Abstract
Saturated fatty acids can activate Toll-like receptor 2 (TLR2) and TLR4 but polyunsaturated fatty acids, particularly docosahexaenoic acid (DHA) inhibit the activation. Lipopolysaccharides (LPS) and lipopetides, ligands for TLR4 and TLR2, respectively, are acylated by saturated fatty acids. Removal of these fatty acids results in loss of their ligand activity suggesting that the saturated fatty acyl moieties are required for the receptor activation. X-ray crystallographic studies revealed that these saturated fatty acyl groups of the ligands directly occupy hydrophobic lipid binding domains of the receptors (or co-receptor) and induce the dimerization which is prerequisite for the receptor activation. Saturated fatty acids also induce the dimerization and translocation of TLR4 and TLR2 into lipid rafts in plasma membrane and this process is inhibited by DHA. Whether saturated fatty acids induce the dimerization of the receptors by interacting with these lipid binding domains is not known. Many experimental results suggest that saturated fatty acids promote the formation of lipid rafts and recruitment of TLRs into lipid rafts leading to ligand independent dimerization of the receptors. Such a mode of ligand independent receptor activation defies the conventional concept of ligand induced receptor activation; however, this may enable diverse non-microbial molecules with endogenous and dietary origins to modulate TLR-mediated immune responses. Emerging experimental evidence reveals that TLRs play a key role in bridging diet-induced endocrine and metabolic changes to immune responses.
Keywords: Toll-like receptor, Inflammation, Saturated fatty acid, Polyunsaturated fatty acid, Docosahexaenoic acid
Chemical compounds studied in this article: Docosahexaenoic acid (PubChem CID: 445580), Palmitic acid (PubChem CID: 985), Pam3Csk4 (CID: 130704), Lipopolysaccharide core (CID: 53481794)
1. Introduction
Toll-like receptors (TLRs) are one of the major pattern recognition receptor families that recognize pathogen associated molecular patterns (PAMPs) and mount innate immune responses for host defense against invading pathogens. However, certain TLRs can be activated by non-microbial endogenous molecules leading to induction of sterile inflammation. TLR4 and TLR2 can be activated by saturated fatty acids (SFAs), but inhibited by omega-3 polyunsaturated fatty acids (PUFAs) in particular docosahexaenoic acid (DHA). Receptor dimerization is a prerequisite and sufficient to induce the activation of TLRs. SFAs induce the dimerization and translocation of TLR4 or TLR2 into lipid raft fractions in plasma membrane where the downstream signaling molecules are recruited and activated. This SFA-induced dimerization of TLR4 or TLR2 reflects ligand independent activation of the receptors. Therefore, SFA-induced activation of TLR4 or TLR2 needs to be assessed in the context of biophysical interaction of fatty acids with TLRs in plasma membrane. Such a mode of activation of TLRs deviates from the conventional view on the key and lock relationship for ligands and receptor activation, but renders a possibility that the ligand independent activation of these TLRs can be modulated by diverse non-microbial agonists or inhibitors with dietary origin. This finding advanced our understanding of the mechanisms by which SFAs activate, and DHA inhibits pro-inflammatory signaling pathways. Understanding the mechanism of such a modulation would help us develop dietary or pharmacological strategy to reduce risk of chronic diseases caused in part by dysregulated TLR-mediated inflammatory responses. In this article, putative molecular mechanisms by which saturated fatty acids and DHA reciprocally modulate the activation of TLR4 and TLR2 are reviewed and discussed. Functional consequences of the modulation of TLR-derived pro-inflammatory signaling pathways by fatty acids with regard to metabolic diseases were reviewed elsewhere (Arranz et al., 2012; Lee et al., 2010).
2. Pro-inflammatory saturated fatty acids and anti-inflammatory n-3 PUFAs
Replacing dietary SFA with PUFA reduces risk of coronary heart disease (Jakobsen et al., 2009). Higher plasma level of C16:0 and C18:0 is associated with increased incidence of type 2 diabetes (Mozaffarian, 2014). A diet high in saturated fat induces insulin resistance, which is associated with low grade inflammation in adipose tissue and liver in experimental animals (Orr et al., 2012; Tsukumo et al., 2007; Yeop Han et al., 2010). By contrast, DHA has anti-inflammatory and insulin sensitizing effects both in animal and human studies (Albert et al., 2014; Fedor and Kelley, 2009). How SFAs exert pro-inflammatory effects and n-3 PUFA DHA exerts anti-inflammatory effects remained as an important question. SFAs activate TLR2/4-derived pro-inflammatory signaling pathways, and TLR2 or TLR4 deficient mice are protected from high saturated fat diet-induced inflammation and insulin resistance (Glass and Olefsky, 2012; Lee et al., 2010). These findings established a causal link of TLR-mediated inflammation to metabolic diseases. However, the mechanism by which saturated fatty acids activate TLR2/4 is still not clearly understood.
3. Lipid binding sites in TLR-MD2 and TLR2/1 or TLR2/6 dimers
Phylogenetic, X-ray crystallographic and amino acid sequence analyses of leucine rich repeat (LRR) domains of TLRs provided an important clue about structural features of the ligands and their interactions with TLRs. Phylogenetic analyses of TLRs revealed TLR1, – 2, – 6, – 10 as the same clade and TLR4 or TLR3, – 5, – 7, – 8, – 9 as two separate clades (Hughes and Piontkivska, 2008). Based on X-ray crystallographic analyses of LRR ectodomains of TLRs and analyses of amino acid sequences, Kang and Lee (Kang and Lee, 2011) divided TLRs into two subgroups: the first group containing LRRs with three-domain fold (TLR 1, – 2, – 4, – 6, –10), and the second group containing LRRs with single-domain fold (TLR3, – 5, – 7, – 8, – 9) (Fig. 1). The LRRs of the first group of TLRs can be divided into distinct three subdomains: N-terminal, central and C-terminal due to the lack of the asparagine networks that are present in the LRR domains of the second group of TLRs. The lack of the asparagine networks creates structural distortions at the boundaries of the subdomains of the first subgroup. Interestingly, the ligand binding pockets of TLR1, – 2, and – 6 are all localized between C-terminal and central domains (Fig. 1C). The ligands for TLR1, – 2, and – 6 contain fatty acyl chains that bind the lipid binding pockets. By contrast, one of the MD2 binding sites in TLR4 is located between the N-terminal and central subdomains. The sequence analysis of the ectodomains of the second group of TLR3, – 5, – 7, – 8, and – 9 revealed that they all have continuous asparagine network exhibiting smooth single-domain fold without having the lipid binding pockets. These single-domain fold TLRs interact with hydrophilic ligands such as nucleic acids through surface-exposed residues. The results demonstrating that SFAs activate TLRs containing lipid binding pockets with three-domain fold group only suggest a direct interaction of SFAs with the lipid binding pockets. More than 100 μM SFAs are required to activate TLR4 or TLR2 in cell culture systems, while pico- or nano-molar ranges of LPS or Pam3CSK4 are enough to stimulate TLR4 or TLR2, respectively. Therefore, it is questionable whether SFAs, without polar head groups present in LPS or lipopetides, act as ligands for TLR4-MD2 and TLR2 dimers. Thus, SFAs should be called as agonists rather than the ligands for the TLRs.
Fig. 1. Arrangement of Toll-like receptor (TLR) domains.
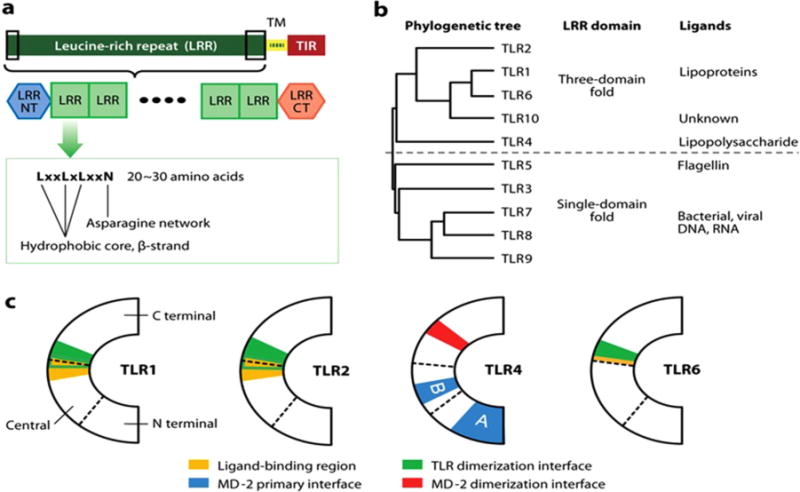
(a) TLRs consist of an extracellular leucine-rich repeat (LRR) domain, a transmembrane (TM) domain, and an intracellular Toll/IL-1R homology (TIR) domain. The extracellular LRR domain contains 20~27 LRR modules. LRRNT and LRRCT modules cover the N and C termini of the LRR modules, respectively. (b) Classification of TLRs: phylogenetic analysis, the structures of the LRR domains, and the chemical properties of the ligands suggest that TLRs can be divided into two major subclasses. (c) Structural boundaries are important for function. Boundaries dividing the N-terminal, central, and C-terminal subdomains are marked by broken lines. Functionally important areas are colored. The A and B patches of the primary TLR4-MD-2 interface are marked. (Kang and Lee, 2011).
4. Toll-like receptors and saturated fatty acids
The conceptual clue that SFA can activate inflammatory signaling pathways was conceived (Hwang, 2001) from the fact that removal of saturated fatty acyl chains in Lipid A causes not only loss of endotoxic activity of LPS but also makes Lipid A act as an antagonist (Kitchens et al., 1992; Munford and Hall, 1986). These results predictively revealed that the saturated fatty acyl chains in LPS are required for the activation of LPS receptor. In addition, the Lipid A derived from Rhodopseudomonas sphaeroides or synthetic Lipid A analogue Eritoran (Fig. 2A) contains one unsaturated fatty acid and acts as endotoxin antagonist in mice (Jin and Lee, 2008; Kim et al., 2007; Krauss et al., 1989; Qureshi et al., 1991). This result further implies that unsaturated fatty acids may interfere with SFA-induced activation of the LPS receptor. The discovery that TLR4 is the LPS receptor (Poltorak et al., 1998) prompted testing whether SFA alone can activate but unsaturated fatty acids inhibit TLR4-mediated signaling pathways (Lee et al., 2001). Subsequently, it was found that saturated fatty acid, lauric acid (C12:0), can activate TLR4 by inducing dimerization of TLR4 (Lee et al., 2001; Wong et al., 2009). Bacterial lipopeptides (ligands for TLR2) also require acylation by SFA for TLR2 activation (Brightbill et al., 1999). Moreover, palmitic acid activates TLR2 by promoting the dimerization of TLR2 with TLR1 (Snodgrass et al., 2013). Thus, acylation of ligands are required for homo-dimerization of TLR4 and hetero-dimerization of TLR2 with TLR1 that are necessary for the activation of receptors (Lee et al., 2010).
Fig. 2. Crystal structures of the TLR4-MD-2-LPS complexes.
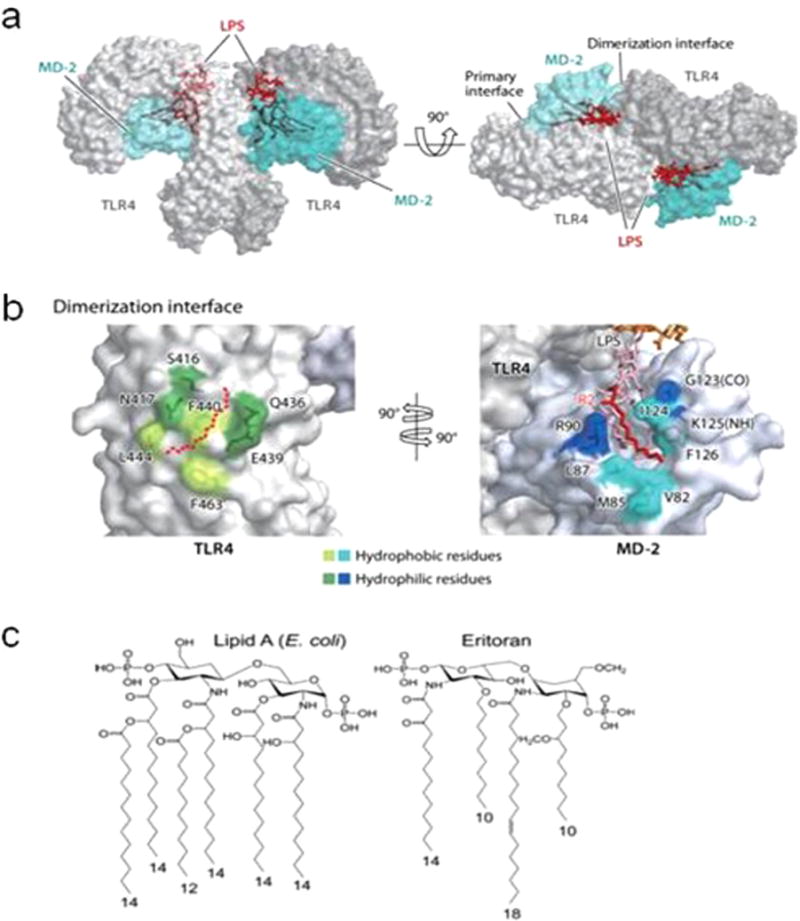
(a) Structure of the TLR4-MD-2-LPS complex. TLR4, MD-2, and LPS are in gray, cyan, and red, respectively. (b) The dimerization interface between TLR4 and MD-2 is split and rotated by 90°. Hydrophilic residues of TLR4 and MD-2 colored dark green and dark blue, respectively, surround this hydrophobic core and form hydrogen or ionic interactions. The R2 lipid chain of LPS is in red. Other parts of lipid A and core carbohydrates are in pink and orange, respectively. (c) Structures of Lipid A and Eritorian. Abbreviations: (CO), backbone carbonyl oxygen; LPS, lipopolysaccharide; (NH), backbone amide nitrogen; TLR, Toll-like receptor. (Jin and Lee, 2008; Kang and Lee, 2011).
5. Homodimerization of TLR4 and heterodimerization of TLR2/1 or TLR2/6 are induced by SFAs
The dimerization of the receptors is the most proximal step in TLR-signaling pathways. X-ray crystallographic analyses revealed that acyl chains of Lipid A occupy the hydrophobic lipid binding pocket in TLR4-MD2 complex leading to homodimerization of TLR4 (Fig. 2) (Park et al., 2009). Heterodimerization of TLR2 with TLR1 or TLR6 is also induced by binding of palmitoyl chain in triacyl lipopeptide or diacyl lipopetide, respectively to the lipid binding sites (Kang and Lee, 2011) (Fig. 3). SFAs can induce homodimerization of TLR4 or heterodimerization of TLR2 with TLR1 within 5–10 min of incubation in cell culture systems (Snodgrass et al., 2013; Wong et al., 2009). At present, it is unclear whether free SFAs without the polar head groups present in Lipid A or lipopetides can directly interact with the lipid binding pocket of TLR4-MD2 complex or TLR2 and TLR1/6.
Fig. 3. Crystal structures of TLR2-TLR1/6 heterodimers induced by binding of triacyl and diacyl lipopeptides.
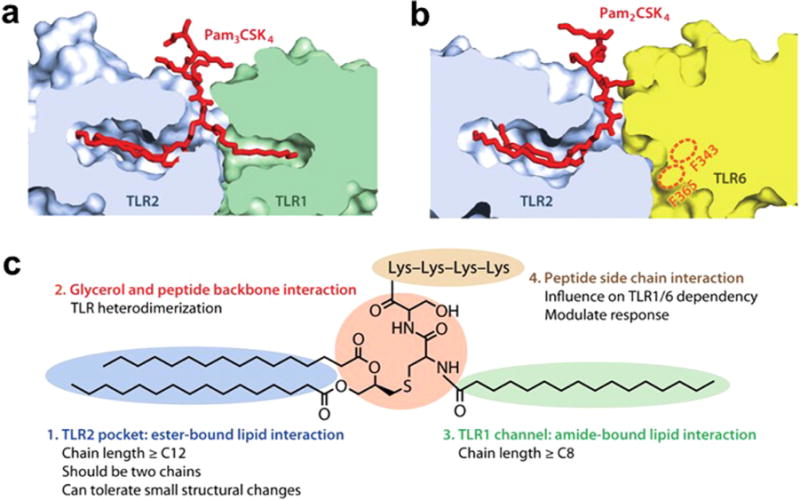
(a) Lipid-binding pocket in the TLR2-TLR1-Pam3CSK4 complex. The structures of the TLRs are dissected to reveal the shape of the lipid pocket. (b) Lipid-binding pocket in the TLR2-TLR6-Pam2CSK4 complex. The putative lipid channel of TLR6 is blocked by phenylalanines F343 and F365. (c) Summary of the lipopeptide patterns recognized by the TLR1-TLR2 and TLR2-TLR6 heterodimers. (Kang and Lee, 2011).
6. Ligand binding and receptor dimerization
Schaeffler et al. (Schaeffler et al., 2009) reported that 14C-stearic acid (C18:0) or 14C-oleic acid (C18:1n-9) does not bind FLAG-tagged extracellular domain of TLR4 fused to full-length MD2. Specific activities of 14C-labeled fatty acids are generally too low for receptor binding assays, and thus, preferably the gamma ray emitting isotope such as 125I-or 3H-labeled compounds with high specific activity have been generally used for receptor binding assay. Therefore, the binding assay alone would not be sufficient to validate the conclusion that the fatty acids do not bind the TLR4-MD2 complex. However, their results also showed that incubation of the fatty acids with the protein containing the ectodomain of TLR4 fused with MD2 did not interfere with the binding of biotinlabeled LPS to the receptor complex (Schaeffler et al., 2009). Based on these results, the authors suggested that the fatty acids may activate TLR4 through unidentified endogenous ligand. Such a suggestion appears to be based on the presumption that SFAs compete with Lipid A for the same binding sites in TLR4-MD2 complex. It was shown that lauric acid potentiates LPS- or palmitoyl-Cys((RS)-2,3-di(palmitoyloxy)-propyl)-Ala-Gly-OH (Pam-CAG)-induced activation of TLR4 or TLR2, respectively (Lee et al., 2003) suggesting that lauric acid potentiates the dimerization of the receptors induced by the ligands (Figs. 4 and 5). Thus, the question is whether or not SFAs promotes the ligand induced dimerization by directly interacting with the lipid binding sites of the receptor complexes.
Fig. 4.
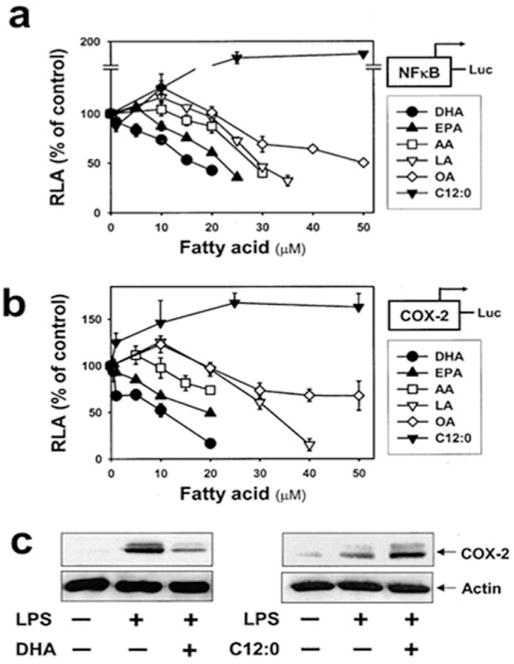
Unsaturated fatty acids inhibit, but saturated fatty acid potentiates, LPS-induced nuclear factor κB (NFκB) activation and COX-2 expression in RAW 264.7 cells. Cells stably transfected with NFκB(5 ×) (a) or COX-2 promoter (b) reporter gene were pretreated with various concentrations of each fatty acid for 3 h. Cells were then treated with LPS (200 ng/ml). After 8 h, cell lysates were prepared and luciferase activities were determined. Data are expressed as a percentage of LPS treatment alone. Values are mean ± SEM (n=3). RLA, relative luciferase activity. (c): Cells were pretreated with docosahexaenoic acid (DHA) (20 μM) or C12:0 (75 μM) for 3h and further stimulated with LPS (200 ng/ml for DHA; 1 ng/ml for C12:0). After 8 h, cell lysates were analyzed by COX-2 and actin immunoblotting. DHA, docosahexaenoic acid (C22:6n-3); EPA, eicosapentaenoic acid (C20:5n-3); AA, arachidonic acid (C20:4n-6); LA, linoleic acid (C18:2n-6); OA, oleic acid (C18:1n-9); C12:0, lauric acid. (Lee et al., 2003).
Fig. 5.
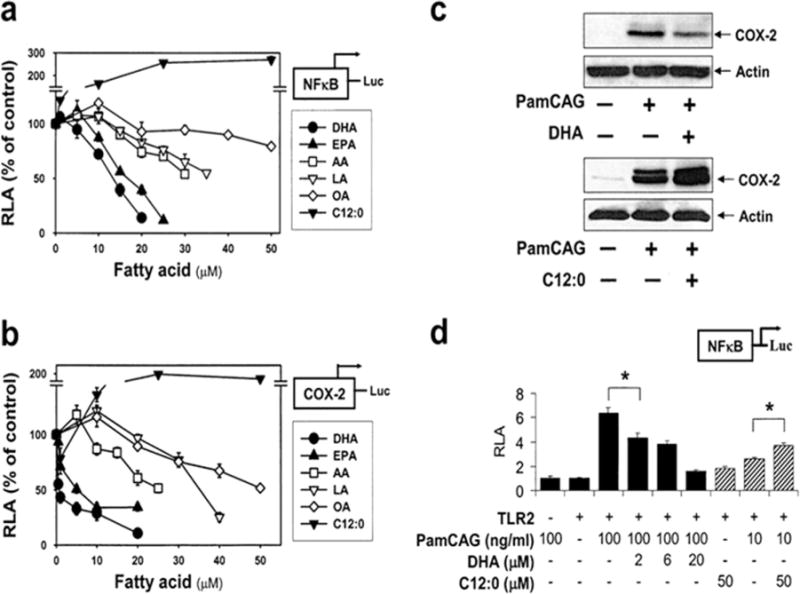
Unsaturated fatty acids inhibit, but saturated fatty acid potentiates, PamCAG-induced NFκB activation and COX-2 expression. RAW 264.7 cells stably transfected with NFκB(5 ×) (a) or COX-2 promoter (b) reporter gene were pretreated with various concentrations of each fatty acid for 3 h. Cells were further stimulated with a synthetic bacterial lipoprotein (PamCAG, 500 ng/ml). After 8 h, luciferase activities were determined. Data are expressed as a percentage of PamCAG treatment alone. (c): RAW 264.7 cells were pretreated with DHA (20 μM) or C12:0 (75 μM) for 3 h and further stimulated with PamCAG (500 ng/ml). After 8 h, cell lysates were analyzed by COX-2 and actin immunoblotting. (d): 293T cells were cotransfected with NFκB-luciferase reporter plasmid and TLR2 expression plasmid, and treated with PamCAG in the presence or absence of DHA or C12:0. Values are mean ± SEM (n=3). RLA, relative luciferase activity. * Significantly different from the respective control (P < 0.05). (Lee et al., 2003).
Fatty acyl chains of a synthetic Lipid A analogue Eritoran containing an unsaturated fatty acyl chain, or Lipid IVa (an intermediate in Lipid A synthesis) can occupy lipid binding pocket of MD2 as does the acyl chains of Lipid A, but cannot induce the dimerization of TLR4-MD2 complex (Kim et al., 2007; Park et al., 2009). Thus, both Eritoran and Lipid IVa act like competitive inhibitors for the wild type Lipid A in activating TLR4. This suggests that binding the receptor complex by agonists alone without inducing receptor dimerization is not sufficient to activate TLR4- mediated signaling pathways. Indeed, truncation of a part of C-terminal LRR domain spanning the dimerization interface of TLR4 blocks LPS-induced dimerization without compromising LPS binding activity (Kim et al., 2007). On the other hand, forced dimerization of TLR4 using chimeric constructs without co-transfection of MD2 can activate downstream NFκB and induces cytokine expression (Medzhitov et al., 1997; Rhee and Hwang, 2000; Zhang et al., 2002). These results suggest that the dimerization of TLR4 is a prerequisite for the receptor activation, and further suggest a possibility that the dimerization of TLRs can occur in a ligand-independent manner. Lipid IVa with four fatty acyl chains (instead of six acyl chains present in mature Lipid A) is an antagonist for human TLR4-MD2 but acts as a weak agonist for murine or equine TLR4 receptor (Scior et al., 2013). Based on the domain swapping experiments between a part of LRR domains of human and equine TLR4 (Scior et al., 2013) and X-ray crystallographic structure of TLR4-MD2 complex, Park et al. (Park et al., 2009) interpreted that Lipid IVa can induce the dimerization of equine TLR4-MD2 in a similar fashion as Lipid A does for human TLR4-MD2 complex. This finding reinforces that the receptor dimerization but not the binding alone of fatty acyl chains to the receptor complex is required for the activation of receptor signaling pathways. Together, these results suggest that dimerization instead of receptor binding would be more faithful readout for assessing the interaction of fatty acids with TLRs to activate the downstream signaling pathways.
7. Dimerization of TLRs can be induced in a ligand independent manner (e.g, translocation into lipid rafts)
Park et al. (Park et al., 2009) revealed that the interaction of the phosphate groups of Lipid A with positively charged residues of TLR4 plays an important role for the dimerization of the ectodomain of TLR4. Then, one can argue that SFAs without the polar head groups may not be able to induce the receptor dimerization or are not as efficient as Lipid A in inducing the receptor dimerization even if they bind the lipid binding pocket of MD2. However, such a mode of the receptor dimerization was based on the X-ray crystallographic study with truncated TLR4 containing only the ectodomain of TLR4 (Park et al., 2009). The receptor dimerization of TLRs involves not only the ectodomain but also cytoplasmic TIR domain of TLRs. The dimerization of Toll/IL-1 receptor (TIR) domains of TLRs or IL-1 receptor is required to recruit the downstream signaling molecule, MyD88. Xu et al. (Xu et al., 2000) showed that the dimerization of TIR domains involves extensive disulfide formation among the TIR domains. At high concentrations, spontaneous formation of dimers and tetramers of the TIR domains occurs in solution. Thus, the dimerization of TIR domains can be induced either by ligand binding or by overexpression in a ligand independent manner.
Gain of function by the truncated Toll proteins lacking the LRR domains was also demonstrated both in Drosophila and in human cells (Medzhitov et al., 1997; Winans and Hashimoto, 1995). The truncated murine TLR4 lacking LRR domains also acts as a constitutively active TLR4 (Rhee and Hwang, 2000). Transfection of cells with the constitutively active TLR4 without MD2 leads to the transactivation of NFκB and COX-2 reporter constructs in a ligand independent manner (Rhee and Hwang, 2000). These results suggest that the gain of function of the truncated TLR4 lacking LRR domains is due to spontaneous dimerization of the receptor. Forced dimerization of TLR4 by replacing the LRR domain with the integrin subunit also confers ligand independent activation of TLR4 (Zhang et al., 2002). Together, these results suggest that the dimerization of TLR4 or TLR2 can be induced by agonists without direct interaction with ligand binding sites. The mechanism for TLR activation in such a ligand independent manner deviates from the conventional view on the key and lock relationship between ligand and receptor activation. However, such a ligand independent activation of TLRs may provide diverse regulatory repertories for the activation or inhibition of TLRs and consequent inflammatory responses by a variety of non-microbial endogenous molecules.
Wong et al. (Wong et al., 2009) showed that lauric acid (C12) induces the dimerization of Flag and GFP-tagged TLR4 stably transfected with MD2 and activates the downstream signaling pathways in murine pro-B cell line. Thus, the question is whether SFAs induce the dimerization of TLR4 with or without binding the ligand binding site of TLR4-MD2 complex.
The concentrations of SFAs to activate TLR4 exceed 100 μM in cell culture systems, whereas those for LPS are in pM ranges. Similarly, the effective concentrations of SFAs in inducing the dimerization of TLR2 with TLR1 exceed 100 μM but those for Pam3CSK4 are in nM ranges (Snodgrass et al., 2013). Preincubation of lauric acid with RAW264.7 cells potentiated LPS- or synthetic TLR2 ligand Pam3CAG-induced NFκB activation (Figs. 4 and, 5). These results suggest that SFAs do not interfere with the binding of TLR4 or TLR2 ligand to the lipid binding pockets of the receptors, but rather, SFAs may promote the ligand binding. The key question is how SFAs can induce the dimerizations of TLR4 and TLR2 without directly binding the ligand binding sites. Several conceptual possibilities for SFAs to induce the dimerization of TLR4 and TLR2 in a ligand independent manner are described below.
8. Saturated fatty acids induce translocation of TLR4 into lipid raft microdomain of the plasma membrane and homodimerization of the receptor
Lipid rafts are microdomains of plasma membrane which can serve as a platform where receptors recruit downstream signaling molecules for activation. Polar lipids in lipid rafts are predominantly acylated by saturated fatty acids, whereas, phospholipids in non-lipid rafts are preferentially acylated by polyunsaturated fatty acids. Receptor dimerization and translocation of TLRs to lipid rafts induced by agonists are the proximal steps required for the activation of downstream signaling pathways (Fernandez-Lizarbe et al., 2008; Nakahira et al., 2006; Olsson and Sundler, 2006; Shin et al., 2008; Triantafilou et al., 2006; Triantafilou et al., 2007; Triantafilou et al., 2004; Triantafilou et al., 2002; Wong et al., 2009). Dimerization allows for the proper orientation of the TIR domains of TLRs to induce the recruitment and interaction of adaptor proteins in lipid rafts. Lauric acid increased the dimerized TLR4 in lipid raft fractions as did LPS in RAW264.7 cells (Wong et al., 2009). The dimerized TLR4 is detected only in lipid raft fractions but not in non-lipid raft fraction. It is not clear whether C12 induces the dimerization of TLR4 in non-lipid rafts and then the dimerized TLR4 is translocated to lipid rafts, or whether C12 stimulates translocation of monomeric TLR4 into lipid rafts and then the dimerization occurs in lipid rafts. The fact that over expression of TLR4 alone without ligand stimulation can induce the dimerization of TLR4 suggests a possibility that C12 can concentrate TLR4 into lipid rafts of the plasma membrane to the point at which spontaneous dimerization can occur; thus, SFA-induced dimerization of TLR4 or TLR2 may be achieved through biophysical interaction of the fatty acids with the TLRs and microdomains of the plasma membrane in a ligand independent manner.
9. Saturated fatty acids induce heterodimerization of TLR2
TLR2 appears to reside predominantly in nonlipid raft fractions; however, a substantial amount of TLR2 is also found in lipid raft fractions isolated from unstimulated THP-1 monocytes (Snodgrass et al., 2013) (Fig. 6). This contrasts to TLR4 which resides mostly in non-lipid rafts in unstimulated cells (Fig. 7). The dimerization of TLR2/1 accompanies the translocation of MyD88 and p47phox, the NADPH oxidase NOX2 organizer protein to lipid raft fractions within 5 min of the treatment with sodium palmitate or Pam3CSK4 in THP-1 monocytes (Fig. 6). No significant translocation of MyD88 or p47phox occurs in unstimulated cells. These results suggest that TLR2 present in lipid raft fractions in unstimulated cells may be monomeric TLR2 or loosely bound inactive dimers with TLR1 or TLR6. In contrast, DHA inhibits both sodium palmitate- and Pam3CSK4-induced recruitment of both MyD88 and p47phox to lipid raft fractions (Fig. 6).
Fig. 6.
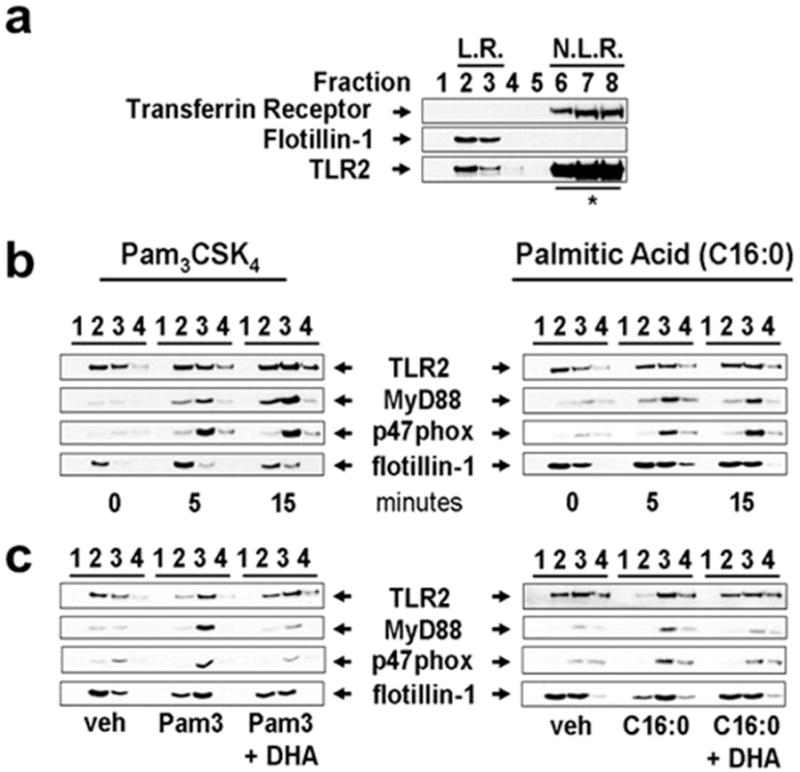
Palmitic acid and Pam3CSK4 induce but DHA inhibits the recruitment of MyD88 and p47 phox (subunit of NADPH oxidase 2) into lipid raft fractions. (a) To demonstrate the separation of lipid rafts (LR) and non-lipid raft (NLR) fractions of plasma membrane, cells were lysed and fractionated by sucrose-gradient ultracentrifugation. LR fractions (fractions 2 and 3) and NLR fractions (fractions 6–8) were identified by the presence of flotillin-1 (LR marker) and transferrin receptor (NLR marker), respectively. (* due to overwhelming expression only 20% of input lysate from fractions 6–8 was subjected to SDS-PAGE and immunoblotted with anti-TLR2 antibodies). (b) To determine whether palmitic acid (C16:0) or Pam3CSK4 induces recruitment of the downstream signaling components of TLR2 into LR fractions, THP-1 cells were serum starved in 1.0% FBS-RPMI-1640 for 12 h, then treated with C16:0 (150 μM) or Pam3CSK4 (100 ng/ml) for indicated time periods. Fractions 1–4 were immunoblotted with anti-TLR2, anti-MyD88, anti-p47phox, and anti-flotillin-1 antibodies. (c) Serum starved THP-1 cells were incubated with DHA (10 μM) for 1 h then treated with C16:0 (150 μM) or Pam3CSK4 (100 ng/ml) for 5 min. Cell lysate was separated by sucrose-gradient ultracentrifugation and fractions were immunoblotted. (Snodgrass et al., 2013).
Fig. 7. Lauric acid induces but DHA inhibits TLR4 homodimerization and association of TLR4 with MD-2 in lipid rafts.
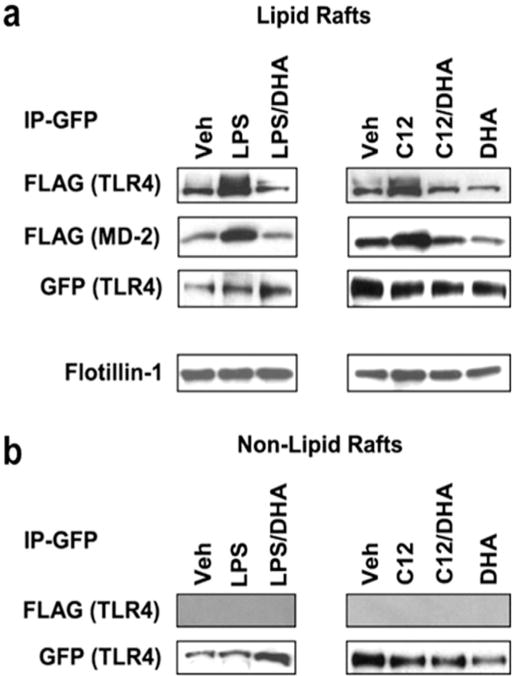
(a) Ba/F3 cells stably transfected with GFP/FLAG-tagged TLR4 and FLAG-tagged MD-2 were treated with LPS or lauric acid in the presence or absence of DHA (20 μM). For the immunoprecipitation, lipid raft Fractions 4 and 5 were pooled from the sucrose gradient. One half of the lipid raft fraction was immunoprecipitated with anti-GFP antibodies and then immunoblotted with anti-FLAG antibodies. The membranes were reprobed with anti- GFP antibodies. The other half of the samples was immunoblotted with anti-flo-tillin-1 antibodies to show the presence of the lipid raft marker. (b) Samples from pooled non-lipid raft Fractions 10–12 were immunoprecipitated and immunoblotted as described above in a. (Wong et al., 2009).
Several TLRs exist as inactive, preformed heterodimers or homodimers in the absence of ligand (Jin et al., 2007; Latz et al., 2007). TLR2 exists as an inactive, loosely bound heterodimer with either TLR1 or TLR6 in the absence of ligand (Tapping and Tobias, 2003; Triantafilou et al., 2006). The X-ray crystallographic study with the ectodomains of the heterodimer of TLR2-TLR1 revealed that two ester-bound fatty acyl (palmitic acid) chains of Pam3CSK4 are inserted into the hydrophobic lipid binding pocket in TLR2, whereas the amide-bound fatty acyl group is inserted into a hydrophobic channel in TLR1 (Jin et al., 2007) (Fig. 3). Thus, binding of palmitoyl moiety of Pam3CSK4 into TLR2 induces heterodimerization of TLR2 with TLR1. Structure-function study for the lipopeptides revealed that the two ester-bound fatty acids are the main determinant for its ligand activity for TLR2, and that the acyl chain with 16 carbons (palmitic acid) provides optimal stimulatory activity compared with fatty acyl chains of different length (Buwitt-Beckmann et al., 2005). It is an interesting question whether palmitic acid itself without the peptide moiety can interact with the hydrophobic lipid binding sites in TLR2 or TLR1 and promote the dimerization of the receptors leading to recruitment of the immediate downstream signaling molecules including MyD88 and p47phox. Upon lipopeptide binding, preformed TLR2/1 dimers undergo rearrangement subsequently bringing TLR2 and TLR1 in much closer proximity and are recruited into lipid rafts (Hoebe et al., 2005; Jin et al., 2007). This ligand-induced conformational change and translocation of the TLR2 heterodimers into lipid rafts lead to the immediate recruitment of downstream signaling molecules including MyD88 and the cytoplasmic NOX2 organizer protein p47phox (Ogier-Denis et al., 2008; Yang et al., 2009; Yang et al., 2008). Therefore, palmitic acid induced heterodimerization of endogenous TLR2 with TLR1 in close proximity can be determined by the time resolved-fluorescence resonance energy transfer technique (TR-FRET). Because FRET occurs only when two receptors are within close proximity, it can be used as a direct readout for the dimerization of the native receptor in the cell membrane. Therefore, TR-FRET allows detection of the dimerization of native full length TLR2 and TLR1 in their biological context unlike X-ray crystallographic study with the truncated receptor without cytoplasmic domain. Snodgrass et al. (Snodgrass et al., 2013) revealed that palmitic acid induces but DHA inhibits the dimerization of TLR2 with TLR1 in THP-1 monocytes as assessed by the TR-FRET (Fig. 8).
Fig. 8. TLR2/1 dimerization determined by TR-FRET in monocytes.
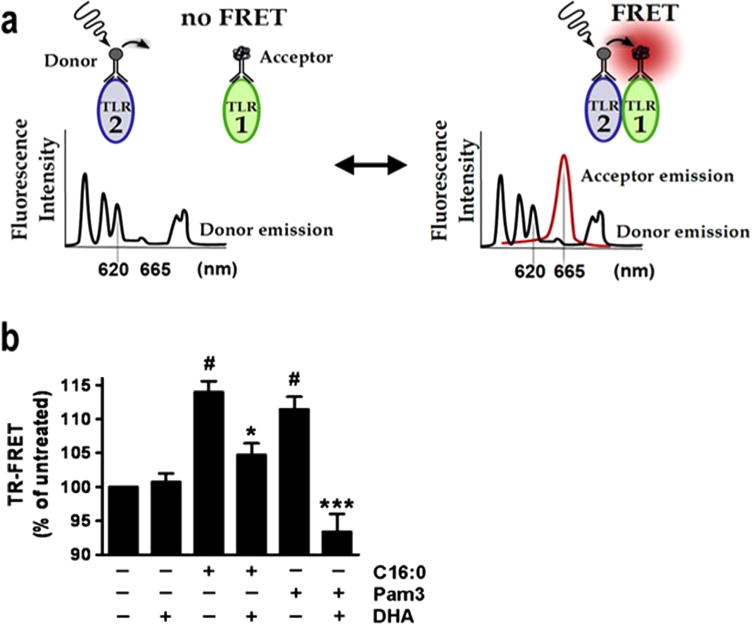
TR-FRET assay was used to detect dimerization of TLR2 with TLR1 using anti-TLR2. Antibodies labeled with europium cryptate as the donor fluorophore and anti-TLR1. Antibodies labeled with d2 as the acceptor fluorophore: an assay to detect the dimerization of native receptors in the context of intact cell membrane, whereas, X-ray crystallography or immunoprecipitation assays to detect dimerization uses truncated or epitope tagged recombinant receptor protein, respectively. (Snodgrass et al., 2013).
Together, these results suggest that saturated fatty acids can induce the homo-dimerization of TLR4 or hetero-dimerization of TLR2 and TLR1 by promoting the formation of lipid rafts and translocation of TLRs into lipid rafts. However, such a mechanism of the actions of SFAs imposes a conceptual dilemma in that if SFAs induce dimerization of TLRs by such a mechanism, why SFAs can activate only TLR4-MD2, TLR2/1 and TLR2/6 dimers containing lipid binding pockets but not other TLRs. Therefore, experimental results still imply potential interactions of SFAs with the lipid binding pockets of TLR4-MD2, TLR2/1, and TLR2/6 to promote the translocation of the TLRs into lipid rafts leading to the dimerization.
10. Potential interactions of SFAs with co-receptors of TLRs
Activation of TLR4 involves not only MD2 but also CD14 and LBP to transfer monomeric LPS to TLR4-MD2 complex. CD14 and CD36 are also involved in TLR2 signaling. Using forward-genetic mutagenesis, Hoebe et al. (Hoebe et al., 2005) demonstrated that TLR2/TLR6 heterodimers require CD36 to sense diacylated lipoproteins, whereas TLR2/TLR1 heterodimers do not. The fatty acyl groups of LPS interact with the N-terminal hydrophobic pocket located in LRR domains of CD14. The predicted fatty acid binding site is also located in CD36. Therefore, it is possible that fatty acids interact with these co-receptors and modulate the propensity of TLR dimerization. However, experimental evidence supporting such a possibility has not been reported so far.
11. Putative endogenous ligand mediating saturated fatty acid-induced activation of TLRs
The activation mode of Toll in Drosophila is different from that of mammalian. PAMPs directly bind mammalian TLRs. Toll receptor in Drosophila is activated by binding of its mature protein ligand Spaetzle. PAMPs induce the precursor of Spaetzle to be proteolytically processed to become an active ligand (Morisato and Anderson, 1994; Schneider et al., 1994). This mode of activation is somewhat similar to that of mammalian IL-1R. The expression of pro-IL-1β is induced by PAMPs and pro-IL-1β is proteolytically cleaved by Caspase-1 or Caspase-4/11 leading to the release of mature IL-1β that can bind IL-1R. However, there is no evidence that mammalian TLRs are activated by PAMPs through protein ligands analogeous to Drosophila spatzel.
Pal et al. (2012) reported that fetuin-A may act as an endogenous ligand of TLR4 for fatty acids mediating lipid-induced insulin resistance. This suggestion is based on the experimental results that fetuin-A knockdown mice are protected from high fat diet induced insulin resistance and that fetuin-A knockdown led to inhibition of TLR4 signaling induced by palmitic acid in cell culture system. Fetuin-A is known to bind free fatty acids as a carrier protein similar to plasma albumin (Cayatte et al., 1990). The composition of fetuin-A bound fatty acids is similar to that of albumin (Cayatte et al., 1990) reflecting the composition of plasma free fatty acids. Thus, it is possible that fetuin-A may efficiently deliver free fatty acids to TLR4-MD2 complex. Since saturated fatty acids can activate TLR2/1 or TLR2/6 dimer, it needs to be determined whether fetuin-A mediates the fatty acid-induced activation of TLR2 dimers as well. In the end, the key question about how saturated fatty acids can induce the dimerization of TLR4 and TLR2 still remains unanswered in such a hypothetical model of TLR activation.
Collectively, the plausible mechanism by which SFAs induce the receptor dimerization favors the interaction of SFAs with the hydrophobic lipid binding pockets in TLR4-MD2 and TLR2/1 or TLR2/6 leading to translocation of the receptors into lipid rafts of plasma membrane.
12. Inhibition of dimerization of TLRs by DHA
DHA inhibits both sodium palmitate- and Pam3CSK4-induced dimerization of TLR2 and TLR1, and recruitment of MyD88 and p47phox (subunit of NADPH dependent oxidase) to lipid raft fractions (Fig. 6). Lipid A containing an unsaturated fatty acid is known to be non-toxic and act as antagonists against endotoxin (Krauss et al., 1989; Qureshi et al., 1991). All PUFAs tested, particularly the n-3 PUFA DHA, inhibit both SFA-induced and TLR4/2 ligand-induced activation of TLR4 or TLR2 (Snodgrass et al., 2013). Therefore, the propensity for the activation of TLR2 and TLR4 may be affected by the delicate balance of relative abundance of the stimulatory SFAs and inhibitory PUFAs. While lauric acid promotes translocation of TLR4 to lipid raft, DHA, an n-3 PUFA, inhibits LPS- or lauric acid-induced dimerization and recruitment of TLR4 into lipid raft fractions. DHA also inhibited SFA-induced or TLR4 or TLR2 ligand-induced ROS generation (Snodgrass et al., 2013; Wong et al., 2009). Consequently, the recruitment of adaptors, MyD88 and TRIF, to TLR4 is attenuated in cells treated with DHA, leading the diminished expression of inflammatory genes such as TNF-α and COX-2. Recruitment of TLR4 to lipid rafts and dimerization are coupled events mediated at least in part by NADPH oxidase-dependent ROS generation. Lauric acid and DHA reciprocally modulate TLR4 activation by regulating the dimerization and recruitment of TLR4 into lipid rafts in a ROS-dependent manner (Wong et al., 2009).
The fact that DHA inhibits the activation of all TLRs tested (both the three domain fold and the single domain fold group without lipid binding pocket) suggests that the target of DHA is not ligand binding sites but rather common downstream step(s) of TLR signaling pathways. The sequence of events for the activation of TLR- mediated signaling pathways is as follows: binding of ligand to receptor complex, dimerization and translocation of the receptor complex into lipid rafts, and recruitment and activation of downstream signaling components leading to modulation of expression of the target genes. DHA can displace T-cell receptor from lipid rafts by altering fatty acid composition of lipid rafts in which saturated fatty acids are the predominant fatty acids (Fan et al., 2004; Stulnig et al., 2001). Therefore, alteration of fatty acid composition in lipid rafts by incorporation of DHA may result in the disruption of TLR4 recruitment to lipid rafts. Together, these results suggest that the target of DHA includes lipid rafts where the dimerization of TLRs occurs. This interpretation is consistent with the experimental results that DHA is a pan inhibitor for all TLRs tested (Lee et al., 2004).
13. Inhibition of the downstream signaling molecule of TLRs by DHA
It was also demonstrated that DHA can inhibit the downstream signaling molecule TAB1 in TLR-mediated signaling pathways (Fig. 9) (Oh et al., 2010). G protein-coupled receptor GPR120 is a sensor for n-3 fatty acids on macrophages and fat cells. Activation of GPR120 by DHA antagonizes the pro-inflammatory effects of TNF-α and lipopolysaccharide in a macrophage cell line. DHA not only blocks the NF-κB and JNK pathways but also prevents expression of cytokines leading to suppression of insulin resistance in obese mice. GPR120 is coupled with the Gq/11 family of G proteins. After ligand binds and Gq/11 is released, G protein receptor kinases phosphorylate the receptor. This generates binding sites for β-arrestins which can interact with downstream signaling molecules (Rajagopal et al., 2010). Oh and colleagues (Oh et al., 2010) demonstrate that β-arrestin2 is essential for the anti-inflammatory effects of n-3 fatty acids in macrophage cells. This β-arrestin2 inhibits both the JNK and NF-κB pathways by sequestering the TAK1 binding protein TAB1. The inhibition of TAB1 prevents phosphorylation and thus activation of IκB kinase upstream of NF-κB and MKK4 (mitogen-activated protein kinase kinase that are upstream of JNK. TAB1 is one of common downstream components of signaling pathways for all TLRs and many cytokines including IL-1β and TNF-α. Therefore, DHA can render suppressive effects on broad pro-inflammatory signaling pathways.
Fig. 9. Schematic diagram of the β-arrestin2 and GPR120-mediated anti-inflammatory mechanism.
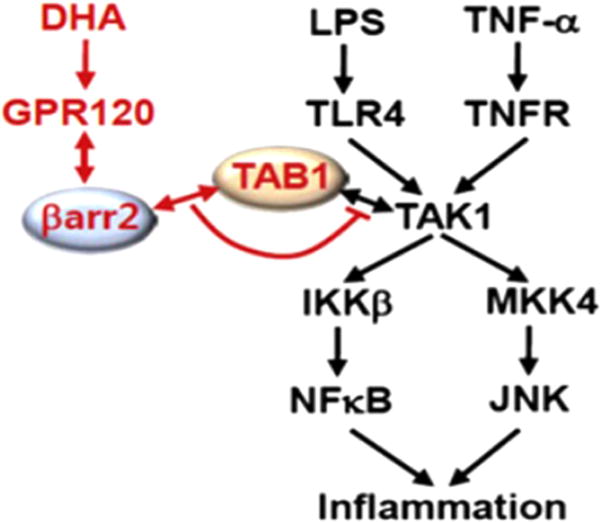
Red colored letters and arrows indicate the DHA-mediated anti-inflammatory effect, and black colored letters and arrows indicate the LPS- and TNF-α-induced inflammatory pathway. (Oh et al., 2010).
14. Pharmacological perspective
Growing evidence suggests that TLR-mediated sterile inflammation is associated with many chronic inflammatory diseases and autoimmune diseases. Therefore, targeting TLR signaling pathways can be a potential strategy in developing preventive and therapeutic measures against chronic inflammatory diseases resulting from dysregulated TLR-mediated immune responses. TLR-mediated immune responses are regulated at multiple levels that include availability of endogenous agonists of TLRs, the expression and activity of TLR complex, and their downstream signaling molecules. Targeting these multiple steps in TLR signaling pathways can be a conceptual strategy in developing small molecule pharmacological agents that inhibit TLR-mediated immune responses. Small molecules that sequester LPS or inhibit LPS binding to CD14 or MD2 have been explored (Peri et al., 2010). A specific inhibitor that binds intracellular domain of TLR4 has been developed (Matsunaga et al., 2011). Activation of TLRs induces the expression of negative regulators of TLR signaling pathways that can assist the resolution of TLR-mediated immune responses (Kawai and Akira, 2010). Dysregulated pathways for the expression of the negative regulators can possibly contribute to exaggerated TLR-mediated immune responses. Thus, the pathways leading to the expression of the negative regulators can also be a conceptual target of modulation for TLR-mediated immune responses. In the context of our current review on the modulation of TLR-mediated signaling pathways, the inhibition of TLR-mediated signaling pathways by dietary components is of particular interest. Some polyphenols which are abundantly present in fruits and vegetables in our diets have been shown to inhibit the dimerization of TLR4 or its downstream signaling molecules (Zhao et al., 2011). Polyphenols are extensively metabolized by gut microbes and liver enzymes (van Duynhoven et al., 2011). Structure function study with the parent dietary polyphenols and their metabolites on TLR activation will help developing safe, specific and potent pharmacological inhibitors.
15. Summary
Saturated fatty acids can activate but DHA inhibits certain TLR-mediated pro-inflammatory signaling pathways. Receptor dimerization is the proximal step and a prerequisite for the activation of TLRs. Bacterial ligands for TLR4 and TLR2/1 or TLR2/6 are acylated with saturated fatty acids. Removal of the fatty acyl moiety from these ligands results in loss of ligand activity indicating that these saturated fatty acyl chains are required for the ligand activity. X-ray crystallographic studies revealed that TLR4 co-receptor MD2 and TLR2, TLR1 or TLR6 contain hydrophobic lipid binding domains, and that occupying these lipid binding domains by acyl- chains of the ligands induce the homodimerization of TLR4 or the heterodimerization of TLR2/1 or TLR2/6. Saturated fatty acids without polar head groups present in lipid A or lipopetides induce homodimerization of TLR4 or heterodimerization of TLR2 with TLR1 or TLR6, albeit at much higher concentrations than those of respective bacterial ligands. The dimerization of TLR4 or TLR2/1 induced by respective bacterial ligands or saturated fatty acids is coupled with the translocation of the receptors into lipid raft fractions of the plasma membrane (e.g., dimerized TLR4 is present only in lipid raft fractions but not in non-lipid raft fractions). Polar lipids in lipid raft fractions are acylated predominantly by saturated fatty acids unlike those of non-lipid raft fractions implying that saturated fatty acids promote, whereas polyunsaturated fatty acids such as DHA inhibit the formation of lipid rafts in the plasma membrane. The experimental results suggest that saturated fatty acids can interact with lipid binding sites of TLR4-MD2 complex or TLR1/2/6 leading to translocation of the receptors into lipid rafts where the receptors can be concentrated to the extent that the dimerization can occur in a ligand independent manner. The lipid raft fractions are the platform where the dimerized receptors recruit and activate the downstream signaling components. DHA inhibits the activation of TLR4 and TLR2/1 or TLR2/6 and other TLRs for which ligands do not possess fatty acyl chains. This suggests that the target of DHA is not the ligand binding sites of TLRs but rather downstream steps where the dimerization of the receptors occurs (e.g., lipid rafts). Receptor dimerization and activation of downstream signaling pathways for TLR4 and TLR2 are illustrated in cartoon below (Fig. 10). In addition, DHA can inhibit the downstream signaling component of the signaling pathways of TLRs and cytokine receptors mediated through GPR120. Together, the reciprocal modulation of TLR signaling pathways by saturated fatty acids and DHA signifies that TLR-mediated innate immune responses can be dynamically regulated by the delicate balance between saturated and unsaturated fatty acids of free fatty acids in tissues and plasma, which in turn, is affected by the kinds of dietary fat we consume.
Fig. 10. Schematic diagram of the translocation of TLR4 and TLR2 into lipid rafts.
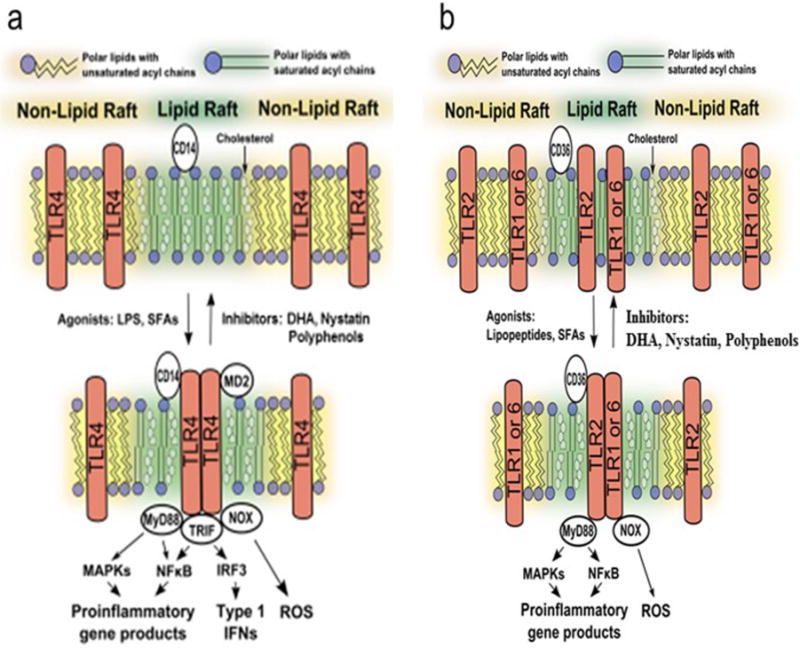
Translocation of TLR4 into lipid rafts and homodimerization of TLR4 induced by LPS or saturated fatty acids, and the inhibition of these steps by DHA (a), or heterodimerization of TLR2 with TLR1 or TLR6 in lipid rafts induced by lipopetides or saturated fatty acids, and its inhibition by DHA (b). For the sake of focusing the dimerization event, the horse shoe shape of TLR4 and exact location of MD2 associated with TLR4 as shown in X-ray crystallographic structure (Fig. 2) are not accurately depicted in this figure.
Acknowledgments
This work was supported by USDA-ARS Program Project (5306-51530-017-00D and USDA/NIFA competitive grant 2013-03477 to D.H.H); NIH (HL128695 to J.K), the American Diabetes Association (1-09-JF-33; 1-12-BS-99 to J.K), American Heart Association (13GRNT17220057 to J. K), UAB diabetes research center sponsored pilot and feasibility program supported by NIH (P30DK079626); grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning of Korea (NRF-2014R1A2A1A11051234 to J. Y. L.)
References
- Albert BB, Derraik JG, Brennan CM, Biggs JB, Smith GC, Garg ML, Cameron- Smith D, Hofman PL, Cutfield WS. Higher omega-3 index is associated with increased insulin sensitivity and more favourable metabolic profile in middle-aged overweight men. Sci Rep. 2014;4:6697. doi: 10.1038/srep06697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arranz S, Chiva-Blanch G, Valderas-Martinez P, Medina-Remon A, Lamuela-Raventos RM, Estruch R. Wine, beer, Alcohol and polyphenols on cardiovascular disease and cancer. Nutrients. 2012;4:759–781. doi: 10.3390/nu4070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Ulmer AJ. Lipopeptide structure determines TLR2 dependent cell activation level. FEBS J. 2005;272:6354–6364. doi: 10.1111/j.1742-4658.2005.05029.x. [DOI] [PubMed] [Google Scholar]
- Cayatte AJ, Kumbla L, Subbiah MT. Marked acceleration of exogenous fatty acid incorporation into cellular triglycerides by fetuin. J Biol Chem. 1990;265:5883–5888. [PubMed] [Google Scholar]
- Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J Immunol. 2004;173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2009;12:138–146. doi: 10.1097/MCO.0b013e3283218299. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Gascon MS, Blanco A, Guerri C. Lipid rafts regulate ethanol-induced activation of TLR4 signaling in murine macrophages. Mol Immunol. 2008;45:2007–2016. doi: 10.1016/j.molimm.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- Hughes AL, Piontkivska H. Functional diversification of the Toll-like receptor gene family. Immunogenetics. 2008;60:249–256. doi: 10.1007/s00251-008-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D. Modulation of the expression of cyclooxygenase-2 by fatty acids mediated through Toll-like receptor 4-derived signaling pathways. FASEB J: Off Publ Fed Am Soc Exp Biol. 2001;15:2556–2564. doi: 10.1096/fj.01-0432com. [DOI] [PubMed] [Google Scholar]
- Jakobsen MU, O’Reilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89:1425–1432. doi: 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a triacylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Jin MS, Lee JO. Structures of the Toll-like receptor family and its ligand complexes. Immunity. 2008;29:182–191. doi: 10.1016/j.immuni.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Kang JY, Lee JO. Structural biology of the Toll-like receptor family. Annu Rev Biochem. 2011;80:917–941. doi: 10.1146/annurev-biochem-052909-141507. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, Lee JO. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Kitchens RL, Ulevitch RJ, Munford RS. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J Exp Med. 1992;176:485–494. doi: 10.1084/jem.176.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss JH, Seydel U, Weckesser J, Mayer H. Structural analysis of the nontoxic lipid A of Rhodobacter capsulatus 37b4. Eur J Biochem FEBS. 1989;180:519–526. doi: 10.1111/j.1432-1033.1989.tb14677.x. [DOI] [PubMed] [Google Scholar]
- Latz E, Verma A, Visintin A, Gong M, Sirois CM, Klein DC, Monks BG, McKnight CJ, Lamphier MS, Duprex WP, Espevik T, Golenbock DT. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat Immunol. 2007;8:772–779. doi: 10.1038/ni1479. [DOI] [PubMed] [Google Scholar]
- Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- Lee JY, Zhao L, Hwang DH. Modulation of pattern recognition receptor- mediated inflammation and risk of chronic diseases by dietary fatty acids. Nutr Rev. 2010;68:38–61. doi: 10.1111/j.1753-4887.2009.00259.x. [DOI] [PubMed] [Google Scholar]
- Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- Matsunaga N, Tsuchimori N, Matsumoto T, Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol. 2011;79:34–41. doi: 10.1124/mol.110.068064. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Morisato D, Anderson KV. The spatzle gene encodes a component of the extracellular signaling pathway establishing the dorsal-ventral pattern of the Drosophila embryo. Cell. 1994;76:677–688. doi: 10.1016/0092-8674(94)90507-x. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D. Saturated fatty acids and type 2 diabetes: more evidence to re-invent dietary guidelines. Lancet Diabetes Endocrinol. 2014;2:770–772. doi: 10.1016/S2213-8587(14)70166-4. [DOI] [PubMed] [Google Scholar]
- Munford RS, Hall CL. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986;234:203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Wang X, Sasidhar M, Nabel EG, Takahashi T, Lukacs NW, Ryter SW, Morita K, Choi AM. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier-Denis E, Mkaddem SB, Vandewalle A. NOX enzymes and Toll-like receptor signaling. Semin Immunopathol. 2008;30:291–300. doi: 10.1007/s00281-008-0120-9. [DOI] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson S, Sundler R. The role of lipid rafts in LPS-induced signaling in a macrophage cell line. Mol Immunol. 2006;43:607–612. doi: 10.1016/j.molimm.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Orr JS, Puglisi MJ, Ellacott KL, Lumeng CN, Wasserman DH, Hasty AH. Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes. 2012;61:2718–2727. doi: 10.2337/db11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- Peri F, Piazza M, Calabrese V, Damore G, Cighetti R. Exploring the LPS/TLR4 signal pathway with small molecules. Biochem Soc Trans. 2010;38:1390–1395. doi: 10.1042/BST0381390. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Qureshi N, Takayama K, Kurtz R. Diphosphoryl lipid A obtained from the nontoxic lipopolysaccharide of Rhodopseudomonas sphaeroides is an endotoxin antagonist in mice. Infect Immun. 1991;59:441–444. doi: 10.1128/iai.59.1.441-444.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Hwang D. Murine Toll-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NF kappa B and expression of the inducible cyclooxygenase. J Biol Chem. 2000;275:34035–34040. doi: 10.1074/jbc.M007386200. [DOI] [PubMed] [Google Scholar]
- Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, Kopp A, Schoelmerich J, Falk W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2009;126:233–245. doi: 10.1111/j.1365-2567.2008.02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DS, Jin Y, Morisato D, Anderson KV. A processed form of the Spatzle protein defines dorsal-ventral polarity in the Drosophila embryo. Development. 1994;120:1243–1250. doi: 10.1242/dev.120.5.1243. [DOI] [PubMed] [Google Scholar]
- Scior T, Alexander C, Zaehringer U. Reviewing and identifying amino acids of human, murine, canine and equine TLR4/MD-2 Receptor complexes conferring endotoxic Innate Immunity Activation by LPS/Lipid A, or antagonistic Effects by Eritoran, in Contrast to Species-Dependent modulation by Lipid IVa. Comput Struct Biotechnol J. 2013;5:e201302012. doi: 10.5936/csbj.201302012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Yang CS, Lee JY, Lee SJ, Choi HH, Lee HM, Yuk JM, Harding CV, Jo EK. Mycobacterium tuberculosis lipoprotein-induced association of TLR2 with protein kinase C zeta in lipid rafts contributes to reactive oxygen species-dependent inflammatory signalling in macrophages. Cell Microbiol. 2008;10:1893–1905. doi: 10.1111/j.1462-5822.2008.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass RG, Huang S, Choi IW, Rutledge JC, Hwang DH. Inflammasome-mediated secretion of IL-1beta in human monocytes through TLR2 activation; modulation by dietary fatty acids. J Immunol. 2013;191:4337–4347. doi: 10.4049/jimmunol.1300298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulnig TM, Huber J, Leitinger N, Imre EM, Angelisova P, Nowotny P, Waldhausl W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J Biol Chem. 2001;276:37335–37340. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- Tapping RI, Tobias PS. Mycobacterial lipoarabinomannan mediates physical interactions between TLR1 and TLR2 to induce signaling. J Endotoxin Res. 2003;9:264–268. doi: 10.1179/096805103225001477. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. Membrane sorting of Toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem. 2006;281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Gamper FG, Lepper PM, Mouratis MA, Schumann C, Harokopakis E, Schifferle RE, Hajishengallis G, Triantafilou K. Lipopolysaccharides from atherosclerosis-associated bacteria antagonize TLR4, induce formation of TLR2/1/CD36 complexes in lipid rafts and trigger TLR2-induced inflammatory responses in human vascular endothelial cells. Cell Microbiol. 2007;9:2030–2039. doi: 10.1111/j.1462-5822.2007.00935.x. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Manukyan M, Mackie A, Morath S, Hartung T, Heine H, Triantafilou K. Lipoteichoic acid and Toll-like receptor 2 internalization and targeting to the Golgi are lipid raft-dependent. J Biol Chem. 2004;279:40882–40889. doi: 10.1074/jbc.M400466200. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- van Duynhoven J, Vaughan EE, Jacobs DM, Kemperman RA, van Velzen EJ, Gross G, Roger LC, Possemiers S, Smilde AK, Dore J, Westerhuis JA, Van de Wiele T. Metabolic fate of polyphenols in the human superorganism. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4531–538. doi: 10.1073/pnas.1000098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans KA, Hashimoto C. Ventralization of the Drosophila embryo by deletion of extracellular leucine-rich repeats in the Toll protein. Mol Biol Cell. 1995;6:587–596. doi: 10.1091/mbc.6.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Tao X, Shen B, Horng T, Medzhitov R, Manley JL, Tong L. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature. 2000;408:111–115. doi: 10.1038/35040600. [DOI] [PubMed] [Google Scholar]
- Yang CS, Shin DM, Kim KH, Lee ZW, Lee CH, Park SG, Bae YS, Jo EK. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol. 2009;182:3696–3705. doi: 10.4049/jimmunol.0802217. [DOI] [PubMed] [Google Scholar]
- Yang CS, Shin DM, Lee HM, Son JW, Lee SJ, Akira S, Gougerot-Pocidalo MA, El-Benna J, Ichijo H, Jo EK. ASK1-p38 MAPK-p47phox activation is essential for inflammatory responses during tuberculosis via TLR2-ROS signalling. Cell Microbiol. 2008;10:741–754. doi: 10.1111/j.1462-5822.2007.01081.x. [DOI] [PubMed] [Google Scholar]
- Yeop Han C, Kargi AY, Omer M, Chan CK, Wabitsch M, O’Brien KD, Wight TN, Chait A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: dissociation of adipocyte hypertrophy from inflammation. Diabetes. 2010;59:386–396. doi: 10.2337/db09-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Tay PN, Cao W, Li W, Lu J. Integrin-nucleated Toll-like receptor (TLR) dimerization reveals subcellular targeting of TLRs and distinct mechanisms of TLR4 activation and signaling. FEBS Lett. 2002;532:171–176. doi: 10.1016/s0014-5793(02)03669-4. [DOI] [PubMed] [Google Scholar]
- Zhao L, Lee JY, Hwang DH. Inhibition of pattern recognition receptor-mediated inflammation by bioactive phytochemicals. Nutr Rev. 2011;69:310–320. doi: 10.1111/j.1753-4887.2011.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


