Abstract
Purpose
Measures of response that are clinically meaningful and occur early are an unmet need in metastatic castration-resistant prostate cancer clinical research and practice. We explored, using individual patient data, week 13 circulating tumor cell (CTC) and prostate-specific antigen (PSA) response end points in five prospective randomized phase III trials that enrolled a total of 6,081 patients—COU-AA-301, AFFIRM, ELM-PC-5, ELM-PC-4, and COMET-1—ClinicalTrials.Gov identifiers: NCT00638690, NCT00974311, NCT01193257, NCT01193244, and NCT01605227, respectively.
Methods
Eight response end points were explored. CTC nonzero at baseline and 0 at 13 weeks (CTC0); CTC conversion (≥ 5 CTCs at baseline, ≤ 4 at 13 weeks—the US Food and Drug Administration cleared response measure); a 30%, 50%, and 70% decrease in CTC count; and a 30%, 50%, and 70% decrease in PSA level. Patients missing week-13 values were considered nonresponders. The discriminatory strength of each end point with respect to overall survival in each trial was assessed using the weighted c-index.
Results
Of the eight response end points, CTC0 and CTC conversion had the highest weighted c-indices, with smaller standard deviations. For CTC0, the mean (standard deviation) was 0.81 (0.04); for CTC conversion, 0.79 (0.03); for 30% decrease in CTC count, 0.72 (0.06); for 50% decrease in CTC count, 0.72 (0.06); for 70% decrease in CTC count, 0.73 (0.05); for 30% decrease in PSA level, 0.71 (0.03); for 50% decrease in PSA level, 0.72 (0.06); and for 70% decrease in PSA level, 0.74 (0.05). Seventy-five percent of eligible patients could be evaluated with the CTC0 end point, compared with 51% with the CTC conversion end point.
Conclusion
The CTC0 and CTC conversion end points had the highest discriminatory power for overall survival. Both are robust and meaningful response end points for early-phase metastatic castration-resistant prostate cancer clinical trials. CTC0 is applicable to a significantly higher percentage of patients than CTC conversion.
INTRODUCTION
The therapeutic landscape for men with metastatic castration-resistant prostate cancer (mCRPC) has changed substantially. Since 2010, six new treatments with diverse mechanisms of action have been approved by the US Food and Drug Administration (FDA). All were based on the demonstration of a survival benefit in large-scale phase III trials. In parallel, new and ongoing molecular profiling studies have led to a more biologically based disease taxonomy identifying subsets of patients likely to respond or not to specific classes of drug.1 Historically, clinical research in the mCRPC population has relied on prostate-specific antigen (PSA) changes, such as the maximal percent or percent at a fixed time point, as indicators of treatment efficacy, although neither is a strong indicator of overall survival.2,3 Other response end points, such as radiographic measures for bone metastases, are problematic because of the difficulty distinguishing whether early unfavorable changes represent worsening or improving disease status. Changes in measurable disease, assessed by Response Evaluation Criteria in Solid Tumors, are also used, although they occur infrequently. With these limitations, along with the increasing number of possible treatment combinations, the unmet need for response indicators that reliably reflect survival and that occur early so trials can be completed in a shorter time frame, has become more urgent.
Most metastasizing cancers spread through the blood as single cells or in clusters. At present, there are a range of devices and assays that enable the detection, enumeration, and biologic characterization of circulating tumor cells (CTCs).4,5 Only one, CellSearch (Menarini Silicon Biosystems, San Diego, CA), has achieved the level of an FDA clearance for the context of use as an “aid in the monitoring of patients with metastatic breast, colorectal, and prostate cancer . . . in conjunction with other clinical methods.”6(p3) Studies in patients with mCRPC have shown that the number of CTCs detected is higher in patients with bone disease relative to lymph node disease and that association with disease burden is modest,7-9 which shows that the ability of a cancer cell to detach, circulate, survive, and colonize a distant site is an intrinsic property of the tumor. It follows that inhibiting the spread of cells through the circulation would represent a therapeutic objective that is clinically meaningful.8,10-14
After the demonstration of CTC conversion rates between 35% and 40% in three phase II studies of abiraterone and enzalutamide,15-17 a collaboration was initiated with the US FDA Center for Diseases and Radiologic Health to study post-treatment CTC-containing end points as potential surrogates for survival. To do so, the CTC biomarker question was embedded in a series of phase III registration trials with a primary end point of overall survival. In this study, we compared the ability of CTC number and PSA as short-term (week 13) response end points to reflect survival in patients with mCRPC treated with systemic therapies. The analyses were performed using data from five independent randomized clinical trials, all completed within the past 6 years, that enrolled diverse populations of patients with mCRPC ranging from chemotherapy naïve to those who experienced treatment failure on one or two approved life-prolonging therapies. Our objective was to generate evidence that an early post-treatment decrease in CTC number is a meaningful indicator of prolonged survival for use in early-phase clinical trials. Finding a robust short-term indicator of prolonged survival would suggest that use of a CTC end point in an early-phase clinical trial could accelerate drug development and aid clinical decision making in clinical practice for the mCRPC population.
METHODS
Patients
The individual patient data from five independent randomized phase III clinical trials for mCRPC were used for this evaluation (Table 1; Fig 1; Appendix Figs A1-A5, online only).10-14 In each trial, the primary end point was overall survival.
Table 1.
Metastatic Castration-Resistant Prostate Cancer Phase III Studies Contributing Data to This Analysis
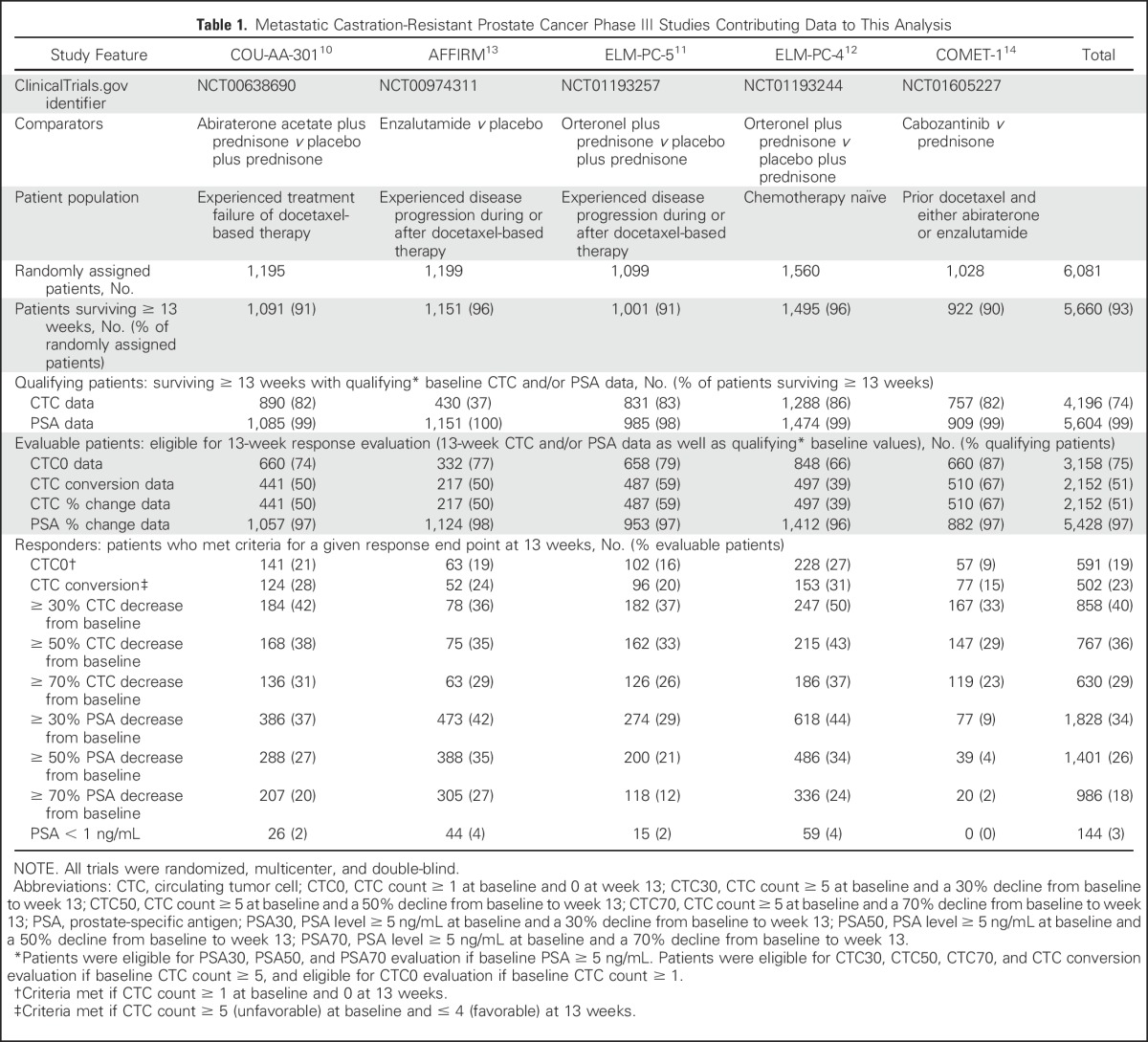
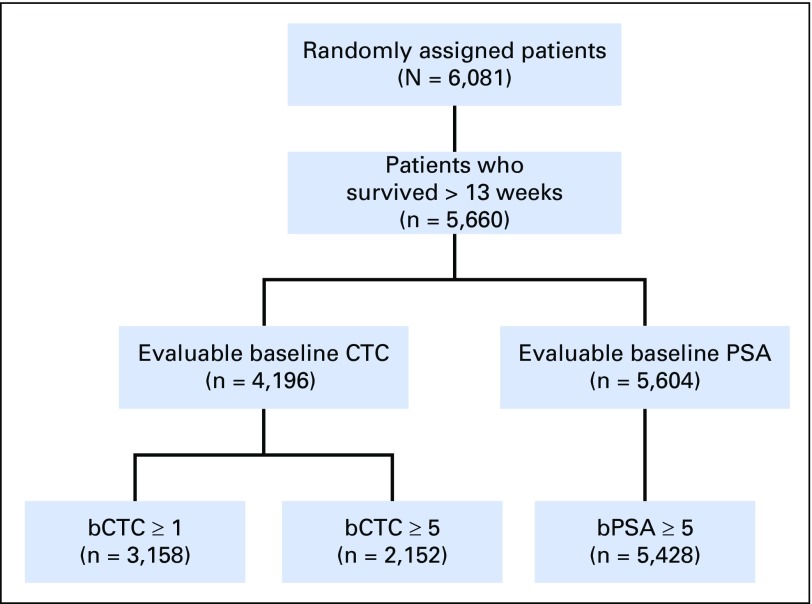
Fig 1.
CONSORT diagram for the five randomized clinical trials combined. bCTC, baseline CTC; bPSA, baseline PSA; CTC, circulating tumor cell; PSA, prostate-specific antigen.
Response Measures
CTC counts and PSA levels at baseline and week 13 were used to define a series of response end points. The evaluable study cohorts from each trial were patients who survived at least 13 weeks and had a recorded baseline CTC or PSA value. The eight CTC and PSA response measures considered were CTC0 (patients with CTC count ≥ 1 at baseline and 0 at week 13); CTC conversion (CTCconv; patients with CTC count ≥ 5 at baseline and ≤ 4 at week 13); percent change in CTC (CTC30, CTC50, CTC70; patients with CTC count ≥ 5 at baseline and a 30%, 50%, or 70% decline from baseline to week 13, respectively); and percent change in PSA (PSA30, PSA50, PSA70; PSA level ≥ 5 ng/mL at baseline and a 30%, 50%, or 70% decline from baseline to week 13, respectively). Patients who achieved these biomarker thresholds were recorded as responders. All other patients were recorded as nonresponders, including those with recorded baseline data who survived more than 13 weeks but dropped out of the biomarker component of the study before week 13.
Missing data.
In all five trials, baseline CTC data were missing for some patients who survived > 13 weeks. Reasons included geographic restrictions, such as the unavailability of the CTC assay in a particular region or country, and patient- and disease-related factors. To ascertain whether the missing data had a significant effect on the analyses, a log-rank test for survival was performed for each study to assess whether the patient populations with missing baseline CTC data differed from the analyzed populations.
Discriminatory value of the response end points.
To evaluate the discriminatory power of the response end points on survival time, the weighted c-index for each of the eight end points was calculated separately for each trial.18 The weighted c-index ranges from 0.5 to 1.0 and represents the likelihood that responders survive longer than nonresponders, with an increasing index indicating a greater probability of longer survival for responders. A weighted c-index near 1.0 would indicate that nearly all patients classified as nonresponders would have died before the shortest survival time among the responding patients. A weighted c-index near 0.5 would signify little discriminatory power—that patients classified as responders and nonresponders would have virtually superimposed survival curves. Because the weighted c-index scale is difficult to discern, Kaplan-Meier estimates of survival for responders versus nonresponders were used to show the magnitude of the gap between the responder and nonresponder survival curves. All survival analyses were landmarked at week 13 to coincide with the response evaluation. To account for the individual effects of the experimental and control arms on discrimination, a supplemental analysis was undertaken based on a stratified-by-treatment-arm weighted c-index analysis.
RESULTS
Patient Populations
A total of 6,081 patients were treated across the five trials; see Table 1 for a synopsis of trials. Table 2 lists summarized baseline and week 13 CTC and PSA data for each trial. These summaries show significant heterogeneity, with a range of prognoses across the studies, consistent with the range of mCRPC states they represent. Noteworthy is that the highest median baseline CTC and PSA values were seen in COMET-1 (ClinicalTrials.gov identifier: NCT01605227), which enrolled patients whose disease had progressed while receiving at least two approved life-prolonging therapies (a taxane-based chemotherapy and either abiraterone or enzalutamide), and the lowest baseline CTC and PSA values occurred in ELM-PC-4 (ClinicalTrials.gov identifier: NCT01193244), which enrolled patients who had not received any prior proven life-prolonging therapy for mCRPC. The other three trials—COU-AA-301, AFFIRM, and ELM-PC-5 (ClinicalTrials.gov identifiers: NCT00638690, NCT00974311, and NCT01193257, respectively)—with baseline values falling in the middle, enrolled patients who had previously received only one life-prolonging therapy, docetaxel.
Table 2.
Median (range) of PSA and CTC Measures
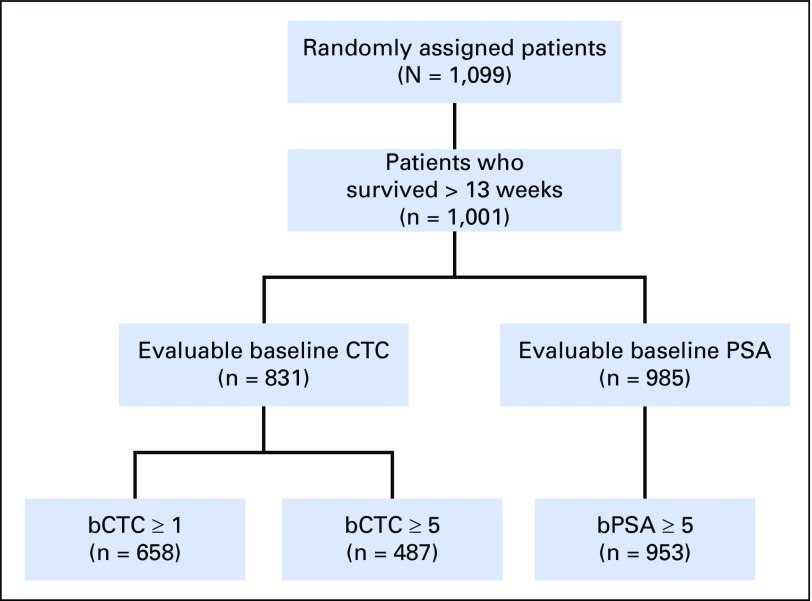
The flow of evaluable patients for each study is shown in Table 1, Figure 1, and Appendix Figures A1-A5. The percentage of patients with qualifying CTC data varied widely (Table 1). For example, in the ELM-PC-4 clinical trial (Fig A4), 1,288 (83%) of the 1,560 randomly assigned patients had baseline CTC data and survived at least 13 weeks. Among the 1,288 patients, 848 (66%) had a baseline CTC value > 0 and were evaluable for the CTC0 response end point. Evaluability for the CTC conversion and percent change in CTC response end points required a baseline CTC value ≥ 5, which only 497 (39%) of the 1,288 patients in the ELM-PC-4 study had. Evaluability of the PSA response end points required a baseline PSA value ≥ 5 ng/mL, which rendered 1,412 (96%) of the 1,474 patients evaluable.
CTC and PSA Response Rate Survival Discrimination
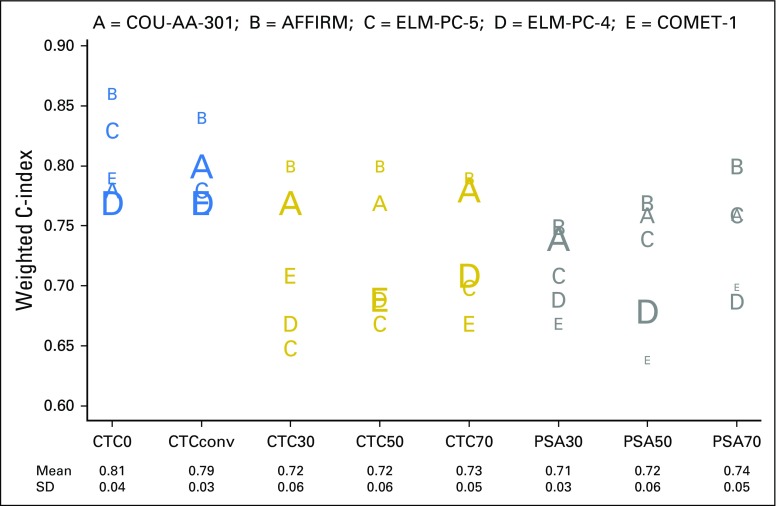
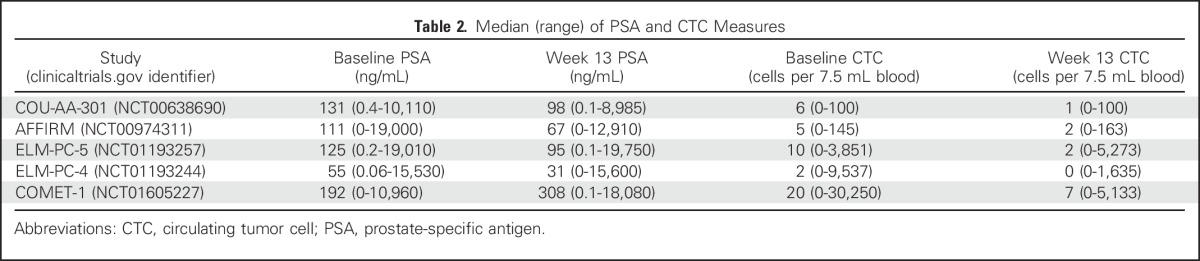
Table 3 and Figure 2 show the strength of the CTC and PSA response end points to discriminate overall survival using the weighted c-index. In Figure 2, the horizontal axis lists the eight end points studied, and the vertical axis indicates the weighted c-index. Each trial is represented by a letter (A through E); the size of the letter is inversely proportional to the SE of the weighted c-index on the study it represents. Using this size distinction, larger letters represent more accurate estimates of discrimination.
Table 3.
Weighted C-Index by Study and Response Indicator End Point
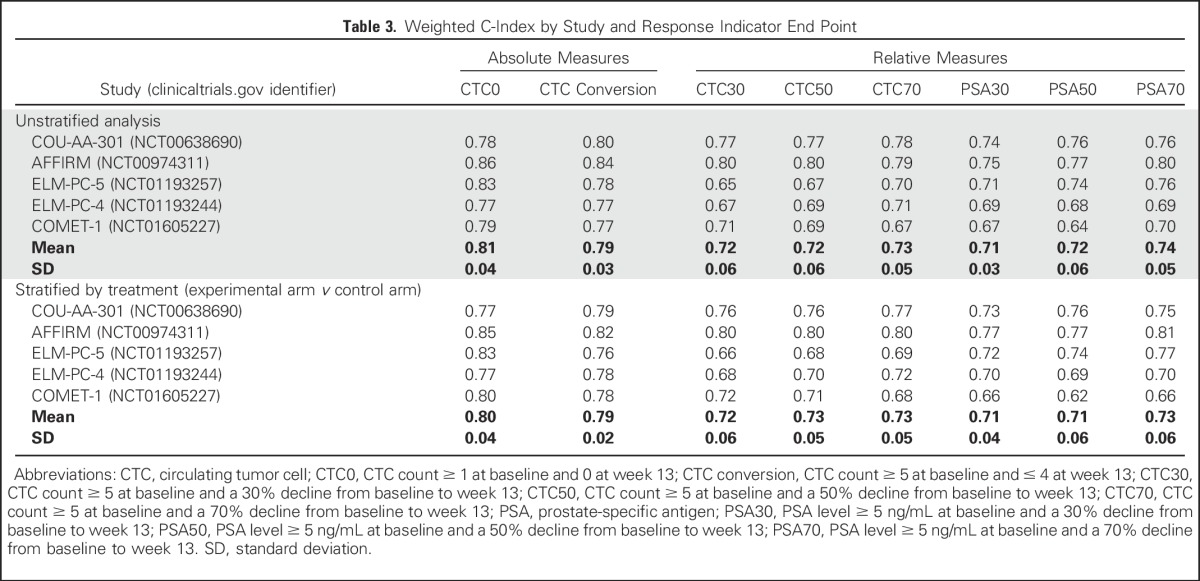
Fig 2.
Discriminatory power of post-therapy circulating tumor cell (CTC) and prostate-specific antigen (PSA) response measures for survival in metastatic castration-resistant prostate cancer registration trials. CTC0, CTC count ≥ 1 at baseline and 0 at week 13; CTCconv, CTC conversion (CTC count ≥ 5 at baseline and ≤ 4 at week 13); CTC30, CTC count ≥ 5 at baseline and a 30% decline from baseline to week 13; CTC50, CTC count ≥ 5 at baseline and a 50% decline from baseline to week 13; CTC70, CTC count ≥ 5 at baseline and a 70% decline from baseline to week 13; PSA30, PSA level ≥ 5 ng/mL at baseline and a 30% decline from baseline to week 13; PSA50, PSA level ≥ 5 ng/mL at baseline and a 50% decline from baseline to week 13; PSA70, PSA level ≥ 5 ng/mL at baseline and a 70% decline from baseline to week 13. SD, standard deviation. ClinicalTrials.gov identifiers for the trials are: COU-AA-301, NCT00638690; AFFIRM, NCT00974311; ELM-PC-5, NCT01193257; ELM-PC-4, NCT01193244; COMET-1, NCT01605227.
As shown, the ability to differentiate the survival outcomes for week-13 responders and nonresponders was greatest using the CTC0 and CTC conversion end points. Finding no CTCs after treatment, or finding that the value had converted from above to below the threshold of five CTCs, provided greater discrimination for patient survival than the percent change in CTC or PSA response end points. The average weighted c-index for the CTC0 and CTC conversion response end points was 0.81 and 0.79, respectively, whereas the average weighted c-indices for the percent change CTC and PSA end points ranged from 0.71 to 0.74 (Table 3). Among the PSA end points, the average weighted c-index for PSA70 was slightly higher than that for PSA50 and PSA30. A test to compare the weighted (by the number of patients) average difference across studies between the CTC0 and PSA70 end points produced a P value of 0.026, which demonstrates improved discriminatory power for the CTC0 end point compared with the PSA70 end point and, by extension, to each of the PSA response end points examined.
In addition to greater discrimination, the CTC0 and CTC conversion response end points were more robust, producing consistent weighted c-indices across the five trials, as shown by the smaller standard deviations in Table 3 and the tighter clustering of letters in Figure 2. A supplemental analysis, on the basis of the stratified (by treatment) weighted c-index, produced comparable results (Table 3).
Evaluability Rates for the CTC0 and CTC Conversion End Points
Although the discriminatory strength of the CTC0 and CTC conversion end points was similar, an important distinction between the two is the percentage of patients for whom these response measures could be used. Overall, 75% of eligible patients were evaluable for the CTC0 end point (CTC value ≥ 1 at baseline), but only 51% were eligible for the CTC conversion end point (CTC value ≥ 5 at baseline; Table 1). In these five studies, the relative increase in the percentage of patients evaluable for the CTC0 end point compared with CTC conversion ranged from 29% to 71%, with the greatest proportional increase (71%, 848 v 497) occurring among patients who were chemotherapy naïve (ELM-PC-4) and the least difference (29%, 660 v 510) occurring among patients who had been exposed to at least two prior treatments (COMET-1; Table 1).
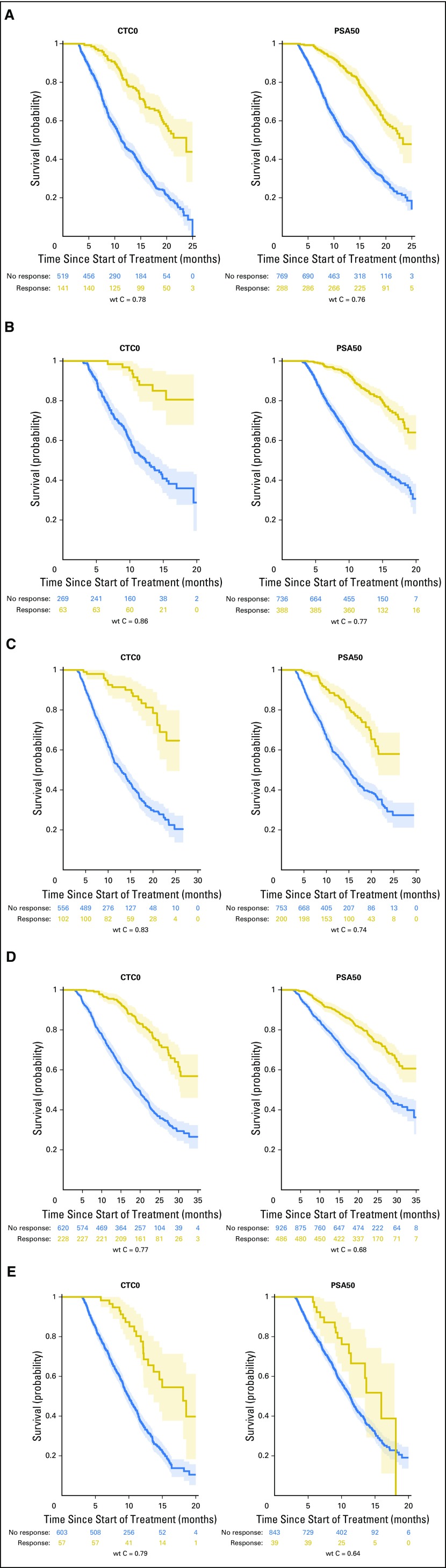
Graphical Interpretation of the Weighted C-Indices
To illustrate how the magnitude of the weighted c-index represents the relationship between the response end point and the survival end point, Kaplan-Meier estimates of survival for responders and nonresponders were generated for each study, along with the associated weighted c-index. Those for CTC0 and PSA50 response are shown in Figures 3A-3E. For example, an examination of the ELM-PC-5 Kaplan-Meier estimates (Fig 3C) clearly depicts an improved survival profile for CTC0 responders relative to CTC0 nonresponders, which is reflected in the large weighted c-index (weighted c-index = 0.83). In contrast, the moderate survival benefit conferred on the PSA50 responders is summarized by a weighted c-index equal to 0.74. In comparison, in the COU-AA-301 Kaplan-Meier estimate (Fig 3A), the difference in discrimination between CTC0 and PSA50 is small, appropriately, because the weighted c-indexes are 0.78 and 0.76, respectively.
Fig 3.
Kaplan-Meier estimates of responders versus nonresponders along with 95% CIs for the circulating tumor cell count ≥ 1 at baseline and 0 at week 13 (CTC0) and prostate-specific antigen level ≥ 5 ng/mL at baseline and a 50% decline from baseline to week 13 (PSA50) response end points at 13 weeks, for the five metastatic castration-resistant prostate cancer registration trials. (A) COU-AA-301 (ClinicalTrials.gov identifier: NCT00638690). (B) AFFIRM (ClinicalTrials.gov identifier: NCT00974311). (C) ELM-PC-5 (ClinicalTrials.gov identifier: NCT01193257). (D) ELM-PC-4 (ClinicalTrials.gov identifier: NCT01193244). (E) COMET-1 (ClinicalTrials.gov identifier NCT01605227). wt C, weighted c-index.
Recognizing that the response analyses could be performed only on eligible patients who survived at least 13 weeks and had baseline marker data, a log-rank test was performed to determine whether the survival rates for each trial differed for the analyzed patient population versus patients who survived more than 13 weeks but were missing baseline CTC or PSA data and were not included in the analysis. The results shown in Appendix Table A1 (online only) indicate that a difference in survival rates was not detectable in any of the five studies.
DISCUSSION
Treatment effects in therapeutic trials are typically assessed using predefined criteria, which represent an early-occurring change in a disease manifestation that was present at the start of a new therapy, or time-to-event measures, which represent the delay or prevention of later-occurring potential disease manifestations that indicate or predict for a deterioration in quality of life or death. For the development of drugs, no single post-treatment early response measure has been established as a true indicator of clinical benefit, with the exception of the palliation or control of pain, for which specific therapies are approved (mitoxantrone) or indicated for use when symptoms of osseous disease are present (radium-223 dichloride). The results presented in this analysis establish that the defined CTC0 end point, a change in the number of CTCs from detectable (present) to undetectable (absent), using the FDA-cleared CellSearch assay, is a response indicator biomarker that is strongly associated with longer survival, an unambiguous clinical benefit to patients. The strength of the CTC0 response end point to reflect a survival improvement was established using individual patient data from > 3,000 men who were evaluable for a CTC response assessment and was consistent across five phase III randomized registration trials powered on survival in which the CTC biomarker question was embedded prospectively. Each of the response measures considered in the individual trials was evaluated independent of the specific intervention under evaluation in the trial and the treatment arm on which a patient was enrolled. The interventions included placebo, prednisone monotherapy, three next-generation androgen receptor signaling inhibitors administered alone or in combination with prednisone, and a signaling inhibitor. The trials were conducted in three distinct populations of patients with mCRPC—patients at the first, second, and third decision point in disease management, who had been previously exposed to either no, one (docetaxel), or two (docetaxel and an approved androgen receptor signaling inhibitor) life-prolonging therapies, respectively. Taken together, the consistency of the outcomes across treatments and disease states shows the generalizability of the results and further supports the CTC0 end point as a measure of clinical benefit for use in clinical trials.
To our knowledge, this is the first reported exploration of CTC0 as a response end point. Of particular note was that the CTC0 end point was superior to the more widely used percent change in PSA end points, which did not discriminate survival to the same degree. Four of the trials included hormonal agents that can in themselves modulate PSA levels independent of an effect on cell kill, thereby limiting post-therapy PSA change measures as a reliable indicator of efficacy. This was one of the reasons CTC number, a measure that is not affected by modulations in androgen receptor signaling, was included in the early phases of development of these agents. Reaching a post-therapy PSA < 1 ng/mL occurred too infrequently in these cohorts to be useful as an outcome (Table 1).
The discriminatory power of CTC0 for survival was matched by the CTC conversion measure.6 The benefit of the CTC0 end point is the increased patient eligibility. The CTC0 end point requires ≥ 1 CTCs at baseline, whereas the CTC conversion end point requires ≥ 5 CTCs at baseline for eligibility. In these five studies, use of the CTC0 end point improved the ability to evaluate response (increases ranging from 29% to 71%), compared with the need to detect ≥ 5 CTCs at baseline. This increase in the percent of evaluable patients, 71% in the first-line, 46% in the second-line, and 29% in the third-line setting, significantly enlarges the patient population, enabling more rapid trial accrual and shorter drug evaluation times in trials while providing greater reliability in studies of treatment efficacy.
A limitation of the study was the number of patients who did not have baseline CTC counts and were therefore not assessable using the CTC response measures proposed here. Excluding the AFFIRM trial, the number of patients lacking baseline CTC counts ranged from 14% to 18% of the patients surviving 13 weeks, which raises the possibility of bias in interpreting the outcome. Sixty-three percent of patients in the AFFIRM trial did not have baseline CTC values, with the majority of CTC samples being obtained in North America. This lack of baseline CTC data most commonly resulted from the unavailability of the assay in the country in which the trial was being conducted or from limitations in access to the reference laboratory performing the assay. To address this, sensitivity analyses were conducted in which we were unable to discern a survival difference for patients with missing baseline counts in any of the five studies. Further questions to be addressed include the reproducibility of the CTC count measured at baseline; specifically, if two samples are drawn, will the results be the same? A second issue is the need for confirmation of the CTC end point measurement, which is traditionally required for blood-based biomarkers such as PSA, and responses by imaging.
To develop new therapeutic agents requires the ability to determine whether a systemic therapy has clinical benefit (eg, improving how a patient feels and functions and how long the patient survives). This seemingly simple need has been one of the most challenging aspects of drug development for patients with mCRPC, because reliable and informative early-occurring indicators of clinical benefit are lacking. Post-therapy PSA changes fall short in prognostic reliability, whereas pretreatment measurable disease that can be objectively assessed post-treatment not only is infrequent but also has not been shown prospectively to associate with an improvement in survival. The CTC0 end point is an indicator that cancer cells that were circulating in the blood are no longer detectable, an easily recognized outcome that is clinically meaningful to patients. It is an outcome that occurs shortly after treatment initiation, providing researchers and practitioners with objective and reliable evidence that the therapy being administered has altered the patient’s prognosis in a favorable way. Taken together, the results of this study support the use of CTC0 as a response end point in early-phase clinical trials.
Appendix
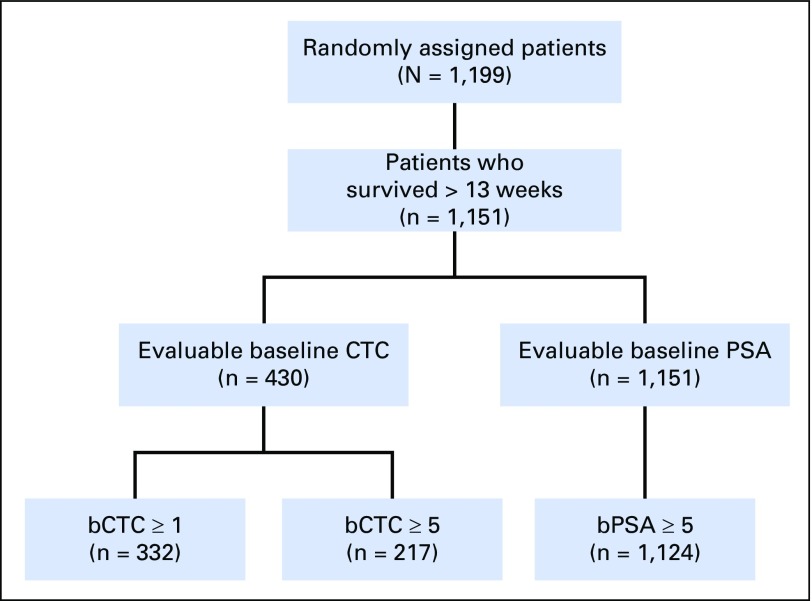
Fig A1.
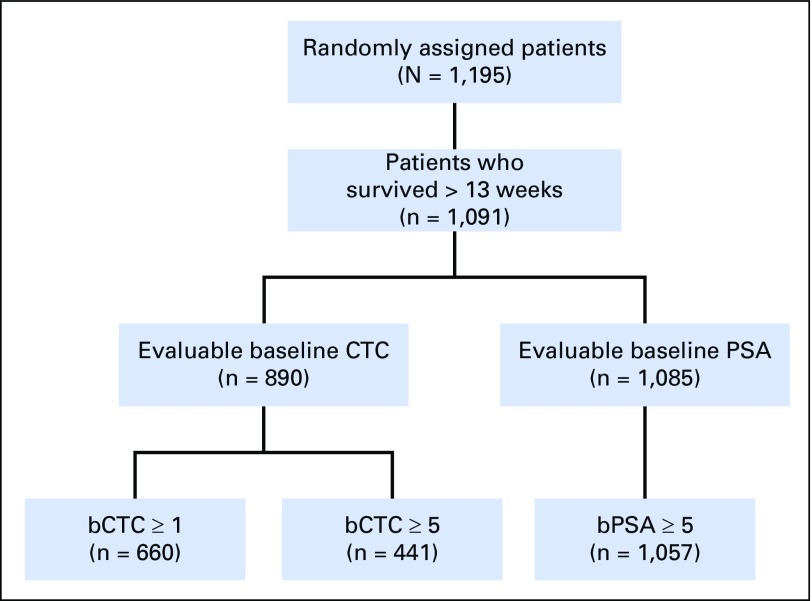
CONSORT diagram for COU-AA-301 (ClinicalTrials.gov identifier: NCT00638690). bCTC, baseline CTC; bPSA, baseline PSA; CTC, circulating tumor cell; PSA, prostate-specific antigen.
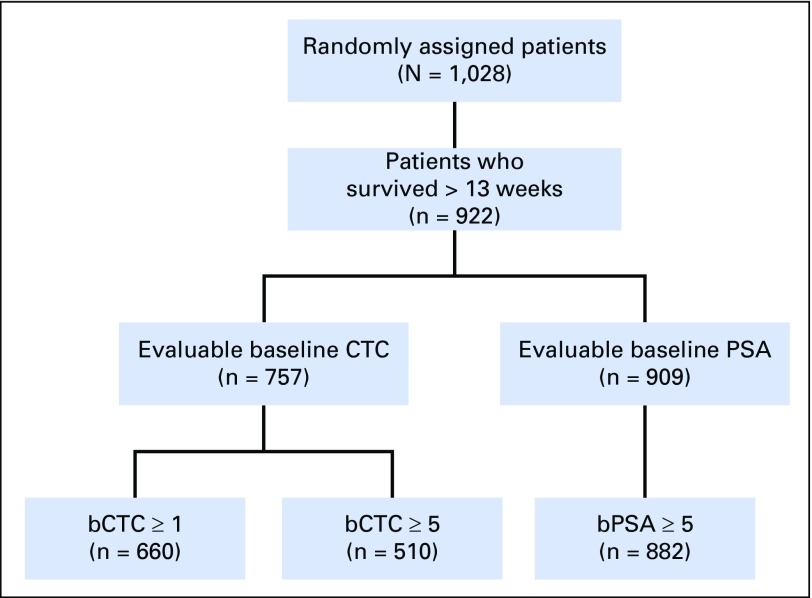
Fig A2.
CONSORT diagram for AFFIRM (ClinicalTrials.gov identifier: NCT00974311). bCTC, baseline CTC; bPSA, baseline PSA; CTC, circulating tumor cell; PSA, prostate-specific antigen.
Fig A3.
CONSORT diagram for ELM-PC-5 (ClinicalTrials.gov identifier: NCT01193257). bCTC, baseline CTC; bPSA, baseline PSA; CTC, circulating tumor cell; PSA, prostate-specific antigen.
Fig A4.
CONSORT diagram for ELM-PC-4 (ClinicalTrials.gov identifier: NCT01193244). bCTC, baseline CTC; bPSA, baseline PSA; CTC, circulating tumor cell; PSA, prostate-specific antigen.
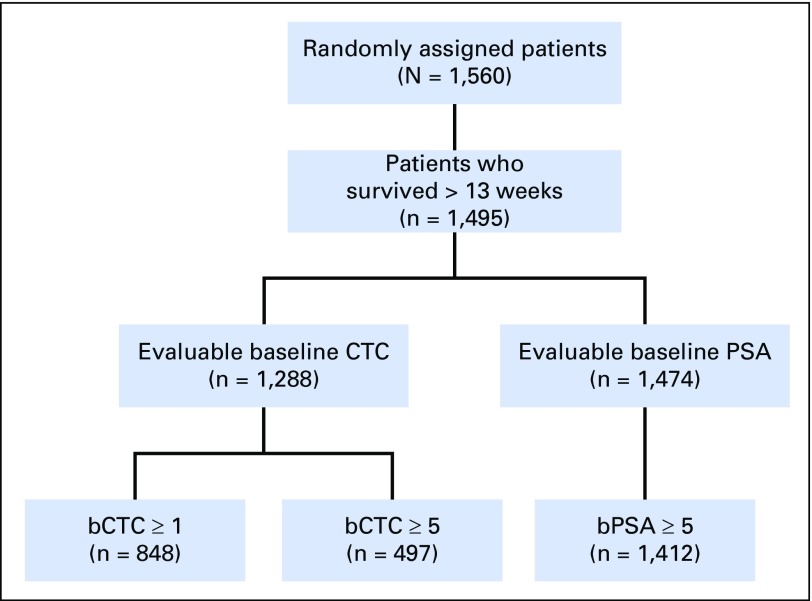
Fig A5.
CONSORT diagram for COMET-1 (ClinicalTrials.gov identifier: NCT01605227). bCTC, baseline CTC; bPSA, baseline PSA; CTC, circulating tumor cell; PSA, prostate-specific antigen.
Table A1.
Sensitivity Analysis for Missing Baseline PSA or CTC Values: Median Survival Time (sample size) for Patients Who Survived ≥ 13 Weeks
Footnotes
Supported by National Institutes of Health (NIH) Grant No. P30-CA008748 (G.H., M.F., and H.I.S. are recipients of the Cancer Center Support Grant); NIH Grant No. P50-CA92629 (G.H. and H.I.S. are recipients of a Specialized Programs of Research Excellence grant); NIH Grant No. R01-CA207220 (G.H. and H.I.S.); Sidney Kimmel Center for Prostate and Urologic Cancers (H.I.S.); Prostate Cancer Foundation (G.H. and H.I.S.); Department of Defense Prostate Cancer Research Program Grant No. PC121111 (G.H. and H.I.S.); and Janssen Diagnostics a division of Janssen Pharmaceutica NV (G.H.). The COU-AA-301 study/publication was supported by Ortho Biotech Oncology Research and Development (a unit of Cougar Biotechnology) and grants from the Medical Research Council of the United Kingdom, Movember Centre of Excellence funding, Experimental Cancer Medical Centre, National Institute for Health Research Biomedical Research Centre, and Prostate Cancer Foundation (H.I.S.). The AFFIRM study/publication was supported by Medivation and Astellas Pharma Global Development. The ELM-PC-5 study/publication was supported by Takeda Pharmaceuticals International. The ELM-PC-4 study/publication was supported by Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical. The COMET-1 study/publication was supported by Exelixis.
Presented in part at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 2-6, 2017.
The project was part of a collaboration with the US Food and Drug Administration to develop intermediate end points for castration-resistant prostate cancer.
See accompanying Editorial on page 525
AUTHOR CONTRIBUTIONS
Conception and design: Glenn Heller, Arturo Molina, Mohammad Hirmand, Johann S. de Bono, Howard I. Scher
Provision of study materials or patients: Matthew R. Smith, Fred Saad, Ronald de Wit, Karim Fizazi, Johann S. de Bono, Howard I. Scher
Collection and assembly of data: Glenn Heller, Robert McCormack, Thian Kheoh, Arturo Molina, Matthew R. Smith, Fred Saad, Dana T. Aftab, Mohammad Hirmand, Ana Limon, Karim Fizazi, Martin Fleisher, Johann S. de Bono, Howard I. Scher
Data analysis and interpretation: Glenn Heller, Matthew R. Smith, Robert Dreicer, Fred Saad, Ronald de Wit, Mohammad Hirmand, Karim Fizazi, Johann S. de Bono, Howard I. Scher
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Circulating Tumor Cell Number as a Response Measure of Prolonged Survival for Metastatic Castration-Resistant Prostate Cancer: A Comparison With Prostate-Specific Antigen Across Five Randomized Phase III Clinical Trials
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Glenn Heller
Research Funding: Janssen Diagnostics
Robert McCormack
Employment: Johnson & Johnson
Stock or Other Ownership: Johnson & Johnson
Thian Kheoh
Employment: Johnson & Johnson
Stock or Other Ownership: Johnson & Johnson
Arturo Molina
Employment: Johnson & Johnson, Sutro Biopharma
Leadership: Sutro Biopharma
Stock or Other Ownership: Sutro Biopharma
Matthew R. Smith
Consulting or Advisory Role: Bayer AG, Janssen Oncology, Astellas Pharma, Amgen, Gilead Sciences
Research Funding: Janssen Oncology (Inst), Gilead Sciences (Inst), Bayer AG (Inst)
Robert Dreicer
Consulting or Advisory Role: Astellas Pharma, Asana Biosciences, Exelixis, AstraZeneca, Bristol-Myers Squibb, Genentech, EMD Serono, Genzyme
Research Funding: Genentech (Inst), Asana Biosciences (Inst)
Fred Saad
No relationship to disclose
Ronald de Wit
Honoraria: Sanofi, Genentech, Merck Sharp & Dohme
Consulting or Advisory Role: Sanofi, Merck Sharp & Dohme, Genentech
Research Funding: Sanofi (Inst)
Dana T. Aftab
Employment: Exelixis
Leadership: Exelixis
Stock or Other Ownership: Exelixis
Patents, Royalties, Other Intellectual Property: Exelixis
Travel, Accommodations, Expenses: Exelixis
Mohammad Hirmand
Employment: Medivation, Peloton Therapeutics
Leadership: Medivation, Peloton Therapeutics
Stock or Other Ownership: Medivation, Peloton Therapeutics
Ana Limon
Employment: Takeda, Celgene (I)
Stock or Other Ownership: Takeda, Celgene
Patents, Royalties, Other Intellectual Property: Celgene (I)
Karim Fizazi
Honoraria: Janssen, Sanofi, Astellas Pharma, Merck, Amgen
Consulting or Advisory Role: Janssen Oncology, Bayer AG, Astellas Pharma, Sanofi, Orion Pharma, CureVac, AstraZeneca, ESSA, Genentech, Clovis Oncology
Travel, Accommodations, Expenses: Amgen
Martin Fleisher
No relationship to disclose
Johann S. de Bono
Honoraria: AstraZeneca, Sanofi, Astellas Pharma, Pfizer, Genentech
Consulting or Advisory Role: AstraZeneca, Sanofi, Genentech, Astellas Pharma
Research Funding: AstraZeneca (Inst), Genentech (Inst), Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: Abiraterone Rewards to Inventors (Inst), PARP inhibitors and DNA repair defects (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Astellas Pharma, GlaxoSmithKline, Orion Pharma , Sanofi, Genmab, Taiho Pharmaceutical, QIAGEN, Vertex Pharmaceuticals
Howard I. Scher
Leadership: Asterias Biotherapeutics
Consulting or Advisory Role: Ferring Pharmaceuticals, WIRB-Copernicus Group, Merck, Clovis Oncology, Janssen Research & Development, Astellas Pharma, Sanofi, Physicians’ Education Resource, OncLive Insights
Research Funding: Janssen (Inst), Innocrin Pharmaceuticals (Inst), Illumina (Inst)
Travel, Accommodations, Expenses: Ferring Pharmaceuticals, Clovis Oncology, WIRB-Copernicus Group, Asterias Biotherapeutics, Physicians’ Education Resource, OncLive Insights
REFERENCES
- 1.Robinson D, Van Allen EM, Wu YM, et al. : Integrative clinical genomics of advanced prostate cancer. Cell 161:1215-1228, 2015. [Erratum: Cell 162:454, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heller G, Fizazi K, McCormack R, et al. : The added value of circulating tumor cell enumeration to standard markers in assessing prognosis in a metastatic castration-resistant prostate cancer population. Clin Cancer Res 23:1967-1973, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scher HI, Morris MJ, Stadler WM, et al. : Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 34:1402-1418, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paoletti C, Hayes DF: Circulating tumor cells. Adv Exp Med Biol 882:235-258, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Parkinson DR, Dracopoli N, Petty BG, et al. : Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med 10:138, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration : CellSearch Circulating Tumor Cell Kit: K073338. Premarket notification—expanded indications for use—metastatic prostate cancer. http://www.accessdata.fda.gov/cdrh_docs/pdf7/K073338.pdf
- 7.Danila DC, Heller G, Gignac GA, et al. : Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res 13:7053-7058, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Goodman OB, Jr, Symanowski JT, Loudyi A, et al. : Circulating tumor cells as a predictive biomarker in patients with hormone-sensitive prostate cancer. Clin Genitourin Cancer 9:31-38, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Hayes DF, Cristofanilli M, Budd GT, et al. : Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12:4218-4224, 2006 [DOI] [PubMed] [Google Scholar]
- 10.de Bono JS, Logothetis CJ, Molina A, et al. : Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364:1995-2005, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fizazi K, Jones R, Oudard S, et al. : Phase III, randomized, double-blind, multicenter trial comparing orteronel (TAK-700) plus prednisone with placebo plus prednisone in patients with metastatic castration-resistant prostate cancer that has progressed during or after docetaxel-based therapy: ELM-PC 5. J Clin Oncol 33:723-731, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saad F, Fizazi K, Jinga V, et al. : Orteronel plus prednisone in patients with chemotherapy-naive metastatic castration-resistant prostate cancer (ELM-PC 4): A double-blind, multicentre, phase 3, randomised, placebo-controlled trial. Lancet Oncol 16:338-348, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Scher HI, Fizazi K, Saad F, et al. : Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367:1187-1197, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Smith M, De Bono J, Sternberg C, et al. : Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol 34:3005-3013, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Danila DC, Morris MJ, de Bono JS, et al. : Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol 28:1496-1501, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid AH, Attard G, Danila DC, et al. : Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol 28:1489-1495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scher HI, Beer TM, Higano CS, et al. : Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1-2 study. Lancet 375:1437-1446, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uno H, Cai T, Pencina MJ, et al. : On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med 30:1105-1117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]