Abstract
Gonadal hormones play a key role in the establishment, activation, and regulation of the hypothalamic–pituitary–adrenal (HPA) axis. By influencing the response and sensitivity to releasing factors, neurotransmitters, and hormones, gonadal steroids help orchestrate the gain of the HPA axis to fine-tune the levels of stress hormones in the general circulation. From early life to adulthood, gonadal steroids can differentially affect the HPA axis, resulting in sex differences in the responsivity of this axis. The HPA axis influences many physiological functions making an organism’s response to changes in the environment appropriate for its reproductive status. Although the acute HPA response to stressors is a beneficial response, constant activation of this circuitry by chronic or traumatic stressful episodes may lead to a dysregulation of the HPA axis and cause pathology. Compared to males, female mice and rats show a more robust HPA axis response, as a result of circulating estradiol levels which elevate stress hormone levels during non-threatening situations, and during and after stressors. Fluctuating levels of gonadal steroids in females across the estrous cycle are a major factor contributing to sex differences in the robustness of HPA activity in females compared to males. Moreover, gonadal steroids may also contribute to epigenetic and organizational influences on the HPA axis even before puberty. Correspondingly, crosstalk between the hypothalamic–pituitary–gonadal (HPG) and HPA axes could lead to abnormalities of stress responses. In humans, a dysregulated stress response is one of the most common symptoms seen across many neuropsychiatric disorders, and as a result, such interactions may exacerbate peripheral pathologies. In this review, we discuss the HPA and HPG axes and review how gonadal steroids interact with the HPA axis to regulate the stress circuitry during all stages in life.
Keywords: Hypothalamic–pituitary–adrenal axis, hypothalamic–pituitary–gonadal axis, gonadal steroids, estrogen, progesterone, testosterone, sex differences, stress circuitry
Introduction
Stress is the natural response of living organisms to environmental perturbations. Appropriate reactivity to an acute stressor and timely cessation of the stress response are critical for an organism’s survival. Although in our society stress has a negative connotation, this is somewhat misleading since the acute activation of stress responses makes an individual more adaptable and allows one to cope with events that jeopardize homeostasis, whether real or perceived, by increasing awareness to our surroundings, and enhancing analgesia, cognition, and euphoria (Charmandari, Tsigos, & Chrousos, 2005). Chronic and traumatic stressors, however, have the opposite effect, causing detrimental changes in the brain and other organs, and have been linked to the development of pathology including neuropsychiatric disorders, such as post-traumatic stress disorder, depression, chronic anxiety (Smith & Vale, 2006), and cardiometabolic diseases (Levine, Levine, & Levine, 2014). Moreover, the mechanisms to cope with stressors differ between males and females (Bale & Epperson, 2015). The basis for these differences is unknown, in part because much of the early work in this field was performed on male rodents, perhaps due to the challenges associated with carrying out experiments that account for fluctuating gonadal hormones in females. Across the estrous cycle of rodents, the changes in progesterone and estrogen levels have been shown to have a direct impact on the way that females respond to stress, with differential responses dependent upon the stage of the cycle (Viau & Meaney, 1991). Thus, the most appropriate study of the neuroendocrine stress response requires that fluctuations in gonadal steroid hormones be taken into account.
Gonadal steroids are largely responsible for the differential response to physical or psychological threats between sexes. Likewise, elevated stress hormones can negatively regulate the reproductive neuroendocrine axis and consequently, the levels of circulating gonadal hormones (Rivier & Rivest, 1991). Biological and physiological differences have been identified between males and females, especially in the way that they react to stress. Exploring these differences using animal models may help explain why psychiatric disorders, like anxiety and major depressive disorder, are more prevalent among females (Bekker & van Mens-Verhulst, 2007). In this review, we will define the HPA axis, identify the differences in its function between sexes, and discuss how gonadal hormones interact with the HPA axis. Finally, we will discuss potential mechanisms by which gonadal hormones may influence the development of the HPA axis.
The HPA axis – overview
The hypothalamic–pituitary–adrenal (HPA) axis is a neuroendocrine axis that utilizes three primary structures, allowing it to respond appropriately to stressful life-events. These include the paraventricular nucleus of the hypothalamus (PVN), the anterior pituitary gland, and the adrenal gland (Figure 1). At its core, the PVN computes and integrates neuronal and humoral inputs to activate a specialized group of cells that synthesize and secrete corticotropin-releasing hormone (CRH) into the hypophyseal portal vasculature. CRH is a 41-amino acid peptide that, at the level of the anterior pituitary gland, stimulates the synthesis and release of adrenocorticotropic hormone (ACTH) into the blood stream. In turn ACTH, upon reaching the adrenal cortex, signals the synthesis of glucocorticoids (Rivier & Vale, 1985; Ulrich-Lai & Herman, 2009). Typical of all steroid hormones, glucocorticoids are immediately released following their synthesis in adrenal cortical cells. The primary glucocorticoids are cortisol in humans and corticosterone in mice and rats. These steroid hormones bind mineralocorticoid (MR) and glucocorticoid (GR) receptors with different affinities to promote an appropriate response to environmental perturbations, whether perceived or psychological (de Kloet, Joels, & Holsboer, 2005; Reul & de Kloet, 1985). The HPA axis is ultimately inhibited by the same hormones acting in different brain regions. This negative feedback process reestablishes the baseline homeostatic state. These responses are known to be adaptive and have effects that influence future HPA axis responses (McEwen & Gianaros, 2010). A dysregulation of these carefully orchestrated interactions can result in pathologies such as immunodeficiency, memory impairment, obesity, and cardiometabolic disorders (Levine, Levine, & Levine, 2014; Munck, Guyre, & Holbrook, 1984; Sapolsky, Romero, & Munck, 2000; Smith & Vale, 2006).
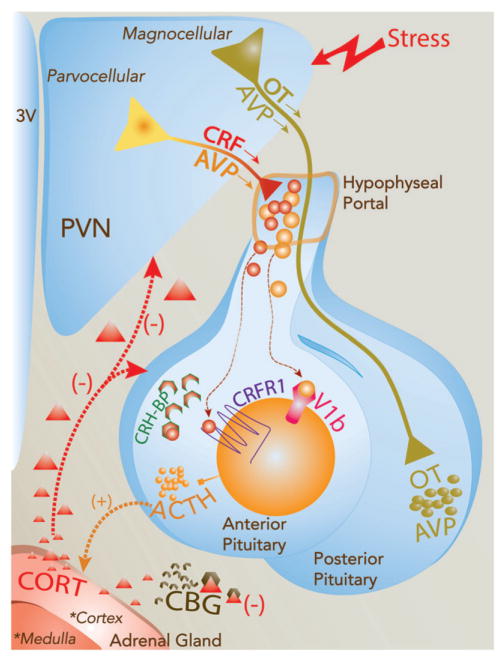
Figure 1.
Schematic diagram of the HPA axis and factors controlling the neuro-endocrine response to stress. Stress signals are relayed to the paraventricular nucleus of the hypothalamus (PVN) where oxytocin (OT), vasopressin (AVP), and corticotropin-releasing hormone (CRH) neurons reside. These neurons are recruited to trigger the HPA axis response. First, a subset of hypophysiotropic PVN neurons releases CRH and AVP into the hypophysial portal vasculature. These neuropeptides are transported to the anterior pituitary to act on corticotropes through their binding to CRHR1 and V1b receptors thereby stimulating the synthesis and release of adrenocorticotropic hormone (ACTH) into the general circulation. CRH activity on corticotropes is also regulated by the CRH-binding protein, which impedes its interaction with CRHR1. OT and AVP also control CRH actions at the pituitary level. In the adrenal cortex, the binding of circulating ACTH to the melanocortin type 2 receptor (MC2R) stimulates the synthesis and secretion of corticosterone (CORT) into the general circulation. Circulating CORT can then modulate behavioral and physiological responses to stress. CORT is also capable of inhibiting HPA axis activation (negative feedback) by acting in brain regions including the PVN, pituitary, hippocampus, and other limbic areas. The activity of circulating CORT is in part regulated by the CORT binding globulin (CBG). In contrast, axons of OT and AVP in the magnocellular division of the PVN project to the posterior pituitary, where they release OT and AVP to the general circulation to regulate the physiological response to stressors, osmoregulation, and reproductive function.
The paraventricular nucleus of the hypothalamus (PVN)
The PVN houses the key neurons controlling the level of activation of the HPA axis, regulation of metabolism, growth, and immune functions, as well as pre-autonomic control of gastrointestinal, cardiovascular and renal functions (Ferguson, Latchford, & Samson, 2008). Close to a century ago, Gurdjian, using the rat model, classified these neurons into two broad groups based on their size (Gurdjian, 1927). The medial parvocellular region has neurons with small nuclei, whereas the lateral magnocellular region houses neurons with large, dense nuclei. Since then, the cyto- and chemo-architecture of the PVN has been studied extensively in the rat. In contrast, the PVN of other species, like the mouse and human, reportedly have noteworthy differences in the chemical and cytological distribution (Biag et al., 2012). Nonetheless, most of the information available regarding the morphology and chemical composition of the PVN is predominantly based on the rat model.
The PVN is composed of two main cellular types classified by their fiber distribution: neuroendocrine and pre-autonomic. Neuroendocrine cells residing in the PVN project to the median eminence or to the posterior pituitary gland. At the median eminence, their chemical content is released into the hypothalamo-hypophyseal portal veins, a series of veins that connect two venous capillary beds, thereby allowing for fast transport and exchange of hormones between the hypothalamus and the anterior pituitary gland (Ferguson, Latchford, & Samson, 2008; Swanson & Sawchenko, 1983). Once these releasing factors reach the anterior pituitary, they trigger the release of hormones into the general circulation. CRH, thyrotropin releasing hormone (TRH), oxytocin (OT), dopamine, somatostatin, and vasopressin (AVP) expressing neurons are among those that project to the median eminence (Ferguson, Latchford, & Samson, 2008). Additionally, OT and AVP magnocellular neurons project to the posterior pituitary and secrete directly into the general circulation (Sawchenko, Imaki, & Vale, 1992). PVN pre-autonomic neurons, on the other hand, directly project to preganglionic autonomic neurons of the brainstem and spinal cord for the regulation of cardiovascular, thermoregulatory, and gastrointestinal functions, to name a few (Geerling, Shin, Chimenti, & Loewy, 2010). In addition to the medial eminence and autonomic nuclei projecting neurons, the PVN contains neurons that project to the forebrain and other hypothalamic nuclei to mediate freezing and other behavioral responses (Fuzesi, Daviu, Wamsteeker Cusulin, Bonin, & Bains, 2016; Knobloch et al., 2012).
The rat neuroendocrine neurons of the PVN can be divided into magno- and parvocellular divisions based on each neuron’s size and projections (Swanson & Kuypers, 1980). OT and AVP neurons are numerous in the magnocellular division, and project to the posterior pituitary gland to release their respective hormones. However, PVN neurons that directly release neuropeptides like CRH, AVP, and TRH into the hypothalamo-hypophyseal portal vasculature are part of the neurosecretory parvocellular division (Cunningham & Sawchenko, 1991; Swanson & Sawchenko, 1980; Wotjak et al., 1996). Of importance for HPA axis regulation, these neuropeptides are carried by the hypothalamo-hypophyseal portal veins to the anterior pituitary, where they activate corticotrophs that release ACTH into the blood stream (Smith & Vale, 2006).
Sex differences it the PVN composition
Interestingly, as far as is known, in rodents and humans, the morphology of the male and female PVN is quite similar, with some exceptions. For instance, women reportedly have larger PVN AVP neurons than men (Ishunina & Swaab, 1999). Otherwise, the lack of sex differences in the morphology of the PVN suggests that the chemical composition or wiring of the PVN is what makes the activation of the HPA axis more robust in females than in males. For example, following exposure to a stressor, the release of CRH from PVN neurons triggers a larger ACTH response in female than in male rats (Le Mevel, Abitbol, Beraud, & Maniey, 1979). Additionally, female rats have a much stronger release of AVP following stress (Williams, Carter, & Lightman, 1985), indicating a robust sexual dimorphism in mounting the HPA response to environmental threats at the PVN level. More on these sex differences will be covered in later sections.
The pituitary gland
The pituitary gland, through its secretion of protein hormones, plays a critical role in the maintenance of homeostasis during and after stress, as well as during other physiological processes, like growth and metabolism (Perez-Castro, Renner, Haedo, Stalla, & Arzt, 2012). This structure serves as the main humoral communication gateway from the brain to peripheral organs. Strategically located at the base of the hypothalamus and enclosed by the bony sella turcica, it interacts with the hypothalamus via compounds that are delivered to it through the hypothalamo-hypophyseal portal veins (Melmed, 2011). The pituitary is divided into two lobes, the anterior lobe (or adenohypophysis) and the posterior lobe (or neurohypophysis). This division is based upon their relative anatomical positions, function, and developmental precursors. The posterior pituitary receives axonal inputs from magnocellular OT and AVP neurons residing in the supraoptic nucleus and PVN (Perez-Castro, Renner, Haedo, Stalla, & Arzt, 2012), where they release their secretory product into the general circulation. In contrast, corticotrophs are the key anterior pituitary cell type involved in HPA axis regulation and the production of ACTH. These cells contain receptors that bind CRH to activate the synthesis of ACTH in response to humoral signals from the hypothalamus. Activation of the CRH receptor type 1 (CRH-R1) (Bale & Vale, 2004) initiates ACTH secretion from the corticotrophs.
There are two main types of the CRH receptor in mammals, although the CRH-R1 is the primary receptor responsible for driving secretion of ACTH from corticotrophs. Both CRH-R1 and CRH-R2 are 7-transmembrane G-protein coupled receptors, which act to increase cAMP activity through stimulation of adenylate cyclase. The release of ACTH is the result of an increase in intracellular calcium driven by the cAMP-protein kinase A pathway (Majzoub, 2006). Corticotrophs in the rat pituitary also express both estrogen receptor subtypes (α and β), but only a pituitary-specific truncated estrogen receptor product (TERP) mRNA transcript changes levels (4-to 7-fold increase) following estradiol treatment (Mitchner, Garlick, & Ben-Jonathan, 1998). Whether these receptors are co-expressed with CRH receptor-containing corticotrophs to regulate the release of ACTH at this level remains unknown. The importance of these receptors in the regulation of the HPA axis has been highlighted recently, especially with its upstream modulatory targets to the PVN, which have been observed to work through CRH receptors. For instance, in a recent article, Henckens et al. (2016) demonstrated that the posterior part of the bed nucleus of the stria terminalis (BnST) modulates the HPA axis (Henckens et al., 2016). They showed this by specifically activating CRHR2-expressing neurons using an optogenetic approach, which resulted in a blunted neuroendocrine response to stress, as well as decreased anxiety and PTSD-like related behaviors. Furthermore, Dabrowska et al. (2011) have shown that not only does the BnST interact with the PVN, but also vice versa, presumably through an OT and CRHR2 mechanism (Dabrowska et al., 2011). They showed that OT and CRHR2 fibers present in the BnST originated in the PVN. These data suggest a potential reciprocal modulation between these two nuclei.
The Crh gene and promoter activity have been shown to be regulated by estrogens (Lalmansingh & Uht, 2008; Miller, Suzuki, Miller, Handa, & Uht, 2004). Estradiol treatment increases Crh gene expression and basal levels of ACTH in ovariectomized rats (Ochedalski, Subburaju, Wynn, & Aguilera, 2007) and CRH neurons in the rat PVN express estrogen receptor β, but not estrogen receptor α (Laflamme, Nappi, Drolet, Labrie, & Rivest, 1998; Mitra et al., 2003). The estradiol-dependent regulation of CRH neurons in the PVN is thought to be regulated by the estrogen receptor β (Lund, Hinds, & Handa, 2006). In vitro studies have shown that the estrogen receptor β, but not α, has a strong stimulatory action on the Crh promoter (Miller, Suzuki, Miller, Handa, & Uht, 2004). These studies suggest that estradiol-dependent effects in the regulation of the HPA axis may be driven by their direct action through the estrogen receptor β in CRH neurons of the PVN. While the size of the pituitary gland has been reported to differ between sexes in humans (MacMaster et al., 2007), and its response to hormones in mice (Sanchez-Cardenas et al., 2010), little is known about the sex differences at this level contributing to the differential response to stressors between males and females.
CRH binding proteins and sex differences in the pituitary gland
CRH-binding protein (CRH-BP) is a secreted protein that binds CRH with similar or greater affinity than the CRH receptor. It is secreted by anterior pituitary corticotropes, and subsequently binds free CRH (Figure 1), thus preventing it from binding to its receptor and thereby blocking the actions of CRH on anterior pituitary corticotrophs (Cortright, Nicoletti, & Seasholtz, 1995; Seasholtz, Burrows, Karolyi, & Camper, 2001). These data suggest a local inhibitory role at the pituitary level.
In female rats, there is a more robust ACTH response to stress as compared to males (Handa et al., 1994; Young, 1996). In a recent report, Stinnett et al. (2015), indicated higher Crh-BP mRNA transcripts in pituitary from female mice versus male mice, even after restraint stress (Stinnett, Westphal, & Seasholtz, 2015). Further, the increase in Crh-BP mRNA levels observed following restraint stress is blunted by adrenalectomy, or depletion of endogenous glucocorticoids, indicating that glucocorticoids play a fundamental role in the regulation of CRH-BP (McClennen, Cortright, & Seasholtz, 1998). The level of CRH-BP is also higher in the murine pituitary and the cells expressing Crh-BP mRNA may also be involved in the regulation of reproductive hormones, such as prolactin and luteinizing hormone β (Speert, McClennen, & Seasholtz, 2002). In fact, on proestrus, 80% of the CRH-BP of the murine female pituitary is present in prolactin-expressing cells, suggesting that high estrogen levels drive the expression of CRH-BP and restrict the amounts of bioactive CRH that can activate corticotrophs. However, this goes against studies showing enhanced secretion of ACTH on proestrus (Viau & Meaney, 1991). Thus, these data may indicate that there are other upstream factors involved. In support of this, the estrogen receptors α and β have been reported to regulate the CRH-BP promoter in vitro, and estradiol has been shown to promote the induction of endogenous CRH-BP (van de Stolpe et al., 2004). Thus, it is possible that CRH-BP could be mediating the levels of CRH sensed by the pituitary gland through an ER-dependent mechanism, bringing CRH sensitivity back to homeostatic levels after stress. However, the actions of CRH-BP on the efficacy of CRH at the level of the pituitary seems to be context specific, or during periods like pregnancy, when exposure to CRH is chronic.
The adrenal gland
The adrenal glands have strategic access to the vascular system allowing them to regulate basal and reactive hormone levels (Rosol, Yarrington, Latendresse, & Capen, 2001). The adrenal cortex is composed of three distinct concentric zones: the zona glomerulosa, zona fasciculata, and zona reticularis from outside to inside. In addition to the cortical zones, the adrenal medulla is also involved in the regulation of homeostasis, as it secretes epinephrine and norepinephrine following autonomic nervous system activation (Mitani, 2014). However, the main adrenal region responsible for glucocorticoid secretion is the zona fasciculata. Cells in the zona fasciculata express the melanocortin receptor-2 (MC2R) (Figure 1). ACTH in the general circulation reaches the adrenal gland to activate MC2R, thereby triggering the synthesis of CORT in the zona fasciculata of the adrenal cortex (Rivier & Vale, 1985; Vale, Spiess, Rivier, & Rivier, 1981).
There are five melanocortin receptor subtypes (MC1R-MC5R), all of which have different expression patterns and specific functions (Yang, 2011). These receptors are part of the G-protein coupled receptor (GPCR) superfamily. MC2R expression is predominantly restricted to the adrenal cortex, pituitary, and adipose tissue (Beuschlein, Fassnacht, Klink, Allolio, & Reincke, 2001; Boston & Cone, 1996; Forti, Dias, & Armelin, 2006). Upon ACTH binding and activating MC2R, genes involved in steroidogenesis are activated by the canonical intracellular molecular pathway involving adenylyl cyclase, cAMP, and protein kinase A (Lefkowitz, Roth, Pricer, & Pastan, 1970; Lehoux, Fleury, & Ducharme, 1998) to allow the synthesis and secretion of adrenal glucocorticoids. The metabolic pathway that gives rise to adrenal steroid hormones has been reviewed in Oyola, Malysz, Mani, and Handa (2016).
Sex differences in adrenal function
The adrenal release of CORT has been shown to differ between the sexes, particularly following stress. CORT increases following a stressor are higher and remain elevated for longer in female rats (Figueiredo, Dolgas, & Herman, 2002). Both estrogen and testosterone have been shown to modulate the levels of CORT, and the degree to which activation of the HPA axis is driven is also influenced by both of these hormones, suggesting an interaction between the HPA and the HPG axes (see below). However, gonadal steroids impact HPA axis reactivity differentially. Gonadectomy of male rats elevates, while androgen replacement blunts the CORT and ACTH response to stress (Handa et al., 1994). In contrast, ovariectomy reduces, while estradiol treatment increases, the gain of the HPA axis (Lund, Munson, Haldy, & Handa, 2004; Viau & Meaney, 1991). The reciprocal relationship between the HPA axis and the regulation of the HPG axis will be further discussed in a later section.
Corticosteroid binding proteins
CORT signaling can be regulated at many levels. The main control of CORT bioavailability is the plasma corticosteroid binding globulin (CBG), which binds CORT in the blood to render it inactive (de Kloet, Joels, & Holsboer, 2005). CBG, or transcortin, is synthesized mainly by hepatocytes and binds CORT with high affinity to transport CORT in blood. This interaction regulates bioavailability during high CORT secretion (Meyer, Nenke, Rankin, Lewis, & Torpy, 2016). Of note, CBG also binds other hormones, including androgens and progesterone, which in humans are thought to displace cortisol from CBG (Dunn, Nisula, & Rodbard, 1981). Moreover, CBG is higher in female than in male rats (Gala & Westphal, 1965), perhaps normalizing the free biological component of circulating CORT to levels observe in males. However, the levels of stress-induced biologically active CORT may be elevated in male rats in part because the CBG is negatively regulated by stress (Tannenbaum et al., 1997), though, it would be interesting to know if the same is true in female rats. Finally, estradiol treatment has been shown to positively regulate CBG in male rats (Gala & Westphal, 1966; Young & Altemus, 2004), but perhaps not over a short time course that is insufficient to increase HPA responses to stress (Lund et al., 2004), indicating that estrogens and androgens may both play a role in the regulation of the HPA axis in part, through modulation of CBG levels.
Negative feedback and corticosteroid receptors
An important step in HPA axis control is its ability to keep its own secretion in check, thereby allowing return to baseline levels. This occurs through inhibition of the entire axis by adrenal glucocorticoids. There are at least two time-domains known to be involved in negative feedback: a time-delayed feedback and a fast acting, non-genomic feedback (Keller-Wood & Dallman, 1984). The more studied of these mechanisms is delayed feedback, which involves corticosteroid receptors and transcriptional regulation in multiple brain regions (Figure 1). Two types of receptors have been described for corticosteroids, the glucocorticoid receptor (GR) and the mineralocorticoid receptor (MR). While GRs are ubiquitously expressed throughout the brain, they are highly expressed in the hippocampus (De Kloet, Vreugdenhil, Oitzl, & Joels, 1998) and hypophysiotropic neurons of the PVN (Kovacs, Foldes, & Sawchenko, 2000; Sawchenko, 1987), two brain regions key to negative feedback. However, because GRs are present throughout the brain, the involvement of many different brain region in negative feedback cannot be overlooked.
CORT can affect glucocorticoid-sensitive brain regions by binding GRs and MRs to directly regulate gene transcription (Reul & de Kloet, 1985). Mineralocorticoid receptors have a much higher affinity (Kd = 0.5 nM) for CORT than do GRs (Kd =5.0 nM) (De Kloet, Veldhuis, Wagenaars, & Bergink, 1984; De Kloet, Vreugdenhil, Oitzl, & Joels, 1998), and consequently, the MRs are mostly occupied when CORT levels are low, such as during the morning trough of CORT in rodents (Bradbury et al., 1991). On the other hand, it is thought that GRs likely become occupied following rises in CORT that can occur after activation of the HPA axis (Reul, van den Bosch, & de Kloet, 1987) and/or at peak concentrations achieved during the diurnal cycle.
Malfunction in the negative feedback loop of the HPA axis results in abnormal CORT levels and can give rise to HPA axis dysregulation. In a series of early studies, PVN infusion of glucocorticoids resulted in decreased activation of the HPA axis, suggesting that this brain nucleus is central to this adaptive process (Kovacs & Makara, 1988; Sawchenko, 1987).
Sex difference in and sites of negative feedback
CRH and AVP neurons of the PVN express GR (Uht, McKelvy, Harrison, & Bohn, 1988) and as a result, corticosterone can act directly on CRH and AVP neurons of the PVN as well as in anterior pituitary corticotropes to regulate synthesis of these key secretagogues. CORT can also act indirectly in extra-PVN regions expressing MR and GR, such as the hippocampus, to inhibit the secretion of CORT (Akana, Cascio, Du, Levin, & Dallman, 1986; Dallman et al., 1987; Kovacs, Foldes, & Sawchenko, 2000; Sawchenko, 1987) through a trans-synaptic mechanism (Herman et al., 2016). Additional evidence for CORT acting directly on PVN neurons comes from studies where targeted deletion of GR in the PVN increased CRH expression and CORT and ACTH levels in male mice (Jeanneteau et al., 2012). Moreover, Solomon et al. (2015) reported that in a transgenic mouse model, where reductions in the levels of GR were targeted to the PVN, there were corresponding increases in ACTH and CORT levels after acute but not chronic stress, thus supporting the hypothesis that PVN GR directly mediates negative feedback. The same reduction in GR in the PVN increased the levels of the diurnal CORT nadir, thus implicating GR in the maintenance of baseline levels of HPA axis control as well. Further, there were suppressed responses to acute and chronic stress-induced ACTH in the transgenic female mice (Solomon et al., 2015), indicating that GR negative feedback occurred predominantly at the hypothalamus. Previous studies by this group showed that forebrain GR does not play as large a role in regulating the HPA of female mice compared to males, since the PVN selective loss of GR caused a striking increase in HPA reactivity and depression-like behaviors in males but not females (Solomon et al., 2012).
Estrogens have also been shown to regulate negative feedback at multiple sites. Weiser and Handa (2009) showed that treatment with estradiol could inhibit the negative feedback effects of the synthetic glucocorticoid, dexamethasone, likely through effects on or near the PVN (Weiser & Handa, 2009). In this work, direct implant of wax pellets containing E2 or the ERα agonist (PPT) proximal to the PVN increased stress-induced and diurnal peak CORT. The opposite was found when pellets with DPN, the ERβ agonist, were placed in the same region. The Peri-PVN region contains, mostly, GABAergic neurons that project into the PVN. Thus, E might be working through activation of ERα in the peri-PVN to increase the HPA axis reactivity, and reduce GR through activation of ERβ. Peripheral administration of the estrogen receptor β agonist, R-DPN, prevented the increased HPA axis reactivity and increased anxiety-like behaviors in female rats in response to the implantation of a wax pellet containing a GR agonist, adjacent to the central nucleus of the amygdala (Weiser, Foradori, & Handa, 2010). Together, such data suggest a possible mechanism for estradiol actions in inhibiting HPA axis reactivity through estrogen receptor β. These results suggest a strong modulation of GR on the HPA axis, marked by a striking sex difference in the negative feedback regulation as a result of estradiol interactions with estrogen receptors.
Gonadal hormone fluctuations and the HPA axis
Fluctuations in gonadal hormones have been shown to modulate the way that males and females react to stress, although the exact mechanism for this has yet to be completely resolved. Changes in gonadal steroid levels are driven by the HPG axis and result in physiological and behavioral changes across the estrous cycle in females. The estrous cycle of the female rodent is characterized by cyclic fluctuations in estradiol and progesterone throughout the reproductive lifespan. For female rodents, there are four stages: proestrus, estrus, metestrus, and diestrus, which can be differentiated by changes in circulating hormone patterns (Levine, 2015). Estradiol begins to rise in the evening of diestrus and peaks near midday on proestrus. This stage is followed by an increase in progesterone in the afternoon of proestrus. These hormonal changes correlate with higher stress hormone levels in female rats noted on proestrus when compared to rats that are in estrus, a time when estradiol and progesterone levels are reduced (Viau & Meaney, 1991). These internal changes are further reflected behaviorally by increased anxiety-like behavioral responses to stressors during proestrus, as compared to estrus (Lovick, 2012). This relationship challenges the experimental approach of using intact females in the study of the stress circuitry and indicates that estrous cyclicity should be monitored in order to allow interpretation of results from females.
In males, the response of the HPA axis to stressors changes dramatically during pubertal development. Testosterone rises shortly before puberty, and subsequently, its levels remain relatively constant. Interestingly, although pre-pubertal and adult male rats exhibit similar CORT and ACTH peak levels following acute stress, pre-pubertal animals take 30–45 min longer to return to baseline levels (Romeo et al., 2006; Romeo, Lee, & McEwen, 2004). These results suggest that changes in the transition from puberty to adulthood have a marked effect on the male HPA axis response to stress. However, whether these changes are driven by the rise in testosterone observed during puberty is not entirely clear. Subsequently, Evuarherhe et al. (2009) tested this hypothesis, and concluded that the rise in testosterone during the pubertal period is indeed key for the proper HPA axis response during adulthood. Thus, gonadal steroids are not only critical for the correct programing of the HPA axis during the perinatal period, but also play a role in establishing the HPA axis response to stress at puberty.
Gonadal hormones and neuropeptidergic regulation of the HPA
Studying the neuropeptidergic expression profile of hypothalamic neurons is key to the understanding of HPA function. PVN neuropeptide neurons are the drivers of the HPA axis, yet, estrogen receptors are expressed in different proportions in CRH, AVP, and OT neurons populating the PVN (Hrabovszky et al., 2004; Laflamme, Nappi, Drolet, Labrie, & Rivest, 1998; Suzuki & Handa, 2004). Such results suggest their involvement in the estrogen-mediated regulation of the HPA axis.
CRH regulation by estrogen and androgen receptors
CRH neurons play a primary role in the integration of limbic and sensory inputs, and through their varied projections can regulate behavior (Fuzesi, Daviu, Wamsteeker Cusulin, Bonin, & Bains, 2016), autonomic, and neuroendocrine responses (Bale & Vale, 2004). As a mediator of estradiol actions, ERβ is expressed at different levels in various subdivisions of PVN CRH neurons in the rat, ranging from 5% co-localization in the medial PVN to 60–80% co-localization in the caudolateral division (Laflamme, Nappi, Drolet, Labrie, & Rivest, 1998). These types of data suggest a potential interaction between these two molecules, where nuclear receptors may act to directly regulate gene transcription (Kushner et al., 2000; Lefstin & Yamamoto, 1998) such as CRH (Miller, Suzuki, Miller, Handa, & Uht, 2004). Examination of the regulation of CRH by estradiol was first described by Vamvakopoulos & Chrousos (1993), who demonstrated that the Crh promoter contains several ERE half sites, suggesting the involvement of ERE-mediated effects of estrogen receptor. In support of this, they showed that estradiol treatment increased human Crh promoter activity by almost twofold in vitro in cells co-transfected with the ER α and a Crh promoter–reporter gene construct.
Later in vitro studies by Miller et al. (2004) used the Crh proximal promoter in transformed rat hypothalamic neurons to show that ERβ can bind the Crh promoter to regulate transcription (Miller, Suzuki, Miller, Handa, & Uht, 2004). In addition, the Crh promoter was differentially regulated by splice variants of ERβ, consistent with the demonstration that a variant that lacks the full DNA binding domain (ERβ1 delta3) could signal through non-classical mechanisms by protein: protein interactions involving an SP1 element rather than an ERE (Price et al., 2001). Of importance, regulation by ERα was weak (Miller, Suzuki, Miller, Handa, & Uht, 2004). Similarly, Crh promoter activity can be augmented by estradiol or the ERβ agonist, DPN, but not the ERα agonist, PPT (Ogura, Kageyama, Hanada, Kasckow, & Suda, 2008). Moreover, the ERβ agonist, 3β-diol, an important metabolite of the non-aromatizable androgen, DHT, can also increase Crh promoter activity in CHO-K1 cell lines overexpressing ERβ (Huang, Zhu, Fischer, & Zhou, 2008). Together, these studies suggest that estrogens are able to modulate Crh gene expression by acting through ERβ directly. The fact that only a relatively low percentage of ERβ cells co-localize with CRH neurons in the PVN, yet treatment with an ERβ agonist is able to decrease the activation of the stress response, is still a conundrum. However, there are a few ways in which these effects could be explained. First, the anxiolytic properties of ERβ might be mediated by extra-PVN regions, which ultimately interact with neurosecretory cell in the PVN. Second, it is possible that the techniques utilized in previous works do not reflect the actual co-localization numbers due to technical limitations (e.g. IHC and microscopy limitations). Third, there are various splice variants of ERβ, which may not be necessarily recognized by the antibodies used for the co-localization studies.
Steroid hormone control of arginine vasopressin and regulation of the HPA
The nanopeptide, AVP, has been shown to be involved in the regulation of the stress response and circadian rhythms, as well as osmoregulation, paternal behaviors, and aggression (Sladek, 2010). Expression of this potent neuromodulator has been found in brain regions including the BST, medial amygdala (MeA), supraoptic nucleus (SON), suprachiasmatic nucleus, and PVN (Dumais & Veenema, 2016; Whitnall, Mezey, & Gainer, 1985). AVP can be co-expressed with CRH in the rat PVN, and can be co-secreted with CRH where it acts at the pituitary to enhance the secretagouge activity of CRH (Rivier, Rivier, Mormede, & Vale, 1984; Rivier & Vale, 1983, 1985). Hypothalamic AVP neurons have been shown to be involved in regulation of the HPA axis. In a study using adrenalectomized rats implanted with pellets delivering physiological CORT levels, Avp transcription and mRNA levels were significantly elevated (Herman, 1995). Restraint stress was shown to cause a similar increase in AVP activity, suggesting a tight relationship between stress, circulating glucocorticoids, and PVN AVP neurons. In particular, AVP has been shown to play a prominent role mediating the glucocorticoid negative feedback upon the HPA axis (Kovacs & Makara, 1988).
Data from our laboratory and others indicate that the estrogen and androgen regulation of AVP expression could be mediated by direct actions of ERβ. For instance, it was shown that the Avp promoter could be differentially regulated by ERα and ERβ in vitro through an upstream ERE. The differential regulatory effects of ERα and ERβ on AVP could be due to the constitutive activity of ERβ on the Avp promoter (Pak, Chung, Hinds, & Handa, 2007; Shapiro, Xu, & Dorsa, 2000). Similarly, the effects of DHT could also be mediated by ERβ or AR. In vivo studies using pre- and post-pubertal gonadectomized female rats showed that the DHT metabolite, 5α androstane 3β-17β-diol (3β-diol), increased the number of Avp mRNA-expressing cells in the BST, although this increment was not as marked as in animals treated with estradiol (Pak, Chung, Hinds, & Handa, 2009). In summary, although the gonadal steroid regulation of AVP is evident, the mechanism by which this is occurring requires further investigation. However, it is plausible that this mechanism involves several intracellular players, such as AR, ERβ, and ERα, acting in parallel, but further regulated by ligand-specific actions.
Gonadal steroid effects on oxytocin and the HPA axis
The nanopeptide hormone, oxytocin, is primarily produced by neurons residing in the PVN and SON (Onaka, 2004). Oxytocin is widely known for its actions on parturition, lactation, and maternal behaviors (Leng, Meddle, & Douglas, 2008). OT is synthesized at two main sites in the hypothalamus: the PVN and the supraoptic nucleus (SON). OT neurons in the PVN can also regulate the HPA axis since PVN OT is released into the hypothalamo-hypophyseal portal vasculature, enhancing the CRH-mediated release of ACTH via binding the V1b type vasopressin receptor (Schlosser, Almeida, Patchev, Yassouridis, & Elands, 1994). Of note, the OT-ergic regulation of the HPA axis has been shown by several laboratories. Although OT can act at the pituitary to enhance CRH action, when delivered to the PVN, OT decreased HPA axis gain, as shown by decreased CORT and ACTH levels (Neumann, Krömer, Toschi, & Ebner, 2000; Windle et al., 2004; Windle, Shanks, Lightman, & Ingram, 1997). Furthermore, global deletion of OT in female mice results in increased stress-induced CORT secretion and Crh mRNA expression in the PVN (Mantella, Vollmer, Rinaman, Li, & Amico, 2004; Nomura et al., 2003), suggesting that OT may regulate HPA axis gain at the PVN level as well as the pituitary.
Gonadal steroids can act in a synergistic manner to decrease HPA axis gain and anxiety-related behaviors (Kudwa, McGivern, & Handa, 2014; McCarthy, McDonald, Brooks, & Goldman, 1996), and this may be mediated through modulation of OT neuron function. OT neurons in the PVN express androgen receptors (Zhou, Blaustein, & De Vries, 1994). However, 85% of OT neurons in the PVN also express ERβ in the rat (Hrabovszky et al., 2004; Suzuki & Handa, 2004) and in mice (Oyola, Thomson, Handa, & Handa, 2017). Interestingly, the ERβ-driven reduction in stress-induced CORT and ACTH levels, and PVN neuronal activation is very similar to that observed following OT treatment (Ochedalski, Subburaju, Wynn, & Aguilera, 2007; Windle et al., 2006, 2004).
Another twist on the potential mechanism for the HPA down-regulation by testosterone is through its conversion to 3β-diol, a weak androgen, which has been shown to bind ERβ [reviewed in Handa, Weiser, and Zuloaga (2009b)]. Supporting this possibility, studies using a rat hypothalamic cell line that constitutively expresses OT, reported that levels of OT mRNA were significantly increased following 3β-diol treatment (Sharma, Handa, & Uht, 2012), and this was accompanied by an increased interaction between the Ot promoter, cAMP response element-binding protein, and the transcriptional regulatory protein, steroid receptor co-activator-1. These findings suggest that 3β-diol aids in the molecular machinery assembly involving cAMP response element and the steroid receptor coactivator-1 to drive the expression of the Ot gene. Consistent with these findings, 3β-diol also increases Ot mRNA expression in the midcaudal, but not the rostral portion, of the rat PVN (Hiroi et al., 2013). This study also reported an increase in Ot mRNA in hypothalamic N38 cells following 3β-diol treatment, indicating it effectiveness in regulating OT in vivo and in vitro. These effects of ERβ agonists were tracked to an ERE half-site in the Ot proximal promoter and thereby verify some molecular mechanisms for Ot gene expression regulation by ERβ. Moreover, the role of OT in mediating the actions of ERβ on the HPA axis can be demonstrated by studies showing that an OT antagonist can partially block the inhibitory effects of an ERβ agonist on HPA axis function (Kudwa, McGivern, & Handa, 2014). These fundamental observations help dissect the molecular mechanisms underlying the actions of ER on OT regulation and may aid our understanding of the complexities of HPA axis regulation and further begin to point out potential therapeutic targets for the control of stress-related pathologies in the future.
The hypothalamic–pituitary–gonadal axis
Reproduction is driven and directed by another neuroendocrine axis, the hypothalamo–pituitary–gonadal (HPG) axis. Like all neuroendocrine axes, the HPG axis consists of interactions between the hypothalamus and the anterior pituitary gland, and in the case of the HPG axis, this drives the growth and maturation of germ cells, and the synthesis of gonadal steroids in both, male and female gonads. At its apex, the decapeptide, gonadotropin releasing hormone (GnRH), is widely accepted as the hypothalamic peptide controlling reproduction. It stimulates the secretion of the anterior pituitary hormones, luteinizing hormone (LH) and follicle stimulating hormone (FSH), that ultimately control the production of gonadal steroids and gametogenesis (Figure 2).
Figure 2.
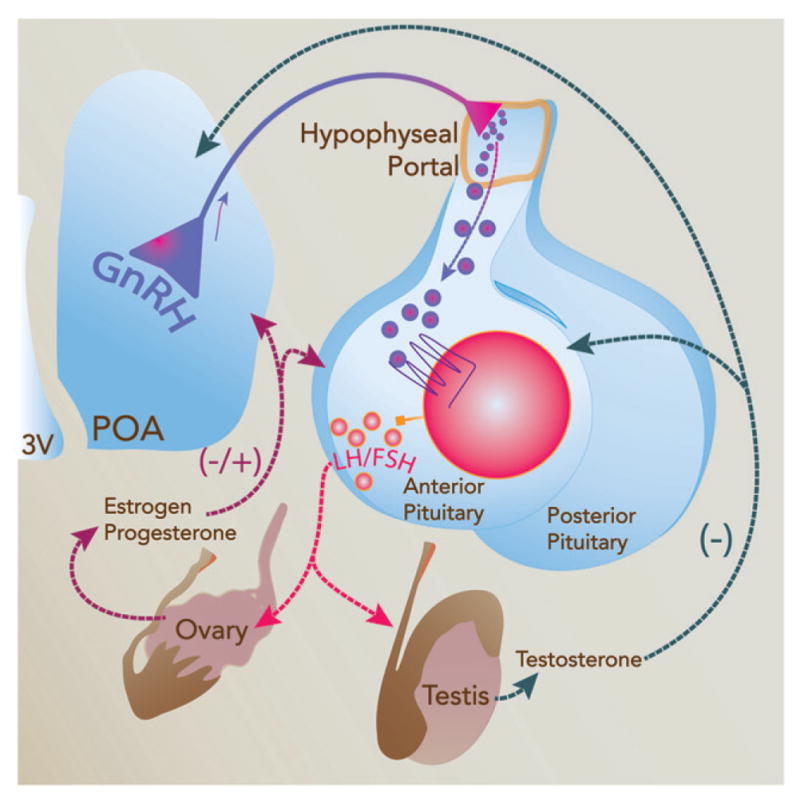
Schematic diagram showing the hypothalamic–pituitary–gonadal (HPG) axis and factors involved in its regulation. Hypophysiotropic neurons in the rodent hypothalamus secrete gonadotropin-releasing hormone (GnRH) into the hypothalamo-hypophyseal portal vasculature at the median eminence, where it is carried to the anterior pituitary. Once in the anterior pituitary, GnRH stimulates gonadotroph cells to synthesize and secrete luteinizing hormone (LH) and follicular stimulating hormone (FSH). LH and FSH act on the ovary and testis to regulate steroidogenesis and gametogenesis. Once released, steroid hormones signal through the anterior pituitary and steroid-sensitive hypothalamic neurons to feedback upon the HPG axis to regulate its activity and to other brain areas thereby controlling reproductive behaviors and functions, including HPA axis activity.
Gonadotropin releasing hormone (GnRH)
GnRH, independently discovered by Andrew Schally and Roger Guillemin and coworkers over 45 years ago (Burgus et al., 1972; Schally, Nair, Redding, & Arimura, 1971), is synthesized and secreted from a subset of neurons in the rostral forebrain of rodents (Jennes & Conn, 1994). Hypothalamic GnRH neurons are specialized neurosecretory cells that release GnRH into the hypothalamo-hypophyseal portal vasculature in a regular pulsatile fashion. GnRH acts on the pituitary to stimulate the secretion of the gonadotropins, LH and FSH, which are also secreted in lock step with the pulsatile release of GnRH from the hypothalamus.
GnRH neurons have a very curious lifespan, arising in the nasal placode in early development and then migrating into the brain along the vomeronasal nerve to eventually take up their adult location in the hypothalamus (Schwanzel-Fukuda & Pfaff, 1989; Tobet & Schwarting, 2006; Wray, Nieburgs, & Elkabes, 1989). In the rodent, GnRH neurons are located in a swath from the medial septum to the medial pre-optic area, whereas in the human and non-human primate they are found further caudal in the medial basal hypothalamus (Rance, Young, & McMullen, 1994; Witkin, Paden, & Silverman, 1982). From there, GnRH neurons extend axons to the hypophyseal portal vasculature. A comprehensive review of the development of GnRH neurons can be found in Wray (2010).
In the rodent, hypophysiotropic GnRH neurons project to the median eminence where they release GnRH in a regular pulsatile manner (Bakker, Rubin, & Baum, 1999; Herbison, 2016; Jimenez-Linan, Rubin, & King, 1997; Silverman, Kokoris, & Gibson, 1988). The organization of these pulses is critical for puberty and reproduction (Herbison, 2016). The activity of GnRH is regulated by neurotransmitters, steroid hormones, and growth factors (Watanabe, Fukuda, & Nabekura, 2014). GnRH pulses are required for reproductive functions in both sexes, and the importance of these pulses in driving reproduction has been described in primates and humans (Tsutsumi & Webster, 2009). In women with hypogonadotrophic hypogonadism, regular ovulatory cycles can be reinstated by the timed pulsatile infusion of GnRH but not by similar levels of GnRH delivered in a constant fashion (Tsutsumi & Webster, 2009). Moreover, studies have demonstrated that slow release of GnRH can selectively drive the synthesis of FSH-β in the anterior pituitary gland, whereas fast pulses of GnRH selectively drive LH-β synthesis (Burger, Dalkin, Aylor, Haisenleder, & Marshall, 2002; Haisenleder, Dalkin, Ortolano, Marshall, & Shupnik, 1991; Kaiser, Jakubowiak, Steinberger, & Chin, 1997).
GnRH action at the anterior pituitary
Mediating the effects of GnRH on anterior pituitary gonadotrophs is the GnRH receptor, a seven transmembrane receptor that is intracellularly coupled to Gαq. Stimulation of GnRH receptors on gonadotrophs results in elevations in intracellular calcium, which drives the release of LH and FSH by exocytosis into the general circulation to eventually reach the gonads (Duran-Pasten & Fiordelisio, 2013). Curiously, GnRH receptors have also been described in numerous tissues throughout the body, including the brain (Cheung & Wong, 2008; Wilson et al., 2006), but their functional significance has not been completely determined.
LH and FSH action on steroidogenesis: males
LH and FSH release is critical for the maintenance of spermatogenesis and steroidogenesis in males. LH receptors are present on Leydig cells in the testis, whereas FSH receptors are expressed predominantly by Sertoli cells. LH stimulates the synthesis of testosterone by Leydig cells (Ramaswamy & Weinbauer, 2014), whereas FSH stimulates sperm cell maturation and supports Sertoli cells. Ultimately, the secretion of testosterone is involved in controlling the HPG axis through its negative feedback action on hypothalamic GnRH neurons and anterior pituitary gonadotrophs. This relationship has been demonstrated numerous times by studies using castrated male rats, and other species, where castration results in increased levels of gonadotropins and testosterone replacement drives secretion back to baseline (Damassa, Kobashigawa, Smith, & Davidson, 1976; Tilbrook & Clarke, 1995).
LH and FSH action on steroidogenesis: females
In females, LH and FSH are essential for the production of estrogens and progesterone. In the ovary, LH controls steroidogenesis by acting upon thecal and granulosa cells. During the follicular phase of the human menstrual cycle, LH acts upon thecal cells to increase synthesis of enzymes involved in the production of the androgen, androstenedione. Androstenedione is released from thecal cells and taken up by adjacent granulosa cells, where it is converted to estrone by the actions of aromatase and then to estradiol by the actions of 17β-HSD-1. In granulosa cells, these two enzymes are regulated by FSH binding to the FSH receptor. The net result of this coordinated action of LH and FSH on thecal and granulosa cells of the ovary gives rise to increases in circulating estradiol and supports the two cell – two gonadotropin hypothesis (Stocco, 2001). While estrogens are made by the conversion of androstenedione into estradiol in ovarian granulosa cells, these cells lack the enzymes required to directly metabolize progesterone into the androgenic precursors of estrogens (Doshi & Agarwal, 2013) and must depend on the-cal cells to provide estrogenic precursors. At mid-cycle, elevated LH levels not only are responsible for ovulation, but also drive the luteinization of the follicle and the development of the corpus luteum. Increases in LH receptor in granulosa cells further stimulate enzymes required for the production of progesterone, which can be now be secreted in large amounts (Kaiser, 2011).
Steroid feedback loop
Under basal conditions, estradiol acts through a negative feedback loop to restrict the secretion of GnRH (Figure 2). This long-loop negative feedback is similar to that of many neuroendocrine axes, where the end product, in this case, estradiol or testosterone, acts upon hypothalamic “motorneurons” that drive the neuroendocrine axis. This system helps maintain relatively constant levels of HPG drive and gonadal hormone secretion.
In rodents, estradiol is also known to have a stimulatory effect on GnRH secretion, and this positive feedback action is essential for ovulation in the female (Evans, Dahl, Mauger, & Karsch, 1995; Levine & Ramirez, 1980; Sarkar & Fink, 1980). As a result of follicle growth and the rising titers of estradiol that culminate late on proestrus morning, a surge of LH is initiated in the afternoon, which drives ovulation. This positive feedback response is a sexually differentiated phenomenon, where male rodents are incapable of responding to elevated estradiol with a surge of LH (Corbier, 1985; Gogan, Beattie, Hery, Laplante, & Kordon, 1980; Olster & Blaustein, 1991). Evidence that positive feedback is sexually differentiated by the neonatal actions of testosterone comes from studies showing that neonatal castration of male rats allows them to respond to estradiol in adulthood with an LH surge (Handa, Corbier, Shryne, Schoonmaker, & Gorski, 1985). Conversely, estrogen or testosterone treatment of neonatal females defeminizes the brain such that they can no longer respond to estradiol in adulthood with a surge of LH (Handa, Corbier, Shryne, Schoonmaker, & Gorski, 1985). Thus, sensitivity of the post-pubertal female brain to respond to elevations in estradiol with a surge of LH is organized by the absence of estradiol derived from testosterone in neonatal females.
The site of negative and positive feedback on the HPG axis is still being explored. Although it would be easy to hypothesize that estradiol acts directly on GnRH neurons to limit their activity, this does not appear to be the case. Early studies failed to detect the presence of estrogen receptor in GnRH neurons, giving rise to the hypothesis that estrogen negative feedback was indirect, perhaps through estrogen receptors that were found in neighboring GABAergic neurons (Shivers, Harlan, Morrell, & Pfaff, 1983). Some studies using immunohistochemistry in rat brain have indeed shown the presence of ERβ in GnRH neuron (Butler, Sjoberg, & Coen, 1999; Hrabovszky et al., 2007; Hrabovszky et al., 2001; Kallo, Butler, Barkovics-Kallo, Goubillon, & Coen, 2001; Navarro et al., 2003; Roy, Angelini, & Belsham, 1999). However, a conundrum arises in that ERβ has not been detected in GnRH neurons of mice, yet they still respond to estradiol (Handa, Ogawa, Wang, & Herbison, 2012).
GnRH neurons do not seem to express ERα either, suggesting that another upstream player is involved in the regulation of GnRH (Herbison & Theodosis, 1992). A relatively recent player implicated in the regulation of GnRH was introduced with the discovery of the RFamide, kisspeptin (Lee et al., 1996). Kisspeptin is a hypothalamic peptide encoded by the KiSS 1 gene (Kotani et al., 2001) and it has been found to be a potent stimulus for GnRH secretion (Herbison, 2016). Kisspeptin neurons have been shown to express ERα (Adachi et al., 2007; Clarkson, d’Anglemont de Tassigny, Moreno, Colledge, & Herbison, 2008; Franceschini et al., 2006) and the kisspeptin receptor GPR54 is expressed in GnRH neurons (Clements et al., 2001; Kotani et al., 2001; Muir et al., 2001; Ohtaki et al., 2001).
Kisspeptin neurons have been found in two main brain areas: the arcuate nucleus and the anteroventral periventricular nucleus (Clarkson, d’Anglemont de Tassigny, Colledge, Caraty, & Herbison, 2009; Clarkson & Herbison, 2006). These two populations of kisspeptin neurons are responsible for vastly different responses in GnRH neurons. Moreover, Clarkson and Herbison (2006) detected a female bias in the levels of kisspeptin expression in the anteroventral and pre-optic periventricular nucleus of the mouse hypothalamus (Clarkson & Herbison, 2006). Rising titers of estradiol can activate kisspeptin neurons in the AVPV causing a concomitant activation of GnRH neurons, which apparently drives the pre-ovulatory surge of GnRH from the hypothalamus. In contrast, activation of kisspeptin neurons in the arcuate nucleus has been shown to directly influence the regulation of pulsatile GnRH release (Han et al., 2005; Millar et al., 2010). The first observations indicating that kisspeptin neurons might be involved in the negative feedback regulation by gonadal steroids were seen in gonadectomized rats, where removal of the gonads significantly increased KiSS 1 mRNA levels, and this effect was reversed by replacement with gonadal steroids (Navarro et al., 2004).
In addition to kisspeptin, mRNA levels of neurokinin B are also upregulated by gonadectomy (Sandoval-Guzman, Stalcup, Krajewski, Voytko, & Rance, 2004). Both, neurokinin B and kisspeptin, have now been shown to have stimulatory effects on the release of GnRH and are both co-localized in some neurons of the arcuate nucleus. A third neuropeptide dynorphin, has also been found in arcuate nucleus kisspeptin neurons but has an inhibitory effect on GnRH secretion (Plant, 2015). Thus, it appears to be the interaction of these three players that sculpt the pulsatile patterns of GnRH secretion. While kisspeptin and neurokinin B can increase the frequency of GnRH pulses, secretion of dynorphin will reduce pulse frequency. These data suggest a complexity whereby estradiol can control both positive and negative feedback action. Arcuate nucleus kisspeptin, neurokinin B, and dynorphin co-expressing neurons initiate pulsatile hormone patterns in the absence of estradiol and are inhibited at low levels of estradiol to account for negative feedback, yet AVPV localized kisspeptin neurons are activated by rising titers of estradiol and initiate the pre-ovulatory surge of LH (Wang, DeFazio, & Moenter, 2016).
Steroid metabolites and the control of the HPA axis
Peripheral steroid hormones are able to cross the brain-blood barrier, due to their lipophilic nature, and can be converted to biologically active metabolites within the brain through actions of several enzymes such as aromatase, 3β-HSD, and 5α-reductase (Lephart, Lund, & Horvath, 2001). These metabolites are thought to regulate cell function by interacting with classical steroid hormone receptors. For example, the effects of testosterone on the brain are often controlled by the intra-cellular conversion of testosterone to estradiol by the aromatase enzyme or to DHT by the 5α-reductase enzyme (Arevalo, Azcoitia, & Garcia-Segura, 2015). Inhibition of 5α-reductase using finasteride abolishes the inhibitory effects of testosterone on the HPA axis in male rats (Handa, Kudwa, Donner, McGivern, & Brown, 2013). 5α-Reductase also converts progesterone and glucocorticoids into their respective metabolites in the brain (Celotti, Negri-Cesi, & Poletti, 1997; Lephart, 1996; Lephart, Lund, & Horvath, 2001). The non-aromatizeable androgen, DHT, may also be further metabolized to the estrogen-like androgen, 3β-diol, via 3β-HSD and then metabolized by the cytochrome P450 enzyme CYP7B1 (Handa, Weiser, & Zuloaga, 2009a). 3β-diol acts by binding and activating estrogen receptor β, rather than an androgen receptor (Weihua et al., 2001). As mentioned in previous sections, these metabolites have regulatory effects on the HPA axis (Figure 3) potentially through the regulation of the Ot or Avp promoter (Hiroi et al., 2013; Pak, Chung, Hinds, & Handa, 2007).
Figure 3.
Schematic diagram demonstrating the reciprocal interaction between the HPA and HPG axes. Panel A: Hormones of the HPG axis are involved in the regulation of the HPA axis at different levels, as delineated by dotted lines. Both axes are influenced by upstream regulatory centers. In the case of the HPA axis, most of these centers release GABA either directly into the PVN or in its immediate periphery. In the case of the HPG axis, upstream centers release kisspeptin to regulate GnRH activity. Panel B: Hormones from the HPA axis are involved in the regulation of the HPG axis at different levels, as delineated by dotted lines. Abbreviations: PVN: paraventricular nucleus of the hypothalamus; AVP: arginine vasopressin; CRH: corticotropin releasing hormone; ACTH: adrenocorticotropic hormone; CORT: corticosterone; POA: pre-optic area; OVLT: organum vasculosum lamina terminalis; GnRH: gonadotropin releasing hormone; LH: luteinizing hormone; FSH: follicle stimulating hormone; E: estrogen; P: progesterone; T: testosterone; DHT: dihydrotestosterone.
Interestingly, the mRNA transcripts levels of aromatase, 5α-reductase, and CYP7B1 are highly present in the PVN, indicating their potential role in the local metabolism of steroids and further regulation of the HPA axis (Lund, Hinds, & Handa, 2006). The presence of these enzymes in stress-regulated brain regions, and their regulatory potential, raises questions regarding the classical view of estrogen modulation of the HPA axis. For example, testosterone in males may work through estrogen receptors, rather than androgen receptors, by its aromatization to estradiol. Moreover, testosterone can be further converted into 3β-diol and thereby act through ERβ to reduce the gain of the HPA axis (Figure 3). Correspondingly, if testosterone is converted into estradiol, both ERs could be activated due to the indiscriminative binding properties of estradiol to both ERs. Since ERs can have opposing effects on HPA activity (ERβ decreases, while ERα increases HPA axis gain), then the gain of the HPA axis in males could be dependent upon the enzymes levels that are present. Moreover, additional regulation could be provided by two other factors. First, the quantity of ERs present in a particular brain region, and, second, the ratio of ERα vs. ERβ at a particular time point. Physiological fluctuations in the ERα: ERβ ratio could dictate how the HPA axis would respond to a stressor. However, this possibility has not been explored in relation to the HPA axis.
Some of the enzymes involved in the local metabolism of estrogens and androgens in the brain are sexually dimorphic. One of the most widely studied of these is aromatase, whose expression levels are much higher in the male stria terminalis, paraventricular pre-optic area, ventromedial nucleus, pre-optic nucleus, and anterior nucleus of the hypothalamus (Roselli, 1991). Also, aromatization of testosterone into estrogen is known to trigger neurite growth and neuronal plasticity in sexually dimorphic brain regions, including the sexually dimorphic nucleus of the rat pre-optic area (Davis, Popper, & Gorski, 1996; Davis, Shryne, & Gorski, 1996). Brain-metabolized estradiol also has a strong influence on stress, reproduction, learning, and memory, as well as sexual behaviors (Blomqvist, 2000; Fink, Sumner, McQueen, Wilson, & Rosie, 1998).
As discussed here, internal cues are critical for the regulation of the HPG axis. However, external signals, like stress, are well known to affect this axis also. Thus, whether through peripheral hormones acting centrally, or metabolism to other steroids through the major metabolic pathways, a mutual relationship exists between the HPA and HPG axes for allo-static regulation and species survival.
Gonadal steroid receptors as regulators of gene transcription
Upon reaching their target cells, steroid hormones readily diffuse through the cell membrane and interact with cytoplasmic or nuclear receptors. The cascade of signaling events triggered by gonadal steroids is regulated by the binding of the hormone to its cognate receptor in a tissue- and region-specific manner. Binding of the steroid hormones, such as corticosterone, to the unliganded steroid receptor induces a conformational change and disassociation from chaperone proteins such as heat shock proteins (HSP90). This modification leads to dimerization of the steroid hormone receptor complex, followed by translocation of the steroid hormone receptor to the target DNA. The steroid hormone receptor complex is accessible to DNA regulatory regions, which contain specific hormone response elements. These hormone response elements have at their core, a palindromic sequence of nucleotides that can bind the hormone receptor dimer. Coactivators or co-regulatory proteins, such as p160 and p300 are also recruited to uncoil the inactive DNA from histone proteins, exposing regulatory regions and transcriptional initiation sites of target genes. Transcription of target genes is initiated through activation of the pre-initiation complex (Beato, 1989; Beato & Klug, 2000; Tyagi et al., 2000). In addition to this classic hormone response element dependent-regulation of gene transcription, steroid receptors can also interact with other transcriptions factor proteins, such as Fos and Jun, that work through other DNA elements such as the AP-1 response element, to regulate gene transcription [for GR and ER actions review see Weikum, Knuesel, Ortlund, & Yamamoto (2017) and Paech et al. (1997) respectively].
Finally, steroid hormones can affect cell function through their interaction with membrane-bound proteins, such as GPR30, also known as GPER [for review, see Prossnitz & Barton (2011)]. Steroid hormone actions through these membrane-bound steroid receptors have rapid effects and involve the alteration in membrane potential and second messenger pathways including extracellular regulate kinase, phospholipase C, Ca2+, mitogen-activated protein, nitric oxide protein kinase A, and protein kinase B. Alternatively, evidence also exists for steroid receptors being localized in caveolae to modulate neurotransmitter receptors, such as mGlu receptors (Meitzen & Mermelstein, 2011). For review of the rapid effects of glucocorticoids see Jiang, Liu, & Tasker (2014).
Sexual differentiation of the rodent brain
The study of sex differences in the brain has unveiled similarities and dissimilarities in the mechanism of neuronal development between sexes (Amateau & McCarthy, 2004; McCarthy & Arnold, 2011; Schwarz, Liang, Thompson, & McCarthy, 2008). Steroid hormones have significant effects on the developing brain and are known to program adult functions required for sex-specific reproductive behaviors. Many behavioral sex differences observed in the adult are the consequences of sex-specific exposure to steroid hormones during critical windows of development. In rodents, male-typical reproductive behaviors, such as mounting, and female-typical behaviors, such as lordosis, are some behaviors organized by gonadal steroid hormones. In the rodent, the absence of testosterone during a critical perinatal period results in the expression of the default program of the brain, which is female-like (McCarthy & Arnold, 2011), whereas perinatal treatment of females with testosterone will defeminize the brain and prevent the expression of female reproductive behaviors in adulthood.
The origin of sexual differences in the brain starts even before gonadal hormones are detected in the plasma (Arnold, 1996; Wade & Arnold, 1996). These changes are thought to begin when genes on the sex chromosomes (X and Y) determine the type of internal and external morphology to be developed, and hence the level of hormones to be present in the fetus. Questions regarding the effects of X and Y gene imbalance have been explored over the past decade, and the data suggests that X and Y gene dose discrepancies account for several sex-biased phenotypes in the brain and periphery (Arnold, 2004; Arnold et al., 2016; Burgoyne et al., 1995). The study of direct sex chromosomes effects in brain sexual differentiation, however, is challenging, mainly because chromosomal manipulation could lead to confounding changes in gonadal steroid levels. Development of mouse models that bypass this issue has been used to further advance our understanding of how sex chromosomes work to influence development of the brain and behavior. For a detailed review on the direct influence of sex chromosomes on brain sexual differentiation see Arnold (2017) and Burgoyne and Arnold (2016).
Sexual differentiation of the HPA axis
The sexually dimorphic behavioral and physiological responses to stress are evident from pre-pubertal stages through adulthood. For instance, basal and stress-induced CORT have been shown to be greater in female than in male rodents (Critchlow, Liebelt, Bar-Sela, Mountcastle, & Lipscomb, 1963; Handa, Burgess, Kerr, & O’Keefe, 1994; Kitay, 1961). Furthermore, these sex-dependent patterns are also observed in the activation of PVN neurons controlling HPA axis reactivity following stress (Larkin, Binks, Li, & Selvage, 2010; Seale, Wood, Atkinson, Harbuz, & Lightman, 2004; Viau, Bingham, Davis, Lee, & Wong, 2005). These responses are thought to be delineated in part by gonadal steroids acting during pre-and perinatal stages as well as at puberty (McCormick & Mathews, 2007). For example, removal of endogenous gonadal hormones by castration of male rats during the peri-natal stage causes a female pattern of ACTH and CORT response to stress during adulthood (McCormick, Furey, Child, Sawyer, & Donohue, 1998).
In line with classical mechanisms for sexual differentiation of the rodent brain, the effects of neonatal gonadectomy on male HPA axis function can be reversed by testosterone treatment during the perinatal period (Bingham & Viau, 2008). Moreover, sex differences in the pulsatile pattern of CORT secretion have been identified by the Lightman group, where females have a higher amplitude and frequency of CORT pulses than males (Lightman & Conway-Campbell, 2010). Interestingly, the CORT pulsatile pattern of adult female rats can be masculinized by treatment with testosterone 24 h after birth, resulting in CORT pulse patterns in adulthood that are similar to those observed in males (Seale, Wood, Atkinson, Harbuz, & Lightman, 2005). On the other hand, aromatization blockade in males immediately after birth results in an augmented CORT and ACTH response to stress in adulthood, similar to that of females (Bingham, Wang, Innala, & Viau, 2012), and akin to that observed following perinatal castration. Thus, these studies further demonstrate the importance of testosterone and its metabolites in the sexual differentiation of the HPA axis.
Summary and conclusion
To increase survival and reproductive success, the HPA and the HPG axes work together to fine-tune each other thereby integrating environmental, psychological, reproductive, and genetic factors. The HPG and HPA axes both exhibit striking sex differences, which are organized and activated by dynamic changes in sex and stress hormones across the lifespan. It can be speculated that, if applicable to humans, the developmental programs determining sex differences in the HPA axis may influence vulnerability to develop neuropathological disorders triggered by stress in adulthood. To maintain homeostasis, the neuroendocrine system continuously monitors the levels of gonadal steroids using estrogen and androgen receptors found in the hypothalamus. Hypothalamic neurons expressing gonadal steroid receptors are of great importance for the proper regulation of the HPA and HPG axes, and thus, the composition of these cells requires further investigation. Dysregulation of either or both of these axes can result in compromised responses to stressful life events. The fluctuations in estradiol and progesterone present across the estrous cycle of adult rodents are arguably responsible for most activational effects in the female brain, thereby regulating the behavioral and physiological repertoire to stress seen during adulthood. In sum, the HPG and HPA axes work in a parallel, and in a carefully choreographed fashion, to successfully maintain species survival and perhaps minimize allostatic load. Although steady advancements have been achieved in the last few years, there are still questions remaining to explore. First, although much has been revealed as to how individual steroid hormones can regulate neuropeptide regulators of the HPA axis from a molecular perspective, the interactions between steroid hormone receptors on specific neuropeptide promoters has not been described and would allow us to better understand the molecular perspective by which specific hormones interact to have distinct and sex specific effects across cell types. Second, elucidating the co-regulatory proteins involved in steroidal regulation of the neuropeptide genes would shed light on specific cell types and intracellular messenger pathways, or brain-specific effects.
Further investigation of how the neonatal hormone milieu influences adult HPA axis function may allow us to understand epigenetic programing of gene expression associated with the HPA axis. It is now known that hormones have specific actions in given brain regions. Thus, the localization, expression levels, and activity of steroid hormone receptors may further help explain why certain hormones and their metabolites have sex-specific effects. On the same note, understanding steroid hormone metabolism and subsequent pre-receptor modulation of the HPA axis is still underway.
Finally, throughout this article we focused on the HPA axis from the perspective of the hypothalamus and its downstream targets. Much less in known about the upstream regulators of the HPA axis. For instance, how do decisional making processing centers, like the prefrontal cortex, interact, either directly or indirectly, with upstream targets of the PVN to regulate the activation of the HPA and HPG axes, and how do extra-hypothalamic regions, such as the BnST help to further coordinate the neuroendocrine stress responses and its response to gonadal steroid hormones? Future research probing these areas will provide new and exciting mechanisms and avenues for exploration of sex differences and steroidal modulation of stress responses.
Acknowledgments
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases [DK105826].
Footnotes
Disclosure statement
The authors report no conflicts of interest.
References
- 1.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, … Maeda K. Involvement of anteroventral periventricular meta-stin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. Journal of Reproduction and Development. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 2.Akana SF, Cascio CS, Du JZ, Levin N, Dallman MF. Reset of feedback in the adrenocortical system: An apparent shift in sensitivity of adrenocorticotropin to inhibition by corticosterone between morning and evening. Endocrinology. 1986;119:2325–2332. doi: 10.1210/endo-119-5-2325. [DOI] [PubMed] [Google Scholar]
- 3.Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nature Neuroscience. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- 4.Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nature Reviews. Neuroscience. 2015;16:17–29. doi: 10.1038/nrn3856. [DOI] [PubMed] [Google Scholar]
- 5.Arnold AP. Genetically triggered sexual differentiation of brain and behavior. Hormones and Behavior. 1996;30:495–505. doi: 10.1006/hbeh.1996.0053. [DOI] [PubMed] [Google Scholar]
- 6.Arnold AP. Sex chromosomes and brain gender. Nature Reviews. Neuroscience. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- 7.Arnold AP. A general theory of sexual differentiation. Journal of Neuroscience Research. 2017;95:291–300. doi: 10.1002/jnr.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold AP, Reue K, Eghbali M, Vilain E, Chen X, Ghahramani N, … Williams-Burris SM. The importance of having two X chromosomes. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2016;371:20150113. doi: 10.1098/rstb.2015.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakker J, Rubin BS, Baum MJ. Changes in mediobasal hypothalamic gonadotropin-releasing hormone messenger ribonucleic acid levels induced by mating or ovariectomy in a reflex ovulator, the ferret. Endocrinology. 1999;140:595–602. doi: 10.1210/endo.140.2.6519. [DOI] [PubMed] [Google Scholar]
- 10.Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nature Neuroscience. 2015;18:1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bale TL, Vale WW. CRF and CRF receptors: Role in stress responsivity and other behaviors. Annual Review of Pharmacology and Toxicology. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 12.Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 13.Beato M, Klug J. Steroid hormone receptors: An update. Human Reproduction Update. 2000;6:225–236. doi: 10.1093/humupd/6.3.225. [DOI] [PubMed] [Google Scholar]
- 14.Bekker MH, van Mens-Verhulst J. Anxiety disorders: Sex differences in prevalence, degree, and background, but gender-neutral treatment. Gender Medicine. 2007;4(Suppl B):S178–S193. doi: 10.1016/s1550-8579(07)80057-x. [DOI] [PubMed] [Google Scholar]
- 15.Beuschlein F, Fassnacht M, Klink A, Allolio B, Reincke M. ACTH-receptor expression, regulation and role in adrenocortial tumor formation. European Journal of Endocrinology. 2001;144:199–206. doi: 10.1530/eje.0.1440199. [DOI] [PubMed] [Google Scholar]
- 16.Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, Hahn JD, … Dong HW. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: A study of immunostaining and multiple fluorescent tract tracing. The Journal of Comparative Neurology. 2012;520:6–33. doi: 10.1002/cne.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bingham B, Viau V. Neonatal gonadectomy and adult testosterone replacement suggest an involvement of limbic arginine vasopressin and androgen receptors in the organization of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2008;149:3581–3591. doi: 10.1210/en.2007-1796. [DOI] [PubMed] [Google Scholar]
- 18.Bingham B, Wang NX, Innala L, Viau V. Postnatal aromatase blockade increases c-fos mRNA responses to acute restraint stress in adult male rats. Endocrinology. 2012;153:1603–1608. doi: 10.1210/en.2011-1749. [DOI] [PubMed] [Google Scholar]
- 19.Blomqvist A. Sex hormones and pain: A new role for brain aromatase? The Journal of Comparative Neurology. 2000;423:549–551. [PubMed] [Google Scholar]
- 20.Boston BA, Cone RD. Characterization of melanocortin receptor subtype expression in murine adipose tissues and in the 3T3-L1 cell line. Endocrinology. 1996;137:2043–2050. doi: 10.1210/endo.137.5.8612546. [DOI] [PubMed] [Google Scholar]
- 21.Bradbury MJ, Akana SF, Cascio CS, Levin N, Jacobson L, Dallman MF. Regulation of basal ACTH secretion by corticosterone is mediated by both type I (MR) and type II (GR) receptors in rat brain. The Journal of Steroid Biochemistry and Molecular Biology. 1991;40:133–142. doi: 10.1016/0960-0760(91)90176-6. [DOI] [PubMed] [Google Scholar]
- 22.Burger LL, Dalkin AC, Aylor KW, Haisenleder DJ, Marshall JC. GnRH pulse frequency modulation of gonadotropin subunit gene transcription in normal gonadotropes-assessment by primary transcript assay provides evidence for roles of GnRH and follistatin. Endocrinology. 2002;143:3243–3249. doi: 10.1210/en.2002-220216. [DOI] [PubMed] [Google Scholar]
- 23.Burgoyne PS, Arnold AP. A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues. Biology of Sex Differences. 2016;7:68. doi: 10.1186/s13293-016-0115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgoyne PS, Thornhill AR, Boudrean SK, Darling SM, Bishop CE, Evans EP. The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1995;350:253–260. doi: 10.1098/rstb.1995.0159. discussion 260–261. [DOI] [PubMed] [Google Scholar]
- 25.Burgus R, Butcher M, Amoss M, Ling N, Monahan M, Rivier J, … Guillemin R. Primary structure of the ovine hypothalamic luteinizing hormone-releasing factor (LRF) (LH-hypothalamus-LRF-gas chromatography-mass spectrometry-decapeptide-Edman degradation) Proceedings of the National Academy of Sciences of the United States of America. 1972;69:278–282. doi: 10.1073/pnas.69.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler JA, Sjoberg M, Coen CW. Evidence for oestrogen receptor alpha-immunoreactivity in gonadotrophin-releasing hormone-expressing neurones. Journal of Neuroendocrinology. 1999;11:331–335. doi: 10.1046/j.1365-2826.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- 27.Celotti F, Negri-Cesi P, Poletti A. Steroid metabolism in the mammalian brain: 5alpha-reduction and aromatization. Brain Research Bulletin. 1997;44:365–375. doi: 10.1016/s0361-9230(97)00216-5. [DOI] [PubMed] [Google Scholar]
- 28.Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annual Review of Physiology. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 29.Cheung LW, Wong AS. Gonadotropin-releasing hormone: GnRH receptor signaling in extrapituitary tissues. The FEBS Journal. 2008;275:5479–5495. doi: 10.1111/j.1742-4658.2008.06677.x. [DOI] [PubMed] [Google Scholar]
- 30.Clarkson J, D’anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. Journal of Neuroendocrinology. 2009;21:673–682. doi: 10.1111/j.1365-2826.2009.01892.x. [DOI] [PubMed] [Google Scholar]
- 31.Clarkson J, D’anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. Journal of Neuroscience. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clements MK, McDonald TP, Wang R, Xie G, O’dowd BF, George SR, … Liu Q. FMRFamide-related neuropeptides are agonists of the orphan G-protein-coupled receptor GPR54. Biochemical and Biophysics Research Communication. 2001;284:1189–1193. doi: 10.1006/bbrc.2001.5098. [DOI] [PubMed] [Google Scholar]
- 34.Corbier P. Sexual differentiation of positive feedback: Effect of hour of castration at birth on estradiol-induced luteinizing hormone secretion in immature male rats. Endocrinology. 1985;116:142–147. doi: 10.1210/endo-116-1-142. [DOI] [PubMed] [Google Scholar]
- 35.Cortright DN, Nicoletti A, Seasholtz AF. Molecular and biochemical characterization of the mouse brain corticotropin-releasing hormone-binding protein. Molecular and Cellular Endocrinology. 1995;111:147–157. doi: 10.1016/0303-7207(95)03558-o. [DOI] [PubMed] [Google Scholar]
- 36.Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. The American Journal of Physiology. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham ET, Jr, Sawchenko PE. Reflex control of magno-cellular vasopressin and oxytocin secretion. Trends in Neurosciences. 1991;14:406–411. doi: 10.1016/0166-2236(91)90032-p. [DOI] [PubMed] [Google Scholar]
- 38.Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, … Rainnie DG. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36:1312–1326. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH secretion: Variations on a theme of B. Recent Progress in Hormone Research. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- 40.Damassa DA, Kobashigawa D, Smith ER, Davidson JM. Negative feedback control of LH by testosterone: A quantitative study in male rats. Endocrinology. 1976;99:736–742. doi: 10.1210/endo-99-3-736. [DOI] [PubMed] [Google Scholar]
- 41.Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the pre-optic area. Brain Research. 1996;734:10–18. [PubMed] [Google Scholar]
- 42.Davis EC, Shryne JE, Gorski RA. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology. 1996;63:142–148. doi: 10.1159/000126950. [DOI] [PubMed] [Google Scholar]
- 43.de Kloet ER, Joels M, Holsboer F. Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 44.De Kloet ER, Veldhuis HD, Wagenaars JL, Bergink EW. Relative binding affinity of steroids for the corticosterone receptor system in rat hippocampus. Journal of Steroid Biochemistry. 1984;21:173–178. doi: 10.1016/0022-4731(84)90380-7. [DOI] [PubMed] [Google Scholar]
- 45.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain cortico-steroid receptor balance in health and disease. Endocrine Reviews. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 46.Doshi SB, Agarwal A. The role of oxidative stress in menopause. Journal of Mid-Life Health. 2013;4:140–146. doi: 10.4103/0976-7800.118990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: Binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. The Journal of Clinical Endocrinology & Metabolism. 1981;53:58–68. doi: 10.1210/jcem-53-1-58. [DOI] [PubMed] [Google Scholar]
- 49.Duran-Pasten ML, Fiordelisio T. GnRH-induced Ca(2+) signaling patterns and gonadotropin secretion in pituitary gonadotrophs. Functional adaptations to both ordinary and extraordinary physiological demands. Frontiers in Endocrinology. 2013;4:127. doi: 10.3389/fendo.2013.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans NP, Dahl GE, Mauger D, Karsch FJ. Estradiol induces both qualitative and quantitative changes in the pattern of gonadotropin-releasing hormone secretion during the presurge period in the ewe. Endocrinology. 1995;136:1603–1609. doi: 10.1210/endo.136.4.7895670. [DOI] [PubMed] [Google Scholar]
- 51.Evuarherhe O, Leggett JD, Waite EJ, Kershaw YM, Atkinson HC, Lightman SL. Organizational role for pubertal androgens on adult hypothalamic-pituitary-adrenal sensitivity to testosterone in the male rat. The Journal of Physiology. 2009;587(Pt 12):2977–2985. doi: 10.1113/jphysiol.2008.168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus – a potential target for integrative treatment of autonomic dysfunction. Expert Opinion on Therapeutic Targets. 2008;12:717–727. doi: 10.1517/14728222.12.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Figueiredo HF, Dolgas CM, Herman JP. Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology. 2002;143:2534–2540. doi: 10.1210/endo.143.7.8888. [DOI] [PubMed] [Google Scholar]
- 54.Fink G, Sumner BE, McQueen JK, Wilson H, Rosie R. Sex steroid control of mood, mental state and memory. Clinical and Experimental Pharmacology & Physiology. 1998;25:764–775. doi: 10.1111/j.1440-1681.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 55.Forti FL, Dias MH, Armelin HA. ACTH receptor: Ectopic expression, activity and signaling. Molecular and Cellular Biochemistry. 2006;293:147–160. doi: 10.1007/s11010-006-9237-0. [DOI] [PubMed] [Google Scholar]
- 56.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neuroscience Letters. 2006;401:225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 57.Fuzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nature Communications. 2016;7:11937. doi: 10.1038/ncomms11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gala RR, Westphal U. Corticosteroid-binding globulin in the rat: Studies on the sex difference. Endocrinology. 1965;77:841–851. doi: 10.1210/endo-77-5-841. [DOI] [PubMed] [Google Scholar]
- 59.Gala RR, Westphal U. Further studies on the corticosteroid-binding globulin in the rat: Proposed endocrine control. Endocrinology. 1966;79:67–76. doi: 10.1210/endo-79-1-67. [DOI] [PubMed] [Google Scholar]
- 60.Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: Axonal projections to the brainstem. The Journal of Comparative Neurology. 2010;518:1460–1499. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gogan F, Beattie IA, Hery M, Laplante E, Kordon D. Effect of neonatal administration of steroids or gonadectomy upon oestradiol-induced luteinizing hormone release in rats of both sexes. The Journal of Endocrinology. 1980;85:69–74. doi: 10.1677/joe.0.0850069. [DOI] [PubMed] [Google Scholar]
- 62.Gurdjian ES. The diencephalon of the albino rat: Studies on the brain of the rat. The Journal of Comparative Neurology. 1927;43:1–114. [Google Scholar]
- 63.Haisenleder DJ, Dalkin AC, Ortolano GA, Marshall JC, Shupnik MA. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: Evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology. 1991;128:509–517. doi: 10.1210/endo-128-1-509. [DOI] [PubMed] [Google Scholar]
- 64.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, … Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. The Journal of Neuroscience. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Handa RJ, Burgess LH, Kerr JE, O’keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Hormones and Behavior. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 66.Handa RJ, Corbier P, Shryne JE, Schoonmaker JN, Gorski RA. Differential effects of the perinatal steroid environment on three sexually dimorphic parameters of the rat brain. Biology of Reproduction. 1985;32:855–864. doi: 10.1095/biolreprod32.4.855. [DOI] [PubMed] [Google Scholar]
- 67.Handa RJ, Kudwa AE, Donner NC, McGivern RF, Brown R. Central 5-alpha reduction of testosterone is required for testosterone’s inhibition of the hypothalamo-pituitary-adrenal axis response to restraint stress in adult male rats. Brain Research. 2013;1529:74–82. doi: 10.1016/j.brainres.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Handa RJ, Nunley K, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiology & Behavior. 1994;55:117–124. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 69.Handa RJ, Ogawa S, Wang JM, Herbison AE. Roles for oestrogen receptor β in adult brain function. Journal of Neuroendocrinology. 2012;24:160–173. doi: 10.1111/j.1365-2826.2011.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5alpha-androstane-3beta,17beta-diol, in modulating oestrogen receptor beta-mediated regulation of hormonal stress reactivity. Journal of Neuroendocrinology. 2009a;21:351–358. doi: 10.1111/j.1365-2826.2009.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5alpha-androstane-3beta,17beta-diol, in modulating oestrogen receptor beta-mediated regulation of hormonal stress reactivity. Journal of Neuroendocrinology. 2009b;21:351–358. doi: 10.1111/j.1365-2826.2009.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Henckens MJ, Printz Y, Shamgar U, Dine J, Lebow M, Drori Y, … Chen A. CRF receptor type 2 neurons in the posterior bed nucleus of the stria terminalis critically contribute to stress recovery. Molecular Psychiatry. 2016 doi: 10.1038/mp.2016.133. Advance online publication. [DOI] [PubMed] [Google Scholar]
- 73.Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nature Reviews. Endocrinology. 2016;12:452–466. doi: 10.1038/nrendo.2016.70. [DOI] [PubMed] [Google Scholar]
- 74.Herbison AE, Theodosis DT. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience. 1992;50:283–298. doi: 10.1016/0306-4522(92)90423-y. [DOI] [PubMed] [Google Scholar]
- 75.Herman JP. In situ hybridization analysis of vasopressin gene transcription in the paraventricular and supraoptic nuclei of the rat: Regulation by stress and glucocorticoids. The Journal of Comparative Neurology. 1995;363:15–27. doi: 10.1002/cne.903630103. [DOI] [PubMed] [Google Scholar]
- 76.Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, … Myers B. Regulation of the hypothalamic-pituitary-adreno-cortical stress response. Comprehensive Physiology. 2016;6:603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hiroi R, Lacagnina AF, Hinds LR, Carbone DG, Uht RM, Handa RJ. The androgen metabolite, 5alpha-androstane-3beta,17beta-diol (3beta-diol), activates the oxytocin promoter through an estrogen receptor-beta pathway. Endocrinology. 2013;154:1802–1812. doi: 10.1210/en.2012-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hrabovszky E, Kallo I, Steinhauser A, Merchenthaler I, Coen CW, Petersen SL, Liposits Z. Estrogen receptor-beta in oxytocin and vasopressin neurons of the rat and human hypothalamus: Immunocytochemical and in situ hybridization studies. Journal of Comparative Neurology. 2004;473:315–333. doi: 10.1002/cne.20127. [DOI] [PubMed] [Google Scholar]
- 79.Hrabovszky E, Kallo I, Szlavik N, Keller E, Merchenthaler I, Liposits Z. Gonadotropin-releasing hormone neurons express estrogen receptor-beta. The Journal of Clinical Endocrinology and Metabolism. 2007;92:2827–2830. doi: 10.1210/jc.2006-2819. [DOI] [PubMed] [Google Scholar]
- 80.Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- 81.Huang Q, Zhu H, Fischer DF, Zhou JN. An estrogenic effect of 5alpha-androstane-3beta, 17beta-diol on the behavioral response to stress and on CRH regulation. Neuropharmacology. 2008;54:1233–1238. doi: 10.1016/j.neuropharm.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 82.Ishunina TA, Swaab DF. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus: Size changes in relation to age and sex. The Journal of Clinical Endocrinology and Metabolism. 1999;84:4637–4644. doi: 10.1210/jcem.84.12.6187. [DOI] [PubMed] [Google Scholar]
- 83.Jeanneteau F, Lambert W, Ismaili N, Bath K, Lee F, Garabedian M, Chao M. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:1305–1315. doi: 10.1073/pnas.1114122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jennes L, Conn PM. Gonadotropin-releasing hormone and its receptors in rat brain. Frontiers in Neuroendocrinology. 1994;15:51–77. doi: 10.1006/frne.1994.1003. [DOI] [PubMed] [Google Scholar]
- 85.Jiang CL, Liu L, Tasker JG. Why do we need nongenomic glucocorticoid mechanisms? Frontiers in Neuroendocrinology. 2014;35:72–75. doi: 10.1016/j.yfrne.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 86.Jimenez-Linan M, Rubin BS, King JC. Examination of guinea pig luteinizing hormone-releasing hormone gene reveals a unique decapeptide and existence of two transcripts in the brain. Endocrinology. 1997;138:4123–4130. doi: 10.1210/endo.138.10.5454. [DOI] [PubMed] [Google Scholar]
- 87.Kaiser UB. Chapter 7 – Gonadotropin hormones. In: Melmed S, editor. The pituitary. 3. Philadelphia (PA): Elsevier Inc; 2011. pp. 205–260. [Google Scholar]
- 88.Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology. 1997;138:1224–1231. doi: 10.1210/endo.138.3.4968. [DOI] [PubMed] [Google Scholar]
- 89.Kallo I, Butler JA, Barkovics-Kallo M, Goubillon ML, Coen CW. Oestrogen receptor beta-immunoreactivity in gonadotropin releasing hormone-expressing neurones: Regulation by oestrogen. Journal of Neuroendocrinology. 2001;13:741–748. doi: 10.1046/j.1365-2826.2001.00708.x. [DOI] [PubMed] [Google Scholar]
- 90.Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocrine Reviews. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- 91.Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- 92.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, … Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 93.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, … Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. Journal of Biological Chemistry. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 94.Kovacs KJ, Foldes A, Sawchenko PE. Glucocorticoid negative feedback selectively targets vasopressin transcription in parvocellular neurosecretory neurons. Journal of Neuroscience. 2000;20:3843–3852. doi: 10.1523/JNEUROSCI.20-10-03843.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kovacs KJ, Makara GB. Corticosterone and dexamethasone act at different brain sites to inhibit adrenalectomy-induced adrenocorticotropin hypersecretion. Brain Research. 1988;474:205–210. doi: 10.1016/0006-8993(88)90435-0. [DOI] [PubMed] [Google Scholar]
- 96.Kudwa AE, McGivern RF, Handa RJ. Estrogen receptor beta and oxytocin interact to modulate anxiety-like behavior and neuroendocrine stress reactivity in adult male and female rats. Physiological Behavior. 2014;129:287–296. doi: 10.1016/j.physbeh.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. The Journal of Steroid Biochemistry and Molecular Biology. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 98.Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: Anatomical evidence of distinct roles of each subtype. Journal of Neurobiology. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 99.Lalmansingh AS, Uht RM. Estradiol regulates corticotropin-releasing hormone gene (crh) expression in a rapid and phasic manner that parallels estrogen receptor-alpha and -beta recruitment to a 3′,5′-cyclic adenosine 5′-monophosphate regulatory region of the proximal crh promoter. Endocrinology. 2008;149:346–357. doi: 10.1210/en.2007-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Larkin JW, Binks SL, Li Y, Selvage D. The role of oestradiol in sexually dimorphic hypothalamic-pituitary-adrena axis responses to intracerebroventricular ethanol administration in the rat. Journal of Neuroendocrinology. 2010;22:24–32. doi: 10.1111/j.1365-2826.2009.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Le Mevel JC, Abitbol S, Beraud G, Maniey J. Temporal changes in plasma adrenocorticotropin concentration after repeated neurotropic stress in male and female rats. Endocrinology. 1979;105:812–817. doi: 10.1210/endo-105-3-812. [DOI] [PubMed] [Google Scholar]
- 102.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. Journal of National Cancer Institute. 1996;88:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 103.Lefkowitz RJ, Roth J, Pricer W, Pastan I. ACTH receptors in the adrenal: Specific binding of ACTH-125I and its relation to adenyl cyclase. Proceedings of the National Academy of Sciences of the United States of America. 1970;65:745–752. doi: 10.1073/pnas.65.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lefstin JA, Yamamoto KR. Allosteric effects of DNA on transcriptional regulators. Nature. 1998;392:885–888. doi: 10.1038/31860. [DOI] [PubMed] [Google Scholar]
- 105.Lehoux JG, Fleury A, Ducharme L. The acute and chronic effects of adrenocorticotropin on the levels of messenger ribonucleic acid and protein of steroidogenic enzymes in rat adrenal in vivo. Endocrinology. 1998;139:3913–3922. doi: 10.1210/endo.139.9.6196. [DOI] [PubMed] [Google Scholar]
- 106.Leng G, Meddle SL, Douglas AJ. Oxytocin and the maternal brain. Current Opinion in Pharmacology. 2008;8:731–734. doi: 10.1016/j.coph.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 107.Lephart ED. A review of brain aromatase cytochrome P450. Brain Research Brain Research Reviews. 1996;22:1–26. [PubMed] [Google Scholar]
- 108.Lephart ED, Lund TD, Horvath TL. Brain androgen and progesterone metabolizing enzymes: Biosynthesis, distribution and function. Brain Research Brain Research Reviews. 2001;37:25–37. doi: 10.1016/s0165-0173(01)00111-4. [DOI] [PubMed] [Google Scholar]
- 109.Levine AB. Neuroendocrine control of the ovarian cycle of the rat. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill’s Physiology of Reproduction. 4. San Diego (CA): Academic Press; 2015. pp. 1199–1257. [Google Scholar]
- 110.Levine AB, Levine LM, Levine TB. Posttraumatic stress disorder and cardiometabolic disease. Cardiology. 2014;127:1–19. doi: 10.1159/000354910. [DOI] [PubMed] [Google Scholar]
- 111.Levine JE, Ramirez VD. In vivo release of luteinizing hormone-releasing hormone estimated with push-pull cannulae from the mediobasal hypothalami of ovariectomized, steroid-primed rats. Endocrinology. 1980;107:1782–1790. doi: 10.1210/endo-107-6-1782. [DOI] [PubMed] [Google Scholar]
- 112.Lightman SL, Conway-Campbell BL. The crucial role of pulsa-tile activity of the HPA axis for continuous dynamic equilibration. Nature Reviews Neuroscience. 2010;11:710–718. doi: 10.1038/nrn2914. [DOI] [PubMed] [Google Scholar]
- 113.Lovick TA. Estrous cycle and stress: Influence of progesterone on the female brain. Brazilian Journal of Medical and Biological Research. 2012;45:314–320. doi: 10.1590/S0100-879X2012007500044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. Journal of Neuroscience. 2006;26:1448–1456. doi: 10.1523/jneurosci.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lund TD, Munson DJ, Haldy ME, Handa RJ. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. Journal of Neuroendocrinology. 2004;16:272–278. doi: 10.1111/j.0953-8194.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- 116.MacMaster FP, Keshavan M, Mirza Y, Carrey N, Upadhyaya AR, El-Sheikh R, … Rosenberg DR. Development and sexual dimorphism of the pituitary gland. Life Sciences. 2007;80:940–944. doi: 10.1016/j.lfs.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Majzoub JA. Corticotropin-releasing hormone physiology. European Journal of Endocrinology. 2006;155:S71–S76. doi: 10.1530/eje.1.02247. [DOI] [Google Scholar]
- 118.Mantella RC, Vollmer RR, Rinaman L, Li X, Amico JA. Enhanced corticosterone concentrations and attenuated Fos expression in the medial amygdala of female oxytocin knockout mice exposed to psychogenic stress. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2004;287:1497–1504. doi: 10.1152/ajpregu.00387.2004. [DOI] [PubMed] [Google Scholar]
- 119.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nature Neuroscience. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiology & Behavior. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- 121.McClennen SJ, Cortright DN, Seasholtz AF. Regulation of pituitary corticotropin-releasing hormone-binding protein messenger ribonucleic acid levels by restraint stress and adrenalectomy. Endocrinology. 1998;139:4435–4441. doi: 10.1210/endo.139.11.6311. [DOI] [PubMed] [Google Scholar]
- 122.McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM. Neonatal sex hormones have ‘organizational’ effects on the hypothalamic-pituitary-adrenal axis of male rats. Brain Research. Developmental Brain Research. 1998;105:295–307. doi: 10.1016/s0165-3806(97)00155-7. [DOI] [PubMed] [Google Scholar]
- 123.McCormick CM, Mathews IZ. HPA function in adolescence: Role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacology, Biochemistry, and Behavior. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 124.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. Journal of Chemical Neuroanatomy. 2011;42:236–241. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Melmed S. Chapter 1 – pituitary development. In: Melmed S, editor. The pituitary. San Diego: Academic Press; 2011. pp. 3–19. [Google Scholar]
- 127.Meyer EJ, Nenke MA, Rankin W, Lewis JG, Torpy DJ. Corticosteroid-binding globulin: A review of basic and clinical advances. Hormone and Metabolic Research. 2016;48:359–371. doi: 10.1055/s-0042-108071. [DOI] [PubMed] [Google Scholar]
- 128.Millar RP, Roseweir AK, Tello JA, Anderson RA, George JT, Morgan K, Pawson AJ. Kisspeptin antagonists: Unraveling the role of kisspeptin in reproductive physiology. Brain Research. 2010;1364:81–89. doi: 10.1016/j.brainres.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 129.Miller WJ, Suzuki S, Miller LK, Handa R, Uht RM. Estrogen receptor (ER)beta isoforms rather than ERalpha regulate corticotropin-releasing hormone promoter activity through an alternate pathway. Journal of Neuroscience. 2004;24:10628–10635. doi: 10.1523/JNEUROSCI.5540-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mitani F. Functional zonation of the rat adrenal cortex: The development and maintenance. Proceedings of the Japan Academy Series B, Physical and Biological Sciences. 2014;90:163–183. doi: 10.2183/pjab.90.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mitchner NA, Garlick C, Ben-Jonathan N. Cellular distribution and gene regulation of estrogen receptors alpha and beta in the rat pituitary gland. Endocrinology. 1998;139:3976–3983. doi: 10.1210/endo.139.9.6181. [DOI] [PubMed] [Google Scholar]
- 132.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, … Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 133.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, … Harrison DC. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. The Journal of Biological Chemistry. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- 134.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrine Reviews. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 135.Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ. Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Molecular Endocrinology. 2003;17:1792–1804. [PubMed] [Google Scholar]
- 136.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, … Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 137.Neumann ID, Krömer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: Involvement of hypothalamic and limbic brain regions. Regulatory Peptides. 2000;96:31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- 138.Nomura M, Saito J, Ueta Y, Muglia LJ, Pfaff DW, Ogawa S. Enhanced up-regulation of corticotropin-releasing hormone gene expression in response to restraint stress in the hypothalamic paraventricular nucleus of oxytocin gene-deficient male mice. Journal of Neuroendocrinology. 2003;15:1054–1061. doi: 10.1046/j.1365-2826.2003.01095.x. [DOI] [PubMed] [Google Scholar]
- 139.Ochedalski T, Subburaju S, Wynn PC, Aguilera G. Interaction between oestrogen and oxytocin on hypothalamic-pituitary-adrenal axis activity. Journal of Neuroendocrinology. 2007;19:189–197. doi: 10.1111/j.1365-2826.2006.01525.x. [DOI] [PubMed] [Google Scholar]
- 140.Ogura E, Kageyama K, Hanada K, Kasckow J, Suda T. Effects of estradiol on regulation of corticotropin-releasing factor gene and interleukin-6 production via estrogen receptor type beta in hypothalamic 4B cells. Peptides. 2008;29:456–464. doi: 10.1016/j.peptides.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 141.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, … Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 142.Olster DH, Blaustein JD. Progesterone facilitates lordosis, but not LH release, in estradiol pulse-primed male rats. Physiology & Behavior. 1991;50:237–242. doi: 10.1016/0031-9384(91)90526-t. [DOI] [PubMed] [Google Scholar]
- 143.Onaka T. Neural pathways controlling central and peripheral oxytocin release during stress. Journal of Neuroendocrinology. 2004;16:308–312. doi: 10.1111/j.0953-8194.2004.01186.x. [DOI] [PubMed] [Google Scholar]
- 144.Oyola MG, Malysz AM, Mani SK, Handa RJ. Steroid hormone signaling pathways and sex differences in neuroendocrine and behavioral responses to stress. In: Shansky RM, editor. Sex differences in the central nervous system. Philadelphia: Elsevier; 2016. pp. 325–364. [Google Scholar]
- 145.Oyola MG, Thomson MK, Handa AZ, Handa RJ. Distribution and chemical composition of estrogen receptor beta neurons in the paraventricular nucleus of the female and male mouse hypothalamus. Journal of Comparative Neurology. 2017 doi: 10.1002/cne.24295. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 147.Pak TR, Chung WC, Hinds LR, Handa RJ. Estrogen receptor-beta mediates dihydrotestosterone-induced stimulation of the arginine vasopressin promoter in neuronal cells. Endocrinology. 2007;148:3371–3382. doi: 10.1210/en.2007-0086. [DOI] [PubMed] [Google Scholar]
- 148.Pak TR, Chung WC, Hinds LR, Handa RJ. Arginine vasopressin regulation in pre- and postpubertal male rats by the androgen metabolite 3beta-diol. American journal of physiology Endocrinology and metabolism. 2009;296:E1409–E1413. doi: 10.1152/ajpendo.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ. The androgen metabolite, 5alpha-androstane-3beta, 17beta-diol, is a potent modulator of estrogen receptor-beta1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146:147–155. doi: 10.1210/en.2004-0871. [DOI] [PubMed] [Google Scholar]
- 150.Perez-Castro C, Renner U, Haedo MR, Stalla GK, Arzt E. Cellular and molecular specificity of pituitary gland physiology. Physiological Reviews. 2012;92:1–38. doi: 10.1152/physrev.00003.2011. [DOI] [PubMed] [Google Scholar]
- 151.Plant TM. 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-pituitary-gonadal axis. The Journal of Endocrinology. 2015;226:T41–T54. doi: 10.1530/JOE-15-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Price RH, Jr, Butler CA, Webb P, Uht R, Kushner P, Handa RJ. A splice variant of estrogen receptor beta missing exon 3 displays altered subnuclear localization and capacity for transcriptional activation. Endocrinology. 2001;142:2039–2049. doi: 10.1210/endo.142.5.8130. [DOI] [PubMed] [Google Scholar]
- 153.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nature Reviews. Endocrinology. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ramaswamy S, Weinbauer GF. Endocrine control of spermatogenesis: Role of FSH and LH/testosterone. Spermatogenesis. 2014;4:e996025. doi: 10.1080/21565562.2014.996025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rance NE, Young WS, III, McMullen NT. Topography of neurons expressing luteinizing hormone-releasing hormone gene transcripts in the human hypothalamus and basal forebrain. The Journal of Comparative Neurology. 1994;339:573–586. doi: 10.1002/cne.903390408. [DOI] [PubMed] [Google Scholar]
- 156.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 157.Reul JM, van den Bosch FR, de Kloet ER. Relative occupation of type-I and type-II corticosteroid receptors in rat brain following stress and dexamethasone treatment: Functional implications. Journal of Endocrinology. 1987;115:459–467. doi: 10.1677/joe.0.1150459. [DOI] [PubMed] [Google Scholar]
- 158.Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: Peripheral and central mechanisms. Biology of Reproduction. 1991;45:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- 159.Rivier C, Rivier J, Mormede P, Vale W. Studies of the nature of the interaction between vasopressin and corticotropin-releasing factor on adrenocorticotropin release in the rat. Endocrinology. 1984;115:882–886. doi: 10.1210/endo-115-3-882. [DOI] [PubMed] [Google Scholar]
- 160.Rivier C, Vale W. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature. 1983;305:325–327. doi: 10.1038/305325a0. [DOI] [PubMed] [Google Scholar]
- 161.Rivier C, Vale W. Effects of corticotropin-releasing factor, neurohypophyseal peptides, and catecholamines on pituitary function. Federation Proceedings. 1985;44(1 Pt 2):189–195. [PubMed] [Google Scholar]
- 162.Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- 163.Romeo RD, Lee SJ, McEwen BS. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004;80:387–393. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- 164.Roselli CE. Sex differences in androgen receptors and aromatase activity in microdissected regions of the rat brain. Endocrinology. 1991;128:1310–1316. doi: 10.1210/endo-128-3-1310. [DOI] [PubMed] [Google Scholar]
- 165.Rosol TJ, Yarrington JT, Latendresse J, Capen CC. Adrenal gland: Structure, function, and mechanisms of toxicity. Toxicologic Pathology. 2001;29:41–48. doi: 10.1080/019262301301418847. [DOI] [PubMed] [Google Scholar]
- 166.Roy D, Angelini NL, Belsham DD. Estrogen directly respresses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-alpha (ERalpha)- and ERbeta-expressing GT1-7 GnRH neurons. Endocrinology. 1999;140:5045–5053. doi: 10.1210/endo.140.11.7117. [DOI] [PubMed] [Google Scholar]
- 167.Sanchez-Cardenas C, Fontanaud P, He Z, Lafont C, Meunier AC, Schaeffer M, … Mollard P. Pituitary growth hormone network responses are sexually dimorphic and regulated by gonadal steroids in adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21878–21883. doi: 10.1073/pnas.1010849107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sandoval-Guzman T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. Journal of Neuroendocrinology. 2004;16:146–153. doi: 10.1111/j.0953-8194.2004.01143.x. [DOI] [PubMed] [Google Scholar]
- 169.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 170.Sarkar DK, Fink G. Luteinizing hormone releasing factor in pituitary stalk plasma from long-term ovariectomized rats: Effects of steroids. The Journal of Endocrinology. 1980;86:511–524. doi: 10.1677/joe.0.0860511. [DOI] [PubMed] [Google Scholar]
- 171.Sawchenko PE. Evidence for a local site of action for glucocorticoids in inhibiting CRF and vasopressin expression in the paraventricular nucleus. Brain Research. 1987;403:213–223. doi: 10.1016/0006-8993(87)90058-8. [DOI] [PubMed] [Google Scholar]
- 172.Sawchenko PE, Imaki T, Vale W. Co-localization of neuroactive substances in the endocrine hypothalamus. Ciba Foundation Symposium. 1992;168:16–30. doi: 10.1002/9780470514283.ch3. discussion 30–42. [DOI] [PubMed] [Google Scholar]
- 173.Schally AV, Nair RM, Redding TW, Arimura A. Isolation of the luteinizing hormone and follicle-stimulating hormone-releasing hormone from porcine hypothalami. The Journal of Biological Chemistry. 1971;246:7230–7236. [PubMed] [Google Scholar]
- 174.Schlosser SF, Almeida OF, Patchev VK, Yassouridis A, Elands J. Oxytocin-stimulated release of adrenocorticotropin from the rat pituitary is mediated by arginine vasopressin receptors of the V1b type. Endocrinology. 1994;135:2058–2063. doi: 10.1210/endo.135.5.7956927. [DOI] [PubMed] [Google Scholar]
- 175.Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- 176.Schwarz JM, Liang SL, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: A mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypo-thalamic-pituitary-adrenal axis activity of male and female rats. Journal of Neuroendocrinology. 2004;16:989–998. doi: 10.1111/j.1365-2826.2004.01258.x. [DOI] [PubMed] [Google Scholar]
- 178.Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Postnatal masculinization alters the HPA axis phenotype in the adult female rat. The Journal of Physiology. 2005;563:265–274. doi: 10.1113/jphysiol.2004.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Seasholtz AF, Burrows HL, Karolyi IJ, Camper SA. Mouse models of altered CRH-binding protein expression. Peptides. 2001;22:743–751. doi: 10.1016/s0196-9781(01)00387-4. [DOI] [PubMed] [Google Scholar]
- 180.Shapiro RA, Xu C, Dorsa DM. Differential transcriptional regulation of rat vasopressin gene expression by estrogen receptor alpha and beta. Endocrinology. 2000;141:4056–4064. doi: 10.1210/endo.141.11.7796. [DOI] [PubMed] [Google Scholar]
- 181.Sharma D, Handa RJ, Uht RM. The ERbeta ligand 5alpha-androstane, 3beta,17beta-diol (3beta-diol) regulates hypothalamic oxytocin (Oxt) gene expression. Endocrinology. 2012;153:2353–2361. doi: 10.1210/en.2011-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shivers BD, Harlan RE, Morrell JI, Pfaff DW. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature. 1983;304:345–347. doi: 10.1038/304345a0. [DOI] [PubMed] [Google Scholar]
- 183.Silverman AJ, Kokoris GJ, Gibson MJ. Quantitative analysis of synaptic input to gonadotropin-releasing hormone neurons in normal mice and hpg mice with preoptic area grafts. Brain Research. 1988;443:367–372. doi: 10.1016/0006-8993(88)91635-6. [DOI] [PubMed] [Google Scholar]
- 184.Sladek CD. Antidiuretic hormone: Synthesis and release comprehensive physiology. New York: John Wiley & Sons, Inc; 2010. [Google Scholar]
- 185.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in Clinical Neuroscience. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Solomon MB, Furay AR, Jones K, Packard AE, Packard BA, Wulsin AC, Herman JP. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–143. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Solomon MB, Loftspring M, de Kloet AD, Ghosal S, Jankord R, Flak JN, … Herman JP. Neuroendocrine function after hypothalamic depletion of glucocorticoid receptors in male and female mice. Endocrinology. 2015;156:2843–2853. doi: 10.1210/en.2015-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Speert DB, McClennen SJ, Seasholtz AF. Sexually dimorphic expression of corticotropin-releasing hormone-binding protein in the mouse pituitary. Endocrinology. 2002;143:4730–4741. doi: 10.1210/en.2002-220556. [DOI] [PubMed] [Google Scholar]
- 189.Stinnett GS, Westphal NJ, Seasholtz AF. Pituitary CRH-binding protein and stress in female mice. Physiology & Behavior. 2015;150:16–23. doi: 10.1016/j.physbeh.2015.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Annual Review of Physiology. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- 191.Suzuki S, Handa RJ. Regulation of estrogen receptor-beta expression in the female rat hypothalamus: Differential effects of dexamethasone and estradiol. Endocrinology. 2004;145:3658–3670. doi: 10.1210/en.2003-1688. [DOI] [PubMed] [Google Scholar]
- 192.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: Cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. The Journal of Comparative Neurology. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 193.Swanson LW, Sawchenko PE. Paraventricular nucleus: A site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- 194.Swanson LW, Sawchenko PE. Hypothalamic integration: Organization of the paraventricular and supraoptic nuclei. Annual Review of Neuroscience. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 195.Tannenbaum B, Rowe W, Sharma S, Diorio J, Steverman A, Walker M, Meaney MJ. Dynamic variations in plasma corticosteroid-binding globulin and basal HPA activity following acute stress in adult rats. Journal of Neuroendocrinology. 1997;9:163–168. doi: 10.1046/j.1365-2826.1997.t01-1-00550.x. [DOI] [PubMed] [Google Scholar]
- 196.Tilbrook AJ, Clarke IJ. Negative feedback regulation of the secretion and actions of GnRH in male ruminants. Journal of Reproduction and Fertility Supplement. 1995;49:297–306. [PubMed] [Google Scholar]
- 197.Tobet SA, Schwarting GA. Minireview: Recent progress in gonadotropin-releasing hormone neuronal migration. Endocrinology. 2006;147:1159–1165. doi: 10.1210/en.2005-1275. [DOI] [PubMed] [Google Scholar]
- 198.Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocrine Journal. 2009;56:729–737. doi: 10.1507/endocrj.k09e-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tyagi RK, Lavrovsky Y, Ahn SC, Song CS, Chatterjee B, Roy AK. Dynamics of intracellular movement and nucleocytoplasmic recycling of the ligand-activated androgen receptor in living cells. Molecular Endocrinology. 2000;14:1162–1174. doi: 10.1210/mend.14.8.0497. [DOI] [PubMed] [Google Scholar]
- 200.Uht RM, McKelvy JF, Harrison RW, Bohn MC. Demonstration of glucocorticoid receptor-like immunoreactivity in glucocorticoid-sensitive vasopressin and corticotropin-releasing factor neurons in the hypothalamic paraventricular nucleus. Journal of Neuroscience Research. 1988;19:405–411. 468–469. doi: 10.1002/jnr.490190404. [DOI] [PubMed] [Google Scholar]
- 201.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 203.Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. Journal of Clinical Investigation. 1993;92:1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.van de Stolpe A, Slycke AJ, Reinders MO, Zomer AW, Goodenough S, Behl C, … van der Saag PT. Estrogen receptor (ER)-mediated transcriptional regulation of the human corticotropin-releasing hormone-binding protein promoter: Differential effects of ERalpha and ERbeta. Molecular Endocrinology. 2004;18:2908–2923. doi: 10.1210/me.2003-0446. [DOI] [PubMed] [Google Scholar]
- 205.Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- 206.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 207.Wade J, Arnold AP. Functional testicular tissue does not masculinize development of the zebra finch song system. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5264–5268. doi: 10.1073/pnas.93.11.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang L, DeFazio RA, Moenter SM. Excitability and burst generation of AVPV kisspeptin neurons are regulated by the estrous cycle via multiple conductances modulated by estradiol action. eNeuro. 2016;3 doi: 10.1523/ENEURO.0094-16.2016. pii: ENEURO.0094-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Watanabe M, Fukuda A, Nabekura J. The role of GABA in the regulation of GnRH neurons. Frontiers in Neuroscience. 2014;8:387. doi: 10.3389/fnins.2014.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Weihua Z, Makela S, Andersson LC, Salmi S, Saji S, Webster JI, … Gustafsson JA. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Weikum ER, Knuesel MT, Ortlund EA, Yamamoto KR. Glucocorticoid receptor control of transcription: Precision and plasticity via allostery. Nature Reviews Molecular Cell Biology. 2017;18:159–174. doi: 10.1038/nrm.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta activation prevents glucocorticoid receptor-dependent effects of the central nucleus of the amygdala on behavior and neuroendocrine function. Brain Research. 2010;1336:78–88. doi: 10.1016/j.brainres.2010.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Whitnall MH, Mezey E, Gainer H. Co-localization of corticotropin-releasing factor and vasopressin in median eminence neurosecretory vesicles. Nature. 1985;317:248–250. doi: 10.1038/317248a0. [DOI] [PubMed] [Google Scholar]
- 215.Williams TD, Carter DA, Lightman SL. Sexual dimorphism in the posterior pituitary response to stress in the rat. Endocrinology. 1985;116:738–740. doi: 10.1210/endo-116-2-738. [DOI] [PubMed] [Google Scholar]
- 216.Wilson AC, Salamat MS, Haasl RJ, Roche KM, Karande A, Meethal SV, … Atwood CS. Human neurons express type I GnRH receptor and respond to GnRH I by increasing luteinizing hormone expression. Journal of Endocrinology. 2006;191:651–663. doi: 10.1677/joe.1.07047. [DOI] [PubMed] [Google Scholar]
- 217.Windle RJ, Gamble LE, Kershaw YM, Wood SA, Lightman SL, Ingram CD. Gonadal steroid modulation of stress-induced hypothalamo-pituitary-adrenal activity and anxiety behavior: Role of central oxytocin. Endocrinology. 2006;147:2423–2431. doi: 10.1210/en.2005-1079. [DOI] [PubMed] [Google Scholar]
- 218.Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. Journal of Neuroscience. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 220.Witkin JW, Paden CM, Silverman AJ. The luteinizing hormone-releasing hormone (LHRH) systems in the rat brain. Neuroendocrinology. 1982;35:429–438. doi: 10.1159/000123419. [DOI] [PubMed] [Google Scholar]
- 221.Wotjak CT, Kubota M, Liebsch G, Montkowski A, Holsboer F, Neumann I, Landgraf R. Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: A novel mechanism of regulating adrenocorticotropic hormone secretion? Journal of Neuroscience. 1996;16:7725–7732. doi: 10.1523/JNEUROSCI.16-23-07725.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wray S. From nose to brain: Development of gonadotrophin-releasing hormone-1 neurones. Journal of Neuroendocrinology. 2010;22:743–753. doi: 10.1111/j.1365-2826.2010.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wray S, Nieburgs A, Elkabes S. Spatiotemporal cell expression of luteinizing hormone-releasing hormone in the pre-natal mouse: Evidence for an embryonic origin in the olfactory placode. Brain Research Developmental Brain Research. 1989;46:309–318. doi: 10.1016/0165-3806(89)90295-2. [DOI] [PubMed] [Google Scholar]
- 224.Yang Y. Structure, function and regulation of the melanocortin receptors. European Journal of Pharmacology. 2011;660:125–130. doi: 10.1016/j.ejphar.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Young EA. Sex differences in response to exogenous corticosterone: A rat model of hypercortisolemia. Molecular Psychiatry. 1996;1:313–319. [PubMed] [Google Scholar]
- 226.Young EA, Altemus M. Puberty, ovarian steroids, and stress. Annals of the New York Academy of Sciences. 2004;1021:124–133. doi: 10.1196/annals.1308.013. [DOI] [PubMed] [Google Scholar]
- 227.Zhou L, Blaustein JD, De Vries GJ. Distribution of androgen receptor immunoreactivity in vasopressin- and oxytocin-immunoreactive neurons in the male rat brain. Endocrinology. 1994;134:2622–2627. doi: 10.1210/endo.134.6.8194487. [DOI] [PubMed] [Google Scholar]