Abstract
Diabetic retinopathy (DR) is a major diabetes complication and the leading cause for vision loss and blindness in the adult human population. Diabetes, being an endocrinological disorder dysregulates a number of hormonal systems including the renin angiotensin system (RAS), which thereby may damage both vascular and neuronal cells in the retina. Angiotensin II (Ang II), an active component of the RAS is increased in diabetic retina, and may play a significant role in neurovascular damage leading to the progression of DR. In this review article, we highlight the role of Ang II in the pathogenesis of retinal damage in diabetes and discuss a newly identified mechanism involving tissue chymase and angiotensin-(1-12) [Ang-(1-12)] pathways. We also discuss the therapeutic effects of potential RAS inhibitors targeting blockade of cellular Ang II formation to prevent/protect the retinal damage. Thus, a better understanding of Ang II formation pathways in the diabetic retina will elucidate early molecular mechanism of vision loss. These concepts may provide a novel strategy for preventing and/or treating diabetic retinopathy, a leading cause of blindness worldwide.
Keywords: Angiotensin II, chymase, diabetic retinopathy, neurodegeneration, oxidative stress, renin-angiotensin system, retina
1. INTRODUCTION
Diabetic retinopathy (DR) is the most common microvascular complication in diabetic subjects and is the leading cause of visual loss and blindness among working adults worldwide. DR develops within 20 years in most patients with Type 1 diabetes and about half of Type 2 diabetes after the onset of diabetes. Globally, the epidemic of diabetes is increasing rapidly and based on the most recent estimate, 415 million have some form of diabetes, suggesting a growing concern of DR [1]. According to the World Health Organization, the prevalence of DR is about 30% among diabetic subjects and the number of people at risk of vision loss is predicted to be double in the next 30 years [2]. The clinical signs of the disease in the retina include blood vessels swelling, leakage, and neovascularization which lead to severe vitreous hemorrhage and/or retinal detachment compromising vision loss. Before any clinical signs of vascular damage, neuronal components of the retina have been found to be compromised with retinal function deficit, suggesting neurodegeneration might be an initiating factor leading to vascular damage in DR [3, 4]. Cellular, molecular and biochemical analyses of diabetic retina revealed that neurovascular damage is caused by a number of mechanisms including oxidative stress, apoptosis, neurodegeneration and inflammation that lead to DR [5, 6].
The prevalence and incidence of DR increase with diabetic duration, and worsen with poor control of hyperglycemia and blood pressure (hypertension) according to two well-known clinical trials: Diabetes, Control and Complications Trial (DCCT) [7], and the UK Prospective Diabetes Study (UKPDS) [8]. Hypertension has been widely recognized as a potential risk factor for the damage of vasculature in diabetic induced complications including retinopathy and nephropathy. In diabetes, the components of the renin angiotensin system (RAS) are found to be increased both systemically and locally in several tissues including the retina. Angiotensin II (Ang II), the principal component of the RAS regulates blood pressure, body fluid, electrolytes balance and influences homeostasis both systemically and at cellular levels [9]. However, elevated Ang II levels in diabetic retina plays a pathogenic role in activating inflammation, oxidative stress, neurodegeneration and endothelial dysfunction, which may lead to DR [10, 11].
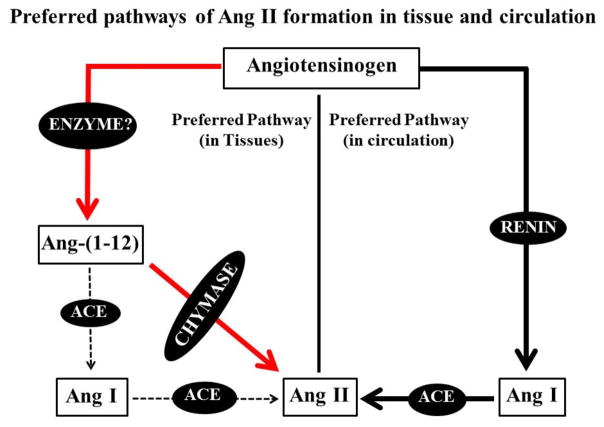
Past studies had concluded that the biochemical mechanisms involved in Ang II formation consisted of a linear sequential processing of the angiotensinogen substrate into angiotensin I (Ang I) by renin which was then acted upon by angiotensin converting enzyme (ACE) to generate Ang II. The singularity of this biotransformation process was first challenged by Ferrario and collaborators with the discovery of an alternate processing of Ang I into angiotensin-(1-7) [Ang-(1-7)] which acted as a potent endogenous Ang II antagonist [12]. The more recent characterization of a shorter form of the Ang II-forming substrate angiotensin-(1-12) [Ang-(1-12)] has now demonstrated a more complex and distinctly discriminative pathway for the generation of the active Ang II and Ang-(1-7) hormones both upstream and downstream of Ang I [13]. Studies demonstrating a direct formation of Ang II from Ang-(1-12) by chymase brought to the forefront past studies which implicated chymase rather than ACE as the major enzyme accounting for processing Ang I into Ang II as depicted in Fig. (1) [14].
Fig. 1.
The preferred pathways of Ang II formation in tissues and circulation. In tissues, Ang II formation is primarily mediated via chymase and Ang-(1-12) [red solid lines] and is independent of renin whereas, Ang II formation in circulation via renin and ACE (black solid lines). Also in tissues, ACE is not preferred pathway to generate Ang II from Ang-(1-12)/Ang I (black dash lines).
Chymase, another RAS enzyme primarily expressed in various tissues and cells converts Ang I into Ang II at 20-fold higher rate than ACE [15]. Ang II is the major effector peptide which acts through RAS receptors, type 1 (AT1R) and type 2 (AT2R). Binding of Ang II to AT1R activates various downstream cellular signaling pathways which subsequently stimulates the cellular factors [16–19]. The functional and biological effect mediated through AT2R is still unclear. However, activation of the AT2R has been documented to oppose the AT1R-mediated actions. Ang II is further cleaved by ACE2 into Ang-(1-7). Ang-(1-7) is a protective molecule which opposes the pathological effects of Ang II via its Mas receptor (MasR).
2. ROLE OF RAS COMPONENTS IN DIABETIC RETINOPATHY
Clinical and experimental studies have suggested that abnormalities of the RAS may play a significant role in the progression of the diabetic retinopathy, presumably through local changes in the blood flow and the production of Ang II primarily by ACE. Besides circulating RAS, this system is also present locally in various eye tissues including retina and occurs independently at the tissue level [20, 21]. Components of RAS are most abundantly expressed both in the neuronal as well as vascular cells of the retina. RAS genes expressions have also been detected in the various eye tissues [22, 23]. Rodents studies suggest that components of RAS including prorenin, angiotensinogen, Ang II, ACE, ACE2, AT1R are increased in the retina with diabetes [24–27]. An increased level of Ang II and other RAS substances have been detected in vitreous pool of proliferative diabetic retinopathy patients compared to non-diabetic subjects [28]. Further, it has been found that the RAS components are produced locally in the tissues and not filtered from the circulation [22, 26]. Numerous studies have shown that RAS plays a pivotal role in the progression of retinal disease, presumably through local changes in RAS level by increasing the Ang II production [24, 29, 30]. They suggested that these local RAS factors, especially Ang II formation are the major source of pathophysiological action in damaging both neuronal and vascular components of the diabetic retina.
3. RAS AND VASCULAR DAMAGE IN DIABETIC RETINA
Retinal microvascular cells play important roles in the regulation of capillary tone and retinal homeostasis [31, 32]. Ang II impacts the retinal microvasculature by damaging both pericytes and endothelial cells [33]. Ang II causes a decrease in pericyte viability by increasing apoptosis which thereby uncouples them from the vasculature to initiate the generation of DR [33–35]. In addition, Ang II induced several biochemical abnormalities in vitreous of humans with proliferative diabetic retinopathy including increased vascular endothelial growth factor (VEGF), deposition of advanced glycation end products (AGEs), matrix metalloproteinase-9 (MMPs) and collagen level [36–40]. AGEs (an important mediator of diabetes-related vascular injury) is produced and deposited as a result of prolonged hyperglycemia. In animal model, it has been observed that exogenous administration of AGEs in vivo promotes atherosclerosis, whereas chemical degradation of AGEs or inhibition of AGE formation decreases microvascular and macrovascular diabetic complications.
In diabetic patients, these vasoactive factors (VEGF, AGEs, MMPs and collagen level) are induced and have been shown to be associated with retinopathy. Studies in diabetic rodents have indicated that ACE inhibitors and AT1R blockers reduced retinal microvascular damage with reductions in vascular leakage, decreased formation of acellular capillaries, and decreased expression of angiogenic factors such as VEGF [41–43]. Retinal leukostasis and the upregulation of adhesion molecules are also reduced with ACE inhibition and AT1R blockade [41, 44, 45]. Thus, increased levels of Ang II is critical for vascular damage in diabetic retina. New approaches to combat Ang II formation or blocking its receptors at an early disease stage may prevent the damages of retinal cells and vessels; thereby may ameliorate the progression of DR.
4. RAS AND NEURONAL DAMAGE IN THE DIABETIC RETINA
A large number of studies show that several prominent neurotrophic factors (such as brain derived neurotrophic factor (BDNF), nerve growth factor (NGF), VEGF, Pigment epithelium-derived factor (PEDF), apoptotic markers (Caspase-3/9) and oxidative stress parameters glutathione (GSH), thiobarbituric acid reactive substances (TBARS) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase have been altered in the retina early after the onset of diabetes [46–52]. These altered factors play a significant role in damaging neuronal cells in the retina that causes the microvascular damage later in DR. In the neural retina, ganglion and Muller cells have been found to express Ang II, AT1R and AT2R [53, 54]. AT1R is expressed in synaptic vesicles in the inner retina which is critical for neurotransmission [54]. Few studies suggest that diabetes induced Ang II activates NADPH oxidase via AT1R stimulation and produces reactive oxygen species, subsequently inducing retinal ganglion cell impairment and death in the diabetic retina [55–58]. AT2R is expressed mostly in the neuronal component of the retina compared to vasculature both in human and rodents model of diabetic retina [30, 53]. In addition, an increased expression level of AT2R was found in diabetic retina [59]. However, the role of AT2R is not yet fully understood in diabetic retina, but some studies suggest its beneficial effects towards cell survival and regeneration in neuronal tissues [60, 61].
Numerous studies reported that an increased level of Ang II is implicated not only in vascular but also in neuronal damage in diabetic retina. Previously, an increased level of AT1R is reported in diabetic retina which resulted in the impaired neuronal function and the AT1R blocker telmisartan suppressed the impaired retinal function [54, 62]. In animal model of glaucoma, AT1R blocker treatment resulted in neuroprotection against retinal ganglion cell loss [63]. In our recent study beneficial effects towards reduction of neurotrophic factors and oxidative stress in the diabetic rat retina were achieved with telmisartan, a drug possessing strong AT1R affinity [64]. The ameliorated level of oxidative stress by the drug may induce an increase in the level of neurotrophic factors and thereby decrease apoptosis in the diabetic retina. In addition, other studies suggested beneficial effects of telmisartan in several brain diseases including the inhibition of cognitive function in the hippocampus of hypertensive rats, lowering the activity of sympathetic nervous system, and in the suppression of impaired retinal function in diabetes [62, 65, 66]. In diabetic animals, ACE inhibition and AT1R blockade have also been reported to attenuate the deficits in retinal function, indicating benefits to neuronal cells. Another study showed that AT1R blockade reduced diabetes-related changes in the electroretinogram and attenuated the level of the synaptic vesicle protein, synaptophysin, showing the effect on retinal neurons in diabetes [62, 67]. In addition, AT1R antagonists reduced the degeneration of dopaminergic neurons [68]. Thus, ameliorating the Ang II level or signaling may protect retinal neurons and thereby retinal function in diabetic retinopathy.
5. ACE2/ANG-(1-7)/MAS RECEPTOR AXIS IN DR
Several studies showed the upregulation of ACE2/Ang-(1-7)/MasR axis which contribute to protective roles associated with complications in diabetic eyes [27, 69–71]. However, the impact of the vasoprotective axis of the ACE2/Ang-(1-7)/MasR in diabetic patient with DR remains poorly understood. Studies suggest that the protective effect of Ang-(1-7) signaling is at least in part mediated via stimulation of nitric oxide production and decreased production of reaction oxygen species, and increased in endothelial nitric oxide synthase [27]. Recent study shows that diabetic microvascular complications can be prevented and reversed by overexpressing the ACE2 level in diabetic rodent retina [72]. Another study reported that the inflammatory response induced by lipopolysaccharide in retinal pigment epithelium (RPE) cells were decreased with diminazene aceturate (DIZE, an ACE2 activator) treatment [73]. They also found that DIZE reduced the expression of Ang II and AT1R, whereas it increased the Ang-(1-7) level in RPE cells. Thus, strategies to enhance the ACE2/Ang-(1-7)/MasR axis in diabetic eye could be a novel therapeutic approach in DR prevention.
6. CLINICAL TRIALS TO BLOCK ANG II OR ITS RECEPTOR IN DIABETIC RETINOPATHY
Studies from clinical and experimental models suggest that the RAS is activated in diabetic retinopathy and its blockade might protect retinal damage [74–80]. Several clinical trials have been done on hypertensive diabetic people (type 1 and type 2 diabetes) to investigate the beneficial effects of antihypertensive drugs (ACE inhibitors and/or ARBs blockers) on DR reduction. We have summarized the results of major clinical trials in Table 1.
Table 1.
List of antihypertensive (ACE, ARBs and diuretic) drugs in clinical trials on DR.
| Trials Name | Group | DR risk factor | References |
|---|---|---|---|
|
| |||
| EUCLID Trial | |||
| Lisinopril (ACE inhibitor) | Insulin dependent | 50% reduction | #74 |
|
| |||
| DIRECT Trial | |||
| Candesartan (AT1R blocker) | Type 1 diabetes | 18% (reduction) | #75 |
| Type 2 diabetes | 34% (reduction) | #76 | |
|
| |||
| RASS (5 year follow-up) | |||
| Enalapril (ACE inhibitor) | Type 1 diabetes | 65% (reduction) | #77,78 |
| Losartan (AT1R blocker) | Type 1 diabetes | 70% (reduction) | #77,78 |
|
| |||
| ABCD Trial | |||
| On blood pressure medications | Hypertensive + Type 2 diabetes | No progression of DR | #79 |
|
| |||
| ADVANCE Trial | |||
| Perindopril (ACE inhibitor) + Indapamide (antihypertensive/diuretic drug) |
Hypertensive + Diabetes | Reduced cardiovascular mortality but no effect on retinopathy risk. | #80 |
(EUCLID: The EURODIAB Controlled trial of Lisinopril in Insulin dependent Diabetes; DIRECTEUCLID: Diabetic Retinopathy Candesartan Trial; RASS: The Renin-Angiotensin System Study; ABCD: The Appropriate Blood Pressure Control in Diabetes; ADVANCE: The Action in Diabetes and Vascular Disease Controlled Evaluation).
These clinical trials show modest benefits of ACE inhibitors and/or ARBs treatments on reduction of DR in hypertensive diabetic people. Further, these clinical trials data indicate that a significant number of diabetic people were unbenefited from the ACE inhibitor and ARB therapy, which suggests that the non-ACE-dependent pathway might also be responsible for the progression of DR. Although, the Ang II receptor antagonist drugs are successfully included in the management of diabetic complications, dismissal of adverse effects of these drugs exposes treated patients to progression of the disease process. Also the ACE drugs have limited efficacy to cross the cell membrane to inhibit the intracellular Ang II formation. Thus, the therapeutic approaches to prevent the progression of DR in diabetic people using the ACE inhibitor require further exploration. Our recent studies show that the chymase/Ang-(1-12) axis is primarily responsible for the generation of cellular Ang II rather than the ACE [81, 82]. These findings further suggest that the combination therapy (chymase and ACE inhibitions), compared to ACE inhibition alone, might be more beneficial to prevent the DR progression in diabetic people.
7. FORMATION OF ANG II BY TISSUE SPECIFIC CHYMASE
Chymase (EC 3.4.21.39, also known as mast cell protease) is a member of the serine class of proteases which are abundantly present in mast cells co-existing with tryptases and carboxypeptidase A [83, 84]. In non-rodent mammals only α-form of the chymase gene is found which selectively hydrolyzes the Phe8-His9 bond of the angiotensin substrates to generate directly Ang II from both Ang-(1-12) and Ang I [85]. However, rodents have several isoforms of β-chymase in addition to the α-form [83, 86]. One of the β-chymase (rat mast cell protease-1, RMCP-1) shows a strong preference to hydrolyze the Tyr4-Ile5 bond, also known as Ang II-destroying β-chymase [87]. Chymases are expressed in various tissues and cells, and the enzyme has been reported to convert Ang I into Ang II at much higher rate compared to ACE [15]. Thus, the classical concept of Ang II formation from Ang I by ACE both in circulation and locally in various organs is amended with the new concept that the intracellular Ang II generation primarily occurs by chymase rather than ACE.
RAS was originally thought to be in a linear pathway which sequentially processes the angiotensinogen to generate biologically active Ang peptides. Researchers believed Ang I as the sole substrate for Ang II production. This concept was changed after the discovery of a new member of the RAS family [Ang-(1-12)] [13]. The amino acid sequence of this novel RAS peptide [Ang-(1-12)] is similar to the sequence of Ang I plus -Val11-Ile12 (in human) and -Leu11-Try12 (in rodent) position of the C-terminus. Compared to Ang I, higher concentration of Ang-(1-12) has been detected in hypertensive rat and diseased human tissues [88, 89].
8. CURRENT CONCEPT OF THE ANG-(1-12)/ CHYMASE AXIS
The Ang II peptide [Ang-(1-8)] is the principal component of RAS which regulates various physiological processes and influences the homeostasis both systemically and at cellular levels. A growing number of studies suggest that Ang-(1-12) plays a significant role in Ang II-formation both at the tissue level and in the circulation [85, 90–93]. Our studies suggest that Ang-(1-12) might serve as an alternate pathway for the generation of Ang II, a pathway that may be of relevance in situations of suppressed renin activity or secretion, as well as functioning as an intracellular precursor for the formation of Ang II [92, 93]. Chymase plays a crucial role in the pathogenesis of organ specific tissue damage, including atherosclerosis, adverse cardiac remodeling, and arrhythmias [85, 94]. Our studies show that human chymase converts Ang-(1-12) and Ang I directly into Ang II with greater efficiency and selectivity than the ACE [85, 92]. More recently Ahmad et al., (2016) demonstrated that chymase has a higher affinity for the Ang-(1-12) compared to Ang I to generate Ang II product in both human and rodents [81].
9. POSSIBLE ROLE OF CHYMASE/ANGIOTENSIN-(1-12) AXIS IN DIABETIC RETINOPATHY
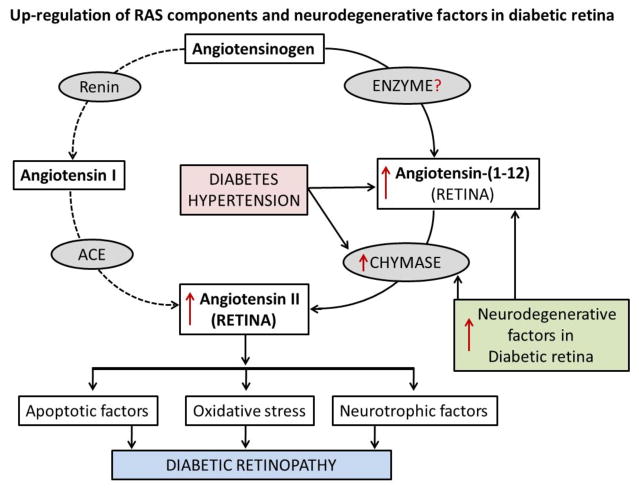
Several recent studies suggest that chymase and RAS components are upregulated in several diseases including diabetes and hypertension in various tissues [85, 92, 95, 96]. Our initial study shows a higher chymase activity in vitreous fluid of diabetic patients compared to non-diabetic subjects [97]. At present, we do not know whether Ang-(1-12) level is also increased along with chymase enzyme and responsible for increased synthesis of Ang II in diabetic retina and vitreous fluids via this non-canonical pathway as depicted in the Fig. (2). The enzyme responsible for the generation of Ang-(1-12) has not yet been identified, but ubiquitous distribution of the Ang-(1-12) has been detected in rodent tissues and diseased human heart [13, 92]. Growing number of studies support a role of a local RAS in retinal damage and progression of retinopathy. Recent studies by our group suggest that intracellular Ang II generation may occur primarily by chymase rather than ACE [81, 92]. Moreover, the affinity of chymase to generate intracellular Ang II is several folds higher than that of ACE in circulation. Addition of AGEs in the cultured human aortic vascular smooth muscle cells (VSMCs) induced a marked expression of chymase mRNA and protein in a time and dose-dependent manner [38]. Studies also show fibrosis and apoptosis in the macular region of the eye after chymase injected into the monkey vitreous [98]. High levels of chymase activity have been detected in the vitreous humor of patients with idiopathic macular hole [95, 96, 98]. Chymase activity was also detected in the anterior uveal tract, choroid and sclera in dog eyes and in the anterior uveal tract of monkey eyes [99]. Thus, cellular Ang II formation by chymase might also be a source for pathophysiological actions and the progression of retinopathy in diabetic subjects. However, no confirmatory studies have been done to show whether the increase in Ang II level in the diabetic eye was due to ACE or chymase. The angiotensin peptides are unable to cross the blood retinal barriers into the circulation unless there is damage in the blood vessels and leakage of blood in the retina (hypertensive retinopathy). Studies show that cellular formation of Ang II via intracellular chymase pathway is independent of ACE and not affected by inhibition of Ang II production by ACE inhibitors as these agents do not penetrate beyond the cell membrane.
Fig. 2.
Possible pathways for the upregulation of RAS components and retinal degenerative factors in diabetic retinopathy. Progression of diabetic retinopathy via classical RAS pathway (dotted lines) and alternate RAS pathway of increased Ang II formation from Ang-(1-12) by chymase, influences retinal damaging factors in the diabetic retina.
10. NEW THERAPEUTIC APPROACH TO BLOCK CELLULAR ANG II FORMATION
Overall, this review article clearly describes the non-canonical pathway of Ang II formation via chymase and from Ang I upstream precursor [Ang-(1-12)], and its importance in progression of retinopathy in diabetic subjects. There is a dire need for new approaches in the management of diabetic retinopathy since current non-surgical approaches using ACE inhibitors and AT1R blockers have relatively partial success in prevention and progression of retinopathy in diabetic subjects. Expanding knowledge of the biochemical pathways including RAS offers an opportunity for novel therapies with the recognition of each new component responsible for diabetic retinopathy. Chymase-mediated Ang II-forming peptide at cellular levels from the novel Ang-(1-12) substrate has generated a new clinical concept of the role of RAS in the pathogenesis and progression of diseases. This working paradigm may resolve the previous observations that Ang II formation in cells is not prevented or partially prevented by ACE inhibitors and Ang II receptors blockers (ARBs). These advances explain the dual role of the RAS as circulating hormonal and tissue-specific system wherein the expression of angiotensin substrate serves not only autocrine/paracrine but also intracrine functions. Research focus on the intracrine synthesis of Ang II produced from a chymase-mediated cleavage of Ang-(1-12) and/or Ang I in the retina of either hypertensive-diabetic human or diabetic rodent models are subjects of further investigation. Selective chymase inhibitors will be helpful to understand the mechanisms of retinopathy progression and also to develop the early treatment. We have evidence that the intracellular formation of Ang II via chymase pathway is independent and not affected by inhibition of Ang II production by ACE or ARBs [12, 100]. However, further research is needed in this regard to investigate the beneficial effects of chymase inhibition over ACE in the treatment/prevention of retinopathy and other eye diseases. Furthermore, studies are also warranted to determine whether systemic reduction of blood pressure or cellular blockage of Ang II would improve neurovascular pathology in diabetic retinopathy.
CONCLUSION
Numerous studies suggest an increased level of Ang II in the diabetic retina which may play a major role in neurovascular damage that leads to DR. Previously, Ang I was considered as the sole source of Ang II formation from sequential cleavage of angiotensinogen protein by renin to Ang I and further by ACE/chymase into Ang II. However, our recent studies in human and rodent tissues showed that Ang-(1-12), an Ang I-upstream precursor was the preferred substrate for intracellular Ang II formation via chymase rather than ACE. Consistent with studies reported on tissue specific increased expression and activity of chymase and Ang II content under pathological conditions, our initial studies also show an increased activity of chymase in the diabetic retina which might be responsible for the retinal damage and DR progression. Moreover, few clinical trials of ACE and ARBs inhibitors showed modest reduction of DR while a significant number of DR patients did not get benefit, suggesting that non-ACE dependent pathway might also be responsible for the progression of DR. Thus, further research is needed to investigate the potential alternate chymase-Ang II pathway and the beneficial effects of chymase inhibition over ACE and/or ARBs in the treatment/prevention of diabetic retinopathy.
Acknowledgments
Author (MSO) would like to thank funding support from King Abdul Aziz City for Science and Technology (KACST), grant number ARP: 30-23.
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
DISCLAIMER: The above article has been published in Epub (ahead of print) on the basis of the materials provided by the author. The Editorial Department reserves the right to make minor modifications for further improvement of the manuscript.
References
- 1.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O’Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY. Global prevalence and major risk factors of diabetic retinopathy. Diabetes care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. The Journal of clinical investigation. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Y, Wang Y, Stock O, Pfister F, Tanimoto N, Seeliger MW, Hillebrands JL, Hoffmann S, Wolburg H, Gretz N, Hammes HP. Vasoregression linked to neuronal damage in the rat with defect of polycystin-2. PloS one. 2009;4:e7328. doi: 10.1371/journal.pone.0007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen SR, Gardner TW. Diabetic Retinopathy and Diabetic Macular Edema. Developments in ophthalmology. 2016;55:137–146. doi: 10.1159/000438970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ola MS, Nawaz MI, Siddiquei MM, Al-Amro S, Abu El-Asrar AM. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. Journal of diabetes and its complications. 2012;26:56–64. doi: 10.1016/j.jdiacomp.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 7.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. The New England journal of medicine. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 8.Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Archives of ophthalmology (Chicago, Ill: 1960) 2004;122:1631–1640. doi: 10.1001/archopht.122.11.1631. [DOI] [PubMed] [Google Scholar]
- 9.Culman J, Hohle S, Qadri F, Edling O, Blume A, Lebrun C, Unger T. Angiotensin as neuromodulator/neurotransmitter in central control of body fluid and electrolyte homeostasis. Clinical and experimental hypertension (New York, NY: 1993) 1995;17:281–293. doi: 10.3109/10641969509087071. [DOI] [PubMed] [Google Scholar]
- 10.Funatsu H, Yamashita H, Nakanishi Y, Hori S. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy. The British journal of ophthalmology. 2002;86:311–315. doi: 10.1136/bjo.86.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manea A, Constantinescu E, Popov D, Raicu M. Changes in oxidative balance in rat pericytes exposed to diabetic conditions. J Cell Mol Med. 2004;8:117–126. doi: 10.1111/j.1582-4934.2004.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrario CM, Ahmad S, Nagata S, Simington SW, Varagic J, Kon N, Dell’italia LJ. An evolving story of angiotensin-II-forming pathways in rodents and humans. Clinical science (London, England: 1979) 2014;126:461–469. doi: 10.1042/CS20130400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochemical and biophysical research communications. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- 14.Wei CC, Hase N, Inoue Y, Bradley EW, Yahiro E, Li M, Naqvi N, Powell PC, Shi K, Takahashi Y, Saku K, Urata H, Dell’italia LJ, Husain A. Mast cell chymase limits the cardiac efficacy of Ang I-converting enzyme inhibitor therapy in rodents. The Journal of clinical investigation. 2010;120:1229–1239. doi: 10.1172/JCI39345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urata H, Boehm KD, Philip A, Kinoshita A, Gabrovsek J, Bumpus FM, Husain A. Cellular localization and regional distribution of an angiotensin II-forming chymase in the heart. The Journal of clinical investigation. 1993;91:1269–1281. doi: 10.1172/JCI116325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert RE, Vranes D, Berka JL, Kelly DJ, Cox A, Wu LL, Stacker SA, Cooper ME. Vascular endothelial growth factor and its receptors in control and diabetic rat eyes. Laboratory investigation; a journal of technical methods and pathology. 1998;78:1017–1027. [PubMed] [Google Scholar]
- 17.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller DN, Fiebeler A, Park JK, Dechend R, Luft FC. Angiotensin II and endothelin induce inflammation and thereby promote hypertension-induced end-organ damage. Clinical nephrology. 2003;60(Suppl 1):S2–12. [PubMed] [Google Scholar]
- 19.Yoshiji H, Kuriyama S, Kawata M, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H. The angiotensin-I-converting enzyme inhibitor perindopril suppresses tumor growth and angiogenesis: possible role of the vascular endothelial growth factor. Clinical cancer research: an official journal of the American Association for Cancer Research. 2001;7:1073–1078. [PubMed] [Google Scholar]
- 20.Brandt CR, Pumfery AM, Micales B, Bindley CD, Lyons GE, Sramek SJ, Wallow IH. Renin mRNA is synthesized locally in rat ocular tissues. Current eye research. 1994;13:755–763. doi: 10.3109/02713689409047011. [DOI] [PubMed] [Google Scholar]
- 21.Van Haeringen NJ. The renin-angiotensin system in the human eye. The British journal of ophthalmology. 1996;80:99–100. doi: 10.1136/bjo.80.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kida T, Ikeda T, Nishimura M, Sugiyama T, Imamura Y, Sotozono C, Nishida K, Kinoshita S, Yoshimura M, Nakamura K, Inokuchi N. Renin-angiotensin system in proliferative diabetic retinopathy and its gene expression in cultured human muller cells. Japanese journal of ophthalmology. 2003;47:36–41. doi: 10.1016/s0021-5155(02)00624-x. [DOI] [PubMed] [Google Scholar]
- 23.Wagner J, Jan Danser AH, Derkx FH, de Jong TV, Paul M, Mullins JJ, Schalekamp MA, Ganten D. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular renin-angiotensin system. The British journal of ophthalmology. 1996;80:159–163. doi: 10.1136/bjo.80.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danser AH, van den Dorpel MA, Deinum J, Derkx FH, Franken AA, Peperkamp E, de Jong PT, Schalekamp MA. Renin, prorenin, and immunoreactive renin in vitreous fluid from eyes with and without diabetic retinopathy. The Journal of clinical endocrinology and metabolism. 1989;68:160–167. doi: 10.1210/jcem-68-1-160. [DOI] [PubMed] [Google Scholar]
- 25.Okada Y, Yamanaka I, Sakamoto T, Hata Y, Sassa Y, Yoshikawa H, Fujisawa K, Ishibashi T, Inomata H. Increased expression of angiotensin-converting enzyme in retinas of diabetic rats. Japanese journal of ophthalmology. 2001;45:585–591. doi: 10.1016/s0021-5155(01)00412-9. [DOI] [PubMed] [Google Scholar]
- 26.Satofuka S, Ichihara A, Nagai N, Noda K, Ozawa Y, Fukamizu A, Tsubota K, Itoh H, Oike Y, Ishida S. (Pro)renin receptor-mediated signal transduction and tissue renin-angiotensin system contribute to diabetes-induced retinal inflammation. Diabetes. 2009;58:1625–1633. doi: 10.2337/db08-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma A, Shan Z, Lei B, Yuan L, Liu X, Nakagawa T, Grant MB, Lewin AS, Hauswirth WW, Raizada MK, Li Q. ACE2 and Ang-(1-7) confer protection against development of diabetic retinopathy. Molecular therapy: the journal of the American Society of Gene Therapy. 2012;20:28–36. doi: 10.1038/mt.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danser AH, Derkx FH, Admiraal PJ, Deinum J, de Jong PT, Schalekamp MA. Angiotensin levels in the eye. Investigative ophthalmology & visual science. 1994;35:1008–1018. [PubMed] [Google Scholar]
- 29.Gao BB, Chen X, Timothy N, Aiello LP, Feener EP. Characterization of the vitreous proteome in diabetes without diabetic retinopathy and diabetes with proliferative diabetic retinopathy. J Proteome Res. 2008;7:2516–2525. doi: 10.1021/pr800112g. [DOI] [PubMed] [Google Scholar]
- 30.Senanayake P, Drazba J, Shadrach K, Milsted A, Rungger-Brandle E, Nishiyama K, Miura S, Karnik S, Sears JE, Hollyfield JG. Angiotensin II and its receptor subtypes in the human retina. Investigative ophthalmology & visual science. 2007;48:3301–3311. doi: 10.1167/iovs.06-1024. [DOI] [PubMed] [Google Scholar]
- 31.Kawamura H, Kobayashi M, Li Q, Yamanishi S, Katsumura K, Minami M, Wu DM, Puro DG. Effects of angiotensin II on the pericyte-containing microvasculature of the rat retina. The Journal of physiology. 2004;561:671–683. doi: 10.1113/jphysiol.2004.073098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otani A, Takagi H, Suzuma K, Honda Y. Angiotensin II potentiates vascular endothelial growth factor-induced angiogenic activity in retinal microcapillary endothelial cells. Circulation research. 1998;82:619–628. doi: 10.1161/01.res.82.5.619. [DOI] [PubMed] [Google Scholar]
- 33.Yamagishi S, Imaizumi T. Pericyte biology and diseases. International journal of tissue reactions. 2005;27:125–135. [PubMed] [Google Scholar]
- 34.Otani A, Takagi H, Oh H, Koyama S, Honda Y. Angiotensin II induces expression of the Tie2 receptor ligand, angiopoietin-2, in bovine retinal endothelial cells. Diabetes. 2001;50:867–875. doi: 10.2337/diabetes.50.4.867. [DOI] [PubMed] [Google Scholar]
- 35.Nadal JA, Scicli GM, Carbini LA, Nussbaum JJ, Scicli AG. Angiotensin II and retinal pericytes migration. Biochemical and biophysical research communications. 1999;266:382–385. doi: 10.1006/bbrc.1999.1834. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes research and clinical practice. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Chibber R, Molinatti PA, Rosatto N, Lambourne B, Kohner EM. Toxic action of advanced glycation end products on cultured retinal capillary pericytes and endothelial cells: relevance to diabetic retinopathy. Diabetologia. 1997;40:156–164. doi: 10.1007/s001250050657. [DOI] [PubMed] [Google Scholar]
- 38.Koka V, Wang W, Huang XR, Kim-Mitsuyama S, Truong LD, Lan HY. Advanced glycation end products activate a chymase-dependent angiotensin II-generating pathway in diabetic complications. Circulation. 2006;113:1353–1360. doi: 10.1161/CIRCULATIONAHA.105.575589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kowluru RA, Zhong Q, Santos JM. Matrix metalloproteinases in diabetic retinopathy: potential role of MMP-9. Expert opinion on investigational drugs. 2012;21:797–805. doi: 10.1517/13543784.2012.681043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mishra M, Flaga J, Kowluru RA. Molecular Mechanism of Transcriptional Regulation of Matrix Metalloproteinase-9 in Diabetic Retinopathy. Journal of cellular physiology. 2016;231:1709–1718. doi: 10.1002/jcp.25268. [DOI] [PubMed] [Google Scholar]
- 41.Mori F, Hikichi T, Nagaoka T, Takahashi J, Kitaya N, Yoshida A. Inhibitory effect of losartan, an AT1 angiotensin II receptor antagonist, on increased leucocyte entrapment in retinal microcirculation of diabetic rats. The British journal of ophthalmology. 2002;86:1172–1174. doi: 10.1136/bjo.86.10.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson-Berka JL, Tan G, Jaworski K, Ninkovic S. Valsartan but not atenolol improves vascular pathology in diabetic Ren-2 rat retina. American journal of hypertension. 2007;20:423–430. doi: 10.1016/j.amjhyper.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 43.Zhang JZ, Xi X, Gao L, Kern TS. Captopril inhibits capillary degeneration in the early stages of diabetic retinopathy. Current eye research. 2007;32:883–889. doi: 10.1080/02713680701584123. [DOI] [PubMed] [Google Scholar]
- 44.Chen P, Scicli GM, Guo M, Fenstermacher JD, Dahl D, Edwards PA, Scicli AG. Role of angiotensin II in retinal leukostasis in the diabetic rat. Experimental eye research. 2006;83:1041–1051. doi: 10.1016/j.exer.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Silva KC, Pinto CC, Biswas SK, Souza DS, de Faria JB, de Faria JM. Prevention of hypertension abrogates early inflammatory events in the retina of diabetic hypertensive rats. Experimental eye research. 2007;85:123–129. doi: 10.1016/j.exer.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Al-Shabrawey M, Rojas M, Sanders T, Behzadian A, El-Remessy A, Bartoli M, Parpia AK, Liou G, Caldwell RB. Role of NADPH oxidase in retinal vascular inflammation. Investigative ophthalmology & visual science. 2008;49:3239–3244. doi: 10.1167/iovs.08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta MM, Chari S. Lipid peroxidation and antioxidant status in patients with diabetic retinopathy. Indian journal of physiology and pharmacology. 2005;49:187–192. [PubMed] [Google Scholar]
- 48.Mohr S, Xi X, Tang J, Kern TS. Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes. 2002;51:1172–1179. doi: 10.2337/diabetes.51.4.1172. [DOI] [PubMed] [Google Scholar]
- 49.Ola MS, Alhomida AS. Neurodegeneration in diabetic retina and its potential drug targets. Current neuropharmacology. 2014;12:380–386. doi: 10.2174/1570159X12666140619205024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ola MS, Berkich DA, Xu Y, King MT, Gardner TW, Simpson I, LaNoue KF. Analysis of glucose metabolism in diabetic rat retinas. American journal of physiology Endocrinology and metabolism. 2006;290:E1057–1067. doi: 10.1152/ajpendo.00323.2005. [DOI] [PubMed] [Google Scholar]
- 51.Ola MS, Nawaz MI, El-Asrar AA, Abouammoh M, Alhomida AS. Reduced levels of brain derived neurotrophic factor (BDNF) in the serum of diabetic retinopathy patients and in the retina of diabetic rats. Cellular and molecular neurobiology. 2013;33:359–367. doi: 10.1007/s10571-012-9901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K, Kobayashi S, Ishida S, Tsubota K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010;53:971–979. doi: 10.1007/s00125-009-1655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Downie LE, Vessey K, Miller A, Ward MM, Pianta MJ, Vingrys AJ, Wilkinson-Berka JL, Fletcher EL. Neuronal and glial cell expression of angiotensin II type 1 (AT1) and type 2 (AT2) receptors in the rat retina. Neuroscience. 2009;161:195–213. doi: 10.1016/j.neuroscience.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 54.Kurihara T, Ozawa Y, Shinoda K, Nagai N, Inoue M, Oike Y, Tsubota K, Ishida S, Okano H. Neuroprotective effects of angiotensin II type 1 receptor (AT1R) blocker, telmisartan, via modulating AT1R and AT2R signaling in retinal inflammation. Investigative ophthalmology & visual science. 2006;47:5545–5552. doi: 10.1167/iovs.06-0478. [DOI] [PubMed] [Google Scholar]
- 55.Chen P, Guo AM, Edwards PA, Trick G, Scicli AG. Role of NADPH oxidase and ANG II in diabetes-induced retinal leukostasis. American journal of physiology Regulatory, integrative and comparative physiology. 2007;293:R1619–1629. doi: 10.1152/ajpregu.00290.2007. [DOI] [PubMed] [Google Scholar]
- 56.Fujita T, Hirooka K, Nakamura T, Itano T, Nishiyama A, Nagai Y, Shiraga F. Neuroprotective effects of angiotensin II type 1 receptor (AT1-R) blocker via modulating AT1-R signaling and decreased extracellular glutamate levels. Investigative ophthalmology & visual science. 2012;53:4099–4110. doi: 10.1167/iovs.11-9167. [DOI] [PubMed] [Google Scholar]
- 57.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circulation research. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 58.Silva KC, Rosales MA, Biswas SK, Lopes de Faria JB, Lopes de Faria JM. Diabetic retinal neurodegeneration is associated with mitochondrial oxidative stress and is improved by an angiotensin receptor blocker in a model combining hypertension and diabetes. Diabetes. 2009;58:1382–1390. doi: 10.2337/db09-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim JH, Yu YS, Cho CS, Kim KW. Blockade of angiotensin II attenuates VEGF-mediated blood-retinal barrier breakdown in diabetic retinopathy. J Cereb Blood Flow Metab. 2009;29:621–628. doi: 10.1038/jcbfm.2008.154. [DOI] [PubMed] [Google Scholar]
- 60.Sarlos S, Rizkalla B, Moravski CJ, Cao Z, Cooper ME, Wilkinson-Berka JL. AT2-RB to attenuate retinal angiogenesis, indicating that the AT2 receptor promotes retinal endothelial cell growth. American Journal of Pathology. 2003;163:879–887. doi: 10.1016/S0002-9440(10)63448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fletcher EL, Phipps JA, Ward MM, Vessey KA, Wilkinson-Berka JL. The renin-angiotensin system in retinal health and disease: Its influence on neurons, glia and the vasculature. Prog Retina Eye Res. 2010;29:284–311. doi: 10.1016/j.preteyeres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 62.Kurihara T, Ozawa Y, Nagai N, Shinoda K, Noda K, Imamura Y, Tsubota K, Okano H, Oike Y, Ishida S. Angiotensin II type 1 receptor signaling contributes to synaptophysin degradation and neuronal dysfunction in the diabetic retina. Diabetes. 2008;57:2191–2198. doi: 10.2337/db07-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang H, Hirooka K, Fukuda K, Shiraga F. Neuroprotective effects of angiotensin II type 1 receptor blocker in a rat model of chronic glaucoma. Investigative ophthalmology & visual science. 2009;50:5800–5804. doi: 10.1167/iovs.09-3678. [DOI] [PubMed] [Google Scholar]
- 64.Ola MS, Ahmed MM, Abuohashish HM, Al-Rejaie SS, Alhomida AS. Telmisartan ameliorates neurotrophic support and oxidative stress in the retina of streptozotocin-induced diabetic rats. Neurochem Research. 2013;38:1572–1579. doi: 10.1007/s11064-013-1058-4. [DOI] [PubMed] [Google Scholar]
- 65.Kishi T, Hirooka Y, Sunagawa K. Sympathoinhibition caused by orally administered telmisartan through inhibition of the AT(1) receptor in the rostral ventrolateral medulla of hypertensive rats. Hypertension Research: Official Journal of the Japanese Society of Hypertension. 2012;35:940–946. doi: 10.1038/hr.2012.63. [DOI] [PubMed] [Google Scholar]
- 66.Konno S, Hirooka Y, Kishi T, Sunagawa K. Sympatho-inhibitory effects of telmisartan through the reduction of oxidative stress in the rostral ventrolateral medulla of obesity-induced hypertensive rats. Journal of hypertension. 2012;30:1992–1999. doi: 10.1097/HJH.0b013e328357fa98. [DOI] [PubMed] [Google Scholar]
- 67.Phipps JA, Wilkinson-Berka JL, Fletcher EL. Retinal dysfunction in diabetic ren-2 rats is ameliorated by treatment with valsartan but not atenolol. Investigative ophthalmology & visual science. 2007;48:927–934. doi: 10.1167/iovs.06-0892. [DOI] [PubMed] [Google Scholar]
- 68.Grammatopoulos TN, Jones SM, Ahmadi FA, Hoover BR, Snell LD, Skoch J, Jhaveri VV, Poczobutt AM, Weyhenmeyer JA, Zawada WM. Angiotensin type 1 receptor antagonist losartan, reduces MPTP-induced degeneration of dopaminergic neurons in substantia nigra. Molecular neurodegeneration. 2007;2:1. doi: 10.1186/1750-1326-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foureaux G, Nogueira BS, Coutinho DC, Raizada MK, Nogueira JC, Ferreira AJ. Activation of endogenous angiotensin converting enzyme 2 prevents early injuries induced by hyperglycemia in rat retina. Braz J Med Biol Res. 2015;48:1109–1114. doi: 10.1590/1414-431X20154583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tikellis C, Johnston CI, Forbes JM, Burns WC, Thomas MC, Lew RA, Yarski M, Smith AI, Cooper ME. Identification of angiotensin converting enzyme 2 in the rodent retina. Curr Eye Res. 2004;29:419–427. doi: 10.1080/02713680490517944. [DOI] [PubMed] [Google Scholar]
- 71.Jarajapu YP, Bhatwadekar AD, Caballero S, Hazra S, Shenoy V, Medina R, Kent D, Stitt AW, Thut C, Finney EM, Raizada MK, Grant MB. Activation of the ACE2/angiotensin-(1-7)/Mas receptor axis enhances the reparative function of dysfunctional diabetic endothelial progenitors. Diabetes. 2013;62:1258–1269. doi: 10.2337/db12-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dominguez JM, Hu P, Caballero S, Moldovan L, Verma A, Oudit GY, Li Q, Grant MB. Adeno-Associated Virus Overexpression of Angiotensin-Converting Enzyme-2 Reverses Diabetic Retinopathy in Type 1 Diabetes in Mice. American Journal Pathology. 2016;186:1688–1700. doi: 10.1016/j.ajpath.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tao L, Qui Y, Fy X, Lin R, Lei C, Wang J, Lei B. Angiotensin-converting enzyme 2 activator diminazene aceturate prevent lipopolysaccharide-induced inflammationby inhibiting MAPK and NF-kB pathways in human retinal pigment epithelium. J Neuroinflammation. 2016;13:35. doi: 10.1186/s12974-016-0489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chaturvedi N, Sjolie AK, Stephenson JM, Abrahamian H, Keipes M, Castellarin A, Rogulja-Pepeonik Z, Fuller JH. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet (London, England) 1998;351:28–31. doi: 10.1016/s0140-6736(97)06209-0. [DOI] [PubMed] [Google Scholar]
- 75.Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J, Parving HH, Bilous R, Sjolie AK. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet (London, England) 2008;372:1394–1402. doi: 10.1016/S0140-6736(08)61412-9. [DOI] [PubMed] [Google Scholar]
- 76.Sjolie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, Bilous R, Chaturvedi N. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet (London, England) 2008;372:1385–1393. doi: 10.1016/S0140-6736(08)61411-7. [DOI] [PubMed] [Google Scholar]
- 77.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R. Renal and retinal effects of enalapril and losartan in type 1 diabetes. The New England journal of medicine. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harindhanavudhi T, Mauer M, Klein R, Zinman B, Sinaiko A, Caramori ML. Benefits of Renin-Angiotensin blockade on retinopathy in type 1 diabetes vary with glycemic control. Diabetes care. 2011;34:1838–1842. doi: 10.2337/dc11-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes care. 2000;23(Suppl 2):B54–64. [PubMed] [Google Scholar]
- 80.Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet (London, England) 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 81.Ahmad S, Varagic J, VonCannon JL, Groban L, Collawn JF, Dell’Italia LJ, Ferrario CM. Primacy of cardiac chymase over angiotensin converting enzyme as an angiotensin-(1-12) metabolizing enzyme. Biochemical and biophysical research communications. 2016;478:559–564. doi: 10.1016/j.bbrc.2016.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reyes S, Varagic J, Ahmad S, VonCannon J, Kon ND, Wang H, Groban L, Cheng CP, Dell’Italia LJ, Ferrario CM. Novel Cardiac Intracrine Mechanisms Based on Ang-(1-12)/Chymase Axis Require a Revision of Therapeutic Approaches in Human Heart Disease. Curr Hypertens Rep. 2017 Feb;19(2):16. doi: 10.1007/s11906-017-0708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lutzelschwab C, Pejler G, Aveskogh M, Hellman L. Secretory granule proteases in rat mast cells. Cloning of 10 different serine proteases and a carboxypeptidase A from various rat mast cell populations. The Journal of experimental medicine. 1997;185:13–29. doi: 10.1084/jem.185.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caughey GH, Leidig F, Viro NF, Nadel JA. Substance P and vasoactive intestinal peptide degradation by mast cell tryptase and chymase. The Journal of pharmacology and experimental therapeutics. 1988;244:133–137. [PubMed] [Google Scholar]
- 85.Ahmad S, Wei CC, Tallaj J, Dell’Italia LJ, Moniwa N, Varagic J, Ferrario CM. Chymase mediates angiotensin-(1-12) metabolism in normal human hearts. Journal of the American Society of Hypertension: JASH. 2013;7:128–136. doi: 10.1016/j.jash.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Advances in immunology. 2007;95:167–255. doi: 10.1016/S0065-2776(07)95006-3. [DOI] [PubMed] [Google Scholar]
- 87.Chandrasekharan UM, Sanker S, Glynias MJ, Karnik SS, Husain A. Angiotensin II-forming activity in a reconstructed ancestral chymase. Science (New York, NY) 1996;271:502–505. doi: 10.1126/science.271.5248.502. [DOI] [PubMed] [Google Scholar]
- 88.Ferrario CM, VonCannon J, Jiao Y, Ahmad S, Bader M, Dell’Italia LJ, Groban L, Varagic J. Cardiac angiotensin-(1-12) expression and systemic hypertension in rats expressing the human angiotensinogen gene. American journal of physiology Heart and circulatory physiology. 2016;310:H995–1002. doi: 10.1152/ajpheart.00833.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nagata S, Varagic J, Kon ND, Wang H, Groban L, Simington SW, Ahmad S, Dell’Italia LJ, VonCannon JL, Deal D, Ferrario CM. Differential expression of the angiotensin-(1-12)/chymase axis in human atrial tissue. Therapeutic advances in cardiovascular disease. 2015;9:168–180. doi: 10.1177/1753944715589717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Ferrario CM. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension (Dallas, Tex: 1979) 2010;55:445–452. doi: 10.1161/HYPERTENSIONAHA.109.145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Westwood BM, Chappell MC. Divergent pathways for the angiotensin-(1-12) metabolism in the rat circulation and kidney. Peptides. 2012;35:190–195. doi: 10.1016/j.peptides.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM. Chymase-dependent generation of angiotensin II from angiotensin-(1-12) in human atrial tissue. PloS one. 2011;6:e28501. doi: 10.1371/journal.pone.0028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahmad S, Varagic J, Westwood BM, Chappell MC, Ferrario CM. Uptake and metabolism of the novel peptide angiotensin-(1-12) by neonatal cardiac myocytes. PloS one. 2011;6:e15759. doi: 10.1371/journal.pone.0015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovascular research. 2009;82:40–50. doi: 10.1093/cvr/cvp003. [DOI] [PubMed] [Google Scholar]
- 95.Maruichi M, Oku H, Takai S, Muramatsu M, Sugiyama T, Imamura Y, Minami M, Ueki M, Satoh B, Sakaguchi M, Miyazaki M, Ikeda T. Measurement of activities in two different angiotensin II generating systems, chymase and angiotensin-converting enzyme, in the vitreous fluid of vitreoretinal diseases: a possible involvement of chymase in the pathogenesis of macular hole patients. Current eye research. 2004;29:321–325. doi: 10.1080/02713680490516161. [DOI] [PubMed] [Google Scholar]
- 96.Nabe T, Kijitani Y, Kitagawa Y, Sakano E, Ueno T, Fujii M, Nakao S, Sakai M, Takai S. Involvement of chymase in allergic conjunctivitis of guinea pigs. Experimental eye research. 2013;113:74–79. doi: 10.1016/j.exer.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 97.Rubino SM, Nae AM, John V, Ahmad S, Ola MS, Ferrario CM, Greven C. Chymase/Angiotensin I axis: A pathway for Angiotensin II generation in diabetic human vitreous. Invest Ophthalmol Vis Sci. 2016;57:5431. [Google Scholar]
- 98.Sugiyama T, Katsumura K, Nakamura K, Kobayashi M, Muramatsu M, Maruichi M, Oku H, Takai S, Miyazaki M, Ikeda T. Effects of chymase on the macular region in monkeys and porcine muller cells: probable involvement of chymase in the onset of idiopathic macular holes. Ophthalmic research. 2006;38:201–208. doi: 10.1159/000093072. [DOI] [PubMed] [Google Scholar]
- 99.Shiota N, Saegusa Y, Nishimura K, Miyazaki M. Angiotensin II-generating system in dog and monkey ocular tissues. Clin Exp Pharmacol Physiol. 1997;24:243–248. doi: 10.1111/j.1440-1681.1997.tb01814.x. [DOI] [PubMed] [Google Scholar]
- 100.Ferrario CM, Ahmad S, Varagic J, Cheng CP, Groban L, Wang H, Collawn JF, Dell Italia LJ. Intracrine angiotensin II functions originate from noncanonical pathways in the human heart. American journal of physiology Heart and circulatory physiology. 2016;311:H404–414. doi: 10.1152/ajpheart.00219.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]