Abstract
Systemic inflammation is involved in vascular calcification and cardiovascular disease which is the leading cause of mortality in rheumatoid arthritis (RA). A high level of serum interleukin (IL)-6 plays a key role in local and systemic inflammation in RA. However, the underlying mechanisms remain unclear. We established a human umbilical artery smooth muscle cell (HUASMC) culturing method to investigate the possible role of IL-6 on vascular calcification. HUASMCs were obtained from umbilical arteries of healthy neonates. To detect calcification effects, HUASMCs were treated with (experimental group) or without (control group) recombinant human (rh) IL-6. The calcium deposition stain and calcium concentrations were measured, as well as the mRNA and protein levels of the regulating factor of osteogenic differentiation-bone morphogenetic protein (BMP) 2 and those calcifying related molecules including bone-specific alkaline phosphatase (BAP), osteoprotegerin (OPG), and osteopontin (OPN). Our study showed that rhIL-6 induced calcification of HUASMCs in a time- and dose-dependent manner, and upregulated expressions of BMP2, BAP, OPG, and OPN of HUASMCs. We then used the anti-BMP2 siRNA to knockdown the expression of endogenous BMP2 to confirm its role. HUASMCs were transfected with negative siRNA (control group) or the valid anti-BMP2 siRNA (experimental group) before they were treated with rhIL-6. Cells transfected with negative siRNA without IL-6 stimulating served as the blank group. The results showed that anti-BMP2 siRNA markedly decreased expressions of BMP2, BAP, OPG, and OPN, and also partly reduced the calcification of HUASMCs induced by rhIL-6. Collectively, according to our study, rhIL-6 could induce the extracellular calcification and osteogenic differentiation of human artery smooth muscle cells through upregulating endogenous BMP2 in vitro. This may be one of the underlying mechanisms of the overwhelming vascular calcification in RA.
Keywords: bone morphogenetic protein 2, interleukin 6, rheumatoid arthritis, RNA interfering, vascular calcification
Rheumatoid arthritis (RA) is a disabling autoimmune disease characterized by chronic erosive arthritis and extra-arthritic manifestations. Recent studies revealed that the mean lifespan of RA patients was a few years less than that of the coetaneous group from the general population despite the improved outcome of arthritis and overall life quality. The leading cause of mortality in RA patients is cardiovascular disease, especially in young adults.1–3 Studies in diabetes mellitus, chronic kidney disease (CKD), other inflammatory arthritis like ankylosing spondylitis, and aging have suggested that the systemic inflammation is associated with vascular calcification and congenital heart failure.4–7 RA is the prototypic inflammatory arthritis with outstanding systemic inflammation. Much research indicated that RA patients are more likely to suffer widely and severe medial artery calcification (MAC). Chronic inflammatory markers are associated with high cardiovascular mortality, high prevalence, and greater extent of peripheral and coronary arterial calcification.7–10 Among these markers, interleukin (IL)-6 is the most prominent one. Tumor necrosis factor (TNF)-α could induce cardiac myocyte apoptosis and remodeling, and was related to congestive heart failure,11 while results of clinical studies in RA patients remained controversial in terms of vascular calcification.12–14
MAC is mainly occurred in vascular smooth muscle cells (VSMCs). Previous studies, including ours, have shown that the concentration of serum IL-6 was positively related to some calcification indicators like bone alkaline phosphatase (BAP), osteopontin (OPN), osteoprotegerin (OPG), osteocalcin (OC), and the ultimate aortic calcification score.6–9,15 Since BAP, OPN, OPG, and OC are all proteins secreted by osteoblasts, it is reasonable to hypothesize that IL-6 is involved in an osteogenic differentiation and calcification of the VSMCs. Pathological studies found that VSMCs in calcified vessels expressed the core regulating factor of osteogenic differentiation-bone morphogenetic protein (BMP) 2, the key transcription factor of osteogenesis-core binding factor α-1 (Cbfa1), and those downstream bone-related proteins.16–19 Therefore, we established a human umbilical artery smooth muscle cell (HUASMC) culturing method to observe the role of IL-6 on vascular calcification in vitro. To confirm the role of endogenous BMP2, small RNA interfering method was used to silence or knockdown the expression of BMP2 of the HUASMCs. Those results may provide a better understanding of the mechanism underlying the overwhelming vessel calcification in RA patients.
Materials and methods
Cell culture and identification
HUASMCs were obtained from artery explants of the umbilical cords of healthy donors. Briefly, arteries of umbilical cords from healthy neonates were separated within 4 h after delivery and thoroughly washed by saline. The sleeve-like adventitia was removed and the remaining tissues were chopped into pieces of 1–2 mm3. The explants were planted on the bottom of T-25 flask in 3–5 pieces/cm2 and merged into the Dulbecco’s modified Eagle’s medium (DMEM) (31600-034, Invitrogen) which was supplemented with 20% fetal bovine serum (Gibco Laboratories), 100 unit/mL penicillin, and streptomycin. The elongate spindle-shaped cells would migrate out from the edge of explants at approximately one week. After 2–3 weeks, cells migrating from the explants would overgrow the plate (Figure 1a). The confluent cells would appear the classic peak-and-valley pattern at prolonged culture (Figure 1b). All cells were synchronically cultured by serum-free DMEM for 24 h before they were used for any experiment (Figure 1c).
Figure 1.
Culture and identification of the human umbilical artery smooth muscle cells. (a) The elongated spindle-shaped cells grew out from the edge of the explants after 2–3 weeks’ culture (100×). (b) Cells appeared the classic peak-and-valley pattern of morphology in prolonged culture (100×). (c) Cells were synchronically cultured by serum-free DMEM for 24 h (100×). (d) The fiber filaments in the cytoplasm were stained with green fluorescence in the indirect immunofluorescence test of α-SM actin (400×).
Cells identified by morphological characters were ultimately confirmed to be VSMCs by indirect immunofluorescence (IIF) test of α-SM actin, the marker of SMCs, using monoclonal antibodies against α-SM actin (A2547, Sigma) (Figure 1d). IIF tests of factor VIII and vetamin with monoclonal antibodies against factor VIII (ab41187, abcam) and vimentin (ab8069, abcam) were also applied to expel contaminations from endothelial cells and fibroblasts.
The protocols were approved by the Ethics Board of the Affiliated Hospital of Qingdao University. All participants provided written informed consent.
Calcification staining and measuring
For calcification staining, Alizarin red S method was used. Cells cultured with rhIL-6 50 ug/L (R&D Systems) for six days served as the experimental group and the routinely cultured cells as the control group. Cells were washed with D-Hank’s solution, fixed with pure iced ethanol, and were then exposed to 0.5% Alizarin red S (PH 7.0) for 15 min at room temperature (red/orange means a positive staining of extracellular matrix). For calcium concentration measuring, O-cresolphthalein complexone method was used. Cells cultured with rhIL-6 10 ug/L or 50 ug/L served as experimental groups and routinely cultured cells served as controls. The calcium measuring was applied at baseline and after being treated for 3, 6, 9, and 12 days. Total calcium in the cell layer was extracted by 0.6 mol/L HCl solution for 24 h at 37°C. The remaining components of cells were dissociated by 1 mL of 0.1 N NaOH/0.1% SDS to obtain the total protein. Protein concentrations were determined using a BCA protein assay kit (Pierce, Cat. no.23227) and final concentrations of calcium were adjusted by the protein content of the cell layer.
Quantitative real-time RT-PCR
The control cells were cultured with regular DMEM and the experimental cells were cultured with extra 10 ug/L rhIL-6. Cells were harvested at 12, 24, and 72 h. Total RNA extraction, reverse-transcription, and real-time RT-PCR reactions were carried out according to the manufacturer’s instructions (TaKaRa, Japan; ABI PRISM 7900, America). All data were analyzed using ABI PRISM SDS 2.0 software (Applied Biosystems). The mRNA expressions of the target genes were represented using the ΔCt method; B-actin was co-amplified as the housekeeping gene.15 Every gene level was transformed into the ratio of experimental group to control group at the same time point during statistical analyzing. The oligonucleotide primers for BMP2, BAP, OPN, and OPG were listed in Table 1.
Table 1.
Primer sequences of the target genes.
| Genes | Upstream primers | Downstream primers |
|---|---|---|
| BMP2 | 5’-AACACTGTGCGCAGCTTCC-3’ | 5’-CCTAAAGCATCTTGCATCTGTTCTC-3’ |
| BAP | 5’-GGACCATTCCCACGTCTTCAC-3’ | 5’-CCTTGTAGCCAGGCCCATTG-3’ |
| OPN | 5’- ATGGAAAGCGAGGAGTTGAATG-3’ | 5’-TGCTTGTGGCTGTGGGTTT-3’ |
| OPG | 5’-AGCTGCAGTACGTCAAGCAGGA-3’ | 5’-TTTGCAAACTGTATTTCGCTCTGG-3’ |
BAP, bone specific alkaline protein; BMP2, bone morphogenetic protein 2; OPG, osteoprotegerin; OPN, osteopontin.
Fluorescent quantitative method
This method was used to detect the BAP protein level. The cell treating process was the same as described above. Total proteins were obtained by 1% triton X-100 NaCl solution. Total protein content was detected by BCA method (see “Calcification staining and measuring” section). BAP protein concentration was assayed by fluorescent quantitation kit (Sigma, Cat. no. APF) and adjusted by total protein content.
Western blot analysis
This method was used to detect the protein levels of BMP2, OPN, and OPG. The cell treating process and total protein extraction were the same as before. Protein sample (25 ug) was loaded to 10% polyacrylamide gels, electro-blotted, and incubated with monoantibodies of OPN (2 mg/L, room temperature, 2 h) (Chemicon, Cat. no. 14331), OPG (0.4 mg/L, room temperature, 2 h) (Chemicon, Cat. no. AB2125P), BMP2 (2 mg/L, 4°C, overnight) (Abcam, Cat. no. ab6285) according to the standard procedure, followed by horseradish peroxidase-conjugated secondary antibodies (PIEREC, Cat. no. 31430; Chemicon, Cat. no. AP132P). The ECL detection system (PIERCE, Cat. no. 34081) was used to detect immune-reactive proteins. The films were scanned and analyzed by density analysis system (GS-8000, Bio-RAD). Equal protein loading for western blots was adjusted by immune-blotting for GAPDH. Every protein level was transformed into the ratio of experimental group to control group at the same time point during statistical analysis.
Anti-BMP2 siRNA interfering
Target gene sequence was obtained through CorNucleotide in the NCBI official website (http://www.ncbi.nlm.nih.gov) and the BMP2 mRNA serial number was NM_001202. Four candidate siRNAs and one negative control siRNA labeled with FAM fluorescein were designed and prepared by Shanghai GenePharma. The transfect agent was Lipofectamine2000 (Invitrogen, Cat. no. 11668-019) 8 uL/well; medium was non-serum OptiMEM (GIBCO, Cat. no. 31985). Optimization of the transfect system and the siRNA validities screening were followed the standard procedure. The negative FAM labeled siRNA of 60 nM achieved the highest efficiency according to visual fluorescence brightness. The following experiments were based on this optimized concentration. One of the four candidate siRNAs showed the lowest expression level of BMP2 mRNA (the mean level was 0.19) and the highest inhibiting efficiency (the mean value was 81%). This anti-BMP2 siRNA was chosen as the valid siRNA: 5’-GUGCUAUCUCGAUGCUGUATT-3’ and 5’-UACAGCAUCGAGAUAGCACTG-3’. The BMP2 mRNA being interfered was: GTGCTATCTCGATGCTGTA.
To test the effect of siRNA on mRNA level, cells were divided into two groups: the control group (transfected with negative siRNA) and experimental group (transfected with the valid anti-BMP2 siRNA). After transfection, the cells of both groups were changed to regular medium culture and both were treated with rhIL-6 10 ng/mL. The cellular mRNA level of BMP2, BAP, OPG, and OPN were tested at 12 h and 48 h.
To observe the effect of siRNA on calcification, 50 ng/mL rhIL-6 was used. Cells were divided into three groups: the control group and the experimental group (the same as described before), and the blank group (transfected with negative siRNA but not treated with rhIL-6). The calcium concentrations of cells were measured at baseline, two and four days after treatment.
Statistical analysis
All tests were repeated three times at cell level. The results of all tests were expressed as mean ± SD. One-way ANOVA was used for data analysis through SPSS 13.0 for windows, and P value <0.05 was considered statistically significant.
Results
RhIL-6 induced calcification of HUASMCs
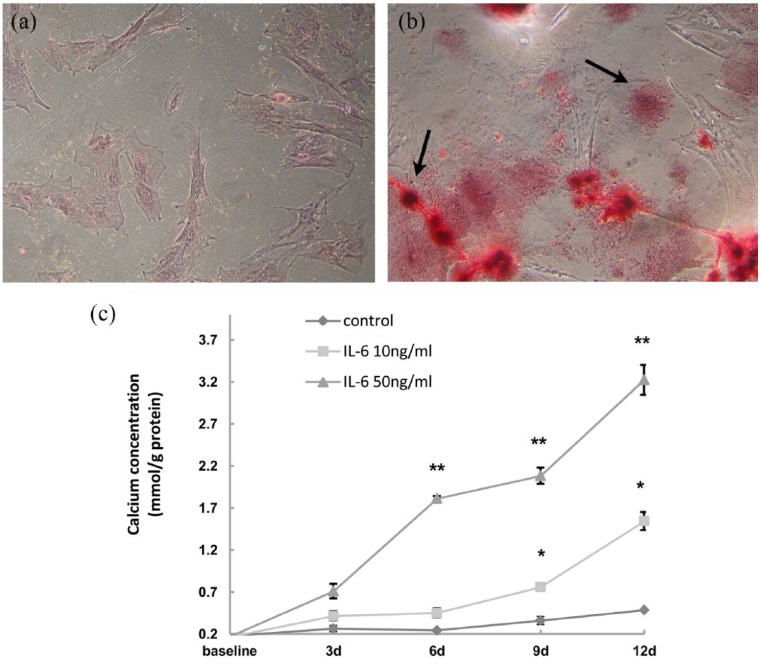
The control cells were negatively stained by Alizarin red S on the extracellular matrix (Figure 2a), while the extracellular matrix of the experimental cells was positively stained in two pattern, sand-like stain and nodular stain (Figure 2b). Calcification measure showed that the calcium concentration of the 50 ng/L group at days 6, 9, or 12 was 1.81 ± 0.03 mmol/g, 2.08 ± 0.10 mmol/g, and 3.22 ± 0.18 mmol/g, respectively, and were all elevated compared to the 10 ng/L group at the same time point (P <0.05). Calcium concentrations of the 10 ng/L group at days 9 and 12 were 0.76 ± 0.02 mmol/g and 1.54 ± 0.11 mmol/L, respectively, and were both higher than the control group at the same time point (P <0.05). The calcium concentrations of all three groups showed a time-dependent elevation in this study (Figure 2c).
Figure 2.
IL-6 induced calcification of the HUASMCs. (a) The control group was treated without rhIL-6 for six days and showed a negative result of calcium stain by alizarin red S staining. (b) The experimental group was treated with rhIL-6 (50 ng/mL) for the same time period and the extracellular matrix was positively stained with orange. The two arrows indicated the sand-like and the nodular calcium stain in the extracellular matrix, respectively. Both panels were magnified 200 times. (c) Cells were treated with different concentrations of rhIL-6 for different durations as indicated. Calcium concentrations were elevated in a time- and dose-dependent manner. *P<0.05 vs. control of the same time point or vs. the sample of the earlier time point within the same group. **P<0.05 vs. control and sample of the other group at the same time point.
RhIL-6 induced expression of BAP, OPN, OPG, and BMP2 in HUASMCs
The experimental group (cells treated with IL-6 10 ng/mL) upregulated gene and protein expression of BMP2, BAP, OPN, and OPG sequentially. Compared with the control group, BAP mRNA levels of the experimental group increased at 12 and 24 h and regressed to baseline at 72 h (2.51 ± 0.11 at 12 h, 1.94 ± 0.03 at 24 h, and 0.90 ± 0.03 at 72 h); BAP protein level only increased at 12 h (3.96 ± 0.54 at 12 h, 1.45 ± 0.46 at 24 h, and 1.27 ± 0.66 at 72 h). OPN mRNA levels of the experimental group increased at 24 and 72 h (1.03 ± 0.09 at 12 h, 1.90 ± 0.10 at 24 h, and 3.14 ± 0.32 at 72 h), while the protein level of OPN only increased at 72 h (0.98 ± 0.32 at 12 h, 1.37 ± 0.47 at 24 h, and 2.57 ± 0.43 at 72 h). OPG mRNA level of the experimental group increased at 24 and 72 h (0.99 ± 0.13 at 12 h, 1.77 ± 0.14 at 24 h, and 4.06 ± 0.24 at 72 h); and the protein level increased at 72 h only (1.25 ± 0.42 at 12 h, 1.46 ± 0.42 at 24 h, and 3.46 ± 0.34 at 72 h), just in the same way of OPN. The mRNA level of BMP2 in experimental group elevated at 12 and 24 h (3.04 ± 0.07 at 12 h, 1.89 ± 0.14 at 24 h, and 0.98 ± 0.14 at 72 h). The protein level of BMP2 in experimental group only elevated at 12 h (8.14 ± 0.41 at 12 h, 1.78 ± 0.76 at 24 h, and 1.14 ± 0.10 at 72 h). Part of the data is shown in Figure 3.
Figure 3.
IL-6 induces the expressions of BAP, OPN, OPG, and BMP2 in the HUASMCs. Cells treated with or without recombinant human IL-6 10 ng/mL served as the experimental (Exp.) group or the control (Con.) group. (a) The mRNA level of every target gene was expressed by the ratio of the experimental group to the control group at the same time point. (b) The proteins detected by western blot were given on the left. The levels of these proteins are shown on the right, which were expressed by the ratio of the experimental group to the control group at the same time point. *P <0.05 vs. control.
Anti-BMP2 siRNA partly reversed the calcification of HUASMCs induced by rhIL-6
The mRNA levels of BMP2 and BAP in the experimental cells at 12 h were 0.02042 ± 0.0069 and 0.1350 ± 0.0093, respectively. Both were significantly lower than those of the control cells (P <0.05) and both were recovered at 48 h, while the mRNA levels of OPG and OPN in the experimental cells were at the same level as the control cells at 12 h and significantly decreased at 48 h to 0.2896 ± 0.0176 and 0.2533 ± 0.0211, respectively (P <0.05). These data suggest that with siRNA interfering, IL-6 failed to induce BMP2 expression at 12 h and the effect on BMP2 downstream genes could last at least 48 h. The statistical results are shown in Figure 4a.
Figure 4.
Anti-BMP2 siRNA partly reversed the IL-6 induced calcification of HUASMCs. Cells transfected with negative siRNA and treated with rhIL-6 served as the control group. Cells transfected with the valid anti-BMP2 siRNA and treated with rhIL-6 served as the experimental group. Cells transfected with negative siRNA but not treated with rhIL-6 served as the blank group. (a) After the BMP2 RNA interfering, rhIL-6 10 ng/mL failed to increase the mRNA levels of BMP2 and BAP at 12 h, and levels of OPG and OPN at 48 h in the experimental group. (b) When observing the calcification effect, 50 ng/mL rhIL-6 was used. The calcium concentration of the experimental group was lower than that of the control group, but higher than that of the blank group at the same time point. *P <0.05 vs. control. **P <0.05 vs. control and the bland group.
The calcium concentrations in the cellular layer had no statistical significance among all three groups at baseline. When incubated with rhIL-6 50 ng/mL, the calcium concentrations in the experimental cells increased to 1.95 ± 0.2472 mmol/g protein at day 2 and 2.17 ± 0.2368 mmol/g protein at day 4, which were both lower than those in the control cells (2.95 ± 0.2241 and 2.89 ± 0.3162, respectively), but higher than those in the blank group cells (1.11 ± 0.2573 and 0.92 ± 0.1073, respectively) at the same time points (P <0.05). The statistical results were showed in Figure 4b. These results indicated that BMP2 RNA interfering could partly reverse the calcification in cell matrix induced by IL-6.
Discussion
Extensive exposure to inflammation of organs all over the body is prominent phenomenon in RA. As a pleiotropic cytokine, IL-6 has been demonstrated to be closely associated with the pathogenesis of RA. Circulating IL-6 is involved in a group of conditions caused by the systemic inflammation of RA, including small-vessel vasculitis, anemia, weight loss, general osteoporosis, extensive artery calcification, and accelerated CVD.20–22 These issues are common in RA patients and are also alarming problems in diabetes, CKD, aging, and chronic stress.23,24 We thus assumed that IL-6 may play a role in systemic inflammation associated vascular calcification. The relationship between IL-6 and vascular calcification has never been proved directly before, in vitro or in vivo. Since vascular smooth muscle cells are the core and origin of vascular calcification tested by several studies in other fields,25–27 we established a system of HUASMCs culturing (Figure 1) to observe the role of IL-6 on vascular calcification in vitro.
The concentration of circulating IL-6 is lower than 10 pg/mL in normal human and is usually above 50 pg/mL in patients with active RA.28 In vitro studies like this would prefer using a high concentration of IL-6 as the stimulator to accomplish the effects in a relatively short time.29,30 Data from our preliminary experiment showed that HUASMCs had normal proliferation curves when co-cultured with IL-6 under 100 ng/mL. We therefore chose up to 50 ng/mL of IL-6 as the treating condition in this study. Our research showed that IL-6 50 ng/mL could induce calcium deposition in extracellular matrix of HUASMCs detected by positive Alizarin red S staining. The calcium concentration increased in both time- and dose-dependent manners. These results indicate precisely that IL-6 can induce calcification in the extracellular matrix of HUASMCs in vitro.
Calcification of the extracellular matrix is an active process carried out by osteoblasts. With the abduction of BMPs, especially BMP2, the precursor cells will differentiate into osteoblasts and express the calcification associated proteins like BAP, OC, OPN, collagen I, and OPG. These proteins will lead into mineral deposition in the extracellular matrix and eventually result in calcification of the bone matrix. Is there any possibility that the cultured HUASMCs trans-differentiated into osteoblasts under stimulating of IL-6? Next, we detected BAP, OPG, and OPN, which are proteins specifically secreted by osteoblasts. We tested all the three proteins in both mRNA and protein levels. The results showed sequential increasing of them. BAP was expressed in early stage, followed by OPN and OPG. High level of BMP2 was also proved to be induced by IL-6 in both mRNA and protein levels. This mimicked what would happen in osteoblasts.31,32
VSMCs and osteoblasts are both derived from the mesenchymal precursor cells. BMP2 is the most important regulatory factor for osteogenic differentiation and osteoblast proliferation, and is speculated as the key factor promoting calcification of human VSMCs in several circumstances like hyperglycemia and hyperphosphatemia.33–36 Several kinds of cells express BMP2, including the bone marrow and mesenchymal stem cells, as well as VSMCs. Based on the results demonstrated above, we thought that BMP2 expressed endogenously by VSMCs initiated osteogenic differentiation of these cells. Pathologic studies revealed that inflammation, uremia, high glucose level, and other conditions could all upregulate the BMP2 expression and ultimately cause calcification.31,37,38 However, the BMP2 gene knockout mouse died because of heart defects after gestating for 7–10 days, which makes the gene knockout an impropriate technique in this kind of research.39 Therefore, we established a BMP2 gene post-transcription silence system in VSMCs by small RNA interference technique, to observe the impact of endogenous BMP2 on VSMCs transform and calcification.
Our research showed that the HUASMCs could upregulate the expression of BMP2 in vitro under the stimulating of rhIL-6 and the expression could be effectively silenced by specific anti-BMP2 siRNA. This silence effect on BMP2 and the downstream genes lasted at least 48 h after the siRNA transfect. We believed that this small RNA interference system could serve as a proper method to explore the role of endogenous BMP2. We then tested the role of the transient endogenous BMP2 gene silencing on extracellular matrix calcium deposition. A high dose of rhIL-6 (50 ng/mL) was chosen as the stimulator to get a detectable calcification effect in HUASMCs as quick as possible. According to our data, IL-6 induced calcification could be partly reversed by BMP2 silencing within four days. These results suggested that: (1) the endogenous BMP2 participated in the osteogenic differentiation of VSMCs and the following calcification under IL-6 inducement; and (2) besides the endogenous BMP2, other mechanism might be involved in IL-6 induced calcification of VSMCs.
Vascular calcification is a major life-threatening condition in several diseases, especially in RA. The underling mechanism is not understood clearly. Possible theories include osteogenic differentiation of VSMCs, high serum calcium and phosphorus products deposition, apoptosis vesicle-based calcification, mechanical stress, and oxidative stress.33,40,41 Through transient silence of BMP2 of the VSMCs, this study confirmed that endogenous BMP2 and osteogenic differentiation was involved in vascular calcification induced by increased IL-6. Thus, the osteogenic differentiation of VSMCs may be one underlying reason of systemic inflammation related vascular calcification in several situations. Furthermore, serial passage culture of the VSMCs manifested senescent characteristics along with the endogenous BMP2 gene expression.42 One study also showed that BMP2 protein was highly expressed at the calcified part of blood vessel along with the prominent phenomenon of apoptosis.43 Another one reported that IL-6 could induce human VSMCs proliferation in vitro.44 These studies imply that IL-6 induces VSMCs proliferation, transgenic differentiation, and apoptosis at the same time. This may explain the results of our study. IL-6 might also induce calcification of VSMCs through apoptosis.
However, a few limitations have to be considered. In this study, we used the RNA interfering method and got the transient inhibitory of the BMP2 expression for about 48 h, so the long-term BMP2 inhibitory effect was not observed. Construction of virus vector of anti-BMP2 siRNA will help in long-term silence and a better understanding of the role of endogenous BMP2 on vascular calcification. The HUASMCs obtained from the primary culture method were heterogenic cells which might include smooth muscle cells, mesenchymal stem cells, and perivascular cells at least. The latter two kinds of cells are multi-lineage potential cells. The endogenous BMP2 may induce these cells to differentiate into osteoblasts via autocrine or paracrine effects. But this problem may need a second thought because the primarily cultured cell system is exactly the imitation of the situation in vivo.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by the grants from National Natural Science Foundation of China (No. 30370653) and the Science and Technology Department of Shandong Province Fund (No. 2013GHY11504).
References
- 1. Lassere MN, Rappo J, Portek IJ, et al. (2013) How many life-years are lost in patients with rheumatoid arthritis? Secular cause-specific and all-cause mortality in rheumatoid arthritis and their predictors in a long-term Australian cohort study. Internal Medicine Journal 43: 66–72. [DOI] [PubMed] [Google Scholar]
- 2. Doran MF, Pond GR, Crowson CS, et al. (2002) Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis and Rheumatism 46: 625–631. [DOI] [PubMed] [Google Scholar]
- 3. Maradit-Kremers H, Nicola PJ, Crowson CS, et al. (2005) Cardiovascular death in rheumatoid arthritis: A population based study. Arthritis and Rheumatism 52: 722–732. [DOI] [PubMed] [Google Scholar]
- 4. Wang Y, Shan J, Yang W, et al. (2013) High mobility group box 1 (HMGB1) mediates high-glucose-induced calcification in vascular smooth muscle cells of saphenous veins. Inflammation 36: 1592–1604. [DOI] [PubMed] [Google Scholar]
- 5. Burgmaier M, Hoppe S, Krüger T, et al. (2013) Serum levels of C-peptide are associated with coronary artery calcification in patients with rheumatoid arthritis. Rheumatology International 35: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 6. Abedin M, Tintut YM, Demer LL. (2004) Vascular calcification: Mechanisms and clinical ramifications. Arteriosclerosis Thrombosis and Vascular Biology 24: 1161–1170. [DOI] [PubMed] [Google Scholar]
- 7. Vasan RS, Sullivan LM, Roubenoff R, et al. (2003) Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: The Framingham Heart Study. Circulation 107: 1486–1491. [DOI] [PubMed] [Google Scholar]
- 8. Navarro-Cano G, Del Rincón I, Pogosian S, et al. (2003) Association of mortality with disease severity in rheumatoid arthritis, independent of comorbidity. Arthritis and Rheumatism 48: 2425–2433. [DOI] [PubMed] [Google Scholar]
- 9. del Rincón I, Haas RW, Pogosian S, et al. (2005) Lower limb arterial incompressibility and obstruction in rheumatoid arthritis. Annals of the Rheumatic Diseases 64: 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giles JT, Szklo M, Post W, et al. (2009) Coronary arterial calcification in rheumatoid arthritis: comparison with the Multi-Ethnic Study of Atherosclerosis. Arthritis Research and Therapy 11: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dessein PH, Lopez-Mejias R, Ubilla B, et al. (2015) TNF-related apoptosis-inducing ligand and cardiovascular disease in rheumatoid arthritis. Clinical and Experimental Rheumatology 33: 491–497. [PubMed] [Google Scholar]
- 12. Chung ES, Packer M, Lo KH, et al. (2003) Randomized, double-blind, placebo-controlled, pilot trial of Infliximab, a chimeric monoclonal antibody to tumor necrosis factor α, in patients with moderate-to-severe heart failure: Results of the Anti-TNF Therapy Against Congestive Heart failure (ATTACH) Trial. Circulation 107: 3133–3140. [DOI] [PubMed] [Google Scholar]
- 13. Mann DL, McMurray JV, Packer M, et al. (2004) Targeted anticytokine therapy in patients with chronic heart failure: Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 109: 1594–1602. [DOI] [PubMed] [Google Scholar]
- 14. Wolfe F, Michaud K. (2004) Heart failure in rheumatoid arthritis: Rates, predictors, and the effect of anti-tumor necrosis factor therapy. American Journal of Medicine 116: 305–311. [DOI] [PubMed] [Google Scholar]
- 15. Zhang F, Luo W, Li Y, et al. (2015) Role of osteopontin in rheumatoid arthritis. Rheumatology International 35: 589–595. [DOI] [PubMed] [Google Scholar]
- 16. Tyson KL, Reynolds JL, McNair R, et al. (2003) Osteo / chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arteriosclerosis Thrombosis and Vascular Biology 23: 489–494. [DOI] [PubMed] [Google Scholar]
- 17. Lyemere VP, Proudfoot D, Weissberg PL, et al. (2006) Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. Journal of Internal Medicine 260: 192–201. [DOI] [PubMed] [Google Scholar]
- 18. Zhu D, Mackenzie NC, Millan JL, et al. (2011) The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS One 6: e19595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim JH, Choi YK, Do JY, et al. (2015) Estrogen-related receptor γ plays a key role in vascular calcification through the upregulation of BMP2 expression. Arteriosclerosis Thrombosis and Vascular Biology 35: 2384–2390. [DOI] [PubMed] [Google Scholar]
- 20. Nikolaisen C, Figenschau Y, Nossent JC. (2008) Anemia in early rheumatoid arthritis is associated with interleukin 6-mediated bone marrow suppression, but has no effect on disease course or mortality. Journal of Rheumatology 35: 380–386. [PubMed] [Google Scholar]
- 21. Nishimoto N. (2006) Interleukin-6 in rheumatoid arthritis. Current Opinion in Rheumatology 18: 277–281. [DOI] [PubMed] [Google Scholar]
- 22. Abdel Meguid MH, Hamad YH, Swilam RS, et al. (2013) Relation of interleukin-6 in rheumatoid arthritis patients to systemic bone loss and structural bone damage. Rheumatology International 33: 697–703. [DOI] [PubMed] [Google Scholar]
- 23. Rho YH, Chung CP, Oeser O, et al. (2009) Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis and Rheumatism 61: 1580–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu X, Jones GW, Choy EH, et al. (2016) The biology behind interleukin-6 targeted interventions. Current Opinion of Rheumatology 28: 152–160. [DOI] [PubMed] [Google Scholar]
- 25. Parhami F, Basseri B, Hwang J, et al. (2002) High-density lipoprotein regulates calcification of vascular cells. Circulation Research 91: 570–576. [DOI] [PubMed] [Google Scholar]
- 26. Mary A, Hénaut L, Boudot C, et al. (2015) Calcitriol prevents in vitro vascular smooth muscle cell mineralization by regulating calcium-sensing receptor expression. Endocrinology 156: 1965–1974. [DOI] [PubMed] [Google Scholar]
- 27. Tintut Y, Alfonso Z, Saini T, et al. (2003) Multilineage potential of cells from the artery wall. Circulation 108: 2505–2510. [DOI] [PubMed] [Google Scholar]
- 28. Shimamoto K, Ito T, Ozaki Y, et al. (2013) Serum linterleukin 6 before and after therapy with Tocilizumab is a principal biomarker in patients with rheumatoid arthritis. Journal of Rheumatology 40: 1074–1081. [DOI] [PubMed] [Google Scholar]
- 29. Nishimoto N, Ito A, Ono M, et al. (2000) IL-6 inhibits the proliferation of fibroblastic synovial cells from rheumatoid arthritis patients in the presence of soluble IL-6 receptor. International Immunology 12: 187–193. [DOI] [PubMed] [Google Scholar]
- 30. Kayakabe K, Kuroiwa T, Noriyuki S, et al. (2012) Interleukin-6 promotes destabilized angiogenesis by modulating angiopoietin expression in rheumatoid arthritis. Rheumatology 51: 1571–1579. [DOI] [PubMed] [Google Scholar]
- 31. Sun M, Guo Y, Zhang M, et al. (2008) Uremic serum induces osteogenic transition and calcification of human umbilical artery smooth muscle cells. Chinese Journal of Nephrology 24: 265–270. [Google Scholar]
- 32. Mizobuchi M, Dwight T, Eduardo S. (2009) Vascular calcification: the killer of patients with chronic kidney disease. Journal of the American Society of Nephrology 20: 1453–1464. [DOI] [PubMed] [Google Scholar]
- 33. Li X, Yang HY, Giachelli CM. (2006) Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circulation Research 98: 857–859. [DOI] [PubMed] [Google Scholar]
- 34. Yan JY, zhou Q, Yu HM, et al. (2015) High glucose promotes vascular smooth muscle cell calcification by activating WNT signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao 35: 29–33. [PubMed] [Google Scholar]
- 35. Willette RN, Gu JL, Lysko PG, et al. (1999) BMP-2 gene expression and effects on human vascular smooth muscle cells. Journal of Vascular Research 36: 120–125. [DOI] [PubMed] [Google Scholar]
- 36. Li X, Yang HY, Giachelli CM. (2008) BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis 199: 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen NX, Duan D, O’Neill KD, et al. (2006) High glucose increases the expression of Cbfa1 and BMP-2 and enhances the calcification of vascular smooth muscle cells. Nephrology Dialysis Transplantation 21: 3435–3442. [DOI] [PubMed] [Google Scholar]
- 38. Moe SM, Chen NX. (2005) Inflammation and vascular calcification. Blood Purification 23: 64–71. [DOI] [PubMed] [Google Scholar]
- 39. Zhang H, Bradley A. (1996) Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122: 2977–2986. [DOI] [PubMed] [Google Scholar]
- 40. Hammoudi TM, Rivet CA, Kemp ML, et al. (2012) Three-dimensional in vitro tri-culture platform to investigate effects of crosstalk between mesenchymal stem cells, osteoblasts, and adipocytes. Tissue Engineering Part A 18: 1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin CP, Huang PH, Lai CF, et al. (2015) Simvastatin attenuates wxidative stress, NF-κB activation, and artery calcification in LDLR-/- mice fed with high fat diet via down-regulation of tumor necrosis factor-α and TNF receptor 1. PLoS One 10: e0143686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Burton DG, Giles PJ, Sheerin AN, et al. (2009) Microarray analysis of senescent vascular smooth muscle cells: A link to atherosclerosis and vascular calcification. Experimental Gerontology 44: 659–665. [DOI] [PubMed] [Google Scholar]
- 43. Maciel TT, Melo RS, Campos AH. (2009) The bone morphogenetic protein antagonist gremlin promotes vascular smooth muscle cell apoptosis. Journal of Vascular Research 46: 325–332. [DOI] [PubMed] [Google Scholar]
- 44. Battiston KG, Ouyang B, Labow RS, et al. (2013) Monocyte/macrophage cytokine activity regulates vascular smooth muscle cell function within a degradable polyurethane scaffold. Acta Biomaterialia 10: 1146–1155. [DOI] [PubMed] [Google Scholar]