Abstract
NASA's Goddard Earth Observing System (GEOS) Earth System Model (ESM) is a modular, general circulation model (GCM), and data assimilation system (DAS) that is used to simulate and study the coupled dynamics, physics, chemistry, and biology of our planet. GEOS is developed by the Global Modeling and Assimilation Office (GMAO) at NASA Goddard Space Flight Center. It generates near‐real‐time analyzed data products, reanalyses, and weather and seasonal forecasts to support research targeted to understanding interactions among Earth System processes. For chemistry, our efforts are focused on ozone and its influence on the state of the atmosphere and oceans, and on trace gas data assimilation and global forecasting at mesoscale discretization. Several chemistry and aerosol modules are coupled to the GCM, which enables GEOS to address topics pertinent to NASA's Earth Science Mission. This paper describes the atmospheric chemistry components of GEOS and provides an overview of its Earth System Modeling Framework (ESMF)‐based software infrastructure, which promotes a rich spectrum of feedbacks that influence circulation and climate, and impact human and ecosystem health. We detail how GEOS allows model users to select chemical mechanisms and emission scenarios at run time, establish the extent to which the aerosol and chemical components communicate, and decide whether either or both influence the radiative transfer calculations. A variety of resolutions facilitates research on spatial and temporal scales relevant to problems ranging from hourly changes in air quality to trace gas trends in a changing climate. Samples of recent GEOS chemistry applications are provided.
Keywords: Earth System Models, GEOS, coupled chemistry
Key Points
The GEOS Earth System Model's architecture is based on ESMF and the GMAO's middleware layer called MAPL
GEOS uses a common code base of interchangeable chemical components for data assimilation, forecasting, and research
GEOS is applied to atmospheric chemistry topics ranging from diurnal air quality variations to climate change
1. Introduction
Among the most important factors influencing the long‐term evolution of the atmosphere are feedbacks between its chemical composition and an array of other physical processes that include, for example, radiative transfer, clouds, and precipitation. The desire to quantify the influence of feedbacks and to identify those that are most important fundamentally motivates our desire to understand atmospheric chemistry and air pollution. To study these complicated and often nonlinear interactions, atmospheric scientists have developed general circulation models of the atmosphere that today are capable of utilizing thousands of processors to accommodate global spatial discretizations down to 10 km or less in the horizontal and tens to hundreds of meters in the vertical. At NASA's Goddard Space Flight Center (GSFC), the Global Modeling and Assimilation Office (GMAO) in partnership with the Atmospheric Chemistry and Dynamics Laboratory (ACDL) instituted a project approximately a decade ago to integrate photochemical mechanisms of increasing complexity into GSFC's atmospheric general circulation model (AGCM), the Goddard Earth Observing System (GEOS) Model. The initial objective was to accurately simulate ozone evolution in a changing environment. Pawson et al. (2008) described the first version of the coupled model, for which a detailed stratospheric chemistry (StratChem) module (Considine et al., 2000; Douglass & Kawa, 1999) was developed. It was used to examine long‐term impacts of the growth and decline of chlorofluorocarbons (CFCs) on the stratosphere's ozone layer (Stolarski et al., 2010) and to study the influence of the Antarctic ozone hole on tropospheric circulation (Perlwitz et al., 2008). Through participation in the Chemistry‐Climate Model (CCM) Validation Activity (CCMVal) (Eyring et al., 2006), GEOS became established as one of the leading CCMs based on several objective, performance‐based metrics (Eyring et al., 2010; Waugh & Eyring, 2008). Other CCM projects in the United States, Europe, and Japan are detailed in the above references and in documents from the Stratospheric Processes and their Role in Climate (SPARC) initiative of the World Climate Research Programme (WCRP).
Successes with GEOS in studying the ozone layer and its relation to climate motivated efforts to enhance its ability to address other topics in chemistry and transport important to NASA's Earth Science Mission (Douglass et al., 2014; Oman & Douglass, 2014; Stolarski et al., 2006). Substantial improvements to the atmospheric physical parameterizations (Molod et al., 2015) were supplemented by introducing aerosol modules (Colarco et al., 2010) and by acquiring modern, highly resolved emissions inventories. The dynamical core has been upgraded so that integrations can be performed on cubed‐sphere grids (Putman & Lin, 2007), which are more scalable and more accurately represent transport than longitude‐latitude grids, especially over the poles. The “stratosphere‐troposphere chemical mechanism” developed by the Global Modeling Initiative (Duncan et al., 2007), hereafter GMI‐STM, was added along with software that enables run‐time selection of an experiment's configuration and discretization. The ability to adapt to and take advantage of ever‐evolving, massively parallel computing architectures and to execute GEOS in multiple environments is reflected in the increasing complexity of its software, whose foundation in the Earth System Modeling Framework (ESMF) (Hill et al., 2004) and the Modeling Analysis and Prediction Layer (MAPL) (Suarez et al., 2007) promotes flexibility and extensibility. In the current era of rapidly evolving trends in emissions, the emphasis of GEOS chemistry‐based research is evolving to include modeling and forecasting tropospheric pollution and air quality, assimilating trace gases, and creating data sets to support observing systems simulations. Each of these topics is driving rapidly expanding resource requirements to accommodate more comprehensive chemical mechanisms than those currently implemented and to enable integrations on scales fine enough to resolve trace constituent gradients near the ground.
The GMAO's model development and validation efforts seek to maximize the use of trace gas information contained in retrievals and radiances from NASA's Earth Observation System (EOS) and subsequent satellites. Since the 1970s, NASA has been mandated to monitor stratospheric ozone, for which global analyses are generated. The GMAO's data assimilation system (DAS) first relied on a series of Solar Backscattered Ultraviolet (SBUV) radiation and Total Ozone Mapping Spectrometers (TOMS) (Heath et al., 1975). Since 2004, these observations have been extended and enhanced by the launch and operation of the EOS Aura platform. On board Aura are the Microwave Limb Sounder (MLS) (Waters et al., 2006), which retrieves ozone profiles in the stratosphere and the upper troposphere down to 261 hectopascals (hPa), and the Ozone Monitoring Instrument (OMI) (Levelt et al., 2006), which provides total column measurements. Information from both instruments is being used by the GMAO to demonstrate the accuracy of simulated springtime Arctic ozone depletion in GEOS when it is configured with StratChem coupled to the GEOS DAS. OMI also measures column nitrogen dioxide (NO2), which has been used to quantify near‐surface pollution and to measure trends during the Aura period (Duncan et al., 2016; Lamsal et al., 2015). As we endeavor to upgrade the aerosol and chemical mechanisms and couple each to the ocean biogeochemistry, land‐surface, and dynamic vegetation components, observations from the above platforms and others will be critical for constraining model performance and guiding improvements. Flemming et al. (2017) describe similar efforts to combine trace constituent assimilation with chemical and aerosol modeling at the European Centre for Medium‐Range Weather Forecasts.
This paper provides documentation of the current version of GEOS from a chemical perspective. Section 2 begins by summarizing the contents of the dynamical core and the physical parameterizations in the AGCM. It then provides a technical description of the chemical components. With the context of the basic elements in place, section 3 introduces key aspects of GEOS's ESMF‐based infrastructure beginning with a description of the GMAO's middleware layer that simplifies software development. It then describes functional units of the hierarchy, and how they fit into the overall structure of the model and promote flexibility in configuring experiments. Section 3 concludes by illustrating and describing the coupling between the chemical components and their connections to the AGCM. Section 4 offers examples that highlight the diverse capabilities of GEOS chemistry. We close by discussing near‐term, chemically oriented GEOS development efforts in section 5.
2. Model Description
This section presents a summary of the building blocks of GEOS with an emphasis on the chemistry components. As summarized below, GEOS has been substantially updated since the version described by Rienecker et al. (2008), most importantly in the moist processes and turbulent diffusion components (Molod et al., 2015, 2012).
2.1. Dynamics
Large‐scale transport and dynamics in GEOS is computed by an adaptation of the flux‐form semi‐Lagrangian (FFSL) finite‐volume (FV) dynamics of Lin (2004) for a cubed sphere horizontal discretization (Putman & Lin, 2007). The FFSL scheme (Lin & Rood, 1996, 1997) is motivated by requirements for accurate and consistent scalar transport of trace constituents, mass, and potential vorticity. FFSL is by design mass conserving, and its local discretization enables it to maintain sharp gradients. FV features a conservative mapping from floating Lagrangian control volumes to GEOS's Eulerian, terrain‐following coordinate. GEOS also contains a separate stand‐alone FV advection module outside of the “dynamical core” for use in offline experiments. In multiple‐model comparison exercises where transport statistics are objectively measured against observations, the FV dynamical core is known to perform well. For example, in integrations spanning several decades, the transport of long‐lived constituents in the stratosphere indicates that the residual mean meridional circulation is accurately simulated (Eyring et al., 2006, 2007; Pawson et al., 2007).
The motivation for the transition to the cubed‐sphere grid is twofold. The first is computational stability. Longitude‐latitude grids are inherently unstable near the poles because cell area diminishes due to the convergence of meridians. The cubed sphere's grid is quasi‐uniform over the entire globe, which eliminates the need to apply a filter over the poles to maintain stability. In addition, because cell areas over the broader polar cap regions are similar to those near the Equator, the cubed sphere is especially advantageous during intervals of strong cross‐polar flow such as sudden stratospheric warmings. The discontinuities at the edges and corners of the cube's six faces present computational challenges. However, Putman and Lin (2007) show that as moving vortices pass over the corners, error growth rates are comparable to those in the original longitude‐latitude implementations, and they decline as resolution is enhanced. The second motivation is scalability. The grid facilitates two‐dimensional domain decomposition on distributed computing architectures, and enables implementation of a completely general algorithm for determining and allocating processor layouts that is extensible to ultra‐high resolutions. The cubed sphere is thus particularly well suited for explicitly resolving previously parameterized processes such as convection (Putman & Suarez, 2011).
GEOS's vertical discretization is a generalized hybrid sigma‐pressure vertical coordinate (Simmons & Burridge, 1981). The current standard configuration is composed of 72 layers, which are terrain‐following at the ground and throughout the lower troposphere. As altitude increases, the layers gradually become isobaric, with the transition completed at approximately 12 km (176 hPa). Layer number 1 is at the model's lid, which lies at 0.01 hPa (approximately 80 km), and layer 72 lies on the ground. Vertical discretization is finest in the lower troposphere, where layer thicknesses are as thin as 60 m at the ground. The layers become thicker with elevation in the troposphere and are approximately 2 km deep in the upper stratosphere and mesosphere.
2.2. Physics
GEOS physics includes parameterizations for convection, large‐scale precipitation and cloud cover, longwave and shortwave radiation, turbulence, gravity wave drag, and land‐surface processes. Convection is parameterized using the Relaxed Arakawa‐Schubert scheme (Moorthi & Suarez, 1992), which contains an updraft‐only cloud model and a quasi‐equilibrium closure. The frequency and intensity of deep convection are governed by a stochastic Tokioka‐type trigger function (Tokioka et al., 1988) as suggested by Bacmeister and Stephens (2011). Prognostic cloud cover and cloud water and ice are determined by the single moment parameterization of Bacmeister et al. (2006), which includes large‐scale condensation, evaporation, autoconversion and accretion of cloud water and ice, sedimentation of cloud ice, and re‐evaporation of falling precipitation. The probability distribution function (PDF) for total water that governs the condensation and evaporation processes is described by Molod (2012). GEOS also has the option to instead execute a two‐moment cloud microphysics module, which improves the simulation of clouds and condensate in many regions (Barahona et al., 2014).
Longwave radiative processes are described by Chou and Suarez (1994), and include absorption due to cloud water, water vapor, aerosols, carbon dioxide (CO2), ozone (O3), nitrous oxide (N2O), methane (CH4), chlorofluorocarbons CFC‐11 and CFC‐12, and hydroclorofluorocarbon HCFC‐22. Shortwave radiative transfer is from Chou (1990, 1992), and includes absorption by water vapor, cloud water, O3, CO2, molecular oxygen (O2), and aerosols, and scattering by cloud water and aerosols. The global concentration of CO2 is specified from Meinshausen et al. (2011), and the shortwave flux reduction due to O2 is derived from a simple function applied to the insolation. The remaining eight constituents listed above are simulated and prognostic, as are the aerosols. Their three‐dimensional, time‐dependent fields are imported into the radiation components, which facilitates chemistry's influence on the dynamics via thermal forcing. The rapid radiative transfer model for GCMs (RRTMG) (Iacono et al., 2000) is also available in GEOS.
The turbulence parameterization is based on the Lock scheme (Lock et al., 2000) interfaced with the Richardson number based algorithm of Louis and Geleyn (1982). The former includes a representation of nonlocal mixing driven by both surface fluxes and cloud‐top processes in unstable layers, either coupled to or decoupled from the surface. It was extended in GEOS to include moist heating and entrainment in the unstable surface parcel calculations which determine the depth of unstable layers. The latter is a first‐order local scheme, and its effect is mostly felt just above the surface layer and in regions of shear‐generated turbulence. The turbulent length scale that governs its behavior is a function of the planetary boundary layer height at the previous time step (Molod et al., 2015), which is diagnosed based on the profile of eddy diffusivity over the ocean and on a bulk Richardson number threshold over land (McGrath‐Spangler & Molod, 2014). The Monin‐Obukhov surface layer parameterization is described by Helfand and Schubert (1995) and includes the effects of a viscous sublayer for heat and moisture transport over all surfaces except land. Ocean surface roughness is determined by a blend of the algorithms of Large and Pond (1981) and Kondo (1975), modified in the midrange wind regime according to Garfinkel et al. (2011) and in the high wind regime according to Molod et al. (2013). The gravity wave drag parameterization computes momentum and heat deposition due to orographic (McFarlane, 1987) and nonorographic (Garcia & Boville, 1994) waves. The background drag profile that generates an internal quasi‐biennial oscillation (QBO) is described by Molod et al. (2015). They demonstrate that downward propagation of the zonal wind anomalies is realistic, but phase speeds are slower and amplitudes are larger than those observed.
2.3. Chemical Mechanisms for Atmospheric Gases
GEOS has several chemical components of varying complexity for the simulation of atmospheric gases. Section 2.3.1 describes the “parameterized chemistry” (PCHEM) module, which executes quickly, is the component that provides radiatively active trace gases in the GMAO's data assimilation system (DAS) production environment, and can be used in simulations and studies that are not specifically targeted at complex chemistry. Section 2.3.2 summarizes the “stratospheric chemistry” (StratChem) package, which provides an economical and accurate mechanism for studies of O3 depletion and recovery and of the influence of O3‐depleting substances (ODSs) and greenhouse gases (GHGs) on the climate of the middle atmosphere. A comprehensive “troposphere‐stratosphere chemistry” mechanism, the subject of section 2.3.3, is available for detailed chemical process studies on diurnal to multidecadal time scales. It is currently too expensive to be employed in DAS operations, but has assumed an important role in the GMAO's chemistry‐climate simulations. It is also used in short‐term, high‐resolution simulations that demonstrate GEOS can generate chemical signatures of important meteorological phenomena such as regional and intercontinental transport, tropical storms, and stratospheric intrusions. Section 2.3.4 describes a new, efficient CH4‐CO‐OH module (Elshorbany et al., 2016) that has recently been developed to investigate the long‐term climate impacts of CH4 emissions with a parameterization that can quickly produce ensembles of century‐length simulations. Section 2.3.5 details the “passive tracer” component, which can be run in tandem with any of the other chemistry components. It instantiates a suite of idealized and realistic tracers that can be used for the diagnosis of GEOS's circulation on both short and long time scales.
2.3.1. Parameterized Chemistry
GEOS's most rapidly executed chemical component parameterizes the stratospheric concentrations of the important radiatively active trace gases: O3, CH4, N2O, two chlorofluorocarbons, CFC‐11 and CFC‐12, hydroclorofluorocarbon HCFC‐22, and water vapor (H2O), for the purpose of establishing heating and cooling rates in the stratosphere. Parameterized chemistry (PCHEM) has two configurations. The first is based on linearized production and loss where a constituent, x, is governed by
where P is the production rate, L is the loss frequency, t is the time, and is the state produced by transport and turbulence. The coefficients P and L are monthly averages derived from an integration of the GSFC two‐dimensional chemistry and transport model (Fleming et al., 2011) to steady state with modern boundary conditions. This is the configuration that is used in the GMAO's DAS forward processing, in the generation of our reanalyses, the Modern‐Era Retrospective Analysis for Research and Applications (MERRA) (Rienecker et al., 2011) and MERRA‐2 (Gelaro et al., 2017), and in operational and seasonal forecasting.
In the second configuration, PCHEM relaxes the concentrations of the seven gases (H2O only above the tropopause) to a prescribed set of monthly zonal averages with a time constant for each gas that is declared at run time and applied globally. This allows the gases to be transported by the large‐scale dynamics and by subgrid‐scale convection and turbulence without substantially drifting from their prescriptions. For a long‐lived gas like CH4 in the stratosphere, experience dictates that time constants between about 3 and 10 days allow the isopleths to align with potential vorticity contours on horizontal projections.
In practice, PCHEM's linearized mode is applied only to present‐day simulations. But its relaxation mode can be used to represent past or future stratospheres by customization of the zonally averaged monthly mean relaxation fields. For example, the GMAO's retrospective climate investigations (Schubert et al., 2014) use a time‐dependent climatology that is generated by a multiple‐step process. First, 5 year running averages of monthly zonal means are computed from a 1950 to 2010 simulation that used an older version of GEOS. Bias correction of N2O, CH4, CFC‐11, CFC‐12, and HCFC‐22 is accomplished by scaling each to their respective surface mixing ratios specified by the fifth phase of the Coupled Model Intercomparison Project (CMIP5) historic midyear concentrations (Meinshausen et al., 2011). The 1951–1955 monthly averages for N2O and CH4 are then copied to each year back to 1870 after scaling them to the CMIP5 concentrations for each respective year. Stratospheric H2O is also projected back to 1870 based on the twice the change in CMIP5 surface CH4 concentration lagged by 5 years, the assumed age‐of‐air (AOA). Finally, for O3, monthly averages from 1870 to 2005 (Cionni et al., 2011) were downloaded from the Earth System Grid Gateway and converted to zonal means.
2.3.2. Stratospheric Chemical Mechanism
The first reactive gases chemistry component in GEOS was originally developed in the 1990s for multidecadal simulations of stratospheric O3 with a chemistry and transport model (CTM, see section 4, paragraph 1 for an example.). Called StratChem, results from early versions are described by Kawa et al. (1995), Douglass et al. (1997), Douglass and Kawa (1999), Kinnison et al. (2001), and Douglass et al. (2004). These studies validated StratChem's performance in configurations that did not allow chemical feedback to the dynamics through radiative forcing, but demonstrated that the mechanism was capable of reproducing realistic polar O3 loss prior to implementing it in GEOS. Using GEOS, Pawson et al. (2008) evaluate O3 and temperature fields from simulations spanning 1980 through 2000 by comparing them to observations, and demonstrate the importance of radiative feedback. Perlwitz et al. (2008) extended simulations to 2100 to show how the growth and decay of the Antarctic O3 hole affects circulation patterns in the southern hemisphere and influences Antarctic surface climate change.
The current equation set consists of 125 thermal and 35 photolytic reactions. StratChem utilizes a family approach that includes Ox [O3 + O( ) + O( )], NOx (NO + NO2 + NO3), ClOx (ClO + OClO + 2Cl2O2), and Brx (Br + BrO). The families are transported. Their members are inferred through steady state partitioning during daylight hours (but not at night) as the solver progresses sequentially through the families first with an estimator step followed by a corrector step. In the mesosphere, five photolysis reactions acting on O2, nitric oxide (NO), and CH4 are added in order to balance the H2O and CH4 budgets. That is, CH4 photolysis yields H2O and is necessary to simulate the observed moistening of the upper atmosphere. Heterogeneous chemical reactions on stratospheric sulfate aerosol as well as Type 1 and Type 2 polar stratospheric clouds (PSCs) are treated as in Considine et al. (2000, 2003). In the troposphere, O3 is relaxed to a zonally averaged climatology constructed from observations. Similarly, the three‐dimensional hydroxyl radical (OH) concentration in the troposphere is borrowed from Spivakovsky et al. (2000). OH acts to restrain the growth of the surface source gases methyl chloroform (CH3CCl3), methyl chloride (CH3Cl), methyl bromide (CH3Br), HCFC, and CH4.
Improvements to StratChem in recent releases of GEOS are described by Oman and Douglass (2014). These include extending the transition from day time to nighttime chemistry from 90° to 94° solar zenith angle, prescribing observed stratospheric sulfate surface area densities (Eyring et al., 2013), updating the GHG concentrations to Representative Concentration Pathway (RCP) 6.0 (Meinshausen et al., 2011), and adding 5 parts per trillion (pptv) to the CH3Br surface mixing ratio to account for short‐lived brominated species. In addition, Aquila et al. (2013) coupled sulfate aerosols from the Goddard Chemistry Aerosol Radiation and Transport (GOCART) component (Chin et al., 2002; Colarco et al., 2010) to StratChem in order to simulate volcano‐induced perturbations to stratospheric trace constituents.
Section 4.3 illustrates a new application GEOS with StratChem. It shows how forecasts of the areal coverage of the Antarctic O3 hole are significantly improved when StratChem replaces PCHEM in the DAS.
2.3.3. Stratosphere‐Troposphere Mechanism
The first comprehensive stratosphere‐troposphere chemical component in GEOS is the GMI‐STM (Duncan et al., 2007), which merges Lawrence Livermore National Laboratory's (LLNL) mechanism developed for the stratospheric version of the GMI model (Douglass et al., 2004) with the existing version of the tropospheric chemical mechanism developed for Harvard University's GEOS‐Chem tropospheric chemistry and transport model (CTM) (Bey et al., 2001; Horowitz et al., 1998) with updated photochemical data. The LLNL code was selected in lieu of StratChem since the former contains the infrastructure to support adding the stratospheric equation set to GEOS‐Chem's SMVGEAR‐II (Jacobson, 1995) solver. GMI‐STM includes 71 transported species, 50 short‐lived nontransported species, 322 gas phase thermal reactions, and 81 photolytic decompositions that account for both stratospheric halogen chemistry and tropospheric non‐CH4 hydrocarbon chemistry. A tracer has been added that measures the age‐of‐air (AOA) with respect to the surface, which is useful for diagnosing the strength of the mean meridional circulation in the stratosphere. Photolytic decomposition uses the Fast‐JX photolysis scheme, which is an outgrowth of the Fast‐J scheme of Wild et al. (2000) for tropospheric photolytic reactions and the Fast‐J2 scheme of Bian and Prather (2002), which treats stratospheric photolytic reactions. Stratospheric heterogeneous chemistry is handled as in StratChem. GMI‐STM also accounts for the heterogeneous effects of aerosols on tropospheric chemistry. The aerosol distributions can be specified from external data sets provided by the GMI project, or can be imported as global distributions from GOCART.
GMI‐STM's default emission scenarios for volatile organic compounds (VOCs), NO, and CO due to biomass burning and from fossil and biofuels are documented in Tables 1 and 2, respectively. Surface fluxes from other CH4 sources, including for example oceans, termites and swamps, amount to 540 Tg yr−1 (Patra et al., 2011), and are ingested from a static data set of 12 monthly averages. An additional source of CO is biogenic emissions, which are parameterized with the Model of Emissions of Gases and Aerosols from Nature (MEGAN) (Guenther et al., 2000). MEGAN generates fluxes of isoprene and monoterpenes after importing the leaf area index, 2 m air temperature, and photosynthetic active radiation from GEOS. The temperature effect on leaf emissions is computed as a function of both the current temperature and the average 2 m air temperature over the previous 15 days (Guenther et al., 1999). Biogenic production of CO is then assumed to be from methanol oxidation, whose flux is scaled from isoprene. In a similar manner, production of CO from monoterpenes is scaled with a constant that assumes instantaneous oxidation even though the lifetime of intermediate species is on the order of hours or days. The scaling is necessary because biogenic sources are not resolved at horizontal resolutions currently used in global chemistry models. In tests conducted with earlier versions of GEOS, isoprene production was 635 Tg yr−1, which compares favorably to Guenther et al. (2006), who estimate a range of 500–750 Tg yr−1.
Table 1.
VOCs, CO, and NO From Biomass Burning in 2012
| Species | Tg yr−1 | Species | Tg yr−1 |
|---|---|---|---|
| Acetaldehyde | 2.7 | Formaldehyde | 3.4 |
| C4,5 alkanes | 0.19 | Methane | 16.8 |
| Ethane | 2.5 | Carbon monoxide | 395 |
| Propene | 1.78 | Methylethylketone | 1.6 |
| Propane | 0.61 | Nitric oxide | 18.2 |
Note. Source: Quick Fire Emission Data set (QFED) v2.4r6. Daily inventories at 5/16° × 1/4°.
Table 2.
CO and NO From Combustion of Fuel
| Species and source | Tg yr−1 | Species and source | Tg yr−1 |
|---|---|---|---|
| CO fossil fuel | 267 | NO other fossil fuel | 27.9 |
| CO biofuel | 233 | NO biofuel | 3.6 |
| NO power plants | 21.3 | NO ship | 10.2 |
Note. Source: Emission Database for Global Atmospheric Research (EDGAR) v4.2 with transportation sector emissions from v4.1. Monthly inventories at 5/16° × 1/4°.
A significant additional nitrogen source is lightning. In GEOS, the column flash rate is coupled directly to the simulated convection, as the parameterization is based on an empirical relationship between the flash rate and five predictors: the positive surface temperature deviation from 283 K, convective cloud‐top pressure, convective available potential energy, convective mass flux, and in‐cloud depth of the 263 K isotherm (Allen et al., 2010). Lightning‐produced reactive nitrogen is added to the NO concentration, and production is specified a priori to generate approximately 5 Tg nitrogen yr−1 (Martini et al., 2011). It is distributed in the vertical between cloud base and cloud top with prescribed profiles that are dependent on type of convection, shallow, or deep, and, for the latter, location, marine, or continental. Peak nitrogen production lies near 400 hPa in the tropics. GEOS was used for the sensitivity study of Liaskos et al. (2015). They demonstrate that column NOx in the model is low‐biased compared to observations when the above production rate is assumed, and examine changes to tropospheric O3 when they enhance the mass of NOx produced by each flash.
To account for nitrogen emitted by soil microbes, GMI‐STM employs the parameterization developed by Yienger and Levy (1995). NO fluxes are computed as a function of vegetation type (Olson, 1992), temperature, precipitation history, and fertilizer applications. A fraction of the soil‐emitted NOx is deposited within the tree canopy as a consequence of the oxidation of NO to NO2, followed by uptake of NO2 by vegetation (Jacob & Bakwin, 1991). A review of published estimates of global soil NOx emissions is given by Vinken et al. (2014), whose estimate of global above‐canopy emissions is approximately 12.9 ± 3.9 Tg yr−1. GMI‐STM's canopy emissions are 15 Tg yr−1, which are modestly high‐biased with respect to the above and to Hudman et al. (2012), and near the top of the range of the models that are quoted.
Section 4 contains several examples of applications of GMI‐STM. The first looks at results from simulations of stratospheric O3 loss in the 20th century and recovery throughout the remainder of the 21st century. The second compares modeled tropospheric column O3 to recent satellite observations. The third and fourth examples demonstrate how horizontal resolution affects results: Better‐resolved dynamics improves O3 concentrations at the ground in a stratospheric intrusion, and high resolution combined with similarly resolved emissions is necessary to drive observed nighttime titration of O3 in high NOx environments.
2.3.4. Parameterized CO, CH4, and OH
A recently added chemistry component encapsulates the scheme of Elshorbany et al. (2016), who describe a computationally efficient module to simulate the atmospheric chemistry of the CH4‐CO‐OH cycle. Called ECCOH (pronounced “echo”), it is used to generate ensembles of sensitivity simulations spanning multiple decades. Such lengthy experiments are necessary for capturing the nonlinear feedbacks in the cycle, and for understanding CH4 perturbations and their impacts on climate. ECCOH separates the influence of various causal factors on OH, including overhead O3 column, NOx, VOCs, and H2O, and to subsequently determine how each affects CH4 and CO. ECCOH gives us the capability to address, for example, the wide variance of simulated OH among various models (Fiore et al., 2009; Naik et al., 2013; Shindell et al., 2006; Voulgarakis et al., 2013).
ECCOH parameterizes the chemistry of tropospheric OH and CH4 and CO. CH4 surface fluxes include annually repeating wetland emissions, biomass burning, and anthropogenic emissions (Patra et al., 2011). Anthropogenic and biomass burning CO emissions are from Strode et al. (2015). Stratospheric concentrations are prescribed (Elshorbany et al., 2016), and CH4 oxidation is based on reactions with OH, atomic chlorine (Cl), and the first excited state of oxygen, O1D. The parameterization of tropospheric OH is based on Spivakovsky et al. (1990a, 1990bb) and Prather and Spivakovsky (1990). Using a set of high‐order polynomials that describe the functional relationship between OH concentrations, meteorological variables (pressure, temperature, cloud albedo, and humidity), solar irradiance (surface albedo, declination angle, and latitude), and the chemical fields, ECCOH reproduces OH predicted by a detailed NOx‐HOx‐VOC‐aerosol photochemical mechanism with an accuracy of 8% in areas with the highest OH abundances (e.g., subtropics and tropics). (Duncan et al., 2000). Before its use in GEOS simulations of the CH4‐CO‐OH cycle itself (Elshorbany et al., 2016), the parameterization was validated in studies of the nonlinear feedbacks of CO and OH (Duncan et al., 2007; Duncan & Logan, 2008).
2.3.5. Passive Tracers
The above chemical components produce distributions of trace gases that can be directed to interact with the other physics components, which in turn, influence GEOS's dynamics. With the passive tracer (TR) component, modelers can instantiate a selection of realistic or idealized tracers that are transported by dynamics, convection, and diffusion, but do not otherwise influence the AGCM's dynamics or physics. Such tracers are useful for studying various aspects of horizontal and vertical transport, including the development and maintenance of interhemispheric gradients, the location and permeability of the tropopause, and origin of air masses (Orbe et al., 2015). TR is designed to be flexible and extensible within each class of tracers so that the tracer instantiations can be configured completely by a set of resource files. This philosophy promotes run‐time decision‐making and reusability of the executable when instantiations are added or removed from the existing tracer classes.
Table 3 lists some examples of the tracer classes currently implemented. Across the classes, sources, and sinks can be obtained by reading external files, by linking with GCM fields, or by using radioactive decay rates of other tracers. Methods for depleting the tracers include using fixed loss rates and rates that vary in both time and space to mirror those of chemically active constituents, and specifying e‐folding and half‐life periods. Tracers can also be subject to wet and dry deposition. Source and sink regions can be configured with masks or enabled in domains bounded by longitude, latitude, and height. For example, the constant‐burden tracer is emitted only at the surface and is constrained to maintain a constant global mass in the presence of a 90 day e‐folding loss rate. The AOA tracer is incremented by the time step length each iteration, except in the surface layer where it is reset to zero. Inventories of anthropogenic CO surface emissions constitute the source fluxes of the surrogate CO tracer, and methyl iodide (CH3I) is instantiated with a constant oceanic emission. Each has a configurable e‐folding loss rate. Finally, the stratospheric source tracer is emitted only at pressures less than 80 hPa. The diagnostic capabilities of TR's tracers are detailed in the references that accompany Table 3.
Table 3.
Some Examples of Optional Passive Tracers
| Tracer description | Purpose | Reference |
|---|---|---|
| Age‐of‐air (AOA) | Generate mean age and age spectra. Constrain diffusive processes. | Waugh and Hall ( 2002) |
| Constant global mean mixing ratio, 90 day lifetime | Differentiate stratospheric and tropospheric air. Find distance from the tropopause. | Prather et al. ( 2011) |
| Radon‐222 | Test convective transport, PBL depths, and continental influence on marine air. | Jacob and Prather ( 1990) |
| Methyl iodide | Diagnose outflows from marine convection and constrain vertical mixing rates. | Bell et al. ( 2002) |
| Lead‐210 | Interpret variations in aerosols due to moist processes. | Considine et al. ( 2005) |
| Stratosphere source, 25 day lifetime | Determine depth of stratospheric intrusions and volume of stratosphere‐troposphere exchange (STE). | Eyring et al. ( 2013) |
| Surrogate CO, 50 day lifetime | Study the impact of circulation changes on pollutant concentrations and transport of emissions. | Shindell et al. ( 2008) |
| Sulfur hexafluoride | Derive transport time scales and identify barriers to large‐scale mixing and transport. | Manzini and Feichter ( 1999) |
| Beryllium radionuclides | Diagnose meridional transport in the stratosphere and STE. | Jordan et al. ( 2003) |
Tracers, whether passively advected or chemically active, that are tied to emissions from selected sources are called “tagged tracers,” or “tags.” Since tags can be multiply instantiated, plumes can be isolated to and followed from certain activities, for example, biomass burning versus combustion of fossil fuels in power plants. Tags can also be configured to capture in‐kind emissions on local, regional, or global scales, which enables geographic attribution downstream. A number of tagged tracers are issued as products from the GEOS DAS forward processing system and are available in the MERRA‐2 reanalysis. An example is given in section 4.4, in which CO loss is parameterized by providing a global OH field and a temperature‐dependent kinetic rate constant. Forecasts of tagged tracers have been instrumental in the GMAO's support of NASA‐sponsored air sampling missions, where they are used by the on‐site science teams to aid flight planning.
2.4. Computing Expenses
Increases in resource utilization that accompany the use of the GMAO's more comprehensive chemical mechanisms are compounded by their application to finer scales. Figure 1 illustrates computing costs, defined as the number of processors allocated to a job multiplied by its wall clock time, in a set of 10 day GEOS experiments with the three indicated mechanisms. The simulations are performed on the cubed sphere at each of five resolutions from c48 to c720, which approximates 2–1/8°. If instead the abscissa is the logarithm of the number of cells, the slopes of the three lines (not shown) connecting the processor hours used for PCHEM, StratChem, and GMI‐STM are 1.077, 1.037, and 1.042, respectively, and the lines are reasonably straight and parallel. Hence, processor hours consumed at c48 can be used to predict the costs of higher resolutions for each of the three mechanisms as long as there are no other changes to the configurations.
Figure 1.
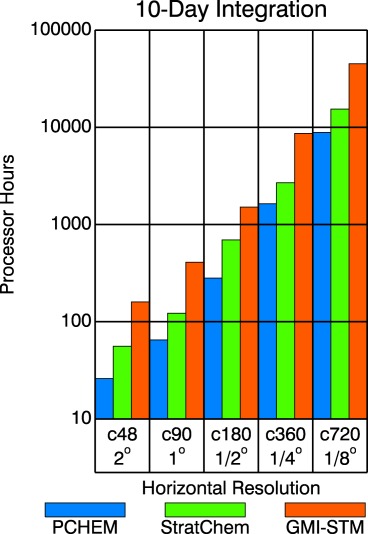
Resource utilization during 10 day GEOS integrations configured to use PCHEM, StratChem, or GMI‐STM at five resolutions on the cubed sphere: c48, c90, c180, c360, and c720. Approximate cell size is labeled on the horizontal axis. Processor hours are given on a logarithmic scale.
The source of cost increases varies depending on the changes made to the configuration. For example, profiling c360 PCHEM and StratChem experiments that use 840 processors shows that the latter chemistry component costs 132% more in this configuration, and since the number of transported species jumps from 7 to 51, the cost for horizontal advection increases by 42%. But, in fact, advection accounts for 59% of the combined cost increase. On the other hand, when transitioning c360, 1680‐processor experiments from StratChem to GMI‐STM, the increases are 623% for chemistry and 4% for advection. In this case, advection accounts for only 2% of the combined change, even though GMI‐STM has 20 more transported constituents.
Other performance tests show that run times from PCHEM experiments conducted at c180 (not shown) fall by a factor of only 2.2 when the number of processors is quadrupled (216–864). This is because components that require interprocessor communication, such as dynamics and transport, and those that lack load balancing, such as the land‐surface, do not scale well. The chemistry components avoid the former because they do not need information from their neighbors on the same layer. So scalability is limited if the time to reach a solution averaged over the cells on each processor is globally nonuniform. We expect spatial inhomogeneities of the chemistry to limit the efficacy of simply adding processors, most notably with StratChem and GMI‐STM. However, the fact that entire columns are processed (because there is no vertical decomposition) preserves the averaging aspect. Long et al. (2015) show that in GEOS the scaling efficiency of complex (tropospheric) chemistry exceeds that of dynamics as the decomposition approaches the maximum possible for a given resolution.
In practice, project objectives combined with resource availability and turnover dictate which resolutions and chemistry components are chosen. For example, PCHEM is used in the DAS, which operates at c720, because it provides an inexpensive, acceptable first guess for stratospheric O3 (see section 4.3). In contrast, GEOS chemistry‐climate simulations with GMI‐STM (see section 4.1) have advanced to 1° but rarely beyond, in part because many of the numerous sequential integrations must wait longer for additional processors to become available.
3. GEOS Infrastructure
GEOS architecture uses the Earth System Modeling Framework (ESMF) (Hill et al., 2004) as the core of its infrastructure. Basic ESMF building blocks are gridded components (GCs), and in GEOS a fine‐grained approach to component design is employed. For ease of use, a middleware (usability) layer that specifies conventions and best practices for utilizing ESMF in integrated ESMs was developed in GMAO. Funded by NASA's Modeling Analysis and Prediction (MAP) program the layer is termed the MAP Layer, or MAPL (Suarez et al., 2007) (pronounced “maple”). MAPL's primary objective is to reduce the labor of constructing ESMF applications without sacrificing ESMF's generality and extensibility. By encapsulating “boilerplate” functions, it aids in coding a GC's “initialize,” “run,” and “finalize” methods, provides tools for describing the contents of the “import,” “export,” and “internal” states (data structures), the latter an MAPL extension of the ESMF_State concept. MAPL also facilitates the use of the ESMF infrastructure layer, and simplifies coupling GCs into complex applications, such as GEOS.
MAPL adopts a hierarchical approach to its architecture, and uses both composite GCs and ESMF coupler components to establish connections between members of the hierarchy. The relationships among the different members of the hierarchy are articulated using an analogy to familial relationships. MAPL adopts ground rules for the behavior of GCs and provides a standard recipe for writing them. For example, MAPL contains generic versions of the different GC “methods” which, among other functions, create, allocate, initialize, and destroy state items, read, and write the elements of the internal and import states, and establish connectivities among other GCs. This approach dramatically reduces the volume of written code while preserving the ESMF flavor of the software. GEOS architecture requires that its components display an MAPL interface.
3.1. Hierarchy
Figure 2 illustrates the hierarchical structure of GEOS as it is configured for the experiments discussed in section 4, with each box in the figure representing a GC. The black lines in the diagram connect “children” to their “parent” composite GCs. At the top of GEOS's hierarchy is the CAP, which is the main program. It contains the time loop that calls the hierarchy of run methods. After all of them have completed, CAP advances the clock and calls HISTORY, the GC that creates and populates output data sets. Upon expiration of the time loop, CAP runs the finalize methods and writes checkpoint files. It also updates the CAP restart file with the date and time of day, which is available to establish the starting time of the next sequential integration in the experiment, whose temporal length is also declared in the CAP resource file.
Figure 2.
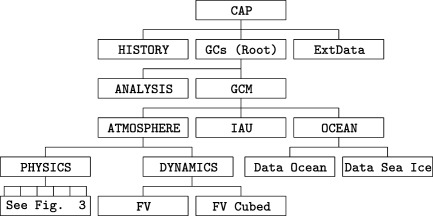
Top‐level GEOS hierarchy with black lines connecting child GCs to their parents. Figure 3 examines the children of PHYSICS.
Further down the hierarchy, for example, are the two children of DYNAMICS, FV, and FV cubed, which host the finite‐volume advection core for regular latitude‐longitude and cubed‐sphere grids, respectively. All the results shown in section 4 are generated on the latter, and, in fact, the former is now rarely used. ATMOSPHERE and OCEAN are two children of GCM. For this paper, all GEOS chemistry experiments are produced with sea surface temperatures and sea ice content prescribed from either observations or data sets archived by atmosphere‐ocean GCMs. In this diagram these are the “Data Ocean” and “Data Sea Ice” children of OCEAN. Version 5 of the Modular Ocean Model (MOM‐5) (Griffies et al., 2005) implemented in GEOS is another child of OCEAN, and is available as an option for coupled chemistry model simulations. For completeness, the incremental analysis update (IAU) GC's is shown as a child of the GCM, but is not discussed. The chemistry GC, which is the focus of the next section, is a child of PHYSICS, whose family is illustrated in Figure 3.
Figure 3.
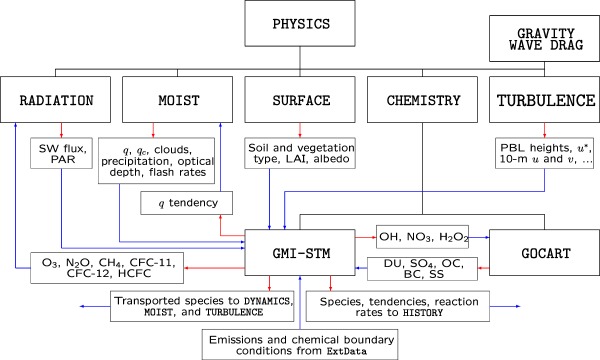
GMI‐STM's relationship to the other children of PHYSICS. GCs and their children are connected by black lines. Red vectors indicate exports and blue vectors imports.
In the following sections, the reader will find references to the HISTORY and ExtData GCs. Each has been developed to simplify the manner in which experimenters interface with GEOS. Both are critical design elements of GEOS's functionality, and help users to quickly instantiate and run experiments at various resolutions. A description of the capabilities of HISTORY and ExtData may be found in Appendix A.
3.2. Coupling the Chemistry
GEOS's chemistry composite GC appears in the hierarchy as a child of the physics composite GC. At the physics level, the software is directed to connect selected export states of various children to import states of other siblings. Figure 3 illustrates which children of physics supply imports to the GMI‐STM, and which of GMI‐STM's exports are available to the physics family. In addition, the transported species are made available to dynamics (horizontal and large‐scale vertical advection), moist processes (convection), and turbulence (boundary layer mixing) by packing them into an ESMF “bundle,” which greatly eases communication. The chemistry “SetServices” method assures that all the species, both transported and nontransported, are included as members of its export state so they are visible to MAPL's history component. For simplicity, other chemistry children, aerosol GCs other than GOCART, and children of radiation are not shown in the figure.
Figure 3 also illustrates GOCART's relationship to GMI‐STM. GOCART generates time‐dependent global distributions of dust (DU), sea‐salt (SS), sulfate (SU or SO4), and organic and black carbon (OC and BC) aerosols. At run time, the experimenter can toggle any of five switches in the GMI‐STM configuration file, one dedicated to each aerosol class, that will establish or break their connectivity. This enables GOCART's predicted aerosols to be imported by GMI‐STM and participate in its photolysis computations. Similarly, three of GMI‐STM's oxidants, OH, nitrate (NO3), and hydrogen peroxide (H2O2) are available for import to the SU component of GOCART. If the connectivities are not active, MAPL will look to ExtData to fill the imports with fields from external data sets.
As with species concentrations from GMI‐STM, the dust and aerosols concentrations from GOCART are bundled for export along with derived aerosol properties, and are imported by the radiative transfer components and by MAPL history, but the connections are not illustrated in Figure 2. To include the effect of scattering due to aerosols, the optical thicknesses are scaled by the single‐scattering albedo and an asymmetry factor.
With the configuration of Figure 3, GMI‐STM is termed the “radiatively active‐trace‐species (RATS) provider” and GOCART is the “aerosol (AERO) provider.” PCHEM and StratChem also function as RATS providers. GMI‐STM or the new “GOCART.data” GC can function as AERO providers, both in which aerosol distributions are imported from external files via ExtData in lieu of simulation. The choices of RATS and AERO providers and, when applicable, of the chemistry child that provides O3 to the DAS, are run‐time decisions, enforced through the top‐level configuration file. That is, the MAPL architecture promotes interchangeability and extensibility. GEOS developers are currently integrating alternative AERO providers, including the Modal Aerosol Model (Liu et al., 2016), and the column‐independent version of GEOS‐Chem (Long et al., 2015). The latter provides a more complete tropospheric chemistry mechanism and contains updates to the biogenic and soil emission modules. Further discussion of projected developments can be found in section 5.
4. Applications
This section describes four applications of the chemical components of GEOS. The object is to highlight the broad range of topics that can be addressed with GEOS chemistry simulations, rather than to offer an in‐depth analysis. Where appropriate, references are provided for more detail. For perspective, Table 4 lists a number of the GMAO's projects that utilize GEOS, which is designed to function seamlessly across many different resolutions and applications. While coupled chemistry is the subject of this paper, a closely related application is the GEOS Chemistry Transport Model (GEOS‐CTM) which uses the advection, chemistry, and physics components of GEOS (plus ESMF and MAPL) to perform uncoupled experiments. The shared code base between the coupled and uncoupled model configurations means that a model user can run parallel online and offline simulations, which are useful for understanding and analyzing the impact of chemistry‐atmosphere feedbacks.
Table 4.
GMAO Activities Supported by GEOS
| Application | Horizontal resolution range |
|---|---|
| Global mesoscale simulations | 7–1.5 km |
| Real‐time analyses and forecasts | 1/8° |
| Long‐term reanalyses | 1/2° |
| Coupled atmosphere‐ocean simulations | 2–1/2° |
| Coupled chemistry simulations | 1° to 7 km |
| Offline chemistry and transport (CTM) | 1° to 7 km |
The discussion of applications is as follows: section 4.1 examines O3 depletion and recovery with GEOS configured in a chemistry‐climate mode, and compares tropospheric column O3 to recent satellite observations; section 4.2 contains two perspectives on coarse‐scale versus fine‐scale simulation. The first demonstrates how better‐resolved dynamics and topography influence O3 mixing ratios during a stratospheric intrusion, and the second shows how fine scales affect the simulated chemistry in polluted environments; section 4.3 illustrates the value of that StratChem brings to forecasts of the size of the Antarctic O3 hole; and section 4.4 demonstrates the utility of employing tagged tracers to track plumes of emissions transported by large‐scale dynamics.
4.1. Chemistry‐Climate
Global AGCMs with coupled chemistry mechanisms, in which the chemical state is influenced by the state of the simulated atmosphere and vice versa, are called Chemistry‐Climate Models (CCMs). CCMs are today's most important tools for understanding the role that atmospheric composition plays in climate change. In GEOS, most CCM applications to date have been run with StratChem or with GMI‐STM in tandem with GOCART (as illustrated in Figure 3). The latter configuration is used for our most recent participation in the Chemistry‐Climate Model Initiative (CCMI, http://www.igacproject.org/CCMI), in which boundary conditions and reference trace gas forcing scenarios were specified by the CCMI Scientific Steering Committee. The supporting experiments span the latter half of the 20th and all of the 21st centuries, which enables simulation of O3 depletion during the 1980s and early 1990s due to the increase in halogens, and the expected O3 recovery over the next several decades as halogen loading declines. The surface concentrations of ODSs and GHGs in this GEOS CCM simulation are from the A1 2014 scenario of Velders and Daniel (2014) and RCP 6.0 (Meinshausen et al., 2011; Moss et al., 2010), respectively.
Figure 4 shows the simulated annually averaged quasi‐global (60°S–60°N) total column ozone (QGTCO3) from 1960 to 2100 from a (REF‐C2) CCMI experiment configured as above, with GOCART aerosols coupled to GMI‐STM. Also plotted are ground‐based (Fioletov et al., 2008) and satellite‐based measurements from NASA's Total and Profile Merged Ozone Data Set version 8.6 (Bhartia et al., 2013; Frith et al., 2014). GMI‐STM captures the halogen‐induced O3 decline through the middle 1990s, though the interannual variations differ from observations because the dynamics in this experiment are not constrained. This version of the GMI‐STM includes solar cycle variability in the Fast‐JX photolysis code, and better simulates the subdecadal O3 variability compared to observations. The scaling of actinic fluxes uses Lean (2000) for 1960 through 2016 and the mean of the most recent four solar cycles for the remainder of the 21st century. A similar solar cycle data set and implementation is described by Swartz et al. (2012).
Figure 4.
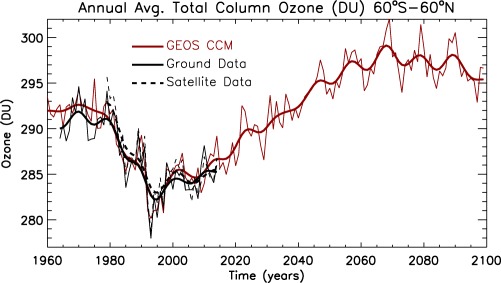
Quasi‐global (60°S–60°N) annual average (thin curves) and low pass filtered (thick curves) total column O3 (Dobson units, DU) from three data sets: (red) GEOS CCM from 1960 to 2100, (solid black) ground‐based observations from 1964 to 2014, and (dashed black) satellite‐based measurements from 1979 to 2015.
Recovery to O3 amounts observed in the 1960s occurs by 2040. But because of cooling in the stratosphere attributable to increases in GHGs, the rates (not shown) of several temperature‐dependent gas phase reactions that destroy O3 slow down (Haigh & Pyle, 1982). Hence, the QGTCO3 in the second half of the 21st century is projected by the simulation to be higher than it was in the 1960s (World Meteorological Organization, 2011). Compared to observations of QGTCO3 over the past 50 years, GEOS is among the best performing CCMs (World Meteorological Organization, 2014). The generally decreasing O3 after 2070 is largely attributable to CH4, which is projected by the RCP 6.0 scenario to decline.
A (REF‐C1) simulation that includes observed sea surface temperature, sea ice concentrations, and trace gas emissions was also performed for CCMI. Figure 5 shows the simulated tropospheric partial column O3 as a function of latitude and month of year compared to an observational data set derived from the residual of OMI and MLS observations (Ziemke et al., 2011). Since the simulation ends in 2010, we compared the 6 year data overlap period of 2005 through 2010 with observational estimates and found that differences are typically less than a few Dobson units (DU). The causes of the 4–6 DU bias in the Northern Hemisphere subtropics and middle latitudes in autumn and winter seen in the bottom plot are under investigation. Uncertainties in the OMI/MLS data set are not much smaller than the bias. The larger negative biases in the Southern Hemisphere high latitudes are due to an issue with the satellite‐derived residuals. In this region, the simulation agrees much better with the ozonesonde‐based climatology of McPeters et al. (2007) as illustrated in Figure 4 of Ziemke et al. (2011).
Figure 5.
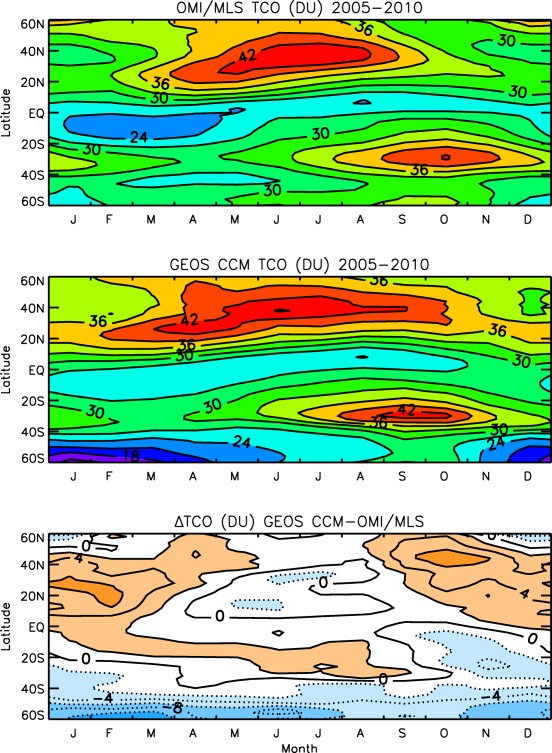
Six year averages of tropospheric partial column ozone (DU) from (top) the OMI/MLS residual (Ziemke et al., 2011), (middle) the REF‐C1 GEOS CCM simulation, and (bottom) their difference.
4.2. Flexible Horizontal Resolution
GEOS allows model users to select a wide range of horizontal resolutions applied globally, including (approximately for the cubed sphere) 2°, 1°, 1/2°, 1/4°, 1/8°, and 7 and 3.5 km. This enables configuration and execution of sets of simulations that utilize a common executable and the same boundary conditions to assess the influence of resolution on transport and chemistry.
For an example of the impact on transport, Figure 6 illustrates GEOS's surface‐layer O3 concentrations during each of four MERRA‐2 meteorology “replay” simulations of a stratospheric intrusion over the western United States on Friday 6 April 2012. Replay is similar to the incremental analysis update method (Bloom et al., 1996) used in the GEOS DAS to apply the analysis as a correction to the background. However it uses pre‐existing analyses to produce the increments. Observed (United States Environmental Protection Agency Clean Air Status and Trends Network) mixing ratios rose to 79 parts per billion by volume (ppbv) in Denver, CO, 84 ppbv in Rocky Mountain National Park, and 76 ppbv at Centennial, WY. As GEOS is configured sequentially toward higher horizontal resolution, it progressively captures more spatial detail in the intrusion's footprint, and it generates concentration maxima that are closer to the surface observations. MERRA‐2 analyses are archived at 1/2° horizontal resolution.
Figure 6.
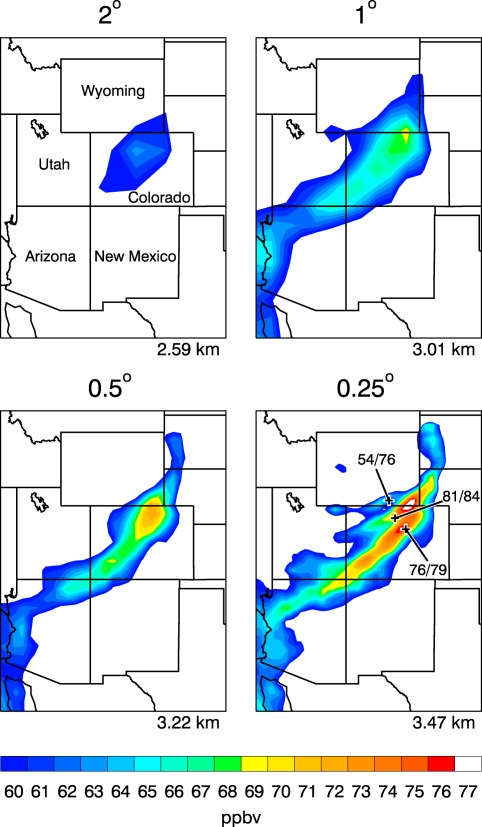
Surface O3 concentration at 6 PM MDT on Friday 6 April 2012 from four simulations of a stratospheric intrusion that are identical except for the labeled horizontal resolution. Observed current/peak‐event volume mixing ratios (ppbv) at the three locations mentioned in the text are drawn on the lower‐right plot. At bottom‐right of each plot, GEOS's highest elevation in Colorado is indicated. 880 m is gained when discretization is enhanced by a factor of eight.
Diagnosis of the O3 budget (not shown) reveals that O3 tendencies in the intrusion are overwhelmingly dominated by transport, especially at high resolution, suggesting that finer discretization improves the representation of jet stream dynamics and downward vertical motion in the intrusion (see Figure 6 in Newton and Trevisan (1984)). Also important is the representation of mountain topography. Peak elevations are more realistic at high resolutions, which makes it more likely that descending O3 will impact the surface in mountainous regions. GMI‐STM is used in these experiments. Ott et al. (2016) provide more details about stratospheric intrusions simulated by GEOS, and discuss the implications for tropospheric O3 budgets.
To demonstrate another aspect of resolution dependence, we focus on the nonlinear behavior of chemical reactions by examining how surface NOx and O3 concentrations evolve during parallel 1/8° and 2° analysis‐driven replay simulations of 8 days length in early May 2011. Both experiments use a copy of the inventory of surface anthropogenic emissions. During each of the 8 days, we determine the maximum NOx concentration that is attained in each surface cell in the 1/8° simulation. At the end of each day (UTC), we extract the locations of the cells with the 15 highest concentrations, with the condition that none are within 2° of each other. The time series of both NOx and O3 concentrations in each of the 15 selected cells is then collected from noon before (local time) to noon after the peak NOx concentration occurred. The sampling yields 120 unique diurnal cycles for each trace gas. A second set of diurnal cycles is obtained from the 2° simulation, wherein each day's chosen cells are collocated with the 15 from the same day of the 1/8° run.
The average diurnal cycles of NOx and O3 from each of the two sample groups are depicted by the blue and orange curves in Figure 7. In both simulations, NOx concentrations increase after sunset, which contributes to the overnight decline of O3. But because the selected 1/8° cells are most often located near strong emitters, and the mass from each is distributed throughout a volume that is approximately 0.4% that of the collocated 2° cell, NOx concentrations on average grow by sunrise to more than an order of magnitude greater than in the 2° simulation. Even though middle‐afternoon O3 concentrations are about the same in both simulations, between 45 and 50 ppbv, the elevated localized NOx drives nighttime O3 to near zero (Brown et al., 2012) at 1/8°, whereas at 2° the average falls no lower than 28 ppbv. A third sample is collected from the 1/8° simulation: the surface concentrations averaged over the 16 × 16 matrix of cells that are collated with each respective member of the sample from the 2° simulation. Their averaged diurnal cycles drawn in green. Across the day, NOx concentrations on average consistently exceed those in their 2° counterparts, but the O3 concentrations do not substantially differ from the 2° values. This example demonstrates that both highly localized emissions and a model discretization that is fine enough to resolve them are required in order simulate NOx concentrations large enough to promote nighttime O3 titration that is observed in polluted air.
Figure 7.
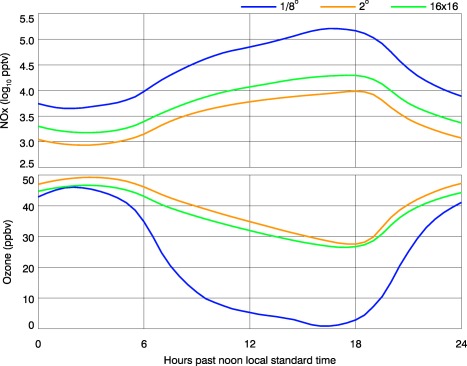
Mean diurnal cycles of (top) NOx and (bottom) O3 surface concentrations from the 15 highest‐NOx locations during each of eight May days of companion 1/8° and 2° simulations. The three sample sets are: (1) high NOx cells from the 1/8° simulation (blue). (2) The collocated cells from the 2° simulation (orange). (3) The average of the concentrations in the 16 × 16 matrix of 1/8° cells with the same footprint as each respective 2° cell (green). Note that the NOx ordinate is scaled logarithmically and O3 is scaled linearly. The sample size is 120.
This result and the intrusion example presented above highlight two aspects of the tropospheric O3 budget that might be important when considering the cost of horizontal resolution. Historically, the expense of discretization has driven chemical modelers investigating this class of phenomena to operate in limited regions with fine meshes that are interfaced with coarser meshes applied globally or with components that provide boundary conditions. In contrast, GEOS is now capable of simulating mesoscale‐dependent chemistry and transport uniformly worldwide with relative efficiency, in part by eliminating the need to engineer the software needed to support embedded meshes and their connecting modules in a general way.
4.3. O3 Depletion in the Data Assimilation System
For the application described here, simulations are performed with the GEOS three‐dimensional variational DAS version 5.13.1, which is based on the Gridpoint Statistical Interpolation (GSI) approach described by Wu et al. (2002). Global analyses are generated using 6 h data windows in which observations are combined with background states produced by the GEOS AGCM. Meteorology is constrained by satellite radiance data and by conventional observations. PCHEM (section 2.3.1) is the default chemical component in the GEOS DAS because it executes quickly and enables the GMAO to meet production schedules, and because it provides an acceptable background stratospheric O3. Driven by efforts to assimilate more trace species, including CO and CO2, and by requirements for generating high‐fidelity and high‐resolution O3 fields for observing system simulation experiments (OSSEs), the GMAO is investigating whether improvements that could come from an operational utilization of StratChem (section 2.3.2) are worth the added computational expense. In the current DAS, there is no covariance structure that relates O3 to other species or to temperature.
The O3 assimilation is described by Wargan et al. (2015), who demonstrate that the GEOS‐5 analysis performs well when compared to ozonesondes in the lower stratosphere where it is strongly constrained by MLS profile data. In addition, Gelaro et al. (2017) show that in MERRA‐2 the size of Antarctic O3 holes, defined as the area of the Earth's surface enclosed by the 220 DU contour of total column O3, agrees well with TOMS and OMI observations from 1980 to 2015.
Figure 8 illustrates results from a 2° GEOS DAS simulation with StratChem that spans September 2015. The time period covers the developing and mature phases of the Antarctic O3 hole. The size of the hole is indicated by the solid black line, which traces the growth of the area in million square kilometers throughout the month. From the analysis, two sets of 10 day forecasts are launched at 21:00 UTC daily between 1 and 20 September. One set of forecasts is generated with PCHEM and the other with StratChem, and the predictions of the O3 hole's size are drawn in red and blue, respectively. Since O3 retrievals from MLS and OMI are assimilated during the DAS, total O3 from the GEOS DAS serves to validate the forecasts.
Figure 8.
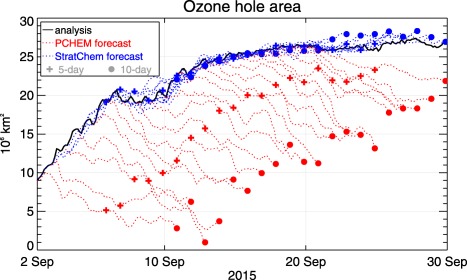
Area of the 2015 Antarctic O3 hole in the DAS (solid black) and during 10 day forecasts with PCHEM (dashed red) and StratChem (dashed blue). Crosses and circles show the areas in each forecast at 5 and 10 days, respectively.
PCHEM's forecasts are poor because its loss coefficients, which are tied to monthly means of a two‐dimensional model's steady state, contain only a weak signal of the Antarctic O3 hole. Thus, immediately after initialization PCHEM drives O3 away from the analysis toward higher mixing ratios in the ozone hole, which raises total O3 and reduces the ozone hole's area. On the other hand, StratChem shows good forecast skill because it explicitly partitions Cl between its active and reservoir species, and generates sufficient concentrations of ClO to drive heterogeneous loss as the sun rises on the pole. In fact, the root‐mean‐square difference from the analyzed area stays within 4%, and the mean difference is less than 1% at 10 days.
Further studies with this configuration of the DAS are being conducted to examine Cl partitioning in two anomalously cold Arctic winters that have long periods of low temperatures and high levels of Cl activation. Early results combined with those above demonstrate that by accepting modest increases in resource utilization, chemical forecasts for the stratosphere can now be a production system reality. In addition, these experiments show that accurate chemistry mechanisms can now be used in the DAS to quantify processes that operate on stratospheric O3 and generate error estimates.
4.4. Tagged Tracers
As mentioned in section 3 GEOS's infrastructure permits multiple instantiations of atmospheric tracers, a feature that is most often used in the “tracer gridded component” (TR) and in GOCART. Using surface sources as an example, the model user can define a tracer with characteristics of a realistic trace gas that has emissions in a specified region, or in any assortment of grid cells, by applying a mask. The evolution of a plume attributable to a particular source, or collectively the plumes from a set of sources, can then be followed by monitoring the “tagged tracer” emitted from the cells particular to each mask. That is, each tagged tracer has its own mask and is independently transported. In principle, hundreds of tagged tracers allocated across a spectrum of emissions can be run simultaneously. Notably, each tagged tracer has its own output stream, so we can examine the evolution of either individual plumes or groups of plumes whose members can be chosen at will.
Tagged tracers are valuable for investigating the spread of pollution and the relative contribution of local and remote sources to the degradation of the air quality. Figure 9a is a map of three tagged CO tracers (advected, but not assimilated) taken from the GEOS DAS forward processing system at 18:00 UTC 29 July 2016. The tags are identified by color. Green shows the surface concentration of CO from biofuel, fossil fuel, and biogenic (nonbiomass) emissions over North America, blue is CO plotted at 500 hPa from Eurasian biomass burning, and orange is CO plotted at 375 hPa from African biomass burning. Because the emissions are transported by analyzed winds, the plumes should bear some resemblance to reality and offer a diagnostic tool that can be used in near‐real‐time to identify remote sources as contributors to local pollution both at the ground and throughout the free troposphere. At the instant of the snapshot, a dense plume from Eurasian biomass burning is transiting Alaska. African emissions are bifurcated, with those close to the equator traversing the Atlantic Ocean to South America and those at more southerly latitudes crossing the Indian Ocean to Australia. Surface winds are strong enough to bring North American emissions to Europe, and, in fact, the pollution is present throughout the Arctic (Shindell et al., 2008; Thomas et al., 2013). In this application, CO loss is parameterized as described in the closing paragraph of section 2.3.5.
Figure 9.
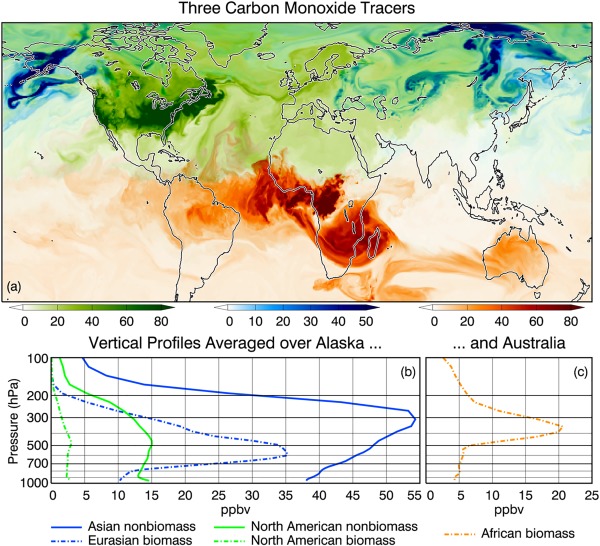
(top) Distribution of three tagged tracers from the GEOS DAS forward processing system. Green: CO from North American nonbiomass burning emissions plotted at the surface. Blue: CO from Eurasian biomass burning plotted at 500 hPa. Orange: CO from African biomass burning plotted at 375 hPa. Darkest shades indicate highest concentrations. (bottom) Vertical profiles of CO tagged tracers averaged over (b) Alaska and (c) Australia.
The instantaneous, area‐averaged vertical distribution of five CO tags, including the three in the snapshot, are plotted in Figures 9b and 9c. The profiles due to North American and Eurasian emissions are averaged over Alaska, and the profile due to African emissions is averaged over Australia. Concentrations peak in the middle and upper troposphere as a result of the dynamics of long‐distance or intercontinental transport (Cooper et al., 2004; Stohl et al., 2002). By vertically integrating the concentrations of both the tagged tracers and the global CO that contains all emissions and then converting to mass, we can obtain the respective contributions of the tags to the total CO. Asian nonbiomass and Eurasian biomass burning taken together account for 60.4% of the total (column) CO over Alaska. North American emissions together contribute substantially less, 14.5%. Over Australia, 12% of the CO is attributable to biomass burning in Africa.
5. Projected Developments
Several updates to StratChem are undergoing testing and validation, and will be included in model versions to be released in the near future. A major upgrade is the addition or reassignment of 18 transported species that include both short‐lived and long‐lived ODSs and GHGs identified by the World Meteorological Organization (WMO) Ozone Assessments as essential to radiative forcing. The first 12 include two CFCs (CFC‐114 and CFC‐115), two HCFCs (141b and 142b), three Halons (1211, 1202, and 2402), and five very‐short‐lived (VSL) bromocarbons [bromoform (CHBr3) and dibromomethane (CH2Br2), CH2BrCl, CHBr2Cl, and CHBrCl2]. The latter are required for an accurate representation of the stratosphere's bromine budget and obviate the need to artificially enhance CH3Br as mentioned in section 2.3.2. The remaining six gases are hydrofluorocarbons (HFC‐23, HFC‐32, HFC‐125, HFC‐134a, HFC‐143a, and HFC‐152) that have strong global warming potentials. In addition to broadening the mechanism, the experimenter can now choose to reference surface flux scenarios in lieu of mixing‐ratio boundary conditions for eight gases: CFC‐11, CFC‐12, CFC‐113, CH3CCl3, carbon tetrachloride (CCl4), HCFC‐22, CHBr3, and CH2Br2. To maintain realistic tropospheric mixing ratios in the presence of emissions, a parameterized surface loss of CH3CCl3 and CCl4 is applied over both land and ocean. Since inorganic bromine formed from the degradation of VSL bromocarbons exists mainly in soluble forms, a new wet scavenging method based on model‐generated precipitation was implemented for HBr, HOBr, and BrONO2. The upgrades are expected to improve StratChem's chemistry‐climate simulations and promote GEOS's continued participation in model comparison exercises.
In cooperation with the GMAO, Long et al. (2015) adapted Harvard University's GEOS‐Chem global atmospheric chemistry model into a grid‐independent (GI), ESMF‐compliant module. This allows GEOS‐Chem to run on unstructured grids, and so is not limited by the domain decomposition for the dynamics. It also allows GEOS‐Chem to be run in three modes from the same code base: (1) as a stand‐alone CTM, (2) as a coupled component of an ESM, and (3) as a CTM within the ESM's infrastructure. The grid‐independent module is now encapsulated in a GEOS GC (a child of CHEMISTRY), and experiments have been conducted with Version 10 of GEOS‐Chem in which the stratosphere is prescribed. It produces realistic tropospheric ozone based on results from a MERRA‐2 replay downscaled to 1/8°. The GMAO is also testing the unified tropospheric‐stratospheric chemistry extension (UCX) (Eastham et al., 2014) in GEOS‐Chem Version 11. By maintaining common‐code repositories with the GEOS‐Chem group at Harvard, the GMAO will have access to the continuous improvements provided by the worldwide GEOS‐Chem community.
The GI GEOS‐Chem provides an opportunity to operate the chemistry on tiled grids that capture the heterogeneity of properties of the Earth's surface not adequately resolved by regular meshes. This will allow solidification of the connections between GEOS‐Chem and other GCs that are exercised on catchments grids and other unstructured grids, including ocean and ice, land‐surface, and dynamic vegetation models.
6. Summary
GEOS, the ESM developed by the GMAO and the subject of this paper, is NASA Goddard's research‐to‐operations platform for data assimilation, numerical weather prediction, and parameterization of geophysical processes, including chemistry. This paper documents the current state of GEOS for configurable coupled chemistry applications, which represents the culmination of 13 years of chemistry‐component development, validation, and integration into the evolving GCM. The effort is conducted by the GMAO in collaboration with partners in the Atmospheric Chemistry and Dynamics Laboratory and the NASA Center for Climate Simulation at NASA Goddard, and recently with colleagues in the Atmospheric Chemistry Modeling Group at Harvard University. Among our principle objectives are to use retrievals and radiances from NASA's satellites to guide development of the model and measure its performance, to create a research tool that enables investigation of topics in atmospheric chemistry on widely varying spatial and time scales, to provide a platform that encourages global mesoscale simulation and assimilation of trace gases, and to enable the GMAO to participate in sensor development through production of chemical OSSEs.
The ESMF‐based infrastructure of GEOS is discussed and elements of the MAPL middleware layer that are useful for designing chemical components and conducting chemical simulations are introduced. MAPL is shown to encapsulate “boilerplate” functionality and moderate the effort needed to integrate new chemistry components and update them. We provide an illustration of how chemistry components are coupled to the AGCM. Our hierarchical approach, with its parent‐child relationships, is shown to provide a straightforward and consistent methodology that promotes extensibility and interoperability as components are added and mature while minimizing the overhead required for connectivity and communication.
Several examples of applications, focusing mostly on O3, highlight the diversity of atmospheric chemistry and transport topics to which GEOS can be applied. We examine two results from a GMI‐STM configuration designed for chemistry‐climate simulations with boundary conditions specified by the CCMI project. GEOS reproduces the decline of global O3 due to halogen loading through the middle 1990s, successfully simulates the beginning of its recovery, and projects its evolution through the remainder of the 21st century. For the present‐day atmosphere, the same configuration is used to compare tropospheric partial column O3 to satellite observations.
With the objective of demonstrating the advantages of fine‐scale discretization, we show how simulated O3 concentrations more closely resemble surface observations in a stratospheric intrusion as resolution increases. We also show that global 1/8° resolution is capable of simulating dramatic nighttime O3 loss in polluted air because high NOx concentrations are maintained near strong emitters that are similarly resolved in the GMAO's emission inventories. Both results suggest that it is important to reevaluate global model‐based tropospheric O3 budgets that are published in the literature since coarser‐scale simulations have to date provided the foundation of global chemistry model bench marking.
We show that forecasts of the size of the Antarctic O3 hole launched from GEOS DAS analyses generate divergent results when either PCHEM or StratChem are used. The ability of the latter to generate reasonably accurate distributions of reservoir and active Cl species proves valuable, justifies the added expense, and demonstrates that detailed prognostications of stratospheric trace species can now be a production reality. Just as important, coupling StratChem to the DAS unlocks the compelling potential to routinely generate trace gas profiles directly comparable to those that are remotely sensed, which opens up new opportunities for model validation and observing system simulation. Finally, in the only example not directly tied to O3, we demonstrate how CO tagged tracers that are incorporated in the operational GEOS DAS forward processing system can be used in real time to identify remote contributors to local pollution.
The infrastructure of GEOS, including the use of ESMF and MAPL and the FV dynamical core on the cubed sphere, make GEOS highly flexible, extensible, and scalable, which enables it to absorb the daunting processor and data flow requirements that will be demanded by NASA‐sponsored Earth Science research during the coming years. To keep pace with shrinking footprints that attend sensor development, global ultra‐fine scale assimilation and simulation will soon be “business as usual,” and several new trace gas data types will be ingested. Grid‐independent chemistry components will be coupled to highly resolved land‐surface and vegetation models operating on irregular grids. The exchange of gases between the atmosphere and ocean and land ecosystems will then be explicit and prognostic. As GEOS evolves, it will create opportunities to more accurately simulate the interplay between seasonal climate variations, VOCs, O3, and regional air quality, and will promote the GMAO's continued participation in model comparison studies focused on determining chemistry's role in climate change.
Acknowledgments
GEOS and the GMAO are supported by NASA's Modeling, Analysis, and Prediction Program under the direction of Dr. David Considine. ESMF is sponsored by the Department of Defense, NASA, the National Science Foundation, and the National Oceanic and Atmospheric Administration. Computations were performed at the NASA Center for Climate Simulation. The GEOS‐5 source code is available under the NASA Open‐Source Agreement at http://opensource.gsfc.nasa.gov/projects/GEOS-5. The version 8.6 reprocessed SBUV merged data set used in Figure 4 is available from the Atmospheric Chemistry and Dynamics Laboratory (ACDL) (https://acd-ext.gsfc.nasa.gov/Data_services/merged/instruments.html), the ground‐based data can be found at the World Ozone and Ultraviolet Radiation Data Centre (http://www.woudc.org/archive/Projects-Campaigns/ZonalMeans), and instructions for obtaining CCMI data sets are available from the British Atmospheric Data Centre (http://blogs.reading.ac.uk/ccmi/badc-data-access). MLS and OMI data used in Figure 5 are available from the Goddard Earth Science Data and Information Services Center as described at https://aura.gsfc.nasa.gov/science/data.html, and the tropospheric column ozone data products are provided by the ACDL (https://acd-ext.gsfc.nasa.gov/Data_services/cloud_slice/index.html). The Environmental Protection Agency surface O3 CASTNET measurements used in Figure 6 are available from https://java.epa.gov/castnet/clearsession.do. The authors thank three reviewers whose comments greatly improved the manuscript.
Appendix A.
A.1. History
GEOS's history GC manages output streams that the experimenter declares at run time via its configuration file, usually named HISTORY.rc. HISTORY sees the pointers to the export states of each component in the GEOS hierarchy, and assembles the experimenter's list of fields from those states into “collections” as GEOS executes. Several collections can be declared, each with specifications of some or all of the following properties or attributes that are coded into the configuration file:
Fields can be instantaneous or time‐averaged, and all fields within a collection use the same time discretization. Time‐averaged data by default are stamped with the central time of the averaging interval.
Collections are given a common name template, usually with calendar date and time included in the template.
A beginning and ending time may be specified for each collection, and each file in the collection has the same fixed number of time groups.
Collections can be written to either flat binary files or to Network Common Data Format (NetCDF) self‐describing format files, though the former is now rarely used.
In addition to time interpolation, HISTORY can subset fields and can apply GEOS's utilities to regrid the fields both horizontally and vertically. Through vertical interpolation, output fields can be written, for example, on a given set of isobaric surfaces instead of on the native eta‐coordinate levels. But, in fact, the routines operate more generally. That is, as long as the desired vertical coordinate is monotonic over the vertical region of interest and is a member of the export state of a GC in the hierarchy, it can be used as the interpolate. In the stratosphere, potential temperature meets these requirements, which enables the experimenter to generate collections on isentropic surfaces.
The remainder of the configuration file contains the list of fields in the collection. Each line consists of a comma‐separated list with the field's name as it appears in the export state of its owner GC, the name of the owner, and an optional alias. If it appears, the alias will replace the export state name when the data set is written. Aliases are useful, for example, when backward compatibility is required for users' applications. Finally, HISTORY allows specification of derived fields that can be readily defined by an arithmetic expression or function that operates on the existing, or primary, exports. The expression is evaluated using the MAPL parser component. Examples include SQRT(U*U + V*V), NO + NO2*2.00E0, and LOG10(HNO3). Since the expression is evaluated element by element, the fields must have the same shape or dimensions, and the order of operations is as in Fortran.
A.2. ExtData
ExtData is an internal MAPL GC, instantiated and run automatically by CAP. It is used to fill import states not satisfied by connectivities in the hierarchy. That is, the pointers to fields that exist in a GC's import state are passed up the GEOS hierarchy until they find an established connectivity to another GC that is able to provide data to fill the pointer. If a connectivity is not found, then the search eventually ascends to CAP, which examines the list of fields in the export state of ExtData for a match. ExtData's export state is established by its configuration file provided by the experimenter at run time, in which each field to be exported is paired with a field variable name on a specified NetCDF data set or data set template. In essence, ExtData is a provider of last resort for gridded, geospatial data. For the chemistry GCs, it provides a flexible, encapsulated way to import, for example, biomass, biofuel, and fossil fuel emissions.
After ExtData acquires a field, it is spatially interpolated (regridded) to GEOS's native resolution. Using a directive in the RC file, the data can be regridded in a mass‐conserving manner, or it will be nonconservatively regridded with bilinear interpolation. When interpolating the horizontal wind fields from their NetCDF grids, the zonal and meridional components are treated not as individual scalars, but rather as vectors that are properly rotated onto the cubed sphere's six faces. For masks, voting‐based directional mapping is used to assure that values on GEOS's native grid remain integers.
For temporal interpolation, ExtData's RC file allows the experimenter to supply a refresh template that describes how often to update the field. ExtData then examines the time variable on the NetCDF files (described by a file template) in search of two that bracket the current time on GEOS's clock. The two time stamps need not be on the same file, as ExtData can interpolate between fields from different files. ExtData provides additional flexibility to specify climatological fields, time offsets, and one‐time updates (for time invariance), the latter established only during the initialize methods. Like HISTORY, ExtData also supports derived exports whose names follow the logical requirements of the MAPL parser component.
Nielsen, J. E. , Pawson, S. , Molod, A. , Auer, B. , da Silva, A. M. , Douglass, A. R. , … Wargan, K. (2017). Chemical mechanisms and their applications in the Goddard Earth Observing System (GEOS) earth system model. Journal of Advances in Modeling Earth Systems, 9, 3019–3044. https://doi.org/10.1002/2017MS001011
References
- Allen, D. , Pickering, K. , Duncan, B. , & Damon, M. (2010). Impact of lightning NO emissions on North American photochemistry as determined using the Global Modeling Initiative (GMI) model. Journal of Geophysical Research, 115, D22301. https://doi.org/10.1029/2010JD014062 [Google Scholar]
- Aquila, V. , Oman, L. , Stolarski, R. , Douglass, A. , & Newman, P. (2013). The response of ozone and nitrogen dioxide to the eruption of Mt. Pinatubo at southern and northern midlatitudes. Journal of the Atmospheric Sciences, 70, 894–900. https://doi.org/10.1175/JAS-D-12-0143.1 [Google Scholar]
- Bacmeister, J. T. , & Stephens, G. L. (2011). Spatial statistics of likely convective clouds in CloudSat data. Journal of Geophysical Research, 116, D04104. https://doi.org/10.1029/2010JD014444 [Google Scholar]
- Bacmeister, J. T. , Suarez, M. J. , & Robertson, F. R. (2006). Rain reevaporation, boundary layer convection interactions, and Pacific rainfall patterns in a AGCM. Journal of the Atmospheric Sciences, 63, 3383–3403. https://doi.org/10.1175/JAS3791.1 [Google Scholar]
- Barahona, D. , Molod, A. , Bacmeister, J. , Nenes, A. , Gettelman, A. , Morrison, H. , … Eichmann, A. (2014). Development of two‐moment cloud microphysics for liquid and ice within the NASA Goddard Earth Observing System Model (GEOS‐5). Geoscientific Model Development, 7, 1733–1766. https://doi.org/10.5194/gmd-7-1733-2014 [Google Scholar]
- Bell, N. , Hsu, L. , Jacob, D. J. , Schultz, M. G. , Blake, D. R. , Butler, J. H. , … Maier‐Reimer, E. (2002). Methyl iodide: Atmospheric budget and use as a tracer of marine convection in global models. Journal of Geophysical Research, 107(D17), 4340 https://doi.org/10.1029/2001JD001151 [Google Scholar]
- Bey, I. , Jacob, D. J. , Yantosca, R. M. , Logan, J. A. , Field, B. D. , Fiore, A. M. , … Schultz, M. G. (2001). Global modeling of tropospheric chemistry with assimilated meteorology: Model description and evaluation. Journal of Geophysical Research, 106, 23,073–23,095. https://doi.org/10.1029/2001JD000807 [Google Scholar]
- Bhartia, P. K. , McPeters, R. D. , Flynn, L. E. , Taylor, S. , Kramarova, N. A. , Frith, S. , … DeLand, M. (2013). Solar backscatter UV (SBUV) total ozone and profile algorithm. Atmospheric Measurement Techniques, 6, 2533–2548. https://doi.org/10.5194/amt-6-2533-2013. [Google Scholar]
- Bian, H. , & Prather, M. J. (2002). Fast‐J2: Accurate simulations of photolysis in global climate models. Journal of Atmospheric Chemistry, 41, 281–296. https://doi.org/10.1023/A:1014980619462 [Google Scholar]
- Bloom, S. , Takacs, L. , DaSilva, A. , & Ledvina, D. (1996). Data assimilation using incremental analysis updates. Monthly Weather Review, 124, 1256–1271. [Google Scholar]
- Brown, S. S. , Dubé, W. P. , Karamchandani, P. , Yarwood, G. , Peischl, J. , Ryerson, T. B. , … Ravishankara, A. R. (2012). Effects of NOx control and plume mixing on nighttime chemical processing of plumes from coal‐fired power plants. Journal of Geophysical Research, 117, D07304. https://doi.org/10.1029/2011JD016954 [Google Scholar]
- Chin, M. , Ginoux, P. , Kinne, S. , Torres, O. , Holben, B. N. , Duncan, B. N. , … Nakajima, T. (2002). Tropospheric aerosol optical thickness from the GOCART model and comparisons with satellite and sunphotometer measurements. Journal of the Atmospheric Sciences, 59, 461–483. [Google Scholar]
- Chou, M.‐D. (1990). Parameterizations for the absorption of solar radiation by O2 and CO2 with applications to climate studies. Journal of Climate, 3, 209–217. [Google Scholar]
- Chou, M.‐D. (1992). A solar radiation model for use in climate studies. Journal of the Atmospheric Sciences, 49, 762–772. [Google Scholar]
- Chou, M.‐D. , & Suarez, M. J. (1994). An efficient thermal infrared radiation parameterization for use in general circulation models (NASA Tech. Memorandum, NASA/TM‐1994–104606, Vol. 3, 85 p.). Greenbelt, MD: NASA Goddard Space Flight Center. [Google Scholar]
- Cionni, I. , Eyring, V. , Lamarque, J. F. , Randel, W. J. , Stevenson, D. S. , Wu, F. , … Waugh, D. W. (2011). Ozone database in support of CMIP5 simulations: Results and corresponding radiative forcing. Atmospheric Chemistry and Physics Discussion, 11, 10,875–10,933. https://doi.org/10.5194/acpd-11-10875-2011 [Google Scholar]
- Colarco, P. R. , da Silva, A. , Chin, M. , & Diehl, T. (2010). On‐line simulations of global aerosol distributions in the NASA GEOS‐4 model and comparisons to satellite and ground‐based aerosol optical depth. Journal of Geophysical Research, 115, D14207. https://doi.org/10.1029/2009JD012820 [Google Scholar]
- Considine, D. B. , Bergman, D. J. , & Liu, H. (2005). Sensitivity of Global Modeling Initiative chemistry and transport model simulations of radon‐222 and lead‐210 to input meteorological data. Atmospheric Chemistry and Physics, 5, 3389–3406. https://doi.org/10.5194/acp-5-3389-2005 [Google Scholar]
- Considine, D. B. , Douglass, A. R. , Connell, P. S. , Kinnison, D. E. , & Rotman, D. A. (2000). A polar stratospheric cloud parameterization for the global modeling initiative three‐dimensional model and its response to stratospheric aircraft. Journal of Geophysical Research, 105, 3955–3974. https://doi.org/10.1029/1999JD900932 [Google Scholar]
- Considine, D. B. , Kawa, S. R. , Schoeberl, M. R. , & Douglass, A. R. (2003). N2O and NOy observations in the 1999/2000 Arctic polar vortex: Implications for transport processes in a CTM. Journal of Geophysical Research, 108(D5), 4170 https://doi.org/10.1029/2002JD002525 [Google Scholar]
- Cooper, O. R. , Forster, C. , Parrish, D. , Trainer, M. , Dunlea, E. , Ryerson, T. , … Moody, J. (2004). A case study of transpacific warm conveyor belt transport: Influence of merging airstreams on trace gas import to North America. Journal of Geophysical Research, 109, D23S08. https://doi.org/10.1029/2003JD003624 [Google Scholar]
- Douglass, A. R. , & Kawa, S. R. (1999). Contrast between 1992 and 1997 high‐latitude spring Halogen Occultation Experiment observations of lower stratospheric HCl. Journal of Geophysical Research, 104, 18,739–18,754. https://doi.org/10.1029/1999JD900281 [Google Scholar]
- Douglass, A. R. , Rood, R. B. , Kawa, S. R. , & Allen, D. J. (1997). A 3D simulation of the evolution of the middle latitude winter ozone in the middle stratosphere. Journal of Geophysical Research, 102, 19,217–19,232. https://doi.org/10.1029/97JD01043 [Google Scholar]
- Douglass, A. R. , Stolarski, R. S. , Strahan, S. E. , & Connell, P. S. (2004). Radicals and reservoirs in the GMI chemistry and transport model: Comparison to measurements. Journal of Geophysical Research, 109, D16302. https://doi.org/10.1029/2004JD004632 [Google Scholar]
- Douglass, A. R. , Strahan, S. E. , Oman, L. D. , & Stolarski, R. S. (2014). Understanding differences in chemistry climate model projections of stratospheric ozone. Journal of Geophysical Research: Atmospheres, 119, 4922–4939. https://doi.org/10.1002/2013JD021159 [Google Scholar]
- Duncan, B. , Portman, D. , Bey, I. , & Spivakovsky, C. (2000). Parameterization of OH for efficient computation in chemical tracer models. Journal of Geophysical Research, 105, 12,259–12,262. https://doi.org/10.1029/1999JD901141 [Google Scholar]
- Duncan, B. N. , Lamsal, L. N. , Thompson, A. M. , Yoshida, Y. , Lu, Z. , Streets, D. G. , … Pickering, K. E. (2016). A space‐based, high‐resolution view of notable changes in urban NOx pollution around the world (2005–2014). Journal of Geophysical Research: Atmospheres, 121, 976–996. https://doi.org/10.1002/2015JD024121 [Google Scholar]
- Duncan, B. N. , & Logan, J. A. (2008). Model analysis of the factors regulating the trends of carbon monoxide, 1988–1997. Atmospheric Chemistry and Physics, 8, 7389–7403. https://doi.org/10.5194/acp-8-7389-2008 [Google Scholar]
- Duncan, B. N. , Logan, J. A. , Bey, I. , Megretskaia, I. A. , Yantosca, R. M. , Novelli, P. C. , … Rinsland, C. P. (2007). The global budget of CO, 1988–1997: Source estimates and validation with a global model. Journal of Geophysical Research, 112, D22301. https://doi.org/10.1029/2007JD008459 [Google Scholar]
- Eastham, S. D. , Weisenstein, D. K. , & Barrett, S. R. H. (2014). Development and evaluation of the unified tropospheric‐stratospheric chemistry extension (UCX) for the global chemistry‐transport model GEOS‐Chem. Atmospheric Environment, 89, 52–63. https://doi.org/10.1016/j.atmosenv.2014.02.001 [Google Scholar]
- Elshorbany, Y. F. , Duncan, B. N. , Strode, S. A. , Wang, J. S. , & Kouatchou, J. (2016). The description and validation of a computationally‐efficient CH4‐CO‐OH (ECCOHv1.01) chemistry module for 3‐D model applications. Geoscientific Model Development, 9, 799–822. https://doi.org/10.5194/gmd-9-799-2016 [Google Scholar]
- Eyring, V. , Butchart, N. , Waugh, D. W. , Akiyoshi, H. , Austin, J. , Bekki, S. , … Yoshiki, M. (2006). Assessment of temperature, trace species and ozone in chemistry‐climate model simulations of the recent past. Journal of Geophysical Research, 111, D22308. https://doi.org/10.1029/2006JD007327 [Google Scholar]
- Eyring, V. , Waugh, D. W. , Bodeker, G. E. , Cordero, E. , Akiyoshi, H. , Austin, J. , … Yoshiki, M. (2007). Multimodel projections of stratospheric ozone in the 21st century. Journal of Geophysical Research, 112, D16303. https://doi.org/10.1029/2006JD008332 [Google Scholar]
- Eyring V., Shepherd T. G., & Waugh D. W. (Eds.) (2010). SPARC report on the evaluation of chemistry‐climate models (SPARC Rep. 5, WCRP‐132, WMO/TD 1526). Retrieved from http://www.atmosp.physics.utoronto.ca/SPARC/ccmval_final
- Eyring, V. , Lamarque, J. F. , Hess, P. , Arfeuille, F. , Bowman, K. , Chipperfield, M. , … Young, P. J. (2013). Overview of IGAC/SPARC Chemistry‐Climate Model Initiative (CCMI) community simulations in support of upcoming ozone and climate assessments (SPARC Newsletter 40, pp. 48–66). Geneva, Switzerland: World Meteorological Organization.
- Fioletov, V. E. , Labow, G. , Evans, R. , Hare, E. W. , Köhler, U. , McElroy, C. T. , … Weber, M. (2008). The performance of the ground‐based total ozone network assessed using satellite data. Journal of Geophysical Research, 113, D14313. https://doi.org/10.1029/2008JD009809 [Google Scholar]
- Fiore, A. M. , F. J., Dentener , O., Wild , C., Cuvelier , M. G., Schultz , P., Hess , C., … Zuber, A. (2009). Multimodel estimates of intercontinental source‐receptor relationships for ozone pollution. Journal of Geophysical Research, 114, D04301. https://doi.org/10.1029/2008JD010816 [Google Scholar]
- Fleming, E. L. , Jackman, C. H. , Stolarski, R. S. , & Douglas, A. R. (2011). A model study of the impact of source gas changes on the stratosphere for 1850–2100. Atmospheric Chemistry and Physics, 11, 8515–8541. https://doi.org/10.5194/acp-11-8515-2011 [Google Scholar]
- Flemming, J. , Benedetti, A. , Inness, A. , Engelen, R. J. , Jones, L. , Huijnen, V. , … Katragkou, E. (2017). The CAMS interim reanalysis of carbon monoxide, ozone, and aerosol for 2003–2015. Atmospheric Chemistry and Physics, 17, 1945–1983. https://doi.org/10.5194/acp-17-1945-2017 [Google Scholar]
- Frith, S. M. , Kramarova, N. A. , Stolarski, R. S. , McPeters, R. D. , Bhartia, P. K. , & Labow, G. J. (2014). Recent changes in total column ozone based on the SBUV Version 8.6 Merged Ozone Data Set. Journal of Geophysical Research: Atmospheres, 119, 9735–9751. https://doi.org/10.1002/2014JD021889 [Google Scholar]
- Garcia, R. R. , & Boville, B. A. (1994). Downward control of the mean meridional circulation and temperature distribution of the polar winter stratosphere. Journal of the Atmospheric Sciences, 51, 2238–2245. [Google Scholar]
- Garfinkel, C. I. , Molod, A. , Oman, L. D. , & Song, I.‐S. (2011). Improvement of the GEOS‐5 AGCM upon updating the air‐sea roughness parameterization. Geophysical Research Letters, 38, L18702. https://doi.org/10.1029/2011GL048802 [Google Scholar]
- Gelaro, R. , McCarty, W. , Suárez, M. J. , Todling, R. , Molod, A. , Takacs, L. , … Zhao, B. (2017). The Modern‐Era Retrospective Analysis for Research and Applications, Version‐2 (MERRA‐2). Journal of Climate, 30, 5419–5454. https://doi.org/10.1175/JCLI-D-16-0758.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffies, S. M. , Gnanadesikan, A. , Dixon, K. W. , Dunne, J. P. , Gerdes, R. , Harrison, M. J. , … Zhang, R. (2005). Formulation of an ocean model for global climate simulations. Ocean Science, 1, 45–79. https://doi.org/10.5194/os-1-45-2005 [Google Scholar]
- Guenther, A. , Baugh, B. , Brasseur, G. , Greenberg, J. , Harley, P. , Klinger, L. , … Vierling, L. (1999). Isoprene emission estimates and uncertainties for the Central African EXPRESSO study domain. Journal of Geophysical Research, 104, 30,625–30,639. https://doi.org/10.1029/1999JD900391 [Google Scholar]
- Guenther, A. , Karl, T. , Harley, P. , Wiedinmyer, C. , Palmer, P. I. , & Geron, C. (2006). Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmospheric Chemistry and Physics, 6, 3181–3210. https://doi.org/10.5194/acp-6-3181-2006 [Google Scholar]
- Guenther, A. C. , Pierce, T. , Lamb, B. , Harley, P. , & Fall, R. (2000). Natural emissions of non‐methane volatile organic compounds, carbon monoxide, and oxides of nitrogen from North America. Atmospheric Environment, 34, 2205–2230. https://doi.org/10.1016/S1352-2310(99)00465-3 [Google Scholar]
- Haigh, J. D. , & Pyle, J. A. (1982). Ozone perturbation experiments in a two‐dimensional circulation model. Quarterly Journal of the Royal Meteorological Society, 108, 5510–5574. https://doi.org/10.1002/qj.49710845705 [Google Scholar]
- Heath, D. F. , Krueger, A. J. , Roeder, H. A. , & Henderson, B. D. (1975). The solar backscatter ultraviolet and total ozone mapping spectrometer (SBUV/TOMS) for Nimbus G. Optical Engineering, 14, 323–331. [Google Scholar]
- Helfand, H. M. , & Schubert, S. D. (1995). Climatology of the simulated Great Plains low‐level jet and its contribution to the continental moisture budget of the United States. Journal of Climate, 8, 784–806. [Google Scholar]
- Hill, C. , DeLuca, C. , Balaji, V. , Suarez, M. , & da Silva, A. (2004). Architecture of the earth system modeling framework. Computing in Science and Engineering, 6(1), 18–28. https://doi.org/10.1109/MCISE.2004.1255817 [Google Scholar]
- Horowitz, L. W. , Liang, J. Y. , Gardner, G. M. , & Jacob, D. J. (1998). Export of reactive nitrogen from North America during summertime: Sensitivity to hydrocarbon chemistry. Journal of Geophysical Research, 103, 13,451–13,476. https://doi.org/10.1029/97JD03142 [Google Scholar]
- Hudman, R. C. , Moore, N. E. , Mebust, A. K. , Martin, R. V. , Russell, A. R. , Valin, L. C. , & Cohen, R. C. (2012). Steps towards a mechanistic model of global soil nitric oxide emissions: Implementation and space based‐constraints. Atmospheric Chemistry and Physics, 12, 7779–7795. https://doi.org/10.5194/acp-12-7779-2012 [Google Scholar]
- Iacono, M. J. , Mlawer, E. J. , Clough, S. A. , & Morcrette, J.‐J. (2000). Impact of an improved longwave radiation model, RRTM, on the energy budget and thermodynamic properties of the NCAR community climate model, CCM3. Journal of Geophysical Research, 105, 14,873–14,890. https://doi.org/10.1029/2000JD900091 [Google Scholar]
- Jacob, D. J. , & Bakwin, P. S. (1991). Cycling of NOx in tropical forest canopies and its implications for the global source of biogenic NOx to the atmosphere In Whitman W. B. (Ed.), Microbial production and consumption of greenhouse gases. Washington, DC: American Society of Microbiology. [Google Scholar]
- Jacob, D. J. , & Prather, M. J. (1990). Radon‐222 as a test of convective transport in a general circulation model. Tellus, Series B, 42, 118–134. https://doi.org/10.1034/j.1600-0889.1990.00012.x [Google Scholar]
- Jacobson, M. Z. (1995). Computation of Global Photochemistry with SMVGEAR‐II. Atmospheric Environment, 29(18), 2541–2546. https://doi.org/10.1016/1352-2310(95)00194-4 [Google Scholar]
- Jordan, C. E. , Dibb, J. E. , & Finkel, R. C. (2003). 10Be/7Be tracer of atmospheric transport and stratosphere‐troposphere exchange. Journal of Geophysical Research, 108(D8), 4234 https://doi.org/10.1029/2002JD002395 [Google Scholar]
- Kawa, S. R. , Kumer, J. B. , Douglass, A. R. , Roche, A. E. , Smith, S. E. , Taylor, F. W. , & Allen, D. J. (1995). Missing chemistry of reactive nitrogen in the upper stratospheric polar winter. Geophysical Research Letters, 22, 2629–2632. https://doi.org/10.1029/95GL02336 [Google Scholar]
- Kinnison, D. E. , Connell, P. S. , Rodriguez, J. M. , Rotman, D. A. , Considine, D. B. , Tannahill, J. , … Prather, M. J. (2001). The Global Model Initiative Assessment Model: Applications to high‐speed civil transport perturbation. Journal of Geophysical Research, 106, 1693–1712. https://doi.org/10.1029/2000JD900406 [Google Scholar]
- Kondo, J. (1975). Air‐sea bulk transfer coefficients in diabatic conditions. Boundary‐Layer Meteorology, 9, 91–112. [Google Scholar]
- Lamsal, L. N. , Duncan, B. N. , Yoshida, Y. , Krotkov, N. A. , Pickering, K. E. , Streets, D. G. , & Lu, Z. (2015). U.S. NO2 trends (2005–2013): EPA Air Quality System (AQS) data versus improved observations from the Ozone Monitoring Instrument (OMI). Atmospheric Environment, 110, 130–143. https://doi.org/10.1016/j.atmosenv.2015.03.055 [Google Scholar]
- Large, W. G. , & Pond, S. (1981). Open ocean momentum flux measurements in moderate to strong winds. Journal of Physical Oceanography, 11, 324–336. [Google Scholar]
- Lean, J. (2000). Evolution of the Sun's spectral irradiance since the Maunder Minimum. Geophysical Research Letters, 27, 2425–2428. [Google Scholar]
- Levelt, P. F. , van den Oord, G. H. J. , Dobber, M. R. , Mälkki, A. , Visser, H. , de Vries, J. , … Saari, H. (2006). The ozone monitoring instrument. IEEE Transactions on Geoscience and Remote Sensing, 44, 1093–1011. https://doi.org/10.1109/TGRS.2006.872333 [Google Scholar]
- Liaskos, C. E. , Allen, D. J. , & Pickering, K. E. (2015). Sensitivity of tropical tropospheric composition to lightning NOx production as determined by replay simulations with GEOS‐5. Journal of Geophysical Research: Atmospheres, 120, 8512–8534. https://doi.org/10.1002/2014JD022987 [Google Scholar]
- Lin, S.‐J. (2004). A vertically Lagrangian finite‐volume dynamical core for global models. Monthly Weather Review, 132, 2293–2307. [Google Scholar]
- Lin, S.‐J. , & Rood, R. B. (1996). Multidimensional flux form semi‐Lagrangian transport schemes. Monthly Weather Review, 124, 2046–2070. https://doi.org/10.1175/1520-0493(1996)124<2046:MFFSLT>2.0.CO;2 [Google Scholar]
- Lin, S.‐J. , & Rood, R. B. (1997). An explicit flux‐form semi‐Lagrangian shallow‐water model on the sphere. Quarterly Journal of the Royal Meteorological Society, 123, 2477–2498. https://doi.org/10.1002/qj.49712354416 [Google Scholar]
- Liu, X. , Ma, P.‐L. , Wang, H. , Tilmes, S. , Singh, B. , Easter, R. C. , … Rasch, P. J. (2016). Description and evaluation of a new four‐mode version of the Modal Aerosol Module (MAM4) within version 5.3 of the Community Atmosphere Model. Geoscientific Model Development, 9, 505–522. https://doi.org/10.5194/gmd-9-505-2016 [Google Scholar]
- Lock, A. P. , Brown, A. R. , Bush, M. R. , Martin, G. M. , & Smith, R. N. B. (2000). A new boundary layer mixing scheme. Part I: Scheme description and single‐column model tests. Monthly Weather Review, 138, 3187–3199. [Google Scholar]
- Long, M. S. , Yantosca, R. , Nielsen, J. E. , Keller, C. A. , da Silva, A. , Sulprizio, M. P. , … Jacob, D. J. (2015). Development of a grid‐independent GEOS‐Chem chemical transport model (v9‐02) as an atmospheric chemistry module for Earth system models. Geoscientific Model Development, 8, 595–602. https://doi.org/10.5194/gmd-8-595-2015 [Google Scholar]
- Louis, J. , & Geleyn, J. (1982). A short history of the PBL parameterization at ECMWF In Proceedings of the ECMWF workshop on planetary boundary layer parameterization (pp. 59–80). Reading, UK: ECMWF. [Google Scholar]
- Manzini, E. , & Feichter, J. (1999). Simulation of the SF6 tracer with the middle atmosphere MAECHAM4 model: Aspects of large‐scale transport. Journal of Geophysical Research, 104, 31,097–31,108. https://doi.org/10.1029/1999JD900963 [Google Scholar]
- Martini, M. , Allen, D. J. , Pickering, K. E. , Stenchikov, G. L. , Richter, A. , Hyer, E. J. , & Loughner, C. P. (2011). The impact of North American anthropogenic emissions and lightning on long‐range transport of trace gases and their export from the continent during summers 2002 and 2004. Journal of Geophysical Research, 116, D07305. https://doi.org/10.1029/2010JD014305 [Google Scholar]
- McGrath‐Spangler, E. L. , & Molod, A. (2014). Comparison of GEOS‐5 AGCM planetary boundary layer depths computed with various definitions. Atmospheric Chemistry and Physics, 14, 6717–6727. https://doi.org/10.5194/acp-14-6717-2014 [Google Scholar]
- McFarlane, N. A. (1987). The effect of orographically excited gravity‐wave drag on the circulation of the lower stratosphere and troposphere. Journal of the Atmospheric Sciences, 44, 1775–1800. [Google Scholar]
- McPeters, R. D. , Labow, G. J. , & Logan, J. A. (2007). Ozone climatological profiles for satellite retrieval algorithms. Journal of Geophysical Research, 112, D05308. https://doi.org/10.1029/2005JD006823 [Google Scholar]
- Meinshausen, M. , Smith, S. J. , Calvin, K. , Daniel, J. S. , Kainuma, M. L. T. , Lamarque, J. F. , … van Vuuren, D. P. P. (2011). The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Climatic Change, 109(1–2), 213–241. https://doi.org/10.1007/s10584-011-0156-z [Google Scholar]
- Molod, A. (2012). Constraints on the total water PDF in GCMs from AIRS data and a high resolution model. Journal of Climate, 25, 8341–8352. https://doi.org/10.1175/JCLI-D-11-00412.1 [Google Scholar]
- Molod, A. , Partyka, G. , & Suarez, M. (2013). The impact of limiting ocean roughness on GEOS‐5 AGCM tropical cyclone forecasts. Geophysical Research Letters, 40, 411–416. https://doi.org/10.1029/2012GL053979 [Google Scholar]
- Molod, A. , Takacs, L. , Suarez, M. , & Bacmeister, J. (2015). Development of the GEOS‐5 atmospheric general circulation model: Evolution from MERRA to MERRA2. Geoscientific Model Development, 8, 1339–1356. https://doi.org/10.5194/gmd-8-1339-2015 [Google Scholar]
- Molod, A. , Takacs, L. , Suarez, M. , Bacmeister, J. , Song, I.‐S. , & Eichmann, A. (2012). The GEOS‐5 Atmospheric General Circulation Model: Mean Climate and Development from MERRA to Fortuna (NASA Tech. Rep. Series on Global Modeling and Data Assimilation, NASA/TM‐2012–104606, Vol. 28, 117 pp.). Greenbelt, MD: NASA Goddard Space Flight Center. [Google Scholar]
- Moorthi, S. , & Suarez, M. J. (1992). Relaxed Arakawa Schubert: A parameterization of moist convection for general circulation models. Monthly Weather Review, 120, 978–1002. [Google Scholar]
- Moss, R. H. , Edmonds, J. A. , Hibbard, K. A. , Manning, M. R. , Rose, S. K. , van Vuuren, D. P. , … Wilbanks, T. J. (2010). The next generation of scenarios for climate change research and assessment. Nature, 463(7282), 747–756. https://doi.org/10.1038/nature08823 [DOI] [PubMed] [Google Scholar]
- Naik, V. , Voulgarakis, A. , Fiore, A. M. , Horowitz, L. W. , Lamarque, J.‐F. , Lin, M. , … Zeng, G. (2013). Preindustrial to present‐day changes in tropospheric hydroxyl radical and methane lifetime from the Atmospheric Chemistry and Climate Model Intercomparison Project (ACCMIP). Atmospheric Chemistry and Physics, 13, 5277–5298. https://doi.org/10.5194/acp-13-5277-2013 [Google Scholar]
- Newton, C. W. , & Trevisan, A. (1984). Clinogenesis and frontogenesis in jet‐stream waves, Part I: Analytical relations to wave structure. Journal of the Atmospheric Sciences, 41, 2717–2734. [Google Scholar]
- Olson, J. (1992). World Ecosystems (WEI.4): Digital raster data on a 10‐minute geographic 1080 × 2160 grid In Global ecosystems database, version 1.0: Disc A. Boulder, CO: NOAA Natl. Geophys. Data Center. [Google Scholar]
- Oman, L. D. , & Douglass, A. R. (2014). Improvements in total column ozone in GEOSCCM and comparisons with a new ozone‐depleting substances scenario. Journal of Geophysical Research: Atmospheres, 119, 5613–5624. https://doi.org/10.1002/2014JD021590 [Google Scholar]
- Orbe, C. , Waugh, D. W. , & Newman, P. A. (2015). Air‐mass origin in the tropical lower stratosphere: The influence of Asian boundary layer air. Geophysical Research Letters, 42, 4240–4248. https://doi.org/10.1002/2015GL063937 [Google Scholar]
- Ott, L. E. , Duncan, B. N. , Thompson, A. M. , Diskin, G. , Fasnacht, Z. , Langford, A. O. , … Yoshida, Y. (2016). Frequency and impact of summertime stratospheric intrusions over Maryland during DISCOVER‐AQ (2011): New evidence from NASA's GEOS‐5 simulations. Journal of Geophysical Research, 121, 3687–3706. https://doi.org/10.1002/2015JD024052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra, P. K. , Houweling, S. , Krol, M. , Bousquet, P. , Belikov, D. , Bergmann, D. , … Wilson, C. (2011). TransCom model simulations of CH4 and related species: Linking transport, surface flux and chemical loss with CH4 variability in the troposphere and lower stratosphere. Atmospheric Chemistry and Physics, 11, 12,813–12,837. https://doi.org/10.5194/acp-11-12813-2011 [Google Scholar]
- Pawson, S. , Stajner, I. , Kawa, S. R. , Hayashi, H. , Tan, W. , Nielsen, J. E. , … Livesey, N. J. (2007). Stratospheric transport using six‐hour averaged winds from a data assimilation system. Journal of Geophysical Research, 112, D23103. https://doi.org/10.1029/2006JD007673 [Google Scholar]
- Pawson, S. , Stolarski, R. S. , Douglass, A. R. , Newman, P. A. , Nielsen, J. E. , Frith, S. M. , & Gupta, M. L. (2008). Goddard Earth Observing System Chemistry‐Climate Model simulations of stratospheric ozone‐temperature coupling between 1950 and 2005. Journal of Geophysical Research, 113, D12103. https://doi.org/10.1029/2007JD009511 [Google Scholar]
- Perlwitz, J. , Pawson, S. , Fogt, R. L. , Nielsen, J. E. , & Neff, W. D. (2008). Impact of stratospheric ozone hole recovery on Antarctic climate. Geophysical Research Letters, 35, L08714. https://doi.org/10.1029/2008GL033317 [Google Scholar]
- Prather, M. J. , & Spivakovsky, C. M. (1990). Tropospheric OH and the lifetimes of hydrochlorofluorocarbons. Journal of Geophysical Research, 95, 18,723–18,729. https://doi.org/10.1029/JD095iD11p18723 [Google Scholar]
- Prather, M. J. , Zhu, X. , Tang, Q. , Hsu, J. , & Neu, J. L. (2011). An atmospheric chemist in search of the tropopause. Journal of Geophysical Research, 116, D04306. https://doi.org/10.1029/2010JD014939 [Google Scholar]
- Putman, W. M. , & Lin, S.‐J. (2007). Finite‐volume transport on various cubed‐sphere grids. Journal of Computational Physics, 227, 55–78. https://doi.org/10.1016/j.jcp.2007.07.022 [Google Scholar]
- Putman, W. M. , & Suarez, M. (2011). Cloud‐system resolving simulations with the NASA Goddard Earth Observing System global atmospheric model (GEOS‐5). Geophysical Research Letters, 38, L16809. https://doi.org/10.1029/2011GL048438 [Google Scholar]
- Rienecker, M. M. , Suarez, M. J. , Todling, R. , Bacmeister, J. , Takacs, L. , Liu, H.‐C. , … Nielsen, J. E. (2008). The GEOS‐5 Data Assimilation System: Documentation of versions 5.0.1 and 5.1.0, and 5.2.0 (NASA Tech. Rep. Series on Global Modeling and Data Assimilation, NASA/TM‐2008–104606, Vol. 27, 92 p.). Greenbelt, MD: NASA Goddard Space Flight Center. [Google Scholar]
- Rienecker, M. M. , Suarez, M. J. , Gelaro, R. , Todling, R. , Bacmeister, J. , Liu, E. , … Woollen, J. (2011). MERRA: NASA's Modern‐Era Retrospective Analysis for Research and Applications. Journal of Climate, 24, 1–25. https://doi.org/10.1175/JCLI-D-11-00015.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert, S. D. , Wang, H. , Koster, R. D. , Suarez, M. J. , & Groisman, P. Y. (2014). Northern Eurasian heat waves and droughts. Journal of Climate, 27, 3169–3207. https://doi.org/10.1175/JCLI-D-13-00360.1 [Google Scholar]
- Shindell, D. T. , Faluvegi, G. , Stevenson, D. S. , Krol, M. C. , Emmons, L. K. , Lamarque, J.‐F. , … Zeng, G. (2006). Multimodel simulations of carbon monoxide: Comparison with observations and projected near‐future changes. Journal of Geophysical Research, 111, D19306. https://doi.org/10.1029/2006JD007100 [Google Scholar]
- Shindell, D. T. , Chin, M. , Dentener, F. , Doherty, R. M. , Faluvegi, G. , Fiore, A. M. , … Zuber, A. (2008). A multi‐model assessment of pollution transport to the Arctic. Atmospheric Chemistry and Physics, 8, 5353–5372. https://doi.org/10.5194/acp-8-5353-2008 [Google Scholar]
- Simmons, A. J. , & Burridge, D. M. (1981). An energy and angular‐momentum conserving vertical finite‐difference scheme and hybrid vertical coordinates. Monthly Weather Review, 109, 758–766 [Google Scholar]
- Spivakovsky, C. , Wofsy, S. , & Prather, M. (1990a). A numerical method for the parameterization of atmospheric chemistry: Computation of tropospheric OH. Journal of Geophysical Research, 95, 18,433–18,439. https://doi.org/10.1029/JD095iD11p18433 [Google Scholar]
- Spivakovsky, C. M. , Logan, J. A. , Montzka, S. A. , Balkanski, Y. J. , Foreman‐Fowler, M. , Jones, D. B. A. , … McElroy, M. B. (2000). Three‐dimensional climatological distribution of tropospheric OH: Update and evaluation. Journal of Geophysical Research, 105, 8931–8980. https://doi.org/10.1029/1999JD901006 [Google Scholar]
- Spivakovsky, C. M. , Yevich, R. , Logan, J. A. , Wofsy, S. C. , McElroy, M. B. , & Prather, M. J. (1990b). Tropospheric OH in a three‐dimensional chemical tracer model: An assessment based on observations of CH3CCl3 . Journal of Geophysical Research, 95, 18,441–18,471. https://doi.org/10.1029/JD095iD11p18441 [Google Scholar]
- Stohl, A. , Eckhardt, S. , Forster, C. , James, P. , & Spichtinger, N. (2002). On the pathways and timescales of intercontinental air pollution transport. Journal of Geophysical Research, 107(D23), 4684 https://doi.org/10.1029/2001JD001396 [Google Scholar]
- Stolarski, R. S. , Douglass, A. R. , Gupta, M. , Newman, P. A. , Pawson, S. , Schoeberl, M. R. , & Nielsen, J. E. (2006). An ozone increase in the Antarctic summer stratosphere: A dynamical response to the ozone hole. Geophysical Research Letters, 33, L21805. https://doi.org/10.1029/2006GL026820 [Google Scholar]
- Stolarski, R. S. , Douglass, A. R. , Newman, P. A. , Pawson, S. , & Schoeberl, M. R. (2010). Relative contribution of greenhouse gases and ozone‐depleting substances to temperature trends in the stratosphere: A chemistry‐climate model study. Journal of Climate, 23, 28–42. https://doi.org/10.1175/2009JCLI2955.1 [Google Scholar]
- Strode, S. A. , Rodriguez, J. M. , Logan, J. A. , Cooper, O. R. , Witte, J. C. , Lamsal, L. N. , … Strahan, S. E. (2015). Trends and variability in regional surface ozone over the United States. Journal of Geophysical Research: Atmospheres, 120, 9020–9042. https://doi.org/10.1002/2014JD022784 [Google Scholar]
- Suarez, M. , Trayanov, A. , Hill, C. , Schopf, P. , & Vikhliaev, Y. (2007). MAPL: A high‐level programming paradigm to support more rapid and robust encoding of hierarchical trees of interacting high‐performance components. In Proceedings of the 2007 symposium on component and framework technology in high‐performance and scientific computing (pp. 11–20). https://doi.org/10.1145/1297385.1297388
- Swartz, W. H. , Stolarski, R. S. , Oman, L. D. , Fleming, E. L. , & Jackman, C. H. (2012). Middle atmosphere response to different descriptions of the 11‐yr solar cycle in spectral irradiance in a chemistry‐climate model. Atmospheric Chemistry and Physics, 12, 5937–5948. https://doi.org/10.5194/acp-12-5937-2012 [Google Scholar]
- Thomas, J. L. , Raut, J. C. , Law, K. S. , Marelle, L. , Ancellet, G. , Ravetta, F. , … Schlager, H. (2013). Pollution transport from North America to Greenland during summer 2008. Atmospheric Chemistry and Physics, 13, 3825–3848. https://doi.org/10.5194/acp-13-3825-2013 [Google Scholar]
- Tokioka, T. , Yamazaki, K. , Kitoh, A. , & Ose, T. (1988). The equatorial 30–60 day oscillation and the Arakawa‐Schubert penetrative cumulus parameterization. Journal of the Meteorological Society of Japan, 66, 883–901. [Google Scholar]
- Velders, G. J. M. , & Daniel, J. S. (2014). Uncertainty analysis of projections of ozone‐depleting substances: Mixing ratios, EESC, ODPs, and GWPs. Atmospheric Chemistry and Physics, 14, 2757–2776. https://doi.org/10.5194/acp-14-2757-2014 [Google Scholar]
- Vinken, G. C. M. , Boersma, K. F. , Maasakkers, J. D. , Adon, M. , & Martin, R. V. (2014). Worldwide biogenic soil NOx emissions inferred from OMI NO2 observations. Atmospheric Chemistry and Physics, 14, 10,363–10,381. https://doi.org/10.5194/acp-14-10363-2014 [Google Scholar]
- Voulgarakis, A. , Naik, V. , Lamarque, J.‐F. , Shindell, D. T. , Young, P. J. , Prather, M. J. , … Zeng G. (2013). Analysis of present day and future OH and methane lifetime in the ACCMIP simulations. Atmospheric Chemistry and Physics, 13, 2563–2587. https://doi.org/ 10.5194/acp‐13–2563‐2013 [Google Scholar]
- Wargan, K. , Pawson, S. , Olsen, M. A. , Witte, J. C. , Douglass, A. R. , Ziemke, J. R. , Strahan, S. E. , & Nielsen, J. E. (2015). The global structure of upper troposphere‐lower stratosphere ozone in GEOS‐5: A multiyear assimilation of EOS Aura data. Journal of Geophysical Research: Atmospheres, 120, 201–2036. https://doi.org/10.1002/2014JD022493 [Google Scholar]
- Waters, J. W. , Froidevaux, L. , Harwood, R. S. , Jarnot, R. F. , Pickett, H. M. , Read, W. G. , … Walch, M. J. (2006). The Earth Observing System Microwave Limb Sounder (EOS MLS) on the Aura satellite. IEEE Transactions on Geoscience and Remote Sensing, 44, 1075–1092. https://doi.org/10.1109/TGRS.2006.873771 [Google Scholar]
- Waugh, D. W. , & Eyring, V. (2008). Quantitative performance metrics for stratospheric‐resolving chemistry‐climate models. Atmospheric Chemistry and Physics, 8, 5699–5713. https://doi.org/10.5194/acp-8-5699-2008 [Google Scholar]
- Waugh, D. W. , & Hall, T. M. (2002). Age of stratospheric air: Theory, observations, and models. Reviews of Geophysics, 40(4), 1010 https://doi.org/10.1029/2000RG000101 [Google Scholar]
- Wild, O. , Zhu, Q. , & Prather, M. J. (2000). Fast‐J: Accurate simulation of in‐ and below‐cloud photolysis in global chemical models. Journal of Atmospheric Chemistry, 37, 245–282. https://doi.org/10.1023/A:1006415919030 [Google Scholar]
- World Meteorological Organization (WMO) (2011). Scientific Assessment of Ozone Depletion: 2010, Global Ozone Research and Monitoring Project (Rep. 52, 516 p.). Geneva, Switzerland: World Meteorological Organization.
- World Meteorological Organization (WMO) (2014), Scientific Assessment of Ozone Depletion: 2014, Global Ozone Research and Monitoring Project (Rep. 55, 416 p.). Geneva, Switzerland: World Meteorological Organization.
- Wu, W.‐S. , Purser, R. J. , & Parrish, D. F. (2002). Three‐dimensional variational analysis with spatially inhomogeneous covariances. Monthly Weather Review, 130, 2905–2916 [Google Scholar]
- Yienger, J. J. , & Levy, H. II (1995). Empirical model of global soil‐biogenic NOx emissions. Journal of Geophysical Research, 100, 11,447–11,464. https://doi.org/10.1029/95JD00370 [Google Scholar]
- Ziemke, J. R. , Chandra, S. , Labow, G. J. , Bhartia, P. K. , Froidevaux, L. , & Witte, J. C. (2011). A global climatology of tropospheric and stratospheric ozone derived from Aura OMI and MLS measurements. Atmospheric Chemistry and Physics, 11, 9237–9251. https://doi.org/10.5194/acp-11-9237-2011 [Google Scholar]


