Abstract
Background
The assessment of fetal growth disorders requires a standard. Current nomograms for the assessment of fetal growth in African American women have been derived either from neonatal (rather than fetal) biometry data or have not been customized for maternal ethnicity, weight, height, parity, and fetal sex.
Objective
We sought to 1) develop a new customized fetal growth standard for African American mothers; and 2) compare such a standard to three existing standards for the classification of fetuses as small (SGA) or large (LGA) for gestational age.
Study Design
A retrospective cohort study included 4,183 women (4,001 African American and 182 Caucasian) from the Detroit metropolitan area who underwent ultrasound examinations between 14 and 40 weeks of gestation (the median number of scans per pregnancy was 5, interquartile range 3-7) and for whom relevant covariate data were available. Longitudinal quantile regression was used to build models defining the “normal” estimated fetal weight (EFW) centiles for gestational age in African American women, adjusted for maternal height, weight, parity, and fetal sex, and excluding pathologic factors with a significant effect on fetal weight. The resulting Perinatology Research Branch/Eunice Kennedy Shriver National Institute of Child Health and Human Development (hereinafter, PRB/NICHD) growth standard was compared to 3 other existing standards—the customized gestation-related optimal weight (GROW) standard; the Eunice Kennedy Shriver National Institute of Child Health and Human Development (hereinafter, NICHD) African American standard; and the multinational World Health Organization (WHO) standard—utilized to screen fetuses for SGA (<10th centile) or LGA (>90th centile) based on the last available ultrasound examination for each pregnancy.
Results
1) First, the mean birthweight at 40 weeks was 133g higher for neonates born to Caucasian than to African American mothers and 150g higher for male than female neonates; maternal weight, height, and parity had a positive effect on birthweight.Second, analysis of longitudinal EFW revealed the following features of fetal growth: (1) all weight centiles were about 2% higher for male than for female fetuses; (2) maternal height had a positive effect on EFW, with larger fetuses being affected more (2% increase in the 95th centile of weight for each 10-cm increase in height); and (3) maternal weight and parity had a positive effect on EFW that increased with gestation and varied among the weight centiles. Third, the screen-positive rate for SGA was 7.2% for the NICHD African American standard, 12.3% for the GROW standard, 13% for the WHO standard customized by fetal sex, and 14.4% for the PRB/NICHD customized standard. For all standards, the screen-positive rate for SGA was at least two-fold higher among fetuses delivered preterm than at term.Fourth, the screen-positive rate for LGA was 8.7% for the GROW standard, 9.2% for the PRB/NICHD customized standard, 10.8% for the WHO standard customized by fetal sex, and 12.3% for the NICHD African American standard. Finally, the highest overall agreement among standards was between the GROW and PRB/NICHD customized standards (Cohen’s inter-rater agreement, kappa=0.85).
Conclusions
We developed a novel customized PRB/NICHD fetal growth standard from fetal data in an African American population without assuming proportionality of the effects of covariates and also without assuming that these effects are equal on all centiles of weight; we also provide an easy-to-use centile calculator. This standard classified more fetuses as being at risk for SGA compared to existing standards, especially among fetuses delivered preterm, but classified about the same number of LGA fetuses. The comparison among the four growth standards also revealed that the most important factor determining agreement among standards is whether they account for the same factors known to affect fetal growth.
Keywords: comparison of fetal growth standards, customized fetal growth standards, ethnic differences, fetal biometry, fetal growth restriction, fetal sex, large for gestational age, maternal height, maternal weight, parity, quantile regression, small for gestational age
Introduction
Growth is a time-dependent change of bodily dimensions1. The human fetus grows at a particularly rapid rate2, 3, and this is important because a principle of developmental biology is that organisms are more susceptible to injury during periods of fast growth4. Birthweight has been used extensively as a parameter to characterize the appropriateness of fetal growth5 and, to date, remains the most frequently used index to assess size as a proxy to growth. Therefore, in clinical practice, many obstetricians rely on the assessment of sonographic estimation of fetal weight to evaluate fetal size and growth6-12. Although the terms “fetal size” and “fetal growth” are not synonymous, there is a relationship between the two, and this is why “fetal size charts” have been referred to as “fetal growth charts.”
Fetal weight is estimated from ultrasound measurements of fetal biometric parameters [e.g. biparietal diameter (BPD), abdominal circumference (AC), femur length (FL), and head circumference (HC)] using one of many mathematical formulas13-16. One widely used equation for estimated fetal weight (EFW) is that proposed by Hadlock et al14, which includes HC, AC, and FL. Assessment of the appropriateness of fetal size is performed by comparing the observed estimated fetal weight (EFW) to a standard. Yet, which standard should be used is a subject of debate.
One issue is whether the same standard, referred to as “population-based,” should be used for all fetuses16, or whether the standard should be customized for physiologic and constitutional factors known to affect neonatal size at birth17-19 as well as EFW20, 21.
One of the most widely used population-based growth charts was proposed by Hadlock et al.22 based on data collected from 392 Caucasian women in the United States. The same investigators suggested using the 10th and 90th centiles of the EFW to evaluate fetal size and growth—adopting the concepts of Battaglia and Lubchenco,5 who classified neonates with a birthweight below the 10th centile as small for gestational age (SGA) and those above the 90th centile as large for gestational age (LGA). However, fetuses with an EFW below the 10th or above the 90th centile are a heterogeneous group: some SGA fetuses have growth deceleration, and others are constitutionally small. Growth-restricted fetuses are those who have deviated from their growth potential, unlike those who are constitutionally small. Similar concepts apply to LGA fetuses, which could experience either fetal growth acceleration or be constitutionally large23.
To address the need for distinguishing between constitutionally small or large fetuses and those affected by growth disorders, Gardosi et al17, 18 proposed to customize the chart of Hadlock et al22 by shifting the normal EFW centiles proportionally up or down so that the mean weight at 40 weeks matches “term optimal weight.” Term optimal weight is personalized for each fetus based on maternal ethnicity, height, weight, parity, and fetal sex, and excludes pathological factors known to affect birthweight, such as smoking. This approach, referred to as “gestation-related optimal weight” (GROW), derives customization coefficients for non-pathologic maternal characteristics and fetal sex by analyzing birthweight data in local populations19, 24, 25.
Other approaches to the customization of growth charts include the Individualized Growth Assessment26-28 that assumes all relevant factors that determine the growth potential of a fetus are captured in the rate of growth during the second trimester. The importance of considering longitudinal measurements to derive fetus-specific growth velocity was also highlighted by Sovio et al.,29 who found that the SGA fetuses identified based on the chart of Hadlock et al.22 were at risk for neonatal morbidity only if their fetal AC growth velocity was in the lowest decile29, 30.
Although several studies suggest that estimates for the association between adverse neonatal outcomes and abnormal birthweight are higher for customized than non-customized (population-based) standards31-37, recent initiatives undertaken to develop growth standards proposed either population-based or only partially customized standards. For example, the INTERGROWTH-21st study16, 38-40 proposed a one-size-fits-all standard derived from a multi-ethnic population. By contrast, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) fetal growth studies21 reported standards specific to four different ethnic-racial groups (non-Hispanic White, Hispanic, African American, and Asian)21, yet customization by factors other than race was not provided. Recently, a study sponsored by the World Health Organization (WHO)20, 41 proposed a multi-ethnic growth standard customized only by fetal sex, despite the observation that other factors (e.g. country of origin, maternal age, height, and parity) had independent effects on EFW. Of interest, by using quantile regression to model EFW data (an approach that does not rely on assuming normal distribution of the data), the investigators reported that the effects of several factors (e.g. maternal height and weight, fetal sex) were graded among the centiles of weight distribution. For example, maternal weight had a higher effect on larger fetuses than on smaller fetuses20.
The most widely adopted customization approach is that of Gardosi et al18, which is based on birthweight data and assumes that the effects of covariates are proportional during gestation (e.g. fetuses of parous mothers will have a higher EFW than those of nulliparous mothers by the same proportion at all gestational ages). However, the assumption of proportionality has not been tested thus far using longitudinal fetal data. Our study is based on a cohort of pregnant women who attended our Center in Detroit, Michigan, where the predominant ethnic group is African American based on self-reporting. The objectives of this study were to 1) develop a new customized fetal growth standard for African American women; and 2) compare the standard derived from our population to three existing standards for the classification of fetuses as SGA and LGA.
Materials and methods
Study population
This retrospective longitudinal cohort study was conducted at the Center for Advanced Obstetrical Care and Research of the Perinatology Research Branch, NICHD, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS). The Center is housed at Hutzel Women’s Hospital in partnership with the Wayne State University (WSU) School of Medicine in Detroit, Michigan, USA. All patients included in this study provided written informed consent for ultrasound examinations and were enrolled in research protocols approved by the Human Investigation Committee of WSU and the Institutional Review Board of NICHD.
From 2002 through 2016, 4,681 pregnant women were enrolled and had ultrasound examinations performed by a maternal-fetal specialist or a senior sonographer with more than three years of experience who performs a minimum of 300 ultrasound scans per year. More than 95% of women were actually enrolled from 2006 through 2015, at an average enrollment of 445 per year, which represents about 25% of the yearly enrollment at our clinic. Women self-reported as African American, 4,239 (90.6%); Caucasian, 197 (4.2%); Hispanic, 31 (0.7%); Asian, 31 (0.7%); and 183 (3.9%) either as “Other” or as “Unknown” race or ethnicity. African American and Caucasian women were included in this study, regardless of pregnancy outcome, if they met the following criteria: 1) age 18-40 years; 2) had at least one ultrasound examination performed between 14-40 weeks of gestation with available measurements of the AC, HC, FL, BPD, and gestational age at each examination. Of the 4,143 African American and 188 Caucasian women who met these inclusion criteria, four were excluded because of outlier fetal biometric measurements, and 144 (3.3%) were excluded because of missing data on maternal weight, height, parity, or fetal sex, resulting in 4,001 African American and 182 Caucasian women (Figure S1).
Ultrasound examinations
Ultrasound studies were performed using the General Electric Voluson Expert and Voluson E8 (GE Healthcare, Milwaukee, WI, USA) ultrasound systems and 5- to 2-MHz probes. Biometric measurements were obtained using methods previously described by Chitty et al42-44 and Altman and Chitty45, which are consistent with recommendations of the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG)46 and the American Institute of Ultrasound in Medicine (AIUM)47. Fetal biometric parameters included: 1) BPD (outer edge to inner edge of the calvarium); 2) HC (ellipse around the outside of the calvarium); 3) AC (ellipse placed at the outer surface of the skin); and 4) FL (calipers placed at the ends of the ossified diaphysis). Estimated fetal weight was computed from the AC, HC, and FL measurements using the formula of Hadlock et al.14 to enable direct comparison to previous standards. The indices of proportionality (HC/AC, FL/AC, and BPD/FL) were also determined. The median number of ultrasound examinations per pregnancy was 5 [interquartile range (IQR) 4-7]. Gestational age was determined based on the last menstrual period and validated during the first ultrasound examination either by crown-rump length or BPD measurement.
Statistical analysis
Effect of covariates on birthweight
We used multi-linear regression with backward elimination as described by Gardosi and Francis19, to assess the effect of covariates on birthweight at 40 weeks of gestation (280 days). The birthweight of neonates born ≥37 gestational weeks was regressed on self-reported ethnicity, height and weight, parity, fetal sex, and gestational age at delivery as well as the following pathological factors: extremely low or high body mass index (BMI) (defined as <20.5 kg/m2 and >40.5 kg/m2, respectively), smoking status, gestational diabetes mellitus, hypertension, preeclampsia, and fetal anomalies. A p-value <0.05 was considered significant.
Development of a customized (PRB/NICHD) fetal growth standard for African American women
We used penalized fixed-effects quantile regression models48, 49 to fit individual centiles (5th, 10th, 50th, 90th, and 95th) of the distributions of fetal biometric parameters, indices of proportionality, and EFW as a function of gestational age. We relied on Bayesian information criteria recommended by Lee et al.50 to determine the “shrinkage parameter” of the fetus-specific fixed effects. The resulting population-level centiles (i.e. non-customized and representing the entire study population) were superimposed on the raw data for visualization purposes and compared to other non-customized standards, such as the NICHD African American standard21 and the WHO standard non-customized by fetal sex.
To determine the effect of covariates on fetal weight centiles, additional covariates were considered for inclusion in the quantile regression models and retained if significant: maternal height, weight, and parity; fetal sex; extremely low or high BMI; smoking status; diabetes; hypertension; preeclampsia; preterm delivery; fetal anomalies; and, importantly, interaction terms between these covariates and gestational age. The 5th, 10th, 50th, 90th, and 95th centiles of EFW were derived from a model that had the same terms but eventually different coefficients for each centile curve. The EFW data was first log transformed; therefore, each covariate without a significant interaction with gestational age had a constant proportional effect on a given EFW centile throughout gestation. The effects of covariates were reported as a percentage of change in estimated weight.
Although fitting of the quantile regression models involved EFW data from all pregnancies regardless of outcome, the prediction of customized “normal” centiles from the quantile regression models was based only on the contribution of non-pathologic factors that affect growth. This is in keeping with the concept proposed by Gardosi et al.18
All statistical analyses were conducted using the R statistical language and environment (www.r-project.org), including the rqpd package for longitudinal quantile regression, available from R-Forge (https://r-forge.r-project.org). Centiles for the customized GROW standard were obtained using the bulk centile calculator version 6.7.8_US from the authors’ website (https://www.gestation.net/).
Results
Maternal characteristics
For the group of 4,001 African American women, the median maternal age, height, and weight were 23 (IQR 20-27) years, 163 (IQR 157-168) cm, and 73 (IQR 61-91) kg, respectively. There were 632 women (15.8%) who delivered preterm (<37 weeks of gestation), and 1,457 (36%) were nulliparous.
For the group of 182 Caucasian women, the median maternal age, height, and weight were 26 (IQR 22-30) years, 163 (IQR 157-168) cm, and 68 (IQR 59-84) kg, respectively. There were 29 women (15.9%) who delivered preterm, and 67 (37%) were nulliparous.
Factors affecting birthweight of neonates delivered at term
Neonatal data were analyzed from 3,368 African American and 152 Caucasian women who delivered at term and had available birthweight data. Table 1 shows the results of multi-linear regression of birthweight on gestational age at delivery, maternal weight, height, and parity, and fetal sex as well as pathologic risk factors: extremely low or high BMI, smoking, and diabetes. All of these variables explained 28% of the variance in birthweight at term (R2=0.28).
Table 1. Effect of covariates on birthweight in women with term delivery.
The analysis involved data from 3,368 AA and 152 Caucasian women who delivered at term and had available birthweight data. In the regression model described below (R2=0.28), the intercept (3223g) represents the mean birthweight at 40 weeks (280 days) of gestation (GA) for a nulliparous African American (AA) mother, having a height of 163 cm, weighing 64 kg at first visit, non-smoking, and without diabetes. The 10th/90th centiles of Body Mass Index (BMI) in AA women in the study population were used to define abnormal low and high BMI, respectively. SE: standard error.
| Birthweight (g) | |||
|---|---|---|---|
|
| |||
| Variable | Coefficient | SE | p-value |
| Intercept | 3223 | 16.3 | <0.001 |
|
| |||
| GA from 40 weeks | |||
| Linear | 144 | 10.3 | <0.001 |
| Quadratic | −15 | 6.0 | 0.02 |
| Cubic | 3 | 2.7 | 0.36 |
|
| |||
| Sex | |||
| Male | 150 | 13.3 | <0.001 |
|
| |||
| Race | |||
| Caucasian | 133 | 32.9 | <0.001 |
|
| |||
| Maternal Height (from 163 cm)+ | 78 | 10.6 | <0.001 |
|
| |||
| Maternal Weight (from 64 kg)++ | 25 | 5.1 | <0.001 |
|
| |||
| Parity | |||
| Para 1 | 58 | 16.6 | <0.001 |
| Para 2 | 96 | 19.6 | <0.001 |
| Para 3 | 85 | 20.5 | <0.001 |
|
| |||
| Low BMI (<20.5 kg/m2) | −81 | 25.4 | 0.001 |
|
| |||
| High BMI (>40.4 kg/m2) | −40 | 32.1 | 0.21 |
|
| |||
| Smoking | −92 | 17.2 | <0.001 |
|
| |||
| Diabetes | 247 | 35.1 | <0.001 |
The effect is estimated for 10 cm increments in maternal height;
The effect is estimated for 10 kg increments in maternal weight.
The mean birthweight at 40 weeks (280 days) was 3,223g for a female fetus born to a nulliparous African American mother, having a height of 163 cm, weighing 64 kg at the first visit, non-smoking, and without diabetes (Table 1). Such a combination of maternal weight and height for the reference pregnancy was used to enable direct comparisons to previously reported effects on birthweight in a different U.S. population19. Independent of all other factors listed in Table 1, mean birthweight was higher for male fetuses (by 150g), Caucasian mothers (by 133g), and parous women (58g, 96g, and 85g for parity 1, 2, and ≥3, respectively). An additional 10 cm in maternal height increased birthweight by 78g, and an additional 10 kg of maternal weight were associated with a 25-g increase in birthweight. Such increments in maternal height and weight were chosen to enable comparison to a previous study20.
A low BMI (<10th percentile, 20.5 kg/m2) was associated with an 81-g decrease in mean birthweight, whereas a high BMI (>90th percentile, 40.4 kg/m2) had a negative effect on the mean birthweight that did not reach statistical significance (40g, p=0.21). Smoking was associated with a 92g decrease in mean birthweight, while diabetes was associated with a 247g increase in mean birthweight. Preeclampsia, gestational hypertension, and fetal anomalies were considered as pathologic covariates, but they did not have a significant effect on term birthweight (all p>0.05) and were not included in the regression model (Table 1). Neonates with congenital anomalies had a lower mean birthweight (71-g difference); however, this was not significant, probably due to the low prevalence of congenital anomalies in our cohort (1.8%). Although more prevalent, preeclampsia (4.8%) and gestational hypertension (13.1%) had a smaller magnitude of effect; hence, they were also non-significant in this analysis.
Customized fetal growth standard for the African American population in Detroit
Given the ethnic differences in EFW reported in the NICHD study21 and in birthweight data reported herein, combined with the limited number of Caucasian women in our study population, we decided to focus on developing a customized fetal growth standard for African American women. Non-customized centiles (5th, 10th, 50th, 90th, and 95th) of fetal biometric parameters, EFW, and indices of proportionality for all 4,001 African American women (regardless of clinical outcome) are shown in Figure S2. The centile curves in Figure S2 can be considered a local reference since about 10% of the data points are below/above the 10th/90th centiles and no pathologic factors were excluded. The local reference for EFW was superimposed onto the non-customized NICHD African American and WHO standards (Figure S3). While the 10th, 50th, and 90th EFW centile curves for our local reference were systematically lower than those of the WHO standard, the variability in estimated weight at 40 weeks (distance between the 10th and 90th centiles) were similar. By contrast, the 10th centile of the NICHD standard was lower (especially close to term), the 50th centile was about the same, and the 90th centile was higher than that of our local reference (Figure S3).
To define a customized EFW chart that corresponds to normal growth, we fitted quantile regression models that included maternal height, weight, and parity and fetal sex, while accounting for and excluding the contribution of pathologic factors with significant effect on at least one of the weight centiles: extremely low and high BMI, smoking, diabetes, preterm delivery and fetal anomalies (Table S1). Figure 1 shows the effects of non-pathologic covariates on the predicted normal fetal weight centiles (10th, 50th and 90th). In Figure 1, the EFW standard used as the baseline (continuous lines) corresponds to a female fetus of a nulliparous African American mother, who is 163 cm in height and 64 kg in weight. Since the effects (derived from the quantile regression models described in Table S1) may vary with gestational age for some covariates, these effects are presented at two gestational ages (30 and 40 weeks) in Table 2 and can be summarized as follows:
Figure 1. Effect of covariates on fetal growth in African American women.
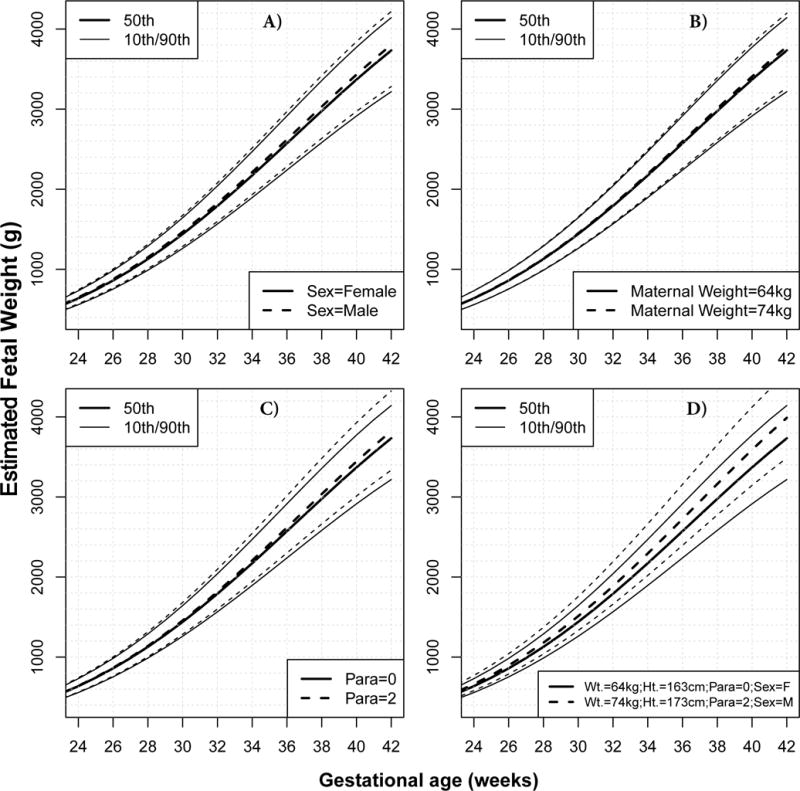
Unless otherwise stated in the legend of each panel, continuous lines represent the estimated fetal weight (EFW) median and the 10th/90th centiles for a female (F) fetus born at term to a nulliparous African American (AA) mother, having a height of 163 cm, weighing 64 kg at first visit. Interrupted lines show how the chart would change for a male fetus (A), for 10 additional kg of maternal weight (B), for a mother in her third pregnancy (Parity=2) (C), and for a combination of factors [10 additional kg in maternal weight (Wt.), 10 additional centimeters in height (Ht.), parity of 2, and a male (M) fetus] (D).
Table 2. Effect of covariates on estimated fetal weight at 30 and 40 weeks of gestation.
The top panel shows the Estimated Fetal Weight (EFW) centiles at 30 weeks (left) and 40 weeks of gestation (right) for a female fetus of a nulliparous African American mother, having a height of 163 cm, weighing 64 kg at first visit, non-smoking, and without diabetes. The middle and bottom panels display the effect of non-pathologic and pathologic covariates, respectively. Effects are expressed as a percentage change in weight. Positive values correspond to an increase while negative values to a decrease in EFW centiles. Values in bold represent significant effects (p<0.05). For e.g. the effect of fetal sex (male vs female) is associated with about 2% increase in EFW, and this effect is the same at 30 weeks and 40 weeks gestation, and affects all centiles similarly. By contrast, maternal height has a stronger positive effect at higher EFW centiles, and this effect does not depend on gestation. The positive effect of 10 additional kg in maternal weight (with normal BMI range) is about twice as large at 40 weeks as is at 30 weeks, for all centiles.
|
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EFW (g) at 30 weeks* | EFW (g) at 40 weeks* | |||||||||
| 5th | 10th | 50th | 90th | 95th | 5th | 10th | 50th | 90th | 95th | |
| 1206 | 1260 | 1440 | 1643 | 1716 | 2777 | 2914 | 3369 | 3773 | 3902 | |
|
|
|
|||||||||
| Effect (% change) | ||||||||||
| Fetal Sex (Male) | 2.3 | 2 | 1.9 | 1.9 | 2.4 | 2.3 | 2 | 1.9 | 1.9 | 2.4 |
| Maternal height (10cm) | 0.9 | 1.0 | 1.2 | 1.8 | 1.9 | 0.9 | 1.0 | 1.2 | 1.8 | 1.9 |
| Maternal weight (10kg) | 0.6 | 0.7 | 0.6 | 0.6 | 0.6 | 1.4 | 1.4 | 1.1 | 1.2 | 1.1 |
| Para 1 | 0.1 | 0.8 | 0.5 | 1.1 | 0 | 1.1 | 2.1 | 0.7 | 2.4 | 0.7 |
| Para 2 | 1.4 | 1.9 | 1.2 | 2.4 | 1.4 | 3.4 | 3.4 | 2.2 | 4.1 | 3.0 |
| Para 3 | 1.0 | 0.9 | 1.5 | 2.3 | 1.8 | 1.1 | 0.2 | 2.4 | 4.0 | 3.7 |
|
|
|
|||||||||
| BMI<20.5 | 1.6 | 0.8 | −1.4 | −2.0 | −2.2 | 1.6 | 0.8 | −1.4 | −2.0 | −2.2 |
| BMI>40.4 | −1.4 | −1.3 | −0.9 | −0.2 | 1.0 | −5.3 | −4.6 | −3.3 | −1.7 | 0.2 |
| Smoking (Yes) | −3.6 | −2.8 | −2.1 | −2.5 | −2.5 | −7.8 | −5.5 | −3.2 | −3.5 | −2.4 |
| Diabetes | 5.4 | 4.6 | 3.4 | 3.7 | 3.1 | 6.5 | 5.6 | 4.2 | 4.6 | 4.5 |
| Preterm Delivery | −12.0 | −9.8 | −2.8 | 0.3 | 1.3 | −14.5 | −11.9 | −3.5 | −0.7 | 1.3 |
| Fetal anomalies | −5.1 | −3.7 | −2 | −0.8 | 0.8 | −5.1 | −3.7 | −2 | −0.8 | 0.8 |
|
|
|
|||||||||
a) Fetal sex
The EFW of male fetuses was about 2% higher than that of female fetuses, independent of all other factors listed in Table 2. This effect was similar among all centiles of the distribution that were evaluated (5th, 10th, 50th, 90th, and 95th). Since no interaction was found between fetal sex and gestational age, customization by fetal sex involves a proportional increase of the entire chart (all centile curves) by about 2% for male fetuses (Figure 1A and Table 2).
b) Maternal height
This covariate had a significant effect on all centiles of EFW, yet the effect was higher for the most extreme centiles. The 95th EFW centile increased by about 2% for each additional 10cm of maternal height while the 5th centile increased by about 1%. The interaction between maternal height and gestational age was not significant; therefore, customization by maternal height involved a proportional shift of the EFW chart for taller mothers, with higher centile curves being shifted more than the lower centiles (Table 2).
c) Maternal weight
For women with a BMI between the 10th and 90th percentiles of the population, the effect of maternal weight on all centiles of EFW at 40 weeks was up to a 1.4% increase for each additional 10kg in maternal weight. However, since the interaction between maternal weight and gestational age was significant for all centiles, the effect of maternal weight increased with gestational age, being about twice as high at 40 weeks as it was at 30 weeks of gestation (Table 2 and Figure 1B).
d) Parity
Fetuses of parous women had a higher EFW than those of nulliparous women, although the magnitude of such an effect varied among centiles and changed with gestational age. For example, compared to nulliparous women, the 90th centile of EFW for women in their third pregnancy (Parity=2) was 4.1% higher at 40 weeks but only 2.4% higher at 30 weeks of gestation (Figure 1C and Table 2).
Figure 1D illustrates the combined effect of change in multiple covariates on the normal growth chart of African American women. For example, at 40 weeks of gestation, the 90th centile of EFW for a male fetus of a mother in her third pregnancy (Para=2), who is 173 cm tall and weighs 74 kg, is 9% higher (4,122g) than for a female fetus of a nulliparous mother who is 10 cm shorter and weighs 10 kg less (3,773g).
The effects of pathologic factors on EFW were higher than those of non-pathologic variables, and such effects also varied across gestation and among the centiles (Table 2). The effect of maternal complications that led to a preterm delivery was associated with a 12% reduction in the 5th centile of EFW at 30 weeks, and with a 5.3% and a 7.8% reduction at 40 weeks for women with a high BMI and those who smoked, respectively.
The equations describing the PRB/NICHD customized chart are provided in Table S1 along with an example of the calculation of centiles. In addition, we provide a user-friendly spreadsheet calculator, available from the authors’ website (http://bioinformaticsprb.med.wayne.edu/). This tool allows: 1) interactive exploration of the effect of covariates on the growth chart; 2) obtaining the customized centile corresponding to an observed EFW value (determined from AC, HC, and FL measurements) for a given gestational age; and 3) printing of the entire customized chart for a given pregnancy.
Comparison of fetal growth standards for classifying fetuses as small or large for gestational age
Our next objective was to determine how different fetal growth standards affect the classification of pregnancies as being at risk for either an SGA or an LGA fetus. Therefore, we applied four different growth standards, including the PRB/NICHD standard developed herein, to classify fetuses of 4,001 African American women based on the observed EFW at the last available ultrasound examination. The median gestational age at the last examination was 36.0 (IQR 33-38) weeks. We determined the overall proportions of fetuses that screened positive for SGA or LGA, but also separately for women with a term or a preterm delivery (Table 3).
Table 3. Percentage of unselected pregnancies predicted at risk of a small- (SGA<10th) or large- (LGA>90th) for-gestational age neonate by different standards.
The standards compared are the NICHD African American (AA) standard, the WHO standard with or without customization by fetal sex, the customized GROW standard, and the PRB/NICHD customized standard for AA women. The SGA and LGA analysis is based on the last available scan of each pregnancy, median GA at last scan 36.0 (Inter Quartile Range 33-38 weeks). The proportion (95% confidence intervals) of fetuses classified as SGA and LGA were determined for pregnancies delivered preterm (<37 weeks), at term, and overall.
| % of fetuses classified as SGA<10th | % of fetuses classified as LGA>90th | |||||
|---|---|---|---|---|---|---|
| Preterm | Term | All | Preterm | Term | All | |
| NICHD AA | 17.6(14.7–20.8) | 5.3(4.6–6.1) | 7.2(6.4–8.1) | 13(10.5–15.9) | 12.2(11.2–13.4) | 12.3(11.4–13.4) |
| WHO | 24.2(21–27.8) | 10(9–11) | 12.2(11.2–13.3) | 10.6(8.4–13.3) | 10(9–11.1) | 10.1(9.2–11.1) |
| WHO by sex | 24.5(21.3–28.1) | 10.8(9.8–11.9) | 13(12–14.1) | 11.9(9.5–14.7) | 10.6(9.6–11.7) | 10.8(9.8–11.8) |
| GROW | 26.3(22.9–29.9) | 9.7(8.7–10.7) | 12.3(11.3–13.4) | 7.8(5.8–10.2) | 8.9(7.9–9.9) | 8.7(7.9–9.6) |
| PRB/NICHD AA | 29(25.5–32.7) | 11.6(10.6–12.8) | 14.4(13.3–15.5) | 8.9(6.8–11.4) | 9.2(8.3–10.3) | 9.2(8.3–10.1) |
The percentage of fetuses classified as SGA (<10th centile) was as follows: 1) NICHD African American standard, 7.2%; 2) GROW standard, 12.3%; 3) WHO standard, 12.2% (13% if customized by fetal sex); and 4) PRB/NICHD standard, 14.4%. All fetal growth standards except the NICHD African American standard classified more SGA fetuses than the expected 10% cut-off.
The proportion of fetuses classified as SGA was 2- to 3-fold higher among women who delivered preterm compared to those who delivered at term, depending upon the standard used. The rate of SGA among fetuses delivered preterm was as follows: NICHD African American standard, 17.6%; WHO standard, 24.2% (24.5% if customized by fetal sex); GROW standard, 26.3%; and PRB/NICHD standard, 29%.
To illustrate the similarity among the four different standards, we constructed a Venn diagram to represent the number of fetuses classified as SGA by each combination of standards (Figure 2A). All fetuses identified as SGA by the NICHD African American standard were also identified by at least two other standards. Of note, the WHO standard classified 71 fetuses as SGA that were not identified as such by any other standard. The highest agreement among standards, as assessed by Cohen’s kappa coefficient, occurred between the PRB/NICHD and GROW standards (kappa= 0.84), followed by the PRB/NICHD standard and the WHO standard customized by fetal sex (kappa=0.79). On the other hand, the lowest agreement, although still substantial51, was between the NICHD African American and PRB/NICHD standards (kappa = 0.63).
Figure 2. Agreement among standards for small- and large for gestational age screening.
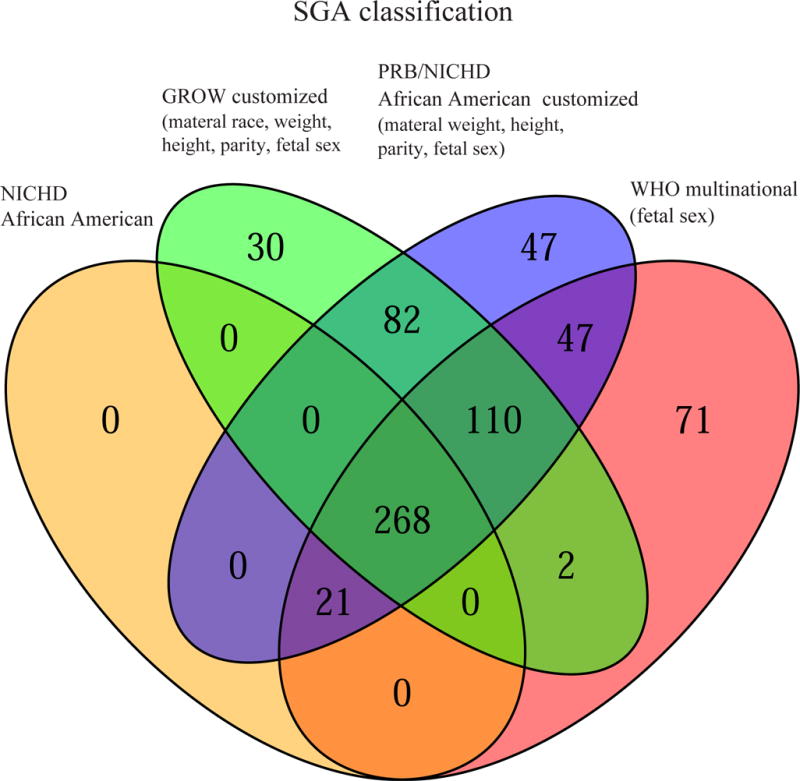
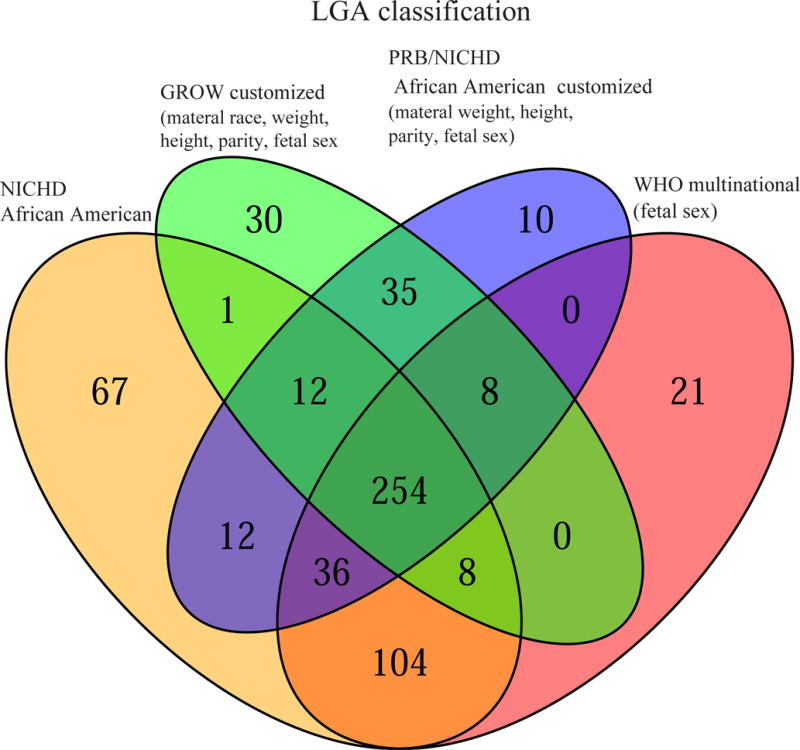
Fetuses of African American women were classified as small (SGA<10th) (A) or large (LGA>90th) (B) for gestational age based on the last available scan before delivery using four standards: NICHD African American (AA) standard, the WHO standard customized by fetal sex, the customized GROW standard, and the PRB/NICHD AA customized standard. For SGA classification, the highest agreement among standards, as assessed by Cohen’s kappa coefficient, was among the PRB/NICHD AA and GROW standards (k= 0.84), followed by PRB/NICHD AA and WHO customized by sex (k=0.79), while the least agreement was between NICHD AA and PRB/NICHD AA (k = 0.63). For LGA classification, the highest agreement among standards was between Detroit African American and GROW standards and between WHO standard customized by sex and NICHD African American standards (both pairs k=0.85).
The percentage of fetuses classified as LGA was: 1) GROW, 8.7%; 2) PRB/NICHD customized, 9.2%; 3) WHO, 10.1% (10.8% if customized by fetal sex); and 4) NICHD African American standards, 12.3%. Of note, the LGA rates for the GROW and NICHD African American standards were significantly lower or higher than the expected 10% cut-off, respectively (Table 3).
Unlike the rate of SGA, the rate of LGA was similar between fetuses delivered preterm or at term, for all fetal growth standards (Table 3).
The agreement among the different standards for LGA classification can be visualized in the Venn diagram in Figure 2B. The PRB/NICHD and GROW standards were in high agreement (kappa=0.85), and the same was true for the WHO standard customized by fetal sex and NICHD African American standards (kappa=0.85). Even the least similar pair of standards (NICHD African American and GROW) was still in substantial agreement for the LGA classification (kappa=0.61).
Comment
The principal findings of the study are as follows. First, the birthweight of a term neonate is affected by maternal ethnicity, weight, height, and parity and fetal sex; 2) longitudinal fetal weight analysis revealed the following features of fetal growth: i) all weight centiles were about 2% higher for male than for female fetuses; ii) maternal height had a positive effect on fetal weight, with larger fetuses being affected more (2% increase in the 95th centile of weight for each 10 cm increase in height); and iii) maternal weight and parity had positive effects on fetal weight that increased with gestation and varied among the weight centiles; 3) the rate of SGA was 7.2% for the NICHD African American standard, 12.3% for the GROW standard, 13% for the WHO standard customized by fetal sex, and 14.4% for the PRB/NICHD customized standard herein. For all standards, the proportion of SGA was at least two-fold higher among fetuses delivered preterm than at term; 4) the rate of LGA was 8.7% for the GROW standard, 9.2% for the PRB/NICHD customized standard, 10.8% for the WHO standard customized by fetal sex, and 12.3% for the NICHD African American standard; and 5) the highest agreement among any two standards was between the GROW and PRB/NICHD standards for both SGA and LGA classifications (Cohen’s inter-rater agreement kappa=0.85).
Factors affecting birthweight in term neonates
We found that the mean birthweight of a female neonate born at 40 weeks to a reference African American mother (nulliparous, 163 cm tall, and weighing 64 kg) was 3223 g, which is similar to the 3226 g reported by Gardosi and Francis19 in a U.S. population. The effects of several non-pathologic and pathologic factors on birthweight were also similar between these two studies, such as 150g versus 132g for fetal sex, 133g versus 161g difference between Caucasian and African American women, and 247g versus 241g for diabetes. Although consistent in terms of significance and direction of effect, the magnitude of the effects of other covariates was somewhat lower in this study compared to those reported by Gardosi and Francis.19 The negative effect of a high BMI (>90th centile) on birthweight in the current study was similar to the one reported by Gardosi and Francis19 (40 g versus 63.4 g), but it did not reach statistical significance. One reason for differences in the magnitude of effects for some covariates is that the U.S. population in the study by Gardosi and Francis19 was comprised mostly of women of European origin, while this study was comprised of mostly African American women.
Ethnic differences in fetal biometric parameters were also recently assessed by other investigators for women with a low-risk pregnancy21. The difference in mean birthweight between African American and Caucasian women at term in the study herein was about one-half (133g) compared to that reported in the NICHD study (246g)21. Possible explanations for this discrepancy are differences in population characteristics and the covariates accounted for in each analysis.
One-size-fits-all versus customized fetal growth standards
There is controversy as to whether a population-based or a customized chart should be used to screen fetuses as being at risk for SGA or LGA. SGA fetuses are at increased risk for fetal death and adverse neonatal outcomes (eg cesarean delivery for non-reassuring fetal heart rate status, neonatal death, and admission to a neonatal intensive care unit)35, 52-55. Although other customization methods exist, such as the Individualized Growth Assessment26-28, the GROW approach of Gardosi et al.18 is the most widely adopted customized standard and has been applied to several populations, including a mostly Caucasian population in the U.S.19, 24, 25. The same authors reported that customization of fetal growth improved the detection of small fetuses that were at risk for fetal death and adverse neonatal outcomes, such as neonatal death and a low five-minute Apgar score56. However, previous comparisons between customized and population-based growth charts for the detection of fetuses at risk for adverse outcome yielded conflicting results10, 53, 54, 57-70. A recent meta-analysis71 reported that the odds ratios of the association between adverse pregnancy outcomes (e.g. perinatal mortality and neonatal intensive care unit admission) and abnormal birthweight were higher for the customized GROW standard compared to the non-customized standards, although the difference was not statistically significant. Reaching a consensus regarding which type of fetal growth standards should be implemented in clinical care remains an important question, as it has a direct effect on patient management and care.
Development of a customized fetal growth standard for the African American population
Previous fetal growth standards were derived from fetal biometric data by excluding patients who developed complications during the current pregnancy21 and/or those with certain risk factors, such as an abnormal BMI, smoking, and adverse perinatal outcomes in previous pregnancies20, 21.
Our approach was to adjust for the presence of pathology in the current pregnancy while assessing the effects of non-pathologic factors on fetal growth. The effects of pathologic variables included in the quantile regression models do not contribute to defining the normal fetal weight chart (eg the chart will not be lowered because of a risk factor, such as smoking), but the additional data from patients with pathologic factors increased the power to dissect the effect of non-pathologic covariates on fetal growth and helped to better calibrate the model so as to distinguish normal from abnormal growth.
Of interest, all variables that had a significant effect on birthweight of neonates delivered at term (Table 1) had also a significant effect on EFW in the longitudinal analysis (Table S1 and Table 2). This is important because it increases the confidence that these variables are indeed needed to define the fetal growth potential, since birthweight data is more reliable than EFW data. In addition, although a high BMI (>90th centile) was not associated with a significant decrease in term birthweight (Table 2), it had a negative effect on the lower centiles of EFW. The 5th and 10th centiles of weight at 40 weeks were about 4.6% lower for women with a BMI >40.4; hence, this group of women are at higher risk of delivering an SGA neonate contrary to other observations72. Similarly, although the negative effect of fetal anomalies on birthweight of neonates delivered at term was not significant, fetal anomalies were associated with up to a 5% reduction in the median, 10th centile, and 5th centile of EFW (Table 2).
While our approach is conceptually similar to Gardosi et al.18, 19, the customization parameters in our study were based directly on EFW data rather than on birthweight. Moreover, instead of assuming that each covariate has a proportionally constant effect on EFW at each gestational age, we have tested for the first time and found significant interactions between parity as well as maternal weight and gestational age (Figure 1 and Table 2). Testing for these interactions would not have been feasible using cross-sectional birthweight data. Additionally, similar to the study by WHO20, we used quantile regression to determine the effect of covariates on each centile of the distribution, rather than assessing the effect on mean fetal weight and assuming a normal distribution of weight around the mean value at each gestational age. Growth chart customization by differentially adjusting the centile curves according to the specific contribution and timing of each factor is novel. Such differences in both study design and analytical approach are reflected in our new customized fetal growth standard and impact the number of fetuses that will screen positive for SGA or LGA as well as who those fetuses are.
SGA and LGA screening rates using different fetal growth standards
The newly developed PRB/NICHD customized growth standard was compared to three existing standards: GROW19, WHO with and without adjustment by fetal sex20, and NICHD African American21. A comparison to the INTERGROWTH-21st standard16 was not performed due to differences in the ultrasound protocols that were previously noted73 (eg the biparietal diameter was measured from the outer to the outer, while we measured from the outer to the inner, borders of the parietal bones) and also due to the different EFW formula used in the INTERGROWTH-21st standard. Among the four standards compared in this study, there were significant differences in the fraction of fetuses classified as SGA (<10th centile) based on the last available ultrasound examination for each pregnancy. The proportion of fetuses that screened positive for SGA in a certain population is determined, in part, by the burden of pregnancy complications present in the population that are related to growth restriction. Indeed, since 15.8% of the African American women in the current study population delivered preterm, it is not surprising that most standards classified significantly more fetuses as SGA (<10th centile) than the expected 10%. For fetuses delivered preterm, the SGA screen positive rate was significantly higher than 10% for all standards, with the GROW and PRB/NICHD customized standards classifying 26.3% and 29% of the preterm population as being at risk, respectively. This finding is consistent with previous reports showing that SGA fetuses are at an increased risk of a preterm delivery74, 75. The rate of fetuses classified as SGA (<10th centile) in women who delivered at term was close to 10% for the WHO and GROW standards, and significantly higher than 10% for the PRB/NICHD (11.6%) and lower for the NICHD African American (5.3%) standards. The fact that the 10th centile of the NICHD African American standard is low for our population, and hence it screens positive for fewer SGA fetuses than expected (7.2%), can also be understood from Figure S3, where the 10th centile curve of the NICHD standard is lower than the corresponding centile of the local reference, especially after 37 weeks of gestation. However, the customized PRB/NICHD standard is built excluding the contribution of fetuses with risk factors associated with lower weight (e.g. preterm delivery), thus the 10th centile of the PRB/NICHD standard is higher than the 10th centile of the local reference shown in Figure S3, therefore classifying 14.4% of fetuses as SGA.
Only the NICHD African American standard classified significantly more fetuses as LGA (12.3%) than the reference cut-off of 10%, which, combined with a lower than expected rate of SGA, suggests that this standard is low for our patient population. The GROW standard identified significantly less than expected (8.7%), while the PRB/NICHD standard identified 9.2% of fetuses as LGA. Since this was an unselected population, it is reasonable to assume that not all fetuses reached their growth potential; hence, standards classifying slightly less fetuses as LGA are actually tracking the growth potential of fetuses rather than being miscalibrated.
In addition to comparing the SGA and LGA screening rates among the four growth standards, we provided complementary information regarding the agreement among the standards in terms of which fetuses are at risk. Using Venn diagrams (Figure 2) and inter-rater agreement statistics for all pairs of standards, we found that the two fully customized standards (GROW and PRB/NICHD) were the most similar, reaching an inter-rater agreement kappa of about 0.85 for both SGA and LGA classifications. Considering the multiple differences in the design of the four standards compared herein, such as the population on which they were based (homogenous versus multi-ethnic), the type of data they were derived from (birthweight versus fetal weight), the analytical assumptions they relied on, and the factors these standards were customized for (ethnicity or fetal sex only versus fully customized), this study suggests that customization by the same set of covariates is key for the reproducibility of growth assessment.
Research and Clinical Implications
This study confirms previous observations that maternal ethnicity, height, weight, and parity and fetal sex are factors affecting birthweight and/or fetal growth76-78; hence, they should be considered when defining fetal growth potential72, 79. Customization of growth charts is commonly performed by assuming a proportionally constant effect of covariates during gestation, and we found that, indeed, this assumption holds for genetically determined (fetal sex) or transmissible traits (height). However, the effects of maternal weight and parity are proportionally graded with gestational age. Additionally, the effects of maternal height and parity in African American women were graded among the different centiles of EFW. The customization approach proposed herein can be applied to other populations as well, provided that ultrasound data and relevant covariate information are available. An easy-to-use implementation of the PRB/NICHD customized growth chart for African American women is freely available from the authors’ website (http://bioinformaticsprb.med.wayne.edu/).
The higher similarity of the two fully customized standards compared to the similarity between the two partially customized standards suggests that the use of customized charts is more likely to lead to reproducible growth assessment across studies.
Depending on the standard used, the rate of fetuses that screened positive for SGA can vary by a factor of two in a given population. The use of fully customized standards in high-risk populations may identify more fetuses as being at risk for growth restriction. However, comparing how the in utero SGA and LGA screenings based on different standards relate to an SGA or LGA diagnosis at birth and to adverse pregnancy outcomes was outside the scope of the current study. Of note, the ability of ultrasound-based estimated fetal weight to predict actual birthweight was described previously14, 29, 73. For example, in a blinded study conducted in a low-risk population, Sovio et al.29 reported that an EFW<10th centile at 36 weeks of gestation correctly identified 57% of fetuses (sensitivity) that were destined to have a birthweight <10th centile, with a specificity of 95%. In their study, a non-customized EFW standard was used for screening while the gold standard for SGA was based on a fetal sex-customized birthweight reference29.
Strengths and Limitations
We conducted the largest longitudinal fetal growth study in an African American population to date. Additional strengths of our study are that all patients were enrolled at a single ultrasound unit and that a consistent protocol was implemented to acquire ultrasound data. Moreover, the large sample size combined with advanced analytical approaches allowed the development of customized fetal growth centiles for an African American population under less-restrictive analytical assumptions than before. Although a possible limitation is that the ultrasound examinations studied herein were not scheduled at fixed gestational-age time points (as was the case for other fetal growth studies), the average number of scans (five) still compares favorably to previous reports21.
Conclusion
We report herein the largest longitudinal fetal growth study of pregnant women self-reported as African American. We found that the effects of maternal weight and parity on estimated fetal weight increase with gestational age and that maternal height and parity affect small or large fetuses differently. The PRB/NICHD African American customized growth chart was designed to account for these features of fetal growth. This standard classified more fetuses as being SGA (14.4%) than other standards, especially among fetuses delivered preterm. Moreover, this standard classified as LGA about the same fraction of fetuses as expected (10%). The comparison among the four growth standards considered herein revealed that the most important factor determining the agreement among standards is whether they account for the same factors known to affect fetal growth.
Supplementary Material
Acknowledgments
Funding: This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Footnotes
Disclosure Statement: The authors report no conflicts of interest.
Condensation: We report a customized fetal growth standard for African American womenthat considers maternal height, weight, parity, and fetal sex. This standard takes into account novel features of fetal growth discovered herein such as timing of the effect of covariates and the differential effect on the different centiles of EFW.
References
- 1.Jeanty P, Cantraine F, Romero R, Cousaert E, Hobbins JC. A longitudinal study of fetal weight growth. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 1984;3(7):321–8. doi: 10.7863/jum.1984.3.7.321. [DOI] [PubMed] [Google Scholar]
- 2.Lampl M, Jeanty P. Timing is everything: a reconsideration of fetal growth velocity patterns identifies the importance of individual and sex differences. American journal of human biology : the official journal of the Human Biology Council. 2003;15(5):667–80. doi: 10.1002/ajhb.10204. [DOI] [PubMed] [Google Scholar]
- 3.Tanner JM. Fetus into man. 1st. Cambridge, MA: Harvard University Press; 1978. [Google Scholar]
- 4.Bornstein MH, Arterberry ME, Lamb ME. Development in infancy: a contemporary introduction. 5th. New York: Psychology Press; 2014. [Google Scholar]
- 5.Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. The Journal of pediatrics. 1967;71(2):159–63. doi: 10.1016/s0022-3476(67)80066-0. [DOI] [PubMed] [Google Scholar]
- 6.Gaccioli F, Aye I, Sovio U, Charnock-Jones DS, Smith GCS. Screening for fetal growth restriction using fetal biometry combined with maternal biomarkers. American journal of obstetrics and gynecology. 2017 doi: 10.1016/j.ajog.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Kalafat E, Morales-Rosello J, Thilaganathan B, Tahera F, Khalil A. Risk of operative delivery for intrapartum fetal compromise in small-for-gestational-age fetuses at term: an internally validated prediction model. American journal of obstetrics and gynecology. 2018;218(1):134.e1–e8. doi: 10.1016/j.ajog.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 8.McEwen EC, Guthridge SL, He VY, McKenzie JW, Boulton TJ, Smith R. What birthweight percentile is associated with optimal perinatal mortality and childhood education outcomes? American journal of obstetrics and gynecology. 2017 doi: 10.1016/j.ajog.2017.11.574. [DOI] [PubMed] [Google Scholar]
- 9.Mendez-Figueroa H, Truong VT, Pedroza C, Khan AM, Chauhan SP. Small-for-gestational-age infants among uncomplicated pregnancies at term: a secondary analysis of 9 Maternal-Fetal Medicine Units Network studies. American journal of obstetrics and gynecology. 2016;215(5):628.e1–e7. doi: 10.1016/j.ajog.2016.06.043. [DOI] [PubMed] [Google Scholar]
- 10.Costantine MM, Mele L, Landon MB, et al. Customized versus population approach for evaluation of fetal overgrowth. American journal of perinatology. 2013;30(7):565–72. doi: 10.1055/s-0032-1329188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson NH, Sadler LC, McKinlay CJ, McCowan LM. INTERGROWTH-21st vs customized birthweight standards for identification of perinatal mortality and morbidity. American journal of obstetrics and gynecology. 2016;214(4):509.e1–7. doi: 10.1016/j.ajog.2015.10.931. [DOI] [PubMed] [Google Scholar]
- 12.Chauhan SP, Beydoun H, Chang E, et al. Prenatal detection of fetal growth restriction in newborns classified as small for gestational age: correlates and risk of neonatal morbidity. American journal of perinatology. 2014;31(3):187–94. doi: 10.1055/s-0033-1343771. [DOI] [PubMed] [Google Scholar]
- 13.Hadlock FP, Deter RL, Harrist RB, Park SK. Estimating fetal age: computer-assisted analysis of multiple fetal growth parameters. Radiology. 1984;152(2):497–501. doi: 10.1148/radiology.152.2.6739822. [DOI] [PubMed] [Google Scholar]
- 14.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements–a prospective study. American journal of obstetrics and gynecology. 1985;151(3):333–7. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 15.Villar J, Knight HE, de Onis M, et al. Conceptual issues related to the construction of prescriptive standards for the evaluation of postnatal growth of preterm infants. Archives of disease in childhood. 2010;95(12):1034–8. doi: 10.1136/adc.2009.175067. [DOI] [PubMed] [Google Scholar]
- 16.Papageorghiou AT, Ohuma EO, Altman DG, et al. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):869–79. doi: 10.1016/S0140-6736(14)61490-2. [DOI] [PubMed] [Google Scholar]
- 17.Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. Customised antenatal growth charts. Lancet. 1992;339(8788):283–7. doi: 10.1016/0140-6736(92)91342-6. [DOI] [PubMed] [Google Scholar]
- 18.Gardosi J, Mongelli M, Wilcox M, Chang A. An adjustable fetal weight standard. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 1995;6(3):168–74. doi: 10.1046/j.1469-0705.1995.06030168.x. [DOI] [PubMed] [Google Scholar]
- 19.Gardosi J, Francis A. A customized standard to assess fetal growth in a US population. American journal of obstetrics and gynecology. 2009;201(1):25 e1–7. doi: 10.1016/j.ajog.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 20.Kiserud T, Piaggio G, Carroli G, et al. The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight. PLoS medicine. 2017;14(1):e1002220. doi: 10.1371/journal.pmed.1002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buck Louis GM, Grewal J, Albert PS, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. American journal of obstetrics and gynecology. 2015;213(4):449 e1–e41. doi: 10.1016/j.ajog.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181(1):129–33. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 23.Vrachnis N, Botsis D, Iliodromiti Z. The fetus that is small for gestational age. Annals of the New York Academy of Sciences. 2006;1092:304–9. doi: 10.1196/annals.1365.028. [DOI] [PubMed] [Google Scholar]
- 24.Figueras F, Meler E, Iraola A, et al. Customized birthweight standards for a Spanish population. European journal of obstetrics, gynecology, and reproductive biology. 2008;136(1):20–4. doi: 10.1016/j.ejogrb.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Unterscheider J, Geary MP, Daly S, et al. The customized fetal growth potential: a standard for Ireland. European journal of obstetrics, gynecology, and reproductive biology. 2013;166(1):14–7. doi: 10.1016/j.ejogrb.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Deter RL. Individualized growth assessment: evaluation of growth using each fetus as its own control. Seminars in perinatology. 2004;28(1):23–32. doi: 10.1053/j.semperi.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Deter RL, Lee W, Sangi-Haghpeykar H, Tarca AL, Yeo L, Romero R. Individualized fetal growth assessment: critical evaluation of key concepts in the specification of third trimester size trajectories. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2013 doi: 10.3109/14767058.2013.833904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker ED, McAuliffe FM, Alderdice F, et al. The role of growth trajectories in classifying fetal growth restriction. Obstetrics and gynecology. 2013;122(2 Pt 1):248–54. doi: 10.1097/AOG.0b013e31829ca9a7. [DOI] [PubMed] [Google Scholar]
- 29.Sovio U, White IR, Dacey A, Pasupathy D, Smith GCS. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet (London, England) 2015;386(10008):2089–97. doi: 10.1016/S0140-6736(15)00131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero R, Deter R. Should serial fetal biometry be used in all pregnancies? Lancet. 2015;386(10008):2038–40. doi: 10.1016/S0140-6736(15)00148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCowan LM, Harding JE, Stewart AW. Customized birthweight centiles predict SGA pregnancies with perinatal morbidity. BJOG : an international journal of obstetrics and gynaecology. 2005;112(8):1026–33. doi: 10.1111/j.1471-0528.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- 32.Johnsen SL, Rasmussen S, Wilsgaard T, Sollien R, Kiserud T. Longitudinal reference ranges for estimated fetal weight. Acta obstetricia et gynecologica Scandinavica. 2006;85(3):286–97. doi: 10.1080/00016340600569133. [DOI] [PubMed] [Google Scholar]
- 33.Odibo AO, Francis A, Cahill AG, Macones GA, Crane JP, Gardosi J. Association between pregnancy complications and small-for-gestational-age birth weight defined by customized fetal growth standard versus a population-based standard. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2011;24(3):411–7. doi: 10.3109/14767058.2010.506566. [DOI] [PubMed] [Google Scholar]
- 34.Cha HH, Kim JY, Choi SJ, Oh SY, Roh CR, Kim JH. Can a customized standard for large for gestational age identify women at risk of operative delivery and shoulder dystocia? Journal of perinatal medicine. 2012;40(5):483–8. doi: 10.1515/jpm-2011-0306. [DOI] [PubMed] [Google Scholar]
- 35.Kase BA, Carreno CA, Blackwell SC. Customized estimated fetal weight: a novel antenatal tool to diagnose abnormal fetal growth. American journal of obstetrics and gynecology. 2012;207(3):218 e1–5. doi: 10.1016/j.ajog.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Sovio U, Smith GCS. The effect of customization and use of a fetal growth standard on the association between birthweight percentile and adverse perinatal outcome. American journal of obstetrics and gynecology. 2017 doi: 10.1016/j.ajog.2017.11.563. [DOI] [PubMed] [Google Scholar]
- 37.Simcox LE, Myers JE, Cole TJ, Johnstone ED. Fractional fetal thigh volume in the prediction of normal and abnormal fetal growth during the third trimester of pregnancy. American journal of obstetrics and gynecology. 2017;217(4):453 e1–e12. doi: 10.1016/j.ajog.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altman DG, Ohuma EO, International F, Newborn Growth Consortium for the 21st C Statistical considerations for the development of prescriptive fetal and newborn growth standards in the INTERGROWTH-21st Project. BJOG : an international journal of obstetrics and gynaecology. 2013;120(Suppl 2):71–6, v. doi: 10.1111/1471-0528.12031. [DOI] [PubMed] [Google Scholar]
- 39.Cheikh Ismail L, Knight HE, Ohuma EO, Hoch L, Chumlea WC. Anthropometric standardisation and quality control protocols for the construction of new, international, fetal and newborn growth standards: the INTERGROWTH-21st Project. BJOG : an international journal of obstetrics and gynaecology. 2013;120(Suppl 2):48–55, v. doi: 10.1111/1471-0528.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villar J, Altman DG, Purwar M, et al. The objectives, design and implementation of the INTERGROWTH-21st Project. BJOG : an international journal of obstetrics and gynaecology. 2013;120(Suppl 2):9–26, v. doi: 10.1111/1471-0528.12047. [DOI] [PubMed] [Google Scholar]
- 41.Merialdi M, Widmer M, Gulmezoglu AM, et al. WHO multicentre study for the development of growth standards from fetal life to childhood: the fetal component. BMC pregnancy and childbirth. 2014;14:157. doi: 10.1186/1471-2393-14-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chitty LS, Altman DG, Henderson A, Campbell S. Charts of fetal size: 2. Head measurements. British journal of obstetrics and gynaecology. 1994;101(1):35–43. doi: 10.1111/j.1471-0528.1994.tb13007.x. [DOI] [PubMed] [Google Scholar]
- 43.Chitty LS, Altman DG, Henderson A, Campbell S. Charts of fetal size: 3. Abdominal measurements. British journal of obstetrics and gynaecology. 1994;101(2):125–31. doi: 10.1111/j.1471-0528.1994.tb13077.x. [DOI] [PubMed] [Google Scholar]
- 44.Chitty LS, Altman DG, Henderson A, Campbell S. Charts of fetal size: 4. Femur length. British journal of obstetrics and gynaecology. 1994;101(2):132–5. doi: 10.1111/j.1471-0528.1994.tb13078.x. [DOI] [PubMed] [Google Scholar]
- 45.Altman DG, Chitty LS. Design and analysis of studies to derive charts of fetal size. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 1993;3(6):378–84. doi: 10.1046/j.1469-0705.1993.03060378.x. [DOI] [PubMed] [Google Scholar]
- 46.Salomon LJ, Alfirevic Z, Berghella V, et al. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2011;37(1):116–26. doi: 10.1002/uog.8831. [DOI] [PubMed] [Google Scholar]
- 47.AIUM practice guideline for the performance of obstetric ultrasound examinations. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2013;32(6):1083–101. doi: 10.7863/ultra.32.6.1083. [DOI] [PubMed] [Google Scholar]
- 48.Koenker R. Quantile regression for longitudinal data. Journal of Multivariate Analysis. 2004;91(1):74–89. [Google Scholar]
- 49.Koenker RW. Quantile Regression. New York: Cambridge University Press; 2005. [Google Scholar]
- 50.Lee ER, N H, Park BU. Model selection via Bayesian information criterion for quantile regression models. Journal of the American Statistical Association. 2014;109:216–29. [Google Scholar]
- 51.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 52.Serena C, Marchetti G, Rambaldi MP, et al. Stillbirth and fetal growth restriction. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2013;26(1):16–20. doi: 10.3109/14767058.2012.718389. [DOI] [PubMed] [Google Scholar]
- 53.Smith NA, Bukowski R, Thomas AM, Cantonwine D, Zera C, Robinson JN. Identification of pathologically small fetuses using customized, ultrasound and population-based growth norms. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2014;44(5):595–9. doi: 10.1002/uog.13333. [DOI] [PubMed] [Google Scholar]
- 54.Agarwal P, Rajadurai VS, Yap F, et al. Comparison of customized and cohort-based birthweight standards in identification of growth-restricted infants in GUSTO cohort study. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2016;29(15):2519–22. doi: 10.3109/14767058.2015.1092956. [DOI] [PubMed] [Google Scholar]
- 55.Moon M, Baek MJ, Ahn E, Odibo AO. Association between small for gestational age and intrauterine fetal death: comparing a customized South Korean growth standard versus a population-based fetal growth chart. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2016;29(6):872–4. doi: 10.3109/14767058.2015.1027189. [DOI] [PubMed] [Google Scholar]
- 56.Clausson B, Gardosi J, Francis A, Cnattingius S. Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. BJOG : an international journal of obstetrics and gynaecology. 2001;108(8):830–4. doi: 10.1111/j.1471-0528.2001.00205.x. [DOI] [PubMed] [Google Scholar]
- 57.De Jong CL, Francis A, Van Geijn HP, Gardosi J. Customized fetal weight limits for antenatal detection of fetal growth restriction. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2000;15(1):36–40. doi: 10.1046/j.1469-0705.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 58.Iraola A, Gonzalez I, Eixarch E, et al. Prediction of adverse perinatal outcome at term in small-for-gestational age fetuses: comparison of growth velocity vs. customized assessment. Journal of perinatal medicine. 2008;36(6):531–5. doi: 10.1515/JPM.2008.100. [DOI] [PubMed] [Google Scholar]
- 59.Larkin JC, Hill LM, Speer PD, Simhan HN. Risk of morbid perinatal outcomes in small-for-gestational-age pregnancies: customized compared with conventional standards of fetal growth. Obstetrics and gynecology. 2012;119(1):21–7. doi: 10.1097/AOG.0b013e31823dc56e. [DOI] [PubMed] [Google Scholar]
- 60.Landres IV, Clark A, Chasen ST. Improving antenatal prediction of small-for-gestational-age neonates by using customized versus population-based reference standards. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2013;32(9):1581–6. doi: 10.7863/ultra.32.9.1581. [DOI] [PubMed] [Google Scholar]
- 61.Costantine MM, Lai Y, Bloom SL, et al. Population versus customized fetal growth norms and adverse outcomes in an intrapartum cohort. American journal of perinatology. 2013;30(4):335–41. doi: 10.1055/s-0032-1324708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaillard R, Jaddoe VW. Assessment of fetal growth by customized growth charts. Annals of nutrition & metabolism. 2014;65(2-3):149–55. doi: 10.1159/000361055. [DOI] [PubMed] [Google Scholar]
- 63.Sjaarda LA, Albert PS, Mumford SL, Hinkle SN, Mendola P, Laughon SK. Customized large-for-gestational-age birthweight at term and the association with adverse perinatal outcomes. American journal of obstetrics and gynecology. 2014;210(1):63 e1–e11. doi: 10.1016/j.ajog.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melamed N, Ray JG, Shah PS, Berger H, Kingdom JC. Should we use customized fetal growth percentiles in urban Canada? Journal of obstetrics and gynaecology Canada : JOGC = Journal d’obstetrique et gynecologie du Canada : JOGC. 2014;36(2):164–70. doi: 10.1016/S1701-2163(15)30663-0. [DOI] [PubMed] [Google Scholar]
- 65.Carberry AE, Gordon A, Bond DM, Hyett J, Raynes-Greenow CH, Jeffery HE. Customised versus population-based growth charts as a screening tool for detecting small for gestational age infants in low-risk pregnant women. The Cochrane database of systematic reviews. 2014;(5):CD008549. doi: 10.1002/14651858.CD008549.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moussa HN, Wu ZH, Han Y, et al. Customized versus Population Fetal Growth Norms and Adverse Outcomes Associated with Small for Gestational Age Infants in a High-Risk Cohort. American journal of perinatology. 2015;32(7):621–6. doi: 10.1055/s-0034-1390343. [DOI] [PubMed] [Google Scholar]
- 67.White SW, Marsh JA, Lye SJ, Briollais L, Newnham JP, Pennell CE. Improving customized fetal biometry by longitudinal modelling. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2016;29(12):1888–94. doi: 10.3109/14767058.2015.1070139. [DOI] [PubMed] [Google Scholar]
- 68.Ghi T, Cariello L, Rizzo L, et al. Customized Fetal Growth Charts for Parents’ Characteristics, Race, and Parity by Quantile Regression Analysis: A Cross-sectional Multicenter Italian Study. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2016;35(1):83–92. doi: 10.7863/ultra.15.03003. [DOI] [PubMed] [Google Scholar]
- 69.Iliodromiti S, Mackay DF, Smith GC, et al. Customised and Noncustomised Birth Weight Centiles and Prediction of Stillbirth and Infant Mortality and Morbidity: A Cohort Study of 979,912 Term Singleton Pregnancies in Scotland. PLoS medicine. 2017;14(1):e1002228. doi: 10.1371/journal.pmed.1002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stock SJ, Myers J. Defining Abnormal Fetal Growth and Perinatal Risk: Population or Customized Standards? PLoS medicine. 2017;14(1):e1002229. doi: 10.1371/journal.pmed.1002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chiossi G, Pedroza C, Costantine MM, Truong VTT, Gargano G, Saade GR. Customized vs population-based growth charts to identify neonates at risk of adverse outcome: systematic review and Bayesian meta-analysis of observational studies. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2017;50(2):156–66. doi: 10.1002/uog.17381. [DOI] [PubMed] [Google Scholar]
- 72.Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. The New England journal of medicine. 1998;338(3):147–52. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 73.Tarca AL, Hernandez-Andrade E, Ahn H, et al. Single and Serial Fetal Biometry to Detect Preterm and Term Small- and Large-for-Gestational-Age Neonates: A Longitudinal Cohort Study. PloS one. 2016;11(11):e0164161. doi: 10.1371/journal.pone.0164161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bukowski R, Gahn D, Denning J, Saade G. Impairment of growth in fetuses destined to deliver preterm. American journal of obstetrics and gynecology. 2001;185(2):463–7. doi: 10.1067/mob.2001.115865. [DOI] [PubMed] [Google Scholar]
- 75.Hawkins LK, Schnettler WT, Modest AM, Hacker MR, Rodriguez D. Association of third-trimester abdominal circumference with provider-initiated preterm delivery. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2014;27(12):1228–31. doi: 10.3109/14767058.2013.852171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abrams BF, Laros RK., Jr Prepregnancy weight, weight gain, and birth weight. American journal of obstetrics and gynecology. 1986;154(3):503–9. doi: 10.1016/0002-9378(86)90591-0. [DOI] [PubMed] [Google Scholar]
- 77.Zhang J, Bowes WA., Jr Birth-weight-for-gestational-age patterns by race, sex, and parity in the United States population. Obstetrics and gynecology. 1995;86(2):200–8. doi: 10.1016/0029-7844(95)00142-e. [DOI] [PubMed] [Google Scholar]
- 78.Ay L, Kruithof CJ, Bakker R, et al. Maternal anthropometrics are associated with fetal size in different periods of pregnancy and at birth. The Generation R Study. BJOG : an international journal of obstetrics and gynaecology. 2009;116(7):953–63. doi: 10.1111/j.1471-0528.2009.02143.x. [DOI] [PubMed] [Google Scholar]
- 79.Gardosi J. Customized fetal growth standards: rationale and clinical application. Seminars in perinatology. 2004;28(1):33–40. doi: 10.1053/j.semperi.2003.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


