Abstract
There is a growing appreciation that our microbial environment in the gut plays a critical role in the maintenance of health and the pathogenesis of disease. Probiotic, beneficial gut microbes, administration can directly attenuate cardiac injury and post-myocardial infarction (MI) remodelling, yet the mechanisms of cardioprotection are unknown. We hypothesised that administration of Bifidobacterium animalis subsp. lactis 420 (B420), a probiotic with known anti-inflammatory properties, to mice will mitigate the pathological impact of MI, and that anti-inflammatory T regulatory (Treg) immune cells are necessary to impart protection against MI as a result of B420 administration. Wild-type male mice were administered B420, saline or Lactobacillus salivarius 33 (Ls-33) by gavage daily for 14 or 35 days, and underwent ischemia/reperfusion (I/R). Pretreatment with B420 for 10 or 28 days attenuated cardiac injury from I/R and reduced levels of inflammatory markers. Depletion of Treg cells by administration of anti-CD25 monoclonal antibodies eliminated B420-mediated cardio-protection. Further cytokine analysis revealed a shift from a pro-inflammatory to an anti-inflammatory environment in the probiotic treated post-MI hearts compared to controls. To summarise, B420 administration mitigates the pathological impact of MI. Next, we show that Treg immune cells are necessary to mediate B420-mediated protection against MI. Finally, we identify putative cellular, epigenetic and/or post-translational mechanisms of B420-mediated protection against MI.
Keywords: cardiovascular disease, inflammation, probiotics, regulatory T cells, B. animalis ssp. lactis
1. Introduction
The bidirectional relationship between commensal gut microbiota and the intestinal immune system regulates the balance of both innate and adaptive immune responses in health and disease (Hakansson and Molin, 2011). By FAO/WHO definition, probiotics are viable microorganisms that confer health benefits to the host once consumed in adequate amounts (FAO/WHO, 2001). Because of their direct contact with the gastrointestinal tract, and given that the main site of immune activity resides in the intestine (Brandtzaeg, 2009), it is apparent why probiotics are predominantly known for their benefits to gut and immune health. However, an emerging paradigm outlines a causal role for harmful gut microbiota and translocation of bacterial components in the development of metabolic disorders, such as diabetes, obesity and cardiovascular disease (CVD) through exacerbation of pro-inflammatory mediators (Cani et al., 2007a,b; De La Serre et al., 2010). Consequently, addressing inflammation is now recognised as tantamount to the treatment of these disorders.
Although many studies in animals and humans have shown that probiotics and probiotic dairy products may protect against CVD, this benefit is typically mediated by lowering CVD risk factors like hypertension or hypercholesterolemia (Chen and Konhilas, 2013; Ebel et al., 2014). Recent work by Hazen and colleagues outlines a congruous story that gut microbiota are active participants in the development of atherosclerosis providing a key mechanistic link between the gut, diet and CVD (Wang et al., 2011). Similarly, in a series of recent experiments, oral administration of vancomycin, a broad-range antibiotic, attenuates cardiac ischemia injury presumably by disruption of gut microbiota (Lam et al., 2012). Further in this study, feeding rats a commercially available probiotic (Lactobacillus plantarum 299v and Bifidobacterium lactis (Bi-07)) decreases infarct size and improves post-myocardial infarction (MI) cardiac function. Similarly, using a rat model of MI, the probiotic Lactobacillus rhamnosus GR-1 protects against cardiac injury (Gan et al., 2014). In these studies, the investigators attribute the protection to an increase in serum leptin levels without additional mechanistic insight.
Cardiac remodelling following MI is initiated by a robust and coordinated inflammatory charge characterised by chemical signals and recruitment of distinct populations of inflammatory cells including monocytes that differentiate into macrophages. This coordinated response is perturbed a priori under heightened inflammatory conditions (Nahrendorf et al., 2007). Consequently, any conditions that elevate peripheral or local inflammation such as high fat diet, hypertension, obesity, diabetes, periodontal disease and now harmful gut microbiota (Cani et al., 2007b; Gomez-Hurtado et al., 2011; Hakansson and Molin, 2011) have the potential to aggravate acute events leading to myocardial damage (Slepian and Gottehrer, 2009; Zuidema and Zhang, 2010). It is now appreciated that probiotics can suppress peripheral inflammation and that this is due to suppression of pro-inflammatory pathways (Toral et al., 2014) and, concurrently, induction of anti-inflammatory pathways (Mengheri, 2008; Smelt et al., 2013).
In this series of experiments, we hypothesised that administration of Bifidobacterium animalis subsp. lactis 420 (B420) or Lactobacillus salivarius-33 (Ls-33) to mice will mitigate the pathological impact of MI, and that anti-inflammatory T regulatory (Treg) immune cells are necessary to impart protection against cardiac injury as a result of B420 or Ls-33 administration. B420 administration has improved metabolic health and reduced markers of tissue inflammation (Amar et al., 2011; Stenman et al., 2014) and Ls-33 is a commercially available strain in wide use known to be beneficial for intestinal and immune disorders (Fedorak, 2008), making them good candidates for cardio-protection in our study. Towards this end, we demonstrate that B420, but not Ls-33 administration attenuates MI injury in hearts of mice following an ischemia/reperfusion (I/R) protocol. We also show that modest Treg depletion eliminates the cardio-protection in B420-treated mice indicating that Treg cells are necessary for the cardio-protection instigated by B420 administration. Finally, we identify putative cellular, epigenetic and/or post-translational mechanisms of B420-mediated protection against MI.
2. Materials and methods
Animals, diet and probiotic administration
All experiments were performed using protocols that adhered to guidelines and approved by the Institutional Animal Care and Use Committee at the University of Arizona and to 2011 NIH guidelines for care and use of laboratory animals. At 10 weeks of age, wild-type male mice (C57Bl/6J; Jackson Laboratories, stock 000664, Bar Harbor, ME, USA) were randomised to either standard rodent chow (NIH-31: 18% fat, 59% carbohydrate, 23% protein; Research Diets, Inc., New Brunswick, NJ, USA) or high-fat diet (#D12331: 58% fat, 25.5% simple carbohydrate, 16.4% protein; Research Diets, Inc.) that was continued throughout the study duration. High fat (HF) feeding was initiated 14 days prior to initiation of gavage protocol and continued throughout the study. Within each dietary group, mice were randomised and administered (daily by gavage; 109 cfu/500 μl saline) B. animalis subsp. lactis 420 (B420) (DuPont Nutrition & Health, Kantvik, Finland; ATCC: SD6685), L. salivarius (Ls-33; a commercially available strain known to be beneficial for intestinal and immune disorders (Collado et al., 2007; Daniel et al., 2006; Fedorak, 2008)) (DuPont Nutrition & Health), or saline (500 μl). We implemented a long-term (28 day) or short-term (10 day) gavage protocol, which is illustrated in the supplemental data (Supplementary Figure S1).
Probiotic batch stability
Probiotic batch stability and potency can vary depending on storage length and conditions. For this reason, we have implemented a quality control in which we determine cfu for each batch of probiotic prior to the initiation of the gavage protocol. This strategy will assure standardisation for each experimental group. To do this, freeze-dried probiotic powder is rehydrated in De Man Rogosa and Sharpe (MRS) broth at room temperature. Serial dilutions are made from this rehydrated powder and then plated on MRS-agar plates in triplicate. Plates are then incubated under anaerobic conditions at 37°C for 72 h. Plates having between 25 and 250 colonies are counted for the determination of cfu/batch.
Surgical procedures
Mice were anesthetised with an intraperitoneal injection of 250 mg/kg tribromoethanol (Sigma, St. Louis, MO, USA), intubated and ventilated with 0.5–2.0% isoflurane (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA). A single injection of Buprenorphine-SR (Reckitt Benckiser Healthcare, Slough, UK) at 1 mg/kg body weight is given prior to surgery. An ischemia reperfusion protocol was utilised in this study. A left anterior thoracotomy was performed to expose the heart and the left coronary artery (LCA) visualised. The LCA was occluded using 8-0 suture compressing a small piece of tubing (PE-10) to prevent vessel damage during occlusion. Blanching of the myocardium was used to confirm ligation and assure similar infarct size. After 30 min of occlusion, the ligature was removed and the animal was allowed to recover for a 3-day reperfusion period.
RT-PCR
Total RNA was isolated from the infarct area of hearts using the RNeasy Midi Kit RNA isolation kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Total cDNA was generated using the NCode™ First-Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA). Maxima™ SYBR Green qPCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) was used for real time PCR reactions. 18S RNA was used as an internal control for real time PCR. RT-PCR specific primer sequences are provided in Supplementary Table S1.
Determination of infarct size and immunohistochemistry
Following the 72-h reperfusion period, mice were anesthetised with an intraperitoneal injection of 250 mg/kg tribromoethanol (Sigma), the LCA was re-occluded at the same original site. Evans Blue (EB) dye (1%; Sigma) was then perfused retrograde through the aorta in situ, staining the whole heart except the left coronary bed and outlining the ischemic zone or area at risk (AAR). The hearts were excised, transversely sectioned at 1 mm thickness, and incubated in 1% 2,3,5-triphenyltetrazoliumchloride stain (TTC; Sigma). After incubation, infarcted myocardium stained white (area of necrosis; AON), viable tissue within AAR stained red, and perfused tissue remained blue. AAR and AON were measured for each section using image analysis software (ImageJ, https://imagej.nih.gov/ij) and infarction size was reported as a percentage of AON to AAR. The sections were then fixed in 10% formalin overnight, paraffin embedded, sectioned at 5 μm, and stained with haematoxylin and eosin (H/E) or with antibodies for immunological and inflammatory markers. The HRP-labelled secondary antibodies were visualised with diaminobenzidine (DAB) and counterstained with haematoxylin (Axio Imager M1; Zeiss, Oberkochen, Germany). The antibodies used are anti-IL-6 (abcam) anti-ICAM(CD54) (Cell Signaling Technologies, Danvers, MA, USA), and anti-IL-10 (LifeSpan BioScience, Seattle, WA, USA). Expansion and/or infiltration of immunohistological markers was determined by measuring the area of markers in each heart section and normalising to the area of the entire heart section (IHC area/total heart area). Next, normalised IHC area was correlated with AAR normalised to total heart area in the same heart section.
Isolation of splenic Treg
Splenic Treg cells were extracted using the EasySep Mouse CD25 Regulatory T cell Positive Selection Enrichment Kit (StemCell Technologies, Vancouver, Canada) according to the manufacturer’s protocol.
Flow cytometry
T cell populations were determined from splenic single cell suspensions. Viability of cell suspensions was determined using LIVE/DEAD Fixable Yellow Dead Cell Stain Kit (Invitrogen) according to manufacturer’s protocol. Non-specific binding was blocked with anti-mouse CD16/32 (eBioscience, San Diego, CA, USA) and then cells were stained with a panel of fluorescently labeled antibodies specific for detecting Treg (anti-mouse CD3 (FITC; BD Biosciences, San Jose, CA, USA), anti-mouse CD4 (PE-Cy7; BD Biosciences), anti-mouse CD8 (Alexa Fluor 700; BioLegend, San Diego, CA, USA), and anti-mouse CD25 (eFluor 450; eBioscience), anti-mouse Foxp3 (Alexa Fluor 647; BD Biosciences), anti-mouse Helios (PE; BD Biosciences), and IL-10 (PE; BioLegend)) After staining, cells were resuspended in cell cytometry staining buffer and analysed by flow cytometry (BD LSRII; BD Biosciences). Results were analysed using FloJo software (FloJo, Ashland, OR, USA).
Depletion of CD25+ cells
Depletion of CD25+ T cells was achieved with an anti-CD25 monoclonal antibody (CD25-mAb; Clone PC61, (BioXCell, West Lebanon, NH, USA)) (Setiady et al., 2010). Using a single IP injection of the CD25-mAb (IgG used as a control) the morning of I/R surgery, CD25+FoxP3+ T cells (CD4+CD3+) were depleted by about 40% 1-day post injection (data not shown). At the end of the 3-day reperfusion period, we were able to achieve a ~35% depletion of CD25+, FoxP3+ T cells (CD4+, CD3+).
Western blot analysis
Treg cell lysates were prepared by mechanical disruption in a protein extraction buffer (in mmol/l): Tris(hydroxymethyl)-aminomethane (50); ethylene glycol-bis(b-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) (0.5); EDTA (1); dithiothreitol (DTT) (0.5) (pH 7.0). The buffer also contained (in mmol/l) leupeptin (0.1), pepstatin (0.1), phenylmethylsulfonyl fluoride (0.1) to prevent non-specific proteolysis. Heart tissue was lysed as described in the multiplex immunoassay section. SDS-PAGE was performed followed by transfer to a membrane (polyvinylidene difluoride [PVDF]) for Western Blot analysis. Membranes were saturated in enhanced chemiluminescence substrate and exposed by Kodak X-OMAT 2000A processor (Eastman Kodak, new York, NY, USA) onto blue basic autoradiography double emulsion film. The processed films were scanned using the Epson Perfection V750 Pro scanner (Epson America, Inc., Long Beach, CA, USA) and SilverFast Ai professional scanning software (LaserSoft Imaging AG, Kiel, Germany). Protein optical densities were quantified using LabImage 1D software (Kapelan Bio-Imaging GmbH, Leipzig, Germany). In addition, prior to immunoblotting, all membranes were stained with Ponceau S acid red and quantified for total protein. Next, total protein measured by Coomassie blue and/or Ponceau S was compared to total protein expression for equal loading. All immunoblot analysis was performed from the semi-quantitation of individual blots and was not compared across blots. All immunoblot images of a given target were taken from the same blot. Antibodies used are anti-histone H3 (Cell Signaling Technologies), anti-acetylated histone H3 (Cell Signaling Technologies), anti-CD206 (R&D Systems, Inc., Minneapolis, MN, USA).
Multiplex immunoassay
Infarct tissue was rapidly excised 24 and 72 h post I/R and snap frozen in liquid nitrogen. Infarct tissue was then immersed in lysis buffer (Sigma CelLytic MT Mammalian Tissue Lysis Reagent containing Sigma protease inhibitor cocktail and Sigma phosphatase inhibitor cocktail 2) homogenised using a Bullet Blender® (NextAdvance, Averill Park, NY, USA). Mouse multiplex kits (Merck Millipore, Darmstadt, Germany) were used to determine cytokine and chemokine levels according to the manufacturer’s recommendations.
Data and statistical analysis
Results are presented as mean ± standard error of the mean. The differences between infarct size between mice eating a HF and normal fat (NF) administered B420, Ls-33 or saline were analysed with a two-way ANOVA followed by a Tukey’s HSD post hoc test. The differences between experimental groups were analysed with a Student’s t-test, one-way ANOVA followed by a Tukey’s HSD post hoc test, or two-way ANOVA followed by a Bonferroni post-hoc test; P<0.05 was considered as significant.
3. Results
B420 administration reduces infarct size following I/R protocol
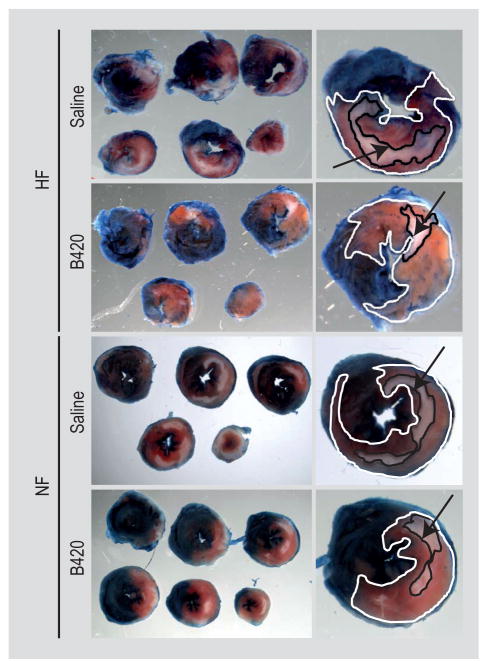
We predicted that probiotic administration would attenuate myocardial damage from ischemia/reperfusion (I/R) injury. Previous work demonstrated that 4–6 weeks of B420 administration reduces inflammatory markers and weight gain related to high-fat feeding (Amar et al., 2011; Stenman et al., 2014). Accordingly, we subjected male mice (10 weeks of age) to 28 days gavage with B420, Ls-33 or saline. Two weeks prior to the initiation of gavage, the HF group was started on the HF diet that was continued throughout the entire study protocol. After 28 days of gavage, mice from the experimental groups described above were subjected to our I/R protocol (Lefer and Bolli, 2011). Myocardial damage was quantified by comparing the AON to the AAR. Percent infarct size is defined as AON (white) relative to the AAR (pink). On average, HF and NF mice treated with B420 displayed a significant (P<0.05) reduction in infarct area when compared to saline- and Ls-33 treated mice (Figure 1; Supplementary Figure S2).
Figure 1.
Bifidobacterium animalis subsp. lactis 420 (B420) reduces infarct size following in ischemia/reperfusion (I/R) compared to saline, Ls-33 treated mice. Serial sections of hearts from high fat (HF) and normal fat (NF) fed mice administered saline or B420. Lactobacillus salivarius-33 administered hearts were omitted for clarity but matched saline controls. Enlarged section image identifying the area at risk (AAR; pink area, outlined in white) and the area of necrosis (AON; white area, outlined in black, indicated by black arrow).
B420 administration attenuates inflammatory transcriptional profile in infarct area
Since cardiac injury after ischemia/reperfusion results in an inflammatory response (Marchant et al., 2012), we wanted to perform a global assessment of inflammation in the infarct area following MI. It is now appreciated that probiotics can suppress peripheral inflammation (Mengheri, 2008) and increase activity of CD4+, CD25+ and FoxP3+ Treg cells (Smelt et al., 2013). Reductions in the Treg population is evident in patients with congestive heart failure (CHF) and lower levels of Treg associate with worse prognosis in CHF patients with reduced cardiac function (Okamoto et al., 2014). Using RT-PCR, we determined gene expression for tumour necrosis factor alpha (TNF-α), interleukin (IL)-6, monocyte chemoattractant protein-1 (MCP-1), forkhead-winged helix P3 transcription factor (FoxP3) and IL-17 in the infarct and peri-infarct area encompassing the border zone region of infarcted hearts.
Sham-operated controls showed minimal (3–6 fold lower expression than post-MI hearts), if any, expression of pro-inflammatory cytokines and were omitted from the bar graph for clarity. As illustrated in Supplementary Figure S3A, the border zone myocardium from HF mice demonstrated a reduction in pro-inflammatory indicators, TNF-α, IL-6, and MCP-1 in B420-treated compared to saline-and Ls33-treated hearts. Although IL-17 trended towards a reduction in B420-treated hearts, this was not statistically significant. Border zone myocardium from NF mice demonstrated a slightly different pattern of cytokine activation with minimal, if any, changes in these same markers (Supplementary Figure S3B). Of particular significance, hearts from B420-administered mice fed either a HF or NF demonstrated an increase in FoxP3 transcriptional expression, suggesting an increase in the Treg population of cells. Incidentally, B420 or Ls33 administration in sham-operated mice did not significantly alter inflammatory markers when compared to sham-operated controls (data not shown).
B420 administration minimises pro-inflammatory cytokines in infarct area
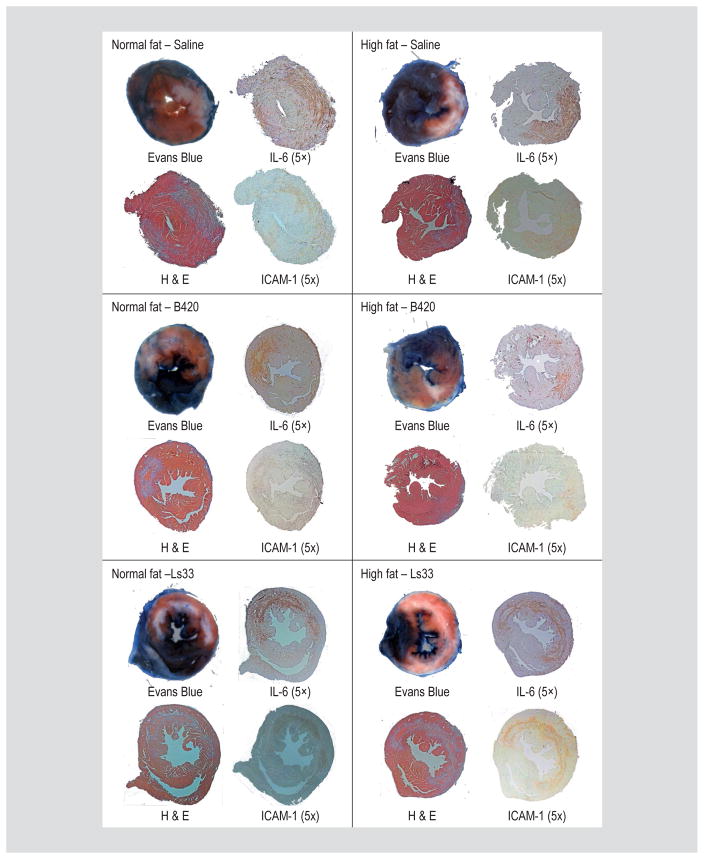
Measures of mRNA transcripts as above only predicts protein expression (Guo et al., 2008). Moreover, therapeutic efficacy is typically evaluated by infarct size and histological assessments (Takagawa et al., 2007). To determine the localisation of the inflammatory response, immunohistochemistry was performed on heart sections for the presence of the inflammatory markers: IL-6 and intercellular adhesion molecule-1 (ICAM-1). H&E staining of infarcted hearts confirmed infarct location and size and matched determinations of EB and TTC staining. In each of the images, IL-6 and ICAM-1 staining paralleled infarct size demonstrating that B420, but not Ls-33 can suppress the absolute amount of IL-6 secretion and subsequent ICAM-1 expression (Figure 2; Supplementary Figure S4).
Figure 2.
Bifidobacterium animalis subsp. lactis 420 (B420) minimises pro-inflammatory cytokines in infarct area. Following EB and TTC staining, hearts were fixed and stained for H&E, IL-6 and ICAM-1 as indicated. In general, the extent of IL-6 and ICAM-1 expression paralleled the size of the AON and is reduced in the hearts of mice administered the B420. Hearts of normal fat and high fat fed mice administered saline (top), B420 (middle), or Lactobacillus salivarius-33 (Ls-33) (bottom).
In order to better quantify IL-6 and ICAM-1 staining, the area of cytokine infiltration was normalised to the total cross-sectional area of each heart section. Next, the normalised cytokine area was plotted against the AON in the identical section, which was also normalised to total cross-sectional area of each heart section. Linear regression analysis represents the ‘correlation’ between infract size as AON to cytokine infiltration. The line of identity indicates a 1:1 correlation between cytokine and AON and is illustrated as the grey line in Supplementary Figure S4. As shown in Supplementary Figure S3, the area of coverage for IL-6 (1.11±0.09; r2=0.918, P<0.0001) more closely matched the AON than the area of coverage for ICAM-1 (0.56±0.15; r2=0.538, P=0.0028).
B420 administration for 7–10 days reduces infarct size following I/R protocol
In order to elevate the clinical and translational potential of these studies, we wished to test a shorter administration period. Since we did not see an effect of Ls-33 on infarct size, we did not include this group in the following studies. We subjected another group of mice to HF feeding and B420 gavage followed by I/R at 7 days from the start of gavage. Following the 3-day reperfusion period brining to the total number of B420 administration to 10 days, hearts were excised and analysed for infarct size (Figure 3). Again, B420-treated mice demonstrated a significant reduction in infarct area when compared to saline-treated animals (Supplementary Figure S5).
Figure 3.
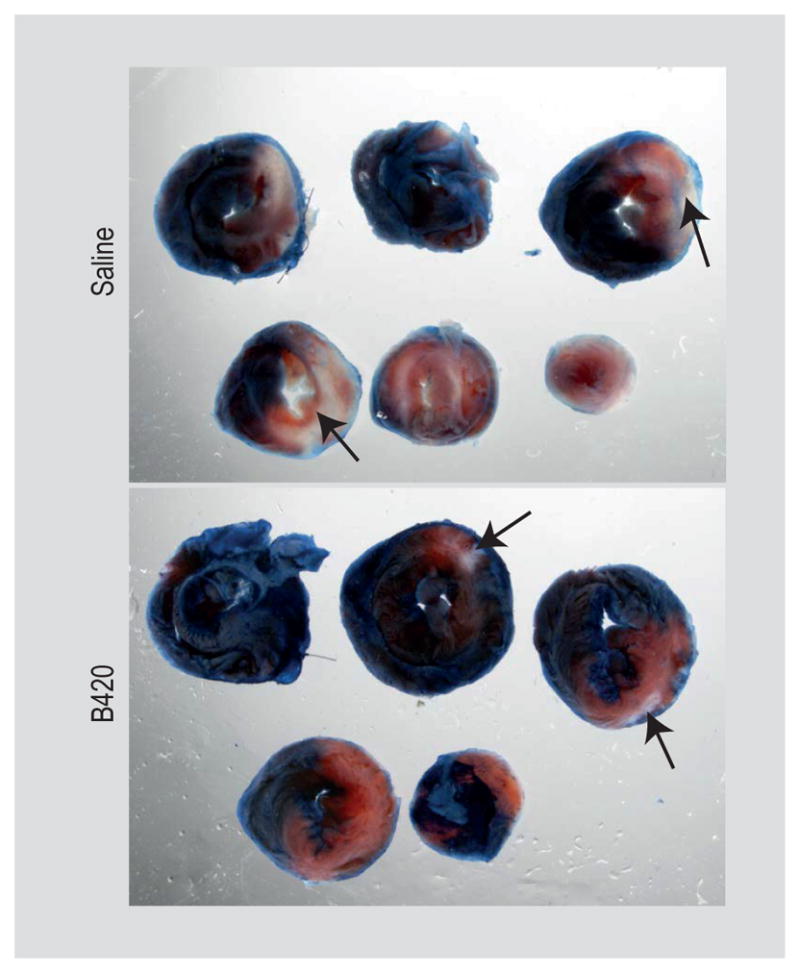
Short-term Bifidobacterium animalis subsp. lactis 420 (B420) administration reduces infarct size following ischemia/ reperfusion (I/R) protocol. Serial sections of hearts from saline and B420 administered group. The area at risk (AAR) is the pink area and the area of necrosis (AON) is the white area (indicated by the black arrow).
B420 administration increases epigenetic and post-translational modifications of Treg cells
The mechanism by which probiotics suppress peripheral inflammation may be due to induction of Treg from dendritic cells in the gut and periphery (Mengheri, 2008). Accordingly, we measured peripheral (splenic, blood, and heart draining mediastinal lymph nodes) Treg populations. Unexpectedly, 4 weeks of B420 administration did not impact Treg cell numbers in the circulation, mediastinum or spleen (Supplementary Figure S6). This suggests that B420 treatment does not increase the quantity of Treg cells. We also established that a HF diet does not increase Treg populations compared to a NF diet (Supplementary Figure S7). Recent work shows that metabolites from the gut may act directly on the Treg population through epigenetically modified conserved non-coding sequences (CNS) in the foxp3 locus or increased acetylation of FoxP3 protein increasing the expression and functionality of Treg cells in vitro and in vivo (Arpaia et al., 2013; Wang et al., 2009). Therefore, we tested the hypothesis that B420 modification of the gut microbiome enhanced global acetylation of Treg cells. For this experiment, splenic Treg were isolated and probed for acetyl-histone 3 (Ac-H3) and total histone (H3) using antibodies specific for Ac-H3 and H3. Figure 4 (and Supplementary Figure S8) illustrates that Ac-H3 normalised against H3 was elevated in B420- compared to saline-treated mice. The indication is an increase in epigenetic and post-translational remodelling in the Treg population.
Figure 4.
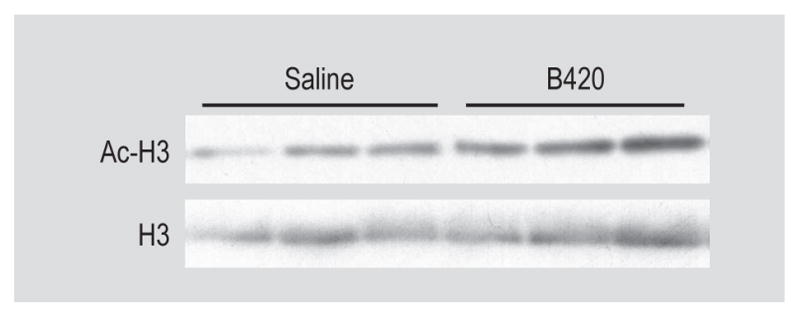
Bifidobacterium animalis subsp. lactis 420 (B420) administration increases epigenetic and post-translational modifications in Treg cells. Western blot images of acetyl-histone 3 (AC-H3) and total histone (H3) from splenic Treg cells.
CD25+FoxP3+ Treg cells are necessary for B420-mediated protection against cardiac injury
The increase in Treg histone H3 acetylation suggests an increase in Treg activity following B420 administration despite no measureable increases in Treg cell numbers. The suggestion is that B420 may expand a subset of the Treg population into a more functional and more immunosuppressive phenotype. Interestingly, complete depletion of the Treg population exacerbates cardiac injury post-MI (Weirather et al., 2014), while superagonism using a CD28 antibody ameliorates pathological remodelling post-MI (Tang et al., 2012). If activated Treg cells are playing a predominant role in gut-induced (following B420 administration) cardio-protection, we predicted that Treg depletion would eliminate cardio-protection in B420-treated and not saline-treated mice. Accordingly, we employed a strategy to moderately deplete the Treg population. We chose a moderate depletion for 2 reasons: (1) complete Treg depletion may mask the protective effect of activated Treg cells, and (2) recruitment of inflammatory cells, specifically Treg, to the site of MI occurs within 3 days (Frangogiannis, 2012; Weirather et al., 2014).
Although a number of strategies have been employed to inhibit or deplete Treg cells (some of which are currently used clinically (Pere et al., 2012)), we used an anti-CD25 monoclonal antibody (CD25-mAb; Clone PC61) to selectively deplete the Treg population (Setiady et al., 2010). Using a single intraperitoneal injection of the anti-CD25-mAb (IgG used as a control) the morning of I/R surgery, CD25+FoxP3+ T cells (CD4+CD3+) were depleted by about ~40% 1 day post injection (data not shown) and at the end of the 3-day reperfusion period, we were able to achieve a ~50–60% depletion of CD25+FoxP3+ T cells (CD4+, CD3+) (Supplementary Figure S9). Therefore, we subjected mice to HF feeding (continued throughout the study protocol), B420 administration (2 weeks), and CD25-mAb or IgG injection prior to I/R. After the 3-day reperfusion period, hearts were excised and analysed for infarct size as before. Again, we observed a clear and significant effect of B420 administration on cardio-protection as above. In support of our prediction, moderate Treg depletion eliminates the cardio-protection in B420- but not saline-treated mice (Supplementary Figure S10). H&E staining of infarcted hearts confirmed infarct location and size and matched determinations of EB and TTC staining. IL-6 and IL-10 staining patterned infarct size, however, there were no detectable differences in staining densities by visualisation (Supplementary Figure S11). These data show that Treg cells are necessary for the cardio-protection instigated by the gut and B420 administration.
B420 administration promotes infarct repair and suppresses infarct expansion
We hypothesised that B420 attenuates pathological and promotes beneficial cardiac remodelling post-MI. In order to better assess the model that B420 attenuates MI expansion and promotes MI repair, we quantified (by multiplex immunoassay) several inflammatory analytes (cytokines and chemokines) in infarct tissue from B420- and saline-treated groups at 24 and 72 h post-I/R. To determine how both the time following the infarct and treatment (Tx) with saline or B420 impact analyte concentration, we performed a 2-way ANOVA followed by a post-hoc Bonferroni analysis (Supplementary Figure S12). First, both factors of time (24 vs 72 h) and Tx (saline vs B420) impacted the amount of interferon γ(IFN-γ) present in the infarct with a significant upregulation of IFN-γ 24 h post-MI in saline-compared to B420-treated mice. More importantly, there was a significant interaction between the factors of time and Tx indicating that B420 administration significantly affects IFN-γ concentration over the 24 to 72 h reperfusion period. There was a clear attenuation of IFN-γ inducible protein 10 (IP-10) in the infarct area of B420-treated mice. In addition, IP-10 showed significant differences in time, Tx and time by Tx (interaction). The chemokine, MCP-1, was impacted by Tx with a significant interaction between time and Tx. Furthermore, MCP-1 was significantly downregulated 72 h post-MI in B420-treated hearts. The patterning of the cytokine, IL-15, exhibited a significant attenuation at 24 and 72 h post-MI in B420-treated mice compared to saline-treated controls. Finally, CD206 expression (an M2 macrophage marker (Lorchner et al., 2015)), as determined by Western blot analysis, showed significant differences in time, Tx and time by Tx (interaction). CD206 expression increases with reperfusion time (24 vs 72 h) in B420-treated compared to saline-treated controls (Supplementary Figure S13). These data suggest that there is an increase in M2 macrophages in the B420 treated group after 72 h of reperfusion post-MI.
4. Discussion
The gut is emerging as a key player in health and disease. Although incremental advances in MI prevention and treatment are continually evolving, we implemented a combinatorial and integrative approach that may accelerate gut and immune-based therapies for MI. We show a marked reduction in MI size following pre-treatment with the probiotic B420. Considering that the last 15–20 years of research has established a mechanistic link between inflammation and every aspect of MI and that B420 treatment reverses CHF-induced inflammation (Amar et al., 2011; Stenman et al., 2014), we predicted that probiotic administration would minimise the impact of MI. In this study, we show that both 4 weeks and 7 days of B420 administration in mice mitigates infarct size from I/R when compared to the saline-treated group. Interestingly, we show that another probiotic strain, Ls-33, has no effect infarct size.
Bifidobacteria and lactobacilli are two distinct genera of bacteria belonging to different phyla (Ventura et al., 2009). Although both strains are considered lactic acid producing, comparative genomics reveal no common genetic signatures and only 7 genes shared between bifidobacteria and lactobacilli (Lukjancenko et al., 2012; Ventura et al., 2009). The authors further suggest that metabolism of complex sugars may underlie Bifidobacterium functionality when compared to other probiotic species, such as Lactobacillus (Ventura et al., 2009). Moreover, the genus of Bifidobacterium is relatively small and may harbour key differences among Bifidobacterium species that drive probiotic efficacy. A further pursuit of this difference in strain and genera efficacy was beyond the scope of this study, but is an interesting direction for further exploration.
In this study, we focused on a model of high-fat feeding (HF) for 2 reasons. First, diabetic and obese patients dominate the clinical ‘high-risk’ MI population. Despite being clinically heterogeneous, the burden of MI patients with diabetes and obesity continues to rise (Fang and Alderman, 2006). We posit that feeding mice a HF models the clinically relevant phenotype of a patient at the highest risk for MI. Second, HF feeding increases low-grade systemic inflammation (Cani et al., 2007b) caused in part by the growth and translocation of harmful bacteria induced by HF feeding (Cani et al., 2008), and B420 treatment reduces weight gain and inflammation in HF fed mice (Stenman et al., 2014). Since B420-mediated cardio-protection occurred in mice eating the standard NF rodent chow we were confident that the HF diet does not introduce significant confounders to the mechanism of B420-mediated protection, and is the most representative of a standard western diet.
Although the cardio-protective effects of probiotics are typically attributed to lowering CVD risk factors like hypertension or hypercholesterolemia (Chen and Konhilas, 2013) and not direct protection against ischemic damage, evidence suggests a direct role for probiotic induced cardio-protection. A recent study demonstrates that altering intestinal microbiota using the antibiotic, vancomycin, diminishes ischemic damage in the rat (Dahl S) heart (Lam et al., 2012). Furthermore, supplementing the rats Goodbelly probiotic juice, which contains 2 probiotic bacteria, L. plantarum (Lp299v) and B. lactis (Bi-07), daily for 14 days also protects against I/R injury. In another study, Sprague-Dawley rats administered L. rhamnosus GR-1 immediately following permanent ligation of the left coronary artery show attenuation of left ventricular hypertrophy and improved haemodynamic and echocardiographic parameters (Gan et al., 2014). It should be noted these effects could be species-specific, since our control strain Ls-33 shows no reduction in infarct size or expression of inflammation markers.
In our study, we pre-treated mice with B420 or Ls-33 that continued throughout the duration of the study protocol. This would be equivalent to more of a preventative measure rather than a therapeutic. To elevate the clinical potential, we initiated B420 administration at an earlier time point with similar results; B420 remained effective at reducing infarct size. While previous work (Gan et al., 2014; Lam et al., 2012) initiated probiotic treatment at the time of infarct, they also used a model of permanent ligation, unlike the current study. Therefore, to elucidate the impact of B420-adminstratin on long-term cardiac remodelling, we induced a moderate MI by distal (permanent) ligation of the LCA following 4 weeks of B420 administration that was continued throughout the study protocol (Supplementary Figure S14). We show attenuation of pathological cardiac remodelling and maintenance of cardiac function in B420-treated compared to saline-treated mice. Therefore, we stipulate that the benefit of B420 administration is cardio-protective based on the extent and not the origin of myocardial damage.
The 2 aforementioned studies (Gan et al., 2014; Lam et al., 2012) provide evidence that Goodbelly or GR-1 administration mediates, at least in part, cardio-protection by lowering levels of leptin, which can exert hypertrophic effects at high levels(Gan et al., 2014; Lam et al., 2012). Our hypothesis, however, focuses on the ability of probiotic administration to modulate the inflammatory response post-MI. As part of cardiac remodelling post-MI, recruitment of distinct populations of inflammatory cells includes monocytes that differentiate into macrophages (Nahrendorf et al., 2007). Macrophages are a heterogeneous cell population central to the acute MI response and critical to post-MI healing (Lorchner et al., 2015). In the infarcted heart, infiltrating monocytes differentiate into cytotoxic, inflammatory macrophages (M1-type) but can be converted to reparative, anti-inflammatory macrophages (M2-type) (Frangogiannis, 2012). Studies indicate an early infiltration of specific monocyte/macrophage (Ly-6Chi) populations to phagocytose necrotic cells with a second wave (Ly-6Clo) to promote infarct healing (Nahrendorf et al., 2007). Evidence points to better clinical outcomes that correlates with the second Ly-6Clo (M2-type; infarct healing) as compared to the initial Ly-6Chi (M1-type; infarct expansion) population (Nahrendorf et al., 2007). Accordingly, the transcriptional profile of infarcted hearts demonstrates a shift towards an anti- from a pro-inflammatory environment post-MI in B420-treated mice. Immunohistochemical staining demonstrates upregulation of IL-6 in response to myocardial injury and is associated with post-MI remodelling (Nian et al., 2004). IL-6 secretion can induce ICAM-1 expression, which promotes the adherence of infiltrating neutrophils following an ischemic episode (Frangogiannis et al., 2002) both of which parallel infarct size demonstrating that B420 can also suppress IL-6 secretion and subsequent ICAM-1 expression.
In addition, T cells are recruited to the site of myocardial injury by these signals where they release pro-inflammatory mediators. At the same time, the immune system produces Treg that suppress the inflammatory responses following an MI (Tang et al., 2012). Treg cells are pleiomorphic regulators of immune homeostasis acting to maintain self-tolerance, control autoimmune deviations, and prevent runaway immunogenic responses to pathogens (Ohkura et al., 2013). They are specialised T cells characterised, in part, by the expression of FoxP3 and cell surface markers, CD4 and CD25 (Sakaguchi et al., 2010). Accordingly, our data indicate an increase in FoxP3 mRNA levels in infarcted regions of the heart in B420 treated animals thus suggesting an increase in Treg populations. A recent study by Weirather et al. (2014) validates a critical role for Treg cells first, by depleting the Treg population, which exacerbates cardiac inflammation and infarct size post-MI and, second, by Treg-cell activation using superagonistic anti-CD28 monoclonal antibody which improves healing and survival post-MI.
For these reasons, we hypothesised that anti-inflammatory Treg cells are necessary to impart protection against an MI as a result of B420 administration. Based on Weirather et al. (2014), complete Treg depletion would exacerbate post-MI remodelling regardless of B420-treatment. Therefore, we employed an anti-CD25-mAb to deplete the Treg population by approximately 50%. This depletion strategy does not significantly worsen infarct size in control animals but eliminates the cardio-protection in B420-treated mice. Supporting our hypothesis, it was recently shown that a combination of B420 and a prebiotic, polydextrose, differentially modulated T cell populations in the small intestinal lamina propria in mice that were fed a HF diet (Garidou et al., 2015). On a further note, the combination of B420 and prebiotic restored the expression of Reg3β which was down regulated by the HF diet. Reg3β is an important regulator of myocardial healing in the ischemic heart (Lorchner et al., 2015). It is unclear, however, how this local modulation would translate into systemic or heart-targeted effects.
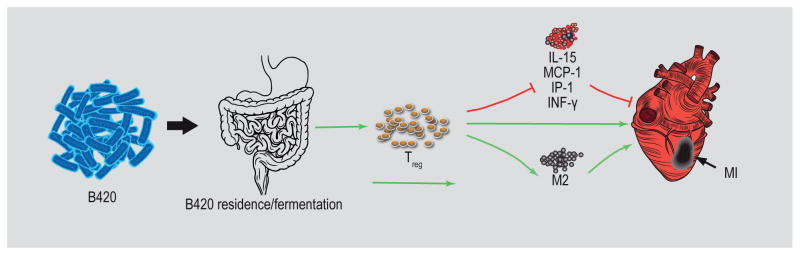
Building upon the model that B420 attenuates MI expansion (M1-type) and promotes MI repair (M2-type), we quantified (by multiplex immunoassay) inflammatory analytes (cytokines and chemokines) in infarct tissue from B420- and saline-treated groups 24 and 72 h post-I/R. The general pattern of analytes (IFN-γ, IP-10, MCP-1, and IL-15) measured in the infarct area supports/validates our prediction that B420 attenuates MI expansion and promotes MI repair. IFN-γ is a cytokine of Th1 cytotoxic T lymphocytes that contributes to macrophage activation by increasing phagocytosis and priming the production of pro-inflammatory cytokines including IP-10 (Bujak et al., 2009). It follows, then, that there is a clear attenuation of IP-10 in the infarct area of B420-treated mice. The chemokine, MCP-1 promotes macrophage differentiation through pro-inflammatory cytokines (Kaikita et al., 2004) and remains lower in B420-treated hearts compared to saline controls. We found the patterning of the cytokine, IL-15, particularly novel and relevant. Monocytes and dendritic cells produce IL-15, which facilitates the activity of cytotoxic natural killer cells, CD8+ T cells and pro-inflammatory macrophages (Huntington, 2014; Ikemizu et al., 2012). Again, 24 h post-MI, B420-treatment mitigates IL-15 that remains attenuated when compared to saline-treated mice. Finally, the enhanced shift towards CD206 positive markers (M2-type) in B420- compared to saline-treated hearts post-MI supports this transition towards reparative mechanisms. The putative mechanisms by which B420 attenuates cardiac injury is outlined in Figure 5.
Figure 5.
Putative mechanism by which Bifidobacterium animalis subsp. lactis 420 (B420) treatment attenuates cardiac injury following myocardial infarction (MI). The putative mechanisms by which B420 may impact cardiac injury based on our data and findings in the literature. The first step is the ingestion of B420 and entry to the gut. It is unclear whether B420 colonises the gut during the administration period, but it is known that B420 fermentation and/or interaction with the gut activates a proportion of the Treg population. Treg activation by B420 administration either directly or through the attenuation of pro-inflammatory cytokines attenuates cardiac injury due to MI. Our data also suggests that B420 administration hastens the transition to reparative, M2 type macrophages, potentially through activated Treg cells. The red arrow indicates a pro-inflammatory impact that would exacerbate MI; the green arrow represents an anti-inflammatory impact that would mitigate MI.
In conclusion, we show that a probiotic, B420, mitigates inflammation-related cardiac conditions in mouse models, namely I/R-induced cardiac injury and permanent ligation of the left coronary artery. In comparison, a Lactobacillus strain showed no effect, demonstrating that the benefit was species-specific. We also demonstrate several aspects of the mechanism, including epigenetic modifications and Treg activation. It is worth commenting on the global implications of these studies in the clinical population. Although the literature cites many examples showing a clinical benefit of specific probiotic strains for human disease, we readily acknowledge that the translation of our findings to a real, clinical benefit for cardio-protection will be challenging. In our study, we administered 109 cfu, which differs from human studies using between 106 up to 1010 cfu per day (Klein et al., 2008; Roessler et al., 2012). Although multiple factors ultimately contribute to the lack of translational clinical efficacy, the predominant conditions impacting probiotic functionality include microbial diversity of the host gut, method of probiotic delivery, and the uncontrolled dietary matrix of the human population. The suggestion is that probiotic strains dosage may need to be titrated based on disease condition. Nevertheless, the finding that probiotics may promote cardiac health with an inflammation-related mechanism provides an entirely novel treatment strategy warrants further investigation in human subjects.
Supplementary Material
Table S1. Primers for RT-PCR.
Figure S1. Two gavage protocols were used to determine the effectiveness of probiotic administration on ischemia/reperfusion injury in the heart.
Figure S2. Comparison of infarct size following ischemia/reperfusion.
Figure S3. RT-PCR of excised heart tissue of infarct and peri-infarct/border zones show differential gene expression for TNF-α, IL-6, MCP-1, FoxP3 and IL-17 of Bifidobacterium animalis subsp. lactis 420-treated compared to saline- and Lactobacillus salivarius-33 treated mice fed high fat or normal fat chow.
Figure S4. The extent of IL-6 and ICAM-1 infiltration compared to the AON for each section normalised to the total heart area.
Figure S5. Infarct size was calculated as a ratio (percentage) of the AON to the AAR.
Figure S6. B420 administration does not impact T cell populations.
Figure S7. High fat feeding does not impact T cell populations.
Figure S8. Averaged Ac-H3 relative to H3 levels.
Figure S9. CD25+FoxP3+ Treg cells are necessary for Bifidobacterium animalis subsp. lactis 420 (B420)-mediated protection against cardiac injury.
Figure S10. Infarct size calculated as a ratio (percentage) of the area of necrosis to the area at risk.
Figure S11. Proinflammatory and anti-inflammatory cytokines patterns of infarct area.
Figure S12. Cytokines and chemokines profile from Bifidobacterium animalis subsp. lactis 420 and saline treated animals collected after 24 or 72 h of reperfusion.
Figure S13. Bifidobacterium animalis subsp. lactis 420 elevates M2 macrophage markers at 72 h, but not at 24 h in infarcted hearts.
Figure S14. B420 administration prevents cardiac dysfunction following permanent coronary ligation.
Acknowledgments
This work was supported by National Institutes of Health grant HL098256, a National Mentored Research Science Development Award (K01 AR052840) and Independent Scientist Award (K02 HL105799) from the NIH to J.P.K., and an Interdisciplinary Training Grant in Cardiovascular Sciences (HL007249). Support was also received from the Sarver Heart Center at the University of Arizona and the Steven M. Gootter Foundation.
Footnotes
Supplementary material can be found online at https://doi.org/10.3920/BM2016.0119.
References
- Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermudez-Humaran LG, Smirnova N, Berge M, Sulpice T, Lahtinen S, Ouwehand A, Langella P, Rautonen N, Sansonetti PJ, Burcelin R. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Molecular Medicine. 2011;3:559–572. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, Van der Veeken J, DeRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scandinavian Journal of Immunology. 2009;70:505–515. doi: 10.1111/j.1365-3083.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, Veeranna V, Tager AM, Luster AD, Frangogiannis NG. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circulation Research. 2009;105:973–983. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani P, Neyrinck A, Fava F, Knauf C, Burcelin R, Tuohy K, Gibson G, Delzenne N. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007a;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007b;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- Chen H, Konhilas JP. Probiotic species on cardiovascular disease: the use of probiotics to reduce cardiovascular disease risk factors. In: Watson RR, Preedy VR, editors. Bioactive food as dietary interventions for cardiovascular disease. Elsevier; Boston, MA, USA: 2013. pp. 303–317. [Google Scholar]
- Collado MC, Meriluoto J, Salminen S. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Letters in Applied Microbiology. 2007;45:454–460. doi: 10.1111/j.1472-765X.2007.02212.x. [DOI] [PubMed] [Google Scholar]
- Daniel C, Poiret S, Goudercourt D, Dennin V, Leyer G, Pot B. Selecting lactic acid bacteria for their safety and functionality by use of a mouse colitis model. Applied and Environmental Microbiology. 2006;72:5799–5805. doi: 10.1128/AEM.00109-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel B, Lemetais G, Beney L, Cachon R, Sokol H, Langella P, Gervais P. Impact of probiotics on risk factors for cardiovascular diseases: a review. Critical Reviews in Food Science and Nutrition. 2014;54:175–189. doi: 10.1080/10408398.2011.579361. [DOI] [PubMed] [Google Scholar]
- Fang J, Alderman MH. Impact of the increasing burden of diabetes on acute myocardial infarction in New York City: 1990–2000. Diabetes. 2006;55:768–773. doi: 10.2337/diabetes.55.03.06.db05-1196. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. 2001 Available at: http://tinyurl.com/8bccc3r.
- Fedorak RN. Understanding why probiotic therapies can be effective in treating IBD. Journal of Clinical Gastroenterology. 2008;42(Suppl 3):S111–115. doi: 10.1097/MCG.0b013e31816d922c. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circular Research. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovascular Research. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, Sidaway JE, Martin G, Gloor GB, Swann JR, Reid G, Karmazyn M. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circulation: Heart Failure. 2014;7:491–499. doi: 10.1161/CIRCHEARTFAILURE.113.000978. [DOI] [PubMed] [Google Scholar]
- Garidou L, Pomie C, Klopp P, Waget A, Charpentier J, Aloulou M, Giry A, Serino M, Stenman L, Lahtinen S, Dray C, Iacovoni JS, Courtney M, Collet X, Amar J, Servant F, Lelouvier B, Valet P, Eberl G, Fazilleau N, Douin-Echinard V, Heymes C, Burcelin R. The gut microbiota regulates intestinal CD4 T cells expressing RORgammat and controls metabolic disease. Cell Metabolism. 2015;22:100–112. doi: 10.1016/j.cmet.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Gomez-Hurtado I, Santacruz A, Peiro G, Zapater P, Gutierrez A, Perez-Mateo M, Sanz Y, Frances R. Gut microbiota dysbiosis is associated with inflammation and bacterial translocation in mice with CCl4-induced fibrosis. PLoS ONE. 2011;6:e23037. doi: 10.1371/journal.pone.0023037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Xiao P, Lei S, Deng F, Xiao GG, Liu Y, Chen X, Li L, Wu S, Chen Y, Jiang H, Tan L, Xie J, Zhu X, Liang S, Deng H. How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochimica et Biophysica Sinica. 2008;40:426–436. doi: 10.1111/j.1745-7270.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- Hakansson A, Molin G. Gut microbiota and inflammation. Nutrients. 2011;3:637–682. doi: 10.3390/nu3060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington ND. The unconventional expression of IL-15 and its role in NK cell homeostasis. Immunology and Cell Biology. 2014;92:210–213. doi: 10.1038/icb.2014.1. [DOI] [PubMed] [Google Scholar]
- Ikemizu S, Chirifu M, Davis SJ. IL-2 and IL-15 signaling complexes: different but the same. Nature Immunology. 2012;13:1141–1142. doi: 10.1038/ni.2472. [DOI] [PubMed] [Google Scholar]
- Kaikita K, Hayasaki T, Okuma T, Kuziel WA, Ogawa H, Takeya M. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. American Journal of Pathology. 2004;165:439–447. doi: 10.1016/S0002-9440(10)63309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Friedrich U, Vogelsang H, Jahreis G. Lactobacillus acidophilus 74-2 and Bifidobacterium animalis subsp lactis DGCC 420 modulate unspecific cellular immune response in healthy adults. European Journal of Clinical Nutrition. 2008;62:584–593. doi: 10.1038/sj.ejcn.1602761. [DOI] [PubMed] [Google Scholar]
- Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, Gross GJ, Salzman NH, Baker JE. Intestinal microbiota determine severity of myocardial infarction in rats. Faseb Journal. 2012;26:1727–1735. doi: 10.1096/fj.11-197921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer DJ, Bolli R. Development of an NIH consortium for preclinicAl AssESsment of CARdioprotective therapies (CAESAR): a paradigm shift in studies of infarct size limitation. Journal of Cardiovascular Pharmacology and Therapeutics. 2011;16:332–339. doi: 10.1177/1074248411414155. [DOI] [PubMed] [Google Scholar]
- Lorchner H, Poling J, Gajawada P, Hou Y, Polyakova V, Kostin S, Adrian-Segarra JM, Boettger T, Wietelmann A, Warnecke H, Richter M, Kubin T, Braun T. Myocardial healing requires Reg3beta-dependent accumulation of macrophages in the ischemic heart. Nature Medicine. 2015;21:353–362. doi: 10.1038/nm.3816. [DOI] [PubMed] [Google Scholar]
- Lukjancenko O, Ussery DW, Wassenaar TM. Comparative genomics of Bifidobacterium, Lactobacillus and related probiotic genera. Microbial Ecology. 2012;63:651–673. doi: 10.1007/s00248-011-9948-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant DJ, Boyd JH, Lin DC, Granville DJ, Garmaroudi FS, McManus BM. Inflammation in myocardial diseases. Circular Research. 2012;110:126–144. doi: 10.1161/CIRCRESAHA.111.243170. [DOI] [PubMed] [Google Scholar]
- Mengheri E. Health, probiotics, and inflammation. Journal of Clinical Gastroenterology. 2008;42(Suppl 3):S177–178. doi: 10.1097/MCG.0b013e31817eedc4. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. Journal of Experimental Medicine. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circular Research. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Noma T, Ishihara Y, Miyauchi Y, Takabatake W, Oomizu S, Yamaoka G, Ishizawa M, Namba T, Murakami K, Iwado Y, Ohmori K, Kohno M. Prognostic value of circulating regulatory T cells for worsening heart failure in heart failure patients with reduced ejection fraction. International Heart Journal. 2014;55:271–277. doi: 10.1536/ihj.13-343. [DOI] [PubMed] [Google Scholar]
- Pere H, Tanchot C, Bayry J, Terme M, Taieb J, Badoual C, Adotevi O, Merillon N, Marcheteau E, Quillien VR, Banissi C, Carpentier A, Sandoval F, Nizard M, Quintin-Colonna F, Kroemer G, Fridman WH, Zitvogel L, Oudard SP, Tartour E. Comprehensive analysis of current approaches to inhibit regulatory T cells in cancer. Oncoimmunology. 2012;1:326–333. doi: 10.4161/onci.18852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler A, Forssten SD, Glei M, Ouwehand AC, Jahreis G. The effect of probiotics on faecal microbiota and genotoxic activity of faecal water in patients with atopic dermatitis: a randomized, placebo-controlled study. Clinical Nutrition. 2012;31:22–29. doi: 10.1016/j.clnu.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nature Reviews Immunology. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Setiady YY, Coccia JA, Park PU. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. European Journal of Immunology. 2010;40:780–786. doi: 10.1002/eji.200939613. [DOI] [PubMed] [Google Scholar]
- Slepian M, Gottehrer NR. Oral-body inflammatory connection. Dent Today. 2009;28:138, 140, 142–133. [PubMed] [Google Scholar]
- Smelt MJ, De Haan BJ, Bron PA, Van Swam I, Meijerink M, Wells JM, Faas MM, De Vos P. Probiotics can generate FoxP3 T-cell responses in the small intestine and simultaneously inducing CD4 and CD8 T cell activation in the large intestine. PLoS ONE. 2013;8:e68952. doi: 10.1371/journal.pone.0068952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman LK, Waget A, Garret C, Klopp P, Burcelin R, Lahtinen S. Potential probiotic Bifidobacterium animalis ssp. lactis 420 prevents weight gain and glucose intolerance in diet-induced obese mice. Beneficial Microbes. 2014;5:437–445. doi: 10.3920/BM2014.0014. [DOI] [PubMed] [Google Scholar]
- Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W, Springer ML. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. Journal of Applied Physiology. 2007;102:2104–2111. doi: 10.1152/japplphysiol.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TT, Yuan J, Zhu ZF, Zhang WC, Xiao H, Xia N, Yan XX, Nie SF, Liu J, Zhou SF, Li JJ, Yao R, Liao MY, Tu X, Liao YH, Cheng X. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Research in Cardiology. 2012;107:232. doi: 10.1007/s00395-011-0232-6. [DOI] [PubMed] [Google Scholar]
- Toral M, Gomez-Guzman M, Jimenez R, Romero M, Sanchez M, Utrilla MP, Garrido-Mesa N, Rodriguez-Cabezas ME, Olivares M, Galvez J, Duarte J. The probiotic Lactobacillus coryniformis CECT5711 reduces the vascular pro-oxidant and pro-inflammatory status in obese mice. Clinical Science. 2014;127:33–45. doi: 10.1042/CS20130339. [DOI] [PubMed] [Google Scholar]
- Ventura M, O’Flaherty S, Claesson MJ, Turroni F, Klaenhammer TR, Van Sinderen D, O’Toole PW. Genome-scale analyses of health-promoting bacteria: probiogenomics. Nature Reviews Microbiology. 2009;7:61–71. doi: 10.1038/nrmicro2047. [DOI] [PubMed] [Google Scholar]
- Wang L, De Zoeten EF, Greene MI, Hancock WW. Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells. Nature Reviews Drug Discovery. 2009;8:969–981. doi: 10.1038/nrd3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circular Research. 2014;115:55–67. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- Zuidema MY, Zhang C. Ischemia/reperfusion injury: the role of immune cells. World Journal of Cardiology. 2010;2:325–332. doi: 10.4330/wjc.v2.i10.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers for RT-PCR.
Figure S1. Two gavage protocols were used to determine the effectiveness of probiotic administration on ischemia/reperfusion injury in the heart.
Figure S2. Comparison of infarct size following ischemia/reperfusion.
Figure S3. RT-PCR of excised heart tissue of infarct and peri-infarct/border zones show differential gene expression for TNF-α, IL-6, MCP-1, FoxP3 and IL-17 of Bifidobacterium animalis subsp. lactis 420-treated compared to saline- and Lactobacillus salivarius-33 treated mice fed high fat or normal fat chow.
Figure S4. The extent of IL-6 and ICAM-1 infiltration compared to the AON for each section normalised to the total heart area.
Figure S5. Infarct size was calculated as a ratio (percentage) of the AON to the AAR.
Figure S6. B420 administration does not impact T cell populations.
Figure S7. High fat feeding does not impact T cell populations.
Figure S8. Averaged Ac-H3 relative to H3 levels.
Figure S9. CD25+FoxP3+ Treg cells are necessary for Bifidobacterium animalis subsp. lactis 420 (B420)-mediated protection against cardiac injury.
Figure S10. Infarct size calculated as a ratio (percentage) of the area of necrosis to the area at risk.
Figure S11. Proinflammatory and anti-inflammatory cytokines patterns of infarct area.
Figure S12. Cytokines and chemokines profile from Bifidobacterium animalis subsp. lactis 420 and saline treated animals collected after 24 or 72 h of reperfusion.
Figure S13. Bifidobacterium animalis subsp. lactis 420 elevates M2 macrophage markers at 72 h, but not at 24 h in infarcted hearts.
Figure S14. B420 administration prevents cardiac dysfunction following permanent coronary ligation.