Key Points
Question
Is there any dietary influence on UV-B–induced changes in skin histological features?
Findings
In this animal study, long-term UV-B irradiation was administered to the dorsal skin in mice fed normal, calorie-restricted, and obesity diets. Histopathological changes were monitored with light microscopic morphometry and immunohistochemistry.
Meaning
Dietary modulation of skin histological response to UV-B irradiation was observed.
Abstract
Importance
Long-term exposure to solar radiation produces deleterious photoaging of the skin. It is not known if diet can influence skin photoaging.
Objectives
To study the influence of a calorie-restricted diet and an obesity diet in mice exposed to long-term UV-B irradiation to assess if there is an association between diet and histopathological response to UV-B irradiation.
Design, Setting, and Participants
In this animal model study in an academic setting, the dorsal skin of SKH1 hairless mice receiving normal, calorie-restricted, and obesity diets was exposed to UV-B irradiation 3 times a week for 10 weeks and were compared with corresponding controls. The mice were placed in the following groups, with 8 animals in each group: (1) intact control (C) with regular diet and no UV-B exposure, (2) intact control with UV-B exposure (CR), (3) calorie-restricted diet (CrC), (4) calorie-restricted diet with UV-B exposure (CrR), (5) obesity diet (OC), and (6) obesity diet with UV-B exposure (OR). The experiment was conducted during October through December 2013. Tissue processing and histological analysis were completed in 2016.
Main Outcomes and Measures
Histomorphometric analysis was performed on paraffin-embedded skin sections stained by histological and immunohistochemical methods for estimation of epidermal thickness, epidermal proliferating cell nuclear antigen index, collagen I, elastic fibers, fibroblasts, mast cells, dermal cellularity, and adipose layer ratio. Changes in wrinkles were noted.
Results
Hairless female mice (age range, 6-8 weeks) were obtained. With a normal diet, changes from UV-B irradiation occurred in epidermal thickness, epidermal proliferating cell nuclear antigen index, collagen I, elastic fibers, fibroblasts, and mast cells, which were modestly influenced by an obesity diet. Calorie restriction influenced the skin in nonirradiated control animals, with higher values for most variables. After UV-B exposure in animals with calorie restriction, epidermal thickness was increased, but other variables were unaffected. Animals receiving the calorie-restricted diet lost weight when exposed to long-term UV-B irradiation. Wrinkles were reduced in the calorie-restricted control group and in UV-B–exposed animals who received the obesity diet.
Conclusions and Relevance
Dietary alterations seem to modify histopathological responses to UV-B exposure in the skin of hairless mice.
Level of Evidence
NA.
This animal model study examines the influence of a calorie-restricted diet and an obesity diet in mice exposed to long-term UV-B irradiation to assess if there is an association between diet and histopathological response to UV-B irradiation.
Introduction
Repeated sun exposure over a lifetime and UV rays from solar radiation induce the generation of free radicals in human skin that damage cellular and connective tissue layers biochemically and morphologically, with the ultimate risk of skin cancer. Clinical signs of aging principally influenced by extrinsic agents appear to be responsible for 80% of visible facial aging manifestations. Photoaging is a serious skin disease that leads to the development of solar keratosis and skin cancer. In human skin, p53 protein (tumor antigen) expression seen with immunohistochemistry has been correlated with different levels of photoaging.
Avoiding exposure to sunlight and other basic strategies to protect from UV-induced free radical skin damage is always desirable, but antioxidant supplement use or dietary modifications are recognized by health practitioners and skin care professionals as affording protection against photoaging. The US Food and Drug Administration does not require documentation of efficacy of dietary supplements, but clinical studies, including randomized clinical trials, have been performed with individual micronutrients for promoting skin health. Calorie-restricted diets in mice have been shown to ameliorate skin tumor formation resulting from short-term UV exposure by modulating activator proteins that have a key role in the development of skin cancer. Similarly, diet-induced obesity can promote progression of melanoma, which is caused by DNA damage from long-term exposure to UV radiation. It is relevant to explore if dietary alterations can modify morphological damage to skin caused by long-term exposure to UV-B irradiation in a laboratory rodent model. The hairless mouse has been established as a suitable animal model of photoaging during the last few decades. The present experiments were conducted to explore the histopathological response of the hairless mouse dorsal skin to UV-B exposure after dietary alteration through calorie restriction (Cr) and the administration of an obesity diet. Histological variables of the diet-altered, UV-B–exposed animals were compared with those of appropriate control groups with light microscopic morphometry and immunohistochemistry.
Methods
Animals, Diet, and UV-B Exposure
All experiments were performed with the approval of the Animal Care Committee at the University of Illinois. Hairless female mice (age range, 6-8 weeks) were obtained from Charles River Laboratories, and the following groups were established, with 8 animals in each group: (1) intact control (C) with regular diet and no UV-B exposure, (2) intact control with UV-B exposure (CR), (3) calorie-restricted diet (CrC), (4) calorie-restricted diet with UV-B exposure (CrR), (5) obesity diet (OC), and (6) obesity diet with UV-B exposure (OR). Control animals were fed a normal diet that was nutritionally complete and purified (TD 99433; Harlan Laboratories). The 2 other diets were a 40% Cr diet (TD 99467; Harlan Laboratories) and the AIN-76A obesity diet (TD 110780; Harlan Laboratories), fed in the form of pellets. Animals were acclimatized to these diets for 2 weeks before initiation of the UV-B exposure protocol. The diets were continued throughout the experimental period.
During the acclimatization, food consumption was monitored, and the mean amount of food per day was determined. For the caloric study, the mean amount of food consumed per day was multiplied by 0.65 g, as indicated by the nutritionist at Harlan Laboratories (written communication from D.H. Robbins, MS, Harlan Laboratories, Madison, Wisconsin, April 2010), and that amount of food was fed daily to Cr mice. All animals were weighed twice a week. The normal diet and obesity diet were fed to mice ad libitum.
The dorsal skin of mice in 3 experimental groups was exposed to a bank of 4 broadband UV-B lamps (Research Radiator; Daavlin), equipped with an electronic controller to regulate UV dosage. The lamps emit UV-B (280-320 nm, with a peak emission at 314 nm), 20% UV-A (320-375 nm), and negligible UV-C. Dosage units are recorded as megajoules per centimeter squared, and exposure is controlled using an integrating dosimeter (Flex Control; Daavlin). The unit was calibrated by engineers from Daavlin before starting the experiments. The UV-B irradiation was administered 3 times a week for 10 weeks to the mice, which were freely moving in cages. The amount of radiation was progressively increased from 45 mJ/cm2 per exposure (for 1 week) to 70 mJ/cm2 (for 1 week), 90 mJ/cm2 (for 2 weeks), 120 mJ/cm2 (for 2 weeks), 140 mJ/cm2 (for 1 week), 160 mJ/cm2 (for 1 week), and 180 mJ/cm2 (for 2 weeks). Animals were humanely killed during the 11th week, and skin specimens were collected for histology following replica preparations.
Histological Features
For processing consistency, all skin samples from each group were embedded in the same paraffin block before microscopic morphometry. For immunostaining, slides from all 6 groups were stained simultaneously in an automated system at the University of Illinois College of Medicine at Chicago. Paraffin sections (5-14 µm) from skin samples were subjected to the following staining methods for analysis of different elements of the dermal matrix: (1) hematoxylin-eosin–phloxine stain to observe dermal cellularity, (2) elastic fiber stain with resorcin-fuchsin and Luna stain for a point counting of fibers, and (3) immunohistochemistry for the identification of epidermal proliferating cell nuclear antigen (PCNA) index, fibroblasts, and collagen I. Methods for microscopic quantitative estimation of epidermal and dermal variables listed in Table 1 have been published elsewhere. All morphometric observations are based on data obtained from 7 to 8 animals. Slide readings were performed in masked fashion by at least 2 observers (T.K.B., Y.H., D.M.W., T.K.D., and J.L.), and the mean values were recorded.
Table 1. Histomorphometric Variables in the Diet and Photoaging Experiment.
| Variable | Group, Mean (SD) | Group, Mean (SD) | Group, Mean (SD) | Comparison of All 6 Groups | Comparison of Control Groups, P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | CR | P Value | CrC | CrR | P Value | OC | OR | P Value | ANOVA F Ratio | P Value | C vs CrC | C vs OC | |
| Wrinkles, Rz value | 103.6 (27.7) | 84.3 (25.1) | NS | 63.4 (15.1) | 79.9 (13.1) | NS | 101.1 (28.9) | 76.1 (25.2) | .03 | 3.48 | <.01 | .001 | NS |
| Epidermal thickness | 9.4 (1.2) | 17.7 (6.7) | <.01 | 21.0 (7.7) | 28.2 (11.8) | <.03 | 7.9 (0.0) | 18.5 (2.8) | <.002 | 7.72 | <.001 | <.001 | NS |
| Epidermal PCNA index | 15.7 (10.0) | 41.1 (2.6) | <.001 | 57.3 (5.0) | 62.4 (5.9) | NS | 17.8 (3.1) | 49.1 (2.6) | <.001 | 3.61 | <.01 | <.01 | NS |
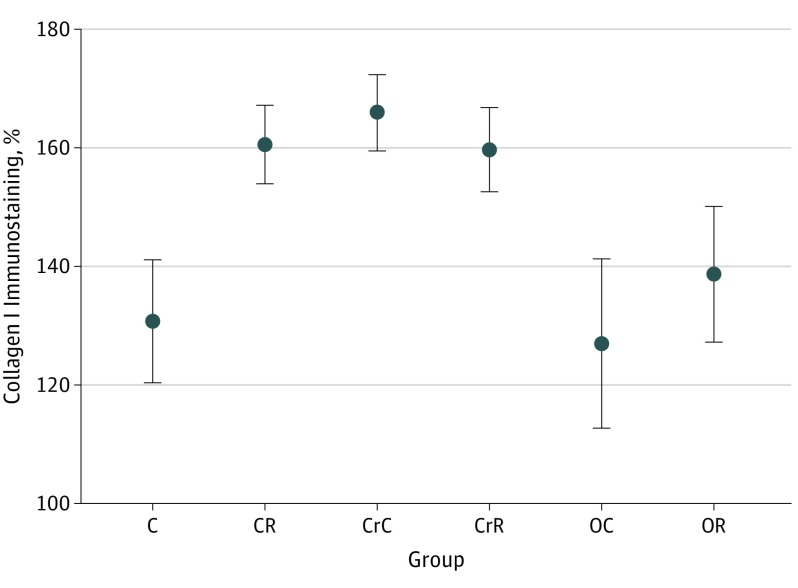
| Collagen I immunostaining, % | 130.7 (10.3) | 160.5 (6.5) | <.01 | 165.9 (6.4) | 159.6 (7.1) | NS | 127.0 (14.2) | 138.6 (11.4) | <.02 | 23 | <.001 | <.01 | NS |
| Elastic fibers, % | 42.3 (8.3) | 51.9 (11.9) | <.02 | 67.5 (8.1) | 60.8 (11.4) | NS | 44.8 (13.5) | 63.5 (15.8) | <.002 | 6.41 | <.001 | <.001 | NS |
| Fibroblasts, No. | 7.3 (4.6) | 25.7 (4.4) | <.001 | 31.0 (2.9) | 21.7 (2.9) | <.001 | 9.1 (1.9) | 15.0 (4.8) | <.003 | 49.7 | <.001 | <.01 | NS |
| Mast cells, No. | 13.8 (1.5) | 19.9 (2.7) | <.01 | 14.5 (3.5) | 10.6 (1.1) | <.01 | 10.3 (2.5) | 17.8 (3.0) | <.01 | 34.52 | <.001 | NS | <.01 |
| Dermal cellularity | 18.5 (3.1) | 21.6 (2.8) | NS | 20.8 (4.0) | 15.4 (1.9) | <.05 | 14.6 (3.2) | 22.5 (4.5) | <.001 | 6.71 | <.001 | NS | <.05 |
| Adipose layer ratio | 0.60 (0.07) | 0.55 (0.06) | NS | 0.63 (0.01) | 0.63 (0.03) | NS | 0.70 (0.06) | 0.64 (0.05) | NS | 3.74 | <.01 | NS | <.05 |
Abbreviations: ANOVA, analysis of variance; C, intact control with regular diet and no UV-B exposure; CR, intact control with UV-B exposure; CrC, calorie-restricted diet; CrR, calorie-restricted diet with UV-B exposure; NS, not significant; OC, obesity diet; OR, obesity diet with UV-B exposure; PCNA, proliferating cell nuclear antigen; Rz, surface roughness.
Replica Preparation
Silicon replicas of skin samples were prepared using resin and rings for the evaluation of skin microrelief by Standard Replica Analysis (BIONET protocol published by CuDerm Corp). Analysis of wrinkles in this study was based on surface roughness (Rz) values. Methodological details have been described in another article.
Statistical Analysis
The quantitative data are expressed as means (SDs). One-way analysis of variance for independent samples was performed on the quantitative data to determine the overall significant difference between all 6 groups. P < .05 was considered statistically significant, and the F ratio was determined. The Fisher least significant difference test was performed for post hoc analysis of pairwise comparisons to detect differences between group means. Statistical tests were performed using commercially available software, including Excel (Microsoft Corp) and SPSS, version 23.0 (SPSS Inc). Table 1 summarizes the results of the F ratio, the level of significance, and comparison of relevant groups.
Results
The quantitative microscopic data are listed in Table 1. Table 2 summarizes the qualitative grading based on the quantitative results.
Table 2. Qualitative Summary of Main Results in the Diet and Photoaging Experiment.
| Variable | Normal Diet and UV-B | Obesity Diet and UV-B | Calorie-Restricted Diet and UV-B | Control Comparison |
|---|---|---|---|---|
| Epidermis | ||||
| Wrinkles, Rz value | NS | ↓ | NS | C vs CrC, ↓ |
| Epidermal thickness | ↑↑ | ↑↑↑ | ↑ | C vs CrC, ↑ |
| Epidermal PCNA index | ↑↑ | ↑↑↑ | NS | C vs CrC, ↑ |
| Dermis | ||||
| Collagen I immunostaining | ↑↑ | ↑ | NS | C vs CrC, ↑ |
| Elastic fibers | ↑ | ↑↑ | NS | C vs CrC, ↑ |
| Fibroblasts | ↑↑ | ↑ | ↓ | C vs CrC, ↑ |
| Mast cells | ↑ | ↑ | ↓ | C vs OC, ↓ |
| Dermal cellularity | NS | ↑ | ↓ | C vs OC, ↓ |
| Adipose layer ratio | NS | NS | NS | C vs OC, ↑ |
Abbreviations: C, intact control with regular diet and no UV-B exposure; CrC, calorie-restricted diet; NS, not significant; OC, obesity diet; PCNA, proliferating cell nuclear antigen; ↑, increased; ↓, decreased; Rz, surface roughness.
Body Weight
Animal body weight data from weekly recordings revealed statistically significant differences. The following trends in body weight changes were seen: (1) higher body weight was noted in the CR group compared with the C group, (2) the OC group gained weight from weeks 7 to 11, (3) UV-B irradiation increased body weight in the OC group vs the OR group, (4) no appreciable difference in body weight was observed in the C group vs the CrC group, and (5) notable weight loss was seen immediately after UV-B exposure in the CrC group vs the CrR group. Toward the end of the experimental period, no appreciable difference was seen between the C, CR, CrC, and OC groups, with the mean body weight of these groups around 30 g. The UV-B irradiation seemed to modestly increase body weight in the C group vs the CR group and in the OC group vs the OR group. Body weight increased from weeks 4 to 11 in the C group vs the OC group.
Wrinkle Analysis
No significant change in Rz values was observed in the C group vs the CR group. A reduction in skin wrinkles was noted when obesity diet animals received UV-B exposure (OC group vs OR group, P < .03). When the 3 control groups (C, CrC, and OC) were compared, the C group and the OC group had identical Rz values. The CrC group showed a reduction in wrinkle formation compared with the C group (P < .001).
Epidermis
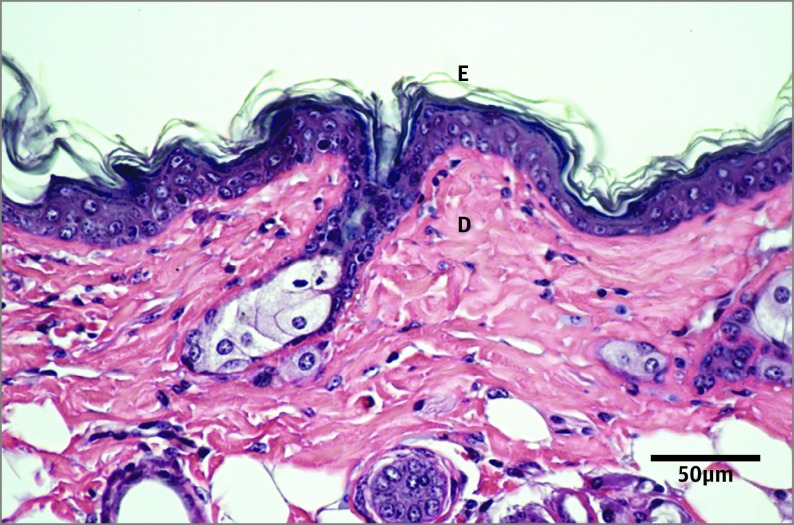
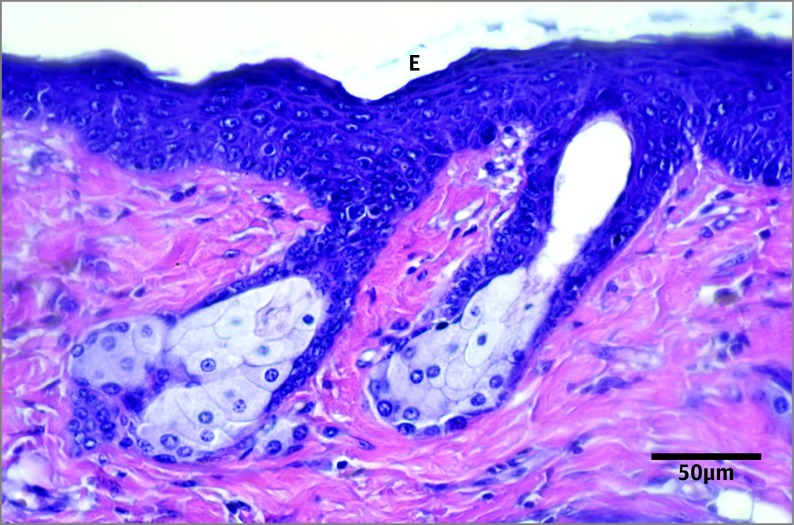
The UV-B irradiation had a greater influence on epidermal thickness in the C group vs the CR group (P < .01) and in the OC group vs the OR group (P < .002). The increase in epidermal thickness was 133% in animals receiving an obesity diet, while the increase was 86% in control animals receiving a normal diet. Calorie-restricted animals had a modest increase (33%) in epidermal thickness after UV-B exposure (CrC group vs CrR group, P < .03). The CrC group had an increased epidermal thickness compared with intact controls receiving a normal diet (Figure 1 and Figure 2); there was no difference between the C group and the CrC group (P = .001) or between the C group and the OC group (not significant [NS]).
Figure 1. Hematoxylin-Eosin–Stained Section of the Dorsal Skin From an Intact Control Animal.
D indicates dermis; E, epidermis.
Figure 2. Hematoxylin-Eosin–Stained Section of the Dorsal Skin From a Calorie-Restricted Control Animal.
Activated epidermis (E) is shown.
The epidermal PCNA index was higher in the C group vs the CR group (P < .001) and in the OC group vs the OR group (P < .001). The index was slightly higher in the OR group (176%) compared with the CR group (161%). No influence of UV-B irradiation on epidermal PCNA index was noted in calorie-restricted animals. Calorie-restricted controls had a significantly higher proliferation rate compared with intact controls; the epidermal PCNA index was identical in the C group vs the CrC group (P < .01) and in the C group vs the OC group (NS).
Dermal Constituents
Collagen I immunostaining is shown in Figure 3. The UV-B exposure increased the immunostaining in the C group vs the CR group (P < .01) and in the OC group vs the OR group (P < .02). No influence of UV-B irradiation was seen on this variable in calorie-restricted animals. Obesity control animals had an intensity of collagen I immunostaining similar to that of intact controls, whereas calorie-restricted controls had a stronger intensity of immunostaining (C group vs CrC group, P < .01).
Figure 3. Immunohistochemistry Results for Collagen I Immunostaining in All Experimental Groups.
Slides were scanned with Leica Aperio Scanscope CS and analyzed in Imagescope 12.2. Error bars show means (SDs). C indicates intact control with regular diet and no UV-B exposure; CR, intact control with UV-B exposure; CrC, calorie-restricted diet; CrR, calorie-restricted diet with UV-B exposure; OC, obesity diet; and OR, obesity diet with UV-B exposure.
Elastic fibers became more abundant after UV-B exposure in the animals receiving a normal diet (C group vs CR group, P < .01). In addition, elastic fibers were more prolific in irradiated obesity diet animals (OC group vs OR group, P < .002). No influence on elastic fibers was seen in irradiated calorie-restricted animals (CrC group vs CrR group, NS). Intact control and obesity control animals had almost identical percentages of elastic fibers. Calorie-restricted control animals had more elastic fibers compared with intact controls (C group vs CrC group, P < .001).
The number of fibroblasts was increased after UV-B exposure in the C group vs the CR group (P < .001) and in the OC group vs the OR group (P < .003). There was a decrease in the number of fibroblasts with UV-B irradiation in calorie-restricted animals (CrC group vs CrR group, P < .001). Calorie-restricted control animals had a much higher number of fibroblasts than intact control animals (C group vs CrC group, P < .01); the number of fibroblasts in intact control animals was not appreciably different from that in the control group receiving an obesity diet (C group vs OC group, NS).
The number of mast cells increased after UV-B irradiation in the C group vs the CR group (P < .01) and in the OC group vs the OR group (P < .01). The number was decreased when calorie-restricted animals were exposed to UV-B irradiation (CrC group vs CrR group, P < .01). Control animals receiving an obesity diet had a slightly lower count of mast cells compared with intact controls (C group vs OC group, P < .01).
Dermal cellularity was unchanged in UV-B–exposed animals receiving a normal diet but was higher in irradiated animals receiving an obesity diet (OC group vs OR group, P < .001). In contrast, less dermal cellularity was observed in UV-B–exposed, calorie-restricted animals (CrC group vs CrR group, P < .05). The receipt of an obesity diet resulted in a lower degree of dermal cellularity in control animals (C group vs OC group, P < .05).
The adipose layer ratio was generally unchanged after UV-B irradiation. The only modification was a greater subdermal fat layer ratio with the obesity diet in control skin specimens (C group vs OC group, P < .05).
Discussion
Numerous histopathological features are observed in human skin that receives long-term exposure to sunlight, and UV-B rays result in epidermal thickening, formation of wrinkles, and dermal alterations, such as collagen I damage, elastic fiber hyperplasia, and fibroblasts, as well as mast cell replication. In the present experiments and in an earlier study, similar UV-B influences were observed; dietary alterations seemed to modify many of these UV-B–mediated responses affecting different components of the mouse skin.
Obesity diet influence was predominantly noted on epidermal thickness and epidermal PCNA index. Epidermal thickness in the obesity group animals showed more aggravated epidermal response, with a greater thickening of the epidermis (133%), while the control group had an 86% increase. The highest increase in epidermal PCNA index (ie, DNA replication) after UV-B irradiation was also noted in obese animals subjected to UV-B exposure, which might indicate activation of signaling pathways that control keratinocyte proliferation with an obesity diet. The release of inflammatory cytokines has been shown in genetically obese mice. In the hairless mouse, body weight gain by feeding a powdered diet enhanced the development of UV-induced skin tumors.
A notable feature of the collagen I immunohistochemistry seen in the present study was a more intense staining reaction of dermal collagen I bundles after induction of UV-B exposure in intact and obesity group animals. The increased immunostaining of collagen I, along with fibroblast proliferation in UV-B–exposed animals fed a normal diet or an obesity diet, presumably indicates hyperactivity or compensatory increase in dermal collagen I synthesis before eventual disorganization and disruption of collagen I bundles in irradiated skin. As reported by different authors (vide infra), collagen I in UV-B–irradiated skin shows a variety of responses, including the absence of an influence, as well as increased or decreased collagen I synthesis that correlates with biochemical and histochemical observations, which may be dependent on the duration and intensity of UV-B exposure. The UV-B irradiation alone induced no change in total collagen or collagen I and collagen III levels in a hairless mouse model exposed to UV-B irradiation for up to 10 weeks. In contrast, long-term UV exposure resulted in increased collagen synthesis until late in the course of irradiation in a hairless mouse model irradiated for up to 24 weeks. Other mouse studies have shown UV-induced damage to mature collagen in the upper dermis or a loss of collagen. In another study, photoaging resulted in increased collagen synthesis and greater matrix metalloproteinase expression in human skin in vivo. Compensatory collagen production may result as a response to UV insults, with the end result being collagen degradation with visible wrinkle damage. In another experiment involving UV exposure at a different dosage, this laboratory observed disruption of collagen bundles and a reduction in collagen I immunostaining.
The UV-B influence was also seen herein in control and obesity diet specimens as mast cell growth, which increased in areas of elastic fiber proliferation. This finding is consistent with the hypothesis that photoaging in skin with long-term exposure to sunlight represents a long-term skin inflammation, and mast cell abundance may contribute to healing of sun-damaged skin.
The increase in epidermal PCNA index observed herein in control animals receiving a Cr diet seems to differ from a previous study reporting that skin mitotic activity is decreased by underfeeding. In another murine species, there was no difference in epidermal thickness between animals receiving a normal diet and a Cr diet. The finding herein that calorie-restricted control animals had greater epidermal thickness and higher epidermal PCNA index than intact control animals may signify that the Cr diet modified physiological changes and inflammatory cytokines, which influenced the epidermis in this animal model. Curiously, a Cr diet has been shown to increase cell division in neural stem cells in the aging brain of mice.
Similar to epidermal measurements and as summarized in Tables 1 and 2, control Cr mice had higher basal values of 3 dermal variables (ie, collagen I immunostaining, elastic fibers, and fibroblasts) compared with control animals receiving a normal diet. Calorie restriction has been described as a “low-intensity stressor,” resulting in an elevated glucocorticoid level, enabling rodents receiving a Cr regimen to cope with adverse situations, suggesting beneficial action from this diet, as well as the concept of hormesis (a detrimental agent that benefits the organism).
A UV-B influence in the CrR group was seen only on epidermal thickness response. Three variables (epidermal PCNA index, collagen I immunostaining, and elastic fibers) had no change after UV-B exposure, while 3 other variables (fibroblasts, mast cells, and dermal cellularity) decreased after UV-B exposure. It may be argued that, because baseline values of some variables were high in control Cr mice, this condition might have prevented further influence by UV-B. However, the epidermal response in UV-B–exposed Cr mice refutes this theory: despite higher epidermal thickness in control Cr mice, the response to UV-B was significant. Furthermore, in our pilot UV-B dosage standardization experiments, the hairless mouse skin exhibited greater morphological response to increasing UV-B insults, with epidermal rupture (blowup) or tumor formation (T.K.B., et al, unpublished observations). Rather, it appears that most dermal structures in the exposed skin from Cr animals were resistant to UV-B damaging influences, presumably due to some inhibition of biochemical pathways, which is consistent with a large number of studies performed with the hairless mouse and other rodents described below.
Calorie restriction in the hairless mouse prevented UV-B influences by controlling DNA binding of activator protein 1 and diminished production of this constituent protein. Furthermore, Cr seemed to afford protection from inflammation injury in another mouse species. In the hairless mouse skin, reactive oxygen species (ROS) generation is increased by UV exposure, which is a major factor in the cascade of skin responses induced by UV radiation. Calorie-restricted animals show less damage caused by ROS. Other Cr-mediated beneficial influences observed in rodents include decreased production of ROS, modulation of endogenous antioxidant systems, decreased oxidative stress, and free radical–induced tissue damage. Calorie restriction has also been shown to retard aging changes in rat skin.
Two other notable results were observed in the present study. The Rz values were decreased in 2 groups: when UV-B exposed, animals receiving an obesity diet demonstrated a lower wrinkle profile, and Cr control animals showed a significantly lower wrinkle profile than intact control animals. One might speculate that proliferation of skin adipose layer in animals receiving an obesity diet might have had a role in decreased wrinkle formation after UV-B exposure. In a study among Japanese women, high fat intake was associated with decreased facial wrinkling. Whether Cr in the native state can reduce the number of wrinkles and if it can be an advantage for the aging skin must be verified in future experimental studies involving suitable animal models of aging. The influence of diet on human facial wrinkling has been explored in some investigations. Also, a dramatic loss in body weight seen herein in UV-B–exposed Cr mice, which may be a stress response from metabolic or endocrine modifications, is comparable to the findings in some published reports on rodents as described below. By itself, UV radiation was reported to cause body weight reduction in mice, and UV radiation in C57/BL/6 mice suppressed body weight gain in animals fed a high-fat diet.
Limitations
The present report is limited in that it is a microscopic and immunohistochemical study without physiological or biochemical validation.
Conclusions
The present experiments explored the complex nexus of diet and photoaging from a histopathological perspective. Dietary and nutritional links to photoprotection have been a focus of much interest to provide endogenous skin protection against lifelong exposure to UV rays from sunlight. Calorie restriction has been a subject of much research in recent years and is a popular topic among health-conscious people aiming to regulate aging or maintain a healthy lifestyle. Poor dietary habits can result in adverse health, and an improved diet can reverse such conditions. Further research will assess whether a nutritionally balanced, calorie-restricted diet can be beneficial to people who are exposed to sunlight for long hours for recreation or in their profession and may prevent the harmful influences of photoaging and photocarcinogenesis.
References
- 1.Flament F, Bazin R, Laquieze S, Rubert V, Simonpietri E, Piot B. Effect of the sun on visible clinical signs of aging in Caucasian skin. Clin Cosmet Investig Dermatol. 2013;6:221-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez D. Evolution of cosmeceuticals and their application to skin disorders including aging and blemishes In: Halters P, Roberts MS, eds. Dermatologic, Cosmeceutic and Cosmetic Development. New York, NY: Informa Health Care; 2008:45-60. [Google Scholar]
- 3.de Castro IA, Schütz L, Capp E, Cartell A, Meurer L, Bakos L. p53 Protein expression in skin with different levels of photoaging. Photodermatol Photoimmunol Photomed. 2009;25(2):106-108. [DOI] [PubMed] [Google Scholar]
- 4.Pandel R, Poljšak B, Godic A, Dahmane R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013;2013:930164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birnbaum J, Le Moigne A, Dispensa L, Buchner L. A review of clinical trials conducted with oral, multicomponent dietary supplements for improving photoaged skin. J Drugs Dermatol. 2015;14(12):1453-1461. [PubMed] [Google Scholar]
- 6.Hopper BD, Przybyszewski J, Chen HW, Hammer KD, Birt DF. Effect of ultraviolet B radiation on activator protein 1 constituent proteins and modulation by dietary energy restriction in SKH-1 mouse skin. Mol Carcinog. 2009;48(9):843-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malvi P, Chaube B, Pandey V, et al. . Obesity induced rapid melanoma progression is reversed by orlistat treatment and dietary intervention: role of adipokines. Mol Oncol. 2015;9(3):689-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balakrishnan KP, Narayanaswamy DP. Botanicals as sunscreens: their role in the prevention of photoaging and skin cancer. Int J Res Cosm Sci. 2011;1(1):1-12. [Google Scholar]
- 9.Kligman LH. The ultraviolet-irradiated hairless mouse: a model for photoaging. J Am Acad Dermatol. 1989;21(3, pt 2):623-631. [DOI] [PubMed] [Google Scholar]
- 10.Sharma MR, Werth B, Werth VP. Animal models of acute photodamage: comparisons of anatomic, cellular and molecular responses in C57BL/6J, SKH-1 and Balb/c mice. Photochem Photobiol. 2011;87(3):690-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JE, Song D, Kim J, et al. . Oral supplementation with cocoa extract reduced UVB-induced wrinkles in hairless mouse skin. J Invest Dermatol. 2016;136(5):1012-1021. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharyya TK, Higgins NP, Sebastian JS, Thomas JR. Comparison of epidermal morphologic response to commercial antiwrinkle agents in the hairless mouse. Dermatol Surg. 2009;35(7):1109-1118. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharyya TK, Thomas JR. Staining properties and distribution of elastic fibers in the pig skin dermis. J Histotechnol. 2007;30(1):15-21. [Google Scholar]
- 14.Bhattacharyya TK, Pathria M, Mathison C, Vargas M, Thomas JR. Cosmeceutical effect on skin surface profiles and epidermis in UV-B–irradiated mice. JAMA Facial Plast Surg. 2014;16(4):253-260. [DOI] [PubMed] [Google Scholar]
- 15.Fisher GJ, Kang S, Varani J, et al. . Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138(11):1462-1470. [DOI] [PubMed] [Google Scholar]
- 16.Ichihashi M, Ando H, Yoshida M, et al. . Photoaging of skin. J Anti-Aging Med. 2009;6(6):46-59. [Google Scholar]
- 17.D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222-12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharyya TK, Hsia Y, Somenek M, Thomas JR. Effect of topical cosmeceuticals in reversing dermal histological injury in photoaged hairless mice. Anti-Aging Med. 2016;2016(6):28-35. [Google Scholar]
- 19.El-Abaseri TB, Putta S, Hansen LA. Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis. 2006;27(2):225-231. [DOI] [PubMed] [Google Scholar]
- 20.Katiyar SK, Meeran SM. Obesity increases the risk of UV radiation–induced oxidative stress and activation of MAPK and NF-κ signaling. Free Radic Biol Med. 2007;42(2):299-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinkova-Kostova AT, Fahey JW, Jenkins SN, et al. . Ultraviolet radiation induced skin carcinogenesis in SKH-1 hairless mice. Nutr Res. 2008;28:539-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaquour B, Seité S, Coutant K, Fourtanier A, Borel JP, Bellon G. Chronic UVB- and all-trans retinoic-acid-induced qualitative and quantitative changes in hairless mouse skin. J Photochem Photobiol B. 1995;28(2):125-135. [DOI] [PubMed] [Google Scholar]
- 23.Kligman LH, Gebre M, Alper R, Kefalides NA. Collagen metabolism in ultraviolet irradiated hairless mouse skin and its correlation to histochemical observations. J Invest Dermatol. 1989;93(2):210-214. [DOI] [PubMed] [Google Scholar]
- 24.Kligman LH. Connective tissue photodamage in the hairless mouse is partially reversible. J Invest Dermatol. 1987;88(3)(suppl):12s-17s. [DOI] [PubMed] [Google Scholar]
- 25.Zhang JA, Yin Z, Ma LW, et al. . The protective effect of baicalin against UVB irradiation induced photoaging: an in vitro and in vivo study. PLoS One. 2014;9(6):e99703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung JH, Seo JY, Choi HR, et al. . Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J Invest Dermatol. 2001;117(5):1218-1224. [DOI] [PubMed] [Google Scholar]
- 27.Poon F, Kang S, Chien AL. Mechanisms and treatments of photoaging. Photodermatol Photoimmunol Photomed. 2015;31(2):65-74. [DOI] [PubMed] [Google Scholar]
- 28.Bosset S, Bonnet-Duquennoy M, Barré P, et al. . Photoageing shows histological features of chronic skin inflammation without clinical and molecular abnormalities. Br J Dermatol. 2003;149(4):826-835. [DOI] [PubMed] [Google Scholar]
- 29.Loewenthal LA, Montagna W. Effects of caloric restriction on skin and hair growth in mice. J Invest Dermatol. 1955;24(4):429-433. [DOI] [PubMed] [Google Scholar]
- 30.Varani J, Bhagavathula N, Aslam MN, et al. . Inhibition of retinoic acid–induced skin irritation in calorie-restricted mice. Arch Dermatol Res. 2008;300(1):27-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell SE, Delville C, Konstantopedos P, et al. . The effects of graded levels of calorie restriction, II: impact of short term calorie and protein restriction on circulating hormone levels, glucose homeostasis and oxidative stress in male C57BL/6 mice. Oncotarget. 2015;6(27):23213-23237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JH, Glass Z, Sayed K, et al. . Calorie restriction alleviates the age-related decrease in neural progenitor cell division in the aging brain. Eur J Neurosci. 2013;37(12):1987-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masoro EJ. Caloric restriction-induced life extension of rats and mice: a critique of proposed mechanisms. Biochim Biophys Acta. 2009;1790(10):1040-1048. [DOI] [PubMed] [Google Scholar]
- 34.Kim HK. Garlic supplementation ameliorates UV-induced photoaging in hairless mice by regulating antioxidative activity and MMPs expression. Molecules. 2016;21(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126(9):987-1002. [DOI] [PubMed] [Google Scholar]
- 36.Helfrich YR, Sachs DL, Voorhees JJ. Overview of skin aging and photoaging. Dermatol Nurs. 2008;20(3):177-183. [PubMed] [Google Scholar]
- 37.Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA. 2007;297(9):986-994. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharyya TK. Skin aging changes in animal models: a histological perspective. In: Farage MA, Miller KW, Maibach HI, eds. Textbook of Aging Skin New York, NY: Springer-Verlag; 2016:1-11. [Google Scholar]
- 39.Nagata C, Nakamura K, Wada K, et al. . Association of dietary fat, vegetables and antioxidant micronutrients with skin ageing in Japanese women. Br J Nutr. 2010;103(10):1493-1498. [DOI] [PubMed] [Google Scholar]
- 40.Pezdirc K, Hutchesson M, Whitehead R, Ozakinci G, Perrett D, Collins CE. Can dietary intake influence perception of and measured appearance? a systematic review. Nutr Res. 2015;35(3):175-197. [DOI] [PubMed] [Google Scholar]
- 41.Blum HF, Grady HG, Kirby-Smith JS. Effect of ultraviolet radiation on body weight of mice. Am J Physiol. 1943;138:378-384. [Google Scholar]
- 42.Geldenhuys S, Hart PH, Endersby R, et al. . Ultraviolet radiation suppresses obesity and symptoms of metabolic syndrome independently of vitamin D in mice fed a high-fat diet. Diabetes. 2014;63(11):3759-3769. [DOI] [PubMed] [Google Scholar]
- 43.Sies H, Stahl W. Nutritional protection against skin damage from sunlight. Annu Rev Nutr. 2004;24:173-200. [DOI] [PubMed] [Google Scholar]
- 44.Denke MA. Changing dietary habits and improving the healthiness of diets in the United States. JAMA. 2016;315(23):2527-2529. [DOI] [PubMed] [Google Scholar]