Abstract
Fire can influence the microclimate of forest habitats by removing understory vegetation and surface debris. Temperature is often higher in recently burned forests owing to increased light penetration through the open understory. Because physiological processes are sensitive to temperature in ectotherms, we expected fire-maintained forests to improve the suitability of the thermal environment for turtles, and for turtles to seasonally associate with the most thermally-optimal habitats. Using a laboratory thermal gradient, we determined the thermal preference range (Tset) of eastern box turtles, Terrapene carolina, to be 27 – 31 °C. Physical models simulating the body temperatures experienced by turtles in the field revealed that surface environments in a fire-maintained longleaf pine forest were 3 °C warmer than adjacent unburned mixed hardwood/pine forests, but the fire-maintained forest was never of superior thermal quality owing to wider Te fluctuations above Tset and exposure to extreme and potentially lethal temperatures. Radiotracked turtles using fire-managed longleaf pine forests maintained shell temperatures (Ts) approximately 2 °C above those at a nearby unburned forest, but we observed only moderate seasonal changes in habitat use which were inconsistent with thermoregulatory behavior. We conclude that turtles were not responding strongly to the thermal heterogeneity generated by fire in our system, and that other aspects of the environment are likely more important in shaping habitat associations.
Keywords: habitat use, Terrapene carolina, temperature, thermal quality, thermoregulation, reptile
1. Introduction
Fire disturbance plays a critical role in the maintenance of structure and function in many habitats worldwide (Wright and Bailey, 1982; Nowacki and Abrams, 2008), with strong implications for the ecology and evolution of biota (Keeley and Rundle, 2005; Beerling and Osborne, 2006; Pausas and Keeley, 2009). Fire can directly kill, injure, or damage biota by exposure to extreme temperatures, but among the most important impacts of fire are indirect changes to the microclimate. For instance, while most prescribed fires do not remove overstory trees, the removal of ground litter, debris, and vegetation can open the forest understory resulting in higher light levels and altered hydric and temperature conditions (Iverson and Hutchinson 2002; Greenburg and Waldrop, 2008; Hossack et al., 2009). The magnitude and duration of such effects and animal responses to them varies depending on habitat, fire frequency, intensity, and whether the fire was naturally ignited or intentionally set for management purposes (i.e., prescribed fire; Pastro et al., 2011; Elzer et al., 2013).
Environmental temperature directly influences the body temperature (Tb) of ectotherms, which in turn strongly affects their physiology, behavior, and performance (Huey, 1982). In environments where temperature varies spatially or temporally, ectothermic vertebrates such as reptiles often behaviorally regulate Tb to maximize time at or near temperatures where performance is optimized (Angilletta et al., 2002). The links between thermoregulatory behavior and temperature-sensitive performance should be strong owing to the influence of temperature on fitness-related activities and processes such as movement, energy and water balance, and reproduction (Huey and Slatkin, 1976; Huey and Bennett, 1987; Congdon, 1989). Canopy openings and understory removal in fire-maintained habitats could thus offer more opportunities to maintain Tb in the optimal performance range (Elzer et al., 2013). Indeed, many ectotherms respond positively to fire disturbance (Mushinsky, 1985; Ashton et al., 2008; Hossack et al., 2009; Matthews et al., 2010; Steen et al., 2013).
The eastern box turtle, Terrapene carolina, provides a useful model to study the effects of post-fire habitat alteration on Tb and habitat use. T. carolina populations are typically found in a variety of forest types, often associating with canopy openings, habitat edges, and early successional or grassland habitat on a seasonal basis (Keister and Willey, 2015). As Tb is strongly associated with environmental temperature in T. carolina (Adams et al., 1989), which in turn influences their performance and energy balance (Adams et al., 1989; Penick et al., 2002), the increased light penetration following fire could offer thermoregulatory opportunities for this small-bodied terrestrial turtle. Alternatively, the increased temperature under open canopies could at times expose them to lethal extremes, requiring movement out of openings and into more shaded forest environments. For instance, in a study on turtle responses to sivlicultural management, T. carolina experienced considerably higher Tb in areas where the canopy was opened by timber harvesting, with associated modifications to their fine-scale movements and activity (Currylow et al., 2012). However, the limited mobility and small home range size of T. carolina may prohibit behavioral modifications over broad spatial scales, though individuals and populations differ in their vagility (Dodd, 2001; Currylow et al., 2012; Greenspan et al., 2015).
A large part of the range of T. carolina is in historically fire-prone habitats, such as the southeastern Coastal Plain and Sandhills ecoregions, which historically burned at a frequency of 1 – 3 and 4 – 6 years, respectively (Frost, 1998). Natural wildfires have been replaced increasingly by prescribed burning in an attempt to mimic this fire return interval for silvicultural, hazard reduction, pest control, grazing, wildlife management, and biodiversity conservation purposes (Haines et al., 2001). However, we have little knowledge of the direct or indirect effects of fire on T. carolina. Here, we investigate the potential for fire to impact the thermal environments of T. carolina, and whether turtles behaviorally respond by seasonal changes in habitat use. We expect the open canopy maintained by fire to increase both environmental temperature and turtle Tb, and that turtles will seasonally associate with the most thermally-optimal habitats.
2. Material and methods
2.1. Study Site
The study was conducted at the Weymouth Woods Sandhills Nature Preserve (hereafter WEWO), a 202-ha state park in the sandhills physiographic region of south-central North Carolina. The habitat is a mosaic of longleaf pine (Pinus palustris), loblolly pine (P. taeda), and hardwood trees in relatively equal proportion (40% longleaf, 33% hardwood, and 27% loblolly; J. Roe, unpubl. data). Longleaf occurs primarily in the xeric uplands, except where fire has been excluded or where loblolly were replanted in remnant forestry plantations. Hardwood forests, including mixed oak (Quercus spp.), hickory (Carya spp.), red maple (Acer rubrum), sweetgum (Liquidambar styraciflua), American holly (Ilex opacum), sassafras (Sassafras albidum), and tulip poplar (Liriodendron tulipifera) trees are patchily distributed in upland habitats, but are primarily restricted to stream margins, bottomland habitats, and park units that have not been part of the prescribed fire program. In the upland areas where fire has been intentionally excluded, a few large living remnant longleaf pine trees and stumps indicate that these habitats were once more populated with longleaf. Prescribed fire has been used in the park since 1974, with 76% of the area being managed using controlled burns. Management units are 12.9 ± 7.8 ha (mean ± standard deviation; 0.9 – 23.9 ha range) in size, with a target burn frequency of 3 – 5 years.
2.2. Temperature preference trials
Fourteen Terrapene carolina carolina adults (11 male, 3 female, carapace length 120.3 – 148.2 mm, mass 340 – 500 g) were captured from 21 August to 16 September 2014 for temperature preference trials. All turtles appeared healthy, were uninjured, and females were unlikely to be gravid at this time of year (typical nesting season is from May – July at this location, J. Roe pers. obs.). Turtles were housed at the University of North Carolina at Pembroke for three to seven days before trials. Individuals were kept in separate rubber bins with constant access to water, but were not fed while in captivity.
Tb was measured along a thermal gradient constructed from plywood (170 cm × 50 cm × 25 cm) with a bottom of aluminum flashing covered by sand 2 cm deep. A temperature gradient was created using an overhead ceramic heat lamp and heat pads (Reptitherm U.T.H., Zoo Med Laboratories, Inc., San Luis Obispo, CA) placed under the aluminum and maintained at constant temperatures by a Herpstat thermostat (Spyder Robotics LLC, Chana, IL). The heat lamp was placed 30 cm over one end of the gradient, with a heat pad maintained at 38 °C approximately 40 cm from the same end. Another heat pad, maintained at 27 °C, was placed halfway down the gradient. The room was constantly maintained at 15 °C, creating a relatively cold end opposite the heat lamp. Overhead lights were set on natural light cycles of 12L:12D (photophase starting at 0700 h and scotophase at 1900 h). Temperatures were spot checked at the beginning and end of each trial with an infra-red thermometer. In addition, five temperature data loggers (Thermocron iButton, Dallas Semiconductor, Dallas, TX) were placed in the sand along the thermal gradient and recorded temperatures every 5 min.
Turtles were fitted with a hermetically sealed tip insulated thermocouple (Omega Engineering, Inc., Norwalk, CT) inserted 2 cm into the cloaca. Temperature was monitored with an EasyLog data logger (Lascar Electronics, Erie, PA) that recorded temperature every 5 min. Turtles were placed individually in the gradient and remained undisturbed for 24 h. The initial three hours of recordings were discarded before analysis to allow turtles to become acclimated to the gradient. We determined the preferred body temperature, or set-point range (Tset) for each turtle from the bounds of the central 50% (i.e., the 25th and 75th quartiles) of selected Tb (Hertz et al., 1993). Given the highly skewed sex ratios, males and females were grouped together in all analyses. Turtles had no access to food or water during the temperature preference trials, and were released at their point of capture following completion of trials.
2.3. Operative environmental temperatures
We estimated the operative environmental temperature (Te), the Tb available to a non-thermoregulating ectotherm (Bakken, 1992), using physical models placed in various habitats in the field. Models were water filled Snapware® plastic bins painted in a flat black and tan mottled pattern of the approximate size, dimensions (135 cm × 85 cm × 58 cm), and color of adult T. carolina in our study system. Internal temperatures were recorded every hour using iButtons sealed in a black rubber coating (Plasti Dip International, Blaine, MN). Models were calibrated alongside two fresh turtle carcasses with iButton dataloggers recording internal Tb and external shell temperature (Ts). One model and carcass pair was placed on the surface in the open for 24 h, while the other model and carcass pair was buried in the nearby litter. Daytime conditions were mostly sunny (0 – 50% cloud cover) during model calibrations.
From May to November 2013, models were placed in five randomly selected locations each in a fire-maintained longleaf forest unit and an adjacent mixed hardwood / non-longleaf pine unburned forest unit where fire has been intentionally excluded at least since the park began the prescribed fire program in 1974. The most recent fire was in May 2012 in the longleaf habitat. Locations were selected using the “create random points” tool in ArcMap 10.1 (Environmental Systems Research Institute, Redlands, CA, USA). Each forest unit was of similar topography (2 – 15% slopes), elevation (364 – 416 m), and soils (combinations of sand, loamy sand, sand clay loam, sandy loam profiles; USDA Natural Resources Conservation Service). Models were placed 50 – 500 m from their counterparts in the adjacent forest units. At each location, one model was placed on the surface, while another was placed under the nearest cover object (leaf litter, logs, grasses, or shrubs) under which we commonly observed T. carolina to seek refuge. Models were rotated to new random locations each week over a period of several weeks during a spring (8 May – 18 June 2013), summer (16 July – 27 Aug 2013), and fall (22 Oct – 12 Nov 2013) sampling period. The rotations resulted in 25 unique locations for each surface-refuge model pair in each habitat type per season in spring and summer, and 15 unique locations in fall. The thermal quality (de) of each habitat type (longleaf or mixed hardwood / non-longleaf pine), position (surface or refuge), and season (spring, summer, fall) combination was calculated as the mean of absolute values of deviations of Te from Tset (Hertz et al., 1993). For surface models, we also calculated the percentage of Te above the lower bound of Tset, (% ≥ Tset) as an index of the degree to which Tset could be maintained in a given habitat by behavioral adjustments (i.e., movement between surface and refuge positions). This final index assumes that suitable refuge microhabitats (litter, shrubs, woody debris) that did not exceed Tset were available for retreat when Te exceeds Tset on the surface.
2.4. Free-ranging turtle temperatures and habitat use
We captured turtles opportunistically and equipped them with radiotransmitters (RI-2B, 10 – 15 g, Holohil Systems Ltd., Carp, ON, Canada) using 5 minute epoxy gel (Devcon, Solon, OH) in the field. We tracked 27 turtles (14 male and 13 female) from WEWO and recorded Ts for a subset of individuals (5 male and 7 female). Hourly Ts was recorded using iButton temperature dataloggers coated in black Plasti Dip and attached to the rear of the carapace. For comparison, we include data from 23 turtles (12 male and 11 female; five of each sex equipped with iButtons) radiotracked at a site 24 km to the south on the border of the Sandhills and Upper Coastal Plain physiographic regions, the Lumber River State Park (LRSP). This park is comprised of mixed pine (39% loblolly, 0.5% longleaf) and hardwood (60%) forests where prescribed fire has not been employed at least since 2001 when the park took over management of the property (J. Roe unpubl. data).
We located telemetered turtles once per week using a receiver (R-1000, Communication Specialists, Orange, CA) and a Yagi antenna. At each location, we assessed the relative composition of tree types in the immediate surrounding area using a CRUZ-ALL angle gauge (Forestry Suppliers, Inc., Jackson, MS, USA). This method involved rotating 360° while holding the angle gauge at head height at a standard length (~ 64 cm) from the observer’s eye, and counting the number of tree trunks that completely filled (or more than filled) the 10-factor gauge opening. Trees were grouped into three categories which included 1) longleaf pine, 2) other non-longleaf pine species, and 3) hardwood species. The number of trees in each category was counted, and the relative proportion of each category was calculated and used as an index of forest microhabitat composition. We assessed canopy openness at a subset of locations (n = 518), which were the first 23 ± 1 locations for the first 23 turtles we studied (ten from LRSP and 13 from WEWO), excluding any locations where the turtle was moving. Canopy openness was assessed with a spherical densiometer facing the four cardinal directions and scores averaged for a single estimate at each location.
2.5. Data analyses
We performed statistical analyses with SPSS 23.0 (SPSS, 2015). Males and females were pooled in all analyses. Where appropriate, we examined the assumptions of homogeneity of variances and normality; when data failed to meet assumptions, they were transformed to approximate normal distributions or equal variances. Statistical significance was accepted at the α < 0.05 level.
We used linear regression to examine the relationships between the temperature of physical models, carcass temperature, and shell temperature. We used paired t-tests to examine the degree to which models and Ts underestimated or overestimated carcass temperature. We examined variation in the estimates derived from physical models using analysis of variance (ANOVA) with mean Te, de, and % ≥Tset as the dependent variables, and habitat type, season, and habitat × season as the independent variables. In the above analyses, both Te and de were log10-transformed, and % ≥Tset was arcsine square root transformed. Data from physical models was not separated based on variation in environmental conditions (e.g., cloud cover, precipitation, and wind) that could influence heating and cooling rates.
We used ANOVA to examine differences in mean monthly Ts between sites (WEWO and LRSP) for each month separately. We also calculated mean canopy openness and mean proportion representation of each tree class (longleaf pine, non-longleaf pines, and hardwood) for each turtle. We used regression analyses to assess the relationships between canopy openness (dependent variable) and the proportional representation of each tree habitat class (independent variables). Both canopy openness and proportion representation values were arcsine square root transformed prior to analyses.
To examine seasonal variation in forest microhabitat use, we used repeated measures ANOVAs with month as the repeated variable and mean proportion of non-longleaf pine and hardwood trees as the dependent variables. We used Friedman tests to examine monthly variation in use of longleaf pine, with month as the repeated variable and mean proportion of longleaf pine as the dependent variable. All proportional values were arcsine square root transformed. We restricted our analysis of seasonal use of forest microhabitats to the active season, between May – October.
3. Results
3.1. Temperature preference
We obtained 3,182 measurements of Tb from turtles in the thermal gradient. Tb ranged from 17.5 – 34.5 °C, with a mean (± SE) of 28.8 ± 0.6 °C. The 25% and 75% quartiles (Tset) averaged across individuals was 27.0 – 31.0 °C (Fig. 1). Available temperatures along the gradient measured by dataloggers under the sand ranged from 18.5 – 37.0 °C, while surface temperature directly under the heat lamp was as high as 45 °C.
Figure 1.
Body temperatures (Tb) of eastern box turtles (Terrapene carolina) on a laboratory thermal gradient.
3.2. Operative environmental temperatures
Temperature of physical models was correlated with Tb of turtle carcasses (R2 = 0.963, F1,158 = 4115.2, P < 0.001), but temperatures of physical models deviated by as much as 6 °C above and 3 °C below Tb. Because models slightly underestimated Tb by 0.41 ± 0.11 °C (paired t = 3.85, P < 0.001), we adjusted temperatures of models using the equation:
We recorded 19,600, 18,270, and 2,012 measures of Te in the spring, summer, and fall seasons respectively. For surface positions, Te, de, and % ≥ Tset varied by habitat, season, and the interaction of these two variables (Table 1). Surface Te ranged from 0 – 62 °C across habitats and seasons and was highest in summer, intermediate in spring, and lowest in fall (Table 2, Fig. 2). Surface Te was approximately 3 °C higher in burned longleaf forests than in adjacent unburned mixed hardwood / non-longleaf pine forests in both spring and summer, but did not differ between habitats in fall (Table 2). Surface de was lowest (i.e., of higher thermal quality) in summer, intermediate in spring, and highest in fall in all habitats (Table 2). Surface de was lower in unburned mixed hardwood / non-longleaf pine forests than adjacent burned longleaf forests in the summer, but did not differ between habitats in spring or fall (Table 2). The amount of time Te was within or above Tset (% ≥ Tset) was 17 – 20 % higher in burned longleaf forests than in unburned mixed hardwood / non-longleaf pine forests in spring and summer, but differences between habitats were minimal in fall (Table 2).
Table 1.
Summary results of ANOVA for the effects of habitat type (longleaf forest or mixed hardwood and pine forest), season (spring, summer, or fall), and their interaction on operative temperature (Te), thermal quality (de), and percentage of Te within or above Tset (% ≥ Tset) in surface and refuge positions.
| position | effect | metric | num df | den df | F | p |
|---|---|---|---|---|---|---|
| surface | habitat | Te | 1 | 24 | 57.6 | < 0.001 |
| de | 1 | 24 | 30.2 | < 0.001 | ||
| % ≥ Tset | 1 | 24 | 99.4 | < 0.001 | ||
| season | Te | 2 | 24 | 2364.1 | < 0.001 | |
| de | 2 | 24 | 2010.4 | < 0.001 | ||
| % ≥ Tset | 2 | 24 | 376.3 | < 0.001 | ||
| habitat × season | Te | 2 | 24 | 36.7 | < 0.001 | |
| de | 2 | 24 | 21.6 | < 0.001 | ||
| % ≥ Tset | 2 | 24 | 11.4 | < 0.001 | ||
| refuge | habitat | Te | 1 | 21 | 4.0 | 0.059 |
| de | 1 | 21 | 10.3 | 0.004 | ||
| season | Te | 2 | 21 | 643.3 | < 0.001 | |
| de | 2 | 21 | 266.3 | < 0.001 | ||
| habitat × season | Te | 2 | 21 | 5.2 | 0.014 | |
| de | 2 | 21 | 4.9 | 0.018 |
Table 2.
Summary of seasonal habitat variation in operative environmental temperatures (Te), thermal quality (de), and percentage of time Te was within or above Tset (% ≥ Tset). The burned forest was predominately longleaf pine, while the unburned forest was a mixture of hardwood and non-longleaf pine species.
| position | season | Habitat | Te °C (se) | de (se) | % ≥ Tset (se) | Te °C range |
|---|---|---|---|---|---|---|
| surface | spring | Burned | 24.7 (0.2) | 5.3 (0.1) | 31.4 (1.3) | 7 – 62 |
| Unburned | 21.8 (0.1) | 5.4 (0.1) | 11.0 (1.5) | 8 – 37 | ||
| summer | Burned | 27.4 (0.2) | 4.0 (0.1) | 41.1 (1.7) | 14 – 55 | |
| Unburned | 24.5 (0.1) | 3.0 (0.1) | 24.3 (1.9) | 11 – 47 | ||
| fall | Burned | 13.0 (0.1) | 14.0 (0.0) | 1.2 (0.3) | 0 – 32 | |
| Unburned | 13.4 (0.3) | 13.5 (0.3) | 0.2 (0.2) | 1 – 31 | ||
| refuge | spring | Burned | 21.5 (0.2) | 5.6 (0.2) | --- | 10 – 37 |
| Unburned | 20.3 (0.2) | 6.7 (0.2) | --- | 10 – 27 | ||
| summer | Burned | 25.2 (0.5) | 2.4 (0.3) | --- | 15 – 35 | |
| Unburned | 23.6 (0.2) | 3.4 (0.2) | --- | 16 – 32 | ||
| fall | Burned | 12.9 (0.2) | 14.3 (0.2) | --- | 4 – 25 | |
| unburned | 13.4 (0.5) | 13.7 (0.4) | --- | 1 – 22 |
Figure 2.
Frequency distribution of seasonal operative environmental temperatures for surface habitats in fire-maintained longleaf pine forests and unburned mixed hardwood/non-longleaf pine forests. Note that the grey color represents areas where the two habitats’ distributionsoverlap. The preferred body temperature, or set-point range (Tset) for eastern box turtles (Terrapene carolina) is 27 – 31 °C.
For refuge positions, Te and de varied by habitat, season, and their interaction, though the effect of habitat was only marginally significant for Te (Table 1). Refuge Te ranged from 1 – 37 °C across habitats and seasons and was higher in burned longleaf forests than unburned mixed hardwood / non-longleaf pine forests in spring and summer, but did not differ between habitats in fall (Table 2, Fig. 3). Refuge de was lowest (i.e., of higher thermal quality) in summer, intermediate in spring, and highest in fall in both habitats, and lower in burned longleaf forests than unburned mixed hardwood / non-longleaf pine forests in all seasons (Table 2).
Figure 3.
Frequency distribution of seasonal operative environmental temperatures for refuge habitats in fire-maintained longleaf pine forests and unburned mixed hardwood/non-longleaf pine forests. Note that the grey color represents areas where the two habitats’ distributions overlap. The preferred body temperature, or set-point range (Tset) for eastern box turtles (Terrapene carolina) is 27 – 31 °C.
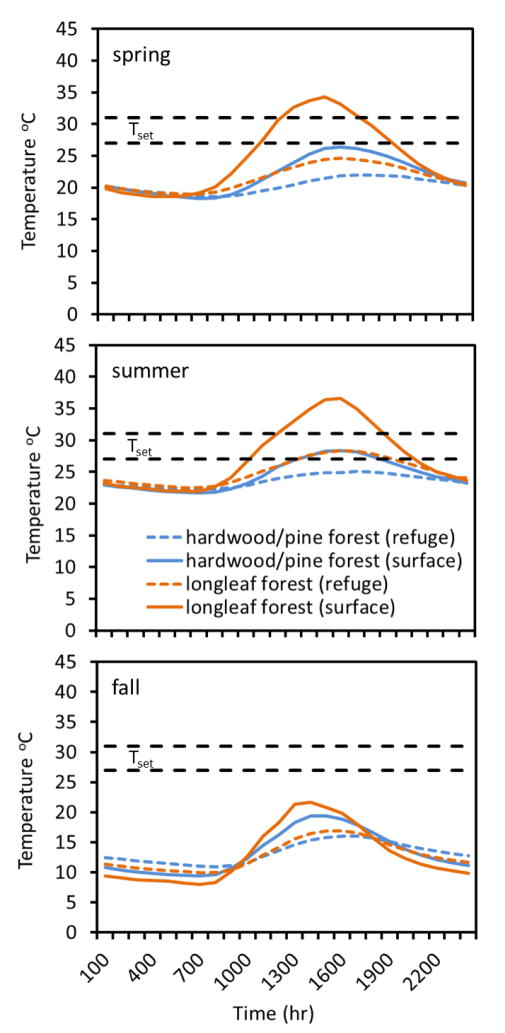
In spring, hourly average Te remained above the minimum Tset threshold (27 °C) from 1100 – 1900 h on the surface in burned longleaf habitat, but neither refuge longleaf nor mixed hardwood / non-longleaf pine (surface or refuge) habitats reached the minimum Tset threshold (Fig. 4). In summer, hourly average Te in burned longleaf habitats reached the minimum Tset threshold from 1000 – 2000 h on the surface, and from 1300 – 2000 h in refuge habitats (Fig. 4). Summer hourly average Te in unburned mixed hardwood / non-longleaf pine habitats reached the minimum Tset threshold from 1300 – 1900 h on the surface, but never reached Tset in refuge habitats (Fig. 4). Fall hourly average Te did not reach Tset in either habitat (Fig. 4).
Figure 4.
Seasonal mean hourly operative environmental temperatures for surface and refuge models in fire-maintained longleaf pine forests and unburned mixed hardwood/non-longleaf pine forests. The preferred body temperature, or set-point range (Tset) for eastern box turtles (Terrapene carolina) is 27 – 31 °C.
3.3. Free-ranging turtle temperatures
Ts was correlated with Tb of carcasses (R2 = 0.912, F1,92 = 948.3, P < 0.001), with Ts overestimating Tb by 0.70 ± 0.34 °C (paired t = 2.06, P = 0.04). However, some measures of Ts deviated by as much as 15 °C above and 3 °C below Tb when on the surface.
From 1 May – 31 October, we obtained 28,896 and 29,640 measures of Ts at WEWO and LRSP, respectively. Ts ranged from 1.5 – 43.5 °C at WEWO and from 1.0 – 42.0 °C at LRSP. Monthly mean Ts was 0.4 – 1.8 °C higher at WEWO than LRSP (Fig. 5), but differed significantly only during August (F1,13 = 12.40, P = 0.004) and October (F1,13 = 8.08, P = 0.014). However, comparisons between sites for most other months approached significance (May: F1,14 = 3.95, P = 0.067, June: F1,15 = 3.87, P = 0.068, July: F1,17 = 4.09, P = 0.059, August: F1,13 = 1.86, P = 0.196). The most pronounced differences in Ts between sites occurred during the day between 1100 – 1700 h, while overnight Ts was similar (Fig. 6).
Figure 5.
Monthly shell temperatures (mean ± SE) for field-active eastern box turtles (Terrapene carolina) at a site managed using prescribed fire (WEWO) compared to turtles at an unburned site (LRSP).
Figure 6.
Hourly mean shell temperatures for field-active eastern box turtles (Terrapene carolina) at a site managed using prescribed fire (WEWO) compared to turtles at an unburned site (LRSP) during May, July, and August.
3.4. Habitat use
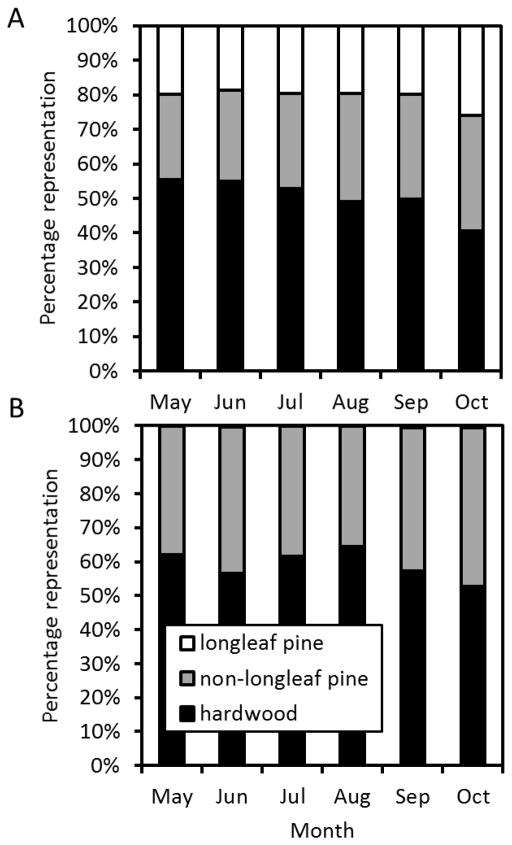
Turtles used habitats with predominantly hardwood trees in all months at both sites, but the relative amounts of each tree class in the nearby habitat varied moderately over the course of the active season (Fig. 7). Turtles at WEWO used forests with increasing percentages of non-longleaf pine from May – October (F5,130 = 2.4, P = 0.042), while using forests with a decreasing percentage of hardwoods over the same time period (F5,130 = 6.1, P < 0.001; Fig. 7). At LRSP, turtles also used forests with increasing percentage of non-longleaf pine from May – October (F5,110 = 2.7, P = 0.046), but the percentage of hardwood trees in used forests did not vary over this time period (F5,130 = 1.8, P = 0.147; Fig. 7). There was no seasonality of use for longleaf habitat at either site (P > 0.334; Fig. 7).
Figure 7.
Seasonal forest habitat use for eastern box turtles (Terrapene carolina) at A) a site managed using prescribed fire (WEWO) compared to B) turtles at an unburned site (LRSP).
Variation in canopy openness depended on tree types in the immediate surrounding area. Canopy openness increased logarithmically with increasing percent longleaf (F1,11 = 25.4, R2 = 0.70, P < 0.001), but decreased linearly with increasing percent hardwood trees (F1,9 = 85.6, R2 = 0.91, P < 0.001; Fig. 8). Canopy openness was not correlated with percent non-longleaf pine species (F1,9 = 1.6, P = 0.233).
Figure 8.

Relationships between canopy openness and relative forest composition of hardwood, longleaf pine, and non-longleaf pine trees at a site managed using prescribed fire.
4. Discussion
The use of prescribed fire in forest habitat management affects the thermal characteristics of the environment, and thus has the potential to indirectly influence the physiology and behavior of ectothermic vertebrates such as turtles. Our results indicate that the burned longleaf habitat experienced considerably higher temperatures than the adjacent unburned mixed hardwood / non-longleaf pine forest, but surface environments in the fire-managed longleaf forest were not of superior thermal quality in any part of the active season. The longleaf forest also had a more variable thermal environment, where surface-active turtles would be regularly exposed to temperatures above their thermal preference range and sometimes exceeding lethal limits. Turtles using fire-managed longleaf forests experienced seasonally higher Ts, but despite the potential thermal costs and benefits associated with the different habitats and seasons, there was little evidence to suggest habitat use behaviors were linked to variation in the habitats’ thermal characteristics.
This is the first quantification of Tset for any subspecies of T. carolina. Our estimates of Tset (27.0 – 31.0 °C) and mean preferred Tb (28.8 °C) are higher than the preferred Tb reported for T. carolina elsewhere (20 – 25 °C; Erskine and Hutchison, 1981; do Amaral et al., 2002a, b). Compared more broadly to other species, our estimates of preferred Tb are within the upper range reported for turtles from temperate regions; 20.7 – 22.4 °C for Sternotherus odoratus (Graham and Hutchison, 1979); 21.1 – 24.9 °C for Clemmys guttata (Graham and Hutchison, 1979); 22.0 – 27.0 °C for Glyptemys insculpta (Dubois et al., 2008); 22.0 – 27.2 °C for Chrysemys picta (Graham and Hutchison, 1979; Edwards and Blouin-Demers, 2007); 24.6 – 29.1 °C for Trachemys scripta (Gatten, 1974); 28.1 °C for Chelydra serpentina (Schuett and Gatten, 1980); 28.3 – 29.8 °C for Terrapere ornata (Gatten, 1974), and 28.7 – 32.5 °C for Graptemys geographica (Bulté and Blouin-Demers, 2010). Temperature preferences in turtles can vary among populations over latitudinal gradients, including in Terrapene spp. (Ellner and Karasov, 1993), and can even differ among individuals within a population exposed to different local thermal environments (Curtin, 1998). Our study populations were from lower latitude and elevation (and thus a warmer climate) compared to most other species for which preferred Tb has been assessed, and the higher preferred Tb in our study is consistent with observations of ectotherms from warmer climates (Ellner and Karasov, 1993). Turtles may also vary in thermal preference due to nutritional status (Gatten, 1974), infection (Monagas and Gatten, 1983; do Amaral et al., 2002b), and perhaps other intrinsic factors. Our sample was limited to apparently healthy and postabsorptive adult turtles in the late active season when potentially confounding variation due to reproductive status would also be minimized. Our estimates of preferred Tb were within the range of temperatures for optimized performance in turtles for feeding (29 – 30 °C, Gatten, 1974; 26 – 29 °C, Parmenter, 1980; 30 °C, Dubois et al., 2008) and locomotion (24 – 32 °C, Adams et al., 1989), further justifying our use of Tset estimates in assessing the suitability of the thermal environment for various important processes in T. carolina.
The long-term use of prescribed fire in the longleaf pine system was likely a strong contributor to the altered thermal environments available to turtles at our site, resulting in part through changes in vegetative structure. Fire reduces vegetative and ground cover, and opens the canopy allowing more direct solar radiation to the forest floor (Mushinsky, 1992; Greenberg and Waldrop, 2008; Matthews et al., 2010), resulting in increased temperature for several years after fire (Hossack et al. 2009). Indeed, in areas where prescribed fire has been regularly employed at our study site, where longleaf pines predominate, canopy openness was higher than in predominantly hardwood forest habitats that are not managed with fire. That the higher mean surface Te in fire-maintained longleaf habitats was closer to Tset at first suggests this would be the most thermally-optimal habitat for T. carolina, yet this habitat was never of superior thermal quality, likely due to its wider Te fluctuations. Minimum Te was similar among habitats, but surface Te exceeded Tset for 20 – 25% of recordings in burned longleaf forests in spring and summer compared to just 1 – 3% in unburned mixed hardwood / non-longleaf pine habitats. Moreover, surface Te in fire-maintained longleaf forests exceeded temperatures presumed to be lethal, or the critical thermal maximum (CTmax), in turtles. While we did not measure CTmax in our study population, based on measures from other studies, we presume it unlikely that T. carolina would tolerate extended exposure to temperatures above 40 °C (Sturbaum, 1981; Plummer et al., 2003; Lagarde et al., 2012). Consistent with this explanation, the only time when thermal quality differed between surface environments was during the hot summer period, when the unburned mixed hardwood / non-longleaf pine forest was of higher thermal quality due to the cooler temperatures. During this period, the more open fire-maintained longleaf forest exceeded CTmax 7% of the time.
It is important to recognize that comparisons of thermal quality only reflect the relative availability of thermally favorable environments without taking into consideration thermoregulatory behaviors. Many reptiles maximize thermal benefits by shuttling among microhabitats or altering position in thermally heterogeneous environments to remain within or closer to Tset for more extended periods (Christian and Weavers, 1996; Edwards and Blouin-Demers, 2007; Dubois et al., 2009; Besson and Cree, 2010). Assuming turtles in our study were behaviorally thermoregulating, fire-maintained longleaf forest could offer more opportunities to maintain Tb closer to or within Tset. For instance, surface Te exceeded the lower bound of Tset for 17 and 20% more of the time than in unburned mixed hardwood / non-longleaf pine forests in summer and spring seasons, respectively, while refuge environments always offered Te below the upper bound of Tset in both forest types at these times. To exploit these opportunities in the fire-maintained longleaf habitat, suitable thermal refuge microsites would need to be readily available, and turtles would need to move between surface and nearby refuge environments. We were not able to assess how carefully turtles thermoregulated in the different habitats because we did not measure Tb in field-active animals. Instead, we measured Ts as an approximation of Tb, as these two measures can be tightly linked in small-bodied turtles (Grayson and Dorcas, 2004). However, the degree to which Ts accurately reflects Tb may differ among species due to shape and size variation and their effects on heating and cooling rates (Polo-Cavia et al., 2009; Bulté and Blouin-Demers, 2010). While our measures of Ts were correlated with Tb, the magnitude of deviations was at times high, so we were cautious in using Ts as an accurate proxy of Tb for all scenarios in field-active turtles. Nevertheless, the higher Ts of turtles at the fire-maintained site (WEWO) suggest they would have also experienced relatively higher Tb than turtles at a nearby site where fire is not used in management (LRSP). However, Ts differences between sites were only moderate (< 2 °C), and whether this difference was a result of habitat alterations from fire, or whether this resulted in measurably different vital rates or performance measures is not yet known. We are currently monitoring growth rates, body condition, survivorship, and other endpoints that could elucidate any temperature effects with fitness consequences.
Turtles often respond to thermally heterogeneous environments by behavioral thermoregulation, including habitat selection to maximize heat gain (Dubois et al., 2009; Picard et al., 2011), alterations to activity timing and use of refuges in times of temperature extremes (Nieuwolt, 1996; Lagarde et al., 2012), and seasonal migration between areas differing in temperature (Swingland and Lessells, 1979). Given the variation in thermal characteristics of habitats at the fire-maintained site, we expected to observe strong links between seasonal habitat use and thermoregulatory behavior in T. carolina. As the fire-maintained and unburned forests occur in close proximity to one another at WEWO, turtles could hypothetically move between the different habitats to maximize thermal benefits (maintain Tb closer to Tset) or minimize costs (avoid overheating) on a temporal basis. Indeed, individual turtles did regularly move between burned and unburned habitats and among different forest types. However, while we did observe some seasonality in habitat use, the magnitude of changes was only moderate, and the directions of change were not consistent with behavioral thermoregulation. For instance, use of the unburned, predominantly hardwood forests should have peaked in summer when this habitat offered superior thermal quality, but associations with such environments decreased moderately (minus 14% from May to October) throughout the active season at WEWO and did not vary seasonally at LRSP. Likewise, if turtles were behaving in ways to minimize risks of exposure to temperature extremes, use of the fire-maintained longleaf forests should have decreased in summer, but we observed no seasonality in use of this habitat. We did observe moderately increased use of forests with more non-longleaf pine species at both sites over the active season (plus 6 – 8% from May to October), but because canopy openness did not vary according to this tree class, it is unlikely that this shift in habitat use was driven by thermoregulation.
Habitat use is shaped by multiple requirements in including hydration, feeding, reproduction, and predator avoidance. While all of these processes are dependent on temperature in ectotherms (Huey, 1982; Angilletta et al., 2002), perhaps the relatively low variation in thermal characteristics among habitats and overall benign thermal environment of our system minimizes the influence of temperature in habitat use “decisions”. Ectotherms must invest more effort into thermoregulation in extreme climates with low thermal quality, such as at higher latitudes and elevations (Blouin-Demers and Nadeau, 2005); as a result, we typically see stronger thermoregulatory behaviors, such as habitat selection and activity timing, in thermally challenging environments (Blouin-Demers and Weatherhead, 2001; Edwards and Blouin-Demers, 2007; Besson and Cree, 2010). In our study, the largest difference in thermal quality (de) between habitats in any season (for comparable positions) was 1.1, and all habitats were of relatively high thermal quality during most of the active season compared to measures from terrestrial habitats in colder climates (Blouin-Demers and Weatherhead, 2001; Blouin-Demers and Nadeau, 2005; Row and Blouin-Demers, 2006). T. carolina may thus be able to more easily maintain Tb close to Tset in both fire-maintained and unburned forests without employing potentially costly thermoregulatory behaviors.
In the only other study of T. carolina in a longleaf system, turtles selected predominantly hardwood and mixed pine-hardwood forests and used predominantly pine forests (including longleaf) less often than expected based upon availability (Greenspan et al., 2015), which is consistent with high use of hardwood forests in all seasons at our sites. However, longleaf pines accounted for approximately 21% of the trees in the microhabitats used by turtles at the fire-maintained site (WEWO), while accounting for less than 1% of forest composition at turtle locations at the unburned site (LRSP). It should be noted that we did not assess habitat selection here, as this would require comparing habitat use with availability. Our approach was simply to examine whether seasonal shifts in the forest composition of the habitats used by turtles could be linked to temporal variation in habitat thermal characteristics. As there were no major temporal or spatial changes in the availability of overstory trees in our study systems, an assessment of changes in habitat use alone should be informative for detecting seasonal shifts in forest habitat associations. Further studies could assess whether associations with (or selection of) other aspects of the habitat structure (e.g., cover objects, water availability, understory vegetation structure) vary seasonally, or whether turtles respond to thermal heterogeneity at broader spatial scales (i.e., macrohabitat or landscape scale distribution). It would also be instructive to determine if turtles in the fire-maintained longleaf forest exhibit different seasonal shifts in surface activity timing to avoid periods of extreme mid-day heat (Te > Tset from 1200 – 1800), as has been observed in other terrestrial turtles in thermally challenging habitats (Meek, 1988; Plummer, 2003). Because no turtles died from overheating nor showed any signs of heat stress (other than some that were burned by fire), we suspect that they exhibited some degree of thermoregulatory behavior, but our sampling design of tracking turtles only once per week was insufficient to detect such fine-scale temporal variation in activity. Our results suggest that T. carolina does not exhibit a strong behavioral response to the thermal heterogeneity created by prescribed fire in our system at the spatial and temporal scales we examined. However, we caution that our observations are from only on a single site where fire disturbance has long been a natural part of the system and where prescribed fire has been used in management for the last several decades. Turtle responses to other fire regimes, habitats, or in other parts of their broad geographic range may be different than observed here.
Our study design was also limited in its ability to disentangle the many environmental factors other than temperature that can influence habitat use in turtles. Moreover, habitat associations and other behaviors could be affected by the direct risks imposed by fire, as T. carolina can suffer injury and mortality from burns (Babbitt and Babbitt, 1951; Platt et al., 2010), as well as decreased body condition following fire (Howey and Roosenburg, 2013). Fire could also indirectly affect habitat use behavior by altering understory vegetation structure, hydric conditions, or food availability (Rossell et al., 2006; Platt et al., 2009; Greenspan et al., 2015). It is clear that as fire becomes a more popular tool for habitat management, much more research is required to understand the responses of T. carolina and other terrestrial ectotherms to both wild and prescribed fires.
Highlights.
Prescribed fire influences the thermal characteristics of forest habitats following burns in south-central North Carolina
The higher and more variable temperatures of fire-maintained forests did not improve the suitability of the thermal environment for eastern box turtles when compared to nearby unburned mixed hardwood and pine forests
Eastern box turtles using fire-maintained forests maintained higher shell temperatures than those in nearby unburned mixed hardwood and pine forests
Eastern box turtles did not seasonally associate with the most thermally optimal habitats, suggesting they were not responding to the thermal heterogeneity generated by fire by habitat selection
Acknowledgments
We would like to thank C. Gause, J. Rowlett, J. Smink, C. Wilson, L. Baxley, G. Hoffmann, Z. Lunn, and C. Haywood for assistance with fieldwork. Research was conducted under protocol Roe-2011 issued by the UNC Pembroke Institutional Animal Care and Use Committee and license SC00506 from the North Carolina Wildlife Resources Commission. This work was supported by the Lucille Stickel Fund, North Carolina Herpetological Society, Pembroke Undergraduate Research Center, UNCP Teaching and Learning Center, UNCP Biology Department, and the UNC Pembroke RISE program under the National Institutes of General Medical Sciences grant #5R25GM077634.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams NA, Claussen DL, Skillings J. Effects of temperature on voluntary locomotion of the eastern box turtle, Terrapene carolina. Copeia. 1989;1989:905–915. [Google Scholar]
- Angilletta MJ, Hill T, Robson MA. Is physiological performance optimized by thermoregulatory behavior?: a case study of the eastern fence lizard, Sceloporus undulatus. Journal of Thermal Biology. 2002;27:199–204. [Google Scholar]
- Ashton KG, Engelhardt BM, Branciforte BS. Gopher tortoise (Gopherus polyphemus) abundance and distribution after prescribed fire reintroduction to Florida scrub and sandhill at Archbold Biological Station. J Herpetol. 2008;42:523–529. [Google Scholar]
- Babbitt LH, Babbit CH. A herpetological study in burned-over areas in Dade County, Florida. Copeia. 1951;1951:79. [Google Scholar]
- Bakken GS. Measurement and application of operative and standard operative temperatures in ecology. Am Zool. 1992;32:194–216. [Google Scholar]
- Beerling DJ, Osborne CP. The origin of the savanna biome. Global Change Biol. 2006;12:2023–2031. [Google Scholar]
- Besson AA, Cree A. A cold-adapted reptile becomes a more effective thermoregulator in a thermally challenging environment. Oecologia. 2010;163:571–581. doi: 10.1007/s00442-010-1571-y. [DOI] [PubMed] [Google Scholar]
- Blouin-Demers G, Nadeau P. The cost-benefit model of thermoregulation does not predict lizard thermoregulatory behavior. Ecology. 2005;86:560–566. [Google Scholar]
- Blouin-Demers G, Weatherhead PJ. Thermal ecology of black rat snakes (Elaphe obsoleta) in a thermally challenging environment. Ecology. 2001;82:3025–3043. [Google Scholar]
- Bulté G, Blouin-Demers G. Implications of extreme sexual size dimorphism for thermoregulation in a freshwater turtle. Oecologia. 2010;162:313–322. doi: 10.1007/s00442-009-1469-8. [DOI] [PubMed] [Google Scholar]
- Christian KA, Weavers BW. Thermoregulation of monitor lizards in Australia: an evaluation of methods in thermal biology. Ecol Monogr. 1996;66:139–157. [Google Scholar]
- Congdon JD. Proximate and evolutionary constraints on energy relations of reptiles. Physiol Zool. 1989;62:356–373. [Google Scholar]
- Currylow AF, MacGowan BJ, Williams RN. Short-term forest management effects on a long-lived ectotherm. PLoS ONE. 2012;7:e40473. doi: 10.1371/journal.pone.0040473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin CG. Plasticity in ornate box turtle thermal preference. J Herpetol. 1998;32:298–301. [Google Scholar]
- do Amaral JPS, Marvin GA, Hutchinson VH. Thermoregulation in the box turtles Terrapene carolina and Terrapene ornata. Can J Zool. 2002a;80:934–943. [Google Scholar]
- do Amaral JPS, Marvin GA, Hutchinson VH. The influence of bacterial lipopolysaccharide on the thermoregulation of the box turtle Terrapene carolina. Physiol Biochem Zool. 2002b;75:273–282. doi: 10.1086/341816. [DOI] [PubMed] [Google Scholar]
- Dodd CK. North American Box Turtles: A Natural History. University of Oklahoma Press; Norman, OK: 2001. [Google Scholar]
- Dubois Y, Blouin-Demers G, Thomas D. Temperature selection in wood turtles (Glyptemys insculpta) and its implications for energetics. Écoscience. 2008;15:398–406. [Google Scholar]
- Dubois Y, Blouin-Demers G, Shipley B, Thomas D. Thermoregulation and habitat selection in wood turtles Glyptemys insculpta: chasing the sun slowly. J Anim Ecol. 2009;78:1023–1032. doi: 10.1111/j.1365-2656.2009.01555.x. [DOI] [PubMed] [Google Scholar]
- Edwards AL, Blouin-Demers G. Thermoregulation as a function of thermal quality in a northern population of painted turtles, Crysemys picta. Can J Zool. 2007;85:526–535. [Google Scholar]
- Ellner LR, Karasov WH. Latitudinal variation in the thermal biology of ornate box turtles. Copeia. 1993;1993:447–455. [Google Scholar]
- Elzer AL, Pike DA, Webb JK, Hammill K, Bradstock RA, Shine R. Forest-fire regimes affect thermoregulatory opportunities for terrestrial ectotherms. Austral Ecol. 2013;38:190–198. [Google Scholar]
- Erskine DJ, Hutchison VH. Melatonin and behavioral thermoregulation in the turtle, Terrapene carolina triunguis. Physiol Behav. 1981;26:991–994. doi: 10.1016/0031-9384(81)90198-0. [DOI] [PubMed] [Google Scholar]
- Frost CC. Presettlement fire frequency regimes of the United States: a first approximation. In: Pruden TL, Brennan LA, editors. Fire in ecosystem management: shifting the paradigm from suppression to prescription. Tall Timbers Fire Ecology Conference Proceedings, No. 20. Tall Timbers Research Station; Tallahassee, FL: 1998. pp. 70–81. [Google Scholar]
- Gatten RE. Effect of nutritional status on the preferred body temperature of the turtles Pseudemys scripta and Terrapene ornata. Copeia. 1974;1974:912–917. [Google Scholar]
- Graham TE, Hutchison VH. Effect of temperature and photoperiod acclimatization on thermal preferences of selected freshwater turtles. Copeia. 1979;1979:165–169. [Google Scholar]
- Grayson KL, Dorcas ME. Seasonal temperature variation in the painted turtle (Chrysemys picta) Herpetologica. 2004;60:325–336. [Google Scholar]
- Greenberg CH, Waldrop TA. Short-term response of reptiles and amphibians to prescribed and mechanical fuel reduction in a southern Appalachian upland hardwood forest. Forest Ecol Manag. 2008;255:2883–2893. [Google Scholar]
- Greenspan SE, Condon EP, Smith LL. Home range and habitat selection of eastern box turtle (Terrapene carolina carolina) in a longleaf pine (Pinus palustris) reserve. Herpetol Conserv Bio. 2015;10:99–111. [Google Scholar]
- Haines TK, Busby RL, Cleaves DA. Prescribed burning in the south: trends, purpose, and barriers. South J Appl For. 2001;25:149–153. [Google Scholar]
- Hertz PE, Huey RB, Stevenson RD. Evaluating temperature regulation by field-active ectotherms: the fallacy of the inappropriate question. Am Nat. 1993;142:796–818. doi: 10.1086/285573. [DOI] [PubMed] [Google Scholar]
- Hossack BR, Eby LA, Guscio CG, Corn PS. Thermal characteristics of amphibian microhabitats in a fire-disturbed landscape. Forest Ecol Manag. 2009;258:1414–1421. [Google Scholar]
- Howey CAF, Roosenburg WM. Effects of prescribed fire on the eastern box turtle (Terrapene carolina carolina) Northeast Nat. 2013;20:493–497. [Google Scholar]
- Huey RB. Temperature, physiology, and the ecology of reptiles. In: Gans C, Pough F, editors. Biology of the Reptilia. Vol. 12. Academic Press; N.Y: 1982. pp. 25–91. [Google Scholar]
- Huey RB, Bennett AF. Phylogenetic studies of coadaptation: preferred temperatures versus optimal performance temperatures of lizards. Evolution. 1987;41:1098–1115. doi: 10.1111/j.1558-5646.1987.tb05879.x. [DOI] [PubMed] [Google Scholar]
- Huey RB, Slatkin M. Costs and benefits of lizard thermoregulation. Q Rev Biol. 1976;51:363–384. doi: 10.1086/409470. [DOI] [PubMed] [Google Scholar]
- Iverson LR, Hutchinson TF. Soil temperature and moisture fluctuations during and after prescribed fire in mixed-oak forests, USA. Nat Area J. 2002;22:296–304. [Google Scholar]
- Keeley JE, Rundel PW. Fire and the Miocene expansion of C4 grasslands. Ecol Lett. 2005;8:683–690. [Google Scholar]
- Keister AR, Willey LL. Terrapene carolina (Linnaeus 1758) – Eastern Box Turtle, Common Box Turtle. In: Rhodin AGJ, Pritchard PCH, van Dijk PP, Saumure RA, Buhlmann KA, Iverson JB, Mittermeier RA, editors. Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. 2015. p. 5. Chelon Res Monogr. [DOI] [Google Scholar]
- Lagarde F, Louzizi T, Slimani T, el Mouden H, Ben Kaddour K, Moulherat S, Bonnet X. Bushes protect tortoises from lethal overheating in arid areas of Morocco. Environ Conserv. 2012;39:172–182. [Google Scholar]
- Matthews CE, Moorman CE, Greenberg CH, Waldrop TA. Response of reptiles and amphibians to repeated fuel reduction treatments. J Wildlife Manage. 2010;74:1301–1310. [Google Scholar]
- Meek R. The thermal ecology of Hermann’s tortoise (Testudo hermanni) in summer and autumn ion Yugoslovia. J Zool. 1988;215:99–111. [Google Scholar]
- Monagas WR, Gatten RE. Behavioral fever in the turtles Terrapene carolina and Chrysemys picta. J Therm Biol. 1983;8:285–288. [Google Scholar]
- Mushinsky HR. Natural history and abundance of southeastern five-lined skinks, Eumeces inexpectatus, on a periodically burned sandhill in Florida. Herpetologica. 1992;48:307–312. [Google Scholar]
- Mushinsky HR. Fire and the Florida sandhill herpetofaunal community: with special attention to responses of Cnemidophorus sexlineatus. Herpetologica. 1985;41:333–342. [Google Scholar]
- Nieuwolt PM. Movement, activity, and microhabitat selection in the western box turtle, Terrapene ornata. Herpetologica. 1996;52:487–495. [Google Scholar]
- Nowacki GJ, Abrams MD. The demise of fire and “mesophication” of forests in the eastern United States. BioScience. 2008;58:123–138. [Google Scholar]
- Parmenter RR. Effects of food availability and water temperature on the feeding ecology of pond sliders (Chrysemys s. scripta) Copeia. 1980;1980:503–514. [Google Scholar]
- Pastro LA, Dickman CR, Letnic M. Burning for biodiversity or burning biodiversity? Prescribed burn vs wildfire impacts on plants, lizards, and mammals. Ecol Appl. 2011;21:3238–3253. [Google Scholar]
- Pausas JG, Keeley JE. A burning story: the role of fire in the history of life. BioScience. 2009;59:593–601. [Google Scholar]
- Penick DN, Congdon JD, Spotila JR, Williams JB. Microclimates and energetics of free-living box turtles Terrapene carolina, in South Carolina. Physiol Biochem Zool. 2002;75:57–65. doi: 10.1086/339219. [DOI] [PubMed] [Google Scholar]
- Picard G, Carrière MA, Blouin-Demers G. Common musk turtles (Sternotherus odoratus) select habitats of high thermal quality at the northern extreme of their range. Amphibia-Reptilia. 2011;32:83–92. [Google Scholar]
- Platt SG, Hall C, Liu H, Borg CK. Wet-season food habitat and intersexual dietary overlap of Florida box turtles (Terrapene carolina bauri) on National Key Deer Wildlife Refuge, Florida. Southeast Nat. 2009;8:335–346. [Google Scholar]
- Platt SG, Liu H, Borg CK. Fire ecology of the Florida box turtle in pine rockland forests of lower Florida Keys. Nat Area J. 2010;3:254–260. doi: 10.1007/s00442-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Plummer MV. Activity and thermal ecology of the box turtle, Terrapene ornata, at its southwestern range limit in Arizona. Chelonian Conserv Biol. 2003;4:569–577. [Google Scholar]
- Plummer MV, Williams BK, Skiver MM, Carlyle JC. Effects of dehydration on the critical thermal maximum of the desert box turtle (Terrapene ornata luteola) J Herpetol. 2003;37:747–750. [Google Scholar]
- Polo-Cavia N, López P, Martin J. Interspecific differences in heat exchange rates may affect competition between introduced and native freshwater turtles. Biol Invasions. 2009;11:1755–1765. [Google Scholar]
- Rossell CR, Rossell IM, Patch S. Microhabitat selection by eastern box turtles (Terrapene c. carolina) in a North Carolina mountain wetland. J Herpetol. 2006;40:280–284. [Google Scholar]
- Row JR, Blouin-Demers G. Thermal quality influences effectiveness of thermoregulation, habitat use, and behavior in milk snakes. Oecologia. 2006;148:1–11. doi: 10.1007/s00442-005-0350-7. [DOI] [PubMed] [Google Scholar]
- Schuett GW, Gatten RE. Thermal preference in snapping turtles (Chelydra serpentina) Copeia. 1980;1980:149–152. [Google Scholar]
- Steen DA, Smith LL, Morris G, Conner LM, Litt AR, Pokswinski S, Guyer C. Response of six-lined racerunner (Aspidoscelis sexlineata) to habitat restoration in fire-suppressed longleaf pine (Pinus palustris) sandhills. Restoration Ecol. 2013;21:457–463. [Google Scholar]
- Sturbaum BA. Responses of the three-toed box turtle, Terrapene carolina triunguis, to heat stress. Comp Biochem Physiol. 1981;70A:199–204. [Google Scholar]
- Swingland IR, Lessells CM. The natural regulation of giant tortoise populations on Aldabra atoll. Movement, polymorphism, reproductive success and mortality. J Anim Ecol. 1979;48:639–654. [Google Scholar]
- Wright HA, Bailey AW. Fire Ecology: United States and Southern Canada. Wiley; New York, N.Y: 1982. [Google Scholar]