Abstract
Biomarker-guided treatments are needed in psychiatry, and previous data suggest oxidative stress may be a target in schizophrenia. A previous add-on trial with the antioxidant N-acetylcysteine (NAC) led to negative symptom reductions in chronic patients. We aim to study NAC’s impact on symptoms and neurocognition in early psychosis (EP) and to explore whether glutathione (GSH)/redox markers could represent valid biomarkers to guide treatment. In a double-blind, randomized, placebo-controlled trial in 63 EP patients, we assessed the effect of NAC supplementation (2700 mg/day, 6 months) on PANSS, neurocognition, and redox markers (brain GSH [GSHmPFC], blood cells GSH levels [GSHBC], GSH peroxidase activity [GPxBC]). No changes in negative or positive symptoms or functional outcome were observed with NAC, but significant improvements were found in favor of NAC on neurocognition (processing speed). NAC also led to increases of GSHmPFC by 23% (P = .005) and GSHBC by 19% (P = .05). In patients with high-baseline GPxBC compared to low-baseline GPxBC, subgroup explorations revealed a link between changes of positive symptoms and changes of redox status with NAC. In conclusion, NAC supplementation in a limited sample of EP patients did not improve negative symptoms, which were at modest baseline levels. However, NAC led to some neurocognitive improvements and an increase in brain GSH levels, indicating good target engagement. Blood GPx activity, a redox peripheral index associated with brain GSH levels, could help identify a subgroup of patients who improve their positive symptoms with NAC. Thus, future trials with antioxidants in EP should consider biomarker-guided treatment.
Keywords: glutathione, glutathione peroxidase, schizophrenia, MRS, prefrontal cortex, neurocognition
Introduction
While early intervention improves the treatment of psychosis patients,1 full benefits of these strategies are hampered by limited biological treatments. If antipsychotics improve positive symptoms, their efficacy on negative symptoms, neurocognition, social functioning, and quality of life is limited,2–5 and their side effects impact treatment adherence negatively.6 Poor knowledge regarding neurobiological mechanisms underlying psychotic disorders has limited pharmacological targets to altered D2 neurotransmission7 rather than more fundamental impairments. Despite improved understanding of the neurobiological bases of schizophrenia, developmentally informed pharmacology8,9 is not yet available. Therefore identification of new drug targets and mechanisms is needed.
Recent data suggest a convergence of redox dysregulation and oxidative stress in promoting the emergence of psychosis. This mechanism, along with neuroinflammation, NMDA receptor hypofunction, and dopamine dysregulation, may belong to a central pathophysiological hub: an imbalance within any of these systems would lead to micro- and macro-circuitry brain defects involving alterations of parvalbumin interneurons and white matter and leading to connectivity and clinical impairments.10–12 Indeed, recent research identified increases in oxidative stress in blood, plasma, cerebrospinal fluid (CSF) and postmortem samples of schizophrenia patients, including increased lipid and protein oxidation and alterations in antioxidant defense systems, such as catalase and superoxide dismutase.13–17 In particular, glutathione (GSH) system deficits are linked to schizophrenia pathophysiology in many ways. GSH levels are lower in chronic schizophrenia, as detected in CSF, in postmortem brain, and by magnetic resonance spectroscopy (MRS)-based in vivo imaging, low cortical GSH levels being associated with worse negative symptoms.18–21 Moreover, the GSH redox enzymes, GSH peroxidase (GPx) and GSH reductase (GR) are also dysregulated.22 Genetic evidence highlights a role for GSH system impairment in schizophrenia etiopathology: copy number and allelic variants of glutathione-s-transferase (GST) genes23 and of key GSH synthesizing genes are associated with the disease24 and lead to functional consequences.25 A randomized controlled add-on trial (RCT) with N-acetylcysteine (NAC), an antioxidant and precursor of GSH, in patients with chronic schizophrenia26 showed that NAC led to significant reductions in negative symptoms and medication side effects. In addition, a study based on a subsample showed that NAC improved EEG local synchronization27 and mismatch negativity.28 An independent add-on trial to risperidone also demonstrated that negative symptoms in patients with chronic schizophrenia improved with NAC.29 While actionable biomarkers in schizophrenia are currently scarce,30 they may allow the identification of patients responding to treatments targeting specific pathophysiological mechanisms.
In genetic or developmental rodent models of schizophrenia presenting oxidative stress-induced parvalbumin interneuron impairment, NAC administered during brain development prevented the deleterious and permanent effects of early insults.31,32 This suggests oxidative stress’ negative impact on parvalbumin interneurons and myelination may occur early in the illness trajectory, thus explaining the modest impact of NAC in chronic schizophrenia patients. The current RCT was therefore launched in early psychosis (EP) patients. Our aims were, first, to determine whether the addition of NAC to standard medication would improve negative symptoms in EP patients; second, to explore the impact of NAC on positive symptoms and neurocognition, the latter being critical for functional outcome33–36; third, to explore whether NAC would modulate brain GSH levels and improve or normalize blood glutathione/redox dysregulation profile, at least in a subgroup of patients, in order respectively to clarify the controversial ability of NAC to cross the blood brain barrier37–40 and to explore whether the response to NAC might be associated with glutathione-related peripheral biomarkers; fourth, to explore impact of NAC treatment on brain levels of glutamate (Glu), glutamine (Gln), Gln/Glu, and myoinositol (Ins), which were normalized by NAC in a previous study of glutathione-deficient schizophrenia mouse model.41
Materials, Subjects, and Methods
Study Design and Participants
The study, conducted in 2009–2014, was a 6-months, randomized, placebo-controlled, double-blind, 2-center trial comparing NAC and placebo as add-on therapy to standard medication (antipsychotics, mood stabilizers, and/or benzodiazepine). Participants were recruited from the Treatment and Early Intervention in Psychosis Program (TIPP, Lausanne University Hospital, Switzerland42) and the Commonwealth Research Center of the Beth Israel Deaconess Medical Center Department of Psychiatry (Harvard Medical School, Boston, USA). Inclusion criteria were (1) male or female aged 18–40; (2) having a psychotic disorder, defined by reaching the “psychosis threshold” subscale on the Comprehensive Assessment of At Risk Mental States scale (CAARMS); (3) less than 12 months of treatment for psychosis; (4) capability to provide informed consent; and (5) sufficient stability to participate in the study. Disease duration is the time between first psychotic symptoms and entry into the trial. The diagnosis was based on DSM-IV criteria.43 (Ethics, power calculation, exclusion criteria: see supplement.)
Study Medication
NAC was provided by Bioadvantex Pharma, Inc. and produced under Good Manufacturing Practice conditions. Stability of the compound was ascertained, and the integrity of the active agent was protected by separate dose packaging. All participants were randomized in a 1:1 allocation ratio to either NAC effervescent tablets (900 mg) at a dosage of 2700 mg/day (morning: 1800 mg; evening: 900 mg) or matching placebo tablets before meals. Tablets were dispensed every month, from visit 1 to 7. At each visit, remaining tablets were counted to assess adherence over the past month; adherence was considered as poor when less than 25% of the monthly dose was taken (less than 675 mg/day) (dose rationale, randomization, and masking: see supplement).
Study Visits, Evaluation, and Outcomes
Eight visits were conducted including a baseline visit (V1) at NAC initiation, monthly visits thereafter (V2–V7), and an additional visit after 1 month postdiscontinuation (V8).
Raters were psychologists with regular training for interrater reliability. The primary outcome was the PANSS negative score (PANSS−). Secondary outcomes, assessed on a monthly basis (V1–V8), included PANSS positive (PANSS+), PANSS general, functioning assessed by the Global Assessment of Functioning (GAF) and Social Functioning Assessment Scale (SOFAS) scales, and relapse rate. After controlling for adherence (see supplement), dosages of antipsychotic medication were converted to chlorpromazine equivalents44,45 and included as a covariate in statistical models. Medication side effects were assessed at V1, V3, V7, and V8 with the Udvalg for Kliniske Undergelser (UKU) scale.46 Adjusted item scores were calculated as differences from baseline values to account for pretreatment differences; we attributed zero to negative differences as recommended.46
Neurocognitive Measures
The MATRICS Consensus Cognitive Battery (MCCB33,34) was assessed at V1 and V7, excluding the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT), which does not “translate” well into French as an index of social cognition. Thus, 9 of 10 subtests were given, comprising 6 factors namely processing speed, sustained attention, working memory, verbal learning, visual learning, and problem solving.
Central and Peripheral Biochemical Assessments
Levels of GSH in medial prefrontal cortex (GSHmPFC) were assessed in a subset of participants by 1H-MRS before (V1) and at the end of 6 months of NAC/placebo supplementation (V7). 1H-MRS measurements were performed as previously described47,48 (supplement).
Blood markers of the glutathione synthesis and redox system (plasmatic Cysteine, CYSPl; blood cells GSH, GSHBC; GPx enzymatic activity in blood cells, GPxBC) were measured at the Lausanne site at V1, V3, and V7 according to standardized procedures described previously49–51 (supplement).
Statistical Analysis
We included 90% observations (27/263 excluded due to nonadherence). We investigated if the missing observations at each time point are “Missing Completely At Random” (MCAR) using Little’s MCAR test in R. Missing data at baseline and at 6 months are MCAR. Missing data at 2 months of treatment are not MCAR, in particular, because the pattern of missing data was different at the 2 sites. But the test supports the MCAR for observations corresponding to 2 months at Lausanne.
Differences in improvement rate during follow-up were estimated using a 2-level random intercept and random slope model, the treatment being tested as a predictor.
A series of marginal tests were performed to investigate associations among demographic variables and baseline scores between groups. Mean values and standard errors are reported for continuous variables; t-tests were applied to assess the significance of differences between groups.
For neurocognitive outcomes, analyses were conducted on MCCB standardized scores (t-scores). Repeated measures ANOVA were performed with group (NAC) as a between-group factor and time as a within-group factor. A significant time × group interaction would indicate that rate of improvement in cognition over assessments differed between groups.
Considering that response to NAC supplementation may be linked to baseline redox status, we performed additional analyses based on Classification and Regression Trees (CARTs)52 to explore whether baseline redox level was benefiting from NAC supplementation. CARTs are powerful and flexible machine learning tools used here to single out which variables are best associated with an improvement in the outcome. Once a potential threshold level was identified, a longitudinal model was fitted to the PANSS scores for patients treated with NAC.
Results
Demographic Characteristics at Baseline
Of 302 patients screened for eligibility, 63 were randomized (figure 1A); 32 to NAC (6 in Boston, 26 in Lausanne) and 31 to placebo (4 in Boston, 27 in Lausanne). After randomization, 2 patients were excluded (one because of white matter lesions detected on baseline MRI, and one withdrew consent). All existing data were used in the estimation of the models including patients with data only at baseline. Missing data were MCAR and imputation methods were not used. Diagnostic repartition was similar in both groups with predominance of schizophrenia (68% and 63%, see supplementary table 1).
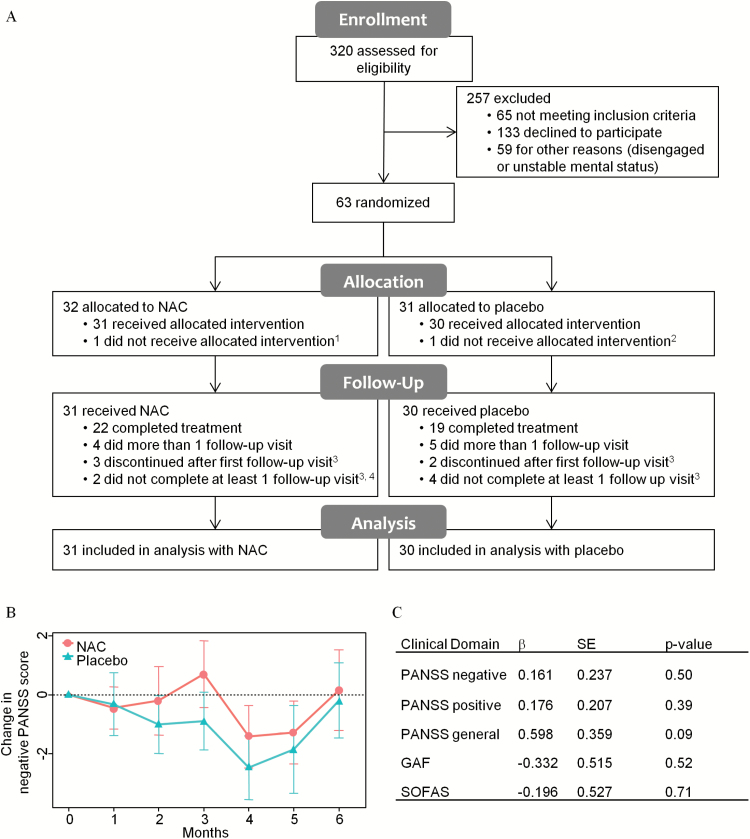
Fig. 1.
CONSORT flow diagram, primary and secondary outcomes. (A) Reasons for withdrawal from trial, 1white matter lesions detected at baseline MRI scan; 2withdrew at baseline; 3lack of motivation; 4side effects. (B) Change in PANSS− score vs baseline in NAC (red circles) and placebo (green triangles) groups at each visit. (C) Comparison of NAC vs placebo in the rate of improvement. PANSS, Positive and Negative Syndrome Scale; GAF, General Assessment of Functioning; SOFAS, Social and Occupational Functioning Assessment Scale; β, an estimate of NAC effect vs placebo; SE, standard error.
Baseline clinical characteristics and antipsychotic dosages are reported in table 1. No significant between-group differences were observed in baseline characteristics including negative symptom levels, which were moderate in severity. For redox markers, basal GSHmPFC levels were lower in NAC than the placebo group. Moreover, values from both sites were within the same range and were thus pooled together (supplementary figure 1). Dosages of antipsychotics expressed in chlorpromazine equivalent, percentage of smokers, and levels of various blood markers were similar in both groups. The numbers of patients for each antipsychotic at baseline are detailed in supplementary table 2.
Table 1.
Clinical Information of Participants at Baseline and Medication Dosage During Trial
| Variable | Total (n = 61) | Treatment Group | Statistics | P-value | |
|---|---|---|---|---|---|
| NAC (n = 31) | Placebo (n = 30) | Test Statistics (df) | |||
| Age, mean (SD), y | 25.4 (6.0) | 26.1 (6.1) | 24.7 (5.9) | t(59) = 0.92 | .36 |
| Male, % (no.) | 77% (47) | 84% (26) | 70% (21) | χ2 (1) = 0.97 | .33 |
| Race (no.) | 47 C, 12Af, 2 Ma | 25 C, 5 Af, 1 Ma | 22 C, 7 Af, 1Ma | χ2 (2) = 0.98 | .61 |
| Smoking, % (no.) | 56% (28) | 45% (11) | 65% (17) | χ2 (1) = 1.94 | .16 |
| Body mass index, mean (SD) | 25.09 (4.35) | 25.3 (3.7) | 24.8 (5.0) | t(56) = 2.22 | .68 |
| Disorder duration, mean (SD), days | 796 (726) | 848 (767) | 747 (693) | t(56) = 0.53 | .60 |
| Antipsychotic medication | |||||
| Chlorpromazine equivalent, mean (SD) | 309 (220) | 309 (252) | 309 (188) | t(52) = 0.01 | .99 |
| Changes of dose, mean (SD) | 1.1 (1.1) | 1.1 (1.1) | 1.0 (1.2) | z = −0.55 | .58 |
| Changes of medication, mean (SD) | 0.2 (0.5) | 0.1 (0.3) | 0.4 (0.6) | z = −1.73 | .08 |
| Clinical scores | |||||
| Positive PANSS, mean (SD) | 14.7 (5.6) | 14.3 (5.4) | 15.0 (5.6) | t(58) = -0.47 | .64 |
| Negative PANSS, mean (SD) | 16.4 (5.7) | 15.6 (5.0) | 17.3 (6.3) | t(55) = −1.2 | .23 |
| GAF, mean (SD) | 52.8 (11.8) | 54.0 (10.8) | 51.6 (12.8) | t(57) = 0.78 | .44 |
| SOFAS, mean (SD) | 54.7 (12.0) | 55.8 (11.0) | 53.5 (13.0) | t(57) = 0.76 | .45 |
| Blood markers | |||||
| Cysteine, mean (SD), uM | 257.7 (35.7) | 261.3 (37.6) | 254.0 (33.6) | t(58) = 0.79 | .43 |
| Glutathione, mean (SD), mM | 0.81 (0.24) | 0.77 (0.21) | 0.84 (0.27) | t(47) = −1.04 | .31 |
| GPx activity, mean (SD), µmol/min/gHb | 21.13 (7.17) | 21.24 (7.50) | 21.01 (6.93) | t(58) = 0.12 | .90 |
| Brain markers | Total (n = 25) | NAC (n = 13) | Placebo (n = 12) | ||
| Glutathione, mean (SD), mM | 0.99 (0.24) | 0.87 (0.23) | 1.12 (0.18) | t(22) = −3.13 | .005 |
| Glutamate, mean (SD), mM | 10.37 (1.04) | 10.12 (0.80) | 10.64 (1.23) | t(19) = −1.23 | .234 |
| Glutamine, mean (SD), mM | 3.09 (0.51) | 3.25 (0.51) | 2.93 (0.48) | t(23) = 1.58 | .128 |
| myo-Inositol, mean (SD), mM | 6.27 (0.85) | 6.25 (1.05) | 6.28 (0.62) | t(20) = −0.07 | .941 |
Note: Antipsychotic medication is expressed in chlorpromazine equivalents. df, degree of freedom; Hb, hemoglobin; SD, standard deviation; no., number; C, Caucasian; Af, African; Ma, Maghreb; PANSS, Positive and Negative Syndrome Scale; GAF, General Assessment of Functioning; SOFAS, Social and Occupational Functioning Assessment Scale.
Clinical Outcome
There were no significant differences between groups on change in negative symptoms (PANSS−; β = 0.161, SE = 0.237, P = .50), positive symptoms (PANSS+; β = 0.176, SE = 0.207, P = .39), general symptomatology (PANSS general score; β = 0.598, SE = 0.359, P = .09), or functional outcome (GAF; β = −0332, SE = 0.515, P = .52 and SOFAS; β = −0.196, SE = 0.527, P = .71) (figure 1B–C). Results remained the same when controlling for age, sex, duration of illness at baseline, and level of co-medication based on chlorpromazine equivalents.
Neurocognition
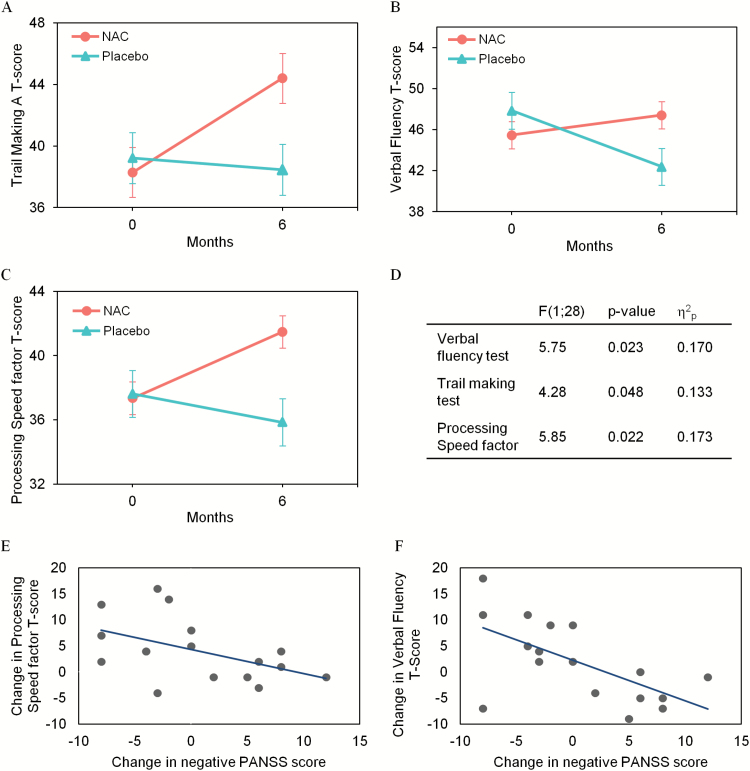
NAC improved cognitive speed in both the verbal fluency (F[1, 28] = 5.75, P = .023, = 0.17) and the trail making A (F[1, 28] = 4.28, P = .048, = 0.13) tests, and in the processing speed (PS) factor containing these tasks (F[1, 28] = 5.85, P = .022, = 0.17; figure 2A–D). Symbol coding, the third task in the PS factor, did not change significantly (F[1, 27] = 0.710, P = .407, = 0.03). Improvement in PS was significantly correlated with a decrease in negative symptoms in NAC-treated patients (PS factor: r = .491, P = .047; verbal fluency: r = .546, P = .023; figure 2E–F). Other cognitive domains were unaffected, and results were the same when controlling for chlorpromazine equivalent antipsychotic dosage (supplementary table 3).
Fig. 2.
Effect of NAC vs placebo on processing speed’s measures. Evolution of trail making test (A), verbal fluency (B), processing speed factor T-scores (C) in NAC (red circles), and placebo (green triangles) groups. (D) Statistical comparison between NAC and placebo groups; partial-eta-square ( ) indicates the effect size. Linear correlations between changes in PANSS− and changes in processing speed factor (E) and verbal fluency T-scores (F) in NAC-treated patients.
Peripheral and Central Biochemical Outcome Measures
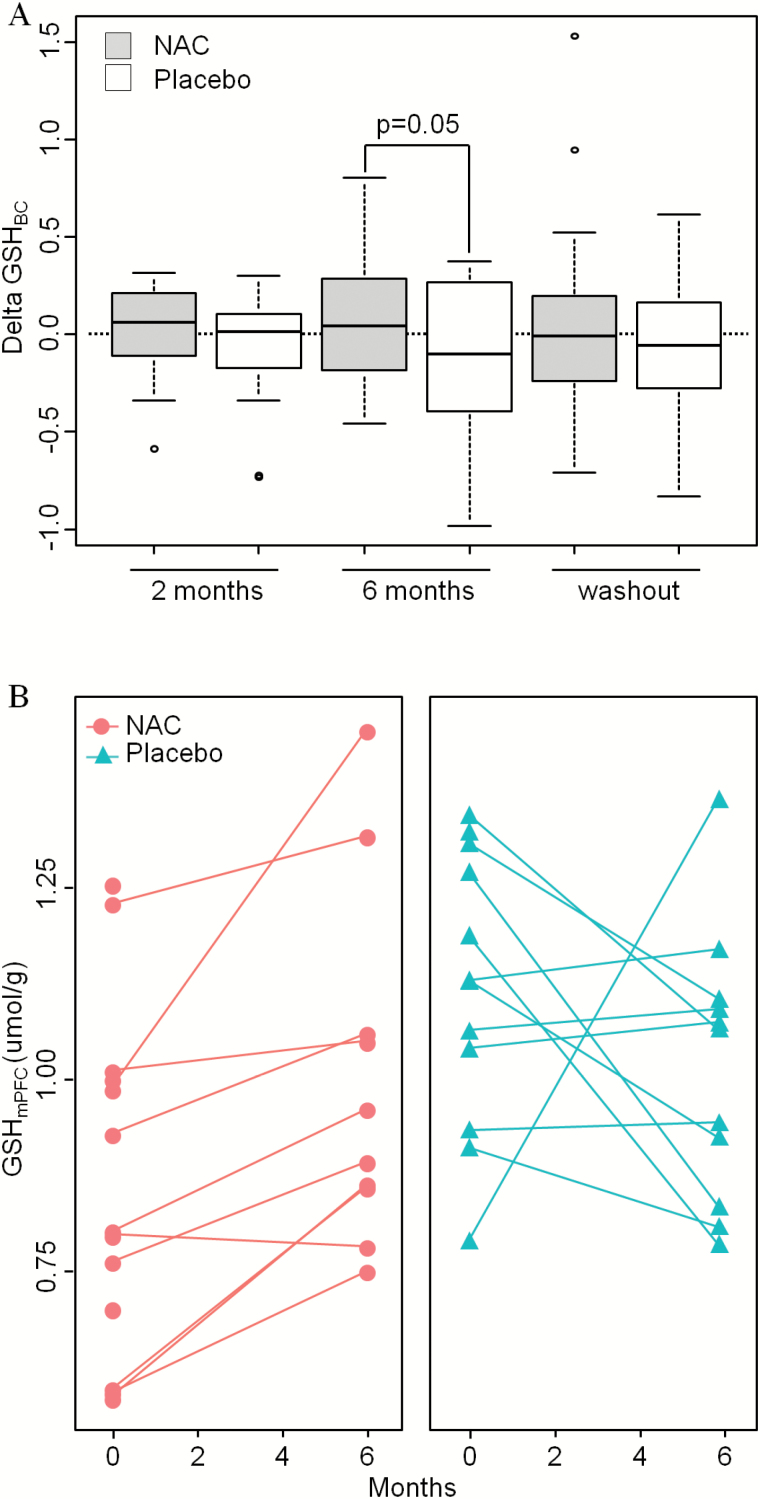
Measurement of changes in GSHBC peripheral blood levels from baseline to month 6, using models adjusted for age and sex, revealed an average increase of 18.8% (SD = 50.2%) in patients receiving NAC while it decreased by 1.9% (SD = 48.7%) in the placebo group, leading to an overall difference of 17% (figure 3A, P = .05). No change in CysPL was found.
Fig. 3.
Effect of NAC vs placebo on GSH levels. (A) Boxplot illustrating changes vs baseline in GSHBC after 2 and 6 months and after washout (7 months) of treatment with NAC (gray boxes) or placebo (white boxes). (B) GSHmPFC levels in patients with NAC (n = 12, red circles, left) or placebo (n = 12, green triangles, right).
Paired t-tests indicate that GSHmPFC levels assessed by 1H-MRS increased after 6 months of NAC administration (mean percentage of change: +22.6%; 7 among 10 patients increased GSHmPFC by more than 10%, P = .005), while it remained stable in the placebo group (mean percentage of change: −4.6%; 1 among 11 patients increased GSHmPFC by more than 10%), leading to an overall difference of 28% (figure 3B). This suggests that oral administration of NAC affects brain GSH levels. However, we cannot rule out that a spontaneous increase of brain GSH contributes to this effect in NAC-treated patients as their basal levels were lower than in the placebo group. Moreover, no changes in Glu, Gln, Gln/Glu, and Ins were observed in NAC or placebo groups (supplementary table 4).
NAC Improves Positive Symptoms in a Subsample of Patients
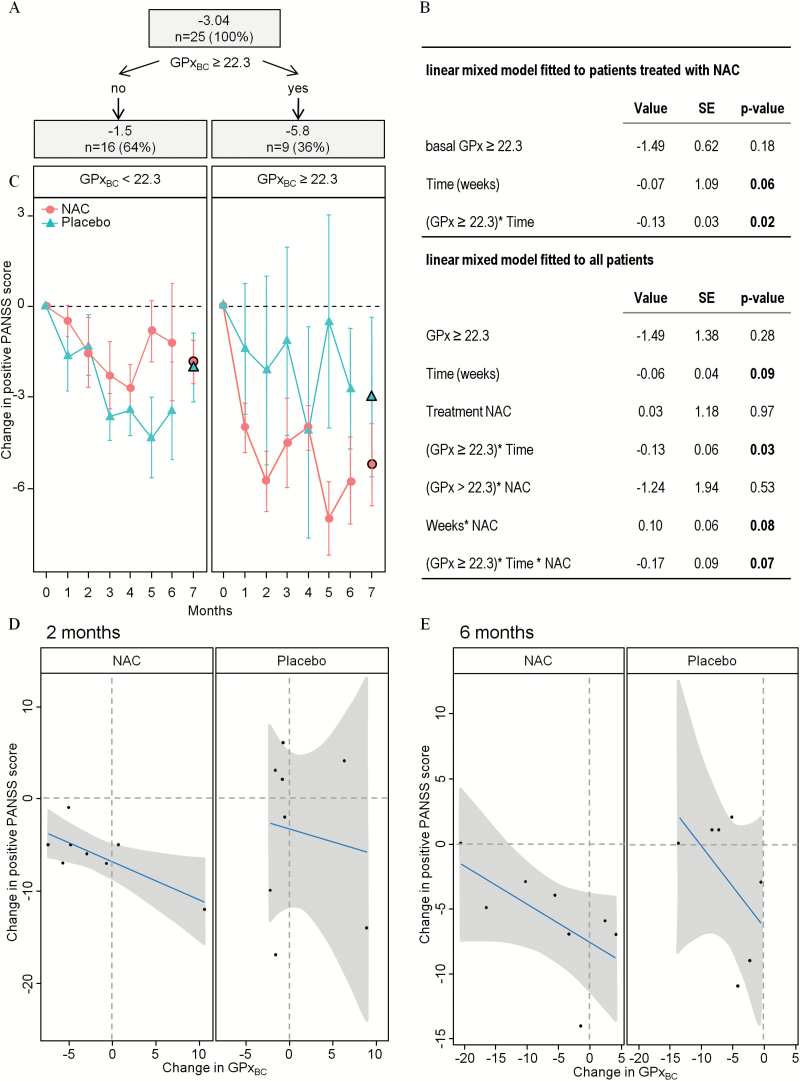
To explore whether patients with baseline peripheral redox dysregulation were more likely to benefit from NAC supplementation, we performed exploratory analyses using CARTs among NAC-treated patients looking for the best predictors of clinical improvement after 2 months among baseline redox activity measures (ie, blood levels of GSHBC cysteine, homocysteine, glutamate, and enzymatic activity of GPxBC, GR and their ratio). Improvement in the positive PANSS score after 2 months of NAC treatment was highly associated with baseline GPxBC activity: patients with baseline GPxBC activity higher than 22.3 U/g of Hb improved their PANSS+ scores at the second month and onwards (figure 4A). None of the tested variables were good predictors of negative symptoms PANSS improvement.
Fig. 4.
Additional analysis by classification and regression tree. (A) Illustration of the classification based on GPxBC enzymatic activity in the NAC group: 36% of patients have an activity at baseline above 22.3 U/g of Hb associated with a mean decrease of PANSS+ score of −5.8 points; 64% of patients have an activity at baseline below 22.3 U/g of Hb associated with a mean improvement of PANSS+ score of −1.5 point. (B) Effect of NAC add-on treatment on PANSS+ scores on patients with baseline activity of GPxBC above 22.3 U/g of Hb. Values are coefficients of each factor in the linear model. SE, standard error. P-values under 0.1 are indicated in bold. (C) Evolution of PANSS+ scores vs baseline in patients with low (left) or high (right) basal activity of GPxBC in NAC (red circles) or placebo (green triangles) groups during the trial (1–6 months) and after washout (7 months). (D, E) Changes in PANSS+ scores vs baseline and changes in GPxBC activity vs baseline is illustrated by linear correlation in patients with basal activity of GPxBC above 22.3 U/g of Hb. Patients under NAC (left) or Placebo (right) at 2 (D) and 6 months (E). Dots depict individual values, gray zones 95% confidence intervals.
A longitudinal model fitted to PANSS+ scores confirmed an improvement over time in NAC-treated patients with high baseline GPxBC compared to low baseline GPxBC (P = .02, figure 4B, model fitted to patients with NAC). This difference remained significant after adjustment for disease duration and chlorpromazine equivalent doses. Among all participants having a high baseline GPxBC activity, a longitudinal improvement in PANSS+ was not observed in placebo, while a trend was seen under NAC (P = .07, figure 4B–C, model fitted to all patients), also after adjusting for disease duration and chlorpromazine equivalent doses. Furthermore, the change in GPxBC activity correlated negatively with the change of PANSS+ score showing that the positive symptoms improved when GPx activity remained stable. This was not observed in the placebo group (figure 4D). The correlation in the NAC group was significant at the second month (r =− .76; P = .03) and a trend remained at the sixth month (r = −.63; P = .09, figure 4E).
Side Effects and Postdiscontinuation Data
There were no significant side effects of NAC as measured by the UKU scale (supplementary table 5). The effect of NAC on GSHBC at 6 months was lost upon washout (7th month, P = .55 after adjustment for age and sex, figure 4C). Mean PANSS+ scores changes were similar in NAC- and placebo-treated groups after treatment discontinuation (P = .52). In patients with high basal GPxBC, mean PANSS+ scores changes were 74% greater after discontinuation of NAC compared to placebo, however, this was not significant (figure 4C, P = .48).
Discussion
This first RCT assessing the impact of NAC treatment in a sample of EP patients and the potential predictive role of peripheral biomarkers of redox dysregulation yielded 3 main findings. First, the addition of NAC to standard treatment in an unselected sample of patients improved neither symptomatic nor functional outcome. Second, oral administration of NAC increased glutathione levels in the brain and improved neurocognition, namely processing speed. Third, redox peripheral index based on blood cells GPx activity identified EP patients displaying a significant response to NAC in their positive symptoms.
Considering the impact observed in a larger sample of 140 chronic patients,26 we hypothesized that NAC would be more beneficial on negative symptoms in an earlier phase of illness, when the adverse impact of redox dysregulation is still unfolding. However, this was not confirmed in this small sample. Besides the obvious limitation of sample size, it must be mentioned that modest baseline negative symptoms levels limited the range for improvement in this domain.
Regarding neurocognition, all significant improvements were observed in the same cognitive domain (processing speed). While multiple comparisons suggest these results need replication, overall 2 out of 9 MCCB tests (1 of 6 factors) were significant in this study which is above what could be expected by chance. Indeed, performing 9 tests with an alpha level set to 0.05 in each case would lead on average to 9 × 0.05 = 0.45, ie, less than one false positive under the null hypothesis (no difference). As 2 cognitive tasks were significant and as they are all in the same cognitive domain, we can argue that there are likely real differences for processing speed. This dimension was assessed by a third task (symbol coding) which was not improved by NAC. However, this task also involves new learning which the 2 other tests do not. Nevertheless, this promising finding must be replicated in a larger sample.
The demonstration of an increase in cerebral GSH levels in vivo after 6 months oral administration of NAC is important considering the low bioavailability of NAC53 and its debated capability to cross the blood–brain barrier.37–40 An increase of cerebral GSH levels has also been confirmed with a single intravenous administration of NAC in healthy controls, patients with Parkinson’s diseases and Gaucher disease.54 NAC is known to be hydrolyzed into cysteine, the limiting precursor for GSH synthesis. Considering GSH’s limited ability to cross the blood–brain barrier and the absence of elevation of cysteine in patients’ blood, increased brain GSH may be linked to transport of NAC rather than cysteine across the blood–brain barrier, an important finding regarding treatment development.
In a previous study of the glutathione-deficient schizophrenia mouse model, NAC treatment also led to neurochemical changes including glutamate (Glu), glutamine (Gln), Gln/Glu and myoinositol.41 However, the effect of NAC on these metabolites was not observed in the current trial, either implying that NAC does not have the same normalizing effect in EP patients or that neurochemical alterations present at this illness stage are not reversible. The observation that NAC normalized most neurochemical alterations in peripubertal animal models to normal levels suggests NAC should be tried in individuals at ultra high risk for psychosis, ie, at an earlier developmental stage.
The correlation between improvement in PANSS+ score and changes of peripheral redox markers such as GPxBC, in association with brain GSH levels are important findings. Indeed, we observed previously a negative correlation between GSHmPFC and GPxBC activity showing that low GSHmPFC was associated with high GPxBC reflecting high oxidation status.55 In many fields of medicine, treatment monitoring is based not only on the symptomatic response, but also on modifications of biological markers.56 We showed here for the first time that NAC administration modified peripheral redox status which paralleled clinical improvement. For clinical characteristics like body mass index and smoking, which are known to influence redox status and cognition, no differences between groups at baseline and at the end of treatment were observed. Moreover, smoking status and NAC add-on treatment seemed to affect different cognitive skills. Our results on the effects of NAC are not accounted for by body mass index or smoking.
Moreover, our results suggest that peripheral redox status allows the identification of a patient subgroup benefiting from NAC supplementation. The best GPxBC blood level to segregate responders from nonresponders in our small sample was 22.3 U/gHb, but this should be refined and confirmed in larger independent cohorts. This replication study should clarify if GPx peripheral redox marker reflects brain function. It is notable that in high GPxBC patients, NAC effect on PANSS+ score was already reached after 2 months, suggesting a 2–3 months supplementation with NAC might be sufficient in a validation study within a larger cohort.
There are limitations to this trial. First, these conclusions come from post hoc analyses, and therefore a prospective hypothesis testing trial is needed to confirm them. Second, the sample size is relatively small, and we may have failed to find significant differences for that reason. The MRS study was limited to 12–13 subjects per group, as many patients declined to participate in this more demanding investigation. Third, the severity of baseline negative symptoms was relatively modest, leaving limited room for improvement. Finally, the finding that only 36% of the patients might respond to NAC based on high GPxBC implies that the power of our study was lower than initially estimated, due to a large prevalence of a nonresponder phenotype. This also suggests that early phases of the disease could be associated with phenotypic heterogeneity which would need valid biomarker-guided treatments.
Conclusion
We believe this to be an important step toward biomarker-guided treatment, as it might allow identifying a subgroup of patients susceptible to benefit from NAC treatment. While this trial of NAC administration as an add-on to standard antipsychotic treatment in a sample of EP patients failed to show global benefits on negative symptoms, secondary analyses showed that NAC improves processing speed and positive symptoms in subjects who display a certain level of redox dysregulation assessed by peripheral markers at baseline. This fits well with the concept that schizophrenia may be composed of various endophenotypes based on different neurobiological mechanisms57 responding to distinct treatments, and represents a relevant step in the development of biomarker-guided treatment with a redox regulator in EP.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
This work was supported by the Swiss National Science Foundation (320030_122419 to P.C. and KQ.D.), National Center of Competence in Research (NCCR) “SYNAPSY—The Synaptic Bases of Mental Diseases” financed by the Swiss National Science Foundation (no. 51AU40_125759).
Supplementary Material
Acknowledgment
We are grateful to Hélène Moser for expert technical assistance, to Tania Teichman for patients’ evaluation, and to all collaborators of the Section Minkowski for their help in patients’ recruitment. We thank the patients for their participation. Magnetic resonance spectroscopy was performed in the Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, and the Leenaards and Jeantet Foundations. We are grateful for support from the Loterie Romande, Avina Foundation, Damm-Etienne Foundation, Stanley Thomas Johnson Foundation, Pro Scientia et Arte Foundation, and Alamaya Foundation. We thank Bioadvantex Pharma, Inc. for providing NAC. We are grateful for support from the Commonwealth of Massachusetts Center of Excellence in Clinical Neuroscience and Psychopharmacological Research (SCDMH82101008006, L.J.S.), a donation supporting the research from the Weisman family (L.J.S.), and to Chelsea Wakeham and Raquelle Mesholam-Gately for their assistance in carrying out the study. Author Contributions: P.C., K.Q.D., M.C., L.J.S., T.B. conceived, planned, and designed the study. C.F., P.S.B., L.A., R.J. (Lausanne site) and A.C., T.-U.W.W., M.S.K., J.W., L.J.S. (Boston site) recruited patients, collected and analyzed clinical data. T.B. monitored the pharmacological aspects of the study. C.B.E. performed the determination of antipsychotic blood levels. R.G. and L.X. developed, performed, and analyzed MRS data. K.Q.D. and M.F. designed, performed, and analyzed biochemical data. M.G. and P.G. performed the statistical analyses. K.Q.D., P.C., M.C., L.J.S., and M.F. interpreted the results. P.C., K.D., M.F., and L.J.S. wrote the manuscript with contributions from all authors. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Robinson DG, Schooler NR, John M et al. Prescription practices in the treatment of first-episode schizophrenia spectrum disorders: data from the national RAISE-ETP study. Am J Psychiatry. 2015;172:237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Möller HJ, Czobor P. Pharmacological treatment of negative symptoms in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2015;265:567–578. [DOI] [PubMed] [Google Scholar]
- 3. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia?Am J Psychiatry. 1996;153:321–330. [DOI] [PubMed] [Google Scholar]
- 4. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”?Schizophr Bull. 2000;26:119–136. [DOI] [PubMed] [Google Scholar]
- 5. Buckley PF, Stahl SM. Pharmacological treatment of negative symptoms of schizophrenia: therapeutic opportunity or cul-de-sac?Acta Psychiatr Scand. 2007;115:93–100. [DOI] [PubMed] [Google Scholar]
- 6. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr Res. 2008;102:1–18. [DOI] [PubMed] [Google Scholar]
- 7. Dunlop J, Brandon NJ. Schizophrenia drug discovery and development in an evolving era: are new drug targets fulfilling expectations?J Psychopharmacol. 2015;29:230–238. [DOI] [PubMed] [Google Scholar]
- 8. Sawa A, Seidman LJ. Is prophylactic psychiatry around the corner? Combating adolescent oxidative stress for adult psychosis and schizophrenia. Neuron. 2014;83:991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Donnell P. Cortical interneurons, immune factors and oxidative stress as early targets for schizophrenia. Eur J Neurosci. 2012;35:1866–1870. [DOI] [PubMed] [Google Scholar]
- 10. Steullet P, Cabungcal JH, Monin A et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology?Schizophr Res. 2016;176:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hardingham GE, Do KQ. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci. 2016;17:125–134. [DOI] [PubMed] [Google Scholar]
- 12. Landek-Salgado MA, Faust TE, Sawa A. Molecular substrates of schizophrenia: homeostatic signaling to connectivity. Mol Psychiatry. 2016;21:10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230. [DOI] [PubMed] [Google Scholar]
- 14. Yao JK, Keshavan MS. Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxid Redox Signal. 2011;15:2011–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martins-de-Souza D, Harris LW, Guest PC, Bahn S. The role of energy metabolism dysfunction and oxidative stress in schizophrenia revealed by proteomics. Antioxid Redox Signal. 2011;15:2067–2079. [DOI] [PubMed] [Google Scholar]
- 16. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coughlin JM, Ishizuka K, Kano SI et al. Marked reduction of soluble superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with recent-onset schizophrenia. Mol Psychiatry. 2013;18:10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Do KQ, Trabesinger AH, Kirsten-Krüger M et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. [DOI] [PubMed] [Google Scholar]
- 19. Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–130. [DOI] [PubMed] [Google Scholar]
- 20. Matsuzawa D, Obata T, Shirayama Y et al. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-MRS study. PLoS One. 2008;3:e1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsuzawa D, Hashimoto K. Magnetic resonance spectroscopy study of the antioxidant defense system in schizophrenia. Antioxid Redox Signal. 2011;15:2057–2065. [DOI] [PubMed] [Google Scholar]
- 22. Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodríguez-Santiago B, Brunet A, Sobrino B et al. Association of common copy number variants at the glutathione S-transferase genes and rare novel genomic changes with schizophrenia. Mol Psychiatry. 2010;15:1023–1033. [DOI] [PubMed] [Google Scholar]
- 24. Tosic M, Ott J, Barral S et al. Schizophrenia and oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene. Am J Hum Genet. 2006;79:586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gysin R, Kraftsik R, Sandell J et al. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci USA. 2007;104:16621–16626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berk M, Copolov D, Dean O et al. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. [DOI] [PubMed] [Google Scholar]
- 27. Carmeli C, Knyazeva MG, Cuénod M, Do KQ. Glutathione precursor N-acetyl-cysteine modulates EEG synchronization in schizophrenia patients: a double-blind, randomized, placebo-controlled trial. PLoS One. 2012;7:e29341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lavoie S, Murray MM, Deppen P et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–2199. [DOI] [PubMed] [Google Scholar]
- 29. Farokhnia M, Azarkolah A, Adinehfar F et al. N-acetylcysteine as an adjunct to risperidone for treatment of negative symptoms in patients with chronic schizophrenia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2013;36:185–192. [DOI] [PubMed] [Google Scholar]
- 30. Hager BM, Keshavan MS. Neuroimaging biomarkers for psychosis. Curr Behav Neurosci Rep. 2015;2015:1–10. [PMC free article] [PubMed] [Google Scholar]
- 31. Cabungcal JH, Steullet P, Morishita H et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci USA. 2013;110:9130–9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cabungcal JH, Counotte DS, Lewis EM et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron. 2014;83:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kern RS, Nuechterlein KH, Green MF et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165:214–220. [DOI] [PubMed] [Google Scholar]
- 34. Nuechterlein KH, Green MF, Kern RS et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. [DOI] [PubMed] [Google Scholar]
- 35. Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. [DOI] [PubMed] [Google Scholar]
- 36. Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, Woodberry KA, Seidman LJ. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des. 2012;18:399–415. [DOI] [PubMed] [Google Scholar]
- 37. Sheffner AL, Medler EM, Bailey KR, Gallo DG, Mueller AJ, Sarett HP. Metabolic studies with acetylcysteine. Biochem Pharmacol. 1966;15:1523–1535. [DOI] [PubMed] [Google Scholar]
- 38. Sunitha K, Hemshekhar M, Thushara RM et al. N-Acetylcysteine amide: a derivative to fulfill the promises of N-acetylcysteine. Free Radic Res. 2013;47:357–367. [DOI] [PubMed] [Google Scholar]
- 39. Farr SA, Poon HF, Dogrukol-Ak D et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173–1183. [DOI] [PubMed] [Google Scholar]
- 40. Shungu DC. N-acetylcysteine for the treatment of glutathione deficiency and oxidative stress in schizophrenia. Biol Psychiatry. 2012;71:937–938. [DOI] [PubMed] [Google Scholar]
- 41. das Neves Duarte JM, Kulak A, Gholam-Razaee MM, Cuenod M, Gruetter R, Do KQ. N-acetylcysteine normalizes neurochemical changes in the glutathione-deficient schizophrenia mouse model during development. Biol Psychiatry. 2012;71:1006–1014. [DOI] [PubMed] [Google Scholar]
- 42. Baumann PS, Crespi S, Marion-Veyron R et al. Treatment and early intervention in psychosis program (TIPP-Lausanne): implementation of an early intervention programme for psychosis in Switzerland. Early Interv Psychiatry. 2013;7:322–328. [DOI] [PubMed] [Google Scholar]
- 43. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 44. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–693. [DOI] [PubMed] [Google Scholar]
- 46. Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. [DOI] [PubMed] [Google Scholar]
- 47. Mekle R, Mlynárik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med. 2009;61:1279–1285. [DOI] [PubMed] [Google Scholar]
- 48. Tkác I, Oz G, Adriany G, Uğurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med. 2009;62:868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vester B, Rasmussen K. High performance liquid chromatography method for rapid and accurate determination of homocysteine in plasma and serum. Eur J Clin Chem Clin Biochem. 1991;29:549–554. [DOI] [PubMed] [Google Scholar]
- 50. Gysin R, Kraftsik R, Boulat O et al. Genetic dysregulation of glutathione synthesis predicts alteration of plasma thiol redox status in schizophrenia. Antioxid Redox Signal. 2011;15:2003–2010. [DOI] [PubMed] [Google Scholar]
- 51. Günzler WA, Kremers H, Flohé L. An improved coupled test procedure for glutathione peroxidase (EC 1-11-1-9-) in blood. Z Klin Chem Klin Biochem. 1974;12:444–448. [DOI] [PubMed] [Google Scholar]
- 52. Breiman L, Friedman J, Stone CJ, Olshen RA.. Classification and Regression Trees.1st ed Monterey, CA: Wadsworth Statistics/Probability; 1984;184. [Google Scholar]
- 53. Sjödin K, Nilsson E, Hallberg A, Tunek A. Metabolism of N-acetyl-l-cysteine. Some structural requirements for the deacetylation and consequences for the oral bioavailability. Biochem Pharmacol. 1989;38:3981–3985. [DOI] [PubMed] [Google Scholar]
- 54. Holmay MJ, Terpstra M, Coles LD et al. N-acetylcysteine boosts brain and blood glutathione in Gaucher and Parkinson diseases. Clin Neuropharmacol. 2013;36:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xin L, Mekle R, Fournier M et al. Genetic polymorphism associated prefrontal glutathione and its coupling with brain glutamate and peripheral redox status in early psychosis. Schizophr Bull. 2016;42:1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Trusheim MR, Berndt ER, Douglas FL. Stratified medicine: strategic and economic implications of combining drugs and clinical biomarkers. Nat Rev Drug Discov. 2007;6:287–293. [DOI] [PubMed] [Google Scholar]
- 57. Clementz BA, Sweeney JA, Hamm JP et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry.2016;173:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.