Abstract
Cloxacillin, a semisynthetic penicillin is a potent inhibitor of most penicillinase-producing Staphylococci. Use of high doses of Cloxacillin for 6 weeks is recommended for the treatment of infective endocarditis caused by methicillin-susceptible Staphylococcus aureus (MSSA). Here, we report a case of Cloxacillin-induced agranulocytosis in a patient treated for MSSA native tricuspid valve endocarditis, which was resolved after discontinuation of the antibiotic. This case report highlights a rare adverse event of a commonly used antibiotic.
Keywords: agranulocytosis, Cloxacillin, infective endocarditis
Introduction
Agranulocytosis is a serious condition with a mortality rate of around 10%. Cloxacillin is commonly prescribed for suspected Gram-positive infection. It is used to treat culture-confirmed methicillin-susceptible Staphylococcus aureus (MSSA) infection.1 Cloxacillin-induced agranulocytosis is a rare but serious adverse event which is preventable if suspected early. Here, we report a case of Cloxacillin-induced agranulocytosis in a patient treated for MSSA native tricuspid valve endocarditis with metastatic infection.
Case presentation
A 21-year-old lady was presented with a 20-day history of high-grade, intermittent (two to three episodes per day) fever, with recorded maximum temperature of 103°F. The fever was associated with chills and rigors, a 10-day history of painful right leg swelling, and a 3-day history of dry cough with breathlessness on exertion; 21 days before the onset of fever, she had taken intramuscular injections in the gluteal region at another hospital for abdominal pain and vomiting. Following this, she had noticed pain and swelling over the same region which progressed over a period of 10 days. She had no other significant systemic symptoms. There was no significant past or family history. She had normal bowel and bladder habits. She had no addictions or co-morbidities. On examination, she was conscious, oriented, and febrile at admission. Her pulse rate was 120 min−1, blood pressure was 110/70 mmHG, and respiratory rate was 25 min−1. All peripheral pulses were felt. She required 2 L of oxygen via nasal prongs to maintain normal saturation. She had pallor and bilateral pitting pedal edema, but no icterus, cyanosis, clubbing, or lymphadenopathy. She had no peripheral signs of infective endocarditis. She had bilateral tender gluteal swelling of 3 × 3 cm with significant warmth and induration at the injection site. She also had a tender fluctuant swelling suggestive of an abscess near the right malleolus. Cardiovascular examination was normal except sinus tachycardia. Respiratory examination revealed bilateral anterior and interscapular mid and late inspiratory crepitations. The rest of the systemic examination was normal.
A clinical diagnosis of probable Gram-positive sepsis originating from a gluteal abscess secondary to an intramuscular injection was considered.
Investigations
Investigations revealed 6.3 g/dL of hemoglobin (microcytic and hypochromic), total white blood cell count of 22,900 cells/mm3, 86% differential white blood cell count of neutrophils, 10% lymphocytes, 4% monocytes, and platelet count of 150,000 cells/mm3. Biochemistry investigations revealed 129 mmol/L of sodium, 3.7 mmol/L of potassium, 17 mmol/L of bicarbonate, 21 mg/dL of urea, and 0.64 mg/dL of creatinine. Liver function test showed 1.4 mg/dL of total bilirubin, 1.2 mg/dL of direct bilirubin, 5.9 g/dL of total protein, 1.9 g/dL of albumin, 18 U/L of aspartate aminotransferase, 12 U/L of alanine aminotransferase, and 189 U/L of alkaline phosphatase. She was initiated on intravenous meropenem after drawing three blood cultures. Transesophageal echocardiography revealed a single 10 × 12 mm freely mobile vegetation in the tricuspid valve with associated tricuspid regurgitation. Chest radiograph revealed multiple nodular opacities in bilateral lung fields suggestive of septic emboli. Ultrasonography of the abdomen showed multiple hypoechoic lesions in the spleen suggestive of abscesses not amenable for aspiration. All three blood cultures grew MSSA. A final diagnosis of MSSA native tricuspid valve endocarditis with metastatic infection was made.
Treatment
She underwent drainage of the abscesses in the gluteal and right malleolar regions. Antibiotics were changed to intravenous Cloxacillin 2 g every 4 h from day 2 of hospitalization, along with intravenous Gentamicin for the first 5 days.
Outcome and follow-up
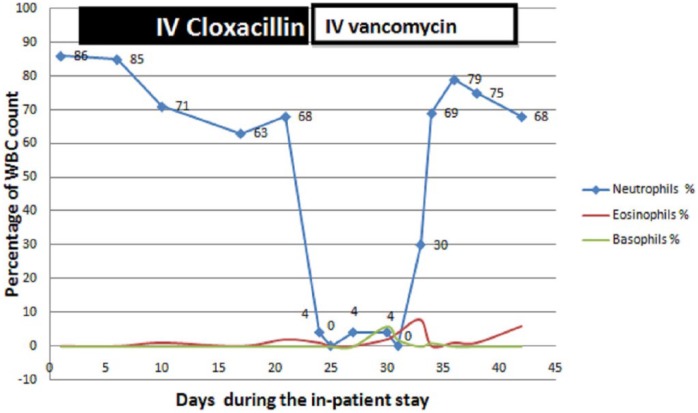
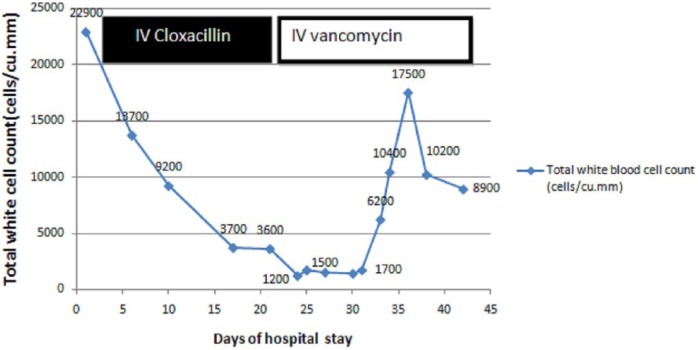
Initially, she had shown clinical improvement with resolution of fever and other clinical symptoms. Her blood cultures became sterile after 7 days of antibiotics; 24 days after starting intravenous Cloxacillin, she developed leucopenia with marked reduction in granulocytes along with high spiking fever but no localizing symptoms. After drawing blood cultures, her antibiotics were changed from Cloxacillin to intravenous meropenem and vancomycin. Repeated blood cultures were sterile. Her symptoms resolved with improvement in total white blood cell and granulocyte count, 8 days after discontinuation of intravenous Cloxacillin. The temporal relationship of Cloxacillin with changes in the total and differential white blood cell counts in the absence of alternate etiologies was suggestive of Cloxacillin-induced agranulocytosis (Figures 1 and 2). This adverse event was classified as possible with a score of 7 according to the Naranjo et al.’s2 Adverse Drug Reaction Probability scale (ADR). She was not rechallenged with Cloxacillin. The antibiotics course of 6 weeks was completed with intravenous Vancomycin. She was asymptomatic and well after 6 months of follow-up.
Figure 1.
Change in granulocyte count during hospital stay in relation to administration of IV Cloxacillin.
Figure 2.
Change in total white blood cell count during hospital stay in relation to administration of IV Cloxacillin.
Discussion
This case report demonstrates the rare adverse event of a commonly prescribed antibiotic. Cloxacillin, a semisynthetic isoxazolyl penicillin, is a potent inhibitor of the growth of most penicillinase-producing Staphylococci. It is rapidly excreted by the kidney. There is also significant hepatic elimination in the bile. Common side effects of Cloxacillin are abdominal pain, nausea, skin rash, and diarrhea. Agranulocytosis is a rare but serious adverse event of Cloxacillin. According to Kramer et al.,3 adverse reactions are considered to be induced by the drug if they develop a reasonable time sequence following administration of the drug and resolve after discontinuation of the same. Our patient fulfilled the above-mentioned criteria. Drugs known to cause agranulocytosis other than chemotherapeutic agents include antibiotics (such as Trimethoprim/Sulfamethoxazole, Cloxacillin, Chloramphenicol, Ceftazidime, and Clindamycin), atypical antipsychotics such as Clozapine; anti-thyroid drugs such as Ticlopidine and Spironolactone; Carbamazepine; Erythromycin; and Diclofenac Sodium.4 A prospective study from Sweden showed that Cloxacillin-induced agranulocytosis occurred in 4% of patients with infective endocarditis who were treated for 10 days or more at the rate of 0.006 per treatment day more than 10 days.5 However, the incidence of adverse events with Cloxacillin-treated episodes was lower than that of Penicillin G and Ampicillin. Following discontinuation of the drug, neutrophil count normalized within 2–4 days. The risk of this adverse event was dependent on the length of treatment and occurred mostly between days 15 and 30. The risk also increased with daily dose of more than 9.8 g of Cloxacillin. The relationship of this adverse event with prolonged duration of antibiotic use has been reported earlier too.6,7 Our patient also developed this adverse event after 24 days of administration of intravenous Cloxacillin at a daily dose of 12 g per day.
A few pathogenic mechanisms are considered for this rare adverse event. One mechanism is the direct toxic effect of β-lactam antibiotics on the bone marrow. Neftel et al.8–10 postulated that there was both in vivo and in vitro inhibition of granulopoiesis by β-lactam antibiotics. Other mechanisms are immune related.11 Early studies by Radermecker and Sanders showed that hemagglutinin antibodies directed against β-lactam antibiotics are demonstrated more in patients who were recently sensitized than non-allergic patients.12,13 This significant correlation between the antibodies and delayed adverse event was also demonstrated later, which favors the mechanism of immune reaction.5 Recent studies on host microbe symbiosis have shown that gut microbiota is an important regulator of hematopoiesis and immunity. When broad-spectrum antibiotics are administered for more than 2 weeks, there is depletion of the gut microbiota leading to decreased hematopoiesis since microbial compounds such as lipopolysaccharides sustain steady-state production of neutrophils.14–16 Furthermore, rare single-nucleotide polymorphisms in genes encoding drug transporters and phase-1 or phase-2 enzymes could modulate response to antibiotics, which may be the reason for inter-individual variation in therapeutic effects and ADRs.17,18 However, this rare adverse event results in good outcome if recognized early and the antibiotic was promptly discontinued.5,6
Agranulocytosis is a rare but serious adverse event of β-lactam antibiotics. The risk increases with higher mean daily dose and increased duration of treatment. Early recognition and discontinuation of the drug are essential to decrease morbidity and mortality.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Patient consent: The patient gave written informed consent for the case to be reported.
References
- 1. Thwaites GE, Edgeworth JD, Gkrania-Klotsas E, et al. Clinical management of Staphylococcus aureus bacteraemia. The Lancet Infectious Diseases 2011; 11(3): 208–222. [DOI] [PubMed] [Google Scholar]
- 2. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clinical Pharmacology & Therapeutics 1981; 30(2): 239–245. [DOI] [PubMed] [Google Scholar]
- 3. Kramer MS, Leventhal JM, Hutchinson TA, et al. An algorithm for the operational assessment of adverse drug reactions. I. Background, description, and instructions for use. Journal of the American Medical Association 1979; 242(7): 623–632. [PubMed] [Google Scholar]
- 4. Ibáñez L, Vidal X, Ballarín E, et al. Population-based drug-induced agranulocytosis. Archives of Internal Medicine 2005; 165(8): 869–874. [DOI] [PubMed] [Google Scholar]
- 5. Olaison L, Belin L, Hogevik H, et al. Incidence of beta-lactam-induced delayed hypersensitivity and neutropenia during treatment of infective endocarditis. Archives of Internal Medicine 1999; 159(6): 607–615. [DOI] [PubMed] [Google Scholar]
- 6. Damali Amiri N, Wijenaike N. Flucloxacillin and fusidic acid-associated neutropenia in a patient with periaortic abscess: Rare side effects of commonly used antibiotics. BMJ Case Reports. Epub ahead of print 25 March 2015. DOI: 10.1136/bcr-2014-208324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shah I, Kumar KS, Lerner AM. Agranulocytosis associated with chronic oral administration of cloxacillin for suppression of staphylococcal osteomyelitis. American Journal of Hematology 1982; 12(2): 203–206. [DOI] [PubMed] [Google Scholar]
- 8. Neftel KA, Hübscher U. Effects of beta-lactam antibiotics on proliferating eukaryotic cells. Antimicrobial Agents and Chemotherapy 1987; 31(11): 1657–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neftel KA, Hauser SP, Müller MR. Inhibition of granulopoiesis in vivo and in vitro by β-lactam antibiotics. Journal of Infectious Diseases 1985; 152(1): 90–98. [DOI] [PubMed] [Google Scholar]
- 10. Neftel KA, Müller MR, Widmer U, et al. Beta-lactam antibiotics inhibit human in vitro granulopoiesis and proliferation of some other cell types. Cell Biology and Toxicology 1986; 2(4): 513–521. [DOI] [PubMed] [Google Scholar]
- 11. Krafft T, Pugin P, Miescher PA. [Agranulocytosis and intravenous cloxacillin]. Schweizerische Medizinische Wochenschrift 1978; 108(46): 1821–1823. [PubMed] [Google Scholar]
- 12. Radermecker M, Salmon J. [Study of sero-agglutination of “penicillin-treated” erythrocytes in humans allergic and not allergic to penicillin]. Acta Allergol 1966; 21(4): 285–298. [PubMed] [Google Scholar]
- 13. Sanders WE, Johnson JE, Taggart JG. Adverse reactions to cephalothin and cephapirin. Uniform occurrence on prolonged intravenous administration of high doses. The New England Journal of Medicine 1974; 290(8): 424–429. [DOI] [PubMed] [Google Scholar]
- 14. Balmer ML, Schürch CM, Saito Y, et al. Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. Journal of Immunology 2014; 193(10): 5273–5283. [DOI] [PubMed] [Google Scholar]
- 15. Iwamura C, Bouladoux N, Belkaid Y, et al. Sensing of the microbiota by NOD1 in mesenchymal stromal cells regulates murine hematopoiesis. Blood 2017; 129(2): 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Theilgaard-Mönch K. Gut microbiota sustains hematopoiesis. Blood 2017; 129(6): 662–663. [DOI] [PubMed] [Google Scholar]
- 17. Kozyra M, Ingelman-Sundberg M, Lauschke VM. Rare genetic variants in cellular transporters, metabolic enzymes, and nuclear receptors can be important determinants of interindividual differences in drug response. Genetics in Medicine 2017; 19(1): 20–29. [DOI] [PubMed] [Google Scholar]
- 18. Fujikura K, Ingelman-Sundberg M, Lauschke VM. Genetic variation in the human cytochrome P450 supergene family. Pharmacogenetics and Genomics 2015; 25(12): 584–594. [DOI] [PubMed] [Google Scholar]