Abstract
Dystonia is a common movement disorder that devastates the lives of many patients but the etiology of this disorder remains poorly understood. Dystonia has traditionally been considered a disorder of the basal ganglia. However, growing evidence suggests that the cerebellum may be involved in certain types of dystonia raising several questions. Can different types of dystonia be classified as either a basal ganglia disorder or a cerebellar disorder? Is dystonia a network disorder that involves the cerebellum and basal ganglia? If dystonia is a network disorder, how can we target treatments to alleviate symptoms in patients? A recent study by Chen et al., using the pharmacological mouse model of Rapid-onset dystonia Parkinsonism (RDP), has provided some insight into these important questions. They showed that the cerebellum can directly modulate basal ganglia activity through a short latency cerebello-thalamo-basal ganglia pathway. Further, this paper and others have provided evidence that in some cases, aberrant cerebello-basal ganglia communication can be involved in dystonia. In this review we examine the evidence for the involvement of the cerebellum and cerebello-basal ganglia interactions in dystonia. We conclude that there is ample evidence to suggest that the cerebellum plays a role in some dystonias, including the early-onset primary torsion dystonia DYT1 and that further studies examining the role of this brain region and its interaction with the basal ganglia in dystonia are warranted.
Introduction
Dystonia is the third most common movement disorder behind only Parkinson’s disease and essential tremor1. In dystonia abnormal muscle contractions result in unwanted movements or postures2. The movements are often patterned and twisting but may be tremulous as well. Further, they are usually exaggerated by voluntary movement and are associated with muscle activation overflow, resulting in the characteristic co-contraction of agonist-antagonist muscle pairs3. Unfortunately, the neural substrates of dystonia are not completely understood and therefore therapeutic options available to patients remain limited4. While dystonia has been considered a disorder of the basal ganglia, there is evidence to suggest that other brain areas may also play a role5–7. Importantly, two recent studies have provided compelling evidence for a cerebello-thalamo basal ganglia pathway.
A 2011 study by Calderon et al., demonstrated that the rare genetic dystonia Rapid-onset dystonia Parkinsonism (RDP) can be pharmacologically modeled in the rodent by targeting both the cerebellum and basal ganglia8. It was also shown that the most common inherited dystonia, DYT1, can be modeled in the mouse by targeting only the cerebellum9. Further, it was suggested that abnormal cerebellar output may cause dystonia by altering basal ganglia activity through a di-synaptic pathway connecting these two brain areas (Figure 1). In 2014, Chen et al., showed that basal ganglia activity can be modulated by input from the cerebellum via this direct cerebello-thalamo-basal ganglia pathway10. In this perspective, we will address the evidence for a role of the cerebellum and cerebello-basal ganglia communication in dystonia. We will also discuss the implications of the paper by Chen et al. and how they add to our understanding of the pathophysiology underlying dystonia. Finally, we will address how these results can improve our therapeutic approach to dystonia.
Figure 1. A disynaptic pathway connects the cerebellum to the basal ganglia.
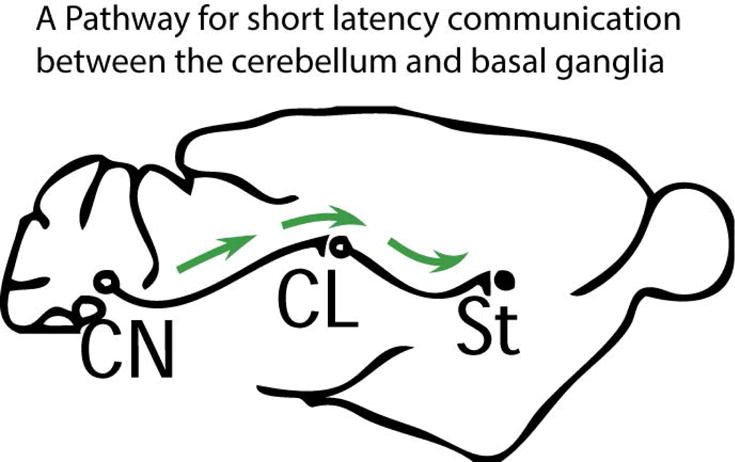
There is a disynaptic cerebello-basal ganglia connection that is conserved between rodents and humans. Fibers from the deep cerebellar nuclei (CN) pass through the intralaminar nuclei of the thalamus (in rodents primarily the centrolateral nucleus, CL) to the dorsolateral striatum (St, top image).
The Basal Ganglia in movement and movement disorders
Clinically, it has been known for a number of years that dysfunction in the basal ganglia can cause movement disorders11. Multiple lines of reasoning suggest that the basal ganglia are involved in dystonia. Because damage to the basal ganglia can lead to secondary dystonia in human patients, this area is traditionally considered to underlie this disorder. Dystonia can be associated with abnormalities in dopamine as seen in patients with DYT5 who are highly responsive to treatment with levodopa12. In addition, some patients with Parkinson’s disease present initially with dystonia13,14. Since normal dopamine signaling is vital to basal ganglia function, dopamine involvement suggests an important link between the basal ganglia and dystonia15,16. Finally, many dystonic patients experience an alleviation of symptoms after lesions or deep brain stimulation (DBS) of nuclei in the basal ganglia17–21.
Animal models have demonstrated that a number of manipulations to the basal ganglia can result in dystonia. Early studies showed that applying a GABAA antagonist to the striatum induced dystonia in cats or rats, indicating that impaired inhibition of the striatum is important in this disorder22–24. Recently, specific pharmacologic block of striatal fast spiking interneurons (FSIs) was performed in rodents and resulted in dyskinesia25. Although the activity of FSIs was uniformly decreased in mice with dyskinesia, the activity change in MSNs was more variable, suggesting that dystonia may not be caused simply by changes in firing rate of MSNs but rather by more complicated changes in firing25,26. Another study showed that abnormal bursting evident in striatal field potentials and the subsequent inhibition of activity in substantia nigra pars reticulata (SNr) were associated with dystonic movements27. In a rat model of kernicterus-induced striatal dystonia, neurons in the globus pallidus (GP), subthalamic nucleus (STN), and entopeduncular nucleus fired in a more irregular manner with many exhibiting bursts of activity uncommon under normal conditions28. Dystonic muscle contractions in this model were shown to correlate with periods of bursting of specific neurons, suggesting that burst firing in the basal ganglia may be an electrophysiological signature of dystonia28.
Studies in humans have corroborated this notion of abnormal firing in the basal ganglia. In a seminal 1999 study, Vitek et al., demonstrated that GPi neurons in patients with dystonia predominantly fire bursts of action potentials with intermittent pauses29. Since then, this finding has been replicated a number of times and burst firing has also been identified in the STN of patients with dystonia30–33. There is also evidence from humans that changes in the rate of firing of the different nuclei of the basal ganglia may contribute to dystonia. Specifically, neurons in the output nuclei of the basal ganglia exhibit a decreased firing rate in dystonic patients29,31,33–35. Still, the effectiveness of pallidotomy in alleviating dystonia rather than worsening it, suggests that a decrease in the rate of basal ganglia output neurons alone does not cause dystonia. Further, whether rate is decreased in the GP at all in dystonia is disputed by some groups36.
The Cerebellum and its role in movement and movement disorders
Cerebellar abnormalities are associated with ataxia in which patients exhibit gross incoordination of muscles37. However, there has been evidence for some time that cerebellar dysfunction and tumors of the cerebellum can result in hyperkinetic disorders as well. Wurffbain probably provided the first description of this phenomenon in 1691 when he identified a child with a cerebellar tumor who exhibited fits of opisthotonus and head retraction. In fact, head retractions and abnormal head postures have been seen numerous times in patients with cerebellar tumors38. There is evidence that cases of secondary focal neck dystonia are most often associated with lesions of the cerebellum and brainstem rather than of the basal ganglia39.
However, the involvement of the cerebellum is not limited to focal neck dystonia. In 1988, a series of patients was presented with degenerative cerebellar ataxias who also displayed prominent dystonia40. It is now clear that in some inherited ataxias, dystonia is a prominent feature37,41,42. In fact, some patients with these disorders present initially with dystonia43–45. Because these disorders are primarily associated with pathology of the cerebellum, the identification of dystonia in these patients is consistent with the hypothesis that dystonia can originate in the cerebellum. Further support came from other patients with familial dystonia associated with cerebellar atrophy46. Based on these findings, it was suggested that at least in some cases abnormalities in the cerebellum might result in dystonia.
There is also evidence that the cerebellum is abnormal in patients with hereditary dystonias. Changes in cerebellar metabolism in patients with inherited dystonia have been reported47. Studies in patients with DYT1, the most common hereditary dystonia, demonstrate that patients exhibit specific changes in cerebellar connectivity compared to controls and non-affected mutation carriers7. A recent study in patients with DYT11 highlighted that there are changes in the metabolism of the cerebellum of patients compared to non-symptomatic carriers6. In fact, there is evidence that transcranial magnetic stimulation of the cerebellum may alleviate symptoms in some dystonic patients48 .
Pioneering work in the 1960s and 1970s demonstrated that in some cases interventions involving the cerebellum could alleviate dystonia and other hyperkinetic disorders49,50. In one case, cerebellar lesion surgery was performed on a patient exhibiting an athetoid movement disorder. During surgery, Purkinje cells exhibited abnormal burst firing with the caveat that the patient was under anesthesia at the time of the recording51.
Further evidence for the role of the cerebellum in hyperkinetic disorders, specifically dystonia, has come from animal models. Both the tottering mouse and the dt rat exhibit dystonia that has been shown to originate in the cerebellum52–54. Also, recent optogenetic studies have shown that stimulation of the cerebellum can elicit involuntary movements55. In the dt rat and tottering mouse, abnormal bursting of cerebellar Purkinje cells and neurons of the CN underlies dystonia, a firing pattern similar to that identified in the athetoid patient of Slaughter, Nashold, and Somjen51,56. In addition, neurons of the deep cerebellar nuclei in cerebellar-induced mouse models of RDP and DYT1 also display abnormal high frequency bursts during dystonic postures9,57,58. Therefore, it is possible that abnormal burst firing may be a common mechanism by which the cerebellum contributes to dystonia.
Evidence for the cerebello-basal ganglia connection
Thus far, compelling evidence has been provided for involvement of the basal ganglia and the cerebellum in dystonia. However, the question still remains whether both areas can be simultaneously involved in dystonia since some inherited dystonias demonstrate abnormal function of both the cerebellum and basal ganglia59. There is also evidence that the cerebellum can affect activity in the basal ganglia. In 1969, Ratcheson and Li demonstrated that electrical stimulation of the CN evoked either excitation or inhibition of activity in neurons of the striatum of the basal ganglia60. This response was dependent on the intralaminar nuclei of the thalamus, areas deep in the thalamus now known to connect a number of regions throughout the brain and thought to be involved in attentional awareness61,62. Nearly 30 years later, this idea was resurrected when a direct di-synaptic connection between the cerebellum and basal ganglia was identified in rodents which could underlie these responses63. The pathway was found to connect the lateral cerebellar nucleus to the striatum of the basal ganglia via the intralaminar nuclei of the thalamus. In rodents, the pathway primarily traversed the centrolateral nucleus of the thalamus (CL), a small area that has been implicated in normal motor coordination62,64. Subsequently, it was shown that this pathway is conserved in primates and recent evidence suggests it is also present in humans65,66. Therefore this pathway could provide a substrate through which abnormal cerebellar output may affect the basal ganglia.
Could abnormal cerebellar output alter activity in the basal ganglia through the cerebello-basal ganglia connection?
There has been a growing interest in understanding whether a cerebello-basal ganglia circuit could play a role in dystonia54,67–70. Using a pharmacologic rodent model of RDP, Calderon et al. addressed this question and systematically demonstrated that the dystonic features in these mice were due to changes in cerebellar output67,71. Further, abnormal bursts of cerebellar EEG activity correlated with dystonic postures. The Parkinsonism-like features of RDP was recapitulated in the rodent model by infusing ouabain to the basal ganglia whereas dystonia was elicited by infusing ouabain to the cerebellum alone. Likewise, the use of shRNAs specifically targeting the α3 isoform of the sodium pump either in the substantia nigra or in the cerebellum produced the same phenotype described above57. Interestingly, dystonia could be alleviated in the pharmacologic rodent model of RDP by preventing cerebellar output, either by electric lesions of the CN, silencing the cerebellum with infusions of tetrodotoxin, or even increasing inhibitory tone by infusing GABA into the cerebellum (Figure 2). Since infusion of ouabain to both the cerebellum and basal ganglia was necessary to recapitulate all the features of RDP, one hypothesis is that abnormal cerebellar output in this pharmacologic model alters activity in the basal ganglia, resulting in dystonia. Additionally, in some cases where dystonia is thought to be secondary to cerebellar dysfunction, DBS of the basal ganglia can alleviate dystonic postures72,73. Although there are multiple interpretations of this phenomenon, it is consistent with the hypothesis that in some cases the cerebellum can drive aberrant activity in the basal ganglia to induce dystonia. Since several lines of evidence already discussed indicate that burst firing of basal ganglia neurons occurs in dystonia, an obvious question is whether or not such firing is present in cerebellum-induced dystonia.
Figure 2. The disynaptic pathway connecting the cerebellum to the basal ganglia is implicated in dystonia.
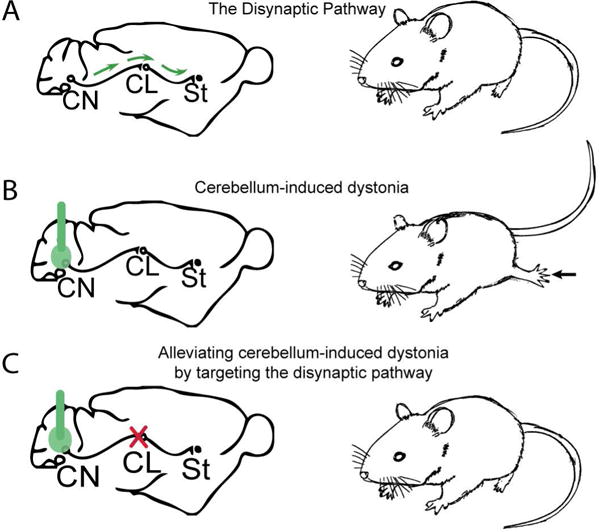
(A) A disynaptic pathway connects the cerebellum to the basal ganglia (B) When the α3 isoform of the sodium potassium ATPase is disrupted pharmacologically (left) with ouabain delivered by an osmotic pump or by shRNA injected stereotaxically in the cerebellum (green), there is a change in cerebellar output and the animal exhibits dystonia. (C) Remarkably, when the disynaptic pathway is disrupted either with (1) electrolytic lesions or (2) optogenetically, dystonic postures in the animal is relieved (right).
Short latency cerebellar modulation of the basal ganglia and its implications in dystonia
Using the established pharmacological model of RDP, Chen et al. recorded neurons in the dorsolateral striatum (DLS) of awake, freely moving animals with cerebellar-induced dystonia10. The DLS receives the majority of input from the di-synaptic pathway connecting the cerebellum to the basal ganglia62–64. They showed that in cerebellar-induced dystonia neurons of the DLS exhibited abnormal high-frequency burst firing (Figure 3). This abnormal firing was exaggerated during periods of severe dystonia. Notably, the abnormal firing pattern observed in the DLS was also recorded in cells of the CN in the RDP mouse model58. Additionally, because the recordings in the DLS were performed using microarrays, there was the added advantage of simultaneously recording cells in close proximity to each other. They found that the activity of nearby neurons in the DLS was highly correlated during cerebellar-induced dystonia, in agreement with the hypothesis that the cells in the DLS receive a strong common drive (Figure 4). To determine whether the cerebellum is the instigator of the aberrant firing in the DLS, the centrolateral nucleus of the thalamus was electrically lesioned or silenced optogenetically, thereby disrupting communication between the cerebellum and basal ganglia. Remarkably, by severing the disynaptic pathway in dystonic mice, Chen et al., found that the dystonic postures were alleviated, suggesting that the cerebellum could be the source of aberrant drive that modifies activity in the striatum thereby resulting in dystonia. Together, these findings support the hypothesis that abnormal cerebellar output drives high-frequency bursting activity in the DLS to cause dystonia. This is the first study showing that abnormal cerebellar output can result in burst firing within the basal ganglia, which has been shown to be a prominent feature of human dystonia29,31.
Figure 3. Electrophysiological signature in the cerebello-basal ganglia connection under physiological and pathological conditions.
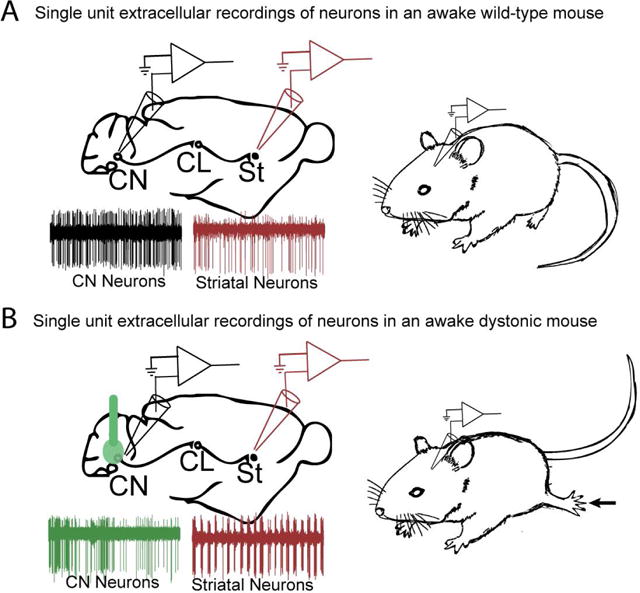
(A) Under physiological conditions cells in the deep cerebellar nuclei (CN) of awake mice fire regularly with a rate of around 40-50 Hz as seen by the raw trace (black), whereas cells in the striatum (St) fire with more variability and at a slower rate (red) compared to cells in the CN. (B) Fremont et al. 2014 showed that when ouabain is infused into the cerebellum, neurons in the CN fire in erratic bursts in the dystonic mouse (green trace). Similarly, Chen et al. 2014 also showed that neurons in the striatum fire in high frequency bursts (red trace) during dystonic postures.
Figure 4. Under pathological conditions simultaneously recorded neighboring neurons in the striatum fire in bursts.
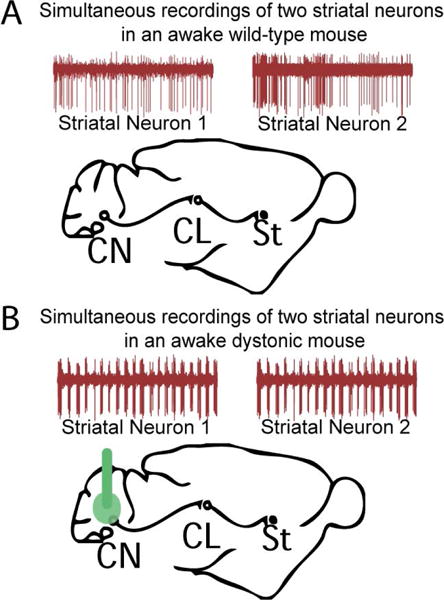
Using a microarray to record single unit activity of neurons in the striatum Chen et al.,2014 found that striatal neurons recorded simultaneously during dystonic postures in the pharmacological model of RDP showed high frequency burst firing, which suggests that these neurons receive a common aberrant input from the cerebellum that could be driving this abnormal activity.
Could interventions targeting a direct cerebello-basal ganglia connection have any therapeutic relevance in dystonia?
In recent years, DBS of the basal ganglia and especially the globus pallidus has become a mainstay of dystonia treatment because of its proven efficacy4,74–77. Although this treatment has been shown to be effective in a variety of different dystonia patients, a common feature of basal ganglia DBS is that it often takes weeks to months for the alleviation of symptoms to occur75,77,78. It is unknown why there is such a marked delay between implantation of DBS stimulating electrodes and the alleviation of symptoms. One hypothesis to explain this phenomena is that dystonia is a disorder of plasticity and the delay in symptom alleviation represents the time it takes for plastic changes in the motor circuitry to occur79. However, it is clear that the delay in alleviation of symptoms is not present for all movement disorders. For example, DBS of GP in Parkinsonian patients has an immediate effect on symptoms80. In patients with tremor, DBS of the ventralis intermedius thalamus can immediately alleviate symptoms81. In fact, early evidence suggested that interventions involving the thalamus could acutely alleviate dystonia as well82. This finding has been recently corroborated for some focal dystonias where lesioning the ventro-oral region of the thalamus results in immediate improvement of symptoms83. Together, these studies suggest that acute alleviation of dystonia can be attained if the right area is targeted.
The findings presented in the ouabain mouse model by Calderon et al, 2011 and Chen et al, 2014 show that acutely silencing or lesioning the centrolateral nucleus of the thalamus (CL) in rodents with cerebellar-induced dystonia alleviates abnormal postures. This suggests that interventions involving the human equivalent of CL may also be effective in immediately alleviating dystonia in some patients. Based on our findings, if the thalamic area analogous to CL in rodents can be identified in humans, then alleviation of dystonia on a fast time scale should be attainable in patients with cerebellar-induced dystonia. Therefore, it would be especially important to examine whether some of the thalamic sites previously used to acutely alleviate dystonia in patients are intermediates in the cerebello-thalamo-basal ganglia pathway in humans. Further investigation into the role of the thalamus as an intermediary in the cerebello-thalamo-basal ganglia pathway and what form this pathway takes in humans is warranted. Therapeutic approaches targeting the cerebellum are also plausible. Calderon et al. 2011 additionally showed that dystonia in their rodent model of RDP could be improved by silencing the cerebellar output either by electrolytic lesions of the CN or by infusions of TTX. Furthermore, GABA infusions into the cerebellum were also sufficient to alleviate the dystonic postures.
In the pharmacologic model of RDP, Chen et al., found that burst firing within the DLS is associated with dystonia. Studies in other rodent models where dystonia is thought to originate from the basal ganglia itself show that burst firing occurs throughout this area and is associated specifically with abnormal muscle contractions28,84–87. Although abnormal bursting activity in DLS is correlated with dystonia, it is unclear whether bursting of the basal ganglia alone is sufficient to cause dystonic postures. However, one study demonstrated that pharmacologically inhibiting FSIs of the striatum results in dystonic-like postures in the rodent25. Recordings from this area showed that while all FSIs demonstrated a decrease in firing rate after infusion of the drug, the response of MSNs was more variable with some cells increasing and others decreasing their firing rate. Although the authors did not specifically test for abnormal burst firing, they suggest in a later publication that abnormal patterns of activity may underlie the dyskinesia generated in this model26. It is clear that we are just beginning to understand the relationship between the cerebellum and the basal ganglia and its role in movement disorders. The fact that aberrant neuronal activity is seen in the cerebellum of two different mouse models of RDP57,58 and in a mouse model of DYT19 and given that there is a disynaptic connection from the cerebellum to the basal ganglia10, it is likely that this irregular activity is also present in the basal ganglia. While the current study addresses some questions, a more thorough understanding of how these areas communicate under physiological and pathological conditions in various mouse models of dystonia is vital.
Concluding Remarks
Recent evidence suggests that the cerebellum and cerebello-basal ganglia interactions have a role in dystonia that may be crucial in understanding this movement disorder. Chen et al., 2014 has shown that the cerebellum can directly modulate the basal ganglia over a short time scale and has provided a mechanism by which abnormal cerebellar output may cause dystonia. Based on the resurgence of interest in the involvement of the cerebellum in dystonia, groups have started reconsidering cerebellar interventions for this disorder. The study by Koch et al., 2014 demonstrated that cerebellar stimulation could alleviate some symptoms in patients with cervical dystonia88. Another study last year by Sokal et al. demonstrated that bilateral deep anterior cerebellar stimulation in patients with secondary dystonia reduces both dystonic symptoms and spasticity89. Future studies should examine whether DBS of the cerebellum may alleviate other types of dystonia as suggested by the results of Calderon et al. in the rodent model of RDP. Further, post mortem studies have suggested that patients with this disorder have marked changes in the cerebellum90. Therefore, it will be important to determine whether patients with RDP and potentially patients with other genetic dystonias could benefit from interventions involving the cerebellum.
There is also a growing body of evidence that interactions between the cerebellum and basal ganglia are important in some dystonias. One case study of a patient with myoclonus dystonia showed that during dystonic episodes there was marked changes in the activity of the cerebellum and thalamus59, suggesting that these areas are involved in this disorder. However, a number of studies have shown that this same syndrome can be alleviated by DBS of the basal ganglia91,92. The recent work by Chen et al., provides one explanation of how dystonic syndromes may be caused by changes in cerebellar output and alleviated by interventions involving the basal ganglia. Whether these same changes are present in other forms of dystonia is at present unknown, and needs to be studied experimentally. Additionally, this study suggests that interventions involving the cerebellum itself or the thalamic intermediate in the di-synaptic cerebello-basal ganglia connection may provide better and faster relief for some dystonic patients. Hopefully, as we discover more about the etiology of dystonia, better interventions can be developed to treat this disorder.
Footnotes
Conflict of Interest: The authors declare no competing financial interests
References
- 1.Stacy MA. Handbook of Dystonia. Informa Health care; New York: 2006. [Google Scholar]
- 2.Albanese A, et al. Phenomenology and classification of dystonia: A consensus update. Mov Disord. 2013;28:863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phukan J, Albanese A, Gasser T, Warner T. Primary dystonia and dystonia-plus syndromes: clinical characteristics, diagnosis, and pathogenesis. Lancet Neurol. 2011;10:1074–1085. doi: 10.1016/S1474-4422(11)70232-0. [DOI] [PubMed] [Google Scholar]
- 4.Balint B, Bhatia KP. Dystonia: an update on phenomenology, classification, pathogenesis and treatment. Curr Opin Neurol. 2014;27:468–476. doi: 10.1097/WCO.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 5.Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- 6.Carbon M, et al. Metabolic changes in DYT11 myoclonus-dystonia. Neurology. 2013;80:385–391. doi: 10.1212/WNL.0b013e31827f0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argyelan M, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29:9740–9747. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calderon DP, Fremont R, Kraenzlin F, Khodakhah K. The neural substrates of rapid-onset Dystonia-Parkinsonism. Nature neuroscience. 2011;14:357–365. doi: 10.1038/nn.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fremont R, Tewari A, Angueyra C, Khodakhah K. A role for cerebellum in the hereditary dystonia DYT1. Elife. 2017;6 doi: 10.7554/eLife.22775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CH, Fremont R, Arteaga-Bracho EE, Khodakhah K. Short latency cerebellar modulation of the basal ganglia. Nature neuroscience. 2014;17:1767–1775. doi: 10.1038/nn.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117(Pt 4):859–876. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- 12.Nygaard TG, Marsden CD, Duvoisin RC. Dopa-responsive dystonia. Advances in neurology. 1988;50:377–384. [PubMed] [Google Scholar]
- 13.Nausieda PA, Weiner WJ, Klawans HL. Dystonic foot response of Parkinsonism. Archives of neurology. 1980;37:132–136. doi: 10.1001/archneur.1980.00500520030003. [DOI] [PubMed] [Google Scholar]
- 14.Lees AJ, Hardie RJ, Stern GM. Kinesigenic foot dystonia as a presenting feature of Parkinson’s disease. Journal of neurology, neurosurgery, and psychiatry. 1984;47:885. doi: 10.1136/jnnp.47.8.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- 16.Haber SN, Fudge JL. The primate substantia nigra and VTA: integrative circuitry and function. Critical reviews in neurobiology. 1997;11:323–342. doi: 10.1615/critrevneurobiol.v11.i4.40. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald JJ, et al. Long-term outcome of deep brain stimulation in generalised dystonia: a series of 60 cases. Journal of neurology, neurosurgery, and psychiatry. 2014 doi: 10.1136/jnnp-2013-306833. [DOI] [PubMed] [Google Scholar]
- 18.Kupsch A, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. The New England journal of medicine. 2006;355:1978–1990. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- 19.Cooper IS. 20-year followup study of the neurosurgical treatment of dystonia musculorum deformans. Advances in neurology. 1976;14:423–452. [PubMed] [Google Scholar]
- 20.Burzaco J. Stereotactic pallidotomy in extrapyramidal disorders. Applied neurophysiology. 1985;48:283–287. doi: 10.1159/000101144. [DOI] [PubMed] [Google Scholar]
- 21.Wichmann T, DeLong MR. Deep Brain Stimulation for Movement Disorders of Basal Ganglia Origin: Restoring Function or Functionality? Neurotherapeutics. 2016 doi: 10.1007/s13311-016-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida M, Nagatsuka Y, Muramatsu S, Niijima K. Differential roles of the caudate nucleus and putamen in motor behavior of the cat as investigated by local injection of GABA antagonists. Neurosci Res. 1991;10:34–51. doi: 10.1016/0168-0102(91)90018-t. [DOI] [PubMed] [Google Scholar]
- 23.Muramatsu S, Yoshida M, Nakamura S. Electrophysiological study of dyskinesia produced by microinjection of picrotoxin into the striatum of the rat. Neurosci Res. 1990;7:369–380. doi: 10.1016/0168-0102(90)90011-3. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura S, Muramatsu S, Yoshida M. Role of the basal ganglia in manifestation of rhythmical jaw movement in rats. Brain research. 1990;535:335–338. doi: 10.1016/0006-8993(90)91620-v. [DOI] [PubMed] [Google Scholar]
- 25.Gittis AH, et al. Selective inhibition of striatal fast-spiking interneurons causes dyskinesias. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:15727–15731. doi: 10.1523/JNEUROSCI.3875-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gittis AH, Kreitzer AC. Striatal microcircuitry and movement disorders. Trends in neurosciences. 2012;35:557–564. doi: 10.1016/j.tins.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada H, Fujimoto K, Yoshida M. Neuronal mechanism underlying dystonia induced by bicuculline injection into the putamen of the cat. Brain research. 1995;677:333–336. doi: 10.1016/0006-8993(95)00190-2. [DOI] [PubMed] [Google Scholar]
- 28.Baron MS, Chaniary KD, Rice AC, Shapiro SM. Multi-neuronal recordings in the Basal Ganglia in normal and dystonic rats. Frontiers in systems neuroscience. 2011;5:67. doi: 10.3389/fnsys.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vitek JL, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol. 1999;46:22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Starr PA, et al. Spontaneous pallidal discharge in 15 cases of dystonia: Comparison with Parkinson’s disease and normal Macaque. Movement Disorders. 2004;19:S90–S90. [Google Scholar]
- 31.Starr PA, et al. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. Journal of neurophysiology. 2005;93:3165–3176. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- 32.Schrock LE, Ostrem JL, Turner RS, Shimamoto SA, Starr PA. The subthalamic nucleus in primary dystonia: single-unit discharge characteristics. Journal of neurophysiology. 2009;102:3740–3752. doi: 10.1152/jn.00544.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang JK, et al. Neuronal firing rates and patterns in the globus pallidus internus of patients with cervical dystonia differ from those with Parkinson’s disease. Journal of neurophysiology. 2007;98:720–729. doi: 10.1152/jn.01107.2006. [DOI] [PubMed] [Google Scholar]
- 34.Zhuang P, Li Y, Hallett M. Neuronal activity in the basal ganglia and thalamus in patients with dystonia. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2004;115:2542–2557. doi: 10.1016/j.clinph.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Lenz FA, et al. Pallidal activity during dystonia: somatosensory reorganisation and changes with severity. Journal of neurology, neurosurgery, and psychiatry. 1998;65:767–770. doi: 10.1136/jnnp.65.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hutchison WD, Lang AE, Dostrovsky JO, Lozano AM. Pallidal neuronal activity: implications for models of dystonia. Annals of neurology. 2003;53:480–488. doi: 10.1002/ana.10474. [DOI] [PubMed] [Google Scholar]
- 37.Seidel K, et al. Brain pathology of spinocerebellar ataxias. Acta neuropathologica. 2012;124:1–21. doi: 10.1007/s00401-012-1000-x. [DOI] [PubMed] [Google Scholar]
- 38.Dow RSaMG. The Physiology and Pathology of the Cerebellum. The University of Minnesota Press; Minneapolis, MN: 1958. [Google Scholar]
- 39.LeDoux MS, Brady KA. Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord. 2003;18:60–69. doi: 10.1002/mds.10301. [DOI] [PubMed] [Google Scholar]
- 40.Fletcher NA, Stell R, Harding AE, Marsden CD. Degenerative cerebellar ataxia and focal dystonia. Movement disorders: official journal of the Movement Disorder Society. 1988;3:336–342. doi: 10.1002/mds.870030410. [DOI] [PubMed] [Google Scholar]
- 41.Manto MU. The wide spectrum of spinocerebellar ataxias (SCAs) Cerebellum. 2005;4:2–6. doi: 10.1080/14734220510007914. [DOI] [PubMed] [Google Scholar]
- 42.Anheim M, Tranchant C, Koenig M. The autosomal recessive cerebellar ataxias. The New England journal of medicine. 2012;366:636–646. doi: 10.1056/NEJMra1006610. [DOI] [PubMed] [Google Scholar]
- 43.Muzaimi MB, Wiles CM, Robertson NP, Ravine D, Compston DA. Task specific focal dystonia: a presentation of spinocerebellar ataxia type 6. Journal of neurology, neurosurgery, and psychiatry. 2003;74:1444–1445. doi: 10.1136/jnnp.74.10.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saunders-Pullman R, et al. Variant ataxia-telangiectasia presenting as primary-appearing dystonia in Canadian Mennonites. Neurology. 2012;78:649–657. doi: 10.1212/WNL.0b013e3182494d51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bodensteiner JB, Goldblum RM, Goldman AS. Progressive dystonia masking ataxia in ataxia-telangiectasia. Arch Neurol. 1980;37:464–465. doi: 10.1001/archneur.1980.00500560094020. [DOI] [PubMed] [Google Scholar]
- 46.Le Ber I, et al. Predominant dystonia with marked cerebellar atrophy: a rare phenotype in familial dystonia. Neurology. 2006;67:1769–1773. doi: 10.1212/01.wnl.0000244484.60489.50. [DOI] [PubMed] [Google Scholar]
- 47.Eidelberg D, et al. Functional brain networks in DYT1 dystonia. Ann Neurol. 1998;44:303–312. doi: 10.1002/ana.410440304. [DOI] [PubMed] [Google Scholar]
- 48.Koch G, et al. Effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stimul. 2014;7:564–572. doi: 10.1016/j.brs.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Cooper IS, Upton AR. Use of chronic cerebellar stimulation for disorders of disinhibition. Lancet. 1978;1:595–600. doi: 10.1016/s0140-6736(78)91038-3. [DOI] [PubMed] [Google Scholar]
- 50.Cooper IS, Upton AR, Amin I. Chronic cerebellar stimulation (CCS) and deep brain stimulation (DBS) in involuntary movement disorders. Appl Neurophysiol. 1982;45:209–217. doi: 10.1159/000101601. [DOI] [PubMed] [Google Scholar]
- 51.Slaughter DG, Nashold BS, Jr, Somjen GG. Electrical recording with micro- and macroelectrodes from the cerebellum of man. J Neurosurg. 1970;33:524–528. doi: 10.3171/jns.1970.33.5.0524. [DOI] [PubMed] [Google Scholar]
- 52.LeDoux MS, Lorden JF, Ervin JM. Cerebellectomy eliminates the motor syndrome of the genetically dystonic rat. Exp Neurol. 1993;120:302–310. doi: 10.1006/exnr.1993.1064. [DOI] [PubMed] [Google Scholar]
- 53.Campbell DB, North JB, Hess EJ. Tottering mouse motor dysfunction is abolished on the Purkinje cell degeneration (pcd) mutant background. Exp Neurol. 1999;160:268–278. doi: 10.1006/exnr.1999.7171. [DOI] [PubMed] [Google Scholar]
- 54.Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heiney SA, Kim J, Augustine GJ, Medina JF. Precise control of movement kinematics by optogenetic inhibition of Purkinje cell activity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:2321–2330. doi: 10.1523/JNEUROSCI.4547-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LeDoux MS, Hurst DC, Lorden JF. Single-unit activity of cerebellar nuclear cells in the awake genetically dystonic rat. Neuroscience. 1998;86:533–545. doi: 10.1016/s0306-4522(98)00007-4. [DOI] [PubMed] [Google Scholar]
- 57.Fremont R, Tewari A, Khodakhah K. Aberrant Purkinje cell activity is the cause of dystonia in a shRNA-based mouse model of Rapid Onset Dystonia-Parkinsonism. Neurobiol Dis. 2015;82:200–212. doi: 10.1016/j.nbd.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fremont R, Calderon DP, Maleki S, Khodakhah K. Abnormal high-frequency burst firing of cerebellar neurons in rapid-onset dystonia-parkinsonism. J Neurosci. 2014;34:11723–11732. doi: 10.1523/JNEUROSCI.1409-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nitschke MF, et al. Functional MRI reveals activation of a subcortical network in a 5-year-old girl with genetically confirmed myoclonus-dystonia. Neuropediatrics. 2006;37:79–82. doi: 10.1055/s-2006-924109. [DOI] [PubMed] [Google Scholar]
- 60.Ratcheson RA, Li CL. Effect of dentate stimulation on neuronal activity in the caudate nucleus. Exp Neurol. 1969;25:268–281. doi: 10.1016/0014-4886(69)90050-8. [DOI] [PubMed] [Google Scholar]
- 61.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain research. Brain research reviews. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 62.Raos VC, Dermon CR, Savaki HE. Functional anatomy of the thalamic centrolateral nucleus as revealed with the [14C]deoxyglucose method following electrical stimulation and electrolytic lesion. Neuroscience. 1995;68:299–313. doi: 10.1016/0306-4522(95)00114-x. [DOI] [PubMed] [Google Scholar]
- 63.Ichinohe N, Mori F, Shoumura K. A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res. 2000;880:191–197. doi: 10.1016/s0006-8993(00)02744-x. [DOI] [PubMed] [Google Scholar]
- 64.Jeljeli M, Strazielle C, Caston J, Lalonde R. Effects of centrolateral or medial thalamic lesions on motor coordination and spatial orientation in rats. Neuroscience Research. 2000;38:155–164. doi: 10.1016/s0168-0102(00)00152-8. [DOI] [PubMed] [Google Scholar]
- 65.Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 66.Pelzer EA, et al. Cerebellar networks with basal ganglia: feasibility for tracking cerebello-pallidal and subthalamo-cerebellar projections in the human brain. The European journal of neuroscience. 2013;38:3106–3114. doi: 10.1111/ejn.12314. [DOI] [PubMed] [Google Scholar]
- 67.Calderon DP, Fremont R, Kraenzlin F, Khodakhah K. The neural substrates of rapid-onset Dystonia-Parkinsonism. Nat Neurosci. 2011;14:357–365. doi: 10.1038/nn.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jinnah HA, Hess EJ. A new twist on the anatomy of dystonia: the basal ganglia and the cerebellum? Neurology. 2006;67:1740–1741. doi: 10.1212/01.wnl.0000246112.19504.61. [DOI] [PubMed] [Google Scholar]
- 69.Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci. 2002;22:7825–7833. doi: 10.1523/JNEUROSCI.22-17-07825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Filip P, Lungu OV, Bares M. Dystonia and the cerebellum: a new field of interest in movement disorders? Clin Neurophysiol. 2013;124:1269–1276. doi: 10.1016/j.clinph.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 71.Fremont R, Calderon DP, Maleki S, Khodakhah K. Abnormal high-frequency burst firing of cerebellar neurons in rapid-onset dystonia-parkinsonism. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:11723–11732. doi: 10.1523/JNEUROSCI.1409-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harries AM, Sandhu M, Spacey SD, Aly MM, Honey CR. Unilateral pallidal deep brain stimulation in a patient with dystonia secondary to episodic ataxia type 2. Stereotact Funct Neurosurg. 2013;91:233–235. doi: 10.1159/000345265. [DOI] [PubMed] [Google Scholar]
- 73.Copeland BJ, et al. Deep brain stimulation of the internal globus pallidus for generalized dystonia associated with spinocerebellar ataxia type 1: a case report. Neuromodulation. 2014;17:389–392. doi: 10.1111/ner.12081. [DOI] [PubMed] [Google Scholar]
- 74.Cif L, et al. Treatment of dystonic syndromes by chronic electrical stimulation of the internal globus pallidus. J Neurosurg Sci. 2003;47:52–55. [PubMed] [Google Scholar]
- 75.FitzGerald JJ, et al. Long-term outcome of deep brain stimulation in generalised dystonia: a series of 60 cases. J Neurol Neurosurg Psychiatry. 2014;85:1371–1376. doi: 10.1136/jnnp-2013-306833. [DOI] [PubMed] [Google Scholar]
- 76.Panov F, et al. Deep brain stimulation in DYT1 dystonia: a 10-year experience. Neurosurgery. 2013;73:86–93. doi: 10.1227/01.neu.0000429841.84083.c8. discussion 93. [DOI] [PubMed] [Google Scholar]
- 77.Vidailhet M, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459–467. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]
- 78.Krauss JK. Deep brain stimulation for dystonia in adults. Overview and developments. Stereotact Funct Neurosurg. 2002;78:168–182. doi: 10.1159/000068963. [DOI] [PubMed] [Google Scholar]
- 79.Peterson DA, Sejnowski TJ, Poizner H. Convergent evidence for abnormal striatal synaptic plasticity in dystonia. Neurobiol Dis. 2010;37:558–573. doi: 10.1016/j.nbd.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Groiss SJ, Wojtecki L, Sudmeyer M, Schnitzler A. Deep brain stimulation in Parkinson’s disease. Ther Adv Neurol Disord. 2009;2:20–28. doi: 10.1177/1756285609339382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Benabid AL, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84:203–214. doi: 10.3171/jns.1996.84.2.0203. [DOI] [PubMed] [Google Scholar]
- 82.Cooper IS. Clinical and physiologic implications of thalamic surgery for disorders of sensory communication. 2. Intention tremor, dystonia, Wilson’s disease and torticollis. J Neurol Sci. 1965;2:520–553. doi: 10.1016/0022-510x(65)90002-x. [DOI] [PubMed] [Google Scholar]
- 83.Horisawa S, Taira T, Goto S, Ochiai T, Nakajima T. Long-term improvement of musician’s dystonia after stereotactic ventro-oral thalamotomy. Ann Neurol. 2013;74:648–654. doi: 10.1002/ana.23877. [DOI] [PubMed] [Google Scholar]
- 84.Bennay M, Gernert M, Richter A. Spontaneous remission of paroxysmal dystonia coincides with normalization of entopeduncular activity in dt(SZ) mutants. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:RC153. doi: 10.1523/JNEUROSCI.21-13-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gernert M, Bennay M, Fedrowitz M, Rehders JH, Richter A. Altered discharge pattern of basal ganglia output neurons in an animal model of idiopathic dystonia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:7244–7253. doi: 10.1523/JNEUROSCI.22-16-07244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gernert M, Richter A, Loscher W. Extracellular electrophysiology of pallidal neurons in a genetic hamster model of idiopathic dystonia. European Journal of Neuroscience. 1998;10:302–302. [Google Scholar]
- 87.Gernert M, Richter A, Loscher W. Alterations in spontaneous single unit activity of striatal subdivisions during ontogenesis in mutant dystonic hamsters. Brain research. 1999;821:277–285. doi: 10.1016/s0006-8993(99)01080-x. [DOI] [PubMed] [Google Scholar]
- 88.Koch G, et al. Effects of Two Weeks of Cerebellar Theta Burst Stimulation in Cervical Dystonia Patients. Brain stimulation. 2014 doi: 10.1016/j.brs.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 89.Sokal P, Rudas M, Harat M, Szylberg L, Zielinski P. Deep anterior cerebellar stimulation reduces symptoms of secondary dystonia in patients with cerebral palsy treated due to spasticity. Clinical neurology and neurosurgery. 2015;135:62–68. doi: 10.1016/j.clineuro.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 90.Oblak AL, et al. Rapid-onset dystonia-parkinsonism associated with the I758S mutation of the ATP1A3 gene: a neuropathologic and neuroanatomical study of four siblings. Acta neuropathologica. 2014 doi: 10.1007/s00401-014-1279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kurtis MM, et al. Clinical and neurophysiological improvement of SGCE myoclonus-dystonia with GPi deep brain stimulation. Clinical neurology and neurosurgery. 2010;112:149–152. doi: 10.1016/j.clineuro.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Magarinos-Ascone CM, Regidor I, Martinez-Castrillo JC, Gomez-Galan M, Figueiras-Mendez R. Pallidal stimulation relieves myoclonus-dystonia syndrome. Journal of neurology, neurosurgery, and psychiatry. 2005;76:989–991. doi: 10.1136/jnnp.2004.039248. [DOI] [PMC free article] [PubMed] [Google Scholar]


