Abstract
This study was designed to evaluate the effect of rutin on hepatotoxicity induced by thioacetamide (TAA) in rats. Four groups of male Wistar rats consisting of six rats each were used: Group I: control group; Group II: rats receiving single injection of 300 mg kg−1 body weight of TAA intraperitoneally; Group III: rats administered rutin (10 mg kg−1 body weight) dissolved in saline orally for 2 weeks; and Group IV: rats administered rutin (10 mg kg−1 body weight) dissolved in saline orally for 2 weeks followed by TAA injection last day of second week. All groups were sacrificed after 24 h of treatment and hepatic toxicity was analyzed with respect to liver toxicity markers, liver DNA fragmentation, and histology of liver tissue. Administration of TAA in Wistar rats resulted in significant increase of hepatic markers, DNA fragmentation in the hepatocytes, and changes in histology. Pretreatment of rats with rutin before 2 weeks of TAA assault resulted in the complete reversal of TAA-mediated hepatic toxicity (P < 0.0001 to P < 0.01) with concomitant restoration of DNA fragmentation. This study suggests rutin as a protective agent for restoration of toxicity caused by TAA.
Keywords: liver enzymes, oxidative stress, rutin, thioacetamide, toxicity
Background
Thioacetamide (TAA) is an organosulfur, white crystalline compound having liver damaging and carcinogenic activity by causing cytomegaly.1 It is used to induce an acute liver injury in rats. TAA gets metabolized to thioacetamide-S-oxide and acetamide immediately after administration to rats. Thioacetamide-S-oxide binds to macromolecules in a cell that are responsible for the change in cell permeability and Ca++ uptake. This interruption of calcium stores increases nuclear volume, enlarges nucleoli, and inhibits mitochondrial activity eventually leading to hepatic necrosis.2,3 The toxicological studies have revealed the neurotoxicity of TAA in humans and animals.4 Generation of a large amount of reactive oxygen species (ROS) due to TAA can overwhelm the antioxidant defense mechanism and damage cellular ingredients such as lipids, proteins, and DNA; this in turn can impair cellular structure and function.5 Regardless of the route of exposure, TAA rapidly distributes to nearby tissues and has also been reported to be a genotoxic, reproductive toxicant and rodent carcinogen.6 It is also reported that liver, kidney, spleen, and erythrocyte have significant binding capacity with TAA. Many studies were done to understand the changes elicited morphologically and biochemically that occur in the liver of TAA-treated rats.7–10
Rutin has a strong antioxidant potential and is a derivative (glycoside) of quercetin. Based on the antioxidant potential of quercetin,11–14 it is hypothesized that rutin can have a significant therapeutic activity in diseases caused due to oxidative stress.15 Rutin is both water-soluble and alcohol-soluble antioxidant found in high amounts in plants maximally in citrus fruits. Rutin attaches to the iron ion Fe2+, preventing its binding to hydrogen peroxide, and hence preventing the generation of a highly reactive free radical that can damage cells.16 Rutin has also been reported to inhibit the vascular endothelial growth factor in vitro.17,18 This in turn decreases the vascular permeability that is primary protection against certain injuries and inflammation. In this study, we, therefore, sought to evaluate the hepatoprotective role of rutin against TAA-induced oxidative stress.
Materials and methods
Chemicals
All the chemicals required in this study, including rutin and TAA, were purchased from Sigma Chemical Co., St Louis, USA. UV-kinetic diagnostic kits were procured from United Diagnostic Industry, Riyadh.
Animals
A total of 24 Wistar rats (male), weighing 100–120 g, were taken from the breeding laboratory of King Saud University and kept in departmental animal care facility. Rats were kept in polypropylene cages at room temperature with a 12 h light–dark cycle and relative humidity of 60% ± 15%. Animals were provided purified water ad libitum with standard laboratory rat chow. The study was approved by the animal ethics committee of Institutional Review Board of King Saud University, and the ethical guidelines for use and care of laboratory animals in experiments were strictly followed.
Experimental protocol
Total 24 rats were divided into four different groups consisting of six rats each. The groups were categorized as: Group I: control group; Group II: rats receiving TAA; Group III: rats receiving rutin; and Group IV: rats receiving rutin before TAA treatment.
Group I: control group received vehicle alone (saline; pH 7.8); Group II: TAA single intraperitoneally injection of 300 mg kg−1 of body weight in week 2; Group III: rutin 10 mg kg−1 of body weight daily for 2 weeks; Group IV: rutin 10 mg kg−1 of body weight daily for 2 weeks followed by TAA injection on last day of rutin treatment. TAA and rutin both were dissolved in 1 mL of saline; TAA was administered intraperitoneally, whereas rutin was administered orally. TAA dosage was selected on the basis of previous literature.19 The rats were sacrificed 24 h after the last treatment by asphyxiation with carbon dioxide.
Sample preparation
After 24 h of treatment, blood from retro orbital plexus under anesthesia was collected in clean tubes, centrifuged at 3000g for 20 min at 4°C and serum was stored at −20°C until further analysis. All the groups of rats were sacrificed and the livers dissected, weighed, and placed in the Petri dishes. Right lobes of the excised livers from all groups were processed for histology; the rest of the liver samples were homogenized and stored in −80°C until further analysis.
Serum enzyme analysis
Measurement of transaminases
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured by method of McPherson and Pincus20 using UV-kinetic diagnostic kits. AST and ALT are both tissue enzymes that catalyze the exchange of amino and keto groups between alpha amino and keto acids. Tissue toxicity releases enzymes to general circulation, hence increasing their levels. AST and ALT activities were determined by the decrease in extinction of NADH followed at 340 nm in a coupling reaction. Each unit of enzyme activity was defined as micromoles of NADH decomposed per minute using molar absorbance of 6.22 × 103 × M−1 Cm−1.
Measurement of alkaline phosphatase
Alkaline phosphatase (ALP) was calculated by the method of Bowers and McComb21 by a colorimetric kinetic diagnostic kit. Hydrolysis of p-nitrophenyl phosphate by ALP produces inorganic phosphate and p-nitrophenol which at alkaline pH yields yellow p-nitrophenoxide ions measured by following the absorbance increase at 405 nm per unit time. One unit of ALP activity was defined as micromoles of p-nitrophenol produced per minute using molar absorbance of 18.75 × 103 × M−1 Cm−1.
Measurement of lactate dehydrogenase
The quantitative determination of lactate dehydrogenase (LDH) is achieved using lactate to pyruvate kinetic method using UV-kinetic diagnostic kit. Activity was evaluated by measuring the extinction decrease in NADH at 340 nm.20 One unit of LDH activity was defined as micromoles of NADH decomposed per minute using molar absorbance of 6.22 × 103 × M−1 Cm−1.
Measurement of total bilirubin
The endpoint determination of total bilirubin was done by the method of Malloy and Evelyn22 using a colorimetric endpoint detection diagnostic. The intensity of purple color was directly proportional to bilirubin concentration in the serum recorded within 1 min for unconjugated bilirubin and after 5 min for conjugated bilirubin after adding methanol at 540 nm. The total bilirubin value was represented as the sum of bilirubin glucuronide (conjugated) and azobilirubin (unconjugated). Values were expressed as milligrams per deciliter.
Protein estimation
The protein content in the samples (both serum and tissue homogenate) was measured by the modified method of Markwell et al.23 Bovine serum albumin (BSA) was used to make the standard curve. The amount of protein was calculated later from the obtained standard curve. Protein values were expressed as milligrams per deciliter.
Tissue analysis
DNA fragmentation assays for apoptosis
Apoptotic changes in the liver were evaluated colorimetrically by DNA fragmentation of known amount of DNA according to the procedure of Perandones et al.24 Liver samples were homogenized in 700 µL hypotonic lysis buffer (0.2% Triton X-100, 10 mM Tris, 1 mM EDTA), incubated at 50°C for 2.4 h and centrifuged for 15 min at 11,000 r/min. The supernatants contained small DNA fragments that were acid extracted and used for quantification by adding two volumes of diphenylamine (DPA) at 600 nm.
Histology
The right lobes of the excised livers from all groups were processed for histology. The processing was done as follows: fixation of the samples in a 10% neutral buffered formalin solution, block preparation in paraffin, section cutting (5–6 µm thick), and staining with hematoxylin–eosin stain. The processed sections were analyzed and photographed by a blinded expert pathologist without any prior information about the experimental groups.
Statistical analysis
Values were expressed as mean ± standard deviation (SD). The data were generally statistically representative in terms of number, mean, and SD. Comparison between different groups was done using independent sample T-test for comparing two groups, and one-way analysis of variance (ANOVA) test was used for comparison between more than two groups with least significance difference as multiple comparison. Correlation between various variables was done by utilizing Pearson correlation coefficient (R) using linear regression with graphic representations. A probability value (P value) less than or equal to (0.05) was deliberated as significant. All statistical calculations were done using computer program SPSS (Statistical Package for Social Science) version (11.0).
Results
In the TAA-treated group (Group: II), the levels of all the liver marker enzymes were markedly elevated and there was an increase in total DNA fragmentation, indicating liver toxicity in this group of animals. In the other group (Group: IV) which received pretreatment of rutin, before TAA, the elevation of all the liver toxicity markers were reversed and DNA fragmentation was markedly reduced. Histopathological examination of liver sections showed the significant protection by rutin with no significant difference between control and rutin groups.
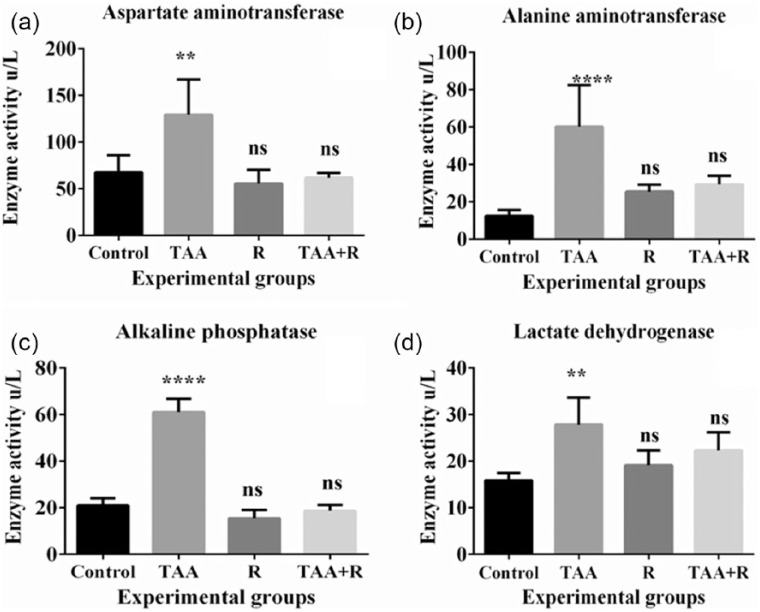
Figure 1(a) shows significant increased levels of AST in TAA-treated rats (P < 0.0001) when compared to control indicating the induction of liver damage. Rutin treatment alone caused non-significant decrease in the AST levels in rutin only treated rat group compared to control. Pretreatment of rutin in TAA group reversed the increased levels of AST and showed significant protection when compared with TAA-treated group. The decreased AST levels in group IV were insignificant compared to control group. Figure 1(b) shows increase in ALT levels by TAA significantly (P < 0.0001) and rutin non-significantly when compared to control. Rutin pretreatment followed by TAA assault reduced the levels when compared with TAA group and significant difference was observed when compared with this group and control group. It was depicted that rutin was completely protective with respect to ALT enzyme.
Figure 1.
(a) ALT, (b) AST, (c) ALP, and (d) LDH enzyme activities in the control, thioacetamide (TAA), rutin (R), and TAA + rutin (TAA + R) groups in rat. Data between the groups were equated with an analysis of variance (ANOVA) and Tukey’s multiple comparison tests.
****P < 0.0001 when equated to control and **P < 0.01 when equated to control (n = 6).
Figure 1(c) shows the significant increased levels of ALP in TAA group when compared to control. Pretreatment of rutin followed by TAA assault showed significant protection as compared to TAA alone group (P < 0.0001). Figure 1(d) shows significant increase in levels of LDH in TAA alone treated groups (P < 0.001) and complete protection of LDH levels was observed in groups pretreated with rutin followed by TAA assault.
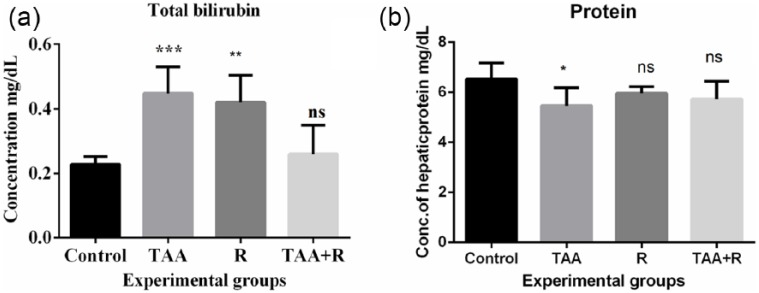
Figure 2(a) shows the concentration of bilirubin in all treated groups. There was significant effect observed on bilirubin content in TAA alone group (P < 0.0001); however, rutin alone treated group also showed significantly increased levels of bilirubin (P < 0.001). The increased levels reached to baseline when TAA was treated in combination with rutin. This further describes rutin as a protective flavonoid. Figure 2(b) shows the effect of TAA and rutin alone as well as together on hepatic protein content in rats of all groups. With the exposure to 300 mg TAA, protein levels were reduced significantly (P < 0.01) when compared to control. Rutin alone as well as along with TAA groups did not show any significant difference in hepatic protein content when compared to control.
Figure 2.
(a) Total bilirubin and (b) protein concentrations in the control, thioacetamide (TAA), rutin (R), and TAA + rutin (TAA + R) groups in rat. Data between the groups were equated with an analysis of variance (ANOVA) and Tukey’s multiple comparison tests.
**P < 0.001 when compared to control (n = 6); ***P < 0.0001 when compared to control.
Figure 3 shows the extent of DNA fragmentation in all treated groups. DNA fragmentation was significantly increased in TAA-treated group, but complete protection of DNA strands was observed in groups pretreated with rutin pretreated group followed by TAA toxicity.
Figure 3.
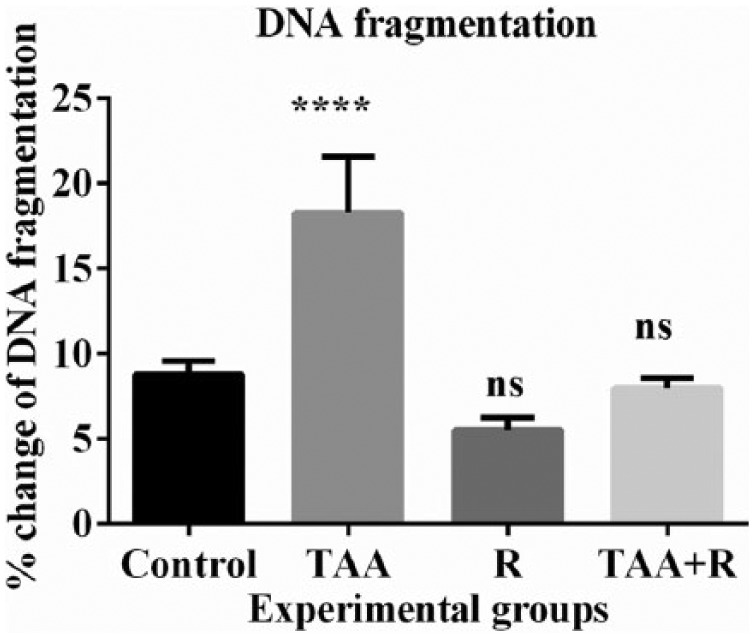
Percentage change of DNA fragmentation in the control, thioacetamide (TAA), rutin (R), and TAA + rutin (TAA + R) groups in rat. Data between the groups were compared with an analysis of variance (ANOVA) and Tukey’s multiple comparison tests.
****P < 0.0001 when compared to control (n = 6).
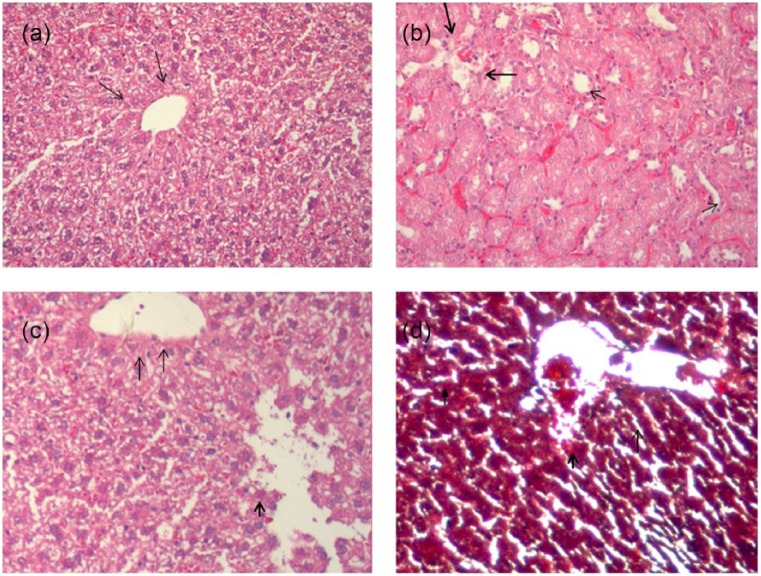
Figure 4 shows the changes produced by treatments with TAA and rutin alone and in combination. Normal rat liver hepatocytes have well-defined cytoplasm and nuclei. Treatment with TAA caused widespread intracellular vacuolization and aster (infiltration of inflammatory cells). Mild congestion was observed with congested portal area. Rutin-treated liver had nodular widened portal area with less inflammatory cell infiltration. Liver section from rutin pretreated group followed by TAA assault exhibited less changes in morphology as compared with TAA alone treated group. This group exhibited mild vacuolization and infiltration of inflammatory cells in portal area. Hepatocytes have dark eosinophilic cytoplasm with cells having heterochromatic nuclei.
Figure 4.
Liver sections of normal and treated groups at scale bar 100 µm. (a) Normal histological appearance of liver tissues with central vein and portal area shown by arrows. Hepatocytes have well-defined nuclei. (b) Thioacetamide-treated liver section with widespread intracellular vacuolization and infiltration of inflammatory cells (aster). Mild congestion is observed with congested portal area (thick arrows). (c) Rutin-treated liver section having nodular widened portal area with less inflammatory cell infiltration. (d) Rutin + TAA-treated liver section with mild vacuolization and mild infiltration of inflammatory cells in portal area (dark arrow). Hepatocytes have dark eosinophilic cytoplasm with cells having heterochromatic nuclei (n = 3).
Discussion
Liver, the largest glandular vital organ, plays various roles in regulating physiological processes. On the other hand, liver diseases have become the major causes of deaths all over world. Among them, drug-induced liver injury is one of the common causative factors that poses a major clinical and regulatory challenge.10,25 TAA at 300 mg kg−1 body weight as a single dose has been reported to cause oxidative stress.19 The aim of this study was to examine the effect of flavonoid rutin against TAA-induced oxidative stress in rats. In this study, rutin caused significant reversal of increased liver enzyme activities, DNA fragmentation, and histopathological changes induced by TAA. Protein levels were reduced significantly in TAA-treated groups possibly due to the formation of protein adducts with TAA. TAA acts as an electrophilic agent and leads to formation of s-oxide that can covalently bind to the lysine residues forming adducts with sulfhydryl groups hence lowering the protein levels and causing significant damage. Protein adducts with TAA were also reported in previous studies.26 This study reported the increase in liver transaminases (AST and ALT) and other enzymes LDH and ALP by TAA treatment which is concomitant to previous literature.5 Rutin exerted a powerful protective effect on the liver toxicity induced by different xenobiotics in other studies.27 This study for the first time reports the significant protection by rutin pretreatment with TAA toxicity. Rutin at 10 mg kg−1 body weight for 2 weeks significantly restored the changes elicited by TAA assault on liver markers of injury and histology. When total bilirubin was evaluated in the control and treated groups, rutin alone group showed significant increase but when treated in combination the increased values reached to baseline showing the protective effect. These results were in accordance with previous study.28 Bilirubin IXα has been recognized as a potent antioxidant. It is found to be toxic in infants but is considered to promote health in adults. Clinical literature from the last 10 years demonstrates that mildly increased serum bilirubin levels are significantly associated with decreased occurrence of chronic diseases, including cardiovascular diseases (CVDs), and CVD-related mortality or risk factors.29 In this study, rutin significantly reversed genotoxicity induced by TAA in liver tissue which was evaluated in terms of DNA fragmentation by DPA. The carcinogenicity of TAA has been demonstrated due to a disruption of function associated with spindle checkpoint immediately ahead of facilitation of cell proliferation.30 However, rutin treatment alone had no effect on DNA fragmentation. Rutin probably caused remarkable changes in tissue morphology; however, it was at the same time protective too. Previously, we have shown quercetin as protective against acrylamide hepatotoxicity.31 In this study, we conclude that rutin which is a glycosidal derivative of quercetin has a hepatoprotective activity against TAA toxicity and prophylactic use of rutin against TAA poisoning is also suggested. These results contribute to the better understanding of rutin in the hepato-protection and reversal of genotoxicity, thus emphasizing its use in the human diet.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This project was funded by Deanship of Scientific Research, King Saud University, Research Group (No. RG-1438-042).
References
- 1. Ichimura R, Mizukami S, Takahashi M, et al. (2010) Disruption of Smad-dependent signaling for growth of GST-P-positive lesions from the early stage in a rat two-stage hepatocarcinogenesis model. Toxicology and Applied Pharmacology 246: 128–140. [DOI] [PubMed] [Google Scholar]
- 2. Hajovsky H, Hu G, Koen Y, et al. (2012) Metabolism and toxicity of thioacetamide and thioacetamide S-oxide in rat hepatocytes. Chemical Research in Toxicology 25: 1955–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruck R, Aeed H, Avni Y, et al. (2004) Melatonin inhibits nuclear factor kappa B activation and oxidative stress and protects against thioacetamide induced liver damage in rats. Journal of Hepatology 40: 86–93. [DOI] [PubMed] [Google Scholar]
- 4. Mladenović D, Hrnčić D, Rašić-Marković A, et al. (2012) Behavioral and electroencephalographic manifestations of thioacetamide-induced encephalopathy: Possible mechanisms of neurotoxic effects. Archives of Biological Sciences 64: 829–841. [Google Scholar]
- 5. Ansil PN, Nitha A, Prabha SP, et al. (2011) Protective effect of Amorphophallus campanulatus (Roxb.) Blume. tuber against thioacetamide induced oxidative stress in rats. Asian Pacific Journal of Tropical Medicine 4: 870–877. [DOI] [PubMed] [Google Scholar]
- 6. De Minicis S, Kisseleva T, Francis H, et al. (2013) Liver carcinogenesis: Rodent models of hepatocarcinoma and cholangiocarcinoma. Digestive and Liver Disease 45: 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al-Bader TC, Mathew M, Koursheed S, et al. (2000) Thioacetamide toxicity and the spleen: Histological and biochemical analysis. Anatomia, Histologia, Embryologia 29: 3–8. [DOI] [PubMed] [Google Scholar]
- 8. Sinha M, Manna P, Sil PC. (2007) Aqueous extract of the bark of Terminalia arjuna plays a protective role against sodium-fluoride-induced hepatic and renal oxidative stress. Journal of Natural Medicines 61: 251–260. [Google Scholar]
- 9. Moreira E, Fontana L, Periago JL, et al. (1995) Changes in fatty acid composition of plasma, liver microsomes, and erythrocytes in liver cirrhosis induced by oral intake of thioacetamide in rats. Hepatology 21: 199–206. [PubMed] [Google Scholar]
- 10. Zargar S. (2014) Protective effect of Trigonella foenum-graecum on thioacetamide induced hepatotoxicity in rats. Saudi Journal of Biological Sciences 21: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ansar S, Siddiqi NJ, Zargar S, et al. (2016) Hepatoprotective effect of Quercetin supplementation against Acrylamide-induced DNA damage in wistar rats. BMC Complementary and Alternative Medicine 6: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zargar S, Siddiqi NJ, Khan TH, et al. (2014) Effect of cadmium fluoride and quercetin on in vivo activity of indoleamine 2, 3-dioxygenase in mice liver and kidney. Fluoride 47: 31–42. [Google Scholar]
- 13. Siddiqi NJ, Zargar S. (2014) Effect of quercetin on cadmium fluoride-induced alterations in hydroxyproline/collagen content in mice liver. Connective Tissue Research 55: 234–238. [DOI] [PubMed] [Google Scholar]
- 14. Zargar S, Siddiqi NJ, Al Daihan SK, et al. (2015) Protective effects of quercetin on cadmium fluoride induced oxidative stress at different intervals of time in mouse liver. Acta Biochimica Polonica 62: 207–213. [DOI] [PubMed] [Google Scholar]
- 15. Zhou YF, Guo B, Ye MJ, et al. (2015) Protective effect of rutin against H2O2-induced oxidative stress and apoptosis in human lens epithelial cells. Current Eye Research 17: 1–10. [DOI] [PubMed] [Google Scholar]
- 16. Yang R, Sun G, Zhang M, et al. (2016) Epigallocatechin gallate (EGCG) decorating soybean seed ferritin as a rutin nanocarrier with prolonged release property in the gastrointestinal tract. Plant Foods for Human Nutrition 71: 77–85. [DOI] [PubMed] [Google Scholar]
- 17. Kim HY, Nam SY, Hong SW, et al. (2015) Protective effects of rutin through regulation of vascular endothelial growth factor in allergic rhinitis. American Journal of Rhinology & Allergy 29: 87–94. [DOI] [PubMed] [Google Scholar]
- 18. Luo H, Jiang BH, King SM, et al. (2008) Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutrition and Cancer 60: 800–809. [DOI] [PubMed] [Google Scholar]
- 19. Abdel Salam OM, Mohammed NA, Sleem AA, et al. (2013) The effect of antidepressant drugs on thioacetamide-induced oxidative stress. European Review for Medical and Pharmacological Sciences 17: 735–744. [PubMed] [Google Scholar]
- 20. McPherson RA, Pincus MR. (2016) Henry’s Clinical Diagnosis and Management by Laboratory Methods. St Louis, MO: Elsevier Health Sciences. [Google Scholar]
- 21. Bowers GN, McComb RB. (1966) A continuous spectrophotometric method for measuring the activity of serum alkaline phosphatase. Clinical Chemistry 12: 70–89. [PubMed] [Google Scholar]
- 22. Malloy HT, Evelyn KA. (1937) The determination of bilirubin with the photoelectric colorimeter. Journal of Biological Chemistry 19: 481–490. [Google Scholar]
- 23. Markwell MAK, Haas SM, Bieber L, et al. (1978) A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Analytical Biochemistry 87: 206–210. [DOI] [PubMed] [Google Scholar]
- 24. Perandones C, Illera V, Peckham D, et al. (1993) Regulation of apoptosis in vitro in mature murine spleen T cells. The Journal of Immunology 151: 3521–3529. [PubMed] [Google Scholar]
- 25. Russmann S, Kullak-Ublick GA, Grattagliano I. (2009) Current concepts of mechanisms in drug-induced hepatotoxicity. Current Medicinal Chemistry 16: 3041–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yakov MK, Sarma D, Hajovsky H, et al. (2013) Protein targets of thioacetamide metabolites in rat hepatocytes. Chemical Research in Toxicology 26: 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luo H, Jiang BH, King SM, et al. (2008) Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutrition and Cancer 60: 800–809. [DOI] [PubMed] [Google Scholar]
- 28. Vítek L, Jirsa M, Brodanová M, et al. (2002) Gilbert syndrome and ischemic heart disease: A protective effect of elevated bilirubin levels. Atherosclerosis 160: 449–456. [DOI] [PubMed] [Google Scholar]
- 29. Wagner KH, Wallner M, Mölzer C, et al. (2015) Looking to the horizon: The role of bilirubin in the development and prevention of age-related chronic diseases. Clinical Science 129: 1–25. [DOI] [PubMed] [Google Scholar]
- 30. Kimura M, Mizukami S, Watanabe Y, et al. (2015) Disruption of spindle checkpoint function ahead of facilitation of cell proliferation by repeated administration of hepatocarcinogens in rats. Journal of Toxicological Sciences 40: 855–871. [DOI] [PubMed] [Google Scholar]
- 31. Zargar S, Siddiqi NJ, Ansar S, et al. (2016) Therapeutic role of quercetin on oxidative damage induced by acrylamide in rat brain. Pharmaceutical Biology 54: 1763–1767. [DOI] [PubMed] [Google Scholar]