ABSTRACT
Cdc20 (cell division cycle 20 homologue) has been reported to exhibit an oncogenic role in human tumorigenesis. However, the function of Cdc20 in osteosarcoma (OS) has not been investigated. In the current study, we aim to explore the role of Cdc20 in human OS cells. Multiple approaches were used to measure cell growth, apoptosis, cell cycle, migration and invasion in OS cells after depletion of Cdc20 or overexpression of Cdc20. We found that down-regulation of Cdc20 inhibited cell growth, induced apoptosis and triggered cell cycle arrest in OS cells. Moreover, Cdc20 down-regulation let to inhibition of cell migration and invasion in OS cells. Consistently, overexpression of Cdc20 in OS cells promoted cell growth, inhibited apoptosis, enhanced cell migration and invasion. Mechanistically, our Western blotting results showed that overexpression of Cdc20 reduced the expression of Bim and p21, whereas depletion of Cdc20 upregulated Bim and p21 levels in OS cells. Altogether, our findings demonstrated that Cdc20 exerts its oncogenic role partly due to regulation of Bim and p21 in OS cells, suggesting that targeting Cdc20 could be useful for the treatment of OS.
KEYWORDS: apoptosis, Cdc20, cell growth, invasion, migration, Osteosarcoma
Introduction
Osteosarcoma (OS), one of the common malignant bone tumors, is often presented in children and youth. More than 400 children and adolescents are diagnosed with OS in the United States every year.1 The common therapies consist of chemotherapy and surgical resection at present, and the patients' survival rate is around 70%.2–4 However, the patients often have a poor prognosis with metastasis, which leads to approximately 20% survival rate at 5 years.5 Therefore, exploration of the molecular mechanism of OS and development of new therapeutic options are warranted to effectively treat OS patients.
The APC (Anaphase Promoting Complex) plays a key role in regulating timely cell cycle progression in both M and G1 phases.6 It is well known that APC is activated by Cdc20 (cell division cycle 20 homologue).7 Cdc20 governs the activation of APC and subsequently regulates the progression of cell cycle.8 The Cdc20 identifies the cell cycle regulators like Securin and Cyclin B to the APC core for ubiquitination and thus controls the transition from the metaphase to anaphase.9,10 A wealth of evidence has demonstrated that Cdc20 targets multiple substrates including Cyclin A,11,12 Nek2A (NIMA related kinase 2),13 p21,14 Mcl-1 (myeloid cell leukemia-1),15 and SMAR1 (scaffold matrix attachment region binding protein 1)16 for destruction to control the process of cell cycle. One recent study showed that prostate cancer-associated mutation in SPOP impaired its ability to target Cdc20 for degradation.17 Mounting evidence has demonstrated that Cdc20 functions as an oncoprotein in tumorigenesis.18‐20 Overexpression of Cdc20 has been observed in various types of human cancers, including lung cancer, glioblastomas, bladder cancer, breast cancer, prostate cancer, colorectal cancer, pancreatic cancer, and hepatocellular cancer.18
Notably, higher expression of Cdc20 is associated with clinicopathological parameters in several human cancers. For instance, high expression of Cdc20 is correlated with poor differentiation and a lower 5-year recurrence-free survival rate in pancreatic cancer.21 Similarly, overexpression of Cdc20 was associated with an aggressive course of breast cancer.22 Consistently, higher level of Cdc20 was correlated with pleural invasion, and shorter 5-year overall survival in lung cancer.23 In line with this, Cdc20 level was correlated with clinical stage and pathological differentiation.24 Patients with higher Cdc20 had a shorter overall survival, indicating that Cdc20 expression was an independent prognostic factor in colorectal cancer.24 Another study showed that overexpression of Cdc20 was positively correlated with tumor differentiation, TNM (tumor, lymph node, metastasis) stage in hepatocellular carcinoma.25 These reports demonstrated that Cdc20 functions as an oncoprotein in a variety of human cancers.
Since it has no reports to show the role of Cdc20 in OS, studies are warrant to appreciate the function of Cdc20 in OS cells. Therefore, our work aimed to explore whether Cdc20 plays an oncogenic role in OS cells. Our results showed that Cdc20 deletion inhibited cell proliferation, induced cell apoptosis and cell cycle arrest, and retarded cell invasion. Moreover, the protein levels of Bim and p21 were increased in Cdc20 knockdown cells. On the other hand, the overexpression of Cdc20 accelerated cell growth and cell invasion. The expression of Bim and p21 was decreased in Cdc20 overexpression cells. In conclusion, our results indicate that Cdc20 may be a novel target for the treatment of OS patients.
Results
Cdc20 shRNA transfection decreased Cdc20 expression
To determine the role of Cdc20 in OS cells, Cdc20 shRNA and empty vector were transfected into MG63 and U2OS cells, respectively. Then we measured the expression of Cdc20 at both mRNA and protein levels in OS cells by real-time RT-PCR and Western blotting analysis. Our real-time RT-PCR data indicated that Cdc20 mRNA level was decreased in MG63 and U2OS cells after Cdc20 shRNA transfection (Fig. 1A). Moreover, our Western blotting results showed that the protein level of Cdc20 was suppressed in both MG63 and U2OS cells transfected with Cdc20 shRNA (Fig. 1B and 1C). These results revealed that Cdc20 shRNA significantly inhibited the Cdc20 expression in OS cells.
Figure 1.
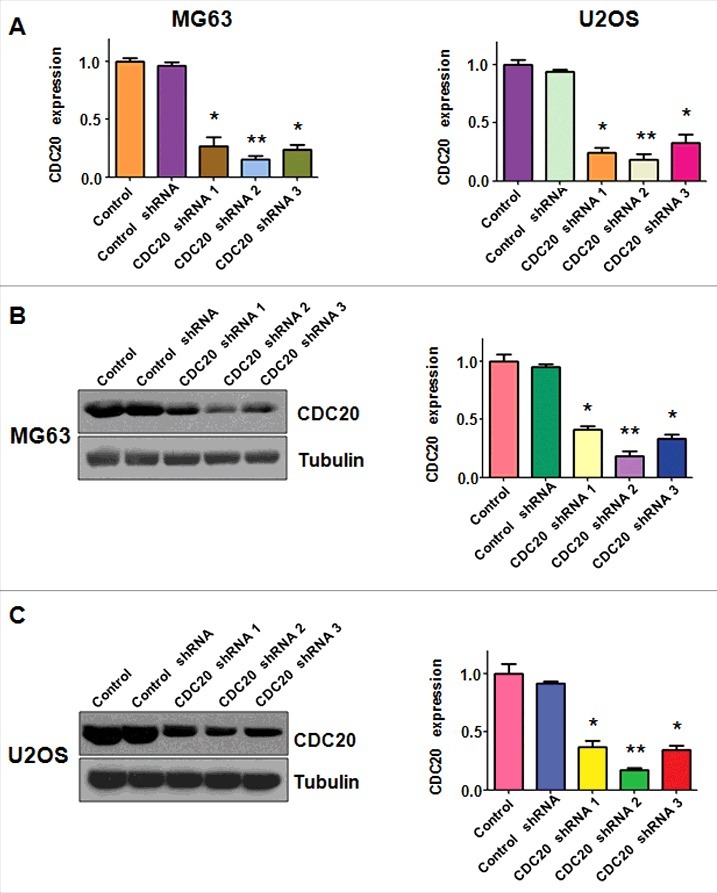
Cdc20 shRNA inhibited the level of Cdc20 in both mRNA and protein. A. The mRNA level of Cdc20 was measured by real-time PCR in both MG63 and U2OS cells with Cdc20 inhibition. * P < 0.05; ** P < 0.01 vs control shRNA.B. Left panel: Western blot was performed to detect the expression of Cdc20 in MG63 cells with Cdc20 shRNA transfection. Right panel: Quantification of the results for left panel. * P < 0.05; ** P < 0.01 vs control shRNA. C. Left panel: Western blotting was conducted to measure the protein level of Cdc20 in U2OS cells with Cdc20 shRNA transfection. Right panel: Quantification of the results for left panel. * P < 0.05; ** P < 0.01 vs control shRNA.
Down-regulation of Cdc20 suppressed cell growth and induced cell apoptosis
To dissect the function of Cdc20 in OS cells, we measured cell viability in OS cells after Cdc20 shRNA transfection. The CTG assays were carried out to detect the ability of cell growth in OS cells with Cdc20 shRNA infection for 48 hours and 72 hours, respectively. Our data demonstrated that down-regulation of Cdc20 suppressed cell proliferation (Fig. 2A). The PI-FITC-annexin assays were performed to explore whether inhibition of Cdc20 could induce cell apoptosis. We observed that apoptosis of OS cells was accelerated in both MG63 and U2OS cells transfected with Cdc20 shRNA (Fig. 2B). Specifically, cell apoptosis was increased from 8.32% to 20.79% after Cdc20 deletion in MG63 cells (Fig. 2B). Similarly, the percentage of cell apoptosis was increased from 9.04% in control shRNA group to 25.38% in Cdc20 shRNA transfection group in U2OS cells (Fig. 2B). Moreover, we found that depletion of Cdc20 induced cell cycle arrest at G2/M phase in OS cells (Fig. 2C). Our work implied that Cdc20 inhibition inhibited cell proliferation and enhanced cell apoptosis in OS cells.
Figure 2.
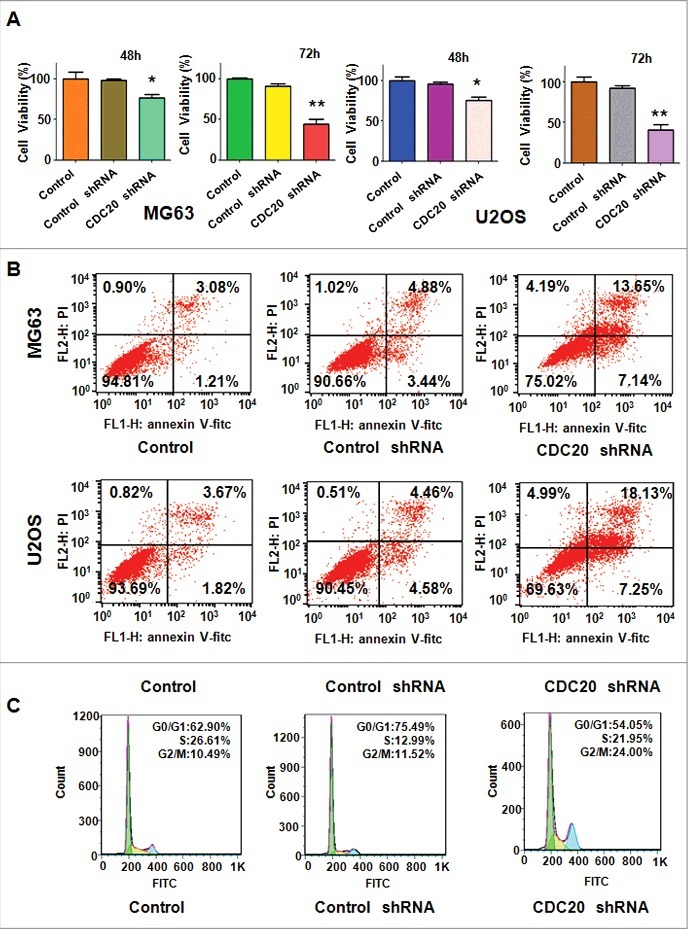
Down-regulation of Cdc20 depressed cell growth and induced apoptosis and cell cycle arrest. A. Cell viability was detected by CTG solution at 48 hours and 72 hours in OS cells after Cdc20 shRNA transfection. * P < 0.05; ** P < 0.01 vs control shRNA. B. Cell apoptosis was determined by Flow cytometry in OS cells transfected with Cdc20 shRNA. C. Flow cytometry was exerted to measure cell cycle in OS cells with Cdc20 shRNA transfection.
Down-regulation of Cdc20 suppressed the ability of cell invasion and migration
The motility of OS cells with Cdc20 inhibition was measured by invasion and migration assays. We found that the cells through the matrigel-coated membrane were decreased markedly in both MG63 and U2OS cells with Cdc20 deletion (Fig. 3A and 3B). The wound healing assays were carried out to investigate the migration of OS cells with Cdc20 shRNA transfection. After 20 hours, the cells with Cdc20 shRNA and control vector were taken photos. Our data showed that migrated OS cells were decreased when Cdc20 was depleted in both MG63 and U2OS cells (Fig. 3C). Taken together, down-regulation of Cdc20 by its shRNA retarded cell motility activity.
Figure 3.
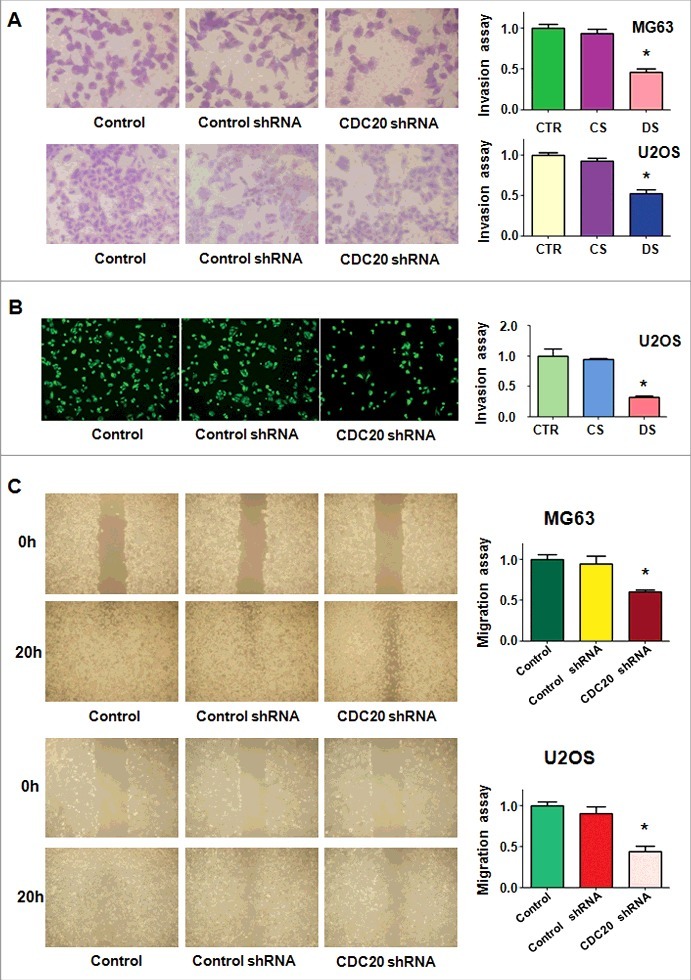
Down-regulation of Cdc20 inhibited cell invasion and migration. A. Left panel: The cell invasiveness was performed in MG63 and U2OS cells with Cdc20 shRNA transfection. Right panel: Quantification of the results for left panel. * P < 0.05 vs control shRNA. B. Left panel: Trans-well assays were conducted to test invasion of U2OS cells after Cdc20 shRNA transfection. Right panel: Quantification of the results for left panel. * P < 0.05 vs control shRNA. C. Left panel: Wound healing assay was performed to measure the migration of MG63 and U2OS cells after Cdc20 shRNA transfection. Right panel: Quantification of the results for left panel. * P < 0.05 vs control shRNA.
Cdc20 overexpression promoted cell proliferation and suppressed cell apoptosis
To further define the role of Cdc20 in OS cells, Cdc20 cDNA and empty vector were transfected into MG63 and U2OS cells, respectively. The CTG assays were exerted to measure the cell viability in OS cells with Cdc20 overexpression. We found that Cdc20 cDNA transfection promoted cell proliferation in OS cells (Fig. 4A). Consistently, the numbers of cell apoptosis were decreased from 20.48% to 5.15% after Cdc20 cDNA transfection in MG63 cells (Fig. 4B). Similarly, the percentage of cell apoptosis in Cdc20 cDNA group was 4.13% compared with 11.18% in control cDNA group in U2OS cells (Fig. 4B). Overexpression of Cdc20 increased S phase in MG63 cells (Fig. 4C). All the data suggested that Cdc20 overexpression promoted cell growth and inhibited cell apoptosis.
Figure 4.
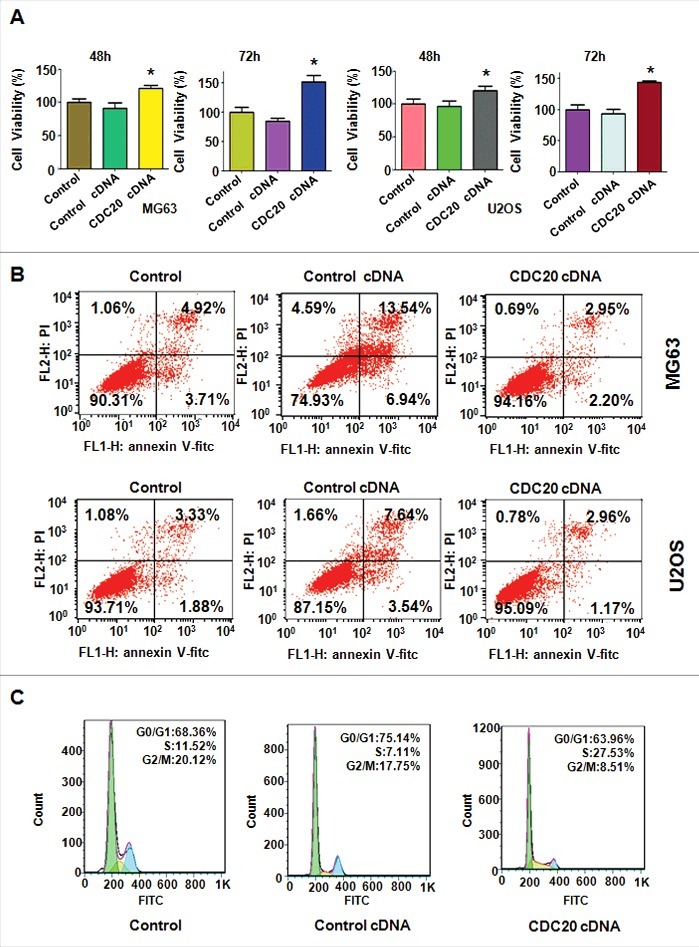
Overexpression of Cdc20 induced cell proliferation and inhibited cell apoptosis. A. Cell viability was detected by CTG solution at 48 hours and 72 hours in MG63 and U2OS cells after Cdc20 overexpression. * P < 0.05 vs control cDNA. B. The Flow cytometry was carried out to detect cell apoptosis in MG63 and U2OS cells with Cdc20 cDNA transfection. C. Cell cycle analysis was performed in U2OS cells with Cdc20 overexpression.
Cdc20 overexpression promoted cell invasion and migration
The trans-well assays were exerted to detect the invasiveness of OS cells with Cdc20 cDNA transfection. We observed that overexpression of Cdc20 enhanced cell invasiveness in both MG63 and U2OS cells (Fig. 5A and 5B). To further explore the migration ability of OS cells with Cdc20 overexpression, wound healing assays were performed. Our wound healing assays displayed that overexpression of Cdc20 led to more numbers of migratory cells compared with the control group cells (Fig. 5 C). Our results exhibited that Cdc20 overexpression enhanced cell invasion and migration in OS cells.
Figure 5.
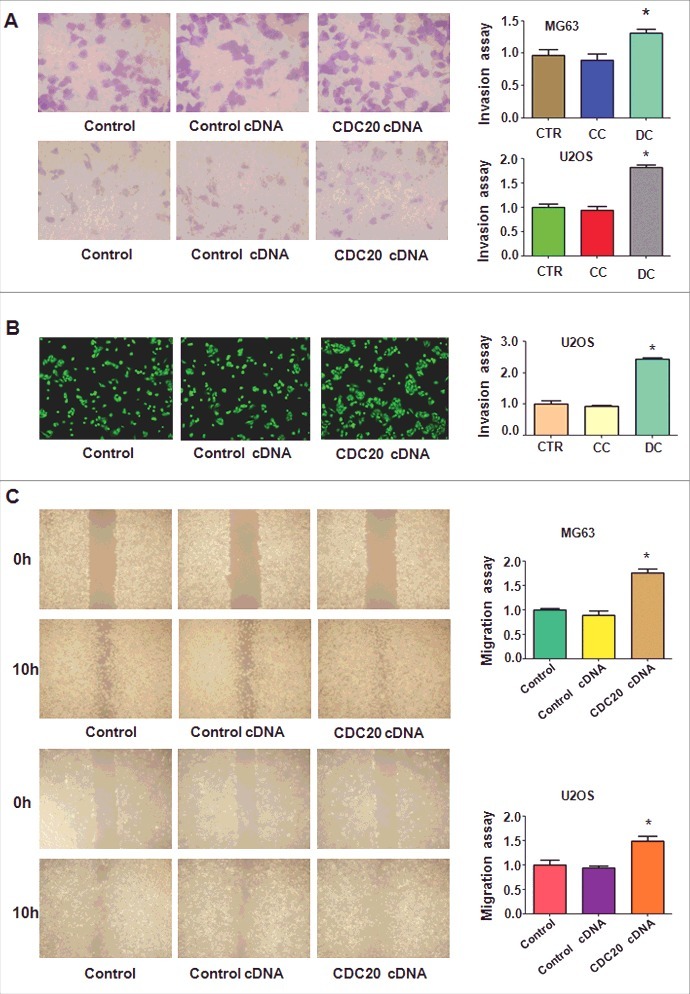
Overexpression of Cdc20 promoted cell invasion and migration. A. Left panel: Trans-well assay was conducted to investigate cell invasion in MG63 and U2OS cells with Cdc20 cDNA transfection. Right panel: Quantification of the results for left panel. * P < 0.05 vs control cDNA. CTR: control; CC: control cDNA; DC: cdc20 cDNA. B. Left panel: Cell invasiveness was detected in U2OS cells with Cdc20 overexpression. Right panel: Quantification of the results for left panel. * P < 0.05 vs control cDNA.C. Left panel: The migratory ability was measured in MG63 and U2OS cells with Cdc20 overexpression. Right panel: Quantification of the results for left panel. * P < 0.05 vs control cDNA.
Cdc20 overexpression inhibited the expression of Bim in OS cells
It has been reported that Cdc20 exerts its oncogenic function partly through regulation of Bim and p21.10‐14 To further explore whether Bim is involved in Cdc20-mediated tumorigenesis in OS cells, we measured the Bim expression in OS cells after Cdc20 depletion. We found that the protein level of Cdc20 was decreased by its shRNA transfection, while the expression of Bim and p21 was up-regulated in both MG63 and U2OS cells (Fig. 4A–4C). To illuminate the molecular mechanism of Cdc20-induced tumorigenesis in OS cells, Western blotting analysis was conducted to measure the expression of Bim in OS cells after Cdc20 cDNA transfection. As expected, Cdc20 expression was increased in OS cells with Cdc20 cDNA treatment (Figure 6). Notably, the expression of Bim and p21 was decreased significantly in both MG63 and U2OS cells (Figure 6). Our results denoted that Cdc20 may target Bim and p21 to play the oncogenic role in OS cells.
Figure 6.
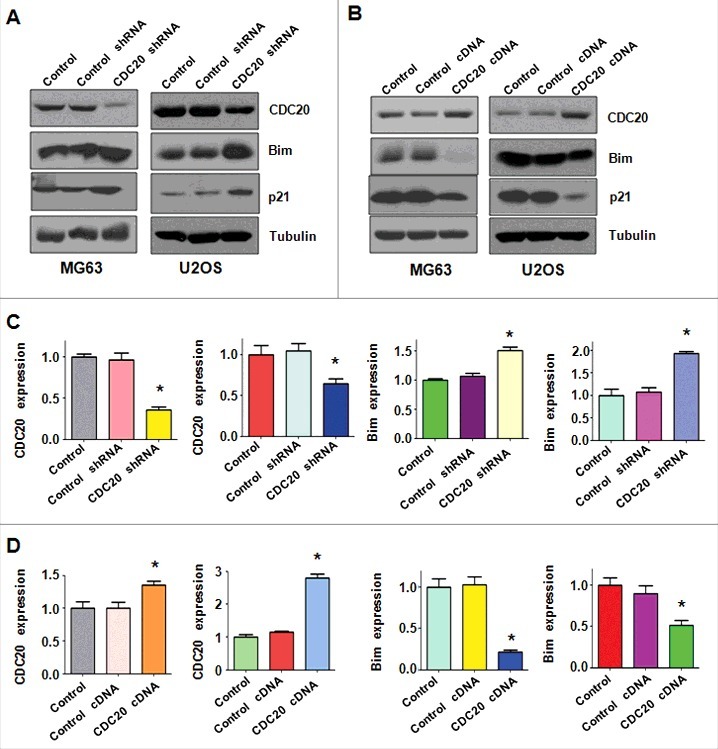
Overexpression of Cdc20 decreased Bim expression. A. The expression of Cdc20, Bim and p21 was detected by Western blotting in OS cells after Cdc20 shRNA transfection. B. The protein levels of Cdc20, p21, and Bim were detected by Western blot in OS cells after Cdc20 overexpression. C. Quantification of the results for panel A. * P < 0.05 vs control cDNA. D. Quantification of the results for panel B. * P < 0.05 vs control cDNA.
Discussion
Emerging evidence has demonstrated that Cdc20 plays oncogenic role in human cancer cells.18 For example, depletion of Cdc20 inhibited cell growth and induced G2/M cell cycle arrest in pancreatic cancer cells.26 Moreover, down-regulation of Cdc20 enhanced pancreatic cancer cells cytotoxicity upon paclitaxel treatment.26 Depletion of Cdc20 suppressed cell growth and induced G2/M cell cycle arrest and inhibited colony formation in lung cancer cells.27 Similarly, hepatocellular carcinoma cells transfected with Cdc20 siRNA exhibited a decrease in cell growth and increase in the number of cells in G2/M phase.25 In line with these findings, we found that depletion of Cdc20 inhibited cell growth in OS cells. Consistently, overexpression of Cdc20 promoted cell growth in OS cells. Altogether, inhibition of Cdc20 could be helpful for the treatment of OS. Studies have implicated that Cdc20 regulated cellular processes mainly through targeting Bim.10 The Bcl-2 (B-cell lymphoma-2) family of proteins contain anti-apoptotic proteins including Bcl-2, Bcl-xL, Bcl-w and Mcl-1,28 and pro-apoptotic members such as Bax, Bak and BH3-only proteins such as Bim.29 Our study indicated that Cdc20 governed cell apoptosis in part via regulation of Bim in OS cells. Cdc20 has been reported to promote cell migration and invasion in human cancers.30 In line with this, we observed that Cdc20 increased cell migration and invasion in OS cells. Recently, one study showed that Cdc20 controlled glioblastoma stem-like cells (GSCs) invasion via up-regulation of transcription factor SOX2 (SRY-box 2).31 Therefore, Cdc20 enhanced OS cell invasion possibly due to upregulation of SOX2. However, it is required to further approach the mechanism of Cdc20-triggered invasion in OS cells.
Due to the key oncogenic role of Cdc20 in tumorigenesis, its inhibitors might provide a therapeutic window in human cancers. One group discovered that TAME (tosyl-L-arginine methyl ester) binds APC and suppresses its activation by Cdc20 and Cdh1. TAME disrupted Cdc20 association with the APC and subsequent suppressed APC E3 ligase activity.32 Moreover, a TAME prodrug (pro-TAME), which is cell permeable and can be processed to yield the active form of TAME, was developed. Mechanistically, TAME inhibits the binding of free Cdc20 to the APC. In addition, in the absence of APC substrates, TAME can promote Cdc20 dissociation from the APC.33 Strikingly, apcin (APC inhibitor) binds Cdc20 and prevents its substrate recognition, resulting in competitively inhibition of the ubiquitination of Cdc20 substrates.34 ProTAME treatment decreased cell viability and increased apoptosis in multiple myeloma.35 The further investigations are necessary to identify Cdc20 inhibitors as safe agents for clinical trials.
Recently, natural compounds have been validated to inhibit the Cdc20 expression. For instance, withaferin A, a bioactive component from Withania somnifera, has been reported to exerts its anti-cancer activity through enhanced degradation of Cdc20.36 Additionally, NAHA, a N-alkylated amino acid-derived sulfonamide hydroxamate, decreased Cdc20 expression, leading to cell growth inhibition.37 Ganodermanontriol (GDNT), a ganoderma alcohol from medicinal mushroom, reduced Cdc20 level, resulting in inhibition of breast cancer cell growth.38 MycoPhyto® Complex (MC), a novel medicinal mushroom blend, inhibited cell proliferation and induced cell cycle arrest via targeting Cdc20 in breast cancer cells.39 Genistein, a phytoestrogenic isoflavonid, exerts its tumor suppressive function partly via inhibition of Cdc20 in glioblastoma, hepatocellular carcinoma, and breast cancer cells.40,41 Recently, rottlerin inhibited cell growth and invasion via suppression of Cdc20 in glioma cells.30 Another natural compound, curcumin, was reported to inhibit Cdc20 in pancreatic cancer cells.42 It is required to determine whether natural compounds inhibit Cdc20 expression in OS cells.
In conclusion, Cdc20 plays oncogenic roles in OS cells via control of cell growth, apoptosis, migration and invasion. Development of Cdc20 inhibitors could possibly be a novel strategy for the treatment of OS. Due to their non-toxic nature, using natural compounds could be a safer approach for the better treatment of OS. Without a doubt, exploring the molecular mechanism by which Cdc20 enhances cell growth and invasion in OS cells could be helpful for discover the novel treatment approach for clinical benefits of OS patients.
Materials and methods
Cell culture and agents
The osteosarcoma MG63 and U2OS cells were cultured in DMEM (Dulbecco's modified Eagle's medium) medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in a 5% CO2 atmosphere at 37°C. Primary antibodies for Cdc20 and Bim were purchased from Cell Signaling Technology Company (Danvers, MA, USA). All second antibodies were purchased from Thermo Scientific (Waltham, MA, USA). Lipofectamine 2000 was obtained from Invitrogen (Carlsbad, CA, USA). Anti-tubulin antibody and CellTiterGlo® luminescence (CTG) were bought from Sigma-Aldrich (St. Louis, MO, USA). Transwell inserts and Matrigel were obtained from BD Biosciences (San Jose, CA, USA).
Transfection
OS cells were seeded into 6-well plates or 96-well plates and transfected with Cdc20 cDNA or Cdc20 shRNA or empty vector through Lipofectamine 2000 following the instruction's protocol.43 After the transfection, the cells were subjected to further analysis as described under the results sections.
Cell viability assay
The OS cells were seeded into 96-well plates, Cdc20 cDNA or Cdc20 shRNA and vector were transfected into cells. After 48 hours and 72 hours, the CTG were added into each well and incubated for 10 min at 37°C atmosphere. Then, the cell viability was assessed using the CTG assay. Each value was normalized to cells transfected with empty vector.
Cell apoptosis assay
The OS cells were seeded in six-well plates and transfected with empty vector and Cdc20 cDNA or Cdc20 shRNA for 48 hours. Then, the cells were harvested and washed with PBS, then resuspended the cells in 500μl binding buffer with 5 μl Propidium iodide (PI) and 5 μl FITC-conjugated anti-Annexin V antibody. The apoptosis was analyzed by a FACScalibur flow cytometer (BD, USA).
Cell cycle analysis
The transfected OS cells were seeded in a six-well plate for overnight. After 48 hours, cells were harvested, washed and resuspended in 70% cold alcohol and kept at 4°C overnight. Then, cells were suspended in 1 × 106 cells/ml in PBS and incubated with 0.1mg/ml RNase I and 50 mg/ml PI at 37°C for 30 min. Cell cycle was further detected with a FACScalibur flow cytometer (BD, USA).
Cell invasion assay
Cell invasion was exerted to test the invasive activity of MG63 and U2OS cells transfected with Cdc20 cDNA or Cdc20 shRNA. Briefly, the cells were seeded in the upper chamber with 200 μl serum-free medium and there is 500 μl complete medium in the under chamber. After incubation for 20 h, the invaded cells were stained with Giemsa and 4 ug/mL calcein AM and photographed with a microscope.44
Cell wound healing assays
The OS cells were seeded in 6-well plates and transfected with Cdc20 cDNA or shRNA and empty vector. After cells almost covered the well, absorbed the supernatant and scratched the cell with a yellow pipette tip. Subsequently, the cells were washed with PBS, and then the scratched zone was taken photos with the microscope at 0 h, 10 h and 20 h, respectively.
Quantitative real-time reverse transcription-PCR analysis
The total RNA from OS transfected cells were extracted with Trizol (Invitrogen, Carlsbad, CA) and reversed-transcribed into cDNA by RevertAid First Strand cDNA Synthesis Kit. PCR were performed using Power SYBR Green PCR Master Mix and the results were calculated by 2-Ct method as described previously.44 The primers used in PCR reaction are: Cdc20, forward primer (5′- GAC CAC TCC TAG CAA ACC TGG -3′) and reverse primer (5′-GGG CGT CTG GCT GTT TTC A-3′);; GAPDH, forward primer (5′- ACC CAG AAG ACT GTG GAT GG -3′) and reverse primer (5′- CAG TGA GCT TCC CGT TCA G- 3′).
Western Blotting analysis
The harvested cells were washed by PBS and lysed with protein lysis buffer. The concentrations of proteins were analyzed with BCA Protein Assay kit. The same amount of protein samples were separated by SDS-PAGE and then transferred into nitrocellulose membranes. Then the membranes were incubated with the primary antibodies (anti-Cdc20: 1:2000; anti-p21: 1:1000; anti-Bim: 1:1000; anti-tubulin: 1:5000) at 4°C overnight. On the second day, the membranes were washed and incubated with the second antibodies for one hour at room temperature. Then the expression of proteins was detected by electrochemiluminescence assay.
Statistic analysis
All statistical analyses were conducted using GraphPad Prism 5.0 (Graph Pad Software, La Jolla, CA). Student t test was exerted to evaluate statistical significance. The results were presented as means ± SD. P < 0.05 was considered as statistically significant.
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- 1.Harrison DJ, Schwartz CL. Osteogenic Sarcoma: Systemic Chemotherapy Options for Localized Disease. Curr Treat Options Oncol. 2017;18:24. doi: 10.1007/s11864-017-0464-2. PMID:28391422 [DOI] [PubMed] [Google Scholar]
- 2.Wu PK, Chen WM, Chen CF, Lee OK, Haung CK, Chen TH. Primary osteogenic sarcoma with pulmonary metastasis: clinical results and prognostic factors in 91 patients. Jpn J Clin Oncol. 2009;39:514-22. doi: 10.1093/jjco/hyp057. [DOI] [PubMed] [Google Scholar]
- 3.Iwamoto Y, Tanaka K, Isu K, Kawai A, Tatezaki S, Ishii T, Kushida K, Beppu Y, Usui M, Tateishi A, et al. Multiinstitutional phase II study of neoadjuvant chemotherapy for osteosarcoma (NECO study) in Japan: NECO-93J and NECO-95J. J Orthop Sci. 2009;14:397-404. doi: 10.1007/s00776-009-1347-6. [DOI] [PubMed] [Google Scholar]
- 4.Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W, Gebhardt M, Goorin AM, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23:2004-11. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 5.Hirotsu M, Setoguchi T, Sasaki H, Matsunoshita Y, Gao H, Nagao H, Kunigou O, Komiya S. Smoothened as a new therapeutic target for human osteosarcoma. Mol Cancer. 2010;9:5. doi: 10.1186/1476-4598-9-5. PMID:20067614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369-81. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 7.McLean JR, Chaix D, Ohi MD, Gould KL. State of the APC/C: organization, function, and structure. Crit Rev Biochem Mol Biol. 2011;46:118-36. doi: 10.3109/10409238.2010.541420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Wan L, Zhong J, Inuzuka H, Liu P, Sarkar FH, Wei W. Cdc20: a potential novel therapeutic target for cancer treatment. Curr Pharm Des. 2013;19:3210-4. doi: 10.2174/1381612811319180005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagting A, Den Elzen N, Vodermaier HC, Waizenegger IC, Peters JM, Pines J. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J Cell Biol. 2002;157:1125-37. doi: 10.1083/jcb.200111001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan L, Tan M, Yang J, Inuzuka H, Dai X, Wu T, Liu J, Shaik S, Chen G, Deng J, et al. APC(Cdc20) suppresses apoptosis through targeting Bim for ubiquitination and destruction. Dev Cell. 2014;29:377-91. doi: 10.1016/j.devcel.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geley S, Kramer E, Gieffers C, Gannon J, Peters JM, Hunt T. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J Cell Biol. 2001;153:137-48. doi: 10.1083/jcb.153.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohtoshi A, Maeda T, Higashi H, Ashizawa S, Hatakeyama M. Human p55(CDC)/Cdc20 associates with cyclin A and is phosphorylated by the cyclin A-Cdk2 complex. Biochem Biophys Res Commun. 2000;268:530-4. doi: 10.1006/bbrc.2000.2167. [DOI] [PubMed] [Google Scholar]
- 13.Hames RS, Wattam SL, Yamano H, Bacchieri R, Fry AM. APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-box. EMBO J. 2001;20:7117-27. doi: 10.1093/emboj/20.24.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amador V, Ge S, Santamaria PG, Guardavaccaro D, Pagano M. APC/C(Cdc20) controls the ubiquitin-mediated degradation of p21 in prometaphase. Mol Cell. 2007;27:462-73. doi: 10.1016/j.molcel.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harley ME, Allan LA, Sanderson HS, Clarke PR. Phosphorylation of Mcl-1 by CDK1-cyclin B1 initiates its Cdc20-dependent destruction during mitotic arrest. EMBO J. 2010;29:2407-20. doi: 10.1038/emboj.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul D, Ghorai S, Dinesh US, Shetty P, Chattopadhyay S, Santra MK. Cdc20 directs proteasome-mediated degradation of the tumor suppressor SMAR1 in higher grades of cancer through the anaphase promoting complex. Cell Death Dis. 2017;8:e2882. doi: 10.1038/cddis.2017.270. PMID:28617439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu F, Dai X, Gan W, Wan L, Li M, Mitsiades N, Wei W, Ding Q, Zhang J. Prostate cancer-associated mutation in SPOP impairs its ability to target Cdc20 for poly-ubiquitination and degradation. Cancer Lett. 2017;385:207-14. doi: 10.1016/j.canlet.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Zhang J, Wan L, Zhou X, Wang Z, Wei W. Targeting Cdc20 as a novel cancer therapeutic strategy. Pharmacol Ther. 2015;151:141-51. doi: 10.1016/j.pharmthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Q, Wu Q, Mack SC, Yang K, Kim L, Hubert CG, Flavahan WA, Chu C, Bao S, Rich JN. CDC20 maintains tumor initiating cells. Oncotarget. 2015;6:13241-54. doi: 10.18632/oncotarget.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji P, Zhou X, Liu Q, Fuller GN, Phillips LM, Zhang W. Driver or passenger effects of augmented c-Myc and Cdc20 in gliomagenesis. Oncotarget. 2016;7:23521-9. doi: 10.18632/oncotarget.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang DZ, Ma Y, Ji B, Liu Y, Hwu P, Abbruzzese JL, Logsdon C, Wang H. Increased CDC20 expression is associated with pancreatic ductal adenocarcinoma differentiation and progression. J Hematol Oncol. 2012;5:15. doi: 10.1186/1756-8722-5-15. PMID:22475564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karra H, Repo H, Ahonen I, Loyttyniemi E, Pitkanen R, Lintunen M, Kuopio T, Soderstrom M, Kronqvist P. Cdc20 and securin overexpression predict short-term breast cancer survival. Br J Cancer. 2014;110:2905-13. doi: 10.1038/bjc.2014.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato T, Daigo Y, Aragaki M, Ishikawa K, Sato M, Kaji M. Overexpression of CDC20 predicts poor prognosis in primary non-small cell lung cancer patients. J Surg Oncol. 2012;106:423-30. doi: 10.1002/jso.23109. [DOI] [PubMed] [Google Scholar]
- 24.Wu WJ, Hu KS, Wang DS, Zeng ZL, Zhang DS, Chen DL, Bai L, Xu RH. CDC20 overexpression predicts a poor prognosis for patients with colorectal cancer. J Transl Med. 2013;11:142. doi: 10.1186/1479-5876-11-142. PMID:23758705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Gao JZ, Du JL, Huang ZX, Wei LX. Increased CDC20 expression is associated with development and progression of hepatocellular carcinoma. Int J Oncol. 2014;45:1547-55. doi: 10.3892/ijo.2014.2559. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi K, Momiyama N, Ueda M, Matsuyama R, Mori R, Fujii Y, Ichikawa Y, Endo I, Togo S, Shimada H. Targeting of CDC20 via small interfering RNA causes enhancement of the cytotoxicity of chemoradiation. Anticancer Res. 2008;28:1559-63. [PubMed] [Google Scholar]
- 27.Kidokoro T, Tanikawa C, Furukawa Y, Katagiri T, Nakamura Y, Matsuda K. CDC20, a potential cancer therapeutic target, is negatively regulated by p53. Oncogene. 2008;27:1562-71. doi: 10.1038/sj.onc.1210799. [DOI] [PubMed] [Google Scholar]
- 28.Cragg MS, Harris C, Strasser A, Scott CL. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nat Rev Cancer. 2009;9:321-6. doi: 10.1038/nrc2615. [DOI] [PubMed] [Google Scholar]
- 29.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647-56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Hou Y, Yin X, Su J, Zhao Z, Ye X, Zhou X, Zhou L, Wang Z. Rottlerin inhibits cell growth and invasion via down-regulation of Cdc20 in glioma cells. Oncotarget. 2016;7:69770-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao DD, Gujar AD, Mahlokozera T, Chen I, Pan Y, Luo J, Brost T, Thompson EA, Turski A, Leuthardt EC, et al. A CDC20-APC/SOX2 Signaling Axis Regulates Human Glioblastoma Stem-like Cells. Cell Rep. 2015;11:1809-21. doi: 10.1016/j.celrep.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng X, Sigoillot F, Gaur S, Choi S, Pfaff KL, Oh DC, Hathaway N, Dimova N, Cuny GD, King RW. Pharmacologic inhibition of the anaphase-promoting complex induces a spindle checkpoint-dependent mitotic arrest in the absence of spindle damage. Cancer Cell. 2010;18:382-95. doi: 10.1016/j.ccr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng X, King RW. An APC/C inhibitor stabilizes cyclin B1 by prematurely terminating ubiquitination. Nat Chem Biol. 2012;8:383-92. doi: 10.1038/nchembio.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sackton KL, Dimova N, Zeng X, Tian W, Zhang M, Sackton TB, Meaders J, Pfaff KL, Sigoillot F, Yu H, et al. Synergistic blockade of mitotic exit by two chemical inhibitors of the APC/C. Nature. 2014;514:646-9. doi: 10.1038/nature13660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lub S, Maes A, Maes K, De Veirman K De Bruyne E, Menu E, Fostier K, Kassambara A, Moreaux J, Hose D, et al. Inhibiting the anaphase promoting complex/cyclosome induces a metaphase arrest and cell death in multiple myeloma cells. Oncotarget. 2016;7:4062-76. doi: 10.18632/oncotarget.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das T, Roy KS, Chakrabarti T, Mukhopadhyay S, Roychoudhury S. Withaferin A modulates the Spindle assembly checkpoint by degradation of Mad2-Cdc20 complex in colorectal cancer cell lines. Biochem Pharmacol. 2014;91:31-9. doi: 10.1016/j.bcp.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 37.Jiang J, Thyagarajan-Sahu A, Krchnak V, Jedinak A, Sandusky GE, Sliva D. NAHA, a novel hydroxamic acid-derivative, inhibits growth and angiogenesis of breast cancer in vitro and in vivo. PLoS One. 2012;7:e34283. doi: 10.1371/journal.pone.0034283. PMID:22479587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang J, Jedinak A, Sliva D. Ganodermanontriol (GDNT) exerts its effect on growth and invasiveness of breast cancer cells through the down-regulation of CDC20 and uPA. Biochem Biophys Res Commun. 2011;415:325-9. doi: 10.1016/j.bbrc.2011.10.055. [DOI] [PubMed] [Google Scholar]
- 39.Jiang J, Sliva D. Novel medicinal mushroom blend suppresses growth and invasiveness of human breast cancer cells. Int J Oncol. 2010;37:1529-36. [DOI] [PubMed] [Google Scholar]
- 40.Regenbrecht CR, Jung M, Lehrach H, Adjaye J. The molecular basis of genistein-induced mitotic arrest and exit of self-renewal in embryonal carcinoma and primary cancer cell lines. BMC Med Genomics. 2008;1:49. doi: 10.1186/1755-8794-1-49. PMID:18847459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, Yang B, Zhou K, Li H, Li D, Gao H, Zhang T, Wei D, Li Z, Diao Y. Potential therapeutic mechanism of genistein in breast cancer involves inhibition of cell cycle regulation. Mol Med Rep. 2015;11:1820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Xue YB, Li H, Qiu D, Wang ZW, Tan SS. Inhibition of Cell Survival by Curcumin Is Associated with Downregulation of Cell Division Cycle 20 (Cdc20) in Pancreatic Cancer Cells. Nutrients. 2017;9. doi: 10.3390/nu9020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Ye X, Cai X, Su J, Ma R, Yin X, Zhou X, Li H, Wang Z. Curcumin suppresses cell growth and invasion and induces apoptosis by down-regulation of Skp2 pathway in glioma cells. Oncotarget. 2015;6:18027-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma J, Fang B, Zeng F, Ma C, Pang H, Cheng L, Shi Y, Wang H, Yin B, Xia J, et al. Down-regulation of miR-223 reverses epithelial-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Oncotarget. 2015;6:1740-9. doi: 10.18632/oncotarget.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]


