Abstract
Intraocular pressure (IOP) elevation is a critical risk factor for development and progression of glaucoma. As such, measuring IOP in animal models of the disease is important for any research work trying to understand the pathophysiologic mechanisms of glaucoma. Non-invasive IOP measurement in animals uses methods that have been adapted from use on humans. Calibration of the instruments used for the specific animal and even strain used is critically important for allowing meaningful comparisons of results. We describe below the methods used for non-invasive IOP measurement in animals that are relevant to glaucoma research.
Keywords: Intraocular pressure, glaucoma, model, methods
Introduction
Intraocular pressure IOP measurement is a critical method for glaucoma research as elevated IOP is a known risk factor for the development and progression of the disease (1–4). In addition IOP is the only modifiable parameter, reduction of which can prevent visual loss from glaucoma (1). Although specific animal models for glaucoma may not necessarily rely on IOP elevation (if for example they only recapitulate the neurodegenerative aspects of the disease), recording IOP during experimental work related to glaucoma is a good practice.
For experiments of relatively short duration IOP can be monitored by direct cannulation of the eye (for examples see (5–8)). However, for chronic experiments direct cannulation for continuous IOP recording is not practical except under special circumstances (9). Repeated cannulations introduce a significant risk for infection or other complications and are generally not practical as well. Thus, for the majority of experimental work, IOP measurement relies on a number of non-invasive methods that indirectly estimate its true value through the wall of the eye. Most of these methods have been adapted from methods used on humans and have specific limitations that one has to keep in mind. Eye characteristics including corneal thickness and elasticity affect almost all of the non-invasive methods for measuring IOP (10–12).
Selection of the most appropriate method for IOP measurement depends on the size of the animal eye, availability of equipment and experimental design. In general, because of diurnal variation, IOP measurements should be performed at approximately the same time of the day. For mice, IOP exhibits an increase at night (13), making differences between groups easier to detect. Maintaining the animals in a reverse light cycle (e.g. lights off at 8AM, on at 8PM) and measurement of IOP under dim light (or using night vision equipment) may be helpful if such a set-up is available. For rats, constant light exposure also seems to elevate IOP making detection of IOP differences easier (14).
The five main methods for measuring IOP non-invasively are: applanation, indentation, pneumotonometry, electronic tonometry, and rebound tonometry. Indentation tonometry has been largely abandoned for both human and animal work as it depends to a large degree on scleral rigidity and it causes a significant change in IOP over time. Indentation is used in a separate glaucoma research method which is called tonography and is infrequently used to measure trabecular outflow.
Of the four remaining methods, applanation tonometry relies on the principle of applying a variable force on the corneal surface to make it flat (plane). At that point the force exerted to the outside the eye is equal in magnitude to the force exerted by IOP. A clever split prism optical system coupled with the use of fluorescein on the corneal surface allows determination of the end-point of measurement (corneal flattening) while the force applied (and by consequence the IOP) can be read off a dial and is expressed in mmHg. Applanation tonometry can be performed in all animal species but for mice and rats special prism tips are needed (15, 16) which are not commercially available at this time. For larger species, applanation tips used in human tonometry can be used. However, it should be pointed out that tips for human tonometry have been designed so that the capillary force exerted by the tear meniscus counteracts the spring force of the cornea as it is being applanated. The latter force differs in other species. This causes a deviation of applanation IOP readings from true IOP that increases with increasing IOP. Typically for animals with larger eyes than humans (e.g. bovine) IOP is significantly lower when measured by applanation and the range of IOPs is dramatically compressed.
In addition for applanation tonometry, the amount of fluorescein applied to the cornea affects the accuracy of measurements. Consistency is important.
Finally, because the visual end-point of these measurements is subjective, when used in research work, applanation tonometry should always be performed in a masked fashion.
Pneumotonometry relies on the principle of applanation but uses pressure generated by a compressed gas (usually air) to flatten the cornea. The pressure is recorded continuously and graphed. Although pneumotonometry is probably the most accurate method for measuring IOP in humans it is not extensively used because it is not integrated with the rest of the ophthalmic examination. The archetypical pneumotonometry instrument is the Mentor 30 tonometer which was introduced in the 60’s. A number of these instruments are still available. Unfortunately newer versions of this instrument, because of FDA regulations, have eliminated the ability to change the gain and offset of the instrument and do not allow recalibration for use on experimental animals. Such recalibration is needed as pneumotonometry suffers similar problems with applanation when it comes to measuring IOP of species with significantly larger eyes. Adjusting the gain of the instrument can significantly expand the scale of the measurements providing expanded IOP resolution. Pneumotonometry typically requires access to electrical power.
Electronic tonometry detects a characteristic change in force applied as the cornea gets pressed by a 1mm diameter plunger, in order to determine IOP. The Tonopen® is the instrument used clinically to perform electronic tonometry. Use of electronic tonometry has been reported for all animal species including the mice (17), rats (18), rabbits (19), cattle (20), dogs (21) and cats (22). However, for tonometry in mice the authors recommended the use of the instrument without the protective latex cover. This results in tear fluid entering between the central plunger and the surrounding collar which necessitates frequent disassembly of the instrument for cleaning and may result in significant variation between readings. For electronic tonometry application of the instrument along the visual axis is critical for obtaining accurate measurements.
Rebound tonometry relies on analysis of the deceleration speed of a probe that impacts the cornea in order to estimate IOP (23). To optimize the detection characteristics two versions of the instrument are available for research work (Tonolab® and Tonovet® for smaller and larger animals respectively) and an additional version is available for human use (iCare tonometer). One of the advantages of rebound tonometry is that it can be performed without the application of topical anesthesia as the contact time of the probe with the eye is in the range of milliseconds (and faster than the blink reflex). In addition, rebound tonometry is fairly insensitive to small off axis deviations in the positioning of the probe in respect to the visual axis (23).
It is important prior to initiation of any experimental work using any of the above methods, to establish the relationship between the true IOP and the IOP measured by the tonometer. Although many of the instruments used to measure IOP provide values in mmHg and can be used without knowing the exact relationship with true IOP (for example in comparative studies) calibrating the instrument for the specific animal species and strain used is important for the following reasons:
To detect small differences in IOP that may be masked by a compressed scale
To accurately estimate the magnitude of IOP difference between groups or over time
To compare IOP values across species or strains
For longitudinal studies (especially when the experimental intervention may affect the ocular characteristics) it is also important to establish the relationship between instrument readings and true IOP at both the beginning and end of the study (and at any point when ocular characteristics change).
We will describe below details of the IOP measurement methods that are used for glaucoma research in various species.
Materials and equipment
For all tonometry methods except rebound tonometry, topical anesthesia (using either proparacaine 0.5%, tetracaine 1% or benoxinate 0.25%) should be used. Although rebound tonometry can be performed without topical anesthesia, it is often easier to perform under topical anesthesia. General anesthesia is not required for any species (or methods) if animals can be restrained adequately. However it is important to avoid compression of the animal neck and thoracic and abdominal cavities while obtaining IOP measurements, as such compression will affect IOP readings. Specific restraining holders have been described for a number of animal species (11, 24, 25). It is also important to allow the animals to relax before initiating IOP measurements. Measuring IOP in rats without the use of general anesthesia can be challenging, unless a prolonged period of acclimation is allowed where the animals get used to being handled over a period of many days. When general anesthesia is required (or elected) it is preferable to use inhalational anesthesia as it seems to affect IOP less than anesthesia involving the use of ketamine (at least in some species) (26). If ketamine is used, IOP measurements should be obtained immediately after induction, as ketamine significantly reduces IOP in a time dependent manner (27).
For IOP measurements the following equipment are needed:
Applanation tonometry: Perkins handheld tonometer
Pneumotonometry: Mentor 30 pneumotonometer
Electronic tonometry: Tonopen tonometer
Rebound tonometry: Tonolab or Tonovet tonometers (depending on species)
Instrument tips should be cleaned after each use with 70% alcohol and dried. For instruments with disposable tips (or covers), a new tip (or cover) should be used for measurements of each animal.
For establishing the relationship between measured and true IOP, tubing (various diameters), syringes, plastic three way valves, needles ranging from 26G to 18G and phosphate buffered saline (or ideally balanced salt solution) are needed. Epoxy glue (5 minute setting) is useful for ensuring lack of leaks.
In-line pressure sensing is preferable to allow confirmation of the intraocular pressure and ensure lack of occlusion of the needle but can be dispensable if the open stopcock method (described below) is used.
Methods
A. Calibration (Establishing the relationship between measured IOP and true IOP)
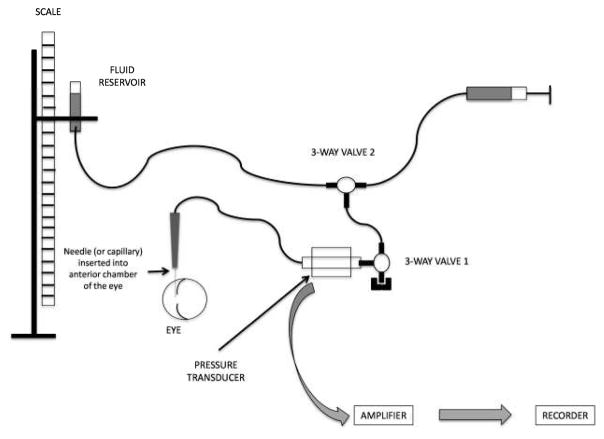
Connect tubing, syringes and pressure sensor as shown in Figure 1. Ensure that no air-bubbles are trapped in the system (see Notes 1 and 2).
Open the three way valve 1 and raise the fluid reservoir so that the free fluid level is ~5cm above the plane of the eye to obtain a very slow fluid flow through the needle.
Cannulate the eye with the needle at approximately 90 degrees to the visual axis through the peripheral cornea (see Notes 3–4).
Dry the cornea around the area of the needle entry using a rolled up kimwipe® (weck® cell sponges can also be used).
Observe under operating microscope to ensure lack of leaks.
For mice or rat eyes apply epoxy glue around the needle entry site after mixing components and wait until the epoxy hardens.
Raise fluid reservoir so that the free fluid surface level is 10cm above the plane of the eye.
Record pressure through the pressure transducer (if pressure transducer is not available proceed to step 17).
Close three-way valve 1, isolating the reservoir from the eye/sensor circuit.
Record pressure through the pressure transducer to ensure that it has not changed.
Measure IOP using the appropriate instrument and record (see below for details) (see Notes 5–6).
Record pressure through the pressure transducer to ensure that it has not changed.
Open the three-way valve 1 allowing flow from the fluid reservoir to the eye/sensor circuit.
Raise reservoir so that free fluid surface level is 5cm higher than before.
Repeat steps 8–14 until you have reached a free fluid surface level that is 60cm above the eye plane.
Graph the recorded IOP values against the recorded pressure values from the pressure transducer at which each IOP reading was obtained. Use this relationship to appropriately adjust IOP reading when reporting for publication (see Note 7).
Measure IOP using the appropriate instrument and record (do so without closing the three way valve–this is the open stopcock method). Note on the record the distance between the free fluid surface level and the eye (see Note 8).
Raise reservoir so that free fluid surface level is 5cm higher than before.
Repeat steps 17–18 until you have reached a free fluid surface level that is 60cm above the eye plane.
Graph the recorded IOP values against the recorded distance between the free fluid surface level and the eye at which each IOP reading was obtained. Use this relationship to appropriately adjust IOP reading when reporting for publication (see Note 7).
Figure 1.
Schematic setup for tonometer calibration. A similar setup can be used for direct IOP measurement. Two 3-way valves are used: 3-way valve 1 has one port permanently closed and allows at will connection between the column of fluid and the eye. 3-way valve 2 allows replenishment of the fluid in the system using a syringe. The port connecting to the syringe is closed during measurements. Pressure transducer is optional if the open stopcock method is used. Components and eye are not to scale.
B. Applanation tonometry
Wear a face mask and head cover. Your face will come very close to the animal head while measuring IOP.
Instill the local anesthetic drops and then the fluorescein. A small amount of fluorescein is needed. Be consistent (see Note 9).
Wipe the tip with an alcohol swab and dry with a kimwipe.
Turn the Perkins tonometer on and hold it with your dominant hand.
Make sure that the tonometer head is perpendicular to the surface of the eye and that the long axis of the prism tip is along the visual axis.
Move the tonometer forward slowly until the prism rests gently on the center of the cornea while observing through the viewfinder of the Perkins tonometer. Use one or two fingers from the other hand to keep the eyelids of the animal open (see Note 10).
With your thumb and index finger turn the calibrated dial on the tonometer clockwise until the two fluorescein semi-circles in the prism head are seen through the viewfinder to touch and form a horizontal ‘S’ shape. The correct end point is when the inner edges of the two fluorescein semi-circle images just touch.
Withdraw the prism from the corneal surface, note the reading on the dial and record it
Wipe the tip, turn the dial to zero and repeat the measurement at least once more (preferably obtain 3 to 5 readings for averaging).
Repeat the procedure for the other eye.
Wipe the prism tip with alcohol, dry and proceed to measure other animals.
After completion of measurements, remove the tip, wipe with a clean, dry swab and place it in a receptacle containing disinfectant (see Note 11).
Turn Perkins tonometer off and store in case.
C. Pneumotonometry
Instill the local anesthetic drops.
Wipe the tip of the pneumotonometer with an alcohol swab. Ensure that the silicone membrane at the tip is present and has no rips.
Turn the Mentor Model 30 tonometer on. Press on the “Zero” button to record the electrical zero baseline. Offset this reading (by turning the “Offset” knob) so that the tracing falls within the marked (graduated) area of the recording paper strip.
Hold the probe with your dominant hand and make sure that the tonometer head is perpendicular to the surface of the eye along the visual axis.
Move the tonometer probe forward slowly while depressing the foot-pedal until the tip of the probe touches the center of the cornea. Use one or two fingers from the other hand to keep the eyelids of the animal open (see Note 10).
Continue to advance the probe until the black line on the central tube of the probe disappears. The red line on the same tube should remain visible at all times. Notice the pitch of the noise as you are recording. It should be stable.
Continue recording for 20–30 seconds.
Withdraw the probe and repeat at least once more.
Review recordings to ensure stable readings and mark the eye and the periods where the actual reading was performed (see Note 12).
Wipe the tip and repeat the procedure for the other eye.
Wipe the prism with alcohol, dry and proceed to measure other animals.
After completion of measurements turn the instrument off and wipe the tip after cleaning with alcohol.
To calculate IOP subtract from the measurement value the electrical zero value. Use the calibration curve created during the instrument calibration (see Note 13) to convert these numbers to IOP in mmHg.
D. Electronic tonometry
Instill the local anesthetic drops.
Turn the Tonopen on and perform the recommended testing by the manufacturer to ensure proper operation (see Note 14).
Place a disposable latex cover over the instrument tip.
Hold the probe with your dominant hand and make sure that the tonometer head is perpendicular to the surface of the eye and the tonometer is along the visual axis.
Move the tonometer forward decisively at moderate speed and touch the cornea. Use one or two fingers from the other hand to keep the eyelids of the animal open (see Note 10). Make sure you hear the characteristic chirp indicating that a reading has been obtained.
Repeat step 5 until the tonometer has accumulated enough readings and has calculated an IOP value which is within acceptable confidence limits. Record that value displayed on the instrument screen.
Repeat measurements at least 5 times per eye (see Note 15) then measure the contralateral eye.
Change tip cover and proceed to measure other animals.
After completion of measurements turn the instrument off and cover the tip with a new cover for storage.
E. Rebound tonometry
Instill the local anesthetic drops (this step is optional).
Turn the instrument on and insert a new probe into the instrument head. Trigger the instrument to ensure that the probe moves freely forward and then is pulled back
Bring the instrument close to the eye and trigger the devise. Ensure that the probe axis is on the visual axis (see Notes 16 and 17).
Record the reading and repeat measurement at least twice more.
Remove the probe from the instrument wipe and replace. Repeat the procedure for measuring IOP in the other eye.
Wipe the probe with alcohol, dry and proceed to measure other animals.
After completion of measurements turn the instrument off remove the probe and wipe the instrument head (see Note 18).
Acknowledgments
Grant support: NEI R01 EY025543
Footnotes
Intravenous (IV) tubing can be used for cannulation of large eyes or when using the open stopcock method. However it does have significant compliance and should be avoided when using the closed stopcock method. Polyethylene tubing (PE) is excellent for this method. When cannulating smaller eyes the tubing can cause significant torqueing which makes positioning often difficult or unstable. Small diameter PE tubing can be used and can be stretched while heated to make it much more pliable.
Air bubbles can occlude tubing and can make measurements unreliable. Ensure that no bubbles are introduced during tube connections (especially when using luer connections). To avoid trapping bubbles fill all tubing with fluid prior to assembling. Fill female luer connectors with fluid using a syringe with 30G needle to inject the fluid in the bottom of the hub prior to assembling male with female luer portions Ideally use degassed fluid (fluid can be easily degassed by applying house vacuum while stirring in a beaker). Be meticulous about inspecting the tubing after assembly and removing any bubbles (even the tiniest ones).
Ideally eyes to be cannulated should not be removed from the orbit and the animal should be deeply anesthetized (but alive) during the procedure. In practice using animals immediately after death is also acceptable. Removal of the eye from the orbit may facilitate positioning but eliminates the cushioning effect of the periorbital fat. Thus for methods that depend on dynamic phenomena (rebound and electronic tonometry) measurements may be affected. Keep that in mind when interpreting data obtained in vivo. For large animals (like bovine) usually only the eye is available when calibrating the instrument. The effect of fat cushioning is less in these eyes.
When cannulating larger eyes use a larger bore needle. For bovine eyes use 18–21G needles for rabbit eyes 21–23G needles and for smaller eyes 25–26G needles. For mice a 26G needle with short bevel is preferable. Enter the eye with the bevel towards the lens to decrease the risk of penetrating the lens capsule. Once in the eye rotate the needle so that the bevel faces anteriorly to avoid occlusion by the iris. In mouse eyes avoid inserting the needle too deeply (across the anterior chamber) as it will affect IOP measurements. Needles can be substituted with pulled capillaries. Pull capillaries in a capillary puller. Ensure that the pulled capillaries have at least 100um diameter. Pulled capillaries may be preferable to needles when cannulating small eyes (especially mouse eyes).
A minimum of 3 to 5 measurements should be obtained at each pressure level when performing calibration and the measurements averaged.
It is critically important to keep the surface of the eye moist while obtaining readings. Re-wet the cornea with PBS and remove excessive fluid prior to obtaining readings at a new pressure level.
Please remember that 1 mmHg = 1.33 mbar or ~1.34cmH2O. Please ensure that all readings are in the correct units when reporting results.
Selection of the open vs closed stopcock methods depends on the availability of equipment. In general when possible the closed stopcock method is preferable. However for methods that depend on dynamic phenomena the open stopcock method is almost equivalent and can be used.
A fixed combination of anesthetic and fluorescein is also commercially available. However the amount of fluorescein included is too much. If you use this preparation allow 3–5 minutes for the tears to flush out the majority of fluorescein before measuring IOP.
Do not apply pressure to the eye itself as this will raise IOP. Hold the upper and if needed lower eyelids against the superior and inferior edges of the orbit to avoid exerting such pressure.
Chlorhexidine can be used for disinfectant. Alternatively a solution of dilute bleach (10% dilution) can be used. Do not leave tip in disinfectant overnight. Remove after 30 minutes, wash with water, dry and store.
Notice that tracings fluctuate with heart cycle and with breathing but the average should remain stable. As the instrument continuously records while the foot-pedal is pressed you will need to mark clearly the portions of the tracing that should be used for IOP calculation.
As mentioned earlier the Mentor 30 instrument is the only pneumotonometer that allows changes in the gain and offset. Changes are made by adjusting two screws in the back of the instrument during the calibration process. Adjusting the gain will expand the scale allowing for finer IOP readings. However excessive increase of the gain will result in noise. Once set during the calibration process do not change the gain during the actual IOP measurements. Although Mentor 30 tonometers are preferable for experimental work, newer pneumotonometers can be used. However it will be harder to detect small IOP differences using these instruments as the scale will be significantly compressed. Newer instruments automatically provide a single IOP value. Do not rely on these values. Instead obtain and inspect the tracings.
If you get a “Bad” reading turn off and try again. It is not unusual to need to turn on and off for a few times before you can get the tonometer to give a “Good” reading.
Tonopen readings are often highly variable. Ensure that the instrument is touching the apex of the cornea at the right angle and that the application of the instrument on the cornea is performed in a consistent way. You may need to collect up to 10 readings per eye and use statistical methods to remove outliers.
For small eyes (e.g. mouse eyes) alignment can be difficult. It can be facilitated by securing the instrument and moving it with the use of a micromanipulator. Using such an approach will also ensure that triggering the device does not inadvertently cause the probe track to change. Alignment should be performed under an operating microscope and verified in the Z-axis through inspection from the side. That is part of the reason that although rebound tonometry can be performed on non-sedated mice many investigators prefer to perform under sedation. Modifications to the instrument to create a detached probe housing and a foot-switch operated triggering mechanism circumvent such problems. For larger animals (using the Tonovet) positioning is less critical in obtaining accurate readings.
For large animals it is not usually necessary to hold the eyelids while recording. If needed use one or two fingers from the other hand to keep the eyelids of the animal open (see Note 10 above). For mice and rats make sure that you apply minimal pressure.
Animal dander and tears often tend to accumulate in the probe shaft or on the probe itself. In addition the probe may become magnetized. If needed demagnetize the probe using a demagnetizer. Also avoid using the instrument next to instruments that can cause strong magnetic interference (including cell phones). Use the manufacturer’s suggestions for keeping the probe shaft clean of debris. Canned compressed air is often useful for cleaning the instrument. Check for the possibility that the probe is bent by rolling the probe on a flat surface. Discard bent probes.
References
- 1.The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130:429–40. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 2.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 3.Nemesure B, Honkanen R, Hennis A, et al. Incident open-angle glaucoma and intraocular pressure. Ophthalmology. 2007;114:1810–5. doi: 10.1016/j.ophtha.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090–5. doi: 10.1001/archopht.1991.01080080050026. [DOI] [PubMed] [Google Scholar]
- 5.Avila MY, Carre DA, Stone RA, et al. Reliable measurement of mouse intraocular pressure by a servo-null micropipette system. Invest Ophthalmol Vis Sci. 2001;42:1841–6. [PubMed] [Google Scholar]
- 6.Kiel JW, Reitsamer HA, Walker JS, et al. Effects of nitric oxide synthase inhibition on ciliary blood flow, aqueous production and intraocular pressure. Exp Eye Res. 2001;73:355–64. doi: 10.1006/exer.2001.1050. [DOI] [PubMed] [Google Scholar]
- 7.Huang W, Fileta JB, Filippopoulos T, et al. Hsp27 phosphorylation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2007;48:4129–35. doi: 10.1167/iovs.06-0606. [DOI] [PubMed] [Google Scholar]
- 8.John SW, Smith RS, Savinova OV, et al. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–62. [PubMed] [Google Scholar]
- 9.Downs JC. IOP telemetry in the nonhuman primate. Exp Eye Res. 2015;141:91–8. doi: 10.1016/j.exer.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chihara E. Assessment of true intraocular pressure: the gap between theory and practical data. Surv Ophthalmol. 2008;53:203–18. doi: 10.1016/j.survophthal.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Nissirios N, Goldblum D, Rohrer K, et al. Noninvasive determination of intraocular pressure (IOP) in nonsedated mice of 5 different inbred strains. J Glaucoma. 2007;16:57–61. doi: 10.1097/IJG.0b013e31802b3547. [DOI] [PubMed] [Google Scholar]
- 12.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44:367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 13.Saeki T, Aihara M, Ohashi M, et al. The efficacy of TonoLab in detecting physiological and pharmacological changes of mouse intraocular pressure--comparison with TonoPen and microneedle manometery. Curr Eye Res. 2008;33:247–52. doi: 10.1080/02713680801919716. [DOI] [PubMed] [Google Scholar]
- 14.Moore CG, Johnson EC, Morrison JC. Circadian rhythm of intraocular pressure in the rat. Curr Eye Res. 1996;15:185–91. doi: 10.3109/02713689608997412. [DOI] [PubMed] [Google Scholar]
- 15.Cohan BE, Bohr DF. Goldmann applanation tonometry in the conscious rat. Invest Ophthalmol Vis Sci. 2001;42:340–2. [PubMed] [Google Scholar]
- 16.Cohan BE, Bohr DF. Measurement of intraocular pressure in awake mice. Invest Ophthalmol Vis Sci. 2001;42:2560–2. [PubMed] [Google Scholar]
- 17.Reitsamer HA, Kiel JW, Harrison JM, et al. Tonopen measurement of intraocular pressure in mice. Exp Eye Res. 2004;78:799–804. doi: 10.1016/j.exer.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Moore CG, Milne ST, Morrison JC. Noninvasive measurement of rat intraocular pressure with the Tono-Pen. Invest Ophthalmol Vis Sci. 1993;34:363–9. [PubMed] [Google Scholar]
- 19.Lim KS, Wickremasinghe SS, Cordeiro MF, et al. Accuracy of intraocular pressure measurements in new zealand white rabbits. Invest Ophthalmol Vis Sci. 2005;46:2419–23. doi: 10.1167/iovs.04-0610. [DOI] [PubMed] [Google Scholar]
- 20.Gum GG, Gelatt KN, Miller DN, et al. Intraocular pressure in normal dairy cattle. Vet Ophthalmol. 1998;1:159–161. doi: 10.1046/j.1463-5224.1998.00017.x. [DOI] [PubMed] [Google Scholar]
- 21.Gelatt KN, MacKay EO. Distribution of intraocular pressure in dogs. Vet Ophthalmol. 1998;1:109–114. doi: 10.1046/j.1463-5224.1998.00024.x. [DOI] [PubMed] [Google Scholar]
- 22.Rusanen E, Florin M, Hassig M, et al. Evaluation of a rebound tonometer (Tonovet) in clinically normal cat eyes. Vet Ophthalmol. 2010;13:31–6. doi: 10.1111/j.1463-5224.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- 23.Kontiola AI, Goldblum D, Mittag T, et al. The induction/impact tonometer: a new instrument to measure intraocular pressure in the rat. Exp Eye Res. 2001;73:781–5. doi: 10.1006/exer.2001.1088. [DOI] [PubMed] [Google Scholar]
- 24.Wang WH, Millar JC, Pang IH, et al. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Invest Ophthalmol Vis Sci. 2005;46:4617–21. doi: 10.1167/iovs.05-0781. [DOI] [PubMed] [Google Scholar]
- 25.Gerometta R, Podos SM, Candia OA, et al. Steroid-induced ocular hypertension in normal cattle. Arch Ophthalmol. 2004;122:1492–7. doi: 10.1001/archopht.122.10.1492. [DOI] [PubMed] [Google Scholar]
- 26.Cone FE, Steinhart MR, Oglesby EN, et al. The effects of anesthesia, mouse strain and age on intraocular pressure and an improved murine model of experimental glaucoma. Exp Eye Res. 2012;99:27–35. doi: 10.1016/j.exer.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia L, Cepurna WO, Johnson EC, et al. Effect of general anesthetics on IOP in rats with experimental aqueous outflow obstruction. Invest Ophthalmol Vis Sci. 2000;41:3415–9. [PubMed] [Google Scholar]