cit2Δ | ACH1 | 0.15 ± 0.37 |
| IMK627 | PDA1 | CIT2 | ach1Δ | 0.16 ± 0.40 |
| IMK629 | PDA1 | cit2Δ | ach1Δ | 0.14 ± 0.45 |
| IMK640 | pda1Δ | CIT2 | ach1Δ | 93.88 ± 4.29 |
| CEN.PK544-4D | pda1Δ | cit2Δ | ACH1 | 0.07 ± 0.21 |
| IMK641 | pda1Δ | cit2Δ | ach1Δ | 92.13 ± 1.85 |
l-Carnitine supplementation enables respiratory growth in glucose-limited cultures of a pda1Δ ach1Δ strain
As the pda1Δ ach1Δ genotype stimulates loss of respiratory competence, IMK640 (pda1Δ ach1Δ) was further characterized in aerobic, glucose-limited chemostat cultures at a dilution rate of 0.05 h−1. Under these conditions, sugar dissimilation of wild-type S. cerevisiae strains is fully respiratory (Van Dijken et al. 2000; Van Hoek, Van Dijken and Pronk 2000). Conversely, strain IMK640 (pda1Δ ach1Δ) showed respirofermentative sugar metabolism under these conditions, as evident from the production of ethanol and a 69% lower biomass yield on glucose compared to the reference strain (Table 4).
Table 4.
Physiology of the S. cerevisiae reference strain CEN.PK113-7D and IMK640 (pda1Δ ach1Δ) in aerobic glucose-limited chemostat cultures with or without 40 mg⋅L−1l-carnitine at a dilution rate of 0.05 h−1. Averages and mean deviations from CEN.PK113-7D and IMK640 were obtained from respectively two and four replicates. The respiratory quotient is the absolute value of qCO2/qO2. Biomass specific consumption (qglucose) and production rates (qproduct) are expressed in mmol⋅gDW−1⋅h−1 and the biomass yield on glucose (Yx/s) in g⋅g−1.
| CEN.PK113-7D (PDA1 ACH1) | IMK640 (pda1Δ ach1Δ) | IMK640 (pda1Δ ach1Δ) | ||
|---|---|---|---|---|
| Units | w/o l-carnitine | w/o l-carnitine | w/ l-carnitine | |
| Biomass dry weight | g⋅L−1 | 3.46 ± 0.12 | 1.07 ± 0.08 | 2.72 ± 0.04 |
| Yx/s | g⋅g−1 | 0.49 ± 0.01 | 0.15 ± 0.01 | 0.37 ± 0.01 |
| Respiratory quotient | 1.06 ± 0.02 | 1.64 ± 0.13 | 0.97 ± 0.08 | |
| qglucose | mmol⋅g−1⋅h−1 | −0.57 ± 0.01 | −1.87 ± 0.11 | −0.74 ± 0.03 |
| qO2 | mmol⋅g−1⋅h−1 | −1.49 ± 0.05 | −2.55 ± 0.19 | −2.24 ± 0.33 |
| qethanol | mmol⋅g−1⋅h−1 | 0.00 ± 0.00 | 1.26 ± 0.16 | 0.03 ± 0.04 |
| qCO2 | mmol⋅g−1⋅h−1 | 1.57 ± 0.03 | 4.17 ± 0.18 | 2.13 ± 0.10 |
| qpyruvate | mmol⋅g−1⋅h−1 | 0.00 ± 0.00 | 0.03 ± 0.01 | 0.00 ± 0.00 |
| qglycerol | mmol⋅g−1⋅h−1 | 0.00 ± 0.00 | 0.03 ± 0.01 | 0.00 ± 0.00 |
| qacetate | mmol⋅g−1⋅h−1 | 0.00 ± 0.00 | 0.15 ± 0.02 | 0.00 ± 0.00 |
| qsuccinate | mmol⋅g−1⋅h−1 | 0.00 ± 0.00 | 0.10 ± 0.05 | 0.02 ± 0.00 |
| qcitrate | mmol⋅g−1⋅h−1 | 0.00 ± 0.00 | 0.07 ± 0.01 | 0.00 ± 0.00 |
| qacetaldehyde | mmol⋅g−1⋅h−1 | N.D.* | 0.13 ± 0.011 | 0.00 ± 0.002 |
| Residual glucose | mmol⋅L−1 | 0.13 ± 0.06 | 0.49 ± 0.02 | 0.18 ± 0.06 |
N.D., not detected.
Average from three replicates.
Average from two replicates.
In aerobic, glucose-limited chemostat cultures, the genes encoding the multiple components of the carnitine shuttle are derepressed (Fig. S1, Supporting Information). When such cultures of strain IMK640 (pda1Δ ach1Δ) were supplemented with l-carnitine, their physiology became fully respiratory, indicated by the absence of ethanol production, a decreased respiratory quotient and an increased biomass yield on glucose (Table 4). The 24% lower biomass yield of strain IMK640 relative to the PDA1 ACH1 reference strain (Table 4) is consistent with increased ATP consumption as a result of redirection of respiratory pyruvate dissimilation via the PDH bypass (Pronk et al. 1994).
Constitutive expression of the carnitine shuttle enables fast growth of a pda1Δ ach1Δ strain on glucose
Under glucose-limited conditions, strain IMK640 (pda1Δ ach1Δ) does not show a mitochondrial acetyl-CoA deficiency when l-carnitine is added to the medium (Table 4). However, in glucose-grown batch cultures of this strain, l-carnitine addition did not support wild-type growth rates (Table 2), suggesting that the flux through the carnitine shuttle was limiting mitochondrial acetyl-CoA provision and thereby growth under these conditions. To test this hypothesis, expression cassettes were constructed with each of the components of the carnitine shuttle (HNM1, AGP2, CRC1, YAT1, YAT2 and CAT2) placed under the control of a strong, constitutive promoter. The resulting six cassettes were integrated into the SGA1 locus of strain IMX710 (pda1Δ ach1Δ) resulting in strain IMX744 (pda1Δ ach1Δ sga1Δ::{CARN}). Enzyme assays showed a high activity of carnitine acetyltransferase activity in cell extracts of glucose-grown batch cultures of the constitutively expressing carnitine shuttle strain IMX744 (3.56 ± 0.20 μmol⋅(mg protein)−1⋅min−1), while activity in reference strain IMX710 (pda1Δ ach1Δ) was below the detection limit (<0.01 μmol⋅(mg protein)−1⋅min−1). Without l-carnitine supplementation, strain IMX744 showed similar growth rates as the pda1Δ ach1Δ reference strain IMX710, irrespective of the nitrogen source (Table 5). However, when l-carnitine was added to the medium, the growth rate of IMX744 reached up to 79% of that of the PDA1 ACH1 reference strain IMX585. These data show that in, the presence of l-carnitine, constitutive expression of the carnitine-shuttle genes indeed enables efficient transport of acetyl-units from the cytosol into the mitochondria in glucose-grown batch cultures.
Table 5.
Effect of the constitutive expression of carnitine shuttle genes on growth of pda1Δ ach1Δ S. cerevisiae. Strains were grown in shake flasks with synthetic medium and 20 g⋅L−1 glucose. As a nitrogen source, either 38 mM urea or 76 mM glutamate was used. Where indicated, l-carnitine was added at a final concentration of 400 mg⋅L−1. The data represent averages of at least two independent experiments. In all cases the mean deviation was ≤ 0.01 h−1.
| Specific growth rate (h−1) | |||||
|---|---|---|---|---|---|
| N-source | |||||
| urea | glutamate | urea | glutamate | ||
| Strain | Relevant genotype | w/o l-carnitine | w/o l-carnitine | w/ l-carnitine | w/ l-carnitine |
| IMX585 | PDA1 ACH1 | 0.35 | 0.37 | 0.34 | 0.37 |
| IMX710 | pda1Δ ach1Δ | 0.10 | 0.10 | 0.13 | 0.13 |
| IMX744 | pda1Δ ach1Δ Carnitine shuttle↑ | 0.08 | 0.09 | 0.27 | 0.25 |
A constitutively expressed carnitine shuttle does not affect respirofermentative metabolism in PDA1 ACH1 S. cerevisiae
Saccharomyces cerevisiae is a Crabtree-positive yeast, using alcoholic fermentation as the predominant catabolic route in aerobic, glucose-grown batch cultures (De Deken 1966). To test whether an alternative entry into the TCA cycle via PDH bypass and carnitine shuttle might affect the distribution of glucose carbon over respiration and fermentation in aerobic, glucose-grown batch cultures of S. cerevisiae, we constructed the PDA1 ACH1 reference strain IMX868, which constitutively expressed the carnitine-shuttle genes. Enzyme assays in glucose-grown batch cultures confirmed overexpression of the carnitine acetyltransferases, showing an activity of 3.01 ± 0.03 μmol⋅rmg protein)−1⋅min−1, while activity in the reference strain IMX585 was below the detection limit (<0.01 μmol⋅(mg protein)−1⋅min−1). Strain IMX868 (PDA1 ACH1 sga1Δ::{CARN}) was grown in aerobic bioreactor batch cultures with glucose as the carbon source, in the presence and absence of l-carnitine (Fig. 2). During growth on glucose, the strain showed similar growth rates (0.39 ± 0.00 h−1) as the reference strain CEN.PK113-7D (0.37 ± 0.01 h−1; Van Maris et al. 2001), when no l-carnitine was added. Upon l-carnitine supplementation, the growth rate slightly decreased, to 0.36 ± 0.00 h−1. Biomass and ethanol yields were highly similar in the absence and presence of l-carnitine, indicating that the presence of an active carnitine shuttle does not affect the balance between respiration and fermentation in aerobic batch cultures of S. cerevisiae.
Figure 2.
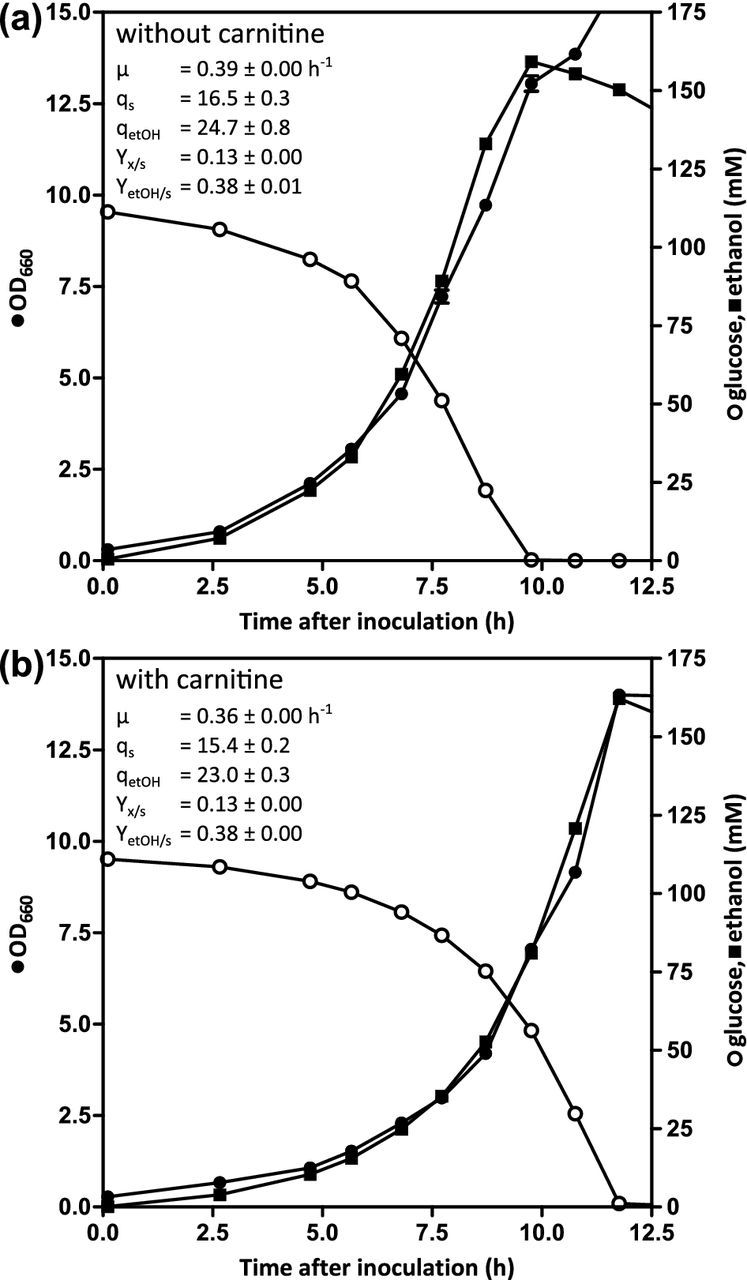
Impact of the constitutive expression of the carnitine shuttle on aerobic growth of S. cerevisiae on glucose in batch cultures. Growth of IMX868 ({CARN}) was analyzed in aerobic bioreactors on synthetic medium with an initial glucose concentration of 20 g⋅L−1 without (a) or with (b) 40 mg⋅L−1l-carnitine. Data shown in the graphs are from single batch experiments for each condition. Independent duplicate experiments for each condition gave essentially the same results. Biomass specific consumption (qs) and production rates (qetOH) are expressed in mmol⋅gDW−1, while yields are expressed in g⋅g−1.
DISCUSSION
Pyruvate and acetyl coenzyme A are key precursors for a wide variety of industrially relevant compounds produced by engineered S. cerevisiae strains (Pronk, Steensma and Van Dijken 1996; Krivoruchko et al. 2015; Lian and Zhao 2015; Sheng and Feng 2015). Design and implementation of metabolic engineering strategies to improve fluxes towards these precursors not only requires knowledge of flux distribution in wild-type strains, but also on compensatory ‘back-up’ pathways that become active when the mechanisms that carry the majority of the flux in wild-type cells are inactivated by genetic modification or by changing process conditions.
In previous work, the PDH complex was shown to be the predominant link between glycolysis and TCA cycle in slow growing, glucose-limited aerobic chemostat cultures, in which sugar dissimilation is exclusively respiratory (Wenzel et al. 1992; Pronk et al. 1994). In aerobic, glucose-grown batch cultures S. cerevisiae employs alcoholic fermentation as the main dissimilatory pathway (Polakis and Bartley 1965; De Deken 1966; Van Dijken, Weusthuis and Pronk 1993). In such cultures, the major role of the TCA cycle is not the dissimilation of pyruvate, but the provision of precursors for assimilation. The near-wild type growth rates of a cit2Δ ach1Δ strain and the impaired growth of a pda1Δ strain in glucose-grown cultures with urea as the nitrogen source show that the combination of mitochondrial pyruvate uptake via the recently discovered Mpc1/Mpc2 ‘MpcFerm’ mitochondrial carrier complex (Bender, Pena and Martinou 2015) and its conversion by the PDH complex to acetyl-CoA is also the main entry into the TCA cycle during aerobic batch cultivation of S. cerevisiae on glucose (Table 2).
By growing S. cerevisiae on glutamate as the sole nitrogen source, we were able to dissect the role of the PDH complex in the biosynthesis of TCA cycle intermediates from its role in the direct provision of intramitochondrial acetyl-CoA. In glucose-grown batch cultures of S. cerevisiae, glucose catabolism is almost completely fermentative (Van Dijken, Weusthuis and Pronk 1993). Consequently, in glucose-urea medium, in which no direct precursors of TCA-cycle intermediates are available, the majority of the flux through the PDH complex will be directed towards the synthesis of TCA cycle-derived metabolic precursors. Under these conditions, cytosolic synthesis of citrate via Cit2 was shown as a key compensatory mechanism in the absence of a functional PDH complex, as evident from the very slow growth of the pda1Δ cit2Δ strain, which could be almost completely restored to wild-type levels when TCA-cycle intermediates were externally supplied by using glutamate instead of urea as the nitrogen source (Table 2). The lack of a marked phenotype of a cit2Δ strain in glucose-grown batch cultures indicates that Cit2 does not have a major role in fueling the TCA cycle in wild-type cells. However, its transcriptional regulation pattern (CIT2 is transcribed in glucose-grown batch cultures but repressed by glutamate; Kim, Rosenkrantz and Guarente 1986) suggests that under certain conditions Cit2 may contribute to synthesis of TCA-cycle intermediates and/or cytosolic acetyl-CoA homeostasis in the presence of a functional PDH complex. This is consistent with previous findings that CIT2 expression is controlled by the retrograde regulation pathway, a communication pathway between the nucleus and mitochondria (Liao et al. 1991; Chelstowska and Butow 1995; Liu and Butow 1999). As a result of retrograde regulation, functional expression of CIT2 is up-regulated when respiratory capacity is reduced or absent, and when the TCA cycle is blocked (Liao et al. 1991; Chelstowska and Butow 1995).
Cit2 is localized to the peroxisomes when proliferation of these organelles is induced by oleate (Lewin, Hines and Small 1990). Its localization under other growth conditions is ambiguous, with several subcellular fractionation studies indicating an at least partial cytosolic localization in glucose-grown cultures (Duntze et al. 1969; Perlman and Mahler 1970; Rickey and Lewin 1986). A clear carbon-source-dependent localization has previously been shown for the MLS1-encoded malate synthase, whose location is peroxisomal in oleate-induced cultures but cytosolic during growth on ethanol (Kunze et al. 2002). Glucose-grown cultures of S. cerevisiae harbor only very few, small peroxisomes (Thieringer et al. 1991). Moreover, Acs2, the only active acetyl-CoA synthetase isoenzyme in the presence of excess glucose due to the glucose repression of ACS1 and glucose catabolite inactivation of Acs1, is nucleocytosolic (Van den Berg et al. 1996; De Jong-Gubbels et al. 1997). Since peroxisomal membranes are impermeable to acetyl-CoA (Van Roermund et al. 1995), our results strongly indicate that, in pda1Δ strains in glucose-grown batch cultures, Cit2 is at least partially localized to the same compartment as Acs2. Although condition-dependent subcellular localization of Cit2 requires further study, the key role of Cit2 as a compensatory enzyme in pda1Δ mutants is fully consistent with a cytosolic localization in glucose-grown cultures.
Previous research on Ach1 mostly focused on its role during growth on ethanol and acetate, either as sole carbon sources or in their metabolism after the diauxic shift in glucose-grown cultures (Lee, Lin and Smith 1990; Buu, Chen and Lee 2003; Fleck and Brock 2009; Orlandi, Casatta and Vai 2012; Eisenberg et al. 2014). Strains with ACH1 deleted show impaired growth at high acetate concentrations, although growth on medium with ethanol is not affected (Buu, Chen and Lee 2003; Fleck and Brock 2009). Post-diauxic shift ach1Δ cultures show increased extracellular acetate accumulation and a reduced life span (Orlandi, Casatta and Vai 2012; Eisenberg et al. 2014). ACH1 has been reported to be repressed by glucose (Lee, Lin and Smith 1990), but in case of the reference strain CEN.PK113-7D, high mRNA levels are found in cultures grown under conditions of glucose repression (Knijnenburg et al. 2009). Our growth experiments with glutamate as the nitrogen source indicated that Ach1 can replace the PDH complex in pda1Δ strains as the source of intramitochondrial acetyl-CoA for synthesis of arginine, leucine and lipoic acid (Table 2). The impact of the impaired mitochondrial acetyl-CoA synthesis in pda1Δ ach1Δ strains became apparent from a strongly reduced growth rate and a spectacularly high incidence of respiratory deficient cells in glucose-grown batch cultures (Table 3). A much less pronounced loss of respiratory deficiency was observed in previous experiments with pda1Δ strains (Wenzel et al. 1992), which can now be interpreted from a partial compensation by Ach1. The strong impact of the pda1Δ ach1Δ strains on respiratory competence resembles the phenotypes of strains impaired in mitochondrial fatty acid synthesis (Harington et al. 1993; Torkko et al. 2001; Hiltunen et al. 2005), which has been proposed to be essential for respiratory complex assembly (Kursu et al. 2013) and requires mitochondrial acetyl-CoA. The frequent, irreversible loss of respiratory competence in these cells requires special measures in genetic modification and maintenance of pda1Δ ach1Δ strains. Use of non-fermentable carbon sources, as applied in this study, and/or inclusion of l-carnitine in growth media offer simple measures to minimize the frequency of respiratory-deficient mutants in stock cultures.
Glutamate also restores growth of Kluyveromyces lactis pda1Δ strains to near-wild type levels (Zeeman et al. 1999), suggesting that this yeast also harbors at least one compensatory pathway for synthesis of intramitochondrial acetyl-CoA. Indeed, the K. lactis genome harbors a gene with 82% sequence identity of its gene product with Ach1 (Dujon et al. 2004). A recent study has demonstrated that, in addition to providing acetyl-CoA in the mitochondrial matrix, Ach1 can also play a role in another compensatory process. In pyruvate-decarboxylase-negative strains, where the PDH bypass cannot meet the cellular demand for cytosolic acetyl-CoA, Ach1 can generate acetate from mitochondrial acetyl-CoA. After its export to the cytosol and activation to acetyl-CoA, this enables growth of Pdc−S. cerevisiae on glucose without addition of a C2 source (Chen et al. 2015). These orthogonal roles suggest that Ach1 can play an important, flexible role in cytosolic and mitochondrial acetyl-CoA homeostasis. The ubiquitous presence of sequences homologous to ACH1 in a large group of fungal genomes (Buu, Chen and Lee 2003; Wapinski et al. 2007; Fleck and Brock 2009; Wapinski 2009), indicates that this role may be widespread among fungi.
An S. cerevisiae strain in which the PDH complex, Ach1 and Cit2 were all absent still showed a low, residual growth rate (Table 2). This observation indicates that S. cerevisiae harbors either another mechanism for intramitochondrial acetyl-CoA synthesis or a pathway that circumvents its necessity. One explanation could be a partial targeting of an acetyl-CoA synthetase isoenzyme to the mitochondrial matrix. Although most studies indicate an extramitochondrial localization of Acs1 and Acs2 (Kispal et al. 1991; Huh et al. 2003; Takahashi et al. 2006; Koh et al. 2015), there are also reports that Acs1 is localized to the mitochondria (Klein and Jahnke 1979; Kumar et al. 2002). However, deletion of the ACS1 ORF in IMK641 (resulting in a pda1Δ cit2Δ ach1Δ acs1Δ genotype) did not result in a lower growth rate than already observed for the pda1Δ cit2Δ ach1Δ strain (data not shown). Based on enzymatic analysis, it was previously hypothesized that S. cerevisiae may harbor a 2-oxoacid dehydrogenase to catabolyse branched-chain amino acids to mitochondrial acetyl-CoA (Dickinson and Dawes 1992). However, after this yeast was sequenced, it became clear that it does not contain the genes encoding the subunits for this complex (Goffeau et al. 1996; Nijkamp et al. 2012). The residual growth rate of the pda1Δ cit2Δ ach1Δ strain illustrates that, even at the interface of the two most intensively studied pathways in central metabolism, our knowledge of pathway topology remains incomplete.
In contrast to the situation in S. cerevisiae (Table 2), l-carnitine addition restored growth of a K. lactis pda1Δ strain to near wild-type levels (Zeeman et al. 1999), suggesting that, in the latter yeast, the carnitine shuttle genes are not, or not as strongly, repressed by glucose as in the former. Indeed, under glucose-derepressed conditions in chemostat cultures, l-carnitine addition could restore respiratory growth of an S. cerevisiae pda1Δ ach1Δ strain in glucose-limited chemostats (Table 4). Constitutive expression of the genes involved in the carnitine shuttle strongly stimulated growth of a pda1Δ ach1Δ strain in the presence of l-carnitine (Table 5), indicating that there are no post-translational or allosteric regulation mechanisms that prevent this shuttle from operating in glucose-containing media. However, the incomplete (77%) recovery of the wild-growth rate may reflect a remaining limitation in this pathway in S. cerevisiae. This study demonstrates, for the first time, that the entire carnitine shuttle in this yeast can be functionally overexpressed. We therefore also investigated its impact in a ‘wild type’ PDA1 ACH1 CIT2 reference strain (Fig. 2). This experiment indicated that a mere facilitation of the entry of acetyl-CoA into the TCA cycle does not affect the balance between respiration and fermentation in aerobic, glucose-grown batch cultures of S. cerevisiae. Glucose repression of other key genes in respiratory glucose metabolism, e.g. those encoding TCA cycle and mitochondrial respiratory chain proteins, constrains the respiratory capacity under these conditions (Gancedo 1998; Fendt and Sauer 2010). We anticipate that S. cerevisiae strains with a constitutively expressed carnitine shuttle will prove to be valuable tools for studies on metabolic engineering of de novol-carnitine synthesis in this yeast (Franken et al. 2015) and on the requirements for in vivo reversibility of the mitochondrial carnitine shuttle (Ventura et al. 1998; Violante et al. 2013).
Supplementary Material
Acknowledgments
We thank Astrid van Uijen for help with initial growth studies, James Dykstra for constructing strain IMX744, Arthur Gorter de Vries for helping in constructing strain IMX868, Ioannis Papapetridis for constructing plasmid pUDR119 and Erik de Hulster for valuable technical assistance.
SUPPLEMENTARY DATA
FUNDING
HMvR, BUK, MSN, MAHL, JMGD, AJAvM and JTP were supported by the BE-Basic R&D Program, which was granted an FES subsidy from the (EL&I) and received additional financial contributions from DSM Biotechnology Center and Amyris Inc.
Conflict of interest. None declared.
REFERENCES
- Aouida M, Rubio-Texeira M, Rubio Texeira M, et al. Agp2, a member of the yeast amino acid permease family, positively regulates polyamine transport at the transcriptional level. PLoS One. 2013;8:e65717. doi: 10.1371/journal.pone.0065717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekers KM, Heijnen JJ, Van Gulik WM. Determination of the in vivo NAD:NADH ratio in Saccharomyces cerevisiae under anaerobic conditions, using alcohol dehydrogenase as sensor reaction. Yeast. 2015;32:541–57. doi: 10.1002/yea.3078. [DOI] [PubMed] [Google Scholar]
- Bender T, Pena G, Martinou J-C. Regulation of mitochondrial pyruvate uptake by alternative pyruvate carrier complexes. EMBO J. 2015;34:911–24. doi: 10.15252/embj.201490197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber LL. Carnitine. Annu Rev Biochem. 1988;57:261–83. doi: 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- Boulton CA, Ratledge C. Correlation of lipid accumulation in yeasts with possession of ATP:citrate lyase. Microbiology. 1981;127:169–76. [Google Scholar]
- Bricker DK, Taylor EB, Schell JC, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S, Oh C, Hoja U, et al. Mitochondrial acyl carrier protein is involved in lipoic acid synthesis in Saccharomyces cerevisiae. FEBS Lett. 1997;408:217–20. doi: 10.1016/s0014-5793(97)00428-6. [DOI] [PubMed] [Google Scholar]
- Buu L-M, Chen Y-C, Lee F-JS. Functional characterization and localization of acetyl-CoA hydrolase, Ach1p, in Saccharomyces cerevisiae. J Biol Chem. 2003;278:17203–9. doi: 10.1074/jbc.M213268200. [DOI] [PubMed] [Google Scholar]
- Chelstowska A, Butow RA. RTG genes in yeast that function in communication between mitochondria and the nucleus are also required for expression of genes encoding peroxisomal proteins. J Biol Chem. 1995;270:18141–6. doi: 10.1074/jbc.270.30.18141. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang Y, Siewers V, et al. Ach1 is involved in shuttling mitochondrial acetyl units for cytosolic C2 provision in Saccharomyces cerevisiae lacking pyruvate decarboxylase. FEMS Yeast Res. 2015;15 doi: 10.1093/femsyr/fov015. [DOI] [PubMed] [Google Scholar]
- De Deken RH. The Crabtree effect: A regulatory system in yeast. J Gen Microbiol. 1966;44:149–56. doi: 10.1099/00221287-44-2-149. [DOI] [PubMed] [Google Scholar]
- De Jong-Gubbels P, Van den Berg MA, Steensma HY, et al. The Saccharomyces cerevisiae acetyl-coenzyme A synthetase encoded by the ACS1 gene, but not the ACS2-encoded enzyme, is subject to glucose catabolite inactivation. FEMS Microbiol Lett. 1997;153:75–81. doi: 10.1111/j.1574-6968.1997.tb10466.x. [DOI] [PubMed] [Google Scholar]
- De Kok S, Nijkamp JF, Oud B, et al. Laboratory evolution of new lactate transporter genes in a jen1Δ mutant of Saccharomyces cerevisiae and their identification as ADY2 alleles by whole-genome resequencing and transcriptome analysis. FEMS Yeast Res. 2012;12:359–74. doi: 10.1111/j.1567-1364.2012.00787.x. [DOI] [PubMed] [Google Scholar]
- De Kok S, Yilmaz D, Suir E, et al. Increasing free-energy (ATP) conservation in maltose-grown Saccharomyces cerevisiae by expression of a heterologous maltose phosphorylase. Metab Eng. 2011;13:518–26. doi: 10.1016/j.ymben.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Dickinson JR, Dawes IW. The catabolism of branched-chain amino acids occurs via 2-oxoacid dehydrogenase in Saccharomyces cerevisiae. J Gen Microbiol. 1992;138:2029–33. doi: 10.1099/00221287-138-10-2029. [DOI] [PubMed] [Google Scholar]
- Dujon B, Sherman D, Fischer G, et al. Genome evolution in yeasts. Nature. 2004;430:35–44. doi: 10.1038/nature02579. [DOI] [PubMed] [Google Scholar]
- Duntze W, Neumann D, Gancedo JM, et al. Studies on the regulation and localization of the glyoxylate cycle enzymes in Saccharomyces cerevisiae. Eur J Biochem. 1969;10:83–9. doi: 10.1111/j.1432-1033.1969.tb00658.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg T, Schroeder S, Andryushkova A, et al. Nucleocytosolic depletion of the energy metabolite acetyl-coenzyme A stimulates autophagy and prolongs lifespan. Cell Metab. 2014;19:431–44. doi: 10.1016/j.cmet.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgersma Y, Van Roermund CW, Wanders RJ, et al. Peroxisomal and mitochondrial carnitine acetyltransferases of Saccharomyces cerevisiae are encoded by a single gene. EMBO J. 1995;14:3472–9. doi: 10.1002/j.1460-2075.1995.tb07353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entian KD, Kötter P. Yeast genetic strain and plasmid collections. Methods Microbiol. 2007;36:629–66. [Google Scholar]
- Fast D. Sporulation synchrony of Saccharomyces cerevisiae grown in various carbon sources. J Bacteriol. 1973;116:925–30. doi: 10.1128/jb.116.2.925-930.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt S-M, Sauer U. Transcriptional regulation of respiration in yeast metabolizing differently repressive carbon substrates. BMC Syst Biol. 2010;4:12. doi: 10.1186/1752-0509-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck CB, Brock M. Re-characterisation of Saccharomyces cerevisiae Ach1p: Fungal CoA-transferases are involved in acetic acid detoxification. Fungal Genet Biol. 2009;46:473–85. doi: 10.1016/j.fgb.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Flikweert MT, De Swaaf M, Van Dijken JP, et al. Growth requirements of pyruvate-decarboxylase-negative Saccharomyces cerevisiae. FEMS Microbiol Lett. 1999;174:73–9. doi: 10.1111/j.1574-6968.1999.tb13551.x. [DOI] [PubMed] [Google Scholar]
- Flikweert MT, Van Der Zanden L, Janssen WM, et al. Pyruvate decarboxylase: An indispensable enzyme for growth of Saccharomyces cerevisiae on glucose. Yeast. 1996;12:247–57. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C247::AID-YEA911%3E3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Franken J, Burger A, Swiegers JH, et al. Reconstruction of the carnitine biosynthesis pathway from Neurospora crassa in the yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2015;99:6377–89. doi: 10.1007/s00253-015-6561-x. [DOI] [PubMed] [Google Scholar]
- Franken J, Kroppenstedt S, Swiegers JH, et al. Carnitine and carnitine acetyltransferases in the yeast Saccharomyces cerevisiae: A role for carnitine in stress protection. Curr Genet. 2008;53:347–60. doi: 10.1007/s00294-008-0191-0. [DOI] [PubMed] [Google Scholar]
- Fritz IB, Schultz SK, Srere PA, et al. Properties of partially purified carnitine acetyltransferase. J Biol Chem. 1963;238:2509–17. [PubMed] [Google Scholar]
- Gancedo JM. Yeast carbon catabolite repression. Microbiol Mol Biol Rev. 1998;62:334–61. doi: 10.1128/mmbr.62.2.334-361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- Goffeau A, Barrell BG, Bussey H, et al. Life with 6000 genes. Science. 1996;274:546, 563–7. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- González-Ramos D, Van den Broek M, Van Maris AJA, et al. Genome-scale analyses of butanol tolerance in Saccharomyces cerevisiae reveal an essential role of protein degradation. Biotechnol Biofuels. 2013;6:48. doi: 10.1186/1754-6834-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadalupe Medina V, Almering MJH, Van Maris AJA, et al. Elimination of glycerol production in anaerobic cultures of a Saccharomyces cerevisiae strain engineered to use acetic acid as an electron acceptor. Appl Environ Microbiol. 2010;76:190–5. doi: 10.1128/AEM.01772-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güldener U, Heck S, Fiedler T, et al. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996;24:2519–24. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harington A, Herbert CJ, Tung B, et al. Identification of a new nuclear gene (CEM1) encoding a protein homologous to a beta-keto-acyl synthase which is essential for mitochondrial respiration in Saccharomyces cerevisiae. Mol Microbiol. 1993;9:545–55. doi: 10.1111/j.1365-2958.1993.tb01715.x. [DOI] [PubMed] [Google Scholar]
- Henriksen P, Wagner SA, Weinert BT, et al. Proteome-wide analysis of lysine acetylation suggests its broad regulatory scope in Saccharomyces cerevisiae. Mol Cell Proteomics. 2012;11:1510–22. doi: 10.1074/mcp.M112.017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Raemy E, Montessuit S, et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337:93–6. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- Hiltunen JK, Okubo F, Kursu VAS, et al. Mitochondrial fatty acid synthesis and maintenance of respiratory competent mitochondria in yeast. Biochem Soc Trans. 2005;33:1162–5. doi: 10.1042/BST20051162. [DOI] [PubMed] [Google Scholar]
- Hoja U, Marthol S, Hofmann J, et al. HFA1 encoding an organelle-specific acetyl-CoA carboxylase controls mitochondrial fatty acid synthesis in Saccharomyces cerevisiae. J Biol Chem. 2004;279:21779–86. doi: 10.1074/jbc.M401071200. [DOI] [PubMed] [Google Scholar]
- Holzer H, Goedde HW. Two ways from pyruvate to acetyl-coenzyme A in yeast. Biochem Z. 1957;329:175–91. [PubMed] [Google Scholar]
- Huh W-K, Falvo J V, Gerke LC, et al. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–91. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- Inoue H, Nojima H, Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1990;96:23–8. doi: 10.1016/0378-1119(90)90336-p. [DOI] [PubMed] [Google Scholar]
- Jauniaux J, Urrestarazu LA, Wlame J. Arginine metabolism in Saccharomyces cerevisiae: Subcellular localization of the enzymes. J Bacteriol. 1978;133:1096–107. doi: 10.1128/jb.133.3.1096-1107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RS, Mayor JA, Gremse DA, et al. High level expression and characterization of the mitochondrial citrate transport protein from the yeast Saccharomyces cerevisiae. J Biol Chem. 1995;270:4108–14. doi: 10.1074/jbc.270.8.4108. [DOI] [PubMed] [Google Scholar]
- Kim KS, Rosenkrantz MS, Guarente L. Saccharomyces cerevisiae contains two functional citrate synthase genes. Mol Cell Biol. 1986;6:1936–42. doi: 10.1128/mcb.6.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispal G, Cseko J, Alkonyi I, et al. Isolation and characterization of carnitine acetyltransferase from Scerevisiae. Biochim Biophys Acta. 1991;1085:217–22. doi: 10.1016/0005-2760(91)90097-2. [DOI] [PubMed] [Google Scholar]
- Kispal G, Evans CT, Malloy C, et al. Metabolic studies on citrate synthase mutants of yeast. A change in phenotype following transformation with an inactive enzyme. J Biol Chem. 1989;264:11204–10. [PubMed] [Google Scholar]
- Klein HP, Jahnke L. Effects of aeration on formation and localization of the acetyl coenzyme A synthetases of Saccharomyces cerevisiae. J Bacteriol. 1979;137:179–84. doi: 10.1128/jb.137.1.179-184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knijnenburg TA, Daran J-MG, Van den Broek MA, et al. Combinatorial effects of environmental parameters on transcriptional regulation in Saccharomyces cerevisiae: a quantitative analysis of a compendium of chemostat-based transcriptome data. BMC Genomics. 2009;10:53. doi: 10.1186/1471-2164-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JLY, Chong YT, Friesen H, et al. CYCLoPs: A comprehensive database constructed from automated analysis of protein abundance and subcellular localization patterns in Saccharomyces cerevisiae. G3. 2015;5:1223–32. doi: 10.1534/g3.115.017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhaw GB, Tan-Wilson A. Carnitine acetyltransferase: Candidate for the transfer of acetyl groups through the mitochondrial membrane of yeast. J Bacteriol. 1977;129:1159–61. doi: 10.1128/jb.129.2.1159-1161.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak BU, Van Rossum HM, Benjamin KR, et al. Replacement of the Saccharomyces cerevisiae acetyl-CoA synthetases by alternative pathways for cytosolic acetyl-CoA synthesis. Metab Eng. 2014a;21:46–59. doi: 10.1016/j.ymben.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Kozak BU, Van Rossum HM, Luttik MAH, et al. Engineering acetyl coenzyme A supply: Functional expression of a bacterial pyruvate dehydrogenase complex in the cytosol of Saccharomyces cerevisiae. MBio. 2014b;5:e01696–14. doi: 10.1128/mBio.01696-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivoruchko A, Zhang Y, Siewers V, et al. Microbial acetyl-CoA metabolism and metabolic engineering. Metab Eng. 2015;28:28–42. doi: 10.1016/j.ymben.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Kumar A, Agarwal S, Heyman JA, et al. Subcellular localization of the yeast proteome. Genes Dev. 2002;16:707–19. doi: 10.1101/gad.970902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze M, Kragler F, Binder M, et al. Targeting of malate synthase 1 to the peroxisomes of Saccharomyces cerevisiae cells depends on growth on oleic acid medium. Eur J Biochem. 2002;269:915–22. doi: 10.1046/j.0014-2956.2001.02727.x. [DOI] [PubMed] [Google Scholar]
- Kursu VAS, Pietikäinen LP, Fontanesi F, et al. Defects in mitochondrial fatty acid synthesis result in failure of multiple aspects of mitochondrial biogenesis in Saccharomyces cerevisiae. Mol Microbiol. 2013;90:824–40. doi: 10.1111/mmi.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FJS, Lin LW, Smith JA. A glucose-repressible gene encodes acetyl-CoA hydrolase from Saccharomyces cerevisiae. J Biol Chem. 1990;265:7413–8. [PubMed] [Google Scholar]
- Lewin AS, Hines V, Small GM. Citrate synthase encoded by the CIT2 gene of Saccharomyces cerevisiae is peroxisomal. Mol Cell Biol. 1990;10:1399–405. doi: 10.1128/mcb.10.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J, Zhao H. Recent advances in biosynthesis of fatty acids derived products in Saccharomyces cerevisiae via enhanced supply of precursor metabolites. J Ind Microbiol Biotechnol. 2015;42:437–51. doi: 10.1007/s10295-014-1518-0. [DOI] [PubMed] [Google Scholar]
- Liao XS, Small WC, Srere PA, et al. Intramitochondrial functions regulate nonmitochondrial citrate synthase (CIT2) expression in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:38–46. doi: 10.1128/mcb.11.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Butow RA. A transcriptional switch in the expression of yeast tricarboxylic acid cycle genes in response to a reduction or loss of respiratory function. Mol Cell Biol. 1999;19:6720–8. doi: 10.1128/mcb.19.10.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lõoke M, Kristjuhan K, Kristjuhan A. Extraction of genomic DNA from yeasts for PCR-based applications. Biotechniques. 2011;50:325–8. doi: 10.2144/000113672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- Mans R, Van Rossum HM, Wijsman M, et al. CRISPR/Cas9: A molecular Swiss army knife for simultaneous introduction of multiple genetic modifications in Saccharomyces cerevisiae. FEMS Yeast Res. 2015;15 doi: 10.1093/femsyr/fov004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashego MR, Van Gulik WM, Vinke JL, et al. Critical evaluation of sampling techniques for residual glucose determination in carbon-limited chemostat culture of Saccharomyces cerevisiae. Biotechnol Bioeng. 2003;83:395–9. doi: 10.1002/bit.10683. [DOI] [PubMed] [Google Scholar]
- Nijkamp JF, Van den Broek M, Datema E, et al. De novo sequencing, assembly and analysis of the genome of the laboratory strain Saccharomyces cerevisiae CEN.PK113-7D, a model for modern industrial biotechnology. Microb Cell Fact. 2012;11:36. doi: 10.1186/1475-2859-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi I, Casatta N, Vai M. Lack of Ach1 CoA-transferase triggers apoptosis and decreases chronological lifespan in yeast. Front Oncol. 2012;2:67. doi: 10.3389/fonc.2012.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oura E. Ph.D. thesis. University of Helsinki; Finland: 1972. The effect of aeration on the growth energetics and biochemical composition of baker's yeast. [Google Scholar]
- Palmieri L, Lasorsa FM, Iacobazzi V, et al. Identification of the mitochondrial carnitine carrier in Saccharomyces cerevisiae. FEBS Lett. 1999;462:472–6. doi: 10.1016/s0014-5793(99)01555-0. [DOI] [PubMed] [Google Scholar]
- Perlman PS, Mahler HR. Intracellular localization of enzymes in yeast. Arch Biochem Biophys. 1970;136:245–59. doi: 10.1016/0003-9861(70)90348-6. [DOI] [PubMed] [Google Scholar]
- Polakis ES, Bartley W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J. 1965;97:284–97. doi: 10.1042/bj0970284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma E, Verduyn C, Scheffers WA, et al. Enzymic analysis of the Crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol. 1989;55:468–77. doi: 10.1128/aem.55.2.468-477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk JT, Steensma HY, Van Dijken JP. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast. 1996;12:1607–33. doi: 10.1002/(sici)1097-0061(199612)12:16<1607::aid-yea70>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Pronk JT, Wenzel TJ, Luttik MA, et al. Energetic aspects of glucose metabolism in a pyruvate-dehydrogenase-negative mutant of Saccharomyces cerevisiae. Microbiology. 1994;140:601–10. doi: 10.1099/00221287-140-3-601. [DOI] [PubMed] [Google Scholar]
- Rickey TM, Lewin AS. Extramitochondrial citrate synthase activity in bakers’ yeast. Mol Cell Biol. 1986;6:488–93. doi: 10.1128/mcb.6.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddles PW, Blakeley RL, Zerner B. Reassessment of Ellman's reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- Schmalix W, Bandlow W. The ethanol-inducible YAT1 gene from yeast encodes a presumptive mitochondrial outer carnitine acetyltransferase. J Biol Chem. 1993;268:27428–39. [PubMed] [Google Scholar]
- Sheng J, Feng X. Metabolic engineering of yeast to produce fatty acid-derived biofuels: Bottlenecks and solutions. Front Microbiol. 2015;6:1–11. doi: 10.3389/fmicb.2015.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis-Escalante D, Kuijpers NGA, Bongaerts N, et al. amdSYM, a new dominant recyclable marker cassette for Saccharomyces cerevisiae. FEMS Yeast Res. 2013;13:126–39. doi: 10.1111/1567-1364.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiegers JH, Dippenaar N, Pretorius IS, et al. Carnitine-dependent metabolic activities in Saccharomyces cerevisiae: Three carnitine acetyltransferases are essential in a carnitine-dependent strain. Yeast. 2001;18:585–95. doi: 10.1002/yea.712. [DOI] [PubMed] [Google Scholar]
- Takahashi H, McCaffery JM, Irizarry RA, et al. Nucleocytosolic acetyl-coenzyme A synthetase is required for histone acetylation and global transcription. Mol Cell. 2006;23:207–17. doi: 10.1016/j.molcel.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Thieringer R, Shio H, Han YS, et al. Peroxisomes in Saccharomyces cerevisiae: Immunofluorescence analysis and import of catalase A into isolated peroxisomes. Mol Cell Biol. 1991;11:510–22. doi: 10.1128/mcb.11.1.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkko JM, Koivuranta KT, Miinalainen IJ, et al. Candida tropicalis Etr1p and Saccharomyces cerevisiae Ybr026p (Mrf1′p), 2-enoyl thioester reductases essential for mitochondrial respiratory competence. Mol Cell Biol. 2001;21:6243–53. doi: 10.1128/MCB.21.18.6243-6253.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg MA, De Jong-Gubbels P, Kortland CJ, et al. The two acetyl-coenzyme A synthetases of Saccharomyces cerevisiae differ with respect to kinetic properties and transcriptional regulation. J Biol Chem. 1996;271:28953–9. doi: 10.1074/jbc.271.46.28953. [DOI] [PubMed] [Google Scholar]
- Van den Berg MA, Steensma HY. ACS2, a Saccharomyces cerevisiae gene encoding acetyl-coenzyme A synthetase, essential for growth on glucose. Eur J Biochem. 1995;231:704–13. doi: 10.1111/j.1432-1033.1995.tb20751.x. [DOI] [PubMed] [Google Scholar]
- Van Dijken JP, Bauer J, Brambilla L, et al. An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enzyme Microb Technol. 2000;26:706–14. doi: 10.1016/s0141-0229(00)00162-9. [DOI] [PubMed] [Google Scholar]
- Van Dijken JP, Weusthuis RA, Pronk JT. Kinetics of growth and sugar consumption in yeasts. Antonie Van Leeuwenhoek. 1993;63:343–52. doi: 10.1007/BF00871229. [DOI] [PubMed] [Google Scholar]
- Van Hoek P, Van Dijken JP, Pronk JT. Regulation of fermentative capacity and levels of glycolytic enzymes in chemostat cultures of Saccharomyces cerevisiae. Enzyme Microb Technol. 2000;26:724–36. doi: 10.1016/s0141-0229(00)00164-2. [DOI] [PubMed] [Google Scholar]
- Van Maris AJA, Bakker BM, Brandt M, et al. Modulating the distribution of fluxes among respiration and fermentation by overexpression of HAP4 in Saccharomyces cerevisiae. FEMS Yeast Res. 2001;1:139–49. doi: 10.1111/j.1567-1364.2001.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Van Roermund CW, Elgersma Y, Singh N, et al. The membrane of peroxisomes in Saccharomyces cerevisiae is impermeable to NAD(H) and acetyl-CoA under in vivo conditions. EMBO J. 1995;14:3480–6. doi: 10.1002/j.1460-2075.1995.tb07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roermund CW, Hettema EH, Van den Berg M, et al. Molecular characterization of carnitine-dependent transport of acetyl-CoA from peroxisomes to mitochondria in Saccharomyces cerevisiae and identification of a plasma membrane carnitine transporter, Agp2p. EMBO J. 1999;18:5843–52. doi: 10.1093/emboj/18.21.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélot C, Lebreton S, Morgunov I, et al. Metabolic effects of mislocalized mitochondrial and peroxisomal citrate synthases in yeast Saccharomyces cerevisiae. Biochemistry. 1999;38:16195–204. doi: 10.1021/bi991695n. [DOI] [PubMed] [Google Scholar]
- Ventura FV, Ijlst L, Ruiter J, et al. Carnitine palmitoyltransferase II specificity towards β-oxidation intermediates: Evidence for a reverse carnitine cycle in mitochondria. Eur J Biochem. 1998;253:614–8. doi: 10.1046/j.1432-1327.1998.2530614.x. [DOI] [PubMed] [Google Scholar]
- Verduyn C, Postma E, Scheffers WA, et al. Effect of benzoic acid on metabolic fluxes in yeasts: A continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast. 1992;8:501–17. doi: 10.1002/yea.320080703. [DOI] [PubMed] [Google Scholar]
- Violante S, IJlst L, Te Brinke H, et al. Carnitine palmitoyltransferase 2 and carnitine/acylcarnitine translocase are involved in the mitochondrial synthesis and export of acylcarnitines. FASEB J. 2013;27:2039–44. doi: 10.1096/fj.12-216689. [DOI] [PubMed] [Google Scholar]
- Wapinski I. Fungal Orthogroups Repository. 2009. [Online] Available from: http://www.broadinstitute.org/regev/orthogroups/ [Google Scholar]
- Wapinski I, Pfeffer A, Friedman N, et al. Automatic genome-wide reconstruction of phylogenetic gene trees. Bioinformatics. 2007;23:549–58. doi: 10.1093/bioinformatics/btm193. [DOI] [PubMed] [Google Scholar]
- Wenzel TJ, Van den Berg MA, Visser W, et al. Characterization of Saccharomyces cerevisiae mutants lacking the E1 alpha subunit of the pyruvate dehydrogenase complex. Eur J Biochem. 1992;209:697–705. doi: 10.1111/j.1432-1033.1992.tb17338.x. [DOI] [PubMed] [Google Scholar]
- Yamashita I, Fukui S. Transcriptional control of the sporulation-specific glucoamylase gene in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1985;5:3069–73. doi: 10.1128/mcb.5.11.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon J, Fried M. Precise gene fusion by PCR. Nucleic Acids Res. 1989;17:4895. doi: 10.1093/nar/17.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman AM, Luttik MAH, Pronk JT, et al. Impaired growth on glucose of a pyruvate dehydrogenase-negative mutant of Kluyveromyces lactis is due to a limitation in mitochondrial acetyl-coenzyme A uptake. FEMS Microbiol Lett. 1999;177:23–8. doi: 10.1111/j.1574-6968.1999.tb13708.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.