Abstract
There are compelling reasons to conduct studies of cancer in Hispanics, the fastest growing major demographic group in the United States (from 15% to 30% of the U.S. population by 2050). The genetically admixed Hispanic population coupled with secular trends in environmental exposures and lifestyle/behavioral practices that are associated with immigration and acculturation offer opportunities for elucidating the effects of genetics, environment, and lifestyle on cancer risk and identifying novel risk factors. For example, traditional breast cancer risk factors explain less of the breast cancer risk in Hispanics than in non-Hispanic whites (NHW), and there is a substantially greater proportion of never-smokers with lung cancer in Hispanics than in NHW. Hispanics have higher incidence rates for cancers of the cervix, stomach, liver, and gall bladder than NHW. With respect to these cancers, there are intriguing patterns that warrant study (e.g., depending on country of origin, the five-fold difference in gastric cancer rates for Hispanic men but not Hispanic women). Also, despite a substantially higher incidence rate and increasing secular trend for liver cancer in Hispanics, there have been no studies of Hispanics reported to date. We review the literature and discuss study design options and features that should be considered in future studies.
Introduction
The Hispanic population is the fastest growing major demographic group in the United States (we use the term Hispanics to refer to Hispanics or Latinos residing in the United States). By 2050, it is expected to triple from 46.7 million to 132.8 million, growing from 15% to 30% of the U.S. population. The Hispanic population suffers from major health disparities relative to non-Hispanic Whites (NHW). They have higher incidence rates of cervical, gall bladder, liver, and gastric cancer, and higher mortality rates for 6 cancer sites than in NHW (see below). Furthermore, they generally have low participation rates in cancer screening and other prevention programs. It is important that we better understand the broad range of causes of cancer in this major, fast-growing segment of the population. Also, the Hispanic population exemplifies experiences of immigration and acculturation, which are taking place worldwide at unprecedented levels. Incorporating the immigrant experience into cancer studies represents both a challenge and an opportunity to better understand important determinants of cancer risk and cancer control. The purpose of this article is to review what is known about cancer in Hispanics and to discuss elements that should be considered in planning future studies of this population.
Overall Summary of Cancer Statistics in Hispanics
Cancer incidence patterns
Figure 1 presents the 15-year (1992–2007) secular trends in incidence for the 4 most common cancers in the general U.S. population (lung, colorectal, breast, and prostate) for Hispanics and NHW and the 4 cancers (cervix, gall bladder, liver, and gastric) where the rates are substantially higher in Hispanics than in NHW. For the 4 most common cancers, the largest relative difference (ratio of incidence rates) is observed for lung cancer, with Hispanics having about a 50% lower incidence rate than NHW. Hispanics have substantially higher incidence rates for cervical, gall bladder, liver, and gastric cancer, with the largest relative difference observed for gall bladder cancer (GBC).
Figure 1.
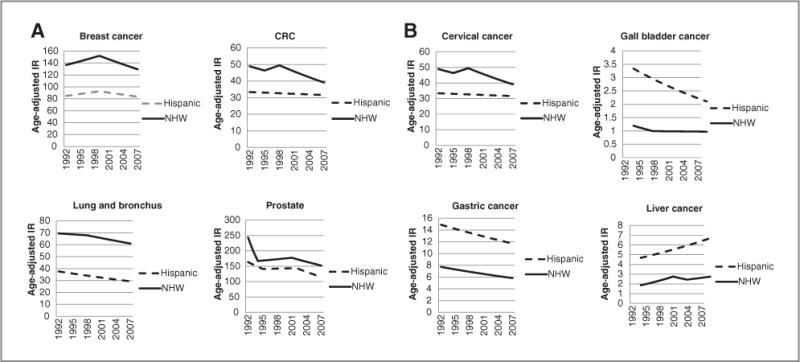
A and B, age-adjusted SEER incidence rates by ethnicity and site. IR, incidence rate. Cancer sites include invasive cases only. Incidence source: SEER 13 area (San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, San Jose-Monterey, Los Angeles, Alaska Native Registry, and Rural Georgia). Rates are per 100,00 and are age-adjusted to the 2000 U.S. Std Population (19 age groups-Census P25-1130). Regression lines are calculated using the Joinpoint Regression Program Version 3.4.3, April 2010, National Cancer Institute. Hispanics are not mutually exclusive from whites, blacks, Asian/Pacific Islanders, and American Indians/Alaska Natives. Incidence data for Hispanics and Non-Hispanics are based on NHIA and exclude cases from the Alaska Native Registry.
Cancer mortality patterns
Hispanics have higher mortality rates than NHW for gastric, cervix, gall bladder, liver and thyroid cancer, and acute lymphocytic leukemia (ALL). Mortality rates for gastric, liver, cervix, and ALL in Hispanics exceed those of NHW by 50% or more (Fig. 2).
Figure 2.
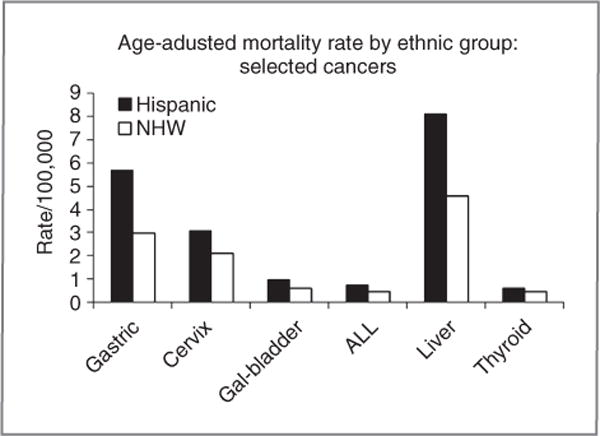
Mortality rates. Average age-adjusted SEER mortality rates (2005–2007) by ethnicity and site. Cancer sites include invasive cases only unless otherwise noted. Incidence source: SEER 13 area (San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, San Jose-Monterey, Los Angeles, Alaska Native Registry, and Rural Georgia). Rates are per 100,000 and are age-adjusted to the 2000 U.S. Std Population (19 age groups-Census P25-1130). Hispanics and NHWs are mutually exclusive. Incidence data for Hispanics and Non-Hispanics are based on NHIA and exclude cases from the Alaska Native Registry.
Cancer screening patterns
Hispanics generally have lower compliance rates with cancer screening guidelines than other racial/ethnic groups (Fig. 3). The likelihood of receiving screening varies according to sociodemographic characteristics such as national origin and access to health care. In a nationally representative sample of Hispanics, those from Mexico, Central America, and South America were less likely to receive cancer screenings relative to other Hispanic subgroups (1). Smaller studies reported similar patterns, with the lowest screening rates typically found among Hispanics from Mexico and the highest among Hispanics from Cuba and the Dominican Republic (2, 3). Acculturation probably affects cancer-screening behavior. Findings, however, are mixed, with some studies reporting positive associations (4), inverse associations (5), or no associations (2, 6) between acculturation and cancer screening, depending on the type of screening test or acculturation measure used. Regardless of country of origin or acculturation, Hispanics are more likely to obtain recommended cancer screenings if they have health insurance and a regular source of health care (1, 3, 4, 7).
Figure 3.
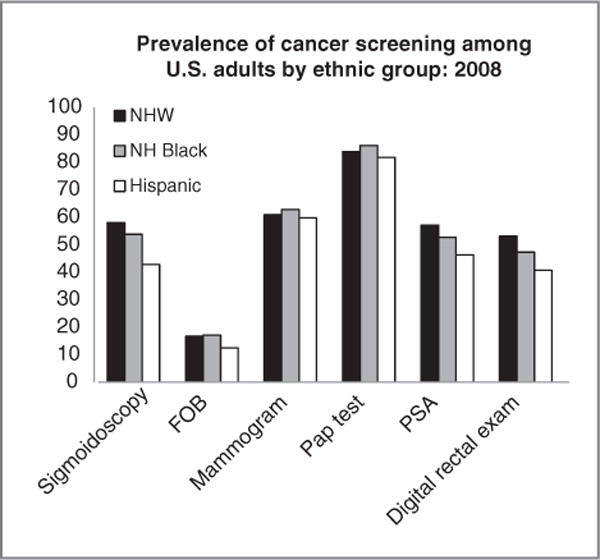
Prevalence of cancer screening among U.S. adults by ethnic group: 2008. Sigmoidoscopy was observed in past 10 years, among men aged 50 years and older. Mammogram: in past year, among women aged 40 years and older. Pap test: Papanicolaou test in past 3 years, among women aged 18 years and older. A digital rectal examination within the past year for men who have not been told they have had prostate cancer. NHW, NH Black, and Hispanic are mutually exclusive. FOB, fecal occult blood test (in past year, among men aged 50 years and older); PSA, prostate-specific antigen test (in past year among men aged 50 years and older). Source: Behavioral Risk Factor Surveillance Survey, 2006 data, reported in ref. 168.
Common Cancers in the General U.S. Population
We examine the 4 most common cancers in the general U.S. population and note key differences between the Hispanic and NHW populations. The general pattern for these 4 cancers is a lower incidence but also a slightly lower cancer-specific survival in Hispanics. Few studies have addressed the determinants of these differences between Hispanics and NHW; they are intriguing and present opportunities for learning more about the causes of these common cancers. Understanding what factors, particularly environmental, lifestyle, and cultural, contribute to the generally lower risk among Hispanics of the 4 most common cancers in the general U.S. population may suggest future avenues of research to prevent these cancers in other populations.
Before proceeding, we comment on the effects of undocumented Hispanics. Although we can speculate that the undocumented Hispanic immigrant population in the United States may artifactually inflate Hispanic cancer incidence rates and artifactually decrease Hispanic cancer mortality and case fatality rates, epidemiologic studies documenting this are lacking. Underrepresentation in population census counting, lack of medical insurance, and fear of visibility and deportation tend to cause greater underestimates of denominators than numerators in population epidemiologic studies. Manifestations of cancer disease would bring some of those not counted in the denominators into the counting system in the numerator only, inflating incidence rates to an unknown but probably small degree. Lack of medical insurance, ineffective doctor/patient/family communication, perception of discrimination, and distrust of allopathic medicine may tend to drive many Hispanics with end-stage cancer away from the U.S. medical care system. Cancer deaths without diagnoses that occur outside the medical care system, plus the common practice of going home to one’s country to die among family, would tend to deflate mortality rate numerators, even among Hispanics who are citizens and permanent residents who were counted in the population or clinical denominators (8, 9).
Breast cancer
Breast cancer is the leading incident cancer and leading cause of cancer death in Hispanic women (10, 11). Although incidence rates are lower in Hispanic women than in NHW women, the rate of decline in incidence rates since 2000 is lower for Hispanics than for NHW (Fig. 1). Hispanic women are more likely to be diagnosed at later stages and with larger tumors (11, 12) and they are 20% more likely to die from their breast cancer than NHW women (13). Inadequate health insurance coverage may explain part of the difference in screening, incidence, and mortality rates (11, 14–18).
Breast cancer risk is higher in United States-born Hispanics than foreign-born Hispanics, and risk increases with increasing duration of residence in the United States and increasing acculturation (19), suggesting that important changes in risk factors occur following immigration. Differences in known risk factors for breast cancer likely contribute to differences in incidence rates. However, among Hispanics, fewer breast cancers can be attributed to known risk factors (20), highlighting the importance of additional research in Hispanic women to identify novel risk factors.
The lower breast cancer survival rates among Hispanics than NHW women have been attributed to poor socioeconomic indicators (21) and a higher prevalence of comorbidities such as diabetes (22, 23). Hispanic women are diagnosed at younger ages (24, 25) and have higher rates of high-grade and estrogen receptor (ER)–negative tumors than NHW women (12, 26–31). It has been postulated that differences in breast cancer incidence rates in the United States between Hispanics and NHW could be explained by the recent increase of ER-positive breast tumors among NHW (32).
Breast cancer risk in Hispanics may be related to the degree of European contribution to their genetic admixture (33, 34), underscoring the importance of understanding the genetic substructure of the Hispanic population (35). Recent data also suggest that Hispanic patients with breast cancer have a slightly higher prevalence of BRCA1 and BRCA2 mutations than NHW (36–38). Overall, little is known about the combined effects of lifestyle (influenced by migration and acculturation) and genetic susceptibility or population mixing on the clinical or molecular characteristics of breast cancer in Hispanic women.
Colorectal cancer
Colorectal cancer (CRC) is the second most common cancer in Hispanic men and women, with incidence rates that are 15% and 19% lower than in NHW men and women, respectively. (11) Incidence rates in U.S. Hispanics are higher than those reported for most Latin American countries (39), suggesting that changes in lifestyle and/or environmental risk factors that occur following migration to the United States contribute to increasing CRC rates. There are, however, exceptions to this general pattern. Relative to NHW, CRC incidence rates are lower in Mexico and among Mexicans living in Florida than in NHW in Florida (40), whereas Puerto Ricans and Cubans living in Florida have higher incidence rates than those living in Puerto Rico or Cuba and NHW in Florida (40). Genetic ancestry, socioeconomic status, and acculturation patterns vary across these Hispanic subgroups and may contribute to differences in CRC incidence.
Despite lower CRC incidence rates compared with NHW, Hispanics are diagnosed at an earlier age and with more advanced disease and have worse survival (41). The later stage at diagnosis is likely related to lower CRC screening rates among Hispanics (47%) and Puerto Ricans (38%) than NHW (59%; refs. 42, 43). However, this does not explain the younger age at diagnosis and the overall lower stage-specific CRC survival for Hispanics and Puerto Ricans. The 5-year CRC survival rates for Puerto Ricans living in Puerto Rico with localized (35%), regional (<20%), and metastatic (<5%) cancer were lower than those in the total U.S. population for the same time period (1992–1996: 90%, 68%, and 10%, respectively; refs. 44, 45). The reasons for these disparities are unknown.
Lung cancer
Lung cancer is the third most common cancer in Hispanic men and women and is the leading cause of cancer death among Hispanic men and the second-leading cause among Hispanic women. The incidence rates of lung cancer among Hispanics are substantially lower than in NHW (Fig. 1). Mortality rates from lung cancer in 2009 were 34.0 per 100,000 in Hispanic men compared with 72.6 per 100,000 in NHW men and 14.5 per 100,000 in Hispanic women compared with 44.0 per 100,000 in NHW women (11). The proportion of cancers diagnosed at distant stage is 59% in Hispanics and 52% in NHW, and the proportions of locally diagnosed cancers are 13% and 17%, respectively, resulting in a lower cancer-specific survival in Hispanics than NHW (46).
The patterns of lung cancer incidence and mortality reflect the pattern of tobacco smoking in the same population decades earlier. In general, Latin American countries have a lower prevalence of smoking compared with the United States. The prevalence of current smokers in U.S. adults has been lower in Hispanics than in NHW: 20.4% versus 25.3% in 1997 and 15.4% versus 22.2% in 2009, respectively (47).
Few studies have investigated tobacco smoking and other risk factors of lung cancer in Hispanics. In a case–control study from New Mexico, the risk of lung cancer did not differ between Hispanics and NHW after adjustment for amount of smoking (48). However, an analysis of the Multiethnic Cohort Study suggested a lower susceptibility to tobacco-related lung cancer in Hispanics compared with NHW (49), possibly related to differences in variants of genes involved in DNA repair (50, 51). Data on other risk factors for Hispanics are very limited, particularly among never-smokers. The latter comprise a relatively large proportion of lung cancer cases in Hispanics (e.g., 18% in Hispanics vs. 6% in NHW; ref. 49); therefore, studies of lung cancer in Hispanics may present opportunities to identify other nonsmoking-related causes of lung cancer.
Prostate cancer
Prostate cancer is the leading incident cancer and the fourth cause of cancer deaths in Hispanic men. It is estimated that more than 11,000 new prostate cancer cases per year are diagnosed in Hispanics, with an estimated 1,500 deaths in 2009. Prostate cancer accounts for almost one quarter of the cancer burden in this population (11). Both incidence and death rates have declined over the past 10 years. For 2002 to 2006, the incidence rate was 131 per 100,000, about 10% lower than in NHW (11). Despite similar distributions by stage at diagnosis, prostate cancer survival rates in Hispanic men are lower than those in NHW men (13, 52). The most important risk factors for prostate cancer in both Hispanics and NHW men are age and a family history of prostate cancer in first-degree relatives (53). While detailed studies of specific environmental risk factors have not been carried out in large populations of Hispanic men, such studies in NHW men are largely inconsistent and uninformative. Notably, many of the risk variants discovered in genome-wide association studies (GWAS) in populations of European ancestry are also positively associated with risk in Hispanics.
Cancers with Elevated Risk in Hispanics
Here, we discuss 4 cancers where the incidence is substantially higher in Hispanics than NHW. Few studies of risk factors have been conducted in this population. Intriguing patterns (e.g., a 5-fold difference in gastric cancer incidence between Hispanic subgroups) offer opportunities for better understanding the causes of these cancers.
Cervical cancer
In the United States, cervical cancer incidence is higher among Hispanics than in all other major racial/ethnic groups. From 1992 to 2007, age-adjusted incidence among Hispanic women fell from 21.0 to 10.1 per 100,000 but remained nearly double than that of NHW women (Fig. 1). During the same period, mortality declined from 4.52 to 3.04 per 100,000 among Hispanic women and from 2.90 to 2.14 per 100,000 among NHW women (46, 54). Higher mortality among Hispanics likely reflects both higher incidence and later detection, as Hispanics are more likely to be diagnosed with advanced cervical cancer than NHW (55, 56).
Disparities in cervical cancer incidence and mortality are also observed between Hispanic subgroups. Incidence rates are higher among Hispanic women living in the United States–Mexico border states than in Hispanics living in other states (57), and cervical cancer mortality of foreign-born Hispanic women is 40% higher than that of United States-born Hispanic women (58). Foreign-born Hispanic women receive Pap screening less frequently than either United States-born Hispanic or NHW women (59, 60).
New technologies such as the human papilloma virus (HPV) vaccine may improve both primary and secondary prevention of cervical cancer (61, 62). Rates of HPV vaccine uptake in the United States remain low, but rates among young Hispanics tend to exceed those in NHW and the proportion of parents accepting vaccination of their daughters by the age of 13 years is higher for Hispanics than other racial/ethnic groups (63). Recently approved DNA-based tests for carcinogenic HPV are more sensitive than traditional cytology for detecting preneoplastic lesions (64, 65). These tests can be adapted for self-administration, which may prove useful in overcoming traditional barriers to screening.
Gastric cancer
Gastric cancer disproportionately affects Hispanics compared with NHW (66). As in other racial/ethnic groups, gastric cancer incidence and mortality rates among Hispanics are higher in men than in women. Incidence rates vary substantially between Hispanic subgroups. Hispanics from Mexico and Puerto Rico have incidence rates 2.2 to 3.6 times higher than those of NHW, whereas Hispanics from South or Central America have rates 4.3 to 5.1 times higher. In contrast, rates in Cubans are almost as low as those of NHW (66). No differences are seen between Hispanics and NHW for either stage at diagnosis (67) and overall or stage-specific survival (68, 69).
Of the 2 main subtypes of gastric cancer, proximal (cardia) cancers have been increasing, especially in developed countries (70) and distal (non-cardia) cancers have declined in both Hispanics and NHW. Each subtype is associated with different etiologic factors. Proximal cancers, which are more frequent in NHW (71), are associated with obesity and gastrointestinal reflux (72). Given the recent increase in obesity in Hispanics, a concern is whether incidence rates of proximal cancers will increase in the future. Helicobacter pylori infection is an established risk factor for distal gastric cancer (66, 73, 74). Infection with H. pylori occurs in early childhood and is likely transmitted person-to-person via fecal-oral or oral-oral routes (75–77). Data from the third National Health and Nutritional Examination Survey show that Hispanics from Mexico had higher rates of infection than NHW (61.6% vs. 26.2%, respectively; ref. 78). In addition, infection prevalence is higher among foreign-born than United States-born Hispanics (79). Recent research has focused on risk factors responsible for progression, from infection to cancer, including bacterial virulence [i.e., cytotoxin-associated gene A (cagA) strains; refs. 80, 81] and host factors such as genetic variants that result in differential expression of proinflammatory cytokines (82). Eradication of H. pylori infection likely represents one of the more promising and achievable strategies for reducing the global impact of this malignancy.
Liver cancer
Liver cancer, primarily hepatocellular carcinoma (HCC), is not a common cancer in the United States; however, both the incidence and mortality rates of liver cancer are on the rise and they are substantially higher in Hispanics than in NHW (Figs. 1 and 2).
Men from Puerto Rico have the highest incidence of liver cancer (19.2 per 100,000), followed by men from Mexico (10.8) and Cuba (10.1; ref. 40). Furthermore, Hispanic men in the United States tend to have higher incidence rates of liver cancer than their counterparts living in Latin America (40). The most striking difference is the 2-fold increased risk for liver cancer among Puerto Rican men living on the U.S. mainland versus those in Puerto Rico (40, 83). Compared with men, women have a substantially lower annual incidence rate (about 5 per 100,000), which does not vary among Hispanic subgroups (40). Despite the substantially higher incidence rate of liver cancer in Hispanics, no epidemiologic studies have ever been done to elucidate the etiology of liver cancer in the Hispanic population and to identify the factors contributing to disparities in liver cancer risk by ethnicity, gender, and Hispanic subgroup.
The risk factors for HCC include hepatitis B virus (HBC) and hepatitis C virus (HCV) infections, heavy alcohol consumption, cirrhosis, nonalcoholic steatohepatitis, tobacco smoking, aflatoxin exposure, and possibly obesity and type 2 diabetes (84, 85). The higher incidence rate of liver cancer in Hispanics than in NHW could partly be due to a greater prevalence of some of these risk factors in Hispanics. Although the prevalence of HBV and HCV seropositivity is similar in Hispanics and NHW (86, 87), Hispanics from Mexico are more likely than NHW to be heavy or binge drinkers (63% vs. 37%); Hispanics from Puerto Rico and Mexico are more likely to be obese (21% vs. 16%) and have diabetes (15% vs. 4%; refs. 87, 88). Hispanics also have a higher frequency of non–alcohol-related hepatic steatosis than NHW (45% vs. 33%; ref. 89). Health care disparities, for example, in access to early detection and receipt of adequate treatment for viral hepatitis, diabetes, and chronic liver diseases may also contribute to the higher liver cancer incidence rate in Hispanics.
The trend of increasing incidence in the United States in general may be partly attributed to the growing problems of obesity and diabetes, as well as improved survival of and hence an increased number of persons living with cirrhosis (90). The proportions of HCV- and HBV-associated HCC almost doubled between 1993 and 1999 indicating that viral hepatitis may contribute to a significant proportion of the increase in HCC (91).
Inequities in health care access do not appear to explain the mortality pattern. The proportions of liver cancer diagnosed at distant stage in 2003 to 2007 were 17% and 18% for Hispanics and NHW, respectively, and the 5-year cause-specific survival rates in the 2 populations were also similar (23% vs. 22%; ref. 54). Thus, the higher liver cancer mortality rate in Hispanics than NHW may largely be due to the disparity in their incidence rates.
Gall bladder cancer
Hispanics have the highest incidence of GBC in the United States. In women, the incidence rate in 2007 was 2 to 2.5 times higher for Hispanics than for NHW, African Americans, or Asian/Pacific Islanders. This rate ratio was about 1.7 for Hispanic men compared with NHW men (54).
The major risk factor for GBC is a history of gallstones, which are much more prevalent in women (92). Worldwide, the highest prevalence of gallstones occurs in various Native American groups (92). High prevalence rates also occur in Native Americans in Central America (93, 94). In Hispanics from Mexico and in Chileans, prevalence rates have been shown to increase with increasing percentage of Native American genetic admixture (94, 95).
Risk factors for gallstone disease and those for GBC largely overlap. The most important risk factors for both diseases are age, gender, parity, obesity, and diabetes mellitus (92, 96, 97). Most studies have also reported a significant increase in risk associated with a family history of either gallstones (98–100) or GBC (101–103). Hispanics have a higher prevalence of both obesity and diabetes, and Hispanic women have higher parity than NHW women (11) which, along with the Native American contribution to their genetic admixture, may explain the high GBC incidence in this population.
The close association between gallstone disease and GBC suggests that gall bladder removal to treat gallstones is a significant preventive measure (92). More limited access to medical insurance among Hispanics than NHW (104) may potentially decrease access to surgical treatment for gallstone disease and effectively decrease primary prevention in this population.
Study Design Considerations
There is an unmet need to conduct studies of cancer etiology and progression in the Hispanic population. Unrealized opportunities offered by the Hispanic population, such as major differences in risk between Hispanic subgroups defined by country of origin or level of acculturation, have the potential to increase our understanding of the causes of cancer. Also, the Hispanic population may be an optimum setting in which to conduct studies of environmental and lifestyle exposures and gene–environment interactions for 2 reasons: (i) Hispanics are experiencing secular trends in exposures that are associated with immigration and acculturation, thus generating a greater range of exposure for selected environmental factors, such as diet, thereby providing greater study power; and (ii) there is a wide range in the underlying genetic ancestry of current Hispanic populations that may be measured with ancestry informative markers (AIM), which will facilitate potentially revealing analyses that incorporate AIMs into studies of genes, environment, and gene–environment interactions.
There is a paucity of even basic descriptive epidemiologic information about the prevalence of risk factors for cancer and determinants of compliance with prevention and screening guidelines and how such factors relate to each other, so surveys designed to characterize the heterogeneity of the Hispanic population (see the Heterogeneity of the Hispanic population section later) would be useful and would help inform the design of larger studies.
Regarding observational studies, a design that is useful for studying the full spectrum of cancer risk and other health outcomes is a cohort study. Advantages of the cohort design, articulated by Manolio and colleagues (105) include: (i) reduction in selection and recall biases, (ii) accuracy and reliability of environmental exposure data, (iii) ability to study predictive biomarkers, and (iv) ability to study a broad range of health outcomes. They suggested that “it is far more likely that environmental and behavioral changes, in interaction with a genetic predisposition, have produced most of the recent increases in chronic disease,” and concluded that “prospective cohort studies provide a valuable, feasible, and, indeed, indispensable means of exploring the genetic basis of complex diseases.”
However, cohort studies are very expensive to establish and take many years before the yield of cases is large enough for informative analyses, particularly for cancer endpoints. The primary motivation for establishing a new cohort of Hispanics would have to come from anticipated studies of more common end points that affect Hispanics at high rates, such as obesity, diabetes, and cardiovascular disease. For cancer, given the importance of the problem (and the opportunities) as described above, it may be that a series of large case– control studies of selected cancers, such as the 4 described above that occur at relatively higher rates in Hispanics, should be considered. While this design is reasonable for genetic association studies (e.g., the recent GWAS) given the availability of genomic controls to “clean up” the signals from controls selected by a variety of ascertainment schemes and varying response rates, case–control studies are becoming problematic for the study of environmental and lifestyle factors because the response rates for controls selected from traditional sources (e.g., neighborhood, friends, random digit dialing) are dropping to worrisome levels, and this is even more of a concern for racial/ethnic minority populations such as Hispanics. The design and generation of proper control series for the study of nongenetic factors in today’s environment is an area that warrants more attention from the epidemiologic community.
As we contemplate the next generation of studies, we should also consider making full use of data that are already available. Two cohorts focused on Hispanics exist in the United States: the NHLBI-sponsored study entitled, “The Hispanic Community Health Study/Study of Latinos” (HCHS/SOL), which includes 16,000 participants recruited from San Diego, Chicago, New York City, and Miami (www.cscc.unc.edu/hchs/), and the ongoing “Mano a Mano” study in Texas, including more than 20,000 Mexican-American participants (www.mdanderson.org/patient-and-cancer-information/cancer-information/cancer-topics/prevention-and-screening/studies-and-programs/prevention-studies-for-men-and-women/mano-a-mano/index.html). In addition, groups of Hispanics are included in several of the cohorts participating in the NCI Cohort Consortium (epi.grants.cancer.gov/Consortia/cohort.html). Table 1 summarizes the data available from cohorts with at least 2,000 Hispanics. Approximately 80,000 individuals are available in 9 cohorts, more than half of whom are in the California component of the Multiethnic Cohort (MEC), which specifically targeted recruitment of a large number of Hispanics. The estimated number of cancer cases that have occurred among Hispanics during the follow-up of these cohorts is about 9,000, although the actual number is not known because not all cohorts have reported the relevant data. Overall, this is a valuable resource for epidemiologic research among Hispanics and an effort to conduct a pooled analysis of these data is encouraged.
Table 1.
Number of Hispanics included in cohorts participating in the NCI Cohort Consortium and expected number of cancer cases (cohorts with <2,000 Hispanic subjects are excluded)
| Cohort | Na | Expected cancer casesb | Cohort website |
|---|---|---|---|
| California Teachers Study | 50,000 | 530 | www.calteachersstudy.org/index.html |
| MEC | 47,000 | 6,340 | www.crch.org/multiethniccohort |
| NIH-AARP | 11,000 | 1,210 | www.dietandhealth.cancer.gov |
| NHS-II | 3,000 | 70 | www.channing.harvard.edu/nhs |
| PLCO | 3,000 | 320 | www.dcp.cancer.gov/ |
| 7th Day Adventistsc | 3,000 | 60 | www.llu.edu/public-health/health/index.page |
| Sister Studyd | 3,000 | 90 | www.sisterstudy.org |
| SCCS | 2,000 | NA | www.southerncommunitystudy.org |
| WHI | 6,000 | 710 | www.whi.org |
| Total | 83,000 | 9,330 |
Abbreviations: MEC, Multiethnic Cohort Study; NIH-AARP, NIH-American Association for Retired Persons study; NHS-II, Nurses Health Study II; PLCO, Prostate Lung Colorectal and Ovarian Cancer Cohort Study; SCCS, Southern Community Cohort Study; WHI, Women’s Health Initiative Study.
Number of Hispanics included in the cohort, based on the data available in the Cohort Consortium database (epi.grants.cancer.gov/Consortia/cohort.html).
Expected number of cancer cases among Hispanics, based on ratio of number of Hispanics over total cohort (assuming comparable age/sex distribution and cancer rates).
7th Day Adventist Health Study.
Sister Study of Breast Cancer.
Elements to Consider in Future Studies of Hispanic Populations
Regardless of design, there are a number of elements to consider as studies of cancer etiology and control in Hispanic populations are planned.
Heterogeneity of the Hispanic population
The tendency to date has been to consider Hispanics as a single category, as if they were a homogeneous population. This is not the case. From a population genetics perspective, U.S. Hispanics are characterized by different admixtures due to the history of the original populating of the Americas, colonization of different regions by different European countries at different times, and differential immigration patterns to the United States. In addition to differences in genetic ancestry, there are differences in environmental exposures and behavioral and lifestyle practices within the Hispanic populations, defined, in part, by their country of origin, degree of acculturation, and region of the United States to which they immigrated. We see this, for example, in differences in dietary practices (see below) and differences in smoking and alcohol consumption between the Hispanic subgroups cited above. Finally, as described above, risk of certain cancers differs 2 to 5-fold within Hispanic population based on country of origin. Future studies should consider means of addressing this heterogeneity.
Genetic factors
AIMs
Genetic studies in admixed populations are particularly vulnerable to confounding due to population stratification, a difference in ancestry between cases and controls (106). Such confounding can be dealt with by estimating individuals’ genetic ancestry using genetic markers and then adjusting the analysis for individual ancestry (86). AIMs can be selected from lists that have been specifically compiled for this purpose (107–111). However, in many instances, finding the ideal “ancestral populations” for the population being considered is difficult. Native American populations have undergone several founder population effects so that Native Americans from Central America, the Caribbean, and different regions of South America may have substantial allele frequency differences (112). Thus, additional work to document the allele frequencies of previously described AIMs in more populations, especially in additional Native American populations, would enhance future studies in admixed populations such as Hispanics.
AIMs have been used to study a variety of Hispanic populations. In general, most Hispanic populations have a combination of European, Native American, and African ancestry, but the proportions vary substantially by country or region of origin. For example, Mexicans and Central Americans are descended mostly from European and Native American populations with a much lower proportion of African ancestry (113). Puerto Rican and other Caribbean populations are mostly descended from European and African populations with a lower proportion of Native American ancestry (113). Even within any particular Latin American country, there may be substantial differences by region. For example, in a recent study in Mexican women, Native American ancestry varied from 54% in Monterrey to 69% in Mexico City (34).
Once individual genetic ancestry is estimated, it can be used just as any other covariate in multivariate models to adjust for genetic associations and examine associations and interactions with environmental factors. For example, among Hispanic women living in California, some reproductive risk factors for breast cancer are associated with higher European and lower Native American ancestry (113). However, even after adjustment for reproductive characteristics and other risk factors, European ancestry is associated with higher risk of breast cancer among Hispanics in California (33) and among Mexican women (34). Thus, genetic ancestry in combination with known environmental risk factors may be used to understand the factors leading to a difference in risk across different Hispanic populations (114).
“Coverage” in GWAS panels and relevant considerations for GWAS in Hispanic populations
GWAS of common cancers have been initiated in Hispanic populations to assess both the pan-ethnic nature of the loci observed in populations of European ancestry and to search for novel risk variants that may be more common or limited to populations with Native American ancestry. Initial scans in breast and prostate cancer are revealing interesting and unexpected results, such as substantially weaker associations at the fibroblast growth factor receptor (FGFR) locus with breast cancer and at 8q24 with prostate cancer (Chris Haiman, unpublished work). Additional large-scale efforts across a wide range of Hispanic subgroups will be needed to better understand the genetic basis of cancer risk in the Hispanic population. The current set of genome-wide scanning arrays mainly includes content for tagging common genetic variation in European populations. The relative coverage in these arrays of genetic variation in Hispanic populations is not known, but ongoing projects should help address this important question. For example, the 1000 Genomes Project is sequencing Hispanic samples (70 each from Mexican Americans in Los Angeles, Puerto Ricans, Colombians, and Peruvians and 100 Iberians from Spain) and will be completed in the near future.
Environmental factors
We made the case above that Hispanics may be an informative group in which to study environmental, behavioral, and lifestyle factors potentially associated with cancer risk. The study of such exposures is not without challenges, however, given the following factors: (i) lower average education; (ii) lack of preliminary data for targeting novel risk factors, and (iii) the need to consider interactions between environmental exposures, ancestry, and acculturation with the consequent loss of statistical power. Below, we address some measurement considerations that may enhance the information we obtain on environmental, behavioral, and lifestyle factors in Hispanics.
Assessment of physical activity
There is evidence for preventive effects of physical activity on breast, colon, endometrial, prostate, and lung cancer (115). The Hispanic population is at significant risk for physical inactivity and obesity. For example, in a nationally representative sample of 68,288 youth in the United States, 22.5% of foreign-born Hispanic youth were physically inactive, defined as having no physical activity in any day in the past week, compared with 9.5% of NHW children (116). Hispanic adults report less time spent in leisure-time physical activity than NHW (117). In Hispanic youth, 38.2% were overweight or obese, compared with 31.7% of all U.S. youth and 76.9% of Hispanic adults were overweight or obese compared with 67.5% of NHW (118–120).
Physical activity is a complex cluster of diverse behaviors that can be measured in many ways including direct observation, objective measures (e.g., pedometers or accelerometers), or questionnaire-based self-reports. Although self-report is the least costly measurement modality, people tend to either misremember or overestimate their own physical activity (121). The complexities involved in measuring physical activity by self-report in Hispanic populations are amplified by the fact that most physical activity measures are not culturally tailored for Hispanic populations. In fact, a recent review could not identify a single Spanish language measure of physical activity that met minimum guidelines for cultural adaptation and translation of measures for use among Hispanics (122). Therefore, objective measurement of physical activity in Hispanic populations is advocated. Accelerometry is the most commonly used objective method of current physical activity assessment in both youth (123) and adults (124) and is not subject to memory or social desirability bias. Recent advances in integrated circuitry and memory capacity have produced sensitive, unobtrusive accelerometers that measure the intensity, frequency, and duration of movement for extended periods (125).
Assessment of diet
Most large studies on dietary etiology of disease use some variation of a food frequency questionnaire (FFQ). However, because the validity of the assessment depends on a predefined food list, the use of an existing FFQ is limited to the group for which it was created. Several questionnaires have been designed for subgroups of the Hispanic population, but most of these only cover the Mexican-American diet (126). Further, FFQ used with multiple Hispanic populations nationally showed low validation coefficients (127). One questionnaire has been designed for Hispanics of Caribbean origin, such as Puerto Ricans, Dominicans, and Cubans (128), but we know of no existing questionnaire that covers all of the diverse Hispanic subgroups in the United States.
Because of the recognized limitations of existing FFQ, the current NHLBI multisite Study of Latinos (SOL) measures dietary intake using two 24-hour recalls (129). This open-ended method is useful for groups for whom there are limited quantitative data from which to develop a FFQ. These data will contribute to a better understanding of the current diets of diverse Hispanic groups in the United States, which have not been available from large-scale surveys since the Hispanic Health and Nutrition Examination Survey (HANES) conducted in the 1980s (130). This method, however, is not without error. In addition to known problems with underreporting, a major limitation of 24-hour recalls is the random error associated with day-to-day variability in intake, which can lead to misclassification.
A valid method should ideally represent an integrated measure of long-term intake with reasonably equal coverage of major foods and preparation methods in population subgroups. For large multicenter studies, it should be conducted efficiently, ideally in a single administration. To avoid cross-study bias, it is important that future studies are planned in advance to use the same dietary assessment tool with appropriate training and standardized implementation. It will be important to develop such a tool for use in studies that include multiple Hispanic subgroups, probably using elements of 24-hour recall responses with an FFQ format to ensure complete and reasonably uniform assessment of usual intake across groups.
Biomarkers of exposure
Biomarkers may contribute to exposure assessment, complementing external monitoring data, questionnaires, and medical and other types of records (131). These biomarkers have the potential to integrate exposures acquired through different routes and to specify subcomponents of exposure. Furthermore, they reflect interindividual differences in uptake, metabolism, and excretion of the relevant agents; these differences can be due to genetic variability but also due to interactions with other exposures. For example, biomarkers of carcinogen metabolites, such as the tobacco-specific N-nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), are proving relevant to tobacco-related carcinogenesis, incorporating differences in smoking habits, type of tobacco, and individual metabolism (132). Temporal relevance is the main drawback of exposure biomarkers, as they typically reflect recent exposure, whereas epidemiologic studies require integration of exposure over a long period of time or time windows relevant to the carcinogenic process.
Only a small number of studies have investigated biomarkers of exposure to carcinogens in Hispanics, but results suggest the usefulness of this approach. In a study of markers of exposure to second-hand smoke in pre-school children from New York, levels of polycyclic aromatic hydrocarbons (PAH)-albumin adducts were lower in Hispanic than in African-American children, after controlling for questionnaire-based exposure to second-hand smoke (133). Similarly, in a study of adult smokers, levels of PAH-DNA adducts were lower in Hispanics than in African Americans, after adjustment for self-reported smoking (134). Despite their limitations, these data support inter-ethnic differences in tobacco-related carcinogenesis. In a study of breast cancer, levels of plasma protein carbonyls, a marker of oxidative damage from both exogenous and endogenous sources, were associated with increased cancer risk and these levels were lower in Hispanic women, again suggesting differences in carcinogenicity in different groups of the population (135).
Metabolomics to complement environmental and genomic studies
With regard to the complex inter- and intraethnic effects on cancer risk of immigration- and acculturation-associated changes in the environment, the emerging field of metabolomics holds promise to enhance the measurement of these factors on individual metabolism and their relationship to disease risk. High-throughput, highly parallel metabolite analyses of complex matrices show early promise to enhance our knowledge of the diversity of the human metabolome (136). This includes measures of metabolites that are derived from or influenced by diet and other environmental exposures (i.e., hormones, toxicants, drugs, infectious agents) and that enable the study of complex host and gut flora “genome” interactions not measured by other approaches (http://www.metabolomics.ca/). Although promising results are emerging for the application of metabolomics to the study of cancer endpoints (137), application to risk assessment in the population setting is in its infancy. In contrast to efforts in the diagnostic and treatment settings, interest in metabolomic cancer risk biomarkers have focused largely on nutrition (138) and hormones, particularly estrogen exposures (e.g., refs. 139, 140). Nutritional “metabolomics,” the profiling of the small molecules of biologics resulting from dietary exposures and the joint metabolism by the host and gut flora, offers new opportunities for more precise biochemical (nongenetic) measurement of “nutritional phenotypes” (141) to study their relationship to cancer risk (138, 142–145). To our knowledge, no studies are currently investigating the impact of environmental change on the nutritional phenotype or other metabolic patterns and cancer risk in Hispanics.
Cultural factors
Hispanic cultural values (e.g., machismo, familism, fatalism) may affect risk of cancer and compliance with screening guidelines (146–150). Key cultural indicators for Hispanics include those commonly studied under the umbrella concept of acculturation, including retention of the Spanish language and Hispanic cultural practices, values, and identifications as well as acquisition of U.S. cultural practices, values, identifications, and English language proficiency (151). These indicators are no longer viewed along a single continuum of acculturation but rather within separate heritage and receiving dimensions of acculturation. This approach enables individuals to be bilingual and bicultural—endorsing nonconflicting practices, values, and identifications from both cultures simultaneously.
The literature is inconsistent about how these dimensions relate to cancer risk and outcomes. Some studies have found that “more acculturated” Hispanics were more likely to seek out cervical cancer screening (152), mammography (153), and genetic cancer risk testing (154), whereas other studies have found that “less acculturated” Hispanics were more likely to present for CRC screening (155). However, the literature is limited by imprecise measures and varying definitions of acculturation as well as by confounding of acculturation with other variables such as socioeconomic status, education, and access to health care.
Moving to multidimensional measures of acculturation might help to elucidate the complex associations between acculturation and cancer risk decisions about cancer screening and treatment seeking.
Few studies have attempted to study the effects of acculturation on cancer incidence or mortality. Several studies have examined proxy measures of acculturation in relation to cancer outcomes. These proxy measures have ranged from simple comparisons such as birth place and length of residence in the United States to more tangential measures such as integration into non-Hispanic neighborhoods. Overall, these studies support the notion that acculturation to the U.S. culture (or loss of ties to traditional Hispanic cultures) is associated with increased incidence of breast cancer (19, 156), CRC (156), and lung cancer (156) and increased cancer mortality (157). However, much of this information is piecemeal and the proxy measures used to represent acculturation may not capture the construct adequately (151). Large longitudinal studies are needed to gain a more complete understanding of the effects of multiple aspects of acculturation on cancer incidence and mortality.
Expanding the focus of acculturation, the interrelation of complex socioeconomic status (SES) factors, especially poverty (158) contribute to observed disparities in cancer incidence and death among racial, ethnic, and underserved groups (159, 160) SES, more than race or ethnicity, predicts likelihood of behavioral risk factors for cancer (e.g., smoking, physical inactivity, obesity, excessive alcohol intake) and having health insurance that provides access to screening for early detection, prevention, and treatment. This holds especially true in the case of Hispanics (11); differences in lifestyle and access to care and treatment weigh most heavily in this disparity (161, 162). Hispanics are also less likely to have health insurance than any other racial or ethnic group (18). Data on whether SES accounts for differences in risk for specific cancers in Hispanics are conflicting and more research is needed to clearly delineate the effects of acculturation and SES (163–167).
Summary
Cancer studies focused on the Hispanic population are warranted because this population represents a major, fast-growing, and understudied segment of the U.S. population and the cancer patterns described above offer some intriguing opportunities for research that may help elucidate the etiology of selected cancers. The issue of heterogeneity of the Hispanic population should be addressed in future studies as this population is highly diverse with respect to genetic ancestry, migration history and acculturation, prevalence and history of exposure to environmental exposures, behavioral and lifestyle practices, and cancer risk. This diversity presents both a challenge and an opportunity.
Footnotes
Disclosure of Potential Conflicts of Interests
No potential conflicts of interests were disclosed.
References
- 1.Vargas Bustamante A, Chen J, Rodriguez HP, Rizzo JA, Ortega AN. Use of preventive care services among Latino subgroups. Am J Prev Med. 2010;38:610–9. doi: 10.1016/j.amepre.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Afable-Munsuz A, Liang SY, Ponce NA, Walsh JM. Acculturation and colorectal cancer screening among older Latino adults: differential associations by national origin. J Gen Intern Med. 2009;24:963–70. doi: 10.1007/s11606-009-1022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zambrana RE, Breen N, Fox SA, Gutierrez-Mohamed ML. Use of cancer screening practices by Hispanic women: analyses by subgroup. Prev Med. 1999;29:466–77. doi: 10.1006/pmed.1999.0566. [DOI] [PubMed] [Google Scholar]
- 4.Gorin SS, Heck JE. Cancer screening among Latino subgroups in the United States. Prev Med. 2005;40:515–26. doi: 10.1016/j.ypmed.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Shah M, Zhu K, Potter J. Hispanic acculturation and utilization of colorectal cancer screening in the United States. Cancer Detect Prev. 2006;30:306–12. doi: 10.1016/j.cdp.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Abraido-Lanza AF, Chao MT, Gates CY. Acculturation and cancer screening among Latinas: results from the National Health Interview Survey. Ann Behav Med. 2005;29:22–8. doi: 10.1207/s15324796abm2901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garbers S, Jessop DJ, Foti H, Uribelarrea M, Chiasson MA. Barriers to breast cancer screening for low-income Mexican and Dominican women in New York City. J Urban Health. 2003;80:81–91. doi: 10.1007/PL00022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campesino M, Ruiz E, Glover JU, Koithan M. Counternarratives of Mexican-origin women with breast cancer. ANS Adv Nurs Sci. 2009;32:E57–67. doi: 10.1097/ANS.0b013e3181a3b47c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith AK, Sudore RL, Perez-Stable MD. Palliative care for Latino patients and their families: whenever we prayed, she wept. JAMA. 2011;301:1047–57. doi: 10.1001/jama.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Center for Chronic Disease Prevention and Health Promotion. Cancer among women. Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion; 2009. [Google Scholar]
- 11.American Cancer Society. Cancer facts & figures for Hispanics/Latinos 2009–2011. Atlanta, GA: American Cancer Society; 2009. [Google Scholar]
- 12.Miller BA, Hankey BF, Thomas TL. Impact of sociodemographic factors, hormone receptor status, and tumor grade on ethnic differences in tumor stage and size for breast cancer in US women. Am J Epidemiol. 2002;155:534–45. doi: 10.1093/aje/155.6.534. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 14.Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16:413–28. doi: 10.1007/s11136-006-9138-4. [DOI] [PubMed] [Google Scholar]
- 15.Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713–9. doi: 10.1200/JCO.2008.17.9234. [DOI] [PubMed] [Google Scholar]
- 16.Maly RC, Liu Y, Leake B, Thind A, Diamant AL. Treatment-related symptoms among underserved women with breast cancer: the impact of physician-patient communication. Breast Cancer Res Treat. 2010;119:707–16. doi: 10.1007/s10549-009-0418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mujahid MS, Janz NK, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. The impact of sociodemographic, treatment, and work support on missed work after breast cancer diagnosis. Breast Cancer Res Treat. 2010;119:213–20. doi: 10.1007/s10549-009-0389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward E, Halpern M, Schrag N, Cokkinides V, DeSantis C, Bandi P, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 19.John EM, Phipps AI, Davis A, Koo J. Migration history, acculturation, and breast cancer risk in Hispanic women. Cancer Epidemiol Biomarkers Prev. 2005;14:2905–13. doi: 10.1158/1055-9965.EPI-05-0483. [DOI] [PubMed] [Google Scholar]
- 20.Hines LM, Risendal B, Slattery ML, Baumgartner KB, Giuliano AR, Sweeney C, et al. Comparative analysis of breast cancer risk factors among Hispanic and non-Hispanic white women. Cancer. 2010;116:3215–23. doi: 10.1002/cncr.25154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vona-Davis L, Rose DP. The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J Womens Health (Larchmt) 2009;18:883–93. doi: 10.1089/jwh.2008.1127. [DOI] [PubMed] [Google Scholar]
- 22.Borrell LN. Self-reported hypertension and race among Hispanics in the National Health Interview Survey. Ethn Dis. 2006;16:71–7. [PubMed] [Google Scholar]
- 23.Patterson RE, Flatt SW, Saquib N, Rock CL, Caan BJ, Parker BA, et al. Medical comorbidities predict mortality in women with a history of early stage breast cancer. Breast Cancer Res Treat. 2010;122:859–65. doi: 10.1007/s10549-010-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miranda PY, Wilkinson AV, Etzel CJ, Zhou R, Jones LA, Thompson P, et al. Policy implication of early onset breast cancer among Mexican-origin women. Cancer. 2011;117:390–7. doi: 10.1002/cncr.25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watlington AT, Byers T, Mouchawar J, Sauaia A, Ellis J. Does having insurance affect differences in clinical presentation between Hispanic and non-Hispanic white women with breast cancer? Cancer. 2007;109:2093–9. doi: 10.1002/cncr.22640. [DOI] [PubMed] [Google Scholar]
- 26.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86:705–12. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 27.Hall MJ, Reid JE, Burbidge LA, Pruss D, Deffenbaugh AM, Frye C, et al. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115:2222–33. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedeen AN, White E. Breast cancer size and stage in Hispanic American women, by birthplace: 1992–1995. Am J Public Health. 2001;91:122–5. doi: 10.2105/ajph.91.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lantz PM, Mujahid M, Schwartz K, Janz NK, Fagerlin A, Salem B, et al. The influence of race, ethnicity, and individual socioeconomic factors on breast cancer stage at diagnosis. Am J Public Health. 2006;96:2173–8. doi: 10.2105/AJPH.2005.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez ME, Nielson CM, Nagle R, Lopez AM, Kim C, Thompson P. Breast cancer among Hispanic and non-Hispanic White women in Arizona. J Health Care Poor Underserved. 2007;18:130–45. doi: 10.1353/hpu.2007.0112. [DOI] [PubMed] [Google Scholar]
- 31.Shavers VL, Harlan LC, Stevens JL. Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer. 2003;97:134–47. doi: 10.1002/cncr.11051. [DOI] [PubMed] [Google Scholar]
- 32.DeSantis C, Howlader N, Cronin KA, Jemal A. Breast cancer incidence rates in U.S. women are no longer declining. Cancer Epidemiol Biomarkers Prev. 2011;20:733–9. doi: 10.1158/1055-9965.EPI-11-0061. [DOI] [PubMed] [Google Scholar]
- 33.Fejerman L, John EM, Huntsman S, Beckman K, Choudhry S, Perez-Stable E, et al. Genetic ancestry and risk of breast cancer among US. Latinas Cancer Res. 2008;68:9723–8. doi: 10.1158/0008-5472.CAN-08-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fejerman L, Romieu I, John EM, Lazcano-Ponce E, Huntsman S, Beckman KB, et al. European ancestry is positively associated with breast cancer risk in Mexican women. Cancer Epidemiol Biomarkers Prev. 2010;19:1074–82. doi: 10.1158/1055-9965.EPI-09-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryc K, Velez C, Karafet T, Moreno-Estrada A, Reynolds A, Auton A, et al. Colloquium paper: genome-wide patterns of population structure and admixture among Hispanic/Latino populations. Proc Natl Acad Sci U S A. 2010;107(Suppl 2):8954–61. doi: 10.1073/pnas.0914618107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.John EM, Miron A, Gong G, Phipps AI, Felberg A, Li FP, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 U.S. racial/ethnic groups. JAMA. 2007;298:2869–76. doi: 10.1001/jama.298.24.2869. [DOI] [PubMed] [Google Scholar]
- 37.Weitzel JN, Lagos V, Blazer KR, Nelson R, Ricker C, Herzog J, et al. Prevalence of BRCA mutations and founder effect in high-risk Hispanic families. Cancer Epidemiol Biomarkers Prev. 2005;14:1666–71. doi: 10.1158/1055-9965.EPI-05-0072. [DOI] [PubMed] [Google Scholar]
- 38.Weitzel JN, Lagos VI, Herzog JS, Judkins T, Hendrickson B, Ho JS, et al. Evidence for common ancestral origin of a recurring BRCA1 genomic rearrangement identified in high-risk Hispanic families. Cancer Epidemiol Biomarkers Prev. 2007;16:1615–20. doi: 10.1158/1055-9965.EPI-07-0198. [DOI] [PubMed] [Google Scholar]
- 39.Curado MP, Edwards B. Cancer incidence in five continents. Lyon, France: IARC; 2007. [Google Scholar]
- 40.Pinheiro PS, Sherman RL, Trapido EJ, Fleming LE, Huang Y, Gomez-Marin O, et al. Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162–9. doi: 10.1158/1055-9965.EPI-09-0329. [DOI] [PubMed] [Google Scholar]
- 41.Stefanidis D, Pollock BH, Miranda J, Wong A, Sharkey FE, Rousseau DL, et al. Colorectal cancer in Hispanics: a population at risk for earlier onset, advanced disease, and decreased survival. Am J Clin Oncol. 2006;29:123–6. doi: 10.1097/01.coc.0000199918.31226.f8. [DOI] [PubMed] [Google Scholar]
- 42.Berry J, Bumpers K, Ogunlade V, Glover R, Davis S, Counts-Spriggs M, et al. Examining racial disparities in colorectal cancer care. J Psychosoc Oncol. 2009;27:59–83. doi: 10.1080/07347330802614840. [DOI] [PubMed] [Google Scholar]
- 43.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 44.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 45.Soto-Salgado M, Suarez E, Calo W, Cruz-Correa M, Figueroa-Valles NR, Ortiz AP. Incidence and mortality rates for colorectal cancer in Puerto Rico and among Hispanics, non-Hispanic whites, and non-Hispanic blacks in the United States, 1998–2002. Cancer. 2009;115:3016–23. doi: 10.1002/cncr.24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Surveillance Epidemiology and End Results (SEER) Research Data, Nov 2009 submission (1992–2007) Bethesda, MD: National Cancer Institute; 2009. Available from: www.seer.cancer.gov. [Google Scholar]
- 47.American Lung Association. Trends in tobacco use. Washington, DC: American Lung Association; 2011. [Google Scholar]
- 48.Humble CG, Samet JM, Pathak DR, Skipper BJ. Cigarette smoking and lung cancer in ‘Hispanic’ whites and other whites in New Mexico. Am J Public Health. 1985;75:145–8. doi: 10.2105/ajph.75.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354:333–42. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 50.Chang JS, Wrensch MR, Hansen HM, Sison JD, Aldrich MC, Quesenberry CP, Jr, et al. Nucleotide excision repair genes and risk of lung cancer among San Francisco Bay Area Latinos and African Americans. Int J Cancer. 2008;123:2095–104. doi: 10.1002/ijc.23801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang JS, Wrensch MR, Hansen HM, Sison JD, Aldrich MC, Quesenberry CP, Jr, et al. Base excision repair genes and risk of lung cancer among San Francisco Bay Area Latinos and African-Americans. Carcinogenesis. 2009;30:78–87. doi: 10.1093/carcin/bgn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Latini DM, Elkin EP, Cooperberg MR, Sadetsky N, Duchane J, Carroll PR. Differences in clinical characteristics and disease-free survival for Latino, African American, and non-Latino white men with localized prostate cancer: data from CaPSURE. Cancer. 2006;106:789–95. doi: 10.1002/cncr.21675. [DOI] [PubMed] [Google Scholar]
- 53.Stone SN, Hoffman RM, Tollestrup K, Stidley CA, Witter JL, Gilliland FD. Family history, Hispanic ethnicity, and prostate cancer risk. Ethn Dis. 2003;13:233–9. [PubMed] [Google Scholar]
- 54.Surveillance, Epidemiology and End Results (SEER) Program SEER*-Stat Database. Bethesda, MD: National Cancer Institute; 2010. [cited 2010 Jun 15]. Available from: www.seer.cancer.gov. [Google Scholar]
- 55.del Carmen MG, Montz FJ, Bristow RE, Bovicelli A, Cornelison T, Trimble E. Ethnic differences in patterns of care of stage 1A(1) and stage 1A(2) cervical cancer: a SEER database study. Gynecol Oncol. 1999;75:113–7. doi: 10.1006/gyno.1999.5543. [DOI] [PubMed] [Google Scholar]
- 56.Saraiya M, Ahmed F, Krishnan S, Richards TB, Unger ER, Lawson HW. Cervical cancer incidence in a prevaccine era in the United States, 1998–2002. Obstet Gynecol. 2007;109:360–70. doi: 10.1097/01.AOG.0000254165.92653.e8. [DOI] [PubMed] [Google Scholar]
- 57.Coughlin SS, Richards TB, Nasseri K, Weiss NS, Wiggins CL, Saraiya M, et al. Cervical cancer incidence in the United States in the US-Mexico border region, 1998–2003. Cancer. 2008;113:2964–73. doi: 10.1002/cncr.23748. [DOI] [PubMed] [Google Scholar]
- 58.Seeff LC, McKenna MT. Cervical cancer mortality among foreign-born women living in the United States, 1985 to 1996. Cancer Detect Prev. 2003;27:203–8. doi: 10.1016/s0361-090x(03)00062-x. [DOI] [PubMed] [Google Scholar]
- 59.Goel MS, Wee CC, McCarthy EP, Davis RB, Ngo-Metzger Q, Phillips RS. Racial and ethnic disparities in cancer screening: the importance of foreign birth as a barrier to care. J Gen Intern Med. 2003;18:1028–35. doi: 10.1111/j.1525-1497.2003.20807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nasseri K, Cress RD, Leiserowitz G. Cervical cancer in California. Santa Barbara, CA: Public Health Institute (Tri-Counties Cancer Surveillance Program); 2006. [Google Scholar]
- 61.Downs LS, Jr, Scarinci I, Einstein MH, Collins Y, Flowers L. Overcoming the barriers to HPV vaccination in high-risk populations in the US. Gynecol Oncol. 2010;117:486–90. doi: 10.1016/j.ygyno.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 62.Scarinci IC, Garcia FA, Kobetz E, Partridge EE, Brandt HM, Bell MC, et al. Cervical cancer prevention: new tools and old barriers. Cancer. 2010;116:2531–42. doi: 10.1002/cncr.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Constantine NA, Jerman P. Acceptance of human papillomavirus vaccination among Californian parents of daughters: a representative statewide analysis. J Adolesc Health. 2007;40:108–15. doi: 10.1016/j.jadohealth.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 64.Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand MH, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26(Suppl 10):K29–41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 65.Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 66.Howe HL, Wu X, Ries LA, Cokkinides V, Ahmed F, Jemal A, et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. 2006;107:1711–42. doi: 10.1002/cncr.22193. [DOI] [PubMed] [Google Scholar]
- 67.Yao JC, Tseng JF, Worah S, Hess KR, Mansfield PF, Crane CH, et al. Clinicopathologic behavior of gastric adenocarcinoma in Hispanic patients: analysis of a single institution’s experience over 15 years. J Clin Oncol. 2005;23:3094–103. doi: 10.1200/JCO.2005.08.987. [DOI] [PubMed] [Google Scholar]
- 68.Al-Refaie WB, Tseng JF, Gay G, Patel-Parekh L, Mansfield PF, Pisters PW, et al. The impact of ethnicity on the presentation and prognosis of patients with gastric adenocarcinoma. Results from the National Cancer Data Base. Cancer. 2008;113:461–9. doi: 10.1002/cncr.23572. [DOI] [PubMed] [Google Scholar]
- 69.Kim J, Sun CL, Mailey B, Prendergast C, Artinyan A, Bhatia S, et al. Race and ethnicity correlate with survival in patients with gastric adenocarcinoma. Ann Oncol. 2010;21:152–60. doi: 10.1093/annonc/mdp290. [DOI] [PubMed] [Google Scholar]
- 70.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11:235–56. doi: 10.1016/s1055-3207(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 71.Wu H, Rusiecki JA, Zhu K, Potter J, Devesa SS. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol Biomarkers Prev. 2009;18:1945–52. doi: 10.1158/1055-9965.EPI-09-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mayne ST, Navarro SA. Diet, obesity and reflux in the etiology of adenocarcinomas of the esophagus and gastric cardia in humans. J Nutr. 2002;132:3467S–70S. doi: 10.1093/jn/132.11.3467S. [DOI] [PubMed] [Google Scholar]
- 73.Brenner H, Arndt V, Stegmaier C, Ziegler H, Rothenbacher D. Is Helicobacter pylori infection a necessary condition for noncardia gastric cancer? Am J Epidemiol. 2004;159:252–8. doi: 10.1093/aje/kwh039. [DOI] [PubMed] [Google Scholar]
- 74.Ekstrom AM, Held M, Hansson LE, Engstrand L, Nyren O. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology. 2001;121:784–91. doi: 10.1053/gast.2001.27999. [DOI] [PubMed] [Google Scholar]
- 75.Garg PK, Perry S, Sanchez L, Parsonnet J. Concordance of Helicobacter pylori infection among children in extended-family homes. Epidemiol Infect. 2006;134:450–9. doi: 10.1017/S0950268805005352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kivi M, Tindberg Y, Sorberg M, Casswall TH, Befrits R, Hellstrom PM, et al. Concordance of Helicobacter pylori strains within families. J Clin Microbiol. 2003;41:5604–8. doi: 10.1128/JCM.41.12.5604-5608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weyermann M, Rothenbacher D, Brenner H. Acquisition of Helicobacter pylori infection in early childhood: independent contributions of infected mothers, fathers, and siblings. Am J Gastroenterol. 2009;104:182–9. doi: 10.1038/ajg.2008.61. [DOI] [PubMed] [Google Scholar]
- 78.Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. 2000;181:1359–63. doi: 10.1086/315384. [DOI] [PubMed] [Google Scholar]
- 79.Tsai CJ, Perry S, Sanchez L, Parsonnet J. Helicobacter pylori infection in different generations of Hispanics in the San Francisco Bay Area. Am J Epidemiol. 2005;162:351–7. doi: 10.1093/aje/kwi207. [DOI] [PubMed] [Google Scholar]
- 80.Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636–44. doi: 10.1053/j.gastro.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 81.Polk DB, Peek RM., Jr Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–14. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 83.Ho GY, Figueroa-Valles NR, De La Torre-Feliciano T, Tucker KL, Tortolero-Luna G, Rivera WT, et al. Cancer disparities between mainland and island Puerto Ricans. Rev Panam Salud Publica. 2009;25:394–400. doi: 10.1590/s1020-49892009000500003. [DOI] [PubMed] [Google Scholar]
- 84.Chuang SC, La Vecchia C, Boffetta P. Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Lett. 2009;286:9–14. doi: 10.1016/j.canlet.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 85.El-Serag HB, Lau M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167:1983–9. doi: 10.1001/archinte.167.18.1983. [DOI] [PubMed] [Google Scholar]
- 86.Chowdhury PP, Balluz L, Okoro C, Strine T. Leading health indicators: a comparison of Hispanics with non-Hispanic whites and non-Hispanic blacks, United States 2003. Ethn Dis. 2006;16:534–41. [PubMed] [Google Scholar]
- 87.Flores YN, Yee HF, Jr, Leng M, Escarce JJ, Bastani R, Salmeron J, et al. Risk factors for chronic liver disease in Blacks, Mexican Americans, and Whites in the United States: results from NHANES IV, 1999–2004. Am J Gastroenterol. 2008;103:2231–8. doi: 10.1111/j.1572-0241.2008.02022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ho GY, Qian H, Kim MY, Melnik TA, Tucker KL, Jimenez-Velazquez IZ, et al. Health disparities between island and mainland Puerto Ricans. Rev Panam Salud Publica. 2006;19:331–9. doi: 10.1590/s1020-49892006000500006. [DOI] [PubMed] [Google Scholar]
- 89.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 90.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 91.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127:1372–80. doi: 10.1053/j.gastro.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 92.Shaffer EA. Gallstone disease: epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981–96. doi: 10.1016/j.bpg.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 93.Covarrubias C, Valdivieso V, Nervi F. Epidemiology of gallstone disease. In: Cappacaccia L, Ricci G, Angelico F, editors. Epidemiology and prevention of gallstone disease. Lancaster, England: MTP; 1984. [Google Scholar]
- 94.Miquel JF, Covarrubias C, Villaroel L, Mingrone G, Greco AV, Puglielli L, et al. Genetic epidemiology of cholesterol cholelithiasis among Chilean Hispanics, Amerindians, and Maoris. Gastroenterology. 1998;115:937–46. doi: 10.1016/s0016-5085(98)70266-5. [DOI] [PubMed] [Google Scholar]
- 95.Diehl AK, Stern MP. Special health problems of Mexican-Americans: obesity, gallbladder disease, diabetes mellitus, and cardiovascular disease. Adv Intern Med. 1989;34:73–96. [PubMed] [Google Scholar]
- 96.Eslick GD. Epidemiology of gallbladder cancer. Gastroenterol Clin North Am. 2010;39:307–30, ix. doi: 10.1016/j.gtc.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 97.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591–602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 98.Gilat T, Feldman C, Halpern Z, Dan M, Bar-Meir S. An increased familial frequency of gallstones. Gastroenterology. 1983;84:242–6. [PubMed] [Google Scholar]
- 99.Sarin SK, Negi VS, Dewan R, Sasan S, Saraya A. High familial prevalence of gallstones in the first-degree relatives of gallstone patients. Hepatology. 1995;22:138–41. [PubMed] [Google Scholar]
- 100.van der Linden W. Genetics of cholelithiasis. New York: Academic Press; 1995. [Google Scholar]
- 101.Fernandez E, La Vecchia C, D’Avanzo B, Negri E, Franceschi S. Family history and the risk of liver, gallbladder, and pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 1994;3:209–12. [PubMed] [Google Scholar]
- 102.Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst. 1994;86:1600–8. doi: 10.1093/jnci/86.21.1600. [DOI] [PubMed] [Google Scholar]
- 103.Hemminki K, Li X. Familial liver and gall bladder cancer: a nationwide epidemiological study from Sweden. Gut. 2003;52:592–6. doi: 10.1136/gut.52.4.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rutledge MS, McLaughlin CG. Hispanics and health insurance coverage: the rising disparity. Med Care. 2008;46:1086–92. doi: 10.1097/MLR.0b013e31818828e3. [DOI] [PubMed] [Google Scholar]
- 105.Manolio TA, Bailey-Wilson JE, Collins FS. Genes, environment and the value of prospective cohort studies. Nat Rev Genet. 2006;7:812–20. doi: 10.1038/nrg1919. [DOI] [PubMed] [Google Scholar]
- 106.Ziv E, Burchard EG. Human population structure and genetic association studies. Pharmacogenomics. 2003;4:431–41. doi: 10.1517/phgs.4.4.431.22758. [DOI] [PubMed] [Google Scholar]
- 107.Parra EJ, Marcini A, Akey J, Martinson J, Batzer MA, Cooper R, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet. 1998;63:1839–51. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paschou P, Ziv E, Burchard EG, Choudhry S, Rodriguez-Cintron W, Mahoney MW, et al. PCA-correlated SNPs for structure identification in worldwide human populations. PLoS Genet. 2007;3:1672–86. doi: 10.1371/journal.pgen.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith MW, Patterson N, Lautenberger JA, Truelove AL, McDonald GJ, Waliszewska A, et al. A high-density admixture map for disease gene discovery in African Americans. Am J Hum Genet. 2004;74:1001–13. doi: 10.1086/420856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tian C, Hinds DA, Shigeta R, Adler SG, Lee A, Pahl MV, et al. A genomewide single-nucleotide-polymorphism panel for Mexican American admixture mapping. Am J Hum Genet. 2007;80:1014–23. doi: 10.1086/513522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang N, Li H, Criswell LA, Gregersen PK, Alarcon-Riquelme ME, Kittles R, et al. Examination of ancestry and ethnic affiliation using highly informative diallelic DNA markers: application to diverse and admixed populations and implications for clinical epidemiology and forensic medicine. Hum Genet. 2005;118:382–92. doi: 10.1007/s00439-005-0012-1. [DOI] [PubMed] [Google Scholar]
- 112.Wang S, Lewis CM, Jakobsson M, Ramachandran S, Ray N, Bedoya G, et al. Genetic variation and population structure in native Americans. PLoS Genet. 2007;3:e185. doi: 10.1371/journal.pgen.0030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ziv E, John EM, Choudhry S, Kho J, Lorizio W, Perez-Stable EJ, et al. Genetic ancestry and risk factors for breast cancer among Latinas in the San Francisco Bay Area. Cancer Epidemiol Biomarkers Prev. 2006;15:1878–85. doi: 10.1158/1055-9965.EPI-06-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burchard EG, Ziv E, Coyle N, Gomez SL, Tang H, Karter AJ, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. 2003;348:1170–5. doi: 10.1056/NEJMsb025007. [DOI] [PubMed] [Google Scholar]
- 115.Miles L. Physical activity and the prevention of cancer: a review of recent findings. Nutr Bull. 2007;32:250–82. [Google Scholar]
- 116.Singh GK, Yu SM, Siahpush M, Kogan MD. High levels of physical inactivity and sedentary behaviors among US immigrant children and adolescents. Arch Pediatr Adolesc Med. 2008;162:756–63. doi: 10.1001/archpedi.162.8.756. [DOI] [PubMed] [Google Scholar]
- 117.Centers for Disease Control and Prevention. Prevalence of leisure-time and occupational physical activity among employed adults– United States, 1990. MMWR Morb Mortal Wkly Rep. 2000;10:141–9. [PubMed] [Google Scholar]
- 118.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 119.Koebnick C, Smith N, Coleman KJ, Getahun D, Reynolds K, Quinn VP, et al. Prevalence of extreme obesity in a multiethnic cohort of children and adolescents. J Pediatr. 2010;157:26–31.e2. doi: 10.1016/j.jpeds.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. CDC growth charts for the United States: methods and development. Vital Health Stat. 2000;11(2002):1–190. [PubMed] [Google Scholar]
- 121.Walsh MC, Hunter GR, Sirikul B, Gower BA. Comparison of self-reported with objectively assessed energy expenditure in black and white women before and after weight loss. Am J Clin Nutr. 2004;79:1013–9. doi: 10.1093/ajcn/79.6.1013. [DOI] [PubMed] [Google Scholar]
- 122.Martinez SM, Ainsworth BE, Elder JP. A review of physical activity measures used among US Latinos: guidelines for developing culturally appropriate measures. Ann Behav Med. 2008;36:195–207. doi: 10.1007/s12160-008-9063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Corder K, Ekelund U, Steele RM, Wareham NJ, Brage S. Assessment of physical activity in youth. J Appl Physiol. 2008;105:977–87. doi: 10.1152/japplphysiol.00094.2008. [DOI] [PubMed] [Google Scholar]
- 124.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 125.Sirard JR, Pate RR. Physical activity assessment in children and adolescents. Sports Med. 2001;31:439–54. doi: 10.2165/00007256-200131060-00004. [DOI] [PubMed] [Google Scholar]
- 126.Block G, Wakimoto P, Jensen C, Mandel S, Green RR. Validation of a food frequency questionnaire for Hispanics. Prev Chronic Dis. 2006;3:A77. [PMC free article] [PubMed] [Google Scholar]
- 127.Block G, DiSogra C. WIC dietary assessment validation study Final report. Alexandria, VA: U.S. Department of Agriculture Food and Nutrition Service; 1994. Available from: www.nutritionquest.com. [Google Scholar]
- 128.Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol. 1998;148:507–18. doi: 10.1093/oxfordjournals.aje.a009676. [DOI] [PubMed] [Google Scholar]
- 129.Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–41. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Department of Health and Human Services. Statistics. Hyattsville, MD: Centers for Disease Control and Prevention; 1984. Hispanic Health and Nutrition Examination Survey 1982–1984. editor. [Google Scholar]
- 131.Nieuwenhuijsen MJ. Exposure assessment. In: Wild C, Vineis P, Garte S, editors. Molecular epidemiology of chronic diseases. Hoboken, NJ: John Wiley & Sons; 2008. pp. 83–96. [Google Scholar]
- 132.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–22. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 133.Tang D, Warburton D, Tannenbaum SR, Skipper P, Santella RM, Cereijido GS, et al. Molecular and genetic damage from environmental tobacco smoke in young children. Cancer Epidemiol Biomarkers Prev. 1999;8:427–31. [PubMed] [Google Scholar]
- 134.Weiserbs KF, Jacobson JS, Begg MD, Wang LW, Wang Q, Agrawal M, et al. A cross-sectional study of polycyclic aromatic hydrocarbon-DNA adducts and polymorphism of glutathione S-transferases among heavy smokers by race/ethnicity. Biomarkers. 2003;8:142–55. doi: 10.1080/1354750031000086269. [DOI] [PubMed] [Google Scholar]
- 135.Zipprich J, Terry MB, Liao Y, Agrawal M, Gurvich I, Senie R, et al. Plasma protein carbonyls and breast cancer risk in sisters discordant for breast cancer from the New York site of the Breast Cancer Family Registry. Cancer Res. 2009;69:2966–72. doi: 10.1158/0008-5472.CAN-08-3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vinayavekhin N, Homan EA, Saghatelian A. Exploring disease through metabolomics. Cambridge, MA: Harvard University; 2010. [DOI] [PubMed] [Google Scholar]
- 137.Spratlin JL, Serkova NJ, Eckhardt SG. Clinical applications of metabolomics in oncology: a review. Clin Cancer Res. 2009;15:431–40. doi: 10.1158/1078-0432.CCR-08-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miller AB, Linseisen J. Achievements and future of nutritional cancer epidemiology. Int J Cancer. 2010;126:1531–7. doi: 10.1002/ijc.25006. [DOI] [PubMed] [Google Scholar]
- 139.Blair IA. Analysis of estrogens in serum and plasma from postmenopausal women: past present, and future. Steroids. 2010;75:297–306. doi: 10.1016/j.steroids.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Faupel-Badger JM, Fuhrman BJ, Xu X, Falk RT, Keefer LK, Veenstra TD, et al. Comparison of liquid chromatography-tandem mass spectrometry, RIA, and ELISA methods for measurement of urinary estrogens. Cancer Epidemiol Biomarkers Prev. 2010;19:292–300. doi: 10.1158/1055-9965.EPI-09-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zeisel SH, Freake HC, Bauman DE, Bier DM, Burrin DG, German JB, et al. The nutritional phenotype in the age of metabolomics. J Nutr. 2005;135:1613–6. doi: 10.1093/jn/135.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dragsted LO. Biomarkers of meat intake and the application of nutrigenomics. Meat Sci. 2010;84:301–7. doi: 10.1016/j.meatsci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 143.Jenab M, Slimani N, Bictash M, Ferrari P, Bingham SA. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum Genet. 2009;125:507–25. doi: 10.1007/s00439-009-0662-5. [DOI] [PubMed] [Google Scholar]
- 144.Stella C, Beckwith-Hall B, Cloarec O, Holmes E, Lindon JC, Powell J, et al. Susceptibility of human metabolic phenotypes to dietary modulation. J Proteome Res. 2006;5:2780–8. doi: 10.1021/pr060265y. [DOI] [PubMed] [Google Scholar]
- 145.Walsh MC, Brennan L, Pujos-Guillot E, Sebedio JL, Scalbert A, Fagan A, et al. Influence of acute phytochemical intake on human urinary metabolomic profiles. Am J Clin Nutr. 2007;86:1687–93. doi: 10.1093/ajcn/86.5.1687. [DOI] [PubMed] [Google Scholar]
- 146.Abraido-Lanza AE, Viladrich A, Florez KR, Cespedes A, Aguirre AN, De La Cruz AA. Commentary: fatalismo reconsidered: a cautionary note for health-related research and practice with Latino populations. Ethn Dis. 2007;17:153–8. [PMC free article] [PubMed] [Google Scholar]
- 147.Coronado GD, Thompson B, Koepsell TD, Schwartz SM, McLerran D. Use of Pap test among Hispanics and non-Hispanic whites in a rural setting. Prev Med. 2004;38:713–22. doi: 10.1016/j.ypmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 148.Goldman RE, Diaz JA, Kim I. Perspectives of colorectal cancer risk and screening among Dominicans and Puerto Ricans: stigma and misperceptions. Qual Health Res. 2009;19:1559–68. doi: 10.1177/1049732309349359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Szapocznik J, Kurtines WM. Family psychology and cultural diversity: opportunities for theory, research and application. Am Psychol. 1993;48:400–7. [Google Scholar]
- 150.Teran L, Baezconde-Garbanati L, Marquez M, Castellanos E, Belkic K. On-time mammography screening with a focus on Latinas with low income: a proposed cultural model. Anticancer Res. 2007;27:4325–38. [PubMed] [Google Scholar]
- 151.Schwartz SJ, Unger JB, Zamboanga BL, Szapocznik J. Rethinking the concept of acculturation: implications for theory and research. Am Psychol. 2010;65:237–51. doi: 10.1037/a0019330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Watts L, Joseph N, Velazquez A, Gonzalez M, Munro E, Muzikansky A, et al. Understanding barriers to cervical cancer screening among Hispanic women. Am J Obstet Gynecol. 2009;201:199 e1–8. doi: 10.1016/j.ajog.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 153.Graves KD, Huerta E, Cullen J, Kaufman E, Sheppard V, Luta G, et al. Perceived risk of breast cancer among Latinas attending community clinics: risk comprehension and relationship with mammography adherence. Cancer Causes Control. 2008;19:1373–82. doi: 10.1007/s10552-008-9209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sussner KM, Thompson HS, Valdimarsdottir HB, Redd WH, Jandorf L. Acculturation and familiarity with, attitudes towards and beliefs about genetic testing for cancer risk within Latinas in East Harlem, New York City. J Genet Couns. 2009;18:60–71. doi: 10.1007/s10897-008-9182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Walsh JM, Salazar R, Kaplan C, Nguyen L, Hwang J, Pasick RJ. Healthy colon, healthy life (colon sano, vida sana): colorectal cancer screening among Latinos in Santa Clara, California. J Cancer Educ. 2010;25:36–42. doi: 10.1007/s13187-009-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eschbach K, Mahnken JD, Goodwin JS. Neighborhood composition and incidence of cancer among Hispanics in the United States. Cancer. 2005;103:1036–44. doi: 10.1002/cncr.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Singh GK, Siahpush M. Ethnic-immigrant differentials in health behaviors, morbidity, and cause-specific mortality in the United States: an analysis of two national data bases. Hum Biol. 2002;74:83–109. doi: 10.1353/hub.2002.0011. [DOI] [PubMed] [Google Scholar]
- 158.Freeman HP. Poverty, culture, and social injustice: determinants of cancer disparities. CA Cancer J Clin. 2004;54:72–7. doi: 10.3322/canjclin.54.2.72. [DOI] [PubMed] [Google Scholar]
- 159.National Cancer Institute. Fact Sheet: Cancer health disparities. 2008 Available from: http://www.cancer.gov/cancertopics/fact-sheet/disparities/cancer-health-disparities.
- 160.Centers for Disease Control and Prevention. CDC features: people without health insurance coverage, by race and ethnicity Atlanta, GA: Centers for Disease Control and Prevention. 2008 Available from: http://www.cdc.gov/Features/dsHealthInsurance/
- 161.American Cancer Society. Cancer facts & figures. Atlanta, GA: American Cancer Society; 2009. [Google Scholar]
- 162.Kinsley C, Bandolin S. Cultural and socioeconomic factors affecting cancer screening, early detection and care in the Latino population. 2011 Available from: http://ethnomed.org.
- 163.Cheng I, Witte JS, McClure LA, Shema SJ, Cockburn MG, John EM, et al. Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California. Cancer Causes Control. 2009;20:1431–40. doi: 10.1007/s10552-009-9369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eggleston KS, Coker AL, Williams M, Tortolero-Luna G, Martin JB, Tortolero SR. Cervical cancer survival by socioeconomic status, race/ethnicity, and place of residence in Texas, 1995–2001. J Womens Health (Larchmt) 2006;15:941–51. doi: 10.1089/jwh.2006.15.941. [DOI] [PubMed] [Google Scholar]
- 165.O’Malley CD, Le GM, Glaser SL, Shema SJ, West DW. Socioeconomic status and breast carcinoma survival in four racial/ethnic groups: a population-based study. Cancer. 2003;97:1303–11. doi: 10.1002/cncr.11160. [DOI] [PubMed] [Google Scholar]
- 166.Vainshtein J. Disparities in breast cancer incidence across racial/ethnic strata and socioeconomic status: a systematic review. J Natl Med Assoc. 2008;100:833–9. doi: 10.1016/s0027-9684(15)31378-x. [DOI] [PubMed] [Google Scholar]
- 167.Zell JA, Rhee JM, Ziogas A, Lipkin SM, Anton-Culver H. Race, socioeconomic status, treatment, and survival time among pancreatic cancer cases in California. Cancer Epidemiol Biomarkers Prev. 2007;16:546–52. doi: 10.1158/1055-9965.EPI-06-0893. [DOI] [PubMed] [Google Scholar]
- 168.Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2008: a review of current American Cancer Society guidelines and cancer screening issues. CA Cancer J Clin. 2008;58:161–79. doi: 10.3322/CA.2007.0017. [DOI] [PubMed] [Google Scholar]


