Abstract
Background:
Aquilaria, a genus belonging to the Thymelaeaceae, produces fragrant resinous agarwood, also known as eaglewood, which has been used as incense since old times. The intense fra-grance is the result of the presence of a wide variety of secondary metabolites.
Objective:
This genus was reported contained sesquiterpenes, chromones, flavonoids, benzophenones, diterpenoids, triterpenoids, and lignans.
Conclusion:
Here, we review the different secondary metabolites that have been identified in Aquilaria to show their diversity and to allow comparison with other Thymelaeaceae genera.
Keywords: Aquilaria, Thymelaeaceae, sesquiterpene, chromone, flavonoid, benzophenone, diterpenoid, triterpenoid, lignan
1. Introduction
The Thymelaeaceae are a family of dicotyledonous plants mainly found in the tropics and subtropics. They are mostly trees and shrubs, but include also a few vines and herbaceous plants. The family is especially diverse in the southern hemisphere, with many different species present in Africa and Australia [1]. Some species can also be found in Europe, and in parts of Asia and South America, but, in these latter regions, their diversity is much less [2]. Some genera are commercially grown for their sweet-scented and fragrant flowers. Other genera are cultivated for their hardwood, for their bark, which is a raw material for paper making, or for their odorous, highly resinous wood (agarwood), which is used for incense and perfume production.
Thymelaeaceae synthesize many, highly diverse secondary metabolites with a wide range of bioactivities, which has led to numerous applications of Thymelaeceae plant extracts in traditional medicine. For instance, in Kampo medicine in Japan, agarwood preparations are used as sedative, analgesic or digestive [3]. In China, Aquilaria leaves are applied topically to treat injuries such as fractures and bruises [4], and, in Korea, agarwood has been used for the treatment of cough, asthma, and as a sedative among others [5].
A completely different application exploits the strong fragrance of Thymeleaeceae plants, in particular of Aquilaria species. In Saudi Arabia and other Arabic countries, the wood of Aquilaria trees is used as incense at important religious occasions [5, 6]. Wood from closely related species is used during Budhist ceremonies in Asian countries such as Japan and India. Interestingly, the fragrant agarwood resin is not produced in normal wood tissues, but it is only formed when the plant is injured, e.g., by wind, lighting, gnawing by ants or insects, or by microbial infection. These natural processes are slow and occur by chance, causing the agarwood to develop very slowly over decades. Therefore, agarwood is also produced artificially by burning, holing, cutting, or deliberate inoculation of the trees with fungi such as Fusarium spp [7-10]. Nevertheless, despite the artificial production, the demand for agarwood far exceeds the available supply, fostering a deep interest in the secondary metabolites that are responsible for the fragrance properties of agarwood
Widely studied Aquilaria species include A. sinensis, A. malaccensis, A. crassna, and A. agallocha. Depending on the region where these species grow, different names are used for the produced agarwood, such as Eaglewood, Gaharu, Kanankoh, Jinkoh, Chen Xiang or Tram [8, 9]. It is also called aloeswood or agalloch [8]. Each species produces agarwood with different fragrance properties, depending on the variety and quantity of the secondary metabolite content, especially sesquiterpenes and chromones. To assist in the search for alternative sources of agarwood-like fragrant resins, we review here the different secondary metabolites that have so far been characterized in Aquilaria.
There are two reviews that have been published that discussed about the same genus [11, 12]. These previous reviews did not discuss several classes of compounds such as diterpenoid, benzophenon, lignan and used references before 2011. Otherwise, this review was compiled using references, mostly published in 2001-2016. Some references which were dated before 2000 showed that the study of this genus had lasted for longtime. Another review published in 2016 discussed more on bioactivity of compounds contained in this genus [13].
2. Phytochemistry aspects
2.1. Sesquiterpenes
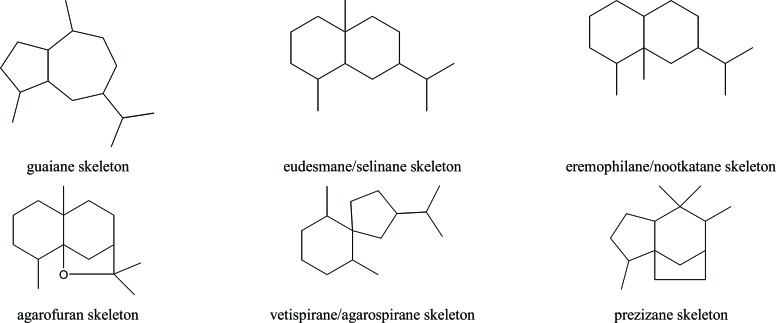
The fragrant sesquiterpenes that have been found in the Aquilaria genus include compounds with a guaiane, eudesmane/selinane, eremophilane/nootkatane, agarofuran, vetispir-ane/agarospirane, or prezizane skeleton (Fig. 1). Most of these sesquiterpenes are oxygenated [14-16].
Fig. (1).
Sesquiterpene skeletons present in the genus Aquilaria.
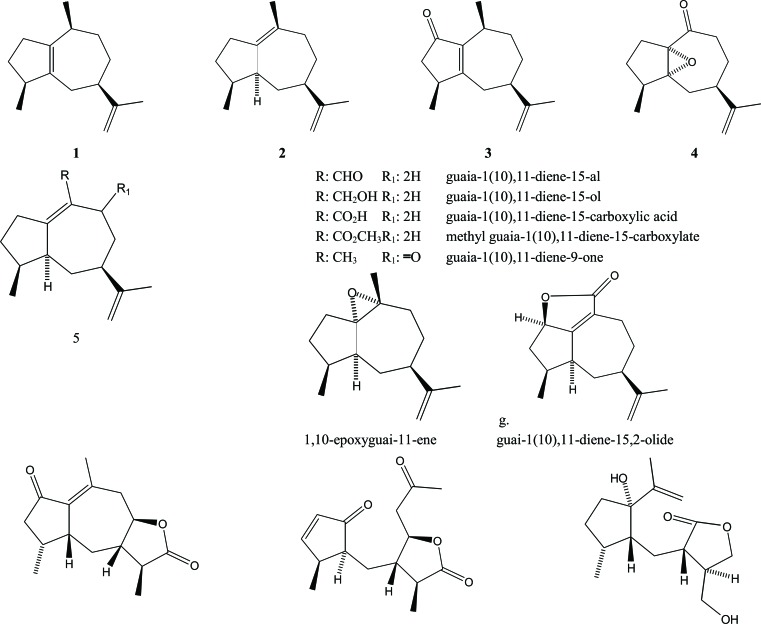
Almost all of these sesquiterpenes have been isolated from agarwood of several species of Aquilaria. For instance, guaiane sesquiterpenes were obtained from the agarwood of A. agallocha including α-guaiene (1), α-bulnesene (2), rotundone (3), 1,5-epoxy-nor ketoguaiene (4), as well as several guaia-1(10),11-diene derivatives (5a-g) [17, 18]. All reported guaiane sesquiterpenes were unsaturated guaianes with some of them being oxygenated.
An interesting guaiane sesquiterpene is 1,10-dioxo-4αH-5αH-7βH-11αH-1, 10-secoguaia-2(3)-en-12, 8β-olide (7), which exhibits anti-inflammatory activity with an IC50 value of 8.1 μM. This compound was isolated from A. sinensis agarwood, together with its derivatives 7βH-guaia-1(10)-en-12,8b-olide (6) and 1β-hydroxy-4βH-5βH-7βH-11αH-8,9-secoguaia-9(10)-en-8,12-olide (8) [19], Fig. (2).
Fig. (2).
Guaiane sesquiterpenes in Aquilaria.
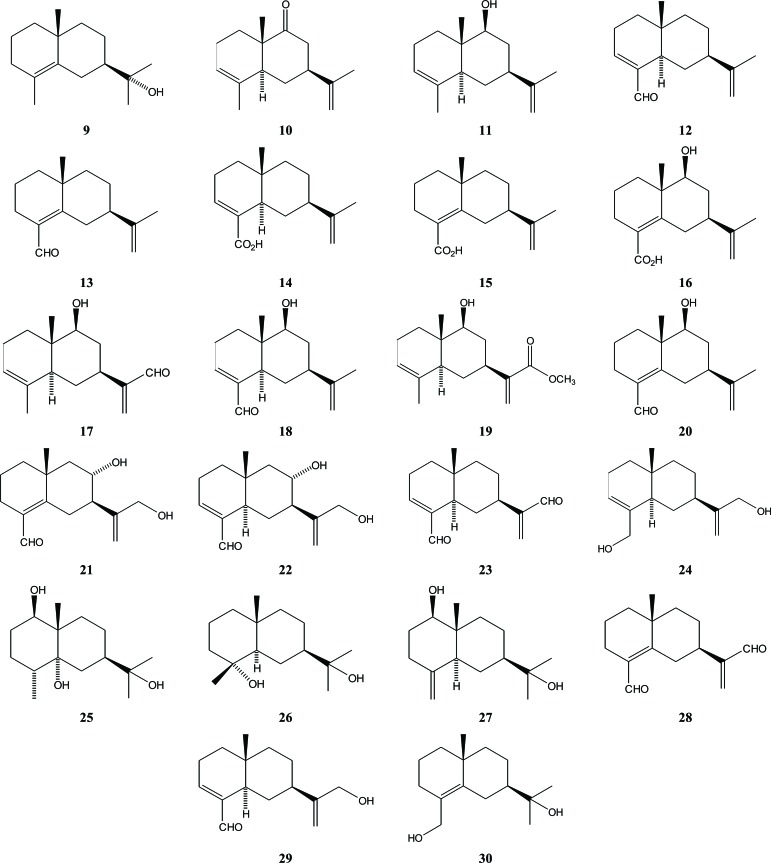
The sesquiterpene 10-epi-γ-eudesmol (9), which has a eudesmane skeleton, has been isolated from the wood of A. malaccensis [20]. Other eudesmanes, including selina-3,11-diene-9-one (10), selina-3,11-diene-9-ol (11), selina-3,11-diene-14-al (12), selina-4,11-diene-14-al (13), selina-3,11-diene-14-carboxylic acid (14), selina-4,11-diene-14- carboxylic acid (15), 9-hydroxyselina-4,11-diene-14-carboxylic acid (16) were isolated from agarwood of A. agallocha [18, 21]. In addition, A. sinensis, which had been subjected to artificial holing, produced several other eudesmane sesquiterpenes, such as 9-hydroxy-selina-3,11-diene-12-al (17), 9-hydroxy-selina-3,11-diene-14-al (18), 9-hydroxy-eudesma-3,11(13)-diene-12-methyl ester (19), 9-hydro-xy-selina-4,11-diene-14-al (20), 8,12-dihydroxy-selina-4,11-diene-14-al (21), 3,4,4α,5,6,7,8,8α-octahydro-7-[1-(hydroxymet-hyl)ethenyl]-4α-methylnaphthalene-1-carboxaldehyde (22), 12, 15-dioxo-α-selinen (23), 15-hydroxyl-12-oxo-α-selinen (24), eudesmane-1β,5α,11-triol (25), 7βH-eudesmane-4α,11-diol (26), and ent-4(15)-eudesmen-1α,11-diol (27) [22]. Compound 23 has also been isolated from A. sinensis by Zhao together with its isomer (28) [19]. In the same year, Wu et al. reported a new eudesmane sesquiterpene (29) that was isolated from 70% MeOH extract of A. malaccensis agarwood chips along with (28) and (30) [23], Fig. (3).
Fig. (3).
Eudesmane sesquiterpenes in Aquilaria.
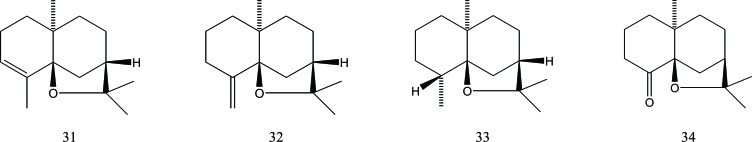
The agarofuran skeleton was the first reported sesquiterpene skeleton found in Aquilaria. Three different sesquiterpenes with this skeleton were isolated from agarwood oil, obtained from fungus-infected A. agallocha Roxb plants, and their structures and absolute configurations were determined by degradative studies and physical measurements. These sesquiterpenes were α-agarofuran (31), β-agarofuran (32) and dihydroagarofuran (33) [24]. β-agarofuran has also been isolated from finely powdered A. agallocha wood originating from Vietnam, together with another agarofuran and nor-ketoagarofuran (34) [25], Fig. (4).
Fig. (4).
Agarofuran sesquiterpenes in Aquilaria.
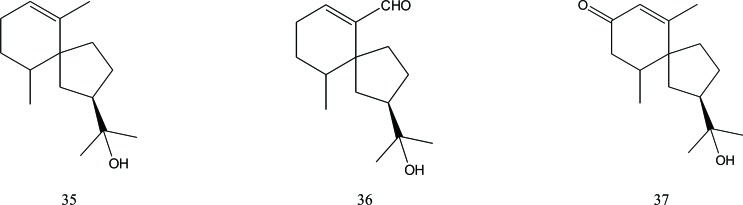
Another sesquiterpene skeleton present in Aquilaria plants is that of agarospirane. Some agarospirane sesquiterpenes that have been identified are agarospirol (35), isolated from agarwood of infected A. agallocha [26], oxo-agarospirol (36), isolated from A. malaccensis [20] and also found in A. agallocha agarwood [18, 25], and 1(10)-spirovetiven-11-ol-2-one (37), isolated from Vietnamese agarwood [8]. Zhao isolated compound (36) from A. sinensis, but named it baimuxinal [19] Fig. (5). This compound was also reported by Wu et al. in 2012, isolated from 70% MeOH extract of A. malaccensis agarwood chips [27], Fig. (5).
Fig. (5).
Agarospiran sesquiterpenes in Aquilaria.
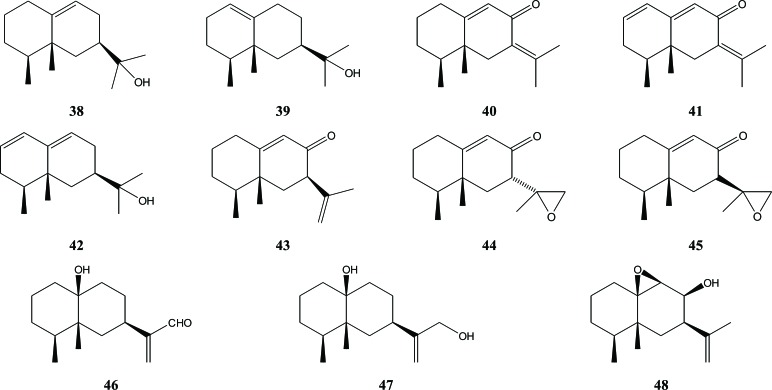
Sesquiterpenes with an eremophilane skeleton have also been identified in A. agallocha agarwood such as jinkoh eremol (38), kusunol (39), and dihydrokaranone (40) [25]. Alkhathlan et al. also successfully isolated the latter compound, but named it dehydrofukinone [6]. Ishihara et al. discovered the presence of the eremophilan sesquiterpene karanone (41) in agarwood from A. agallocha [17]. Two other eremophilan sesquiterpenes from A. agallocha were reported by Ishihara et al. in 1993, namely dehydrojinkoh-eremol (42) and neopetasane (43) [21]. The last compound, also reported by Wu et al. was isolated from 70% MeOH extract of A. malaccensis agarwood chips [27]. In 2014, Yang et al. published their research on the isolation and identification of compound (43), 7β-H-9(10)-ene-11,12-epoxy-8-oxoeremo-philane (44) and 7α-H-9(10)-ene-11,12-epoxy-8-oxoeremo-philane (45) from Chinese agarwood (A. sinensis (Lour.) Gilg.) [16]. In 2012, Wu et al. reported the presence of three eremophilane sesquiterpenes (46), (47), and (48) isolated from 70% MeOH extract of A. malaccensis agarwood chips [23], Fig. (6).
Fig. (6).
Eremophilane sesquiterpenes in Aquilaria.
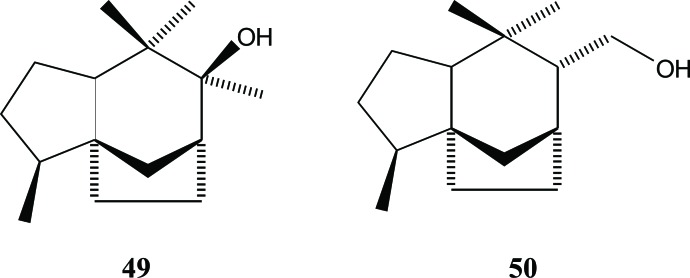
The presence of prezizane sesquiterpenes in Aquilaria appears to be limited. Jinkohol (49) and jinkohol II (50) were present in agarwood from A. malaccensis. This finding was reported by Yoneda et al. in 1984 [25], Fig. (7). Previously, Nakanishi et al.. had already reported the existence of jinkohol in Aquilaria sp.(Indonesian agarwood) [14].
Fig. (7).
Prezizane sesquiterpenes in Aquilaria.
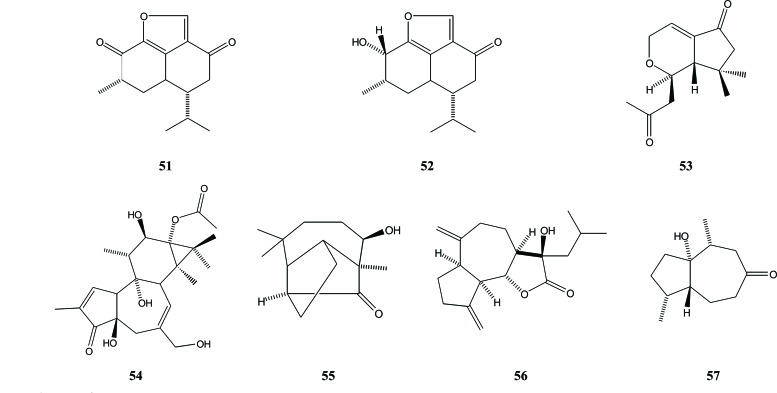
Miscellaneous sequiterpenes found in Aquilaria are gmelofuran (51) and 8β-dihydrogmelofuran (52), which were isolated from wood of A. agallocha [27]. Aquilarin B, a degraded sesquiterpene (53), as well as another sesquiterpene (54), both with a guaiane-like skeleton, were identified from the stem of A. sinensis [28, 29]. In addition, Yang et al. obtained two other compounds from Chinese eaglewood, with one of them also having a guaiane-like skeleton. These two compounds are 8β-hydroxy-longicamphenylone (55) and 11β-hydroxy-13-isopropyl-dihydro-dehydrocostus lactone (56) [30]. Another compound identified in Chinese eaglewood, A. sinensis (Lour.) Gilg., is the sesquiterpenoid derivative 1α-hydroxy-4α,10α-dimethyl-5βH-octahydro-azulen-8-one (57), which was isolated from a 95% ethanolic extract [19], Fig. (8).
Fig. (8).
Miscellaneous sesquiterpenes in Aquilaria.
2.2. Chromones
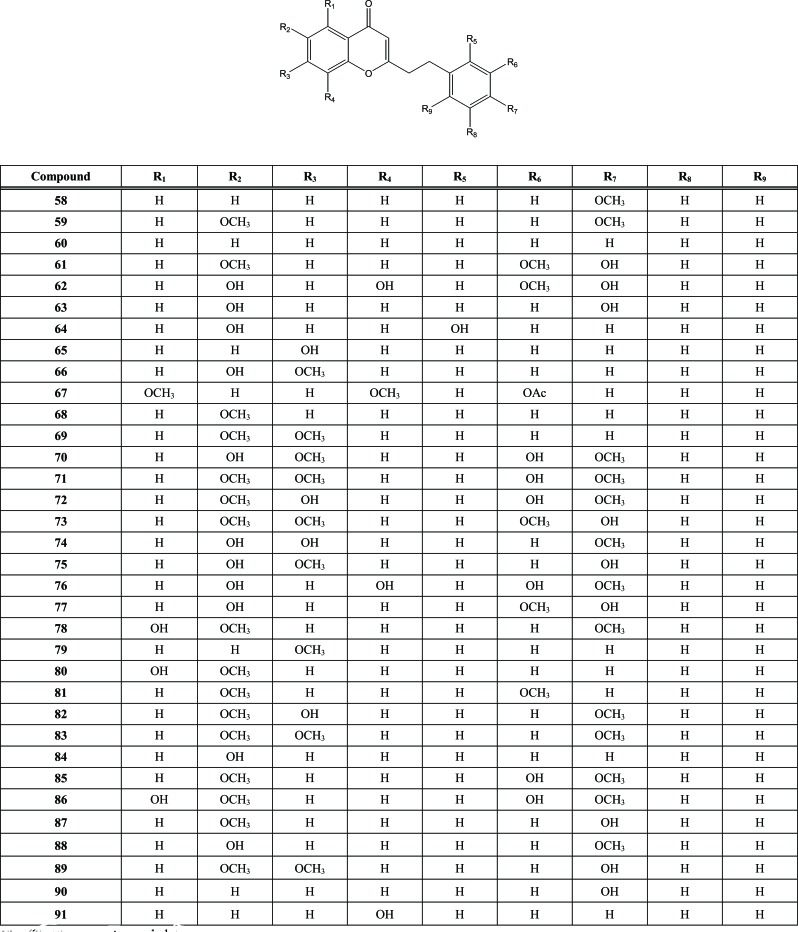
Another class of extensively reported secondary metabolites that have been isolated from Aquilaria species is the chromones, Fig. (9). All isolated chromones are derivatives of 2-(2-phenylethyl) chromone. The first two chromones identified (58 and 59) were isolated from A. agallocha wood [31], but the most simple chromone reported was 2-(2-phenylethyl)chromone or flidersiachromone (60) which was isolated for the first time from the ether extract of powdered agarwood of A. malaccensis along with 6-methoxy-2-[2-(3-methoxy-4-hydroxyphenyl)ethyl]chromone (61), 6,8-dihydroxy-2-(2-phenylethyl)chromone (62), 6-hydroxy-2-[2-(4-hydroxyphenyl)ethyl]chromone (63), 6-hydroxy-2-[2-(2-hydroxyphenyl)ethyl]chromone (64); 7-hydroxy-2-(2-phenylethyl)chromone (65), and 6-hydroxy-7-methoxy-2-(2-phenylethyl)chromone (66) [32]. Another 2-(2-phenylethyl) chromone derivative, namely 7,8-dimethoxy-2-[2-(3’-acetoxyphenyl)ethyl] chromone (67), was isolated from an acetone extract of Cambodian agarwood of A. agallocha along with two other chromones, 6-methoxy-2-(2-phenylethyl) chromone (68) and 6,7-dimethoxy-2-(2-phenylethyl) chromone (69) [6]. Then, Yang et al. obtained eight 2-(2-phenylethyl) chromone derivatives (70-77) from an EtOH extract of Chinese eaglewood from the A. sinensis [33]. Six 2-(2-phenylethyl) chromones were isolated from a 70% MeOH extract of A. malaccensis agarwood chips [23]. Two chromones was reported before and identified as compound (58) and (59) and four others were compound 78-81. In the same year, 2012, Wu published again the report about some compounds contained in 70% MeOH extract of A. malaccensis agarwood chips. In that report, six chromones was isolated and identified [27]. Three compounds were reported before (60), (68) and (69), whereas three others had not been reported yet (82-84). In 2014, Li et al. reported the isolation of four 2-(2-phenylethyl)chromones (85-88), as well as other compounds that had been reported before, such as compounds 59, 61, 69, 76, 78, 79 and 84 from the EtOAc extract of Chinese agarwood induced by artificial holing originating from A. sinensis (Lour.) Gilg [9]. Phytochemical analysis of high quality Chinese agarwood from A. sinensis led to the isolation of three new 2-(2-phenylethyl) chromone derivatives (89-91) and two compounds that had been reported before [7]. In 2015, three 2-(2-phenylethyl) chromone derivatives were also isolated from the petioles and leaves of A. sinensis. The structures of these three chromones were elucidated and its structure were identical to compounds 58, 61 and 63 [34].
Fig. (9).
Chromone basic skeleton.
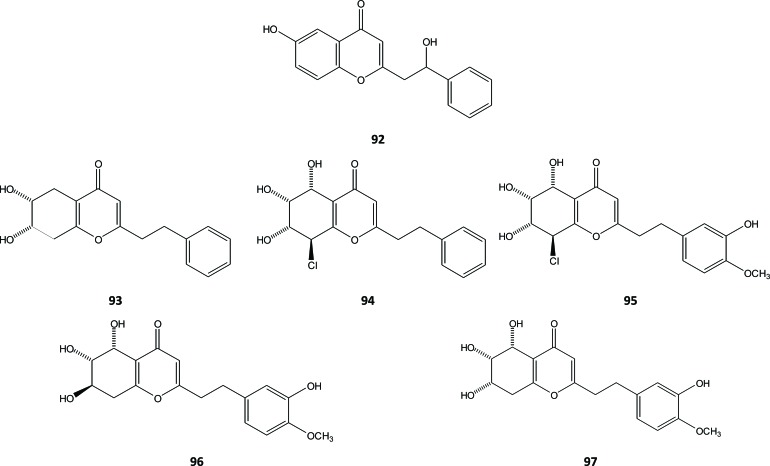
In addition to having been isolated from A. agallocha, compound 68 was also obtained from withered wood of A. sinensis, together with three other chromones (92, 93 and 94) [3]. The agarwood of the same plant gave also the 8-chloro-5,6,7-dihydroxy-2(3-hydroxy-4-methoxyphenylethyl)-5,6,7,8-tetrahydro-4H-chromen-4-one chromone (95), which was isolated and identified by a Chinese research team [35]. Compound 93, 94 and 95 belong to tetrahydrochromones. However, compound 94 and 95 are tetrahydrochromones that substituted by chloro in its structure. The presence of chloro in secondary metabolites structure is very rare. Meanwhile, compound 92 is unique because it is the only one chromone isolated and identified from Aquilaria that is hydroxylated in ethyl moiety. Subsequently, Dai et al.. isolated and structurally characterized two isomers of another tetrahydrochromones (96 and 97) from the EtOH extract of the withered wood of A. sinensis [36], Fig. (10).
Fig. (10).
Some tetrahydrochromones from A. sinensis.
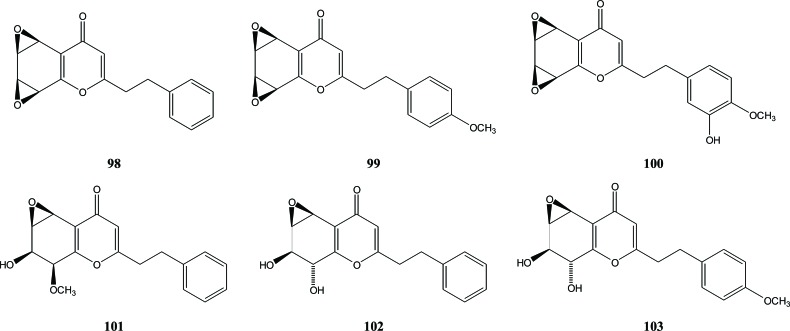
Three di-epoxy-tetrahydrochromones - oxidoagarochromones A (98), B (99), and C (100) - were isolated from agarwood that was artificially produced by intentional wounding of A. crassna [37]. Compounds 98 and 99 have also been obtained from Chinese agarwood of A. sinensis wounded by artificial holing [9]. In 2014, Li et al. reported the isolation of mono-epoxy-tetrahydrochromones (101, 102 and 103) [9], Fig. (11).
Fig. (11).
Epoxy-tetrahydrochromones from Aquilaria.
2.3. Flavonoids
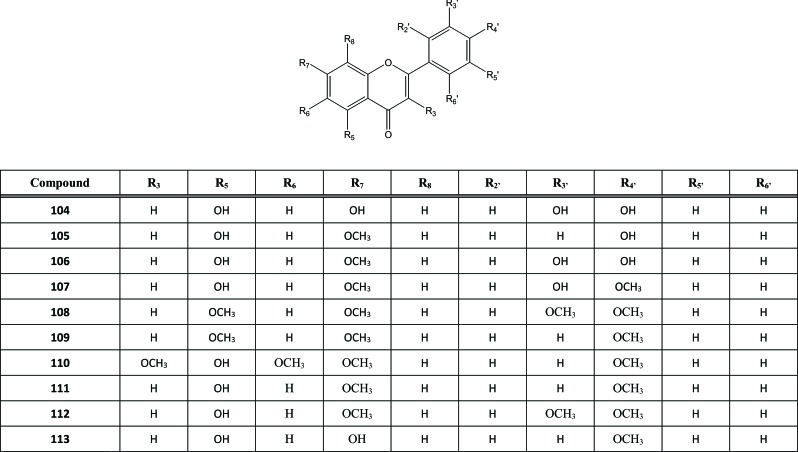
From the leaves of A. sinensis, several flavonoids, both as glycoside or aglycon, have been isolated and identified. Some aglycon flavonoids found in Aquilaria were luteolin (104), genkwanin (105), and hydroxygenkwanin (106) that were isolated by Qi et al. [38]. Cheng et al. identified several other aglycon flavonoids, such as 7,4’-dimethyl luteolin (107), 5,7,3’,4’-tetramethoxy–flavone (108), 5,7,4’-trimeth-oxyflavone (109) and 5-hydroxy-3,4’,6,7-tetramethoxy-flavone (110) [39]. Furthermore, several aglycon flavonoids were also isolated from the stem of this plant, such as 5-hydroxyl-7,4’-dimethoxyflavone (111), 7, 3’, 4’-trimethyl luteolin (112) and 5, 7-dihydroxyl-4’-methoxyflavone (113) [40], Fig. (12). All of aglycone flavonoids that has been reported belong to flavone, except compound 110 that was part of flavonol.
Fig. (12).
Flavonoid basic skeleton.
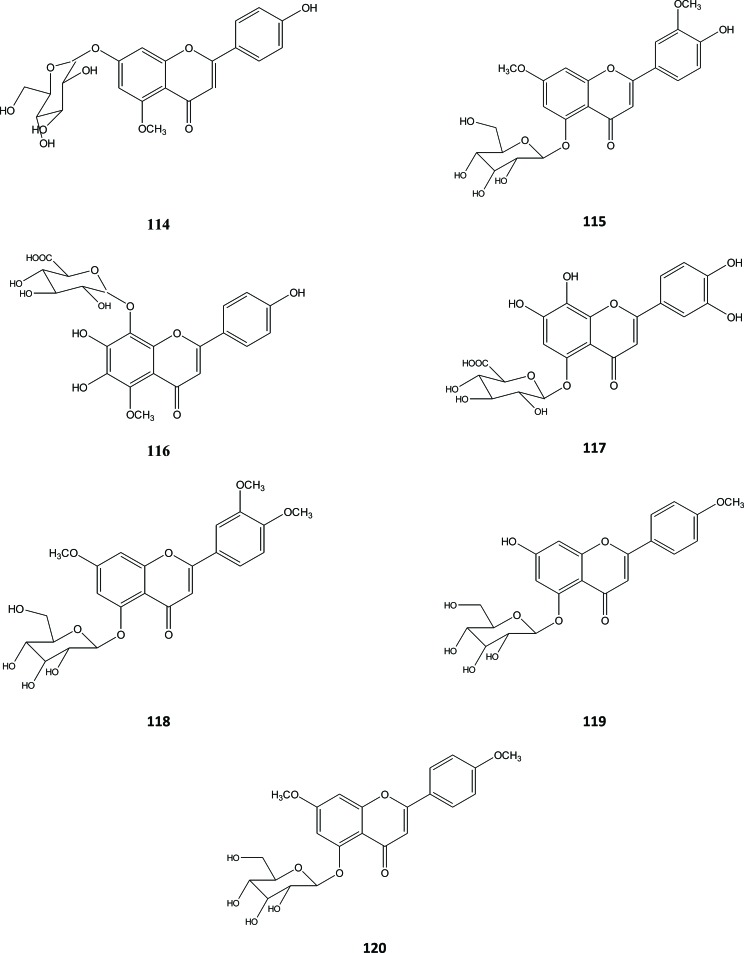
Flavonoids glycoside identified from A. sinensis were mono- or di-glycoside. This sugar moiety most often substituted the hydroxy group at position 5, but substitutions can also occur in positions 7 and 8. These flavonoids mono-glycoside were the 7−β−D-glucoside of 5-O-methylapigenin (114), the 5-β-D-glucoside of 7,3-di-O-methylluteolin (115) [38], aquilarisin (116), and hypolaetin 5-O-β-D-glucuronopyranoside (117) [41]. Several flavonoids glycoside were also isolated from the stem of this plant, such as 5-O-glucosides of 7,3‘,4‘-tri-O-methylluteolin (lethedoside A) (118), 7-hydroxyl-4’-methyl-5-O-glucosideflavonoid (119) and 7, 4’-dimethyl-5-O-glucosideflavonoid (120) [40], Fig. (13).
Fig. (13).
Mono-glycoside flavonoids in A. sinensis.
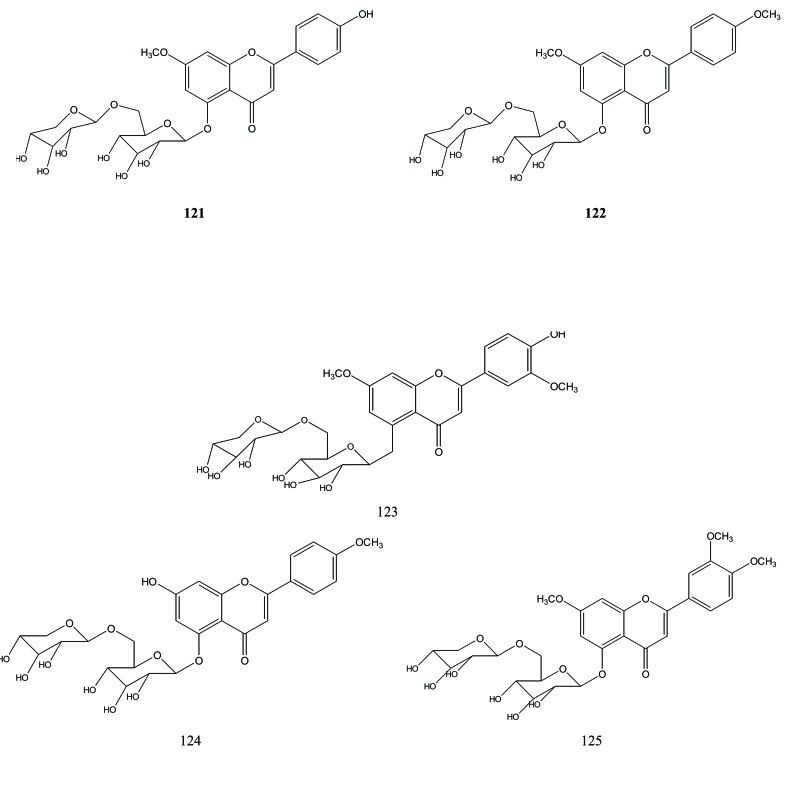
Flavonoid di-glycoside reported from Aquilaria were 5-O-xylosylglucoside of 7-O-methylapigenin (121), 5-O-xylosylglucoside of 7,4'-di-O-methylapigenin (122) [38], aquisiflavoside (123) [42], aquilarinoside A1 (124) and lethedioside A (= 5-O-xylosylglucosides of 7,3‘,4‘-tri-O-methylluteolin) (125) [40], Fig. (14).
Fig. (14).
Di-glycoside flavonoid in A. sinensis.
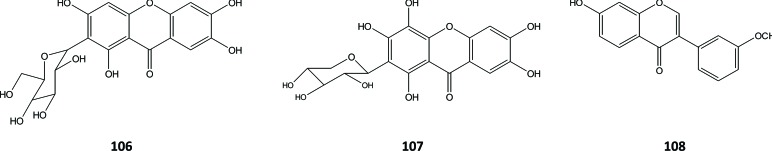
Besides flavonoids, the presence of several xanthons has been reported, particularly mangiferin (106) and aquilarixanthon (107) in A. sinensis [38, 41]. One isoflavonoid, formononetin (108), was also isolated and identified from the stem of this plant [40], Fig. (15).
Fig. (15).
Xanthones and isoflavonoid in A. sinensis.
2.4. Benzophenones
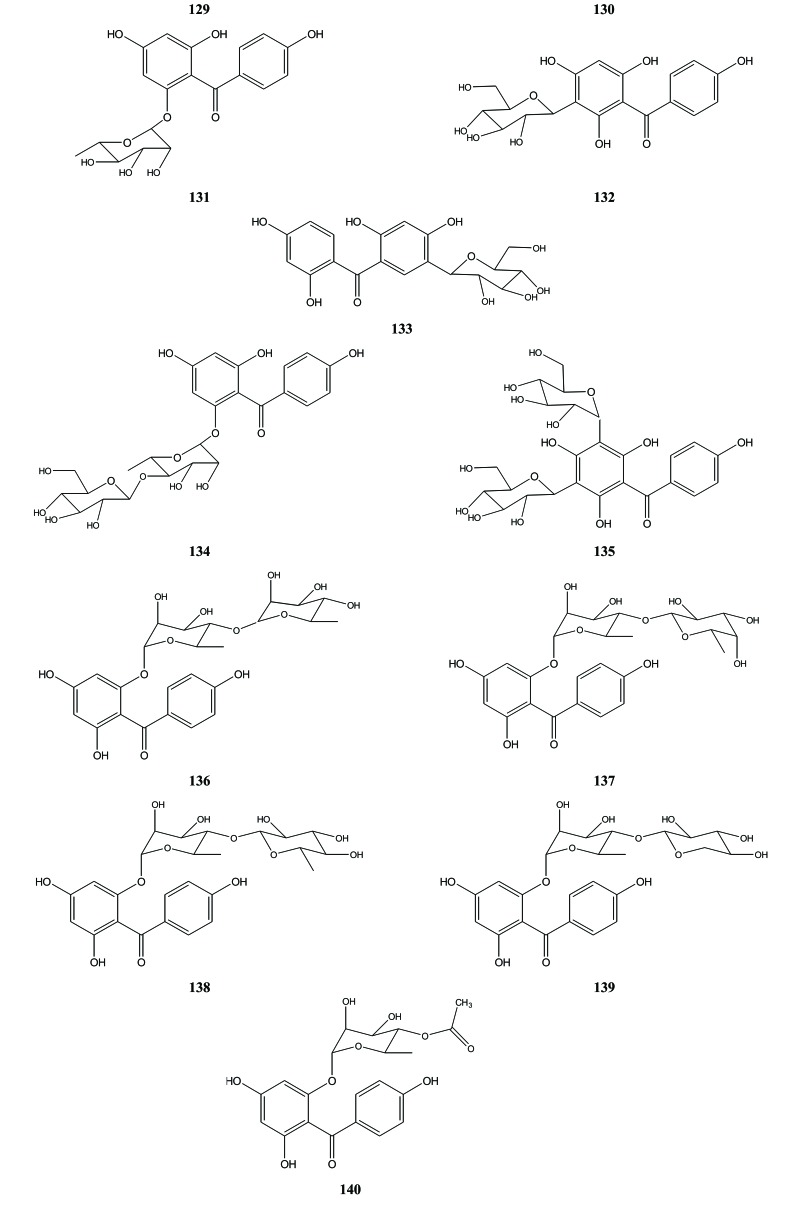
Such as flavonoids, Aquilaria benzophenone was also divided over the benzophenone aglycone and benzophenone glycoside. All benzophenone glycoside identified in Aquilaria are derivate of benzophenone iriflophenone (129) that was isolated from the leaves of A. sinensis along with the flavonoids described above [38]. An iriflophenone derivative, aquilarinoside A (130), was also obtained from the leaves of A. sinensis. Aquilarinoside A (130) was a benzophenone mono-glycoside with α-fructofuranose as a sugar moiety [38]. Some others iriflophenone mono-glycoside were iriflophenone 2-O-α-L-rhamnopyranoside (131) that was isolated from the leaves of A. sinensis, iriflophenone 3-C-β-D-glucoside (132) that were isolated from the petioles and leaves of A. sinensis [34] and another benzophenon that have been reported by other researchers and were identified as benzophenone C-glycoside (= 3C-β-D-glucopyranosyl-4’,2,4,6-tetrahydroxybenzophenone (133) [39, 43]. Aquilarisinin (134) and iriflophenone 3,5-C-β-D-diglucopyranoside (135) that were isolated from the petioles and leaves of A. sinensis were exemples benzophenon iriflophenone di-glycoside [34]. In 2014, Sun et al. published four benzophenone glycosides, the aquilarinensides A-D (136-139), which had also been isolated from the leaves of the same plant [44]. In addition to these four compounds, an iriflophenone glycoside with acetyl group (140) was also reported. Based on these structures, it can be seen that there are iriflophenone-O-glycoside and iriflophenone-C-glycoside. A sugar moiety could substitute hydroxyl group either at position 2 or 6, Fig. (16). Meanwhile for iriflophenone-C-glycoside, a sugar moiety could be substituted at position 3, 5 or 5’.
Fig. (16).
Benzophenones in Aquilaria.
2.5. Diterpenoids
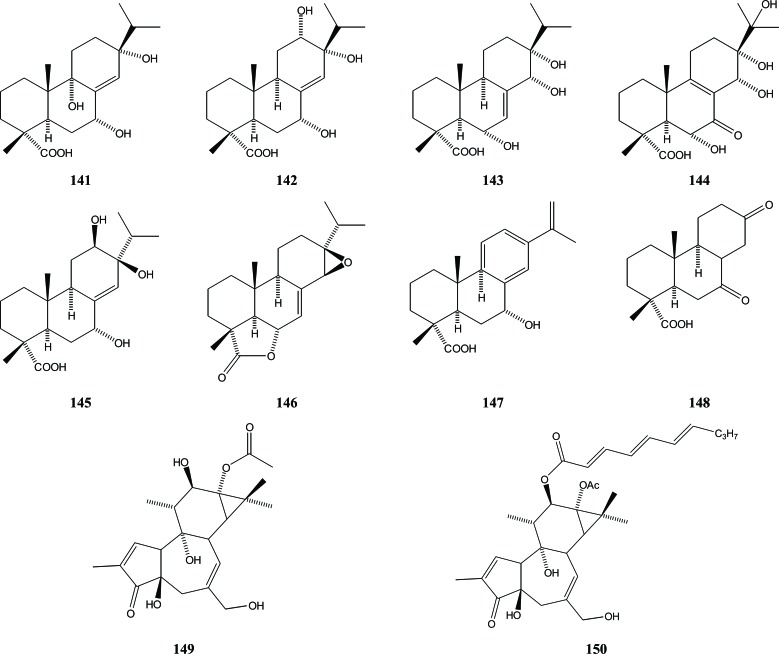
All diterpenoid compounds that so far have been isolated from Aquilaria species have an abietane and podocarpane skeleton. Seven abietane diterpenoids (aquilarabietic acids 141-147) and one podocarpane diterpenoid (148) were obtained from the Chinese eaglewood, A. sinensis [45]. Meanwhile, a phorbol derivative - phorbol-13-acetate (149) - was isolated from the EtOH extract of the fresh stem of A. sinensis (Lour.) Gilg. [29]. Phorbol is a member of the tigliane family of diterpenes. Another phorbol compound had previously been isolated from the stem bark of the Thai A. malaccensis tree, namely 12-O-n-deca-2,4,6-trienoylphor-bol-13-acetate (150) [46], Fig. (17).
Fig. (17).
Diterpenoides in Aquilaria.
2.6. Triterpenoids
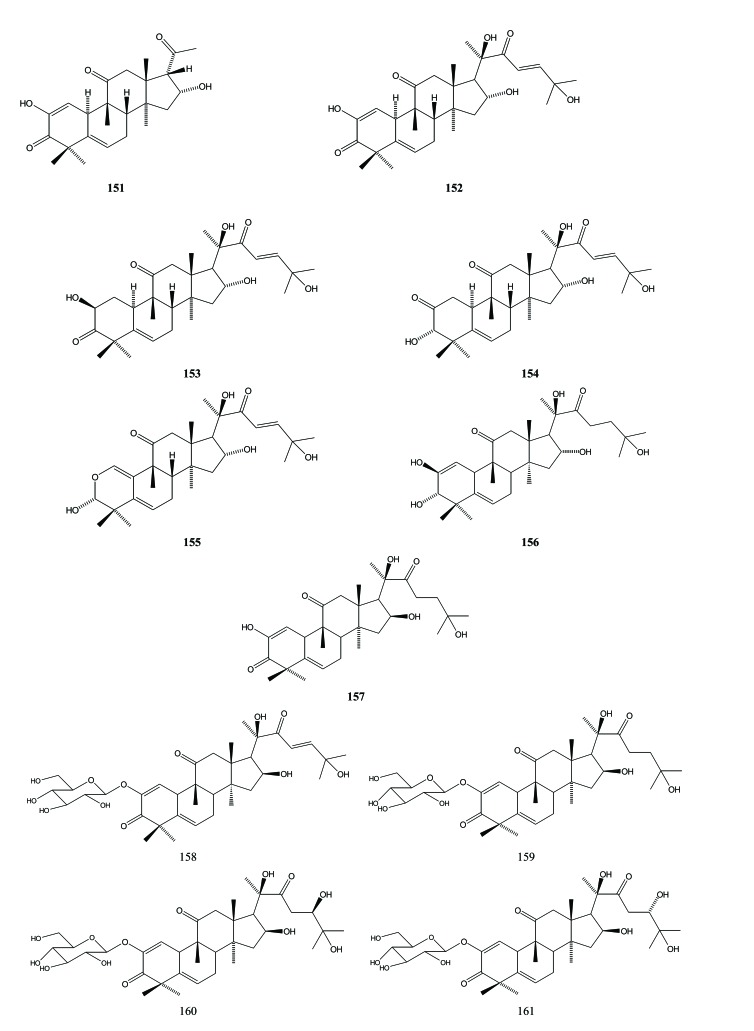
From the fruits of A. sinensis, five cucurbitacine triterpenoid compounds were isolated and identified as hexanocucurbitacin I (151), cucurbitacin I (152), cucurbitacin D (153), isocucurbitacin D (154), and neocucurbitacin B (155) [47]. Another cucurbitacine triterpenoid, namely dihydrocucurbitacine F (156), was isolated from the EtOH extract of the fresh stem of A. sinensis (Lour). Gilg. [29]. Furthermore, an aglycon cucurbitacine triterpenoid (157) were reported by Wang along with some cucurbitane triterpene glycosides (158-161) [34], Fig. (18).
Fig. (18).
Cucurbitacine triterpenoid in Aquilaria.
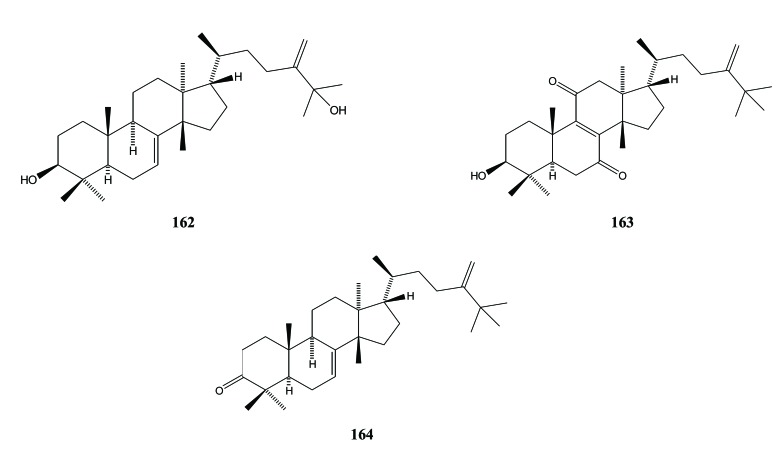
Three triterpenoids with a tirucallane skeleton have also been identified in A. sinensis. They are aquilacalane A (24-methylenetirucall-7(8)-en-3β,25-diol) (162), aquilacallane B (24-methylene-25-methyltirucall-8(9)-en-3β-ol-7,11-dione (163), and 24-methylene-25-methyltirucall-7-en-3-one (wallenone) (164)). They were isolated from the leaves of A. sinensis [39, 48]. The presence of these two tirucallane triterpenoids (162 and 163) in the petioles and leaves of. A. sinensis have also reported by Wang [34], Fig. (19).
Fig. (19).
Tirucallane triterpenoid in Aquilaria.
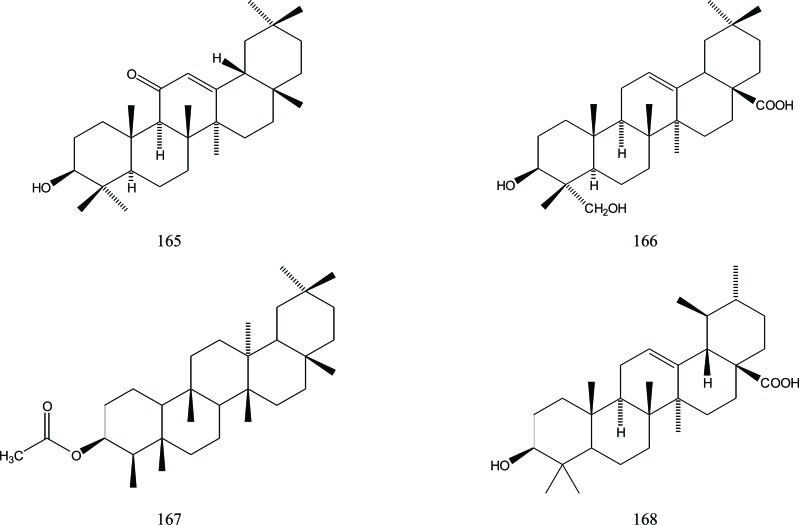
Oleanane triterpenoid skeletons were also found in A. sinensis such as those of 11-oxo-β-amyrin (165), hederagenin-an (166), 3β-acetoxyfriedelane (167) and ursolic acid (168). Their presence was reported by Cheng [39], Fig. (20).
Fig. (20).
Oleanane triterpenoides in Aquilaria.
2.7. Lignans
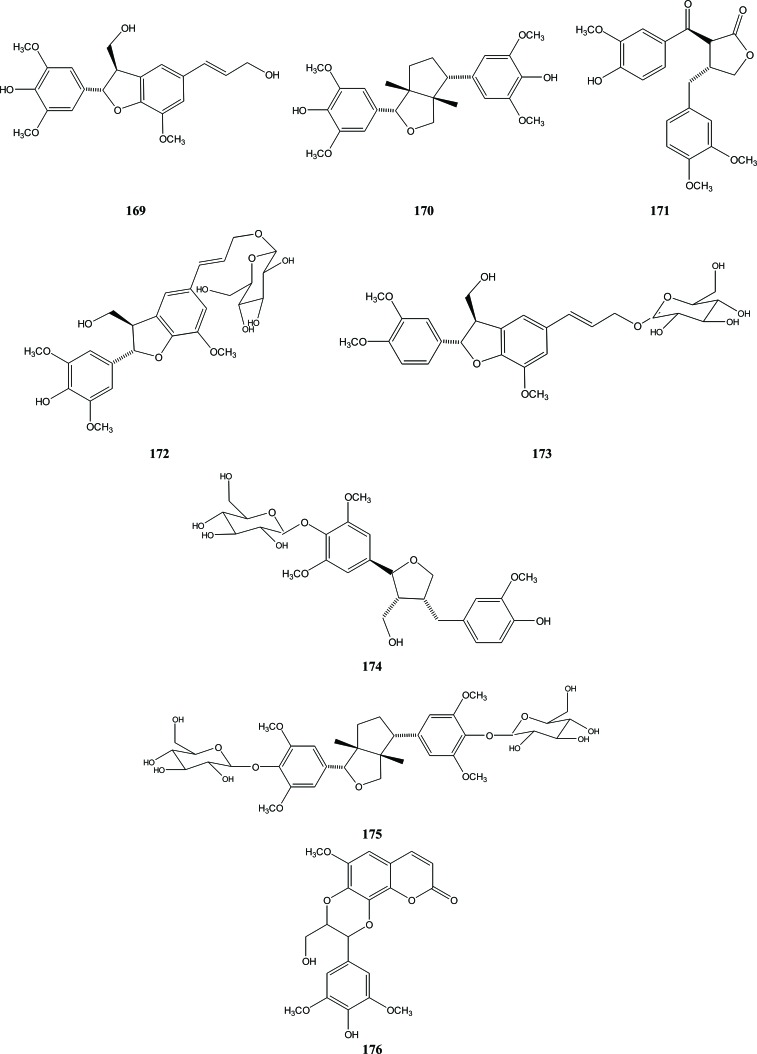
From the dried stems of A. sinensis that were collected in Qingyuan, Guangdong Province of China, seven lignans were isolated, including lignan aglycon and lignan glycoside [49]. They were identified as simulanol (169), syringaresinol (170), conicaol B (171), aquilaroside A (172), longifloroside A (173), conicaoside (174) and liriodendrin (175). These compounds are lignan derivatives with different skeletons, which are widely distributed in higher plants. Compounds 169, 172 and 173 are common benzofuran-type lignan derivatives, but compounds 170 and 175 are di-tetrahydrofuran lignans, while compounds 174 and 171 represent tetrahydrofuran and dibenzylbutyrolactone types, respectively. A coumarinolignan, namely aquillochin (176), was isolated from the whole plant of A. agallocha. The structure was proposed by Bhandari et al. on the basis of chemical and physical characterization [50], Fig. (21).
Fig. (21).
Lignans in Aquilaria.
2.8. Miscellaneous
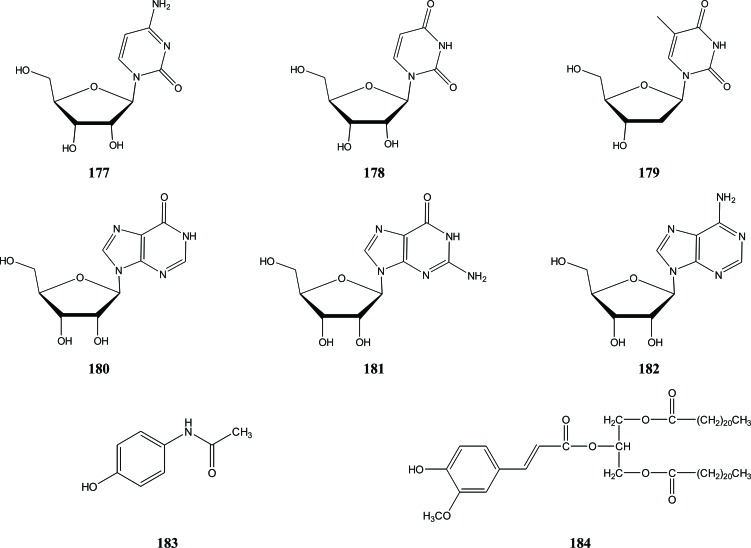
Several other compounds outside the compound classes discussed above are also found in Aquilaria. They include several nucleosides (177-182) and 4-hydroxyacetanilide (183). The nucleosides were isolated from the petioles and leaves of A. sinensis, whereas 4-hydroxyacetanilide (183) was obtained from a leaves extract of A. malaccencis [34, 51]. From the stem bark of A. malaccensis tree, the glyceride 1,3-dibehenyl-2-ferulyl glyceride (184) was isolated and identified [46], Fig. (22).
Fig. (22).
Miscellaneous compounds in Aquilaria.
As Aquilaria plants are known as producers of high-quality fragrant material, then it is to be expected that these plants produce essential oils. 4-Phenyl-2-butanone, α-bulnesene, α-guaiene, agarospirol, ledene oxide-(II), elemol and γ-eudesmol were identified as the major chemical constituents of Malaysian agarwood (A. malaccensis) oils [52]. The composition of essential oils can be used to determine the quality of agarwood obtained from healthy, naturally infected, or artificially wounded trees. Such research has been done with agarwood from A. agallocha Roxb. [53].
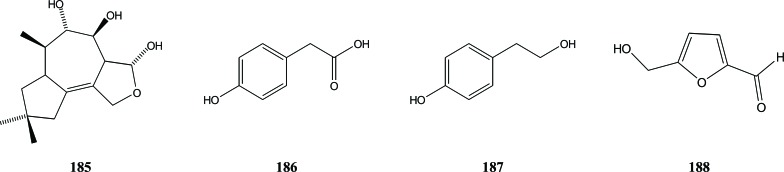
Not only Aquilaria species, but also the fungi infecting them may produce fragrant compounds. For instance, from a fermentation of the endophytic Chinese eaglewood fungus HP-1, four compounds were isolated that were identified as 3α, 3β, 10β-trimethyl-decahydroazuleno [6, 7] furan-8, 9, 14-triol (185), 4-hydroxyphenylacetic acid (186), 4-hydroxyphenethyl alcohol (187) and 5-hydroxymethyl-2-furancarboxaldehyde (188) [54], Fig. (23).
Fig. (23).
Compounds producing by endophytic Chinese eaglewood fungus HP-1.
Table 1 provides a summary of secondary metabolites have been reported, along with parts of the plant and the species from which they were isolated.
Table 1.
Summary of secondary metabolites isolated from some Aquilaria species.
| Secondary Metabolites | Part | Species | References |
|---|---|---|---|
| Guaiane Sesquiterpenes | Agarwood | Aquilaria agallocha | Ishihara, M. et al. |
| Aquilaria sinensis | Zhao, H. et al. | ||
| Eudesmane Sesquiterpenes | Wood | Aquilaria malaccensis | Nakanishi, T. et al. |
| Agarwood | Aquilaria agallocha | Ishihara, M. et al. | |
| Aquilaria sinensis | Li, W. et al.Zhao, H. et al. | ||
| Aquilaria malaccensis | Wu, B. et al. | ||
| Agarofuran Sesquiterpenes | Agarwood | Aquilaria agallocha | Maheswari, M.L. et al. |
| Wood | Yoneda, K. et al. | ||
| Secondary Metabolites | Part | Species | References |
| Agarospirane Sesquiterpenes | Agarwood | Aquilaria agallocha | Varma, K.R. et al.Ishihara, M. et al.Yoneda, K. et al. |
| Aquilaria malaccensis | Nakanishi, T. et al. | ||
| Aquilaria sp. (Vietnam) | Ueda, J. et al. | ||
| Aquilaria sinensis | Zhao, H. et al.Wu, B. et al. | ||
| Eremophilane Sesquiterpenes | Agarwood | Aquilaria agallocha, | Yoneda, K. et al.Alkhathlan, H.Z. et al.Ishihara, M. et al. |
| Aquilaria malaccensis | Wu, B. et al. | ||
| Aquilaria sinensis | Yang, D.L. et al. | ||
| Prezizane Sesquiterpenes | Agarwood | Aquilaria malaccensis, | Yoneda, K. et al. |
| Aquilaria sp. (Indonesia) | Nakanishi, T. et al. | ||
| Miscellaneous Sesquiterpenes | Wood | Aquilaria agallocha | Wu, B. et al. |
| Stem | Aquilaria sinensis | Pant, P. et al. | |
| Agarwood/Eaglewood | Yang, L. et al.Zhao, H. et al. | ||
| Chromones | Wood | Aquilaria agallocha | Nakanishi, T. et al. |
| Agarwood/ Eaglewood | Aquilaria malaccensis, | Konishi, T. et al.Wu, B. et al. | |
| Aquilaria agallocha, | Alkhathlan, H.Z. et al. | ||
| Aquilaria sinensis | Yang, L. et al.Li, W. et al.Yang, D.L. et al. | ||
| Petioles And Leaves | Aquilaria sinensis | Wang, S.C. et al. | |
| Withered Wood | Yagura, T. et al. | ||
| Tetrahydrochromone | Withered Wood | Aquilaria sinensis | Yagura, T. et al.Dai, H.F. et al. |
| Agarwood/ Eaglewood | Liu, J. et al. | ||
| Di-Epoxy-Tetrahydrochromone | Agarwood | Aquilaria crassna, | Yagura, T. et al. |
| Aquilaria sinensis | Li, W. et al. | ||
| Mono-Epoxy-Tetrahydrochromone | Agarwood | Aquilaria sinensis | Li, W. et al. |
| Aglycon Flavonoids | Leaves | Aquilaria sinensis | Qi, J. et al.Cheng, J.T. et al. |
| Stem | Chen, D. et al. | ||
| Mono-Glycoside Flavonoids | Leaves | Aquilaria sinensis | Qi, J. et al.Feng, J. et al. |
| Stem | Chen, D. et al. | ||
| Secondary Metabolites | Part | Species | References |
| Di-Glycoside Flavonoids | Leaves | Aquilaria sinensis | Qi, J. et al.Yang, X.B. et al. |
| Stem | Chen, D. et al. | ||
| Xanthons | Leaves | Aquilaria sinensis | Qi, J. et al.Cheng, J.T. et al. |
| Isoflavonoid | Stem | Aquilaria sinensis | Wu, Y. et al. |
| Aglycon Benzophenones | Leaves | Aquilaria sinensis | Qi, J. et al. |
| Mono-Glycoside Benzophenone | Leaves | Aquilaria sinensis | Qi, J. et al.Cheng, J.T. et al. |
| Leaves and petioles | Wang, S.C. et al. | ||
| Di-Glycoside Benzophenone | Petioles and leaves | Aquilaria sinensis | Wang, S.C. et al. |
| Leaves | Sun, G.J. et al. | ||
| Abietane And Podocarpane Diterpenoid | Agarwood | Aquilaria sinensis | Yang, L. et al. |
| Tigliane Diterpenoids | Stem | Aquilaria sinensis | Peng, K. et al. |
| Stem bark | Aquilaria malaccensis | Gunasekera, S.P. et al. | |
| Cucurbitacine Triterpenoids | Fruits | Aquilaria sinensis | Mei, W.L. et al. |
| Stem | Peng, K. et al. | ||
| Aglycon And GlycosideCucurbitane Triterpenoid | Petioles and leaves | Aquilaria sinensis | Wang, S.C. et al. |
| Tirucallane Triterpenoid | Petioles and leaves | Aquilaria sinensis | Cheng, J.T. et al.Wang, S.C. et al. |
| Oleanane Triterpenoid | Leaves | Aquilaria sinensis | Cheng, J.T. et al. |
| Benzofuran-Type Lignan(Aglycon And Glycoside) | Stem | Aquilaria sinensis | Wu, Y. et al. |
| Coumarinolignan | Whole plant | Aquilaria agallocha | Bhandari, P. et al. |
| Nucloesides | Petioles and leaves | Aquilaria sinensis | Wang, S.C. et al. |
| Acetanilide | Leaves | Aquilaria malaccensis | Afiffudden, S.K.N. et al. |
| Glyceride | Stem bark | Aquilaria malaccensis | Mei, W.L. et al. |
| Essential Oil | Agarwood | Aquilaria malaccensis | Tajuddin, S.N. et al. |
| Aquilaria agallocha | Bhuiyan, M.N.I. et al. |
CONCLUSION
The Aquilaria genus is very rich in different classes of natural products, such as sesquiterpenes, chromones, flavonoids, benzophenones, diterpenoids, triterpenoids and lignans. Hundreds of compounds have been identified in extracts from these plants, with A. sinensis as the most intensively studied source. Almost all parts of the A. sinensis plant have been investigated. Knowing the content of secondary metabolites from each part will be able to help understand the diversity of the usefulness of this plant. An example is the use of leaves that were reported to be used locally in trauma-related diseases such as fracture, bruise etc. It was also reported that agarwood has significant anticancer activities, analgesic and anti-inflammatory activities and anti-depressant activities [55]. Research on the phytochemicals from this genus (Aquilaria) will continue certainly because there are still some species that have not been studied. There is a high possibility to find other compounds, even new compounds, from species that have not yet been studied. In addition, knowledge of the fragrant constituents of agarwood may be useful for the development of new fragrance products from other natural sources in the future.
ACKNOWLEDGEMENTS
The authors are thankful to the Directorate General of Higher Education, Ministry of Education and Culture, Republic of Indonesia, for supporting the research through funding of the PUPT Airlangga University research scheme year 2016.
The authors are also grateful to Prof. Bauke W. Djikstra from University of Groningen, Netherlands for manuscript correction.
Consent for Publication
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Heywood V.H., Brummitt R.K., Culham A., Seberg O. Flowering Plant Families of The World. Ontario, Canada: Firefly Books; 2007. [Google Scholar]
- 2.Heywood V.H. Les Plantes à Fleurs. Paris: Editions Nathan; 1996. [Google Scholar]
- 3.Yagura T., Iro M., Kiuchi F., Honda G., Shimada Y. Four new 2-(2-phenylethyl) chromone derivatives from withered wood of Aquilaria sinensis. Chem. Pharm. Bull. (Tokyo) 2003;51(5):560–564. doi: 10.1248/cpb.51.560. [DOI] [PubMed] [Google Scholar]
- 4.Zhou M., Wang H., Suolangjiba Kou, J., Yu B. Antinociceptive and anti-inflammatory activities of Aquilaria sinensis (Lour.) Gilg. leaves extract. J. Ethnopharmacol. 2008;117:345–350. doi: 10.1016/j.jep.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y.C., Lee E.H., Lee Y.M., Kim H.K., Song B.K., Lee E.J., Kim H.M. Effect of the aqueous extract of Aquilaria agallocha stems on the immediate hypersensitivity reactions. J. Ethnopharmacol. 1997;58:31–38. doi: 10.1016/s0378-8741(97)00075-5. [DOI] [PubMed] [Google Scholar]
- 6.Alkhathlan H.Z., Al-Hazimi H.M., Al-Dhalaan F.S., Mousa A.A. Three 2-(2-phenylethyl) chromones and two terpenes from agarwood. Nat. Prod. Res. 2005;19(4):367–372. doi: 10.1080/14786410412331280122. [DOI] [PubMed] [Google Scholar]
- 7.Yang D.L., Wang H., Guo Z.K., Dong W.H., Mei W.L., Dai H.F. A new 2-(2-phenylethyl) chromone derivative in Chinese agarwood “Qi-Nan” from Aquilaria sinensis. J. Asian Nat. Prod. Res. 2014;16(7):770–776. doi: 10.1080/10286020.2014.896342. [DOI] [PubMed] [Google Scholar]
- 8.Ueda J., Imamura L., Tezuka Y., Tran Q.L., Tsuda M., Kadota S. New sesuiterpene from Vietnamese agarwood and its induction effect on brain-derived neurotrophic factor mRNA expression in vitro. . Bioorg. Med. Chem. 2006;14:3571–3574. doi: 10.1016/j.bmc.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Li W., Cai C.H., Dong W.H., Guo Z.K., Wang H., Mei W.L., Dai H.F. 2-(2-phenylethyl) chromone derivatives from Chinese agarwood induced by artificial holing. Fitoterapia. 2014;98:117–123. doi: 10.1016/j.fitote.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Gao X., Xie M., Liu S., Guo X., Chen X., Zhong Z., Wang L., Zhang W. Chromatographic fingerprint analysis of metabolites in natural and artificial agarwood using gas chromatography-mass spectrometry combined with chemometric methods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014;967:264–273. doi: 10.1016/j.jchromb.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 11.Naef R. The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: A review. Flavour Fragrance J. 2011;26:73–89. [Google Scholar]
- 12.Chen H.Q., Wie J.H., Yang J.S., Zhang Z., Yang Y., Gao Z.H., Sui C., Gong B. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem. Biodivers. 2012;9:236–250. doi: 10.1002/cbdv.201100077. [DOI] [PubMed] [Google Scholar]
- 13.Hashim Y.Z., Kerr P.G., Abbas P., Salleh H.M. Aquilaria spp. (agarwood) as a source of health beneficial compounds: A review of traditional use, phytochemistry and pharmacology. J. Ethnopharmacol. 2016;189:331–360. doi: 10.1016/j.jep.2016.06.055. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi T., Yamagata E., Yoneda K., Miura I. Jinkohol, a prezizane sesquiterpene alcohol from agarwood. Phytochemistry. 1981;20(7):1597–1599. [Google Scholar]
- 15.Bajaj Y.P. Medicinal and aromatic plant VIII (Biotechnology in agriculture and forestry). Berlin: Springer-Verlag; 2013. [Google Scholar]
- 16.Yang D.L., Wang H., Guo Z.K., Li W., Mei W.L., Dai H.F. Fragrant agarofuran and eremophilane sesquiterpenes in agarwood “Qi-Nan” from Aquilaria sinensis. Phytochem. Lett. 2014;8:121–125. [Google Scholar]
- 17.Ishihara M., Tsuneya T., Uneyama K. Guaiane sesquiterpenes from agarwood. Phytochemistry. 1991;30(10):3343–3347. [Google Scholar]
- 18.Ishihara M., Tsuneya T., Shiga M., Uneyama K. Three sesquiterpenes from agarwood. Phytochemistry. 1991;30(2):563–566. [Google Scholar]
- 19.Zhao H., Peng Q., Han Z., Yang L., Wang Z. Three new sesquiterpenoids and one new sesquiterpenoid derivative from Chinese eaglewood. Molecules. 2016;21(3):281–288. doi: 10.3390/molecules21030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakanishi T., Yamagata E., Yoneda K., Nagashima T., Ichiro K., Yoshida T., Mori H., Miura I. Three fragrant sesquiterpenes of agarwood. Phytochemistry. 1984;23(9):2066–2067. [Google Scholar]
- 21.Ishihara M., Tsuneya T., Uneyama K. Fragrant sesquiterpenes from agarwood. Phytochemistry. 1993;33(5):1147–1155. [Google Scholar]
- 22.Li W., Cai C.H., Guo Z.K., Wang H., Zuo W.J., Dong W.H., Mei W.L., Dai H.F. Five new eudesmane-type sesquiterpenoids from Chinese agarwood induced by artificial holing. Fitoterapia. 2015;100:44–49. doi: 10.1016/j.fitote.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Wu B., Lee J.G., Lim C.J., Jia S.D., Kwon S.W., Hwang G.S., Park J.H. Sesquiterpenoids and 2-(2-phenylethyl)-4H-chromen-4-one (=2-(2-phenylethyl)-4H-1-benzopyran-4-one) derivatives from Aquilaria malaccensis agarwood. Helv. Chim. Acta. 2012;95:636–642. [Google Scholar]
- 24.Maheswari M.L., Jain T.C., Bates R.B., Bhattacharyya S.C., Terpenoids X.L. Structure and absolute configuration of α-agarofuran, β-agarofuran and dihydroagarofuran. Tetrahedron. 1963;19:1079–1090. [Google Scholar]
- 25.Yoneda K., Yamagata E., Nakanishi T., Nagashima T., Kawasaki I., Yoshida T., Mori H., Miura I. Sesquiterpenoids in two different kinds of agarwood. Phytochemistry. 1984;23(9):2068–2069. [Google Scholar]
- 26.Varma K.R., Maheshwarsi M.L., Bhattacharyya S.C. The constituent of Agarospirol, a sesquiterpenoid with a new skeleton. Tetrahedron. 1965;21:115–138. [Google Scholar]
- 27.Wu B., Kwon S.W., Hwang G.S., Park J.H. Eight new 2-(2-phenylethyl) chromone (=2-(2-phenylethyl)-4H-1-benzopyran-4-one) derivatives from Aquilaria malaccensis agarwood. Helv. Chim. Acta. 2012;95:1657–1665. [Google Scholar]
- 28.Pant P., Rastogi R.P. Agarol, a new sesquiterpene from Aquilaria agallocha. Phytochemistry. 1980;19:1869–1870. [Google Scholar]
- 29.Peng K., Mei W.L., Zhao Y.X., Tan L.H., Wang Q.H., Dai H.F. A novel degraded sesquiterpene from fresh stem of Aquilaria sinensis. J. Asian Nat. Prod. Res. 2011;13(10):951–955. doi: 10.1080/10286020.2011.598860. [DOI] [PubMed] [Google Scholar]
- 30.Yang L., Qiao L.R., Zhang J.J., Dai J.G., Guo S.X. Two new sesquitrepene derivatives from Chinese eaglewood. J. Asian Nat. Prod. Res. 2012;14(11):1054–1058. doi: 10.1080/10286020.2012.704910. [DOI] [PubMed] [Google Scholar]
- 31.Nakanishi T., Inada A., Nishi M., Yamagata E., Yoneda K. A new and a known derivatives of 2-(2-phenylethyl) chromone from a kind of agarwood (“kanankoh” in Japanese) originating from Aquilaria agallocha. J. Nat. Prod. 1986;49(6):1106–1108. [Google Scholar]
- 32.Konishi T., Konoshima T., Shimada Y., Kiyosawa S. Six new 2-(2-phenylethyl) chromones from agarwood. Chem. Pharm. Bull. (Tokyo) 2002;50(3):419–422. doi: 10.1248/cpb.50.419. [DOI] [PubMed] [Google Scholar]
- 33.Yang L., Qiao L., Xie D., Yuan Y., Chen N., Dai J., Guo S. 2-(2-Phenylethyl) chromones from Chinese eaglewood. Phytochemistry. 2012;76:92–97. doi: 10.1016/j.phytochem.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Wang S.C., Wang F., Yue C.H. Chemical constituents from the petioles and leaves of Aquilaria sinensis. Biochem. Syst. Ecol. 2015;61:458–461. [Google Scholar]
- 35.Liu J., Wu J., Zhao Y.X., Deng Y.Y., Mei W.L., Dai H.F. A new cytotoxic 2-(2-phenylethyl) chromone from Chinese eaglewood. Chin. Chem. Lett. 2008;19:934–936. [Google Scholar]
- 36.Dai H.F., Liu J., Han Z., Zeng Y.B., Wang H., Mei W.L. Two new 2-(2-phenylethyl) chromones from Chinese eaglewood. J. Asian Nat. Prod. Res. 2010;12(2):134–137. doi: 10.1080/10286020903508424. [DOI] [PubMed] [Google Scholar]
- 37.Yagura T., Shibayama N., Ito M., Kiuchi F., Honda G. Three novel diepoxy tetrahydrochromones from agarwood artificially produced by intentional wounding. Tetrahedron Lett. 2005;46:4395–4398. [Google Scholar]
- 38.Qi J., Lu J.J., Liu J.H., Yu B.Y. Flavonoids and a rare benzophenone glycoside from the leaves of Aquilaria sinensis. Chem. Pharm. Bull. (Tokyo) 2009;57(2):134–137. doi: 10.1248/cpb.57.134. [DOI] [PubMed] [Google Scholar]
- 39.Cheng J.T., Han Y.Q., He J., WU X.D., Dong L.B., Peng L.Y., Li Y., Zhao Q.S. Two new tirucallane triterpenoids from the leaves of Aquilaria sinensis. Arch. Pharm. Res. 2013;36:1084–1089. doi: 10.1007/s12272-013-0088-4. [DOI] [PubMed] [Google Scholar]
- 40.Chen D., Bi D., Song Y.L., Tu P.F. Flavonoids from the stems of Aquilaria sinensis. Chin. J. Nat. Med. 2012;10(4):287–291. [Google Scholar]
- 41.Feng J., Yang X.W., Wang R.F. Bio-assay guided isolation and identification of a-glucosidase inhibitors from the leaves of Aquilaria sinensis. Phytochemistry. 2011;72:241–247. doi: 10.1016/j.phytochem.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 42.Yang X.B., Feng J., Yang X.W., Zhao B., Liu J.X. Aquisiflavoside, a new nitric oxide production inhibitor from the leaves of Aquilaria sinensis. J. Asian Nat. Prod. Res. 2012;14(9):867–872. doi: 10.1080/10286020.2012.701209. [DOI] [PubMed] [Google Scholar]
- 43.Severi J.A., Lima Z.P., Kushima H., Monteiro A.R., Brito S., dos Santos L.C., Vilegas W., Hiruma-Lima C.A. Polyphenols with antiulcerogenic action from aqueous aecoction of Mango leaves (Mangifera indica L.). Molecules. 2009;14:1098–1110. doi: 10.3390/molecules14031098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun G.J., Wang S., Xia F., Wang K.Y., Chen J.M., Tu P.F. Five new benzophenone glycosides from the leaves of Aquilaria sinensis (Lour.). Chin. Chem. Lett. 2014;25:1573–1576. [Google Scholar]
- 45.Yang L., Qiao L., Ji C., Xie D., Gong N.B., Lu Y., Zhang J.D., Guo S. Antidepressant abietane diterpenoids from Chinese eaglewood. J. Nat. Prod. 2013;76:216–222. doi: 10.1021/np3006925. [DOI] [PubMed] [Google Scholar]
- 46.Gunasekera S.P., Kinghorn A.D., Cordell G.A., Farnsworth N.R. Plant anticancer agents. XIX. Constituents of Aquilaria malaccensis. J. Nat. Prod. 1981;44(5):569–572. doi: 10.1021/np50017a010. [DOI] [PubMed] [Google Scholar]
- 47.Mei W.L., Lin F., Zuo W.J., Wang H., Dai H.F. Cucurbitacins from fruits of Aquilaria sinensis. Chin. J. Nat. Med. 2012;10(3):234–237. [Google Scholar]
- 48.Schun Y., Cordell G.A., Cox P.J., Howie R.A. Wallenone, a C32 triterpenoid from the leaves of Gyrinops walla. Phytochemistry. 1986;25:753–755. [Google Scholar]
- 49.Wu Y., Liu C., Li H.F., Sun J.B., Li Y.Y., Gu W., Wang D.Y., Liu J.G., Hu Y.L. A novel neolignan glycoside from Aquilaria sinensis. Biochem. Syst. Ecol. 2014;55:41–45. [Google Scholar]
- 50.Bhandari P., Pant P., Rastogi R.P. Aquillochin, a coumarinolignan from Aquilaria agallocha. Phytochemistry. 1982;21(8):2147–2149. [Google Scholar]
- 51.Afiffudden S.K., Alwi H., Hamid K.H. Determination of 4-hydroxyacetanilide in leaves extract of Aquilaria malaccensis by high pressure liquid chromatograph. Procedia Soc. Behav. Sci. 2015;195:2726–2733. [Google Scholar]
- 52.Tajuddin S.N., Muhamad N.S., Yarmo M.A., Yusoff M.M. Characterization of the chemical constituents of agarwood oils from Malaysia by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. Mendeleev Commun. 2013;23:51–52. [Google Scholar]
- 53.Bhuiyan M.N., Begum J., Bhuiyan M.N. Analysis of essential oil of eaglewood tree (Aquilaria agallocha Roxb.) by gas chromatography mass spectrometry. Bangladesh J. Pharmacol. 2009;4:24–28. [Google Scholar]
- 54.Zuo W.J., Jin P.F., Dong W.H., Dai H.F., Mei W.L. Metabolites from the endophytic fungus HP-1 of Chinese eaglewood. Chin. J. Nat. Med. 2014;12(2):151–153. doi: 10.1016/S1875-5364(14)60025-X. [DOI] [PubMed] [Google Scholar]
- 55.Kang Y.F., Chien S.L., Wu H.M., Li W.J., Chen C.T., Li H.T., Chen H.L., Chao D., Chen S.J., Huang C.T., Chen C.Y. Secondary metabolites from the leaves of Aquilaria sinensis. Chem. Nat. Compd. 2014;50(6):1110–1112. [Google Scholar]