Abstract
Purpose
Gene expression profile (GEP) testing can support chemotherapy decision making for patients with early-stage, estrogen receptor–positive, human epidermal growth factor 2–negative breast cancers. This study evaluated the cost effectiveness of one GEP test, Oncotype DX (Genomic Health, Redwood City, CA), in community practice with test-eligible patients age 40 to 79 years.
Methods
A simulation model compared 25-year societal incremental costs and quality-adjusted life-years (QALYs) of community Oncotype DX use from 2005 to 2012 versus usual care in the pretesting era (2000 to 2004). Inputs included Oncotype DX and chemotherapy data from an integrated health care system and national and published data on Oncotype DX accuracy, chemotherapy effectiveness, utilities, survival and recurrence, and Medicare and patient costs. Sensitivity analyses varied individual parameters; results were also estimated for ideal conditions (ie, 100% testing and adherence to test-suggested treatment, perfect test accuracy, considering test effects on reassurance or worry, and lowest costs).
Results
Twenty-four percent of test-eligible patients had Oncotype DX testing. Testing was higher in younger patients and patients with stage I disease (v stage IIA), and 75.3% and 10.2% of patients with high and low recurrence risk scores received chemotherapy, respectively. The cost-effectiveness ratio for testing (v usual care) was $188,125 per QALY. Considering test effects on worry versus reassurance decreased the cost-effectiveness ratio to $58,431 per QALY. With perfect test accuracy, the cost-effectiveness ratio was $28,947 per QALY, and under ideal conditions, it was $39,496 per QALY.
Conclusion
GEP testing is likely to have a high cost-effectiveness ratio on the basis of community practice patterns. However, realistic variations in assumptions about key variables could result in GEP testing having cost-effectiveness ratios in the range of other accepted interventions. The differences in cost-effectiveness ratios on the basis of community versus ideal conditions underscore the importance of considering real-world implementation when assessing the new technology.
INTRODUCTION
Gene expression profile (GEP) tests, such as Oncotype DX (Genomic Health, Redwood City, CA), have been recommended for use to support treatment decision making for patients with early-stage, node-negative, estrogen receptor (ER)–positive, human epidermal growth factor 2 (HER2)–negative cancers.1-3 The primary goal of GEP testing is to identify patients at high recurrence risk who will benefit from chemotherapy, while allowing patients with a low recurrence risk to forego chemotherapy, potentially offsetting the test costs with savings from reductions in chemotherapy use.
To date, use of GEP testing in community practice remains low, ranging from 22% to 42% of test-eligible patients.4-9 Moreover, chemotherapy use is sometimes discordant with test results, with 17% to 26% of patients with high recurrence risk scores not receiving chemotherapy and 8% of patients with low recurrence risk scores receiving chemotherapy.10
Prior economic analyses of GEP evaluated hypothetical cohorts under ideal conditions and concluded that it had low costs relative to its benefits.11-17 However, given the divergence of community testing and chemotherapy use from the ideal, it is possible that the expected clinical and economic benefits of GEP are not being fully realized. In this study, we conducted an analysis of the likely cost effectiveness of Oncotype DX testing on the basis of community practice patterns.
METHODS
We constructed a discrete-time state transition simulation model to estimate the likely incremental costs per quality-adjusted life-year (QALY) of community use of Oncotype DX testing versus usual care from a societal perspective. The Georgetown University Oncology and Kaiser Permanente Northern California (KPNC) institutional review boards approved the research.
Intervention and Patients
We selected Oncotype DX because it is the most commonly used GEP test in the United States10 and the primary focus of prior economic analyses.11-17 The population included test-eligible patients age 40 to 79 years diagnosed with stage I or IIA, ER-positive, HER2-negative breast cancer between 2000 and 2012. Costs and effects for patients diagnosed from 2000 to 2004 (ie, pre–Oncotype DX period, usual care) were compared with those among patients diagnosed from 2005 to 2012 (ie, period when Oncotype DX testing was used in community practice).
Model Overview
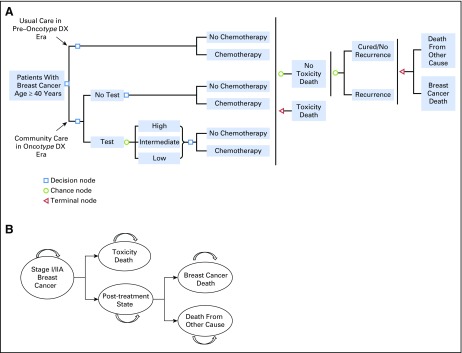
The model was developed using TreeAge Pro 2015 (TreeAge Software, Williamstown, MA). The model captured a 25-year time horizon from diagnosis because the median age of diagnosis is > 60 years18 and almost all distant recurrences (and deaths from recurrences) occur within 25 years of diagnosis.19 Events (eg, chemotherapy use or toxicity) were tallied at 1-year transition intervals. The model decision pathways and health states are summarized in Figures 1A and 1B, respectively.
Fig 1.
Decision tree and state transitions for patients with stage I or IIA, node-negative, estrogen receptor (ER)–positive, human epidermal growth factor receptor 2 (HER2)–negative breast cancer. (A) Simulation model schema. The model was developed to compare cost effectiveness of community practice with use of Oncotype DX test versus usual care without the test. The community practice arm included observed testing and chemotherapy use in 2005 to 2012. The usual care arm included chemotherapy use patterns in the pre–Oncotype DX era (2000 to 2005). Testing probabilities were conditional on age and stage. The test results affected the probability of chemotherapy use. (B) State transition. All simulated patients were newly diagnosed with ER-positive, HER2-negative, node-negative, stage I or IIA breast cancer. If death from chemotherapy toxicity did not happen at initial treatment, all simulated patients transitioned to the post-treatment state until breast cancer death (if recurrence occurred) or death from other cause (if no recurrence occurred or death from other cause occurred before recurrence). Patients without distant recurrence only died of non–breast cancer causes or chemotherapy toxicity. Patients remain in the same state until the time of a transition event.
Briefly, the model began with the generation of simulated patients with breast cancer by age and stage on the basis of national incidence rates. In the usual care scenario, patients could receive chemotherapy or not on the basis of their age and stage. In the Oncotype DX testing period, patients were tested or not, and received chemotherapy on the basis of age, stage, and test use and results. If recurrence occurred, it was assumed to progress to breast cancer death within 25 years, unless death occurred earlier as a result of chemotherapy toxicity or other causes. Without recurrence, patients died of other causes, and if chemotherapy was received, it did not provide benefit but could have resulted in toxicity.
Tracking variables built into the model were used to tally starting age, current age, recurrence status, testing status, test result, chemotherapy use, toxicity grade, and cause of death. These tracking variables were used in postprocessing analyses using SAS 9.4 (SAS Institute, Cary, NC), including calculation of life-years for each simulated patient, application of utility weights, cost allocation to each event, and discounting of costs and effects.
Input Parameters
Model inputs used to estimate costs and effects were derived from national data, published research, and KPNC electronic records linking registry data, treatment, and GEP testing (Table 1). Incidence rates were based on SEER data from 2000 to 2012.21 Oncotype DX testing and chemotherapy use rates were based on age- and stage-specific use at KPNC.8 The marginal distribution of risk score categories and the probability of recurrence conditional on each risk score category were based on published data.3 Using Bayes’ theorem, these data were used to calculate the probability of having each risk score category conditional on whether or not recurrence occurred, as a measure of the test accuracy.
Table 1.
Model Input Parameters for Estimation of Costs and Effects Among Patients With ER-Positive, HER2-Negative, Lymph Node–Negative, Stage I or IIA Breast Cancer
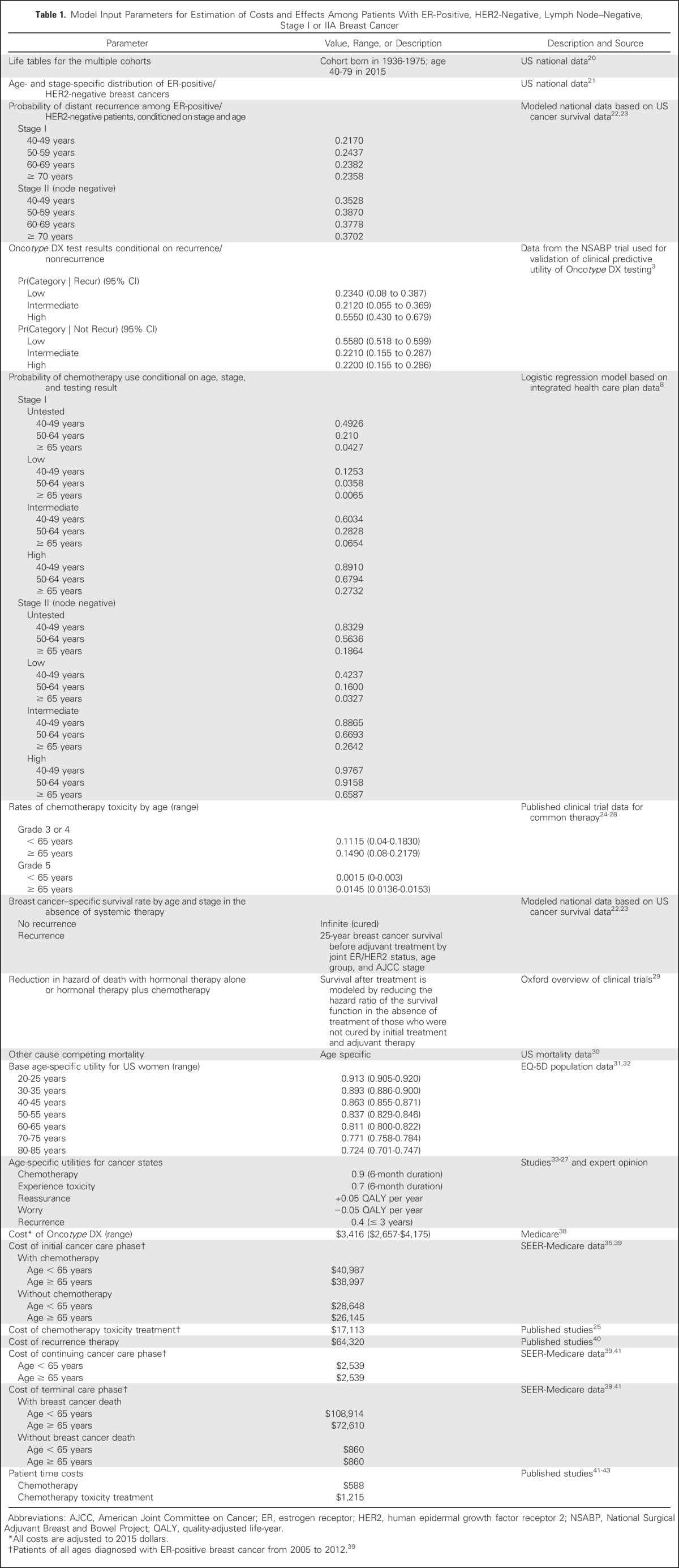
The underlying age- and stage-specific 25-year breast cancer survival in the absence of treatment of ER-positive, HER2-negative, stage I and IIA cancers was based on prior Cancer Intervention and Surveillance Modeling Network analyses.22,23 This overall survival was partitioned into survival among those who experienced distant recurrence and those who did not and was used to calculate annual risk of breast cancer death given recurrence status in the absence of any adjuvant treatment.
To isolate the effects of Oncotype DX on chemotherapy-related outcomes, we assumed that 100% of patients received hormonal therapy and that adherence was independent of Oncotype DX testing. Treatment with hormonal therapy alone or hormonal therapy and chemotherapy reduced the risk of death among those destined to have distant recurrences but had no effect on breast cancer mortality among those who would never experience recurrence. Treatment effects were based on the most recent meta-analysis from the Early Breast Cancer Collaborative Trialists’ Group.29 The probability of experiencing chemotherapy toxicity and toxicity grade were based on published trials.24-28 Non–breast cancer mortality was based on US data.30
Survival was weighted by utility values for each health state to estimate QALYs. Utilities were based on female population age-specific values from the EQ-5D reported on the Medical Expenditure Panel Survey.31,32 Among those who received chemotherapy, utility was further adjusted for the 6 months of administration.33-35 Patients experiencing recurrence had further decrements in utility (Table 1).
The costs of the Oncotype DX test ($3,416) were based on the Medicare reimbursement rate.38 Age- and stage-specific cancer care costs were based on published national estimates.38-40,44 Initial care costs were separated into initial care with and without chemotherapy (including toxicity) on the basis of age- and stage-specific proportions of patients receiving chemotherapy nationally. Costs of treatment of chemotherapy toxicity were assumed to include a short hospitalization and emergency room visits for evaluation of adverse events.25
Patients who experienced a distant recurrence were assumed to incur new chemotherapy costs.2 On the basis of a median overall survival after distant recurrence of 36 months,45,46 recurrence costs included 1 year of chemotherapy, 1 year of continuing care, and 1 year of terminal care. Patients without recurrence incurred continuing care costs until the last year of life; they then incurred terminal care costs on the basis of those of the noncancer population.39
Patient time costs for chemotherapy were based on travel and time for standard regimens.41,42 Time costs for the treatment of toxicity were based on the average length of a hospital stay (eg, for febrile neutropenia) and/or number of emergency room visits. Patient time was valued using the average 2012 US female hourly wage rate.43 All costs were updated to 2015 US dollars (the most current year available) using the medical care component of the Consumer Price Index.47 Future costs and QALYs were discounted at 3%.
Analyses
One hundred million simulations were conducted to reduce Monte Carlo error in the estimation of costs and effects. We calculated the incremental cost-effectiveness ratio for community Oncotype DX test and chemotherapy treatment patterns versus usual care in the pre–Oncotype DX era.
Accounting for Uncertainty
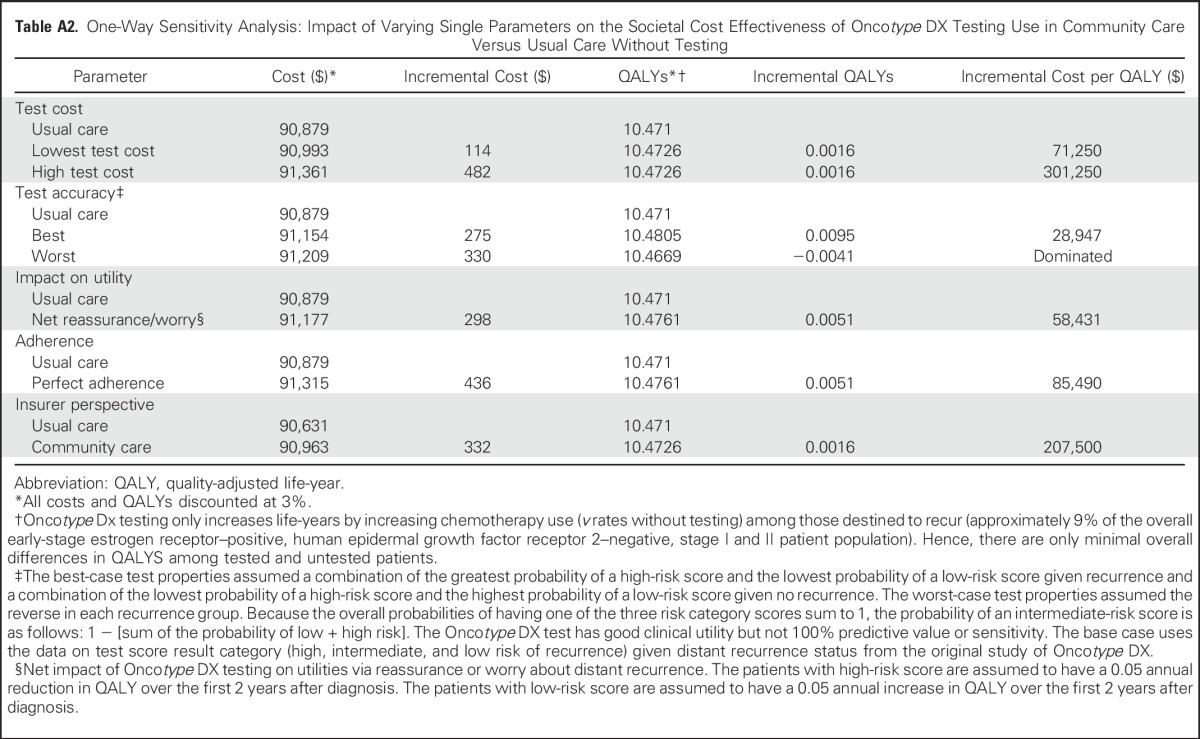
To evaluate the impact of uncertainty, we conducted several one-way sensitivity analyses. First, we examined the impact of test misclassification of distant recurrence by varying results across the upper and lower 95% CIs of the derived test operating characteristics for accuracy. Because there were three possible categories of recurrence risk scores (low, intermediate, and high) conditional on two recurrence possibilities (yes or no), to estimate the least misclassification of low-risk scores given no recurrence, the highest probability of having a low-risk score was combined with the lowest probability for having a high-risk score. For the least misclassification of high-risk given recurrence, the highest probability of having a high-risk score was combined with the lowest probability of having a low-risk score. In each calculation, the total is constrained to equal 1, so that the probability of intermediate risk was 1 minus the sum of the probability of the high-risk and low-risk scores.
Next, we varied the cost of the Oncotype DX test from $2,657 to $4,175 on the basis of the difference (± $759) between the retail price of $4,175 and the Medicare reimbursement rate ($3,416). To assess the impact of perfect patient adherence to test-suggested treatment, 100%, 50%, and 0% chemotherapy use was assumed among those with high-, intermediate-, and low-risk scores, respectively.
Scenario Analyses
We assessed the following two alternative scenarios to the base-case analyses: using the insurer (ie, Medicare) perspective by excluding patient time costs, and including the net impact of Oncotype DX testing on possible reassurance or worry about distant recurrence through further utility weighting. For the latter, we assumed that over the first 2 years after diagnosis, patients with low-risk scores gained 0.05 QALY as a result of a decrease in worry, whereas patients with high-risk scores had a 0.05 reduction in QALYs as a result of increased worry about recurrence.48
Finally, we conducted a multiway sensitivity analysis of a scenario with the following idealized conditions: 100% test rates and adherence to test-suggested chemotherapy treatment, best test accuracy, inclusion of the impact of testing on utility, and lowest costs. We did not perform a probabilistic sensitivity analysis because the computational burden exceeded available computing resources.
Model Validation
To evaluate the validity of the model outcomes, the code was verified by confirming that results varied in expected directions using extreme values of parameters. Face validity was evaluated by comparing life-years saved among clinically relevant patient subgroups on the basis of age, stage, recurrence, and chemotherapy use (Appendix Table A1, online only).
RESULTS
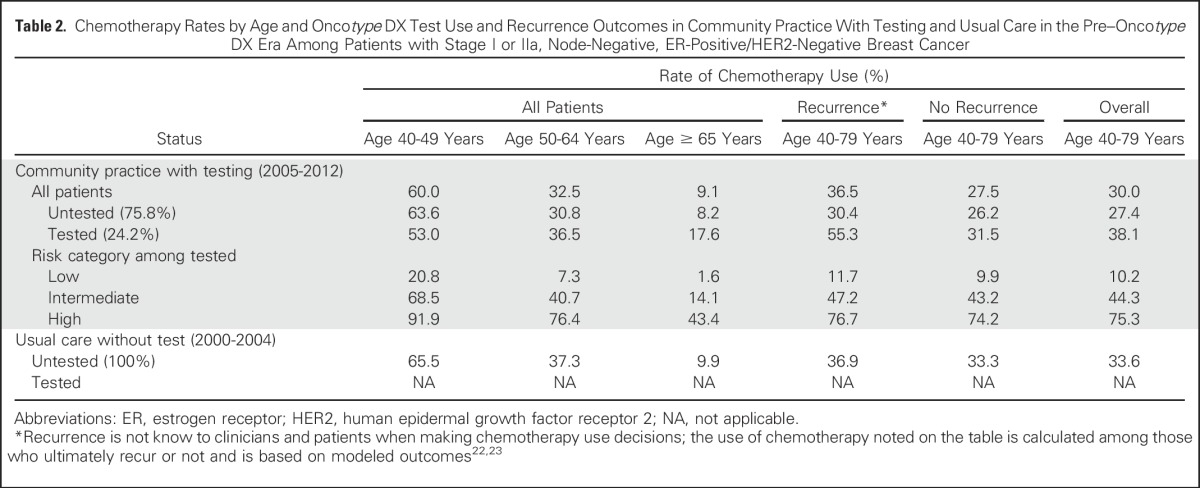
Community practice Oncotype DX test and chemotherapy rates between 2005 and 2012 were 24.2% and 30.0%, respectively. Tested patients were younger than nontested patients (mean age, 56.2 years [standard deviation, 8.9 years] v 60.7 years [standard deviation, 10.1 years], respectively) and more likely to have stage I disease than stage II disease (data not shown). Tested patients younger than age 50 years had lower chemotherapy rates than untested patients in the same age group (53.0% v 63.6%, respectively). Among older patients, there was more chemotherapy use among tested than untested patients (age 50 to 64 years: 36.5% v 30.8%, respectively; age ≥ 65 years: 17.6% v 8.2%, respectively; Table 2). These patterns resulted in a greater proportion of tested than untested patients who were destined to have distant recurrences receiving chemotherapy (55.3% v 30.4%, respectively).
Table 2.
Chemotherapy Rates by Age and Oncotype DX Test Use and Recurrence Outcomes in Community Practice With Testing and Usual Care in the Pre–Oncotype DX Era Among Patients with Stage I or IIa, Node-Negative, ER-Positive/HER2-Negative Breast Cancer
Incremental Cost Effectiveness
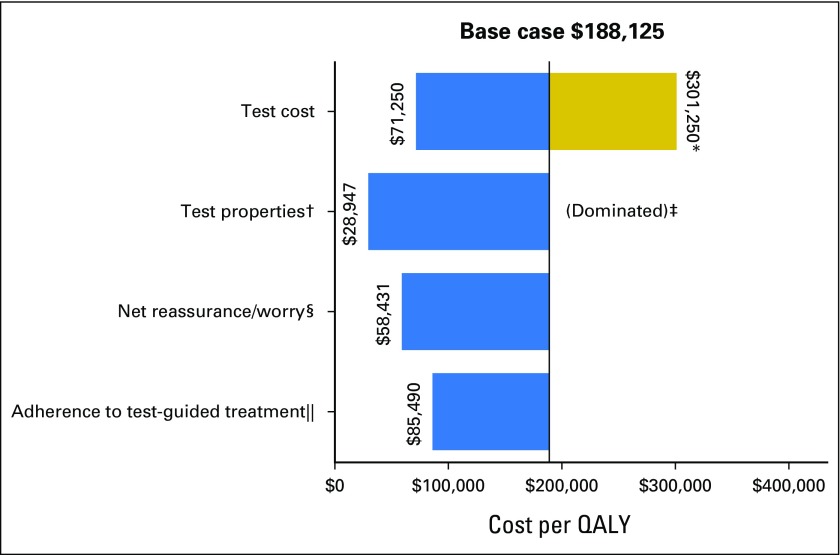
The incremental cost-effectiveness ratio of breast cancer management using Oncotype DX testing as observed in community practice versus usual care without testing was $188,125 per QALY (Table 3). However, varying the values of several factors changed the results substantially, in several cases decreasing the costs to < $75,000 per QALY (Fig 2 and Appendix Table A2, online only). For instance, if Oncotype DX costs were decreased from current Medicare reimbursement rates of $3,416 to $2,657, then the incremental cost-effectiveness ratio of community practice versus usual care decreased to $71,250 per QALY. If Oncotype DX test properties improved, the incremental cost-effectiveness ratio decreased to $28,947 per QALY. If testing had the worst-case accuracy, testing would be dominated (ie, costs more and saves fewer lives than usual care).
Table 3.
Societal Perspective Incremental Cost-Effectiveness Ratio of Oncotype Testing in Community Practice Versus Usual Care Without Testing Among Patients With Stage I or II, Node-Negative, ER-Positive, HER2-Negative Breast Cancer
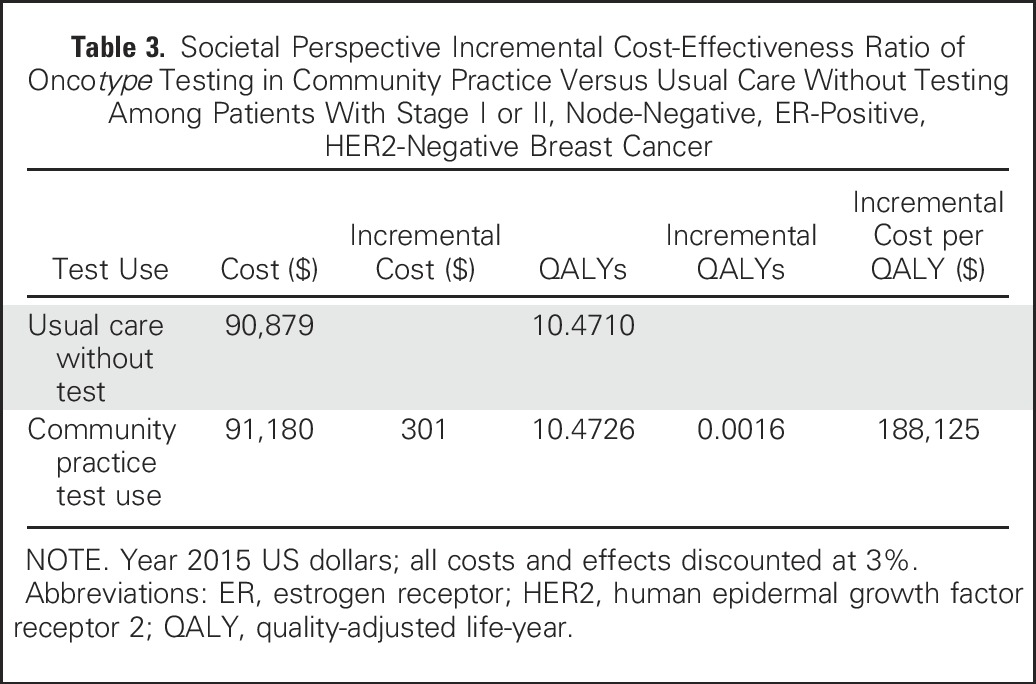
Fig 2.
Impact of varying single parameters on the societal cost-effectiveness ratios for Oncotype DX testing in community practice versus usual care without testing among patients with stage I or II, node-negative, estrogen receptor–positive, human epidermal growth factor receptor 2–negative breast cancer. This diagram illustrates the changes in the incremental cost-effectiveness ratio (ICER) for the costs per quality-adjusted life-year (QALY) under various parameter values and alternative assumptions. The solid vertical line represents the base-case ICER result comparing community practice with Oncotype DX testing versus usual care without Oncotype DX. The horizontal bars indicate the change from the base ICER when the one individual parameter is varied. If the bar goes to the right of the base case, it indicates that the alternative value or assumption costs more per QALY than the base case, where bars that go to the left indicate that the alternative value or assumption costs less per QALY than the base case. (*) Test costs were varied from the base case of $3,416 to $2,657 and $4,175. The large difference in cost per QALY when test costs were varied is a result of these effects being magnified by the small incremental QALYs between usual care and community care. (†) The accuracy of the test represents the probability of a test score, conditional on actual distant recurrence. The best test accuracy reflects a greater proportion of women who actually experience recurrence having high-risk scores and a smaller proportion having low-risk scores, and among those who do not experience recurrence, fewer have high-risk scores and more have low-risk scores than in the base case on the basis of observed performance in the original validation study. (‡) The worst testing accuracy was dominated. That is, it resulted in community practice being more costly and producing fewer QALYs than usual care without testing. (§) Net reassurance/worry is based on gaining 0.5 QALY or losing 0.5 QALY over the first 2 years after diagnosis with low- and high-risk recurrence scores, respectively. (‖) One hundred percent adherence to test-guided treatment assumes 100% chemotherapy use among patients with high-risk score on gene expression profile testing, 50% chemotherapy use for intermediate risk, and 0% chemotherapy use for low risk.
Under the assumption that having information about recurrence risk affects utilities via worry or reassurance, the incremental cost-effectiveness ratio for Oncotype DX testing as it occurred in community practice versus usual care was $58,431 per QALY gained (Fig 2 and Appendix Table A2). Adherence to test-concordant treatment lowered the cost-effectiveness ratio to $85,490 per QALY, but the insurers’ perspective had less of an effect on the cost-effectiveness ratio ($207,500 per QALY). Finally, in the multiway scenario analyses of ideal circumstances, the likely cost-effectiveness ratio for Oncotype DX testing would be $39,496 per QALY compared with usual care without testing (not shown).
DISCUSSION
This study evaluated the likely cost effectiveness of Oncotype DX testing as integrated into breast cancer care in community practice versus usual care without testing for patients diagnosed with early-stage, ER-positive, HER2-negative breast cancer. The patterns of Oncotype DX use in community practice suggest that there was selection of patients to testing where results may have been most likely to affect treatment decisions. Although Oncotype DX testing has high costs relative to its benefits as deployed in community practice, realistic variations in assumptions about key variables could result in testing having cost-effectiveness ratios in the range of other generally accepted interventions. The variables that resulted in lower cost-effectiveness ratios for community use of Oncotype DX than seen in the base case included lower test costs, higher test accuracy, greater adherence to test-suggested treatment, and consideration of the benefits of testing on quality of life.
GEP testing is primarily recommended to support decisions about adjuvant chemotherapy. Although only 22% to 42% of test-eligible patients undergo Oncotype DX testing in the United States,4-9 the patterns of care in our study suggest that testing is being used in situations where results are most likely to change management. For instance, although older women were less likely to be tested than younger women, older women who were tested were twice as likely to receive chemotherapy as those who were not tested, especially when they had high recurrence risk scores. In addition, among younger patients in whom chemotherapy is typically recommended, many who were tested and had low-risk results avoided chemotherapy.
The cost-effectiveness ratio in this study is substantially higher than that reported in past analyses of Oncotype DX.12-14,16 This difference is likely to be the result of several factors. First, past studies assumed ideal conditions and/or large reductions in chemotherapy use with testing.12-14,16 We found that although rates of chemotherapy decreased in community practice after the introduction of Oncotype DX testing,8 testing did not change decisions about chemotherapy as dramatically as earlier analyses assumed it would. Second, in contrast to the assumptions in prior analyses, not all patients who were tested followed the test-suggested decision about chemotherapy.11,16 Moreover, in community practice, fewer women were receiving chemotherapy under usual care before the introduction of testing than assumed in the earlier studies.
This study was unique in considering the impact of test properties on cost-effectiveness ratios, whereas past analyses generally assumed perfect prediction of recurrence.12,14,16 In fact, the original validation study found that 70% of patients with high-risk scores did not develop distant recurrence and 7% of patients at low risk had distant recurrences at 10 years.3 When we examined idealized conditions, including perfect test accuracy, the cost-effectiveness ratio decreased to $39,496 per QALY, which is more similar to earlier estimates, given inflation.13,14
We examined Oncotype DX in this study, but there are several other GEP tests being promoted for clinical use.49-53 Consequently, it is possible that market forces will decrease future GEP test costs. This analysis demonstrated that if Oncotype DX test costs were lower than present Medicare reimbursement rates, it would have cost-effectiveness ratios similar to many currently covered services.54,55
A novel contribution of this analysis is the consideration of the impact on the cost-effectiveness ratio of the potential ability of GEP testing to provide reassurance if results indicate a low risk of recurrence (or to increase worry with high-risk results). Given that the majority of patients for whom testing is currently recommended will have low recurrence risk scores, the increase in QALYs from reassurance outweighed any decrease as a result of increasing worry among those with high-risk scores. Consideration of these effects lowered the cost-effectiveness ratio to $58,431. Because our result was based on expert opinion, further research is warranted to determine patient utility and willingness to pay related to this aspect of GEP testing. Furthermore, because selection of test result–concordant therapy affects cost-effectiveness ratios, future studies should explore reasons for discordance between treatment prescribed by GEP results and actual treatments received.
There are several caveats that should be considered in evaluating our results. First, the cost-effectiveness results for community practice used data from a large integrated health plan for GEP testing and chemotherapy rates because there is no national source of community data with registry information, GEP results, and complete chemotherapy data. The data used in this analysis may not generalize to other community settings if financial barriers and other practice factors cause different patterns of patient selection to testing and/or differentially affect events downstream from the decision to use GEP testing. Therefore, costs and effects in other community settings could be better or worse than estimated in this analysis. However, data from the patients included in the integrated health plan have been shown to be representative of the US population in terms of sociodemographic and cancer characteristics,8,56,57 and the patterns of Oncotype DX use and treatment are similar to those reported in other care settings.58
Second, GEP testing does not have a direct effect on survival. GEP testing can only affect QALYs by guiding a greater use of chemotherapy to the small proportion of women at highest risk of recurrence who would not otherwise be treated without testing. Hence, the difference in QALYs between tested and untested patients in this analysis is small. In these situations, factors that lead to even small differences in QALYs between community practice and usual care can magnify differences in the cost-effectiveness ratios. Finally, it will be important to reassess the cost-effectiveness ratios for GEP testing as results of the predictive validity for intermediate-risk scores59,60 and node-positive disease become available.59-61
Overall, this economic analysis found that the likely cost-effectiveness ratio for Oncotype DX testing in community practice versus usual care without testing was higher than the ratios for most commonly accepted diagnostic and preventive interventions. However, plausible changes in several factors could change the results and lead to Oncotype DX testing having a cost-effectiveness ratio similar to other commonly accepted practices. The substantial differences in conclusions about cost-effectiveness ratios on the basis of community practice versus more idealized practice underscore the importance of considering real-world implementation when assessing the costs and survival associated with new diagnostic (or treatment) technology.
ACKNOWLEDGMENT
We thank Yan Li, MD, Laurie Habel, PhD, and Lawrence Kushi, ScD, for prior work integrating Oncotype DX results with Kaiser Permanente Northern California data and for thoughtful suggestions on earlier versions of this article.
Appendix
Table A1.
Model Outcomes for Life-Years Among Patients With Stage I or II, Node-Negative, ER-Positive, HER2-Negative Breast Cancer by Chemotherapy, Age, Stage, and Recurrence
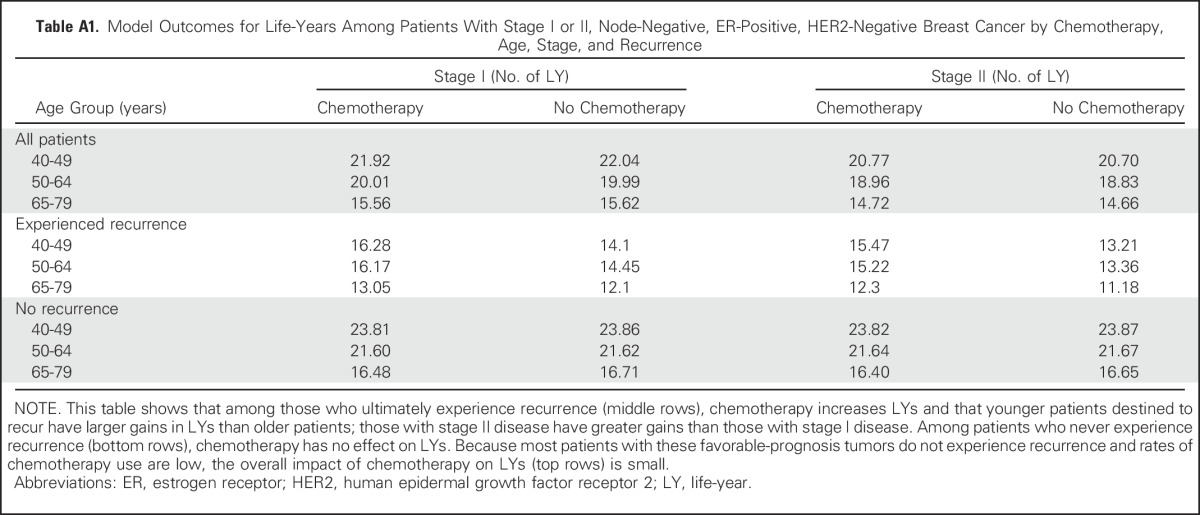
Table A2.
One-Way Sensitivity Analysis: Impact of Varying Single Parameters on the Societal Cost Effectiveness of Oncotype DX Testing Use in Community Care Versus Usual Care Without Testing
Footnotes
Supported by National Cancer Institute Grant No. UO1 CA183081 (J.M., T.A.L., and S.R.) and American Cancer Society Mentored Research Scholar Grant No. 14-027-01-CPHPS (Y.C.). Also supported, in part, by Grant No. U01 CA152958 from the National Cancer Institute as part of the Cancer Intervention and Surveillance Modeling Network, Grant No. R35CA197289 (J.M.) from the National Cancer Institute, Grant No. UC2 CA148471 (Katrina Goddard, Lawrence Kushi, and Evelyn Whitlock) from the National Cancer Institute, Grant No. R01 CA105274 (Lawrence Kushi) from the National Cancer Institute, Lombardi Comprehensive Cancer Center American Cancer Society Young Investigator Award No. ACS IRG 92-152-20 (J.J.), and a Cancer Prevention Research Fellowship sponsored by the American Society of Preventive Oncology and the Breast Cancer Research Foundation (ASPO-17-001; J.J.).
The content is solely the responsibility of the authors and does not represent the official views of the National Cancer Institute at the National Institutes of Health or the American Cancer Society.
AUTHOR CONTRIBUTIONS
Conception and design: Young Chandler, Clyde B. Schechter, Charles E. Phelps, Tracy A. Lieu, Scott Ramsey, Jeanne S. Mandelblatt
Administrative support: Aimee Near, Jeanne S. Mandelblatt
Collection and assembly of data: G. Thomas Ray, Tracy A. Lieu, Jeanne S. Mandelblatt
Data analysis and interpretation: Young Chandler, Clyde B. Schechter, Jinani Jayasekera, Aimee Near, Suzanne C. O'Neill, Claudine Isaacs, Charles E. Phelps, Tracy A. Lieu, Scott Ramsey, Jeanne S. Mandelblatt
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cost Effectiveness of Gene Expression Profile Testing in Community Practice
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Young Chandler
No relationship to disclose
Clyde B. Schechter
Consulting or Advisory Role: COHRDATA
Jinani Jayasekera
No relationship to disclose
Aimee Near
No relationship to disclose
Suzanne C. O'Neill
No relationship to disclose
Claudine Isaacs
Consulting or Advisory Role: Pfizer, Genentech, Novartis, AstraZeneca, Medivation, NanoString Technologies
Speakers' Bureau: Genentech, Celgene, Pfizer, AstraZeneca
Research Funding: Novartis (Inst), Pfizer (Inst), Genentech (Inst), Tesaro (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Travel, Accommodations, Expenses: Caris Life Sciences
Charles E. Phelps
No relationship to disclose
G. Thomas Ray
Employment: The Permanente Medical Group
Research Funding: Pfizer (Inst)
Tracy A. Lieu
No relationship to disclose
Scott Ramsey
Consulting or Advisory Role: Bayer, Genentech, Bristol-Myers Squibb
Research Funding: Genentech
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Bayer
Jeanne S. Mandelblatt
No relationship to disclose
REFERENCES
- 1.ASCO Institute for Quality : Breast cancer treatment plan and summary resources. http://www.instituteforquality.org/breast-cancer-treatment-plan-and-summary-resources
- 2. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer. Version 2.2017. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 3.Paik S, Shak S, Tang G, et al. : A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817-2826, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Afghahi A, Mathur M, Thompson CA, et al. : Use of gene expression profiling and chemotherapy in early-stage breast cancer: A study of linked electronic medical records, cancer registry data, and genomic data across two health care systems. J Oncol Pract 12:e697-e709, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein AJ, Wong YN, Mitra N, et al. : Adjuvant chemotherapy use and health care costs after introduction of genomic testing in breast cancer. J Clin Oncol 33:4259-4267, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Neill SC, Isaacs C, Chao C, et al. : Adoption of gene expression profiling for breast cancer in US oncology practice for women younger than 65 years. J Natl Compr Canc Netw 13:1216-1224, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orucevic A, Heidel RE, Bell JL: Utilization and impact of 21-gene recurrence score assay for breast cancer in clinical practice across the United States: Lessons learned from the 2010 to 2012 National Cancer Data Base analysis. Breast Cancer Res Treat 157:427-435, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray GT, Mandelblatt J, Habel LA, et al. : Breast cancer multigene testing trends and impact on chemotherapy use. Am J Manag Care 22:e153-e160, 2016 [PMC free article] [PubMed] [Google Scholar]
- 9.Su KW, Hall J, Soulos PR, et al. : Association of 21-gene recurrence score assay and adjuvant chemotherapy use in the Medicare population, 2008-2011. J Geriatr Oncol 7:15-23, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Carlson JJ, Roth JA: The impact of the Oncotype Dx breast cancer assay in clinical practice: A systematic review and meta-analysis. Breast Cancer Res Treat 141:13-22, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hornberger J, Cosler LE, Lyman GH: Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care 11:313-324, 2005 [PubMed] [Google Scholar]
- 12.Blohmer JU, Rezai M, Kümmel S, et al. : Using the 21-gene assay to guide adjuvant chemotherapy decision-making in early-stage breast cancer: A cost-effectiveness evaluation in the German setting. J Med Econ 16:30-40, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Hall PS, McCabe C, Stein RC, et al. : Economic evaluation of genomic test-directed chemotherapy for early-stage lymph node-positive breast cancer. J Natl Cancer Inst 104:56-66, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Klang SH, Hammerman A, Liebermann N, et al. : Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value Health 13:381-387, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Kondo M, Hoshi SL, Ishiguro H, et al. : Economic evaluation of 21-gene reverse transcriptase-polymerase chain reaction assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer in Japan. Breast Cancer Res Treat 112:175-187, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Lyman GH, Cosler LE, Kuderer NM, et al. : Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: An economic analysis based on prognostic and predictive validation studies. Cancer 109:1011-1018, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Tsoi DT, Inoue M, Kelly CM, et al. : Cost-effectiveness analysis of recurrence score-guided treatment using a 21-gene assay in early breast cancer. Oncologist 15:457-465, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Cancer Institute: SEER Program: Cancer Stat Facts: Female Breast Cancer. https://seer.cancer.gov/statfacts/html/breast.html.
- 19.Esserman LJ, Moore DH, Tsing PJ, et al. : Biologic markers determine both the risk and the timing of recurrence in breast cancer. Breast Cancer Res Treat 129:607-616, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter SB, Gartner SS, Haines MR, et al. : Historical Statistics of the United States: Millennial Edition (Online Version). Volume One: Population. New York, NY, Cambridge University Press, 2006 [Google Scholar]
- 21. National Cancer Institute: SEER Program: SEER*stat databases: Incidence - SEER 9 regs research data, November 2015 submission (1973-2013). https://seer.cancer.gov/data/seerstat/nov2015/
- 22. doi: 10.1177/0272989X17743236. Munoz D, Plevritis SK: Estimating breast cancer progression features and survival by molecular subtype in the absence of screening and treatment. Med Decis Making (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz D, Near AM, van Ravesteyn NT, et al. : Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst 106:dju289, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Maio E, Gravina A, Pacilio C, et al. : Compliance and toxicity of adjuvant CMF in elderly breast cancer patients: A single-center experience. BMC Cancer 5:30, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassett MJ, O’Malley AJ, Pakes JR, et al. : Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst 98:1108-1117, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Jones S, Holmes FA, O’Shaughnessy J, et al. : Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol 27:1177-1183, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Muss HB, Berry DA, Cirrincione C, et al. : Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: The Cancer and Leukemia Group B experience. J Clin Oncol 25:3699-3704, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Muss HB, Berry DA, Cirrincione CT, et al. : Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med 360:2055-2065, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peto R, Davies C, Godwin J, et al. : Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379:432-444, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. doi: 10.1177/0272989X17717981. Gangnon RE, Stout NK, Alagoz O, et al: Contribution of breast cancer to overall mortality for U.S. women. Med Decis Making (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agency for Healthcare Research and Quality : Medical expenditure panel survey. https://meps.ahrq.gov/mepsweb/
- 32.Hanmer J, Lawrence WF, Anderson JP, et al. : Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making 26:391-400, 2006 [DOI] [PubMed] [Google Scholar]
- 33.de Haes JC, de Koning HJ, van Oortmarssen GJ, et al. : The impact of a breast cancer screening programme on quality-adjusted life-years. Int J Cancer 49:538-544, 1991 [DOI] [PubMed] [Google Scholar]
- 34.Earle CC, Chapman RH, Baker CS, et al. : Systematic overview of cost-utility assessments in oncology. J Clin Oncol 18:3302-3317, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Stout NK, Lee SJ, Schechter CB, et al. : Benefits, harms, and costs for breast cancer screening after US implementation of digital mammography. J Natl Cancer Inst 106:dju092, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandelblatt JS, Stout NK, Schechter CB, et al. : Collaborative modeling of the benefits and harms associated with different U.S. breast cancer screening strategies. Ann Intern Med 164:215-225, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stout NK, Rosenberg MA, Trentham-Dietz A, et al. : Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst 98:774-782, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Centers for Medicaid and Medicare Services : Clinical laboratory fee schedule. https://www.cms.gov/medicare/medicare-fee-for-service-payment/clinicallabfeesched/
- 39.Mariotto AB, Yabroff KR, Shao Y, et al. : Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 103:117-128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stokes ME, Thompson D, Montoya EL, et al. : Ten-year survival and cost following breast cancer recurrence: Estimates from SEER-Medicare data. Value Health 11:213-220, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Yabroff KR, Davis WW, Lamont EB, et al. : Patient time costs associated with cancer care. J Natl Cancer Inst 99:14-23, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Secker-Walker RH, Vacek PM, Hooper GJ, et al. : Screening for breast cancer: Time, travel, and out-of-pocket expenses. J Natl Cancer Inst 91:702-708, 1999 [DOI] [PubMed] [Google Scholar]
- 43.US Bureau of Labor Statistics : Median weekly earnings of full-time wage and salary workers by detailed occupation and sex. https://www.bls.gov/cps/cpsaat39.htm
- 44.Yabroff KR, Lamont EB, Mariotto A, et al. : Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst 100:630-641, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Finn RS, Crown JP, Lang I, et al. : The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol 16:25-35, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Zeichner SB, Herna S, Mani A, et al. : Survival of patients with de-novo metastatic breast cancer: Analysis of data from a large breast cancer-specific private practice, a university-based cancer center and review of the literature. Breast Cancer Res Treat 153:617-624, 2015 [DOI] [PubMed] [Google Scholar]
- 47.US Bureau of Labor : Consumer Price Index. https://www.bls.gov/cpi/home.htm
- 48.Raldow AC, Sher D, Chen AB, et al. : Cost effectiveness of the Oncotype DX DCIS Score for guiding treatment of patients with ductal carcinoma in situ. J Clin Oncol 343963–3968.2016 [DOI] [PubMed] [Google Scholar]
- 49.Cardoso F, van’t Veer LJ, Bogaerts J, et al. : 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375:717-729, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Esteva FJ, Sahin AA, Cristofanilli M, et al. : Prognostic role of a multigene reverse transcriptase-PCR assay in patients with node-negative breast cancer not receiving adjuvant systemic therapy. Clin Cancer Res 11:3315-3319, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Ignatiadis M, Azim HA, Jr, Desmedt C, et al. : The genomic grade assay compared with Ki67 to determine risk of distant breast cancer recurrence. JAMA Oncol 2:217-224, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Martin M, Brase JC, Calvo L, et al. : Clinical validation of the EndoPredict test in node-positive, chemotherapy-treated ER+/HER2- breast cancer patients: Results from the GEICAM 9906 trial. Breast Cancer Res 16:R38, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sgroi DC, Sestak I, Cuzick J, et al. : Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: A prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 14:1067-1076, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Institute for Clinical and Economic Review: ICER value assessment framework: 1.0 to 2.0. http://icer-review.org/wp-content/uploads/2016/02/Value-Assessment-Framework-slides-for-July-29-webinar-FINAL.pdf.
- 55.Neumann PJ, Sanders GD: Cost-Effectiveness Analysis 2.0. N Engl J Med 376:203-205, 2017 [DOI] [PubMed] [Google Scholar]
- 56.Kurian AW, Munoz DF, Rust P, et al. : Online tool to guide decisions for BRCA1/2 mutation carriers. J Clin Oncol 30:497-506, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oehrli MD, Quesenberry CP, Leyden W: 2006 annual report on trends, incidence, and outcomes. Kaiser Permanente, Northern California Cancer Registry. https://www.scienceopen.com/document?vid=993b2113-bcec-4a0e-b017-617fa63dcbd7.
- 58.Potosky AL, O’Neill SC, Isaacs C, et al. : Population-based study of the effect of gene expression profiling on adjuvant chemotherapy use in breast cancer patients under the age of 65 years. Cancer 121:4062-4070, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jasem J, Fisher CM, Amini A, et al. : The 21-gene recurrence score assay for node-positive, early-stage breast cancer and impact of RxPONDER trial on chemotherapy decision-making: Have clinicians already decided? J Natl Compr Canc Netw 15:494-503, 2017 [DOI] [PubMed] [Google Scholar]
- 60.Paik S: Development and clinical utility of a 21-gene recurrence score prognostic assay in patients with early breast cancer treated with tamoxifen. Oncologist 12:631-635, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Ramsey SD, Barlow WE, Gonzalez-Angulo AM, et al. : Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating OncotypeDX-guided management for women with breast cancer involving lymph nodes. Contemp Clin Trials 34:1-9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]