Abstract
Canada and the USA differ in their recommendations for the use of live attenuated influenza vaccine (LAIV). The Canadian National Advisory Committee on Immunization (NACI) continues to recommend LAIV as one of the influenza vaccines available for use in children 2 to 17 years of age. The US Advisory Committee on Immunization Practices (ACIP) made an interim recommendation against the use of LAIV for the 2016 to 2017 influenza season in response to low LAIV effectiveness observed in the USA during the 2013 to 2014 to 2015 to 2016 seasons. The recommendation has been continued for the 2017 to 2018 season. In response, NACI undertook a review of available LAIV effectiveness data in children and adolescents from Canada, the USA and a number of European countries. This commentary by Canada’s Chief Public Health Officer summarizes the findings of that review and provides the rationale for Canada’s current continued recommendation for LAIV use.
Live attenuated influenza vaccine (LAIV) is administered by intranasal spray rather than by intramuscular injection. The influenza strains in LAIV are attenuated so that they do not cause influenza and are cold-adapted and temperature sensitive, so that they replicate in the nasal mucosa, rather than in the lower respiratory tract (1). In Canada and the USA, LAIV is manufactured by MedImmune (a subsidiary of AstraZeneca) and marketed as FluMist®. The trivalent formulation of FluMist® was approved by Health Canada for use in persons 2 to 59 years of age in June 2010 and was first used in publicly funded immunization programs in Canada for the 2012 to 2013 influenza season. FluMist® Quadrivalent was first used in Canada for the 2014 to 2015 season. The trivalent vaccine is no longer available in Canada.
Influenza vaccine effectiveness (VE) is a measure of the per cent reduction in the frequency of influenza among persons who have been immunized against influenza compared to those who have not. Observational methods, such as case–control and cohort study designs, are commonly used for estimating influenza VE. In recent years, influenza VE has been generally evaluated using the test-negative design (TND), which is a type of case–control study. Briefly, in the TND, patients seeking care for acute respiratory infection (ARI) or influenza-like illness (ILI) are enrolled and tested for influenza virus infection. The patient’s influenza immunization history is determined through self-report or verification through medical records or immunization registries, depending upon the study. As for any observational study design, there is potential for biases, but by enrolling only patients seeking care for ARI or ILI, the TND implicitly controls for selection bias associated with healthcare seeking behaviour. These studies also usually attempt to adjust for other potential biases (e.g., age group, chronic medical conditions) in their analysis.
Influenza VE estimates from the US Influenza Vaccine Effectiveness Network (US Flu VE Network) showing that quadrivalent LAIV provided no evidence of effectiveness during the influenza A(H1N1) dominant 2015 to 2016 influenza season (2) or against the dominant circulating strains in the two prior influenza seasons (2013 to 2014 and 2014 to 2015) led to the US Advisory Committee on Immunization Practices (ACIP) interim recommendation against its use during the 2016 to 2017 influenza season (3). This recommendation has been continued for the 2017 to 2018 influenza season (4). In light of the ACIP recommendation, it is important to provide Canadian paediatricians, family physicians, nurse practitioners, pharmacists and other child and youth health professionals with the rationale for why the current recommendation for LAIV use in Canada differs from the USA.
In Canada, the National Advisory Committee on Immunization (NACI) provides the Public Health Agency of Canada (PHAC) with medical, scientific and public health advice related to immunization. NACI recommends annual influenza immunization for all Canadians 6 months of age and older who do not have contraindications to the vaccine (5). However, the effectiveness of influenza vaccines can vary from season to season, so it is important to continually evaluate their effectiveness to inform Canadian influenza immunization program recommendations.
In 2011, NACI recommended the preferential use of LAIV in children and adolescents 2 to 17 years of age, in anticipation of the availability of trivalent LAIV for the 2012 to 2013 influenza season. The recommendation was based on randomized placebo controlled studies and postmarketing safety data, which showed LAIV to be safe, efficacious, and immunogenic and to provide better protection against influenza in children than trivalent inactivated influenza vaccine (TIV) (6). The recommendation was continued when the trivalent formulation was replaced by FluMist® Quadrivalent for the 2014 to 2015 season.
In response to the US LAIV effectiveness results and publication of the interim ACIP recommendation against the use of LAIV for the 2016 to 2017 influenza season, NACI undertook a review of available national and international data on LAIV and inactivated influenza vaccine (IIV) VE. Data were reviewed for the 2010 to 2011 to 2015 to 2016 influenza seasons in children and adolescents 2 to 17 years of age.
As a result of this review, NACI continues to recommend LAIV, concluding that the current evidence no longer supports a recommendation for the preferential use (as previously recommended) of LAIV in children 2 to 17 years of age, but that the findings are consistent with LAIV providing comparable protection against influenza to that afforded by IIV in various jurisdictions, including Canada. Given this, LAIV remains one of the influenza vaccines available for use in this age group, along with quadrivalent inactivated influenza vaccine (QIV) and TIV (5).
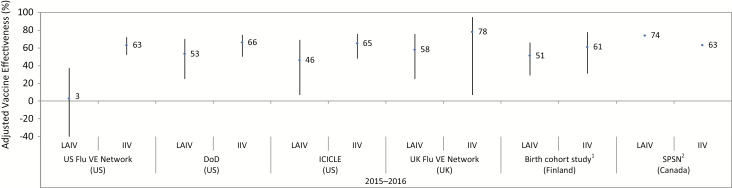
As an example of the analysis undertaken as part of the review, in the influenza A(H1N1) predominant 2015 to 2016 influenza season, NACI reviewed LAIV VE data from studies conducted in the USA (7,8), the UK (9), Finland (10) and Canada (11). The NACI review found that four of the five studies conducted in other countries observed LAIV to have moderate and statistically significant effectiveness against any influenza (7–10) (Figure 1). In Canada, the Canadian Sentinel Practitioner Surveillance Network (SPSN) also observed significant LAIV effectiveness against any influenza, although this point estimate had wide confidence intervals (11). In contrast to these other studies, the US Flu VE Network found a low, nonsignificant LAIV effectiveness against any influenza (8).
Figure 1.
Adjusted vaccine effectiveness estimates against any influenza by study and vaccine type for the 2015 to 2016 influenza season in children and adolescents 2 to 17 years of age#. DoD United States Department of Defense; ICICLE Influenza Clinical Investigation for Children study; IIV inactivated influenza vaccine; LAIV live attenuated influenza vaccine; SPSN Canadian Sentinel Practitioner Surveillance Network; UK Flu VE Network United Kingdom Influenza Vaccine Effectiveness Network; US FLU VE Network United States Influenza Vaccine Effectiveness Network; %, percentage. #For each study, the black circles represent the vaccine effectiveness point estimates for LAIV and IIV and the vertical bars represent the 95% confidence interval around the point estimates. The 95% confidence interval lower limits are truncated at –40%. A vaccine with a confidence interval that includes zero is considered ineffective. Vaccines whose estimates have overlapping confidence intervals are considered not to be significantly different in effectiveness. 1The Finland birth cohort study reported vaccine effectiveness in children 2 years of age. 2The Canadian SPSN reported wide and overlapping 95% confidence intervals (exact values not publicly available at time of writing).
Looking specifically at influenza A(H1N1) in the 2015 to 2016 influenza season, the US Flu VE Network (8) and data on US Air Force dependents from the US Department of Defense (8) found lower, nonsignificant estimates of LAIV VE that were more consistent with no vaccine protection. In contrast, LAIV VE estimates from the UK (9) and Finland (10), which have since been published, found moderate LAIV VE (42% to 48%) that was similar to estimates of LAIV VE from Canada’s SPSN (11) and the manufacturer’s postmarketing study of LAIV VE in the USA (7) (about 50%). However, only the LAIV VE estimate from the Finish study was statistically significant.
All six studies also allowed for a comparison of the observed VE of LAIV and IIV against any influenza and against influenza A(H1N1). Although all of the studies found the estimated effectiveness of LAIV to be lower than for IIV against any influenza (Figure 1) and against influenza A(H1N1) (7–11), only in the US Flu VE Network were these differences statistically significant.
The findings of this review and NACI’s recommendations for LAIV use were published in a NACI Addendum (12) and subsequently summarized in both the NACI Statement on Seasonal Influenza Vaccine 2017–2018 (5) and in a Canada Communicable Disease Report summary of the statement (13). The NACI decision to continue to recommend the use of LAIV for the 2016 to 2017 influenza season is consistent with decisions made in the UK and Finland (9,10).
We do not fully understand why LAIV VE estimates in the US Flu VE Network differ from those in Canada, the UK and Finland. Some of the theories are that they may reflect biological mechanisms, methodological issues or both. The manufacturer of FluMist® is currently conducting an investigation focusing on the ability of the H1N1pdm09 LAIV strain in the vaccine to reproduce in human cells and whether the other virus strains in the vaccine may in some way interfere with the immune response elicited by the H1N1pdm09 LAIV strain. Preliminary findings suggest a lower reproductive ability of the H1N1pdm09 component of the vaccine; the evaluation of vaccine virus interference is ongoing (14). There are also potential differences in the study populations and study methodologies that could in part explain the different VE estimates between studies.
Vaccination remains the most effective way to prevent influenza and its complications, such as in young children who are an identified high-risk group for influenza-related complications and hospitalization. Health care providers are encouraged to take every opportunity to review a child’s influenza vaccination status and offer influenza vaccination as appropriate.
The Public Health Agency of Canada will continue to monitor the effectiveness of influenza vaccines, including LAIV, to inform influenza immunization program recommendations in Canada. Based on NACI’s recent careful review of the evidence, the PHAC continues to support NACI’s recommendation that LAIV is one of the influenza vaccines recommended for use in children 2 to 17 years of age for the upcoming 2017 to 2018 influenza season, along with QIV and TIV. In children 6 to 23 months of age TIV, QIV and adjuvanted TIV vaccines are available. Given the burden of influenza B disease in children and the potential for lineage mismatch between the predominant circulating strain of influenza B and the strain in a trivalent vaccine, NACI continues to recommend that a quadrivalent formulation of influenza vaccine be used in children. If a quadrivalent vaccine is not available, a trivalent formulation should be used.
References
- 1. AstraZeneca Canada. Product Monograph: FLUMIST® QUADRIVALENT Influenza Vaccine (live, attenuated) Intranasal spray (ATC Code: J07BB03) https://pdf.hres.ca/dpd_pm/00036926.PDF (Accessed August 25, 2017).
- 2. Jackson ML, Chung JR, Jackson LA et al. . Influenza vaccine effectiveness in the united states during the 2015-2016 season. N Engl J Med 2017;377(6):534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grohskopf LA, Sokolow LZ, Broder KR et al. . Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016;65(5):1–54. [DOI] [PubMed] [Google Scholar]
- 4. Grohskopf LA, Sokolow LZ, Broder KR et al. . Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practice – United States, 2017–18 Influenza Season. MMWR Recomm Rep 2017;66(No. RR–2). https://www.cdc.gov/mmwr/volumes/66/rr/rr6602a1.htm (Accessed August 25, 2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Advisory Committee on Immunization. Statement on Seasonal Influenza Vaccine for 2017–2018 https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/healthy-living/canadian-immunization-guide-statement-seasonal-influenza-vaccine/naci-stmt-2017-2018-eng.pdf (Accessed: August 22, 2017).
- 6. National Advisory Committee on Immunization. Recommen dations on the use of live, attenuated influenza vaccine (FluMist®): Supplemental Statement on Seasonal Influenza Vaccine for 2011–2012. CCDR. 2011;37:ACS-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ambrose C. 2015–16 US influenza vaccine effectiveness: Influenza Clinical Investigation for Children (ICICLE) Study. Presented to Advisory Committee on Immunization Practices, Atlanta 2016. [Google Scholar]
- 8. Flannery B, Chung J. Influenza vaccine effectiveness, including LAIV vs IIV in children and adolescents, US Flu VE Network, 2015–16. Presented to Advisory Committee on Immunization Practices, Atlanta 2016. [Google Scholar]
- 9. Pebody R, Warburton F, Ellis J et al. . Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 end-of-season results. Euro Surveill. 2016;21(38):pii=30348 10.2807/1560-7917.ES.2016.21.38.30348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nohynek H, Baum U, Syrjanen R et al. . Effectiveness of the live attenuated and the inactivated influenza vaccine in two-year-olds – a nationwide cohort study Finland, influenza season 2015/16. Euro Surveill. 2016;21(38):pii=30346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skowronski DM. Live attenuated influenza vaccine (LAIV) vs. inactivated influenza vaccine (IIV): Summary of effectiveness evidence since 2009. Presented to NACI Influenza Working Group, Ottawa 2016. [Google Scholar]
- 12. National Advisory Committee on Immunization. Addendum – LAIV use in children and adolescents https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/canadian-immunization-guide-chapter-on-influenza-statement-on-seasonal-influenza-vaccine-2016-2017-addemdum-laiv-use-children-adolescents.html (Accessed August 23, 2017).
- 13. Vaudry W, Stirling R on behalf of the National Advisory Committee on Immunization Summary of the NACI Statement on Seasonal Influenza Vaccine for 2017–2018. Can Commun Dis Rep. 2017;43(5):96–103. https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2017–43/ccdr-volume-43-5-may-4-2017/summary-naci-statement-seasonal-influenza-vaccine-2017–2018.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bright H, Mallory R. Update on Status of Investigation of Reduced LAIV Effectiveness. Presented to Advisory Committee on Immunization Practices, Atlanta 2017. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/influenza-04-bright-mallory.pdf (Accessed August 23, 2017). [Google Scholar]