Abstract
A key role in the pathogenesis of rheumatoid arthritis (RA) and psoriatic arthritis (PsA) is played by inflammatory cytokines, including tumor necrosis factor-α (TNF-α), which are also involved in inducing inflammatory anemia. We have followed 67 RA patients and 64 PsA patients for 1 year to evaluate the effects of TNF-α inhibitors on disease activity and on inflammatory anemia. Patients were divided into three different treatment groups, according to a randomized assignment to receive therapy with etanercept, adalimumab, or infliximab. Treatment with anti-TNF-α resulted in a significant reduction in disease activity score-28 (DAS28) values both in RA and PsA patients, already from the third month of treatment (P = 0.01). In both populations, there was an increase in hemoglobin (HB) levels already after 3 months of treatment (P = 0.001), and HB levels were inversely proportional to the disease activity, regardless of the type of medication used. The increased HB values and the reduction of DAS28 values during the observation period suggest the existence of a negative correlation between them both in RA and PsA, regardless of the type of anti-TNF-α used. Our data suggest a pleiotropic action of anti-TNF-α, such as the well-known action on the activity of the disease, and the improvement in inflammatory anemia.
Keywords: anemia, anti-TNF-α, arthritis, hemoglobin, inflammation
Introduction
Inflammatory cytokines play a crucial role in the pathogenesis of chronic inflammatory diseases, such as rheumatoid arthritis (RA) and psoriatic arthritis (PsA). The block of these agents, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), represents an important therapeutic opportunity against chronic inflammatory diseases with an inhibitory effect on inflammatory cells recruitment, on migration of circulating leucocytes into inflamed joints, on angiogenesis, and on tissue damage by decreasing expression of adhesion molecules and chemokines.1
Moreover, proinflammatory cytokines, primarily TNF-α and IL-6, play an important role in inducing inflammatory anemia. In particular, IL-6 induces the synthesis of hepcidin, the key regulator of iron metabolism in anemia of chronic diseases (ACD), which is involved in reducing the intestinal iron absorption and blocking its release from deposits;2 TNF-α, on the contrary, is probably involved in altering erythropoiesis.3 Thus, anemia is an important extra-articular manifestation of RA and PsA, correlated to physical disability and increased mortality.4,5
Since TNF-α and IL-6 cytokines are involved in the pathogenesis of ACD, the use of biotechnological drugs, such as tocilizumab (humanized monoclonal antibody directed against the receptor of the IL-6) and inhibitors of TNF-α, could potentially lead to increased levels of hemoglobin (HB). In fact, in several studies in patients with RA and other chronic inflammatory diseases, increased HB levels were observed after treatment with inhibitors of IL-6 and TNF-α.6,7
This study wants to evaluate the effects of three different TNF-α inhibitors, etanercept (ETN), adalimumab (ADA), infliximab (IFX), in terms of clinical efficacy, effects on HB, iron, and ferritin levels, in patients affected by PsA or RA, not suffering from other co-morbidities and not treated with erythropoiesis-stimulating agents or iron. We want to compare these three anti-TNF-α agents among them and to assess if there is a correlation between their effects on HB and the disease activity.
Patients and methods
RA patients, fulfilling the American College of Rheumatology (ACR) criteria 2010 and patients affected by PsA according to the CASPAR criteria (Classification of Psoriatic Arthritis Study), afferent to our Rheumatology Clinic, were recruited and followed prospectively for 1 year.8,9 The study protocol was approved by the local ethics committee. Written informed consent was obtained from all participants.
The two populations were homogeneous for age and sex. Exclusion criteria were age <18 years and pregnancy. All patients were receiving methotrexate and were naive to biological drugs. Each patient has given his or her consent through a written form. The patients not responsive to methotrexate and with high (disease activity score-28 (DAS28) >5.1) or moderate disease activity (DAS28 >3.2 and ⩽5.1) were candidated for treatment with biologic drugs.
Patients with RA and patients with PsA were divided into three different treatment groups, according to a randomized assignment to receive therapy with ETN, ADA, or IFX.
Age, duration of disease, C-reactive protein (CRP), DAS28, HB (g/dL), serum iron (µg/dL), and ferritin (ng/mL) were evaluated for each patient in each group at baseline and after 3, 6, 9, and 12 months of therapy.
Anemia has been defined according to World Health Organization (WHO), which establishes the values of HB threshold for anemic state corresponding to <12 g/dL for women and <13 g/dL for men.10
The study excluded patients who had extra-articular involvement (lung, heart, intestine); HB levels below 8.5 g/dL; creatinine levels >1.5 mg/dL; history of cancer, liver, kidney, and endocrine disease; and neurological and psychiatric disorders. We also excluded patients treated with hemodialysis or continuous treatment with erythropoietin or iron and patients with active gastrointestinal bleeding in the 2 months prior to enrollment and during the entire duration of the study. We also excluded from the study patients with deficiency anemia, by differentiating iron-deficiency anemia from the typical inflammatory anemia through the evaluation of serum ferritin values; therefore, we have considered only patients with HB values below the threshold levels dictated by WHO and ferritin levels >200 ng/mL, assuming these levels as suggestive of inflammatory anemia (reduced serum iron, normal or reduced transferrin, reduced transferrin saturation index).
The clinical response to inhibitors of TNF-α was assessed by the reduction in DAS28 from baseline values up to 12 months of therapy.
Continuous variables were expressed as mean ± standard deviation (SD). Differences between groups were assessed using the non-parametric Mann–Whitney U test for continuous variables; the Wilcoxon rank test was used within the continuous variables. Pearson correlation coefficient was used to evaluate the correlation between selected continuous variables. Values of P < 0.05 were considered as statistically significant.
In all, 67 patients with RA and 64 patients with PsA were included in the study. In PsA group, 23 patients started therapy with ADA, 20 with IFX, and 21 with ETN. In RA group, 22 patients started therapy with ADA, 21 with IFX, and 24 with ETN.
All patients were treated with methotrexate (dosage between 10 and 15 mg/week) and steroid (5 mg/day of prednisone). None of them have stopped or modified methotrexate treatment during the observation. TNF-α inhibitors dosage remained stable during the study (ADA 40 mg every 2 weeks; IFX 5 mg/kg at week 0, 2, 6, and subsequently every 8 weeks; ETN 50 mg/week).
Results
The main demographic and clinical characteristics are shown in Table 1. At baseline (before to start therapy with anti-TNF-α agents), HB values were significantly lower in RA patients than in PsA patients; on the contrary, CRP values were significantly higher in RA patients than in PsA patients. There were no significant differences between groups for the other evaluated parameters (age, DAS28, iron, ferritin), even if values of DAS28 were higher in RA patients than in PsA patients, without reaching statistical significance.
Table 1.
Demographic and clinical characteristics at baseline.
| PsA | RA | P value | |
|---|---|---|---|
| Patients (n = 131, %) | 64 (49) | 67 (51) | |
| Age (range) | 50.9 (40–60) | 53.4 (40–61) | |
| F (n and %) | 47 (74) | 51 (77) | |
| M (n and %) | 17 (26) | 16 (23) | |
| Age at diagnosis (range) | 42.6 (38–46) | 41.3 (40–54) | |
| CRP (mean ± SD) | 0.64 ± 1.44 | 1 ± 0.85 | 0.04 |
| DAS28 (mean ± SD) | 3.87 ± 0.49 | 4.45 ± 0.68 | 0.09 |
| HB (mean ± SD) | 11.67 ± 0.9 | 10.78 ± 0.88 | 0.0001 |
| Ferritin (mean ± SD) | 227 ± 66.37 | 241.43 ± 53.25 | 0.3 |
| Iron (mean ± SD) | 31.9 ± 4.63 | 30 ± 4.73 | 0.1 |
| ETN | 21 (33) | 24 (36) | |
| ADA | 23 (36) | 22 (33) | |
| IFX | 20 (31) | 21 (31) |
PsA: psoriatic arthritis; RA: rheumatoid arthritis; F: female; M: male; CRP: C-reactive protein; DAS28: disease activity score-28; HB: hemoglobin; ETN: etanercept; ADA: adalimumab; IFX: infliximab; SD: standard deviation.
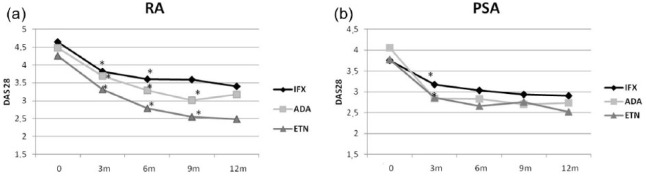
Disease activity in the different treatment groups in RA and PsA
Treatment with anti-TNF-α agents resulted in a significant reduction in disease activity both in RA patients and in PsA patients, in terms of DAS28 already after 3 months (P = 0.01) (Figure 1(a) and (b)). The decreasing trend of DAS28 has regarded the overall length of the study in almost all treatment subgroups with anti-TNF-α, except for the ADA subgroup, which showed a loss of efficacy after 12 months both in RA and PsA patients, with higher DAS28 values at 12 months than the ninth month, but still significantly lower than at baseline (P = 0.01).
Figure 1.
Anti-TNF-α effect on DAS28 in (a) RA and (b) PSA. P values are compared to the time point before. *P < 0.05.
At baseline, RA patients in subgroups randomized to ADA and IFX showed higher DAS28 values than those obtained in patients randomized to receive treatment with ETN, but without statistical significance (P > 0.5). However, after 9 months, the ETN subgroup showed a significantly lower DAS28 compared with IFX and ADA subgroups (P < 0.001 and P = 0.04, respectively; Figure 1(a)).
Among patients with PsA (Figure 1(b)), at baseline, DAS28 value was significantly higher in the ADA subgroup than in the ETN subgroup (P = 0.03) and in the IFX subgroup (P = 0.01). After 3 months of treatment, DAS28 values were significantly reduced in all treatment subgroups and continued to reduce also for subsequent observation months. The response, in terms of clinical efficacy, at the treatment with biotechnological drugs was observed for all three TNF-α inhibitors from the third month of therapy. Subsequently, it has been constantly maintained for all the length of the study with the exception of the ADA group which at the twelfth month showed a lag in clinical efficacy. Patients treated with ETN showed an inconstant disease activity; up to 6 months of therapy, there was a significant reduction in DAS28 values, followed by an increase in DAS28 values at 9 months of treatment, with a reduction at 12 months. At 12 months, ETN showed a more efficient effect in reducing disease activity compared to ADA (P = 0.9) and IFX (P = 0.3).
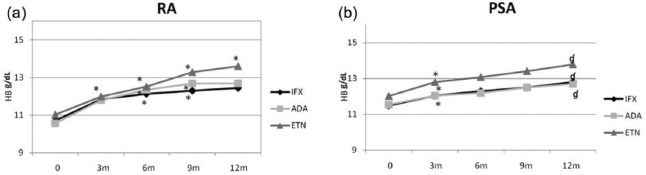
Effects of different treatments with anti-TNF-α agents in RA and PsA on HB levels
At baseline, RA patients had higher ferritin values than PsA patients (P = 0.3). During the treatment with anti-TNF-α agents, there was a gradual reduction in ferritin values in both populations (Figure 2).
Figure 2.
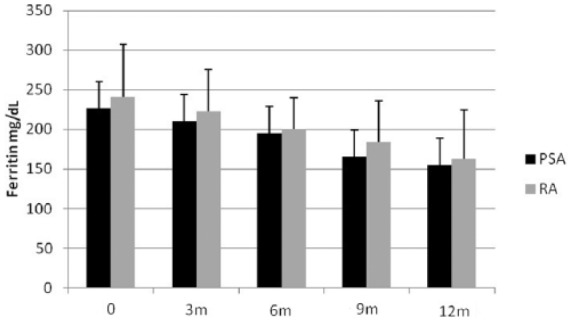
Ferritin values in RA and PSA at baseline (range, 95–330), at 3 months (range, 50–130), at 6 months (range, 50–120), at 9 months (range, 47–135), and at 12 months (range, 50–126). Values are given as mean ± SD.
In patients with RA (Figure 3(a)), there were no differences between the three different treatments (ADA, IFX, and ETN) in increasing HB values, which were significant for all three drugs from the third month. After 12 months, the levels of HB in patients treated with ETN were significantly higher than those of patients treated with IFX (P = 0.01). If we consider data expressed as percentage increase, we found that the HB percentage increase in ADA group was higher than in ETN (P = 0.018) and IFX (P = 0.2) groups until the ninth month of observation; the percentage increase of HB reached a plateau at the ninth month for ADA and IFX, while in ETN group HB percentage increased progressively; thus, after 12 months, percentage variations in ETN group were higher than in the other treatment groups, reaching significance compared with IFX group (similar to what was observed when data were analyzed in absolute values).
Figure 3.
Anti-TNF-α effect on HB over time in HB in (a) RA and (b) PSA. P values are compared to the time point before. *P < 0.05; ɠP ⩽ 0.01.
HB values analysis in the three subgroups of patients with PsA (Figure 3(b)) showed that in all three subgroups HB values increased from T1. The HB increase from baseline to the twelfth month was 1.3 g/dL for IFX group, 1.17 g/dL for ADA group, and 1.78 for ETN group. Percentage increase of HB in ETN group was higher than in ADA and IFX groups at the third month (P = 0.04) (Figure 3b); however, in subsequent observations, the percentage HB increase in ETN group was lower than in the follow-up in the other groups.Furthermore, percentage increase of HB from baseline to the twelfth month was almost identical in all three treatment subgroups.
Correlation between changes in HB and DAS28
HB levels were inversely proportional to the disease activity both in RA patients (r = −0.5, P < 0.0001) and PsA patients (r = −0.5, P < 0.001).
Discussion
Many studies have shown the role of IL-6 inhibition in improving HB levels in RA patients by interfering hepcidin production and increasing iron bioavailability. Nevertheless, less clear is the role of TNF-α inhibition in improving HB levels.3,6,11–14 In our study, DAS28 and CRP values were significantly higher in RA patients than in PsA patients. Moreover, HB levels were significantly lower in RA patients than in PsA patients. These data can be explained by considering that the putative more intense inflammatory status may influence HB levels in RA. We have observed increased HB levels in patients with RA for all three anti-TNF-α agents. At the twelfth month, patients treated with ETN had HB levels higher than patients treated with IFX and ADA. We have hypothesized that anti-drug antibodies probably play a role in reducing the efficacy of monoclonal antibodies, such as IFX and ADA, on anemia compared to a soluble TNF receptor fusion protein, such as ETN.15 The increased HB values and the reduction in DAS28 values suggest the existence of a negative correlation between HB levels and DAS28 values both in RA and PsA, regardless of the type of anti-TNF-α agent used. Our data confirm the well-known action of anti-TNF-α agents on the disease activity, and the improvement in inflammatory anemia, demonstrated by the reduction in serum ferritin values. Ferritin value in this study was considered as discriminating between iron-deficiency anemia and inflammatory anemia and, given its function of acute phase reactant, the reduction of its value in the course of treatment with TNF-α inhibitors does not contradict the expected results. Anti-inflammatory effects of anti-TNF-α therapy may explain both the tendency of ferritin levels to decrease and the tendency of HB levels to increase. Ferritin is a cellular iron storage protein and increased ferritin levels usually are related to excessive iron storage as commonly found in inflammatory anemia. As demonstrated by Song et al.,12 serum ferritin levels in chronic arthritis show a direct correlation with serum hepcidin levels, suggesting that increased hepcidin is responsible for increasing iron storage and for reducing the amount of serum iron. Thus, it is conceivable that anti-TNF-α treatment may be involved in reducing both hepcidin and ferritin levels and that improvement in anemia may be related to an improvement in serum iron available for HB synthesis and erythrocyte production.
In view of the fact that the literature is quite abundant about anemia in RA, improvement of HB values obtained in PsA patients was most surprising. Nevertheless, further studies are needed in order to better define the role of anemia even in course of PsA.
Acknowledgments
All data are provided in the results section of this paper.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Maini RN, Feldman M. (2002) How does infliximab work in rheumatoid arthritis? Arthritis Research 4(Suppl. 2): S22–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raj DSC. (2009) Role of interleukin-6 in the anemia of chronic disease. Seminars in Arthritis and Rheumatism 38: 382–388. [DOI] [PubMed] [Google Scholar]
- 3. Papadaki HA, Kritikos HD, Valatas V, et al. (2002) Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: Improvement following anti-tumor necrosis factor-alpha antibody therapy. Blood 100: 474–482. [DOI] [PubMed] [Google Scholar]
- 4. Weiss G, Goodnough LT. (2005) Anemia of chronic disease. New England Journal of Medicine 352: 1011–1023. [DOI] [PubMed] [Google Scholar]
- 5. Han C, Rahman MU, Doyle MK, et al. (2007) Association of anemia and physical disability among patients with rheumatoid arthritis. Journal of rheumatology 34: 2177–2182. [PubMed] [Google Scholar]
- 6. Doyle MK, Rahman MU, Han C, et al. (2009) Treatment with infliximab plus methotrexate improves anemia in patients with rheumatoid arthritis independent of improvement in other clinical outcome measures—A pooled analysis from 3 large, multicenter, double-blind, randomized clinical trials. Seminars in Arthritis and Rheumatism 39: 123–131. [DOI] [PubMed] [Google Scholar]
- 7. Doyle MK, Rahman MU, Frederick B, et al. (2013) Effects of subcutaneous and intravenous golimumab on inflammatory biomarkers in patients with rheumatoid arthritis: Results of a phase 1, randomized, openlabel trial. Rheumatology 52: 1214–1219. [DOI] [PubMed] [Google Scholar]
- 8. Taylor W, Gladman D, Helliwell P, et al. CASPAR Study Group (2006) Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis and Rheumatism 54: 2665–2673. [DOI] [PubMed] [Google Scholar]
- 9. Aletaha D, Neogi T, Silman AJ, et al. (2010) 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Annals of the Rheumatic Diseases 69: 1580–1588. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization (2008) Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. In: de Benoist B, McLean E, Egli I, et al. (eds). Available at: http://apps.who.int/iris/bitstream/10665/43894/1/9789241596657_eng.pdf
- 11. Hashimoto M, Fujii T, Hamaguchi M, et al. (2014) Increase of hemoglobin levels by anti-IL-6 receptor antibody (tocilizumab) in rheumatoid arthritis. PLoS ONE 9: e98202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song SN, Iwahashi M, Tomosugi N, et al. (2013) Comparative evaluation of the effects of treatment with tocilizumab and TNF-α inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Research & Therapy 15: R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davis D, Charles PJ, Potter A, et al. (1997) Anaemia of chronic disease in rheumatoid arthritis: In vivo effects of tumour necrosis factor alpha blockade. British Journal of Rheumatology 36: 950–956. [DOI] [PubMed] [Google Scholar]
- 14. Furst DE, Kay J, Wasko MC, et al. (2013) The effect of golimumab on haemoglobin levels in patients with rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis. Rheumatology 52: 1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thomas SS, Borazan N, Barroso N, et al. (2015) Comparative immunogenicity of TNF inhibitors: Impact on clinical efficacy and tolerability in the management of autoimmune diseases. A systematic review and meta-analysis. BioDrugs 29: 241–258. [DOI] [PubMed] [Google Scholar]