Abstract
Purpose of review
Type 1 diabetes (T1D) is now predictable by measuring major islet autoantibodies (IAbs) against insulin (IAA) and other pancreatic β-cells proteins including GAD65 (GADA), islet antigen 2 (IA-2A) and zinc transporter 8 (ZnT8A). The assay technology for IAbs has made great progress; however, several important aspects still need to be addressed and improved.
Recent findings
Currently a radio-binding assay (RBA) has been well established as the ‘gold’ standard assay for all 4 IAbs. New generation of non-radioactive IAb assay with electrochemiluminescence technology has been shown to further improve sensitivity and disease specificity. Recently, multiplexed assays have opened the possibility of more efficient screening in large populations. Identification of potential new autoantibodies to neo-antigens or neo-epitopes post-translational modification is a new important field to be explored.
Summary
Individuals having a single positive autoantibody are at low risk for progression to T1D, while individuals expressing two or more positive autoantibodies, especially on multiple tests over time, have nearly 100% risk of developing clinical T1D when followed for over 2 decades. More efficient and cost effective IAb assays will hopefully lead to point-of-care screening in the general population.
Keywords: autoantibodies, type 1 diabetes, biomarkers, prediction, assays
Introduction
Type 1 diabetes (T1D) is one of the most common and serious long-term diseases often beginning in childhood. The incidence of T1D is increasing worldwide at 3–5% each year and has doubled in the last two decades [1,2]. Although T1D is mainly a T-lymphocytes mediated -destruction of insulin producing beta cells within pancreatic islets, appearance of islet autoantibodies (IAbs) in peripheral blood is currently the most reliable marker to assess the autoimmune process leading to T1D; it marks the onset of beta cell autoimmunity and determines the disease risk. IAbs usually appear years before overt clinical disease and children with 2 or more of four major islet autoantibodies to insulin (IAA), GAD65 (GADA), islet antigen 2 (IA-2A), and zinc transport 8 (ZnT8A), have a 70% risk of developing clinical T1D in 10 years and a nearly 100% progression to clinical disease when followed for over 2 decades [3]. Many national and international screening studies for T1D have being launched in relatives and general population, and these longitudinal studies on pre-clinical T1D have helped to 1) better understand the natural history of T1D development; 2) define the onset of islet autoimmunity to identify potential triggers; 3) identify individuals for current and upcoming trials to prevent T1D; and finally 4) prevent life-threatening diabetic ketoacidosis.
Through the laboratory proficiency programs and harmonization efforts [4–7], a radio-binding assay (RBA) has been well established as the current ‘gold’ standard assay for all 4 IAbs. A newly validated nonradioactive IAb assay using electrochemiluminescence (ECL) detection for both IAA and GADA demonstrated higher sensitivity and disease specificity than RBA in several clinical studies, with superior detection of early seroconversion to islet autoimmunity and better discrimination of high-affinity, high-risk autoantibodies from those low-affinity, low risk signals less or non-relevant to the disease [8,9,10,11**,12**,13*,14**]. With the platforms of ECL assay and liquid-phase ELISA recently published, multiplexed assays combining multiple antibody assays in one well are currently being tested [15**,16**,17,18*]. However, improvements in IAbs assays are still needed to help with large scale screening in general populations, more efficiently at a lower cost. In this review, we will further discuss two fundamental aspects, sensitivity and disease specificity of autoantibodies for prediction of the risk and the rate of T1D progression. Potential autoantibodies to modified epitopes on protein molecules, post translationally modified and chimeric peptides may be a new interesting field to explore. Finally, we will briefly discuss the new generation of autoantibody assay, a multiplexed assay, which will hopefully allow large scale population screening and point-of-care clinical needs.
Text of review
Early identification of T1D autoimmunity
IAbs are currently the most reliable biomarkers of the autoimmune process leading to T1D and almost all individuals with 2 or more IAbs will develop clinical T1D over time. The IAbs usually develop sequentially, not simultaneously, and IAA is usually the first IAb to appear in young children followed from birth, which has been further confirmed by recent data from clinical studies [19]. Seroconversion with appearance of the first IAb in the peripheral blood circulation may mark the initiation of islet autoimmunity, an important check point as prospective studies such as The Environmental Determinants of Diabetes in the Young (TEDDY) analyze possible environmental factors contributing to the start of the autoimmune process. Children followed from birth, either from relatives of patients with T1D or general population, usually present with IAA as their first IAb, but older children and young adults often present with GADA first, especially subjects with DR3-DQ2 haplotype [20*], which has led to the current idea of heterogeneous etiology of T1D development, with different primary β-cell autoantigens, varying according to age and genetic background. The current standard RBA for IAbs have been greatly improved through the laboratory proficiency programs and harmonization efforts. However, the IAA assay has proven the most difficult to standardize with relatively wide discrepancies between laboratories in Islet Autoantibody Standardization Program (IASP) workshop, and has not yet achieved a satisfactory level of sensitivity and specificity. A newly developed IAA assay with ECL technology has been validated in multiple clinical studies with higher sensitivity and earlier detection of seroconversion by years than RBA. In the Diabetes Autoimmunity Study in the Young (DAISY), all pre-diabetic children who were followed to clinical T1D from birth were found almost to have IAA as their first IAb by ECL assay at seroconversion (9); as the data is limited to only one study, it needs to be further validated in other large prospective studies. In addition, IAA has been found to correlate inversely with age in both prevalence and level, with lower frequency and lower levels in older children and adults, which obviously adds more difficulties to effectively identify IAA among individuals beyond childhood. In summary, IAA is an important early marker for initiation of islet autoimmunity and the sensitivity of IAA assay might be a key issue in resolving the current puzzle of T1D etiology in terms of primary autoantigen(s). This will be an essential question to be answered in the near future to better understand the underlying mechanisms of initiation of the autoimmune process leading to T1D in both children and adults with different genetic backgrounds. It is also critical to precisely pinpoint the exact time of initial seroconversion as studies such as TEDDY investigate potential environmental triggering factors involved in T1D pathogenesis. In addition, all current IAA assays are not able to distinguish natural IAA from induced insulin antibodies (IA) by exogenous insulin usage, and both IAA and IA are high affinity antibodies, although the epitope binding sites might be different. It would be helpful to have an assay that distinguishes IAA from induced IA to further aid T1D research as current T1D trials, often require the confirmatory presence of at least one IAb.
Predicting rate of T1D progression
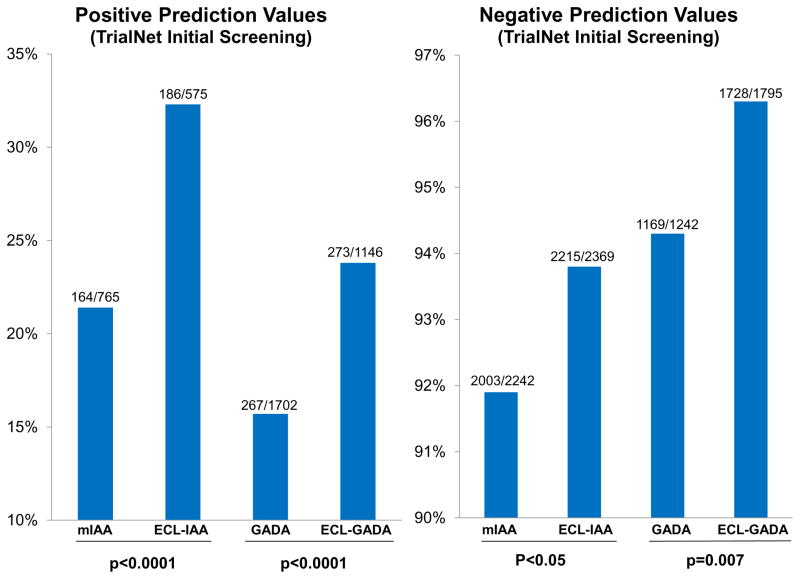
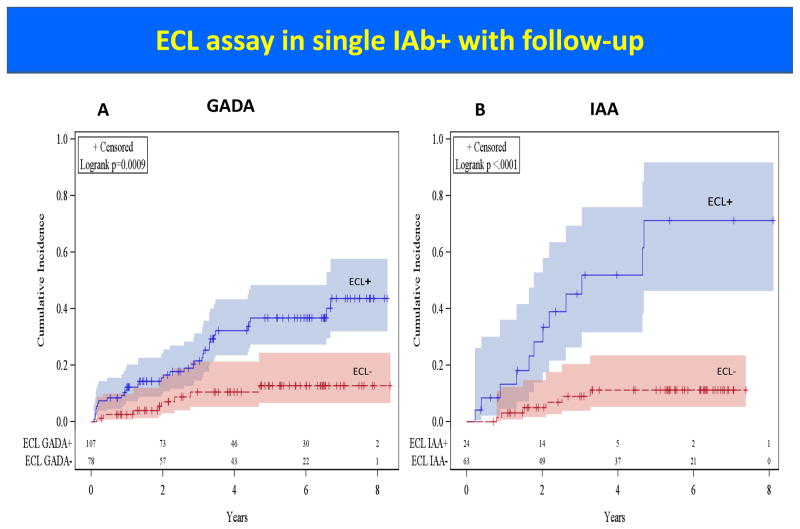
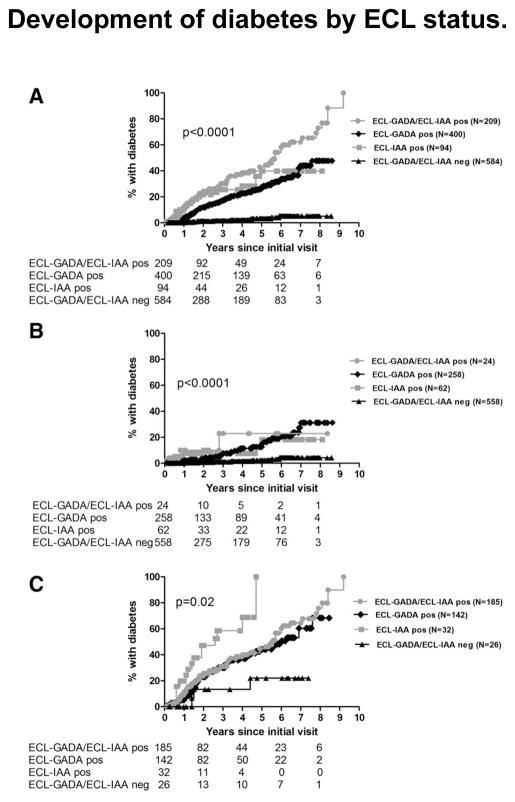
Natural history studies of T1D have shown that T1D may begin with seroconversion of single IAb, conversion to multiple IAbs, and finally progression to overt clinical diabetes. At initial screening either in first degree relatives of patients with T1D or in general population, IAA or GADA are often detected in isolation while IA-2A and ZnT8A are rarely present as a single IAb. Children with single IAb only have a 15% risk of developing T1D [3] after 15 years of follow-up and predictive values with either single IAA or single GADA are low. In several longitudinal studies including DAISY, TrialNet, and TEDDY, our group has shown that prediction of T1D risk in subjects with single IAb can be improved by using ECL assay, which is more disease specific [8,10,11**]. Figure 1 illustrates better positive prediction values of single IAb by ECL assay compared to RBA for both IAA and GADA (both p< 0.0001) in the TrialNet Pathway to Prevention study. Cumulative risk of T1D development in subjects with single IAb by ECL assay is significantly higher than RBA for both GADA (p= 0.0009) and IAA (p< 0.0001) (Figure 2). Furthermore recent evidence suggests that subjects with either single IAA or single GADA confirmed by ECL assay have metabolic changes of impaired glucose tolerance [14**] comparable to subjects with multiple IAbs, while ECL negative subjects showed no signs of impaired glycemia during a median follow-up of 4.7 years. TrialNet Pathway to Prevention subjects who were ECL-positive had an overall risk of progression to diabetes within 6 years of 58% compared to 5% for those ECL-negative subjects (Figure 3A) (p<0.0001). Survival curves analyzing participants positive for 1 IAb by RBA showed a much higher risk for development of diabetes by year 6 for subjects who were ECL positive (23% if both ECL-IAA and ECL-GADA positive, 18% if only ECL-IAA positive and 21% if only ECL-GADA positive) compared to those subjects who were ECL negative (4%) (Figure 3B) (p<0.0001). When looking at subjects positive for ≥2 IAbs by RBA, the risk for development of diabetes was again much higher for those subjects who were ECL positive: 61% by 6 years if both ECL-IAA and ECL-GADA positive, 69% by 4 years if only ECL-IAA positive and 51% by 6 years if only ECL-GADA positive compared to 22% for those subjects who were ECL negative (Figure 3C) (p=0.02).
Figure 1.
Comparison of predictive values between ECL and radioassays on 2,944 subjects for their very first initial screening samples in TrialNet Pathway to Prevention Study.
Panel A: Comparison of positive predictive values between RBA-IAA (mIAA) and ECL-IAA (p<0.0001), and between RBA-GADA and ECL-GADA (p<0.0001).
Panel B: Comparison of negative predictive values between RBA-IAA (mIAA) and ECL-IAA (p<0.05), and between RBA-GADA and ECL-GADA (p=0.007).
[11] Diabetes Technol Ther. 2015;17:119–27.
Figure 2.
Cumulative incidence of T1D development in subjects with single IAb in TrialNet Pathway to Prevention Study.
Panel A: Cumulative incidence of T1D development in subjects with single GADA positive by RBA, divided with ECL-GADA positive vs ECL-GADA negative (p= 0.0009).
Panel B: Cumulative incidence of T1D development in subjects with single IAA positive by RBA, divided with ECL-IAA positive vs ECL-IAA negative (p< 0.0001).
Figure 3.
Development of diabetes by ECL status. A: All subjects (n = 1,287). B: Subjects positive for one autoantibody by RIAs (n = 902). C: Subjects positive for two or more autoantibodies by RBAs (n = 385). Survival analysis was performed for the development of diabetes since initial visit according to ECL positivity using the log-rank test.
ECL-GADA/ECL-IAA pos, positive for both ECL-GADA and ECL-IAA; ECL-GADA pos, positive for ECL-GADA only; ECL-IAA pos, positive for ECL-IAA only; ECL-GADA/ECL-IAA neg, negative for both ECL-GADA and ECL-IAA.
[13] Diabetes Care. 2016;39:1738–44.
Progression to diabetes diagnosis after seroconversion varies tremendously from several months to over a decade. Factors involved in rate of progression are poorly understood, although some determinants have been observed to be associated with rate of progression including younger age at seroconversion, number of autoantibodies, higher levels of IAA [21] and more recently higher levels of IA-2A [22*], but not GADA. In children with confirmed positive IAbs, the time of progression from single to multiple IAbs is influenced by family history of T1D and the presence of the high-risk HLA-DR3-DQ2/DR4-DQ8 genotype, while family history and HLA genotypes did not influence the time of progression to T1D in children with multiple IAbs [23**]. The rate of conversion from single to multiple IAbs was significantly higher in children with younger age less than 5 years in a recent study of the BABYDIAB/BABYDIET and the rate of conversion from single to multiple IAbs was highest in the first 2 years after seroconversion and declined rapidly after 4 years of follow-up (p = 0.003) [23**]. Development of diabetes was also faster in children who became multiple IAbs within 2 years of initial seroconversion than in children who became multiple IAbs above 2 years after seroconversion. Although positivity for multiple IAbs is accurate in predicting risk for T1D, we currently do not have the ability to predict the rate of progression to diabetes in individuals with islet autoimmunity. Adding other biomarkers to IAbs such as age, T and B or other cellular immune markers, metabolic changes, and genetic background, will likely help to predict rate of progression from islet autoimmunity to clinical T1D.
Neo-antigens and neo-epitopes
There is increasing evidence both in animal models and in humans that post-translational modification (PTM) is a characteristic of antigens in many autoimmune diseases. A recent study [24**] published in Science showed that the proteasome-generated spliced peptide pool accounts for one-third of the entire HLA class I immunopeptidome in terms of diversity and one-fourth in terms of abundance, which may have profound implications for the concept of self/nonself peptide presentation, derived from human or pathogen proteomes, with direct implications for autoimmunity. The β-cell is highly susceptible to endoplasmic reticulum (ER) and oxidative stress and is potentially a site of alteration in protein alternative splicing and PTMs [25,26]. There is supporting evidence in T1D that β-cell antigens undergo modifications through these mechanisms, which might be involved in the killing of β-cells [27–31]. Very recently, diabetes-inducing CD4 T cell clones isolated from NOD mice were found to recognize epitopes formed by covalent cross-linking of proinsulin peptides to chromogranin A and islet amyloid polypeptide present in β-cell secretary granules [32**, 33**,34*]. These hybrid proinsulin peptides are antigenic for CD4 T cells from both mice and human, and CD4 T cells from the residual pancreatic islets of organ donors who had T1D were also found to recognize these proinsulin hybrids. The studies of modification of β-cell antigens have been limited to T cells so far with no current studies reported on B cells and autoantibodies. If PTM plays a critical role in initiation and development of the disease, identification of autoantibodies to these neo-antigens or neo-epitopes will be an important approach to gain new evidence of T1D pathogenesis and to help understand the underlying mechanism of loss of immune tolerance in T1D. These autoantibodies will also be new valuable biomarkers for T1D.
General population screening
Large screening of IAbs in general population, especially in young children, is currently in progress in the research setting [35*,36*]. Four biochemically defined IAbs are available and important in prediction and evaluation of risk of progression to T1D in both relatives of patients with T1D and general population. The screening methods using current standard RBA with single IAb measurement are laborious and inefficient for large scale screening. With the platforms of ECL assay and liquid-phase ELISA recently published [15–18], multiplexed assays combining multiple antibody assays in one well are being tested to help with large scale screening in general populations. There are at least 4 advantages with multiplex assays (using 4-plex assay as example) compared with current single radioassays: 1) higher throughput capacity: one lab technician is able to complete the assays for 3 to 4 times the number of samples that can be handled with current single RBA; 2) lower volume of serum: multiplex assay needs 3 to 4 times less serum than current RBA; 3) lower cost: multiplex assay will cost 30% less than total cost for current 4 single RBAs; 4) non-radioactive: as the multiplex ECL assay does not require radioactivity, we envision increased access to IAbs measurement with this methodology across academic and commercial laboratories.
Large scale screening can be hindered by geographical limitations, with families having to travel significant distances to study centers, as well as aversion to venous blood sampling particularly in young children. A simple, home-based procedure, without the need of a healthcare professional, could improve access to screening and potential prevention studies. Through the TrialNet study group, two pilot studies of feasibility for sampling on both dried blood spots (DBS) [37] and capillary blood samples (CBS) [38*] were performed. The IAbs were analyzed and compared with venous blood serum samples. Self-collected DBS or CBS were shown to be a feasible option, preferred by families over venous sampling, with the potential to facilitate screening for T1D risk. In the feasibility study on DBS samples, IAA was not performed since IAA assay on DBS samples was historically challenging with less sensitivity. In a very recent study [39*], conditions to elute autoantibodies from DBS on filter paper were optimized and stability of the DBS samples was assessed over time. Eluted DBS IAb measurements were performed for all 4 IAbs in new-onset T1D patients and in controls and compared to serum measurements using standard radioassays. Our results demonstrated that IAb measurements, including IAA, from DBS on filter paper strongly correlate to serum levels. Validation studies will be warranted to expand this approach into a general population screening test for T1D risk in the near future.
Conclusion
IAbs are currently the best biomarkers for T1D and have greatly contributed to the understanding of the natural history of the T1D autoimmune process and the prediction of risk in both first-degree relatives of T1D patients and general population. There are still many challenges and room for improvements, especially in 1) understanding the etiology of T1D in terms of primary autoantigens and to more precisely pinpoint the time of initiation of islet autoimmunity; 2) combining other biomarkers to predict or estimate rate of progression to disease; 3) exploring potential T1D autoantibodies to neo-antigens and neo-epitopes created by PTM mechanism; finally 4) developing an efficient and affordable tool for general population screening and point-of care.
Key points.
IAA is an important early marker for initiation of islet autoimmunity and the sensitivity of IAA assay might be a key issue in resolving the current puzzle of T1D etiology in terms of primary autoantigen(s).
Adding other biomarkers to IAbs such as age, cellular immune markers, metabolic changes, and genetic background, will likely help to predict rate of progression from islet autoimmunity to clinical T1D.
Identification of autoantibodies to neo-antigens or neo-epitopes post translational modification (PTM) is a new important field which will likely help understand the underlying mechanism of loss of immune tolerance in T1D and provide new potential biomarkers for T1D.
Multiplexed IAb assay combining multiple antibody assays in one well will be a useful tool for general population screening
Acknowledgments
Financial support and sponsorship
This work was supported by NIH grant DK32083, Diabetes Autoimmunity Study in the Young Grant DK32493, JDRF Grant 2-SRA-2015-51-Q-R, ADA Grant 1-14-CD-17.
Funding received for this work from
National Institute of Health (NIH)
Juvenile Diabetes Research Foundation (JDRF)
American Diabetes Association (ADA)
Footnotes
Conflicts of interests
None.
References
- 1.Harjutsalo V, Sjoberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777–82. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- 2.Vehik K, Hamman RF, Lezotte D, et al. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care. 2007;30:503–9. doi: 10.2337/dc06-1837. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–9. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bingley PJ, Bonifacio E, Mueller PW. Diabetes Antibody Standardization Program: first assay proficiency evaluation. Diabetes. 2003;52:1128–36. doi: 10.2337/diabetes.52.5.1128. [DOI] [PubMed] [Google Scholar]
- 5.Torn C, Mueller PW, Schlosser M, et al. Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia. 2008;51:846–52. doi: 10.1007/s00125-008-0967-2. [DOI] [PubMed] [Google Scholar]
- 6.Schlosser M, Mueller PW, Torn C, et al. Diabetes Antibody Standardization Program: evaluation of assays for insulin autoantibodies. Diabetologia. 2010;53:2611–20. doi: 10.1007/s00125-010-1915-5. [DOI] [PubMed] [Google Scholar]
- 7.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab. 2010;95:3360–7. doi: 10.1210/jc.2010-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu L, Miao D, Scrimgeour L, et al. Distinguishing persistent insulin autoantibodies with differential risk: nonradioactive bivalent proinsulin/insulin autoantibody assay. Diabetes. 2012;61:179–86. doi: 10.2337/db11-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu L, Dong F, Miao D, et al. Proinsulin/Insulin autoantibodies measured with electrochemiluminescent assay are the earliest indicator of prediabetic islet autoimmunity. Diabetes Care. 2013;36:2266–70. doi: 10.2337/dc12-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miao D, Guyer KM, Dong F, et al. GAD65 autoantibodies detected by electrochemiluminescence assay identify high risk for type 1 diabetes. Diabetes. 2013;62:4174–8. doi: 10.2337/db13-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Miao D, Steck AK, Zhang L, et al. Electrochemiluminescence assays for insulin and glutamic acid decarboxylase autoantibodies improve prediction of type 1 diabetes risk. Diabetes Technol Ther. 2015;17:119–27. doi: 10.1089/dia.2014.0186. Using samples from subjects followed in the TrialNet Pathway to Prevention study, authors further confirmed their previous studies that the ECL assay is more disease-specific and better able to predict the risk of progression to type 1 diabetes than the current standard radio-binding assay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Steck AK, Fouts A, Miao D, et al. ECL-IAA and ECL-GADA Can Identify High-Risk Single Autoantibody-Positive Relatives in the TrialNet Pathway to Prevention Study. Diabetes Technol Ther. 2016;18:410–4. doi: 10.1089/dia.2015.0316. ECL assay identify high affinity islet autoantibodies. This study provides strong evidence that ECL assay is associated with high affinity at initial screening visit. Affinity stayed consistent and no converting events from low to high or high to low affinity were seen over time. ECL assay is able to help staging of risk for type 1 diabetes in single autoantibody-positive subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Fouts A, Pyle L, Yu L, et al. Do Electrochemiluminescence Assays Improve Prediction of Time to Type 1 Diabetes in Autoantibody-Positive TrialNet Subjects? Diabetes Care. 2016;39:1738–44. doi: 10.2337/dc16-0302. Compared with current radioassay, ECL assay improved the ability to predict time to diabetes in subjects with positive islet autoantibodies, which might be helpful in the design and eligibility criteria for prevention trials in the future. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Sosenko JM, Yu L, Skyler JS, et al. The Use of Electrochemiluminescence Assays to Predict Glycemic and Autoantibody Progression to Type 1 Diabetes in Individuals with Single Autoantibodies. Diabetes Technol Ther. 2017;19:183–7. doi: 10.1089/dia.2016.0243. Early assessment of risk for type 1 diabetes among subjects with single islet autoantibody, either single GADA or single IAA, is currently problematic since most of single positives are ‘false positive’ biologically. This study uniquely showed that single positivity confirmed by ECL assay defines risk for developing multiple autoantibodies and for glycemic progression toward type 1 diabetes, whereas those ECL negative are at very low risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Zhao Z, Miao D, Michels A, et al. A multiplex assay combining insulin, GAD, IA-2 and transglutaminase autoantibodies to facilitate screening for pre-type 1 diabetes and celiac disease. J Immunol Methods. 2016;430:28–32. doi: 10.1016/j.jim.2016.01.011. This is a revolutionary new generation of autoantibody assay combining multiple autoantibody assays in one well and allowing high throughput screening simultaneously for multiple autoantibodies and for multiple autoimmune diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Zhao Zhiyuan, Miao Dongmei, Michels Aaron, et al. A multiplex assay for simultaneous screening type 1 diabetes and multiple autoimmune diseases. Diabetes. 2016;65(suppl 1):1419. This study is a new progress of the study in reference 15, expanding multiplex from 4 to 8, possibly combining up to 10 autoantibody assays in one well with a drop of blood. [Google Scholar]
- 17.Mathew A, Wang M, Barbero S, et al. Multiplex assay panels for prediction/diagnosis of type 1 diabetes (T1D) and comorbid autoimmune diseases. Diabetes. 2016;65(suppl 1):1673. [Google Scholar]
- 18*.Ziegler AG, Haupt F, Scholz M, et al. 3 Screen ELISA for High-Throughput Detection of Beta Cell Autoantibodies in Capillary Blood. Diabetes Technol Ther. 2016;18:687–693. doi: 10.1089/dia.2016.0199. This is the first application of multiplex islet autoantibody assay in screening for general population children. [DOI] [PubMed] [Google Scholar]
- 19.Elding Larsson H, Vehik K, Gesualdo P, et al. Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatr Diabetes. 2014;15(2):118–26. doi: 10.1111/pedi.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Krischer JP, Lynch KF, Schatz DA, et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58:980–7. doi: 10.1007/s00125-015-3514-y. It is a very important question whether insulin molecule is a common primary autoantigen for type 1 diabetes or whether there is heterogeneity. With currently used standard radio-binding assays for identifying seroconversion of islet autoantibodies, this observational study suggests the order of appearance of islet autoantibodies is heterogeneous, related to HLA-DR-DQ genotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steck AK, Johnson K, Barriga KJ, et al. Age of Islet Autoantibody Appearance and Mean Levels of Insulin, but Not GAD or IA-2 Autoantibodies, Predict Age of Diagnosis of Type 1 Diabetes: Diabetes Autoimmunity Study in the Young. Diabetes Care. 2011;34:1397–9. doi: 10.2337/dc10-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Steck AK, Vehik K, Bonifacio E, et al. Predictors of Progression from the Appearance of Islet Autoantibodies to Early Childhood Diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY) Diabetes Care. 2015;38:808–13. doi: 10.2337/dc14-2426. Progression to diabetes diagnosis after seroconversion varies tremendously from several months to over a decade. This study was trying to identify possible factors involved in rate of progression to type 1 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Chmiel R, Giannopoulou EZ, Winkler C, et al. Progression from single to multiple islet autoantibodies often occurs soon after seroconversion: implications for early screening. Diabetologia. 2015;58:411–3. doi: 10.1007/s00125-014-3443-1. Factors involved in rate of progression to type 1 diabetes are poorly understood. This is an important observational study regarding rate of progression from single to multiple islet autoantibodies. [DOI] [PubMed] [Google Scholar]
- 24**.Liepe J, Marino F, Sidney J, et al. A large fraction of HLA class I ligands are proteasome-generated spliced peptides. Science. 2016;354:354–358. doi: 10.1126/science.aaf4384. The widespread appearance and abundance of proteasome-catalyzed peptide splicing events from this study may provide a previously untapped source of neo-antigens/neo-epitopes common in multiple cell types and may have implication for immunobiology and autoimmunity theories. [DOI] [PubMed] [Google Scholar]
- 25.Ortis F, Naamane N, Flamez D, et al. Cytokines interleukin-1beta and tumor necrosis factor-alpha regulate different transcriptional and alternative splicing networks in primary beta-cells. Diabetes. 2010;59:358–74. doi: 10.2337/db09-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Hertog W, Maris M, Ferreira GB, et al. Novel insights into the global proteome responses of insulin-producing INS-1E cells to different degrees of endoplasmic reticulum stress. J Proteome Res. 2010;9:5142–52. doi: 10.1021/pr1004086. [DOI] [PubMed] [Google Scholar]
- 27.Dogra RS1, Vaidyanathan P, Prabakar KR, et al. Alternative splicing of G6PC2, the gene coding for the islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP), results in differential expression in human thymus and spleen compared with pancreas. Diabetologia. 2006;49:953–7. doi: 10.1007/s00125-006-0185-8. [DOI] [PubMed] [Google Scholar]
- 28.Yip L, Su L, Sheng D, et al. Deaf1 isoforms control the expression of genes encoding peripheral tissue antigens in the pancreatic lymph nodes during type 1 diabetes. Nat Immunol. 2009;10:1026–33. doi: 10.1038/ni.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mannering SI, Harrison LC, Williamson NA, et al. The insulin A-chain epitope recognized by human T cells is posttranslationally modified. J Exp Med. 2005;202:1191–7. doi: 10.1084/jem.20051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skowera A1, Ellis RJ, Varela-Calviño R, et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest. 2008;118:3390–402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Lummel M, Duinkerken G, van Veelen PA, et al. Posttranslational modification of HLA-DQ binding islet autoantigens in type 1 diabetes. Diabetes. 2014;63:237–47. doi: 10.2337/db12-1214. [DOI] [PubMed] [Google Scholar]
- 32**.Jin N, Wang Y, Crawford F, et al. N-terminal additions to the WE14 peptide of chromogranin A create strong autoantigen agonists in type 1 diabetes. Proc Natl Acad Sci U S A. 2015;112:13318–23. doi: 10.1073/pnas.1517862112. This study gives strong experimental evidence that mimotope peptides of natural chromogranin A-processed peptide, WE14, are much more potent than WE14 in T-cell stimulation and activate a diverse population of CD4(+) T cells. Such posttranslational modification may occur uniquely in the pancreas or pancreatic lymph nodes and could account for the escape of these T cells from thymic negative selection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Delong T, Wiles TA, Baker RL, et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science. 2016;351:711–4. doi: 10.1126/science.aad2791. Hybrid insulin peptides (HIPs) by covalent cross-linking of proinsulin peptides to other peptides present in β cell secretory granules are shown to be antigenic for CD4 T cells isolated from non-obese diabetic (NOD) mice and the study further demonstrates CD4 T cells from the residual pancreatic islets of two organ donors who had type 1 diabetes also recognize HIPs. The study may help understand the underlying mechanism of loss of immune tolerance in type 1 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Wiles TA, Delong T, Baker RL, et al. An insulin-IAPP hybrid peptide is an endogenous antigen for CD4 T cells in the non-obese diabetic mouse. J Autoimmun. 2016 doi: 10.1016/j.jaut.2016.10.007. pii: S0896–8411. A hybrid insulin peptide (HIP) formed by linkage of an insulin C-peptide fragment and a fragment of islet amyloid polypeptide (IAPP) was discovered as an endogenous islet antigen for a T cell clone isolated from a non-obese diabetic (NOD) mouse, a new evidence of HIP as possible diabetogenic neo-antigen/neo-epitope. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Raab J, Haupt F, Scholz M, et al. Capillary blood islet autoantibody screening for identifying pre-type 1 diabetes in the general population: design and initial results of the Fr1da study. BMJ Open. 2016;6(5):e011144. doi: 10.1136/bmjopen-2016-011144. doi:10.1136. It is a ground breaking study of staging for early type 1 diabetes within a public health setting at a general population-based level. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Gesualdo PD, Bautista KA, Waugh KC, et al. Feasibility of screening for T1D and celiac disease in a pediatric clinic setting. Pediatr Diabetes. 2016;17:441–8. doi: 10.1111/pedi.12301. It is a pilot study of screening for type 1 diabetes and celiac disease. The study concluded that it is feasible to conduct this type of screening in a pediatric clinic and that it could lead to increased disease awareness and benefits from early detection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bingley PJ, Rafkin LE, Matheson D, et al. Use of Dried Capillary Blood Sampling for Islet Autoantibody Screening in Relatives: A Feasibility Study. Diabetes Technol Ther. 2015;17:867–71. doi: 10.1089/dia.2015.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Liu Y, Rafkin LE, Matheson D, et al. Use of self-collected capillary blood samples for islet autoantibody screening in relatives: a feasibility and acceptability study. 2017 doi: 10.1111/dme.13338. The feasibility of self-collected capillary blood sampling (CBS) was evaluated and the study concluded that CBS offers a feasible alternative to venous sampling, with the potential to facilitate autoantibody screening for Type 1 diabetes risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Simmons KM, Alkanani AK, McDaniel KA, et al. Islet Autoantibody Measurements from Dried Blood Spots on Filter Paper Strongly Correlate to Serum Levels. PLoS One. 2016;11(11):e0166213. doi: 10.1371/journal.pone.0166213. doi:10.1371. Success in measuring islet autoantibodies from dried blood spots (DBS) on filter paper has been limited and IAA has historically been found less sensitive on DBS. This study with the assay conditions optimized successfully achieved the same sensitivity/specificity as for serum samples for all four islet autoantibody assays with DBS including IAA. [DOI] [PMC free article] [PubMed] [Google Scholar]