Abstract
Importance
Cryolipolysis is a popular, well-tolerated nonsurgical procedure that uses controlled cooling to selectively destroy fat cells. Central submental cryolipolysis has been reported to be safe and effective, but many patients would benefit from extending this treatment over the entire submental region.
Objective
To investigate the safety and efficacy of cryolipolysis for reduction of lateral and central submental fat.
Design, Setting, and Participants
The study population consisted of 14 participants who were treated from January 22 to June 30, 2016, in the lateral and central submental area to reduce unwanted subcutaneous fat. A small-volume cup applicator was used to administer 2 cryolipolysis treatments, delivered in 45-minute treatment cycles in 2 sessions. For the first treatment session, all participants received bilateral treatments with approximately 20% overlap of the treatment area. At the 6-week follow-up visit, participants were reassessed to determine whether they would benefit from a second treatment and to determine the number of cycles needed to achieve the optimal aesthetic result, and then they were treated a second time.
Main Outcomes and Measures
Participant surveys assessed tolerability and treatment satisfaction at 12 weeks following the second treatment. Clinicians monitored adverse events to assess safety. Caliper measurements were recorded to assess fat thickness reduction. Treatment efficacy was objectively evaluated using 2-dimensional and 3-dimensional imaging.
Results
Among the 14 participants (12 women and 2 men; mean [SD] age, 50.5 [10.4] years), the adverse effects of the procedure were typically mild and included numbness and tingling, which resolved without intervention by the final 12-week follow-up visit. An independent review of digital photographs revealed an 81.0% (95% CI, 65.9%-91.4%; P = .02) correct identification rate (34 of 42 images) of the pretreatment and posttreatment images. Caliper measurements demonstrated a mean (SD) fat layer reduction of 2.3 (0.8) mm (range, 0.7-3.5 mm). Three-dimensional imaging revealed a mean (SD) reduction in fat volume of 4.82 (11.42) cm3 (from a reduction of 32.69 cm3 to an increase of 13.85 cm3), in skin surface area of 1.29 (1.42) cm2 (from a reduction of 3.18 cm2 to an increase of 0.99 cm2), and in fat thickness of 3.77 (3.59) mm (from of reduction of 13.10 mm to an increase of 0.47 mm). Results of participant surveys indicated that 13 participants (93%) were satisfied with the cryolipolysis treatment.
Conclusions and Relevance
Although safe and efficacious central submental cryolipolysis has been reported, this is the first clinical study of cryolipolysis for treatment of the entire submental area using overlapping bilateral treatments and a shorter treatment duration. The study demonstrates that bilateral submental cryolipolysis is well tolerated and produces visible and significant fat layer reduction.
Level of Evidence
4.
This nonrandomized interventional cohort study investigates the safety and efficacy of cryolipolysis for reduction of lateral and central submental fat.
Key Points
Question
Is cryolipolysis treatment of the lateral and central submental area using bilateral, overlapping treatment cycles safe, effective, and well tolerated?
Findings
This nonrandomized interventional cohort study showed that bilateral submental cryolipolysis treatment of 14 participants resulted in a statistically significant reduction in fat thickness (3.77 mm) and skin surface area (1.29 cm2) and yielded high participant satisfaction (93%). Side effects of the procedure were typically mild and included numbness and tingling, which resolved without intervention.
Meaning
Bilateral submental cryolipolysis is safe and well tolerated and produces visible and significant fat layer reduction.
Introduction
Cryolipolysis uses controlled cooling to noninvasively damage adipocytes. The selective fat damage results from the greater susceptibility of lipid-rich adipocytes to cold injury compared with surrounding water-rich cells. Cryolipolysis has been shown to safely and effectively reduce subcutaneous fat and currently has US Food and Drug Administration clearance for treatment of the flanks, abdomen, thighs, submental area, back, bra area, underneath the buttocks, and arms.
Clinical studies have investigated the safety and efficacy of cryolipolysis treatments for subcutaneous fat reduction in numerous areas of the body, including the abdomen, flanks, inner thighs, outer thighs, arms, and chest. Noninvasive reduction of submental fat has currently been an area of interest in aesthetic medicine owing to recent advances in technologies, increased public awareness, and a strong record of safety and efficacy. A previous study of cryolipolysis of the submental area demonstrated that the procedure was safe and well tolerated, produced visible improvement to the neck contour, and generated high patient satisfaction.
Our study sought to further initial successes by performing cryolipolysis beyond the area treated in the earlier study, which used a single –10°C, 60-minute cryolipolysis cycle in the center of the submental area administered on 2 separate treatment visits. Studies have shown that multiple cryolipolysis applicator placements and repeated treatment sessions produce greater fat reduction and more aesthetically pleasing results than does a single applicator placement on a solitary treatment day. Thus, our treatment protocol used 2 overlapping applications, incorporating 2 treatment visits to investigate the efficacy of such an approach at reducing submental fat. Many patients with unwanted submental fullness could benefit from the greater reduction in submental fat afforded by using bilateral cryolipolysis applicator placement with overlap in the central region as opposed to placement of a single applicator cycle.
We investigated a commercially available, small-volume vacuum cup cryolipolysis applicator with an automated treatment control system to evaluate its safety and efficacy at reducing lateral and central submental fat. We report the results of this study investigating bilateral overlapping cryolipolysis treatment cycles for submental fat reduction using 2-dimensional (2-D) and 3-dimensional (3-D) imaging to quantify reduction in fat thickness, fat volume, and skin surface area.
Methods
This was a prospective, nonrandomized interventional cohort study conducted from January 22 to June 30, 2016. The clinical study protocol was approved by the Salus Institutional Review Board (Austin, Texas). All participants provided written informed consent.
Participants
Of the 14 participants who enrolled in the study, 12 were female and 2 were male. They ranged in age from 25 to 63 years (mean [SD] age, 50.5 [10.4] years). Participants’ Fitzpatrick skin type ranged from I to VI, with 1 participant having type I skin, 9 participants having type II skin, 2 participants having type III skin, and 2 participants having type VI skin. Their weights ranged from 67.3 to 122.4 kg (from 149.6 to 272.1 lb) (mean, 92.7 kg [206.1 lb]). Their body mass indices (calculated as weight in kilograms divided by height in meters squared) ranged from 24.9 to 45.3 (mean, 33.1). Eligible participants were male or female, between 22 and 65 years of age, and with clearly visible submental skin fold thickness greater than 1 cm as measured with calipers. Candidates were examined for soft, pliable tissue that could be drawn by vacuum suction into the cryolipolysis cup applicator. For the duration of the study, participants were instructed to avoid implementing major diet or exercise changes in order to maintain their weight within 5% of the baseline measurement.
Cryolipolysis Treatment
Each participant underwent up to 2 treatment cycles per visit to the lateral and central submental areas based on the investigators’ assessment of the submental area, with 2 treatment visits spaced 6 weeks apart. Each treatment consisted of a maximum of 2 and a minimum of one –11°C, 45-minute cooling cycles using a commercially available, small-volume vacuum cup cryolipolysis applicator (CoolMini Applicator, CoolSculpting System; ZELTIQ Aesthetics). The applicator opening is approximately 2.5 × 7.5 cm and has a concave shape to match the typical curvature of the submental tissue. To mark the treatment areas, the participant’s head was positioned in a neutral position, and a treatment area template was positioned to achieve approximately 20% overlap at the center of the submental area. The method of administration of cryolipolysis treatment was similar to the procedure used in a prior study. A protective transparent coupling gel was applied to the skin, and then the small-volume cup applicator was fitted with a new geltrap (a disposable component of the applicator that prevents gel from being drawn into the vacuum line). After vacuum suction was initiated, the applicator was positioned over the marked treatment area and then placed onto the skin. The vacuum drew the target tissue into the cup, filling it the full depth of 2 cm. The vacuum force adhered the applicator to the treatment area with minimal discomfort, and cloth adhesive straps were attached to a pillow to provide additional support throughout the cooling treatment. At the conclusion of the treatment cycle, the applicator was removed and the treatment area was manually massaged for 2 minutes, allowing the tissue to rewarm and regain its original shape. On the first treatment visit, all 14 participants received 2 bilateral treatment cycles, overlapping approximately 20%. On the second treatment visit, 12 participants received 2 bilateral cycles, and 2 participants received 1 centered cycle because they were deemed not to have sufficient remaining submental fat to allow placement of 2 treatment cycles.
Participant Satisfaction Ratings
Participant satisfaction data were collected by use of a written questionnaire at the 12-week posttreatment follow-up visit. This questionnaire used a 5-point Likert scale to elicit responses but also allowed for free-text responses.
Caliper Measurement
Prior to treatment and at the 6- and 12-week follow-up visits, caliper measurements were made with the participant’s head maintained in a neutral position. The caliper (Defender Body Fat Caliper, Sequoia Fitness Products) was positioned to pinch the skin at 15 mm on either side of the center point of the submental area. Three measurements were made and averaged at each of the baseline and final follow-up visits. The mean fat layer reduction was calculated, and statistical significance was determined by a paired 2-tailed t test. P < .05 was considered significant.
2-D Photographic Evaluation
Standardized 2-D studio photographic images and 3-D stereophotogrammetric images were taken at the baseline visit and 12 weeks after the final treatment. Frontal, left lateral, and right lateral 2-D images were captured using the IntelliStudio (Canfield Scientific, Inc). Three-dimensional face and neck images were captured using the Vectra XT system (Canfield Scientific, Inc). Prior to photography, all makeup and jewelry were removed, hair was pulled away from the participant’s face using a hair band, and a black tank top was worn to standardize background color. In addition, to ensure standardized jaw positioning, a bite plate mold was created out of wax for each participant and worn for both pretreatment and posttreatment photographs. Participants were instructed to stand erect with their heads adjusted, making their Frankfort horizontal plane, or ear-eye plane, parallel to the floor. For the final 2-D images taken 12 weeks after the final treatment, the live preview image of the participant was registered to the baseline image using the MatchPose ghosting capture software (Canfield Scientific, Inc). The mean fat volume, fat thickness, and skin surface area reduction were calculated, and statistical significance was determined by a paired t test. Two-dimensional digital images taken at the 12-week posttreatment visit were compared with those taken at baseline by a blinded independent panel of 3 physicians who were board certified in either dermatology or plastic surgery. Independent photographic review data were generated by randomizing pretreatment and posttreatment pairs of photographs of each participant and then asking the reviewers to determine which image was the pretreatment image. Statistical significance of the independent photographic review data was determined using a 1-sided exact binomial test with statistical significance set at α = 0.025.
3-D Image Evaluation
Both a volumetric and a surface analysis were performed on the 3-D image data sets. Volume difference, height difference, and surface area reduction measures were calculated using VECTRA Analysis Module software (Canfield Scientific, Inc). A trained and certified image analysis technician registered the follow-up 3-D image to precisely match the position and orientation of the baseline image using a mathematical surface-correspondence algorithm referencing common rigid facial surfaces (eg, nose, forehead, and temple). Once registration was complete, the image analysis technician created the submental area of interest from which the measurements were calculated using prespecified anatomical landmarks (right mandible point, right sternocleidomastoid, laryngeal prominence, left sternocleidomastoid, left mandible point, and menton). Automated image analysis algorithms were then run within the VAM software to produce the volume difference from baseline to follow-up, the maximum and mean height difference within the area of interest from the baseline surface to the follow-up surface, and the surface area reduction by tracking the skin deformation within the area of interest from the baseline surface to the follow-up surface.
Adverse Events
Safety was monitored by documentation of adverse events and clinical assessment of the treatment site. Participants were assessed throughout the study for adverse events. Procedural pain was assessed during treatment and immediately after treatment, prior to discharge, and at the 1-week, 6-week, and 12-week follow-up visits using a scale of 0 (no pain) to 10 (worst possible pain). A clinical assessment of the treatment sites was performed immediately after treatment and at 3 days, 1 week, and 6 weeks after the first treatment visit, and these assessments were performed immediately after treatment and at 3 days, 1 week, and 12 weeks after the second treatment visit. At each time point, participants were assessed for common side effects including erythema, edema, bruising, numbness, and tingling at the treatment site, as well as any other reported side effects.
Results
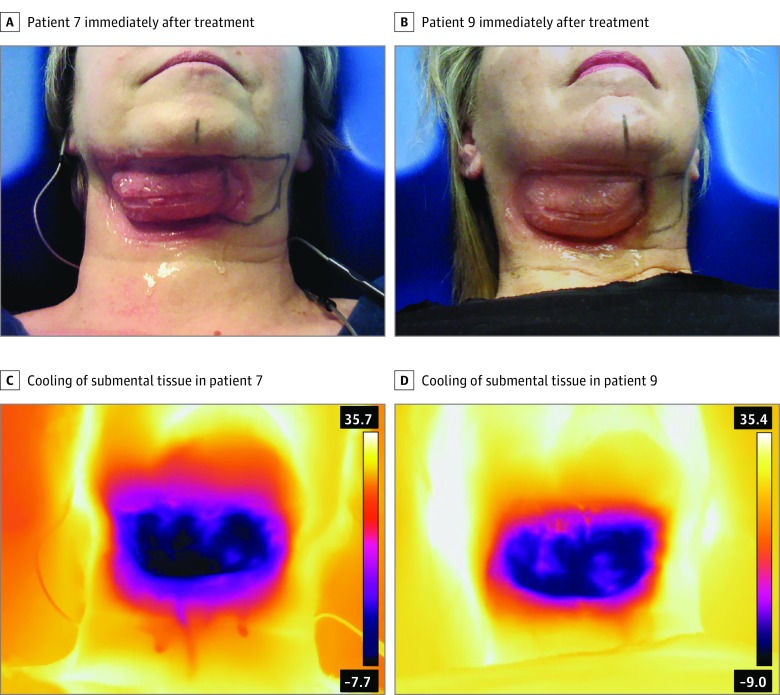
All participants remained within the allowed ±5% weight change limit; therefore, no participants were excluded from the treatment efficacy analysis owing to weight change. The weight change from the first treatment visit to the 12-week follow-up visit was a mean (SD) decrease of 0.5 (2.3) kg (1.2 [5.0] lb). On completion of the treatment cycle and immediately following removal of the cryolipolysis applicator, photographs were taken prior to manual massage of the treatment area. The immediate posttreatment images in Figure 1 demonstrate the typical firm, solidified tissue for the first of 2 bilateral treatments. The tissue was stiff and erythematous immediately after treatment but quickly softened and rewarmed with manual massage. As shown in the infrared images in Figure 1, the entire tissue within the treatment cup attained uniform cooling, and localized warming is evident presumably owing to blood flow.
Figure 1. Immediate Posttreatment Images.
A, Participant 7 immediately after treatment, demonstrating solidified submental tissue. B, Participant 9 immediately after treatment, demonstrating solidified submental tissue. C, Infrared image of participant 7 immediately after treatment, showing uniform cooling of submental tissue. D, Infrared image of participant 9 immediately after treatment, showing uniform cooling of submental tissue.
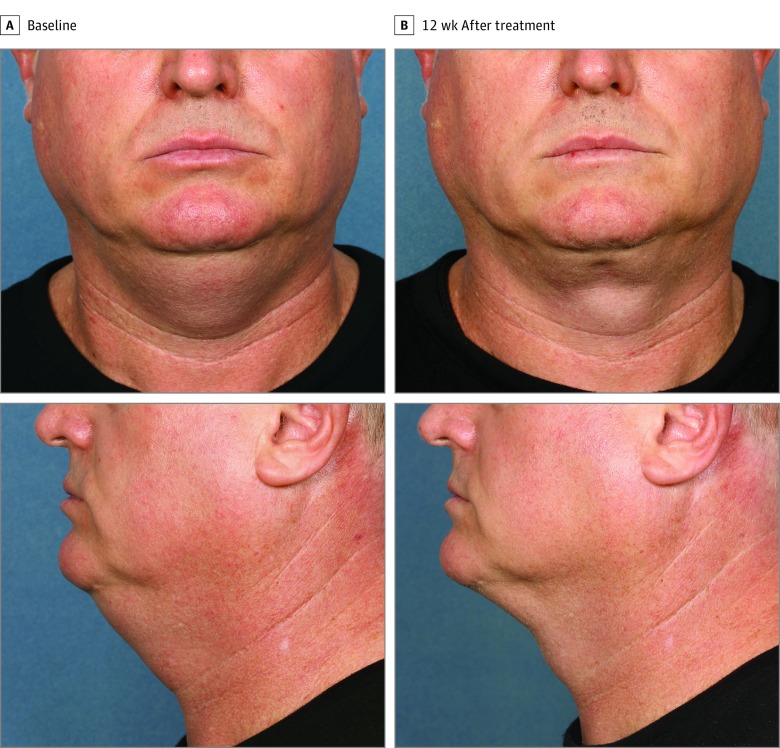
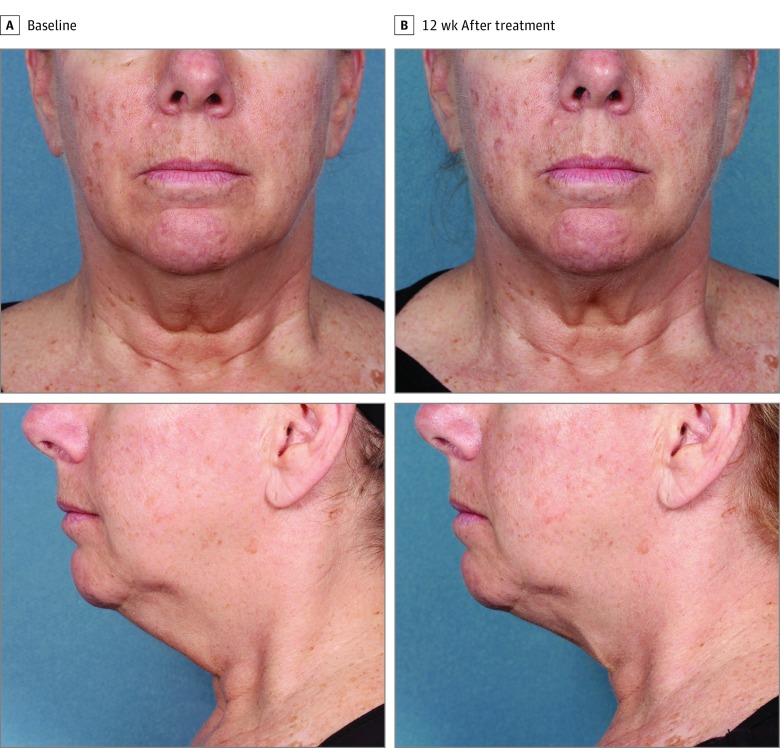
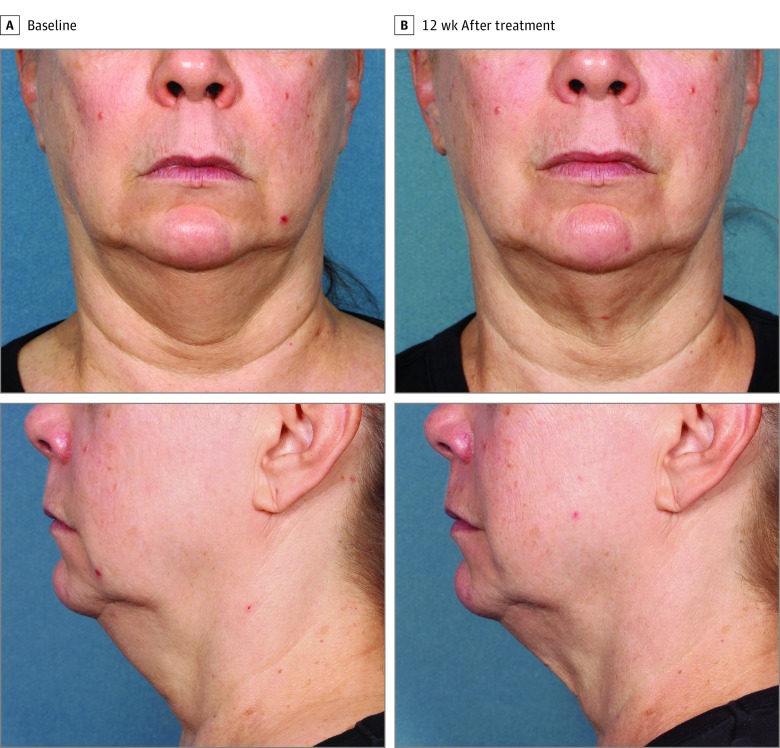
Figure 2, Figure 3, and Figure 4 show representative participants at baseline and 12 weeks after the final treatment. Visible reduction in submental fullness is demonstrated from the pretreatment and posttreatment photographs. For the independent photographic review, 3 blinded, independent physicians reviewed the photographs in randomized pairs. The overall correct identification rate was 81.0% (95% CI, 65.9%-91.4%; P = .02) (34 of 42 photographs), with the 3 reviewers each correctly identifying 92.9% (13 of 14), 78.6% (11 of 14), and 71.4% (10 of 14) of paired photographs. At least 2 of the 3 reviewers correctly identified 78.6% (11 of 14) of the pretreatment images.
Figure 2. Participant 14 at Baseline and 12 Weeks After Treatment.
Weight change, 0.05 kg (0.1 lb) (0.05%) from baseline.
Figure 3. Participant 9 at Baseline and 12 Weeks After Treatment.
Weight change, 1.5 kg (3.4 lb) (1.7%) from baseline.
Figure 4. Participant 3 at Baseline and 12 Weeks After Treatment.
Weight change, 0.3 kg (0.6 lb) (0.3%) from baseline.
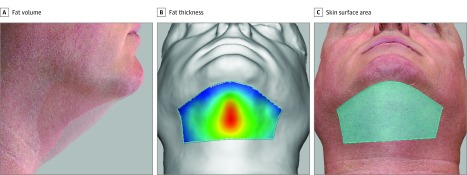
Three-dimensional images were analyzed to quantify changes in the treatment area (Figure 5). The difference in subcutaneous fat volume, maximum fat thickness in the center of the submental area, and skin surface area were quantified. Participant 12 was excluded from the efficacy analysis because of inconsistent neck position between the baseline and follow-up images; thus, 3-D imaging data were pooled for 13 participants. The mean (SD) fat volume reduction was 4.82 (11.42) cm3 (95% CI, –1.39 to 11.02 cm3) (from a reduction of 32.69 cm3 to an increase of 13.85 cm3). The maximum height difference was calculated to determine the fat layer reduction in the center of the submental area. The mean (SD) central submental fat thickness reduction was 3.77 (3.59) mm (95% CI, 1.82-5.72 mm; P < .001) (from a reduction of 13.10 mm to an increase of 0.47 mm). The mean (SD) skin surface area reduction was 1.29 (1.42) cm2 (95% CI, 0.51-2.06 cm2; P < .001) (from a reduction of 3.81 cm2 to an increase of 0.99 cm2). Reductions in fat thickness and skin surface area were statistically significant (P < .001), whereas reduction in volume was not (P = .13).
Figure 5. Three-Dimensional Images.
Images were quantified to evaluate changes between baseline and 12 weeks after treatment.
Skinfold caliper data were analyzed to assess treatment efficacy. Caliper measurements demonstrated a mean (SD) fat layer reduction of 2.3 (0.8) mm (95% CI, 1.9-2.7 mm; P < .001) (range, 0.7-3.5 mm).
Survey data were tabulated for all 14 participants. From the surveys, 13 participants (93%) were satisfied with the procedure, 13 (93%) would recommend submental cryolipolysis to a friend, 12 (86%) felt the procedure improved the contour of their chin and neck, 11 (79%) felt less self-conscious about chin fat following the procedure, and 11 (79%) felt that their appearance improved following the treatment.
Pain was assessed on a scale from 0 to 10 during and after treatment. The procedural pain scores were averaged, and the mean pain score during the first treatment visit was 2.6 for the first cycle and 1.4 for the second cycle. During the second treatment visit, the mean pain score was 2.7 for the first cycle and 1.8 for the second cycle. Posttreatment pain scores were averaged following the first treatment visit; the mean pain score was 1.5 at 1 day, 0.7 at 2 days, 0.4 at 3 days, 0.1 at 1 week, and 0 at 6 weeks after treatment. Following the second treatment visit, the mean pain score was 1.0 at 1 day, 0.4 at 2 days, and 0 at 3 days, 1 week, and 12 weeks after treatment.
Adverse effects of the procedure were typically mild and consisted mainly of numbness and tingling, which resolved without intervention by the final follow-up visit. Immediately after the treatment, the most common effects within the treatment area were erythema, edema, numbness, and tingling. At the 1-week visit after the first treatment, there were 2 incidents of mild swelling, 10 incidents of mild numbness, 1 incident of moderate numbness, 5 incidents of mild tingling, and 1 incident of mild bruising, all of which resolved without intervention by the 6-week follow-up visit. After the second treatment, there was 1 incident of mild swelling, 6 incidents of mild numbness, 3 incidents of mild tingling, and 1 incident of mild pruritus at the 1-week follow-up visit. By the 12-week follow-up visit after the second treatment, all adverse effects had resolved.
There were 2 adverse events associated with the device and/or the procedure. One was an incident of intermittent sharp pain in the left ear that was treated with 400 mg of ibuprofen every 4 to 6 hours and resolved 5 days after treatment. There was 1 incident of tongue tingling following the procedure that resolved without intervention 7 days after the treatment. The primary safety end point for the study was satisfied, and there were no device- or procedure-related serious adverse events. No unanticipated adverse device effects occurred during the study.
Discussion
This study demonstrates the safety, efficacy, tolerability, and participant satisfaction of cryolipolysis for treatment of lateral and central submental fat. A prior study investigated cryolipolysis treatment in the center of the submental area using a prototype applicator and a 60-minute treatment cycle; our study extends the treatment from the center to the lateral submental area and uses a commercial version of the small-volume cup applicator with a 45-minute treatment cycle (CoolMini). The placement of the bilateral applicator in this study allowed treatment of lateral submental fat to achieve a more aesthetically pleasing result for participants with fuller chins and necks. This study used a “treatment to transformation” approach that assessed each individual’s submental area to develop a treatment plan, used multiple applicator placements during the treatment visit, and performed the treatment on multiple visits.
The efficacy of this bilateral submental cryolipolysis procedure was similar to that found in the prior study of central submental cryolipolysis since fat layer reduction was measured in the center of the submental region. The previous central submental study found a mean fat layer reduction of 2.0 mm as measured by ultrasonography. For this bilateral submental treatment study, skinfold caliper data demonstrated a mean (SD) fat layer reduction of 2.3 (0.8) mm. The mean (SD) fat thickness reduction measured via 3-D imaging was 3.77 (3.59) mm in the central submental area. Both of these fat thickness reduction measurements are greater than the central submental cryolipolysis fat layer reduction; the difference is likely because of applicator overlap, resulting in greater efficacy in the central treatment area. For participants with little excess submental fat in the central area, care should be taken to minimize treatment area overlap to avoid excessive fat reduction and irregular contours.
Questionnaires were administered at the final follow-up visit and demonstrated high satisfaction with the bilateral submental treatment procedure. The surveys found that 93% of participants were satisfied, 93% would recommend submental cryolipolysis to a friend, and 86% felt the procedure improved the contour of their chin and neck. These participant surveys show higher satisfaction than the previous central submental study, which determined that 83% of participants were satisfied with submental cryolipolysis and 77% reported visible fat reduction. The increased satisfaction in the present study may be a result of enhanced fat reduction since both the central and lateral portions of the submental area were treated, leading to more aesthetically pleasing chin and neck contouring, and to the fact that participants started with more submental fat than the participants in the previous study. However, the present study did not have a single-application control in the central submental area for direct comparison, and these survey results are being compared across studies.
Injury to the marginal mandibular nerve, a motor branch of the facial nerve, could cause an asymmetrical smile owing to paresis of lip depressor muscles and, therefore, was assessed because the marginal mandibular nerve is located in the lateral mandibular region. Anatomically, the marginal mandibular branch of the facial nerve typically descends about 1 to 4 cm below the angle of the mandible in the lateral neck but is always located above the inferior border of the mandible anterior to the antegonial notch, where it crosses with the facial artery and is away from the submental area. Applicators were placed well away from the location of the marginal mandibular nerve, and no evidence of an asymmetrical smile was noted or reported in any participant. Typical side effects of lateral and central submental cryolipolysis included mild erythema, edema, numbness, and tingling, all of which resolved by the final follow-up visit. There were 2 adverse events associated with the device and/or procedure consisting of 1 participant each with pain and tingling, which resolved shortly over a period of days. There were no serious adverse events, and bilateral submental cryolipolysis was determined to be safe and effective. The procedural and posttreatment pain scores also demonstrated that the bilateral submental cryolipolysis procedure is well tolerated.
The 3-D image analysis from this study demonstrated significant fat layer reduction. The mean (SD) fat volume reduction was 4.82 (11.42) cm3 and ranged from a 13.85-cm3 increase to a 32.69-cm3 reduction. The participant who experienced the 13.85-cm3 increase did not have weight gain (0.6-kg decrease [1.4-lb decrease]) that would contribute to submental volume and did not have increased skinfold caliper measurement (2.2-mm decrease); photographs did not show a clearly visible volume increase in her submental area; there was no evidence of any changes, such as paradoxical adipose hyperplasia, that would have been detected on physical examination at the 12-week visit; and her survey responses indicated that she was very satisfied and felt the overall treatment effect exceeded her expectations. The cause of the increased fat volume for this participant is not known but may be owing to participant positioning inconsistency between the baseline and final 3-D images. The 3-D image analysis also allowed assessment of skin surface area reduction in the treatment region. The quantified image analysis found a mean surface area reduction of 1.29 cm2 of 47.09 cm2, or 2.7%. This skin surface area reduction has been discussed in previous publications as skin tightening. To our knowledge, this is the first publication of quantified skin tightening as a result of cryolipolysis treatment. Further clinical studies should be done to quantify skin tightening and verify if this result is reproducible in other areas of the body following cryolipolysis.
Limitations
The study is limited by its relatively small size (14 participants). The clinical study design was not powered to detect rare adverse events, such as nerve injury and paradoxical adipose hyperplasia.
Conclusions
This study used a bilateral treatment approach to noninvasively reduce submental fat with cryolipolysis. Fourteen participants received bilateral submental cryolipolysis and were reassessed and retreated after 6 weeks. This study shows that lateral and central submental fat can be safely and effectively treated with a small-volume cryolipolysis applicator with high tolerability. The bilateral submental treatments safely and effectively contoured the chin and neck without surgery and produced high participant satisfaction.
References
- 1.Manstein D, Laubach H, Watanabe K, Farinelli W, Zurakowski D, Anderson RR. Selective cryolysis: a novel method of non-invasive fat removal. Lasers Surg Med. 2008;40(9):595-604. [DOI] [PubMed] [Google Scholar]
- 2.Zelickson B, Egbert BM, Preciado J, et al. . Cryolipolysis for noninvasive fat cell destruction: initial results from a pig model. Dermatol Surg. 2009;35(10):1462-1470. [DOI] [PubMed] [Google Scholar]
- 3.Avram MM, Harry RS. Cryolipolysis for subcutaneous fat layer reduction. Lasers Surg Med. 2009;41(10):703-708. [DOI] [PubMed] [Google Scholar]
- 4.Zelickson BD, Burns AJ, Kilmer SL. Cryolipolysis for safe and effective inner thigh fat reduction. Lasers Surg Med. 2015;47(2):120-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens WG, Bachelor EP. Cryolipolysis conformable-surface applicator for nonsurgical fat reduction in lateral thighs. Aesthet Surg J. 2015;35(1):66-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munavalli GS, Panchaprateep R. Cryolipolysis for targeted fat reduction and improved appearance of the enlarged male breast. Dermatol Surg. 2015;41(9):1043-1051. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein EF, Bloom JD, Basilavecchio LD, Plugis JM. Non-invasive fat reduction of the flanks using a new cryolipolysis applicator and overlapping, two-cycle treatments. Lasers Surg Med. 2014;46(10):731-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SJ, Jang HW, Kim H, Suh DH, Ryu HJ. Non-invasive cryolipolysis to reduce subcutaneous fat in the arms. J Cosmet Laser Ther. 2016;18(3):126-129. [DOI] [PubMed] [Google Scholar]
- 9.Wanitphakdeedecha R, Sathaworawong A, Manuskiatti W. The efficacy of cryolipolysis treatment on arms and inner thighs. Lasers Med Sci. 2015;30(8):2165-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilmer SL. Prototype CoolCup cryolipolysis applicator with over 40% reduced treatment time demonstrates equivalent safety and efficacy with greater patient preference. Lasers Surg Med. 2017;49(1):63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derrick CD, Shridharani SM, Broyles JM. The safety and efficacy of cryolipolysis: a systematic review of available literature. Aesthet Surg J. 2015;35(7):830-836. [DOI] [PubMed] [Google Scholar]
- 12.Ingargiola MJ, Motakef S, Chung MT, Vasconez HC, Sasaki GH. Cryolipolysis for fat reduction and body contouring: safety and efficacy of current treatment paradigms. Plast Reconstr Surg. 2015;135(6):1581-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki GH, Abelev N, Tevez-Ortiz A. Noninvasive selective cryolipolysis and reperfusion recovery for localized natural fat reduction and contouring. Aesthet Surg J. 2014;34(3):420-431. [DOI] [PubMed] [Google Scholar]
- 14.Stevens WG, Pietrzak LK, Spring MA. Broad overview of a clinical and commercial experience with CoolSculpting. Aesthet Surg J. 2013;33(6):835-846. [DOI] [PubMed] [Google Scholar]
- 15.Dierickx CC, Mazer JM, Sand M, Koenig S, Arigon V. Safety, tolerance, and patient satisfaction with noninvasive cryolipolysis. Dermatol Surg. 2013;39(8):1209-1216. [DOI] [PubMed] [Google Scholar]
- 16.Kilmer SL, Burns AJ, Zelickson BD. Safety and efficacy of cryolipolysis for non-invasive reduction of submental fat. Lasers Surg Med. 2016;48(1):3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dingman RO, Grabb WC. Surgical anatomy of the mandibular ramus of the facial nerve based on the dissection of 100 facial halves. Plast Reconstr Surg Transplant Bull. 1962;29:266-272. [DOI] [PubMed] [Google Scholar]
- 18.Baker DC, Conley J. Avoiding facial nerve injuries in rhytidectomy: anatomical variations and pitfalls. Plast Reconstr Surg. 1979;64(6):781-795. [DOI] [PubMed] [Google Scholar]
- 19.Carruthers J, Stevens WG, Carruthers A, Humphrey S. Cryolipolysis and skin tightening. Dermatol Surg. 2014;40(suppl 12):S184-S189. [DOI] [PubMed] [Google Scholar]
- 20.Stevens WG. Does cryolipolysis lead to skin tightening? a first report of cryodermadstringo. Aesthet Surg J. 2014;34(6):NP32-NP34. [DOI] [PubMed] [Google Scholar]