Abstract
Background
Malaria is an important cause of illness and death across endemic regions. Considerable success against malaria has been achieved within the past decade mainly through long‐lasting insecticide‐treated nets (LLINs). However, elimination of the disease is proving difficult as current control methods do not protect against mosquitoes biting outdoors and when people are active. Repellents may provide a personal protection solution during these times.
Objectives
To assess the impact of topical repellents, insecticide‐treated clothing, and spatial repellents on malaria transmission.
Search methods
We searched the following databases up to 26 June 2017: the Cochrane Infectious Diseases Group Specialized Register; the Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library; MEDLINE; Embase; US AFPMB; CAB Abstracts; and LILACS. We also searched trial registration platforms and conference proceedings; and contacted organizations and companies for ongoing and unpublished trials.
Selection criteria
We included randomized controlled trials (RCTs) and cluster‐randomized controlled trials of topical repellents proven to repel mosquitoes; permethrin‐treated clothing; and spatial repellents such as mosquito coils. We included trials that investigated the use of repellents with or without LLINs, referred to as insecticide‐treated nets.
Data collection and analysis
Two review authors independently reviewed trials for inclusion, extracted the data, and assessed the risk of bias. A third review author resolved any discrepancies. We analysed data by conducting meta‐analysis and stratified by whether the trials had included LLINs. We combined results from cRCTs with individually RCTs by adjusting for clustering and presented results using forest plots. We used GRADE to assess the certainty of the evidence.
Main results
Eight cRCTs and two RCTs met the inclusion criteria. Six trials investigated topical repellents, two trials investigated insecticide‐treated clothing, and two trials investigated spatial repellents.
Topical repellents
Six RCTS, five of them cluster‐randomized, investigated topical repellents involving residents of malaria‐endemic regions. Four trials used topical repellents in combination with nets, but two trials undertaken in displaced populations used topical repellents alone. It is unclear if topical repellents can prevent clinical malaria (RR 0.65, 95% CI 0.4 to 1.07, very low certainty evidence) or malaria infection (RR 0.84, 95% CI 0.64 to 1.12, low‐certainty evidence) caused by P. falciparum. It is also unclear if there is any protection against clinical cases of P. vivax (RR 1.32, 95% CI 0.99 to 1.76, low‐certainty evidence) or incidence of infections (RR 1.07, 95% CI 0.80 to 1.41, low‐certainty evidence). Subgroup analysis of trials including insecticide‐treated nets did not show a protective effect of topical repellents against malaria. Only two studies did not include insecticide‐treated nets, and they measured different outcomes; one reported a protective effect against clinical cases of P. falciparum (RR 0.40, 95% CI 0.23 to 0.71); but the other study measured no protective effect against malaria infection incidence caused by either P. falciparum or P. vivax.
Insecticide‐treated clothing
Insecticide‐treated clothing were investigated in trials conducted in refugee camps in Pakistan and amongst military based in the Colombian Amazon. Neither study provided participants with insecticide‐treated nets. In the absence of nets, treated clothing may reduce the incidence of clinical malaria caused by P. falciparum by approximately 50% (RR 0.49, 95% CI 0.29 to 0.83, low‐certainty evidence) and P. vivax (RR 0.64, 95% CI 0.40 to 1.01, low‐certainty evidence).
Spatial repellents
Two cluster‐randomized RCTs investigated mosquito coils for malaria prevention. We do not know the effect of spatial repellents on malaria prevention (RR 0.24, 95% CI 0.03 to 1.72, very low certainty evidence). There was large heterogeneity between studies and one study had high risk of bias.
Authors' conclusions
There is insufficient evidence to conclude topical or spatial repellents can prevent malaria. There is a need for better designed trials to generate higher certainty of evidence before well‐informed recommendations can be made. Adherence to daily compliance remains a major limitation. Insecticide‐treated clothing may reduce risk of malaria infection in the absence of insecticide‐treated nets; further studies on insecticide‐treated clothing in the general population should be done to broaden the applicability of the results.
2 April 2019
Up to date
All studies incorporated from most recent search
All eligible published studies found in the last search (26 Jun, 2017) were included and four ongoing studies have been identified (see 'Characteristics of ongoing studies' section)
Keywords: Animals; Culicidae; Insect Repellents; Protective Clothing; Incidence; Insecticide‐Treated Bednets; Malaria, Falciparum; Malaria, Falciparum/epidemiology; Malaria, Falciparum/prevention & control; Malaria, Vivax; Malaria, Vivax/epidemiology; Malaria, Vivax/prevention & control; Randomized Controlled Trials as Topic
Plain language summary
Mosquito repellents for malaria prevention
What was the aim of this review?
The aim of this Cochrane Review was to find out if mosquito repellents — topical repellents (applied to the skin); insecticide‐treated clothing; or spatial repellents such as mosquito coils — can prevent malaria. We collected and analysed the results of all relevant studies to answer this question and found data from ten trials: six on topical repellents, two on insecticide‐treated clothing, and two on spatial repellents.
Key messages
We do not know if the use of repellent lotions or burning of mosquito coils can provide protection from malaria to communities living in endemic regions. In situations where long‐lasting insecticide‐treated bed nets (LLINs) cannot be rolled out, such as after a natural disaster or amongst displaced populations, the use of insecticide‐treated clothing may reduce the risk of malaria infection by 50%. Most studies included in our analysis were poorly designed and had high risk of bias. In order to draw well‐informed conclusions, further high‐quality studies must be conducted to improve the certainty of the evidence. However, it is questionable if topical repellents can be used for malaria prevention in the general population as daily compliance and poor standardization (amount of repellent used, surface area applied, time of application, and period between repeated applications) are major limitations of this intervention.
What was studied in this review
Mosquito repellents provide protection from mosquito bites. There are three different types of repellents: topical repellents, which can be applied on the skin; insecticide‐treated clothing, through impregnation of clothing with repellent compounds; and spatial repellents, such as mosquito coils. Malaria has decreased in many countries because people have been given highly effective LLINs. However people are still being bitten before they go to bed. There is a need to find a way to offer protection from malaria during these hours. Mosquito repellents may address this gap.
What are the main results of the review?
A total of six trials investigated the use of topical repellents for malaria prevention. The trials took place in different malaria‐endemic regions across South America, Asia, and sub‐Saharan Africa. The topical repellents tested included lotions, treated soap, and local cosmetics. We analysed the studies in groups according to LLIN inclusion. Most studies rolled out LLINs to the population and investigated topical repellents as a complementary intervention to the treated bed‐nets. The poor design of the included studies provided low to very low certainty evidence, consequently we do not know if there is a benefit of using topical repellents in addition to LLINs to prevent malaria. The compliance of participants to adhere to the daily application of repellents remains a challenge to further research.
Insecticide‐treated clothing was investigated in two trials conducted with refugees in Pakistan and military deployed in the Amazon; neither study rolled out or reported the use of bed‐nets. In the absence of LLINs, there is some evidence that insecticide‐treated clothing may reduce the risk of malaria infection by 50%. Given that the findings relate to special populations living in particularly harsh conditions it is unclear if the results are applicable to the general population. Further studies involving civilian populations should be done to improve the certainty of these findings.
Two studies investigated the practice of burning mosquito coils to reduce malaria infections. One study was conducted in China and the other in Indonesia. The study designs were substantially different and one study had high risk of bias leading to very low certainty evidence. We do not know if mosquito coils offer protection against malaria. The findings underline the need for further research.
How up to date is this review?
The review authors searched for studies that had been published up to 26 June 2017.
Summary of findings
Summary of findings for the main comparison. Topical repellents compared to placebo or no treatment for malaria prevention.
| Topical repellents compared to placebo or no treatment for malaria prevention | ||||||
| Patient or population: malaria prevention Setting: malaria‐endemic regions Intervention: topical repellents Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Placebo or no treatment | Risk with Topical repellents | |||||
| Clinical malaria: P. falciparum | 39 per 1000 | 25 per 1000 (15 to 41) | RR 0.65 (0.40 to 1.07) | 4450 (3 studies) | ⊕⊝⊝⊝
VERY LOW1,2,3 Due to risk of bias, inconsistency and imprecision |
We do not know if topical repellents have an effect on malaria cases caused by P. falciparum. We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. |
| Parasitaemia: P. falciparum | 15 per 1000 | 12 per 1000 (9 to 17) | RR 0.84 (0.64 to 1.12) | 13,310 (4 studies) | ⊕⊕⊝⊝
LOW4,5 Due to risk of bias and imprecision |
Topical repellents may or may not have a protective effect against P. falciparum parasitaemia. Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimation of the effect. |
| Clinical malaria: P. vivax | 36 per 1000 | 48 per 1000 (36 to 64) | RR 1.32 (0.99 to 1.76) | 3996 (2 studies) | ⊕⊕⊝⊝
LOW6,7 Due to risk of bias and imprecision |
Topical repellents may increase the number of clinical cases caused by P. vivax. Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimation of the effect. |
| Parasitaemia: P. vivax | 18 per 1000 | 19 per 1000 (14 to 25) | RR 1.07 (0.80 to 1.41) | 9434 (3 studies) | ⊕⊕⊝⊝
LOW7,8 Due to risk of bias and imprecision |
Topical repellents may or may not have a protective effect against P. vivax parasitaemia Our confidence in the effect estimation is limited. The true effect may be substantially different from the estimation of the effect. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by 1 for risk of bias: Sangoro 2014a used alternate allocation and reported a baseline imbalance; random sequence generation and allocation concealment were not described by Rowland 2004; and Sluydts 2016 did not have a placebo so the intervention was not blinded. 2Downgraded by 1 because of the large heterogeneity between the 3 trials. The I² statistic, which quantifies the proportion of the variation in the point estimates due to among‐study differences, was considered substantial at 50%. The subgroup analysis to some extent explained the heterogeneity but we do not believe that there is enough evidence to suggest there is a true subgroup effect given that there is no heterogeneity in the outcome parasitaemia caused by P. falciparum where studies with and without LLINs were also analysed. 3Downgraded by 1 for imprecision because the sample size is too small, the CIs are wide, the pooled effect (0.40 to 1.07) overlaps a risk ratio (RR) of 1.0 (no effect) and presents an estimate of effect ranging between beneficial and harmful. 4Downgraded by 1 for risk of bias: Hill 2007 used alternate allocation and reported a baseline imbalance; random sequence generation and allocation concealment were not described by McGready 2001. 5Downgraded by 1 for imprecision because the sample size is too small, the CIs are very wide, the pooled effect (0.62 to 1.12) overlaps a risk ratio (RR) of 1.0 (no effect) and presents an estimate of effect ranging between beneficial and harmful. 6Downgraded by 1 for risk of bias: random sequence generation and allocation concealment were not described by Rowland 2004; Sluydts 2016 was not placebo‐controlled and intervention was not blinded. 7Downgraded by 1 for imprecision because the CIs are very wide, the pooled effect (0.80 to 1.41) overlaps a risk ratio (RR) of 1.0 (no effect) and presents an estimate of effect ranging between beneficial and harmful. 8Downgraded by 1 for risk of bias: random sequence generation and allocation concealment were not described by McGready 2001.
Summary of findings 2. ITC compared to placebo or no treatment for malaria prevention.
| ITC compared to placebo or no treatment for malaria prevention | ||||||
| Patient or population: malaria prevention Setting: malaria‐endemic regions Intervention: ITC Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with ITC | |||||
| Clinical malaria: P. falciparum | 35 per 1000 | 17 per 1000 (10 to 29) | RR 0.49 (0.29 to 0.83) | 997 (2 studies) | ⊕⊕⊝⊝
LOW1,2 Due to risk of bias and imprecision |
Insecticide‐treating clothing may have a protective effect against malaria caused by P. falciparum. Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. |
| Clinical malaria: P. vivax | 116 per 1000 | 74 per 1000 (47 to 117) | RR 0.64 (0.40 to 1.01) | 997 (2 studies) | ⊕⊕⊝⊝
LOW1,2 Due to risk of bias and imprecision |
Insecticide‐treated clothing may have a protective effect against malaria caused by P. vivax. Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by 1 for risk of bias: Soto 1995 did not describe how randomization and allocation concealment was assured; and had unclear risk of baseline bias because did not report how long soldiers in each arm were deployed to malaria endemic areas. Rowland 1999 did not describe the method used for allocation concealment. 2Downgraded by 1 for imprecision: the sample sizes and number of events are very small.
Summary of findings 3. Spatial repellents compared to placebo or no treatment for malaria prevention.
| Spatial repellents compared to placebo or no treatment for malaria prevention | ||||||
| Patient or population: malaria prevention Setting: malaria‐endemic regions Intervention: spatial repellents Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with Spatial repellents | |||||
| Parasitaemia Plasmodium spp. | 10 per 1000 |
2 per 1000 (0 to 18) |
RR 0.24 (0.03 to 1.72) |
6683 (2 studies) |
⊕⊝⊝⊝
VERY LOW1,2,3 Due to risk of bias, imprecision and inconsistency |
We do not know if spatial repellents protect against malaria. We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by 1 for risk of bias: Hill 2014 was not blinded. 2Downgraded by 1 for imprecision: Hill 2014 was underpowered and reported very few events (1/3349 in the intervention and 11/3270 in the control), and the CIs ranged from no effect to large benefits. Both studies were underpowered. 3Downgraded by 1 for inconsistency: there is considerable unexplained heterogeneity between trials (I² statistic = 46%)
Background
Description of the condition
Malaria is caused by protozoan parasites of the genus Plasmodium. The most severe form of the disease is caused by Plasmodium falciparum. Other Plasmodium species known to cause milder cases of malaria include Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae. The parasites are transmitted to people through the bite of an infected Anopheles mosquito. Malaria is widespread in tropical and subtropical regions and is considered endemic in 91 countries worldwide (WHO 2017). Symptoms of malaria include fever, chills, headache, and vomiting, and usually appear between 10 to 15 days after the bite of an infected mosquito. If left untreated, the person may develop severe complications and malaria can quickly become life‐threatening by disrupting the blood supply to vital organs. Diagnosis is done through identification of the Plasmodium parasite in the patient's bloodstream, usually by microscopic examination of a blood slide or malaria rapid diagnostic tests (mRDTs).
In the past decade, great advances have been made in the fight against malaria. From 2000 to 2016 global incidence of malaria fell by 40% and related mortality by 62% (WHO 2017). This is due to massive scale‐up of vector control interventions using long‐lasting insecticide‐treated bed nets (LLINs) and indoor residual spraying (IRS), as well as the introduction of mRDTs for better malaria diagnosis and use of highly effective artemisinin‐based combination therapies (ACTs). Despite these developments, an estimated three billion people living in 91 countries are still at risk of contracting malaria and 1200 children under five years old die every day in malaria‐endemic regions (WHO 2017). The World Health Organization's (WHO) Global Technical Strategy (GTS) aims to reduce global malaria incidence and mortality rates by 90% by 2030, with a milestone of at least 40% reduction by 2020. The GTS also set targets to eliminate the disease in at least 10 countries by 2020 and 35 countries by 2030. While the vector control component of most national malaria control programmes concentrates on distribution of LLINs and IRS, there is substantial malaria transmission within and outside Africa at times when people are outdoors (Durnez 2013). Recent estimates are that 10% of global malaria burden occurs outside Africa, with approximately 58% of P. vivax cases occurring in the WHO South‐East Asia Region (WHO 2017), where vectors are primarily early evening feeders (Sinka 2010; Sinka 2011). In order to achieve sustained malaria control and move towards malaria elimination, new tools will be required to interrupt transmission in environments where existing tools are not completely effective (malERA 2011). Residual malaria transmission is maintained by the presence of asymptomatic carriers, the significant number of non‐compliant LLIN users, early evening outdoor‐feeding Anopheles mosquitoes, and the spread of drug and insecticide resistance (White 2014). As well as preventing early evening bites, mosquito repellents may be suitable for people who have a high occupational risk of contracting malaria, such as: those working at night particularly in mining; soldiers; people in close contact with forest ecosystems; and migrants (Sangoro 2014b). It is well known that these high‐risk individuals 're‐seed' malaria in areas where vector control activities are carried out (Tatem 2010). With the impetus for malaria eradication of the past decade and the realization that the existing control tools alone cannot achieve this, mosquito repellents are increasingly being considered as supplementary tools in some malaria‐endemic settings (Sturrock 2013).
Description of the intervention
Personal protection has been used for centuries to prevent mosquito bites (Herodotus 1996). Historically, people burned repellent plants and applied essential oils directly to their skin or clothing. In recent times, manufacturers have developed more effective products that have largely replaced traditional methods. These products include mosquito coils, long‐lasting formulated repellent lotions, and insecticide treatments for clothing. Mosquito repellents are currently recommended by the WHO as the first‐line malaria‐prevention tool for travellers (WHO 2012), and they are commonly used by expatriates in tropical developing countries.
There are three main interventions that help prevent mosquito bites:
applying topical repellents directly to the skin;
wearing insecticide‐treated clothing (ITC);
using spatial repellents.
The mode of action of these three interventions on the mosquito is not the same; however they all result in preventing mosquito bites outside sleeping hours and so potentially reduce transmission of Plasmodium parasites from infected mosquitoes to humans.
Topical repellents
Topical repellents may contain a wide range of active ingredients and are available in various formulations in lotions, gels, roll‐ons, and on wipes. Repellents interfere with mosquitoes' olfactory reception, affecting their ability to locate and feed on a human host. Approved active ingredients for mosquito‐borne disease prevention are DEET (chemical name: N,N‐diethyl‐m‐toluamide or N,N‐diethyl‐3‐methyl‐benzamide); icaridin (KBR 3023 [Bayrepel] and picaridin inside the USA; chemical name: 2‐2‐hydroxyethyl‐1‐piperidinecarboxylic acid 1‐methylpropyl ester); PMD (para‐methane‐3,8‐diol); and IR3535 (chemical name: 3‐[N‐butyl‐N‐acetyl]‐aminopropionic acid, ethyl ester) (CDC 2014; WHO 2012). The Environmental Protection Agency (EPA) estimates that approximately 200 million people use DEET worldwide every year (WHOPES 1998).
ITC
ITC is widely used by military personnel to protect against vector‐borne diseases and biting nuisance (Kitchen 2009). The synthetic pyrethroid permethrin (2 g/m²) is used most commonly for treatment of clothing. Permethrin is approved by the WHO for this purpose because of its low dermal absorption, low mammalian toxicity, lack of odour and minimal irritation (WHOPES 2006). The mode of action of ITC is through contact irritancy, whereby mosquitoes make oriented movement away from the person after physical contact with the treated clothing surface; it also affects mosquitoes' feeding response. Both of these modes of action result in a reduction in mosquito bites to the person using the treated material.
Spatial repellents
Spatial repellents disperse active ingredients into the surrounding air that interfere with the mosquito's ability to find a host, thus preventing mosquitoes from taking a blood meal. They may interfere with host detection; or cause insects to fly in an undirected manner until they eventually move away from the source of repellent vapour (excito‐repellency). Spatial repellents create a protective area within a given radius and can be used to protect more than one person at the same time. Dispersal of the active ingredient can be done in two ways:
through heat, for example mosquito coils and electric emanators; or
through evaporation, for example passive emanators made of paper or agarose gel.
The most popular format is the mosquito coil and an estimated 45 to 50 billion mosquito coils are used annually by approximately two billion people worldwide, mainly in Southeast Asia (Zhang 2010). Mosquito coils are made from a mixture of inert ingredients, such as sawdust or coconut husks, and pigment. The coils burn at a low temperature dispersing the active ingredient, usually a volatile pyrethroid with a quick knock‐down action (for example, pyrethrin, D‐allethrin, transfluthrin, or metofluthrin). The smoke produced by the burning of mosquito coils can cause indoor air pollution.
Electric emanators consist of an electrical heating agent that vaporizes insecticide that has been impregnated into a pad or wick. These produce no smoke but require a source of electricity, which is not available in a large proportion of homes in malaria‐endemic countries.
Passive emanators do not require a source of heat or combustion. They have a large surface area which allows the passive dispersal of the volatile active ingredient into the air by evaporation. The chosen active ingredients are predominantly less polar compounds that are easily volatilized: examples include volatile pyrethroids such as metofluthrin and transfluthrin.
How the intervention might work
During the first Global Malaria Eradication Campaign the concept of vectorial capacity was developed and validated to mathematically evaluate the impact of mosquito‐control interventions on malaria transmission using several measurable field parameters (Garrett‐Jones 1964). Vectorial capacity is defined as: "the daily rate at which future inoculations of a parasite arise from a currently infective case, provided that all female vectors biting that case become infected" (Garrett‐Jones 1964). The original validation demonstrated that by reducing man‒vector contact (mosquito bites) by 50% there was a consequent 75% reduction in vectorial capacity. Man‒vector contact can be reduced by using repellents. Mosquitoes will be repelled or disabled from feeding on a person while being exposed to the repellent. These personal protective measures can be used at any time or location, and so are suitable for controlling mosquitoes biting outdoors and during early evening hours before people go to bed. Repellents might also protect individuals from other mosquito‐borne diseases such as dengue, Zika, and chikungunya.
Why it is important to do this review
The wide distribution of LLINs in malaria‐endemic countries has resulted in a considerable reduction of malaria incidence and prevalence throughout affected areas (WHO 2017). However residual malaria transmission, defined as the malaria transmission occurring despite universal coverage with effective IRS or LLINs, requires other vector control interventions, particularly outdoors and outside sleeping hours. It is estimated that in South America and Southeast Asia 80% of malaria transmission occurs before sleeping hours. Even in Africa, where Anopheles mosquitoes are traditionally late feeders, up to 20% of malaria transmission takes place during early evening and early morning hours (Sangoro 2014b). During this time the only available means of protection are repellents or ITC, thus these interventions might have the potential to reduce residual transmission. This Cochrane Review aimed to measure the effectiveness of these interventions — either alone or when combined with LLINs — in reducing the incidence of malaria, to facilitate decision makers considering the inclusion of repellents in national malaria control programmes. In addition, we believe that this review may be helpful in the pursuit of Goal 3 of the United Nations Sustainable Development Goals (SDGs), to ensure healthy lives and promote well‐being for all at all ages. The specific SDG 3 targets that this review addresses include:
by 2030, reduce the global maternal mortality ratio to less than 70 per 100,000 live births: pregnant women are more attractive to mosquitoes and therefore at a higher risk of infection than when the same women are not pregnant. In addition, pregnant women are particularly susceptible to complications of malaria. Modern repellents are safe to use among pregnant women and therefore have the potential to confer protection to a high‐risk group;
by 2030, end preventable deaths of newborns and children under five years of age, with all countries aiming to reduce neonatal mortality to at least as low as 12 per 1000 live births and under‐five mortality to at least as low as 25 per 1000 live births. Reducing the number of mosquito bites a child receives has been shown to lower the morbidity from malaria (Snow 1998). Repellents may also reduce other vector‐borne diseases as the most widely used repellents are broad spectrum and prevent bites from a range of disease vectors;
by 2030, end the epidemics of AIDS, tuberculosis, malaria, and neglected tropical diseases, and combat hepatitis, water‐borne diseases, and other communicable diseases: by directly reducing the human‐vector biting rate and reducing malaria transmission.
Objectives
To assess the impact of topical repellents, insecticide‐treated clothing (ITC), and spatial repellents on malaria transmission.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs) or cluster randomized controlled trials (cRCTs) with more than two units of randomization.
Types of participants
We included all adults and children living in malaria‐endemic areas.
Types of interventions
We included trials with or without LLINs in both trial arms.
Intervention
ITC impregnated with permethrin; or
topical repellents including DEET, icaridin, picardin, IR3535, and PMD; or
spatial repellents including transfluthrin coils, metofluthrin coils, D‐allethrin coils, pyrethrin coils, metofluthrin emanators, and transfluthrin emanators.
Control
Individuals given a placebo or no treatment.
Types of outcome measures
Primary outcomes
Clinical malaria: confirmed through blood smears or rapid diagnostic tests (P. falciparum or P. vivax);
malaria parasitaemia (malaria infection incidence): confirmed through thick or thin blood smears, mRDTs, or polymerase chain reaction (PCR) (P. falciparum or P. vivax).
Secondary outcomes
Anaemia (haemoglobin < 10 g/dL);
time to first infection (days);
all‐cause fever;
adherence to regular usage of the intervention measured through spot‐checking per period of time;
reduction in mosquitoes attempting to feed on humans;
recorded adverse events such as skin irritation, irritation of upper airways, nausea, and headache.
Search methods for identification of studies
We identified all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress) (Lefebvre 2011).
Electronic searches
We searched the following databases using the search terms and strategy described in Appendix 1: the Cochrane Infectious Diseases Group Specialized Register (up to 23 June 2017); MEDLINE (PubMed, 1966 to 26 June 2017); Embase (OVID, 1974 to 26 June 2017); CAB Abstracts (Web of Science, 1910 to 26 June 2017), and LILACS (1982 to 26 June 2017). We also searched the United States Armed Forces Pesticide Management Board website (US AFPMB; www.acq.osd.mil/eie/afpmb) on 12 August 2016; the WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/trialsearch); and ClinicalTrials.gov on 26 June 2017, using 'randomised controlled Trial', 'controlled clinical trial', 'mosquito*', 'Anopheles', 'malaria', 'DEET', 'PMD', 'IR3535', 'Icaridin', 'Metofluthrin', 'Transfluthrin', 'vaporizer mat*', 'electric emanator', insecticide treated clothing', 'ITC', 'personal protection', and 'repellen*' as search terms.
Searching other resources
Conference proceedings
We searched the following conference proceedings of the relevant abstracts:
MIM conference abstract booklets (2008 to present);
Annual ASTMH conference (2008 to present);
Entomological Society of America (2008 to present);
Society of Vector Ecology of America (2008 to present).
Organizations and pharmaceutical companies
We contacted organizations (including the WHO, Centers for Disease Control and Prevention (CDC), United States Department of Agriculture (USDA), United States Agency for International Development (USAID), US AFPMB, and Deployed War Fighter Protection Program (DWFP)) and chemical companies (including Bayer, Sumitomo, Vestergaard‐Frandsen, BASF, SC Johnson, Insect Shield, Mosiguard, Sara Lee, and Syngenta) for ongoing and unpublished trials.
Reference lists
We also checked the reference lists of all included trials for further relevant studies.
Data collection and analysis
Selection of studies
Two review authors (MM and MK) independently assessed the titles and abstracts of trials identified by the searches. The same two review authors assessed full‐text copies of potentially relevant trials for inclusion using an eligibility form based on inclusion criteria. They compared included trials, and resolved any disagreements by discussion and consensus, with arbitration when necessary by one or two more review authors (SJM and CL). We ensured that multiple publications of the same trial were only included once. We listed excluded studies, together with their reasons for exclusion, in table format.
Data extraction and management
Two review authors (MM and MV) independently extracted information from the trials using pre‐piloted, electronic data extraction forms. Differences in extracted data were discussed between both authors until a consensus was reached. In cases where a consensus could not be reached, further discussions were held involving one or two more authors (SJM and CL). In cases where missing data were identified, we contacted the original trial author(s) for clarification.
We extracted data on the following:
trial design: type of trial; method of participant selection; unit of randomization (for RCTs); adjustment for clustering for cRCTs; sample size; method of blinding of participants and personnel; diagnostic method; primary vector; vector biting time; malaria endemicity; Plasmodium species;
participants: trial settings and population characteristics; recruitment rates; withdrawal and loss to follow‐up;
intervention: description of intervention; co‐interventions; description of controls; time of follow‐up; passive or active case detection; compliance;
outcomes: definition of outcome; number of events; number of participants; power; unit of analysis; incomplete outcomes/missing data.
For dichotomous outcomes, we extracted the number of patients experiencing each outcome and the number of patients in each treatment group. For continuous outcomes, we extracted the mean and a measure of variance (standard error) for each treatment group.
For cRCTs we recorded the number of clusters randomized; number of clusters analysed; measure of effect (such as risk ratio, odds ratio, or mean difference) with confidence intervals (CIs) or standard deviations; number of participants; and the intra‐cluster correlation coefficient (ICC) value.
Assessment of risk of bias in included studies
Two review authors (MM and MK) independently assessed risk of bias for each included trial using the Cochrane's 'Risk of bias' tool (Higgins 2011). Any discrepancies were resolved through discussion or by consulting one or two more review authors (SJM and CL). We classified judgements of risk of bias as either 'low', 'high' or 'unclear', using summary graphs ('Risk of bias' summary and 'Risk of bias' graph) to display results.
We assessed each of the following components for each included RCT randomized by the individual and by cluster.
Sequence generation
We described the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it produced comparable groups. We regarded a trial as having a low risk of selection bias if the sequence generation was truly random (for example computer‐generated table of random numbers, tossing a coin); a high risk of bias if sequence generation was non‐random (for example alternate randomization, randomization by birth date); or an unclear risk of bias if the randomization process was not clearly described.
Balance
We assessed if both arms of the trial were equally balanced at baseline using criteria including age, gender, malaria indicators, socioeconomic status, housing, use of other interventions, knowledge about malaria transmission, and occupation.
Allocation concealment
We described the method used to conceal allocation to treatment groups before assignment. We regarded trials as having a low risk of selection bias if allocation was truly concealed (for example central allocation of participants; use of sequentially numbered, opaque, sealed envelopes; lottery system); a high risk of bias if the allocation process was not concealed (for example open randomization, unsealed or non‐opaque envelopes); or an unclear risk of bias if the process of concealing allocation was not described sufficiently to make a judgement.
Blinding of participants and personnel
We described whether blinding was present, who was blinded, and the methods used to blind trial participants and personnel. We regarded a trial as having a low risk of performance bias if blinding was present, or if the absence of blinding was unlikely to affect the outcomes; a high risk of bias if blinding was absent and likely to affect the results; or an unclear risk of bias if blinding was not clearly described.
Blinding of outcome assessors
Regarding blinding of outcome assessors: we described whether blinding of outcome assessors was present, and how they were blinded. We regarded a trial as having a low risk of detection bias if they were blinded to knowledge about which intervention the participants received; a high risk of bias if blinding was absent; or an unclear risk if blinding was not clearly described.
Incomplete outcome data
We described the percentage and proportion of patients who were lost to follow‐up; reasons for attrition; and whether attrition was balanced across groups or related to outcomes. We regarded trials as having a low risk of attrition bias if there were no missing data or if missing data were balanced across groups or clusters; high risk of bias if there were missing data or if missing data were more prevalent in one of the groups; or unclear risk of bias if it is unclear whether outcome data are missing.
Selective outcome reporting
We recorded any discrepancies between the pre‐specified outcomes in the Methods section and the outcomes reported, and identified outcomes that were measured but not reported on. We regarded a trial as having low risk of reporting bias if it was evident that all pre‐specified outcomes were reported on; high risk of bias if it was evident that not all pre‐specified outcomes were reported on; and unclear risk of bias if it was unclear whether all outcomes were reported on.
Incorrect analysis
We described whether the analysis was appropriate; whether an analysis plan was followed; and if it was adjusted for clustering.
Other bias
We described any important feature of included trials that could have affected the result.
In addition to the above, we assessed the following for each included cRCT.
Recruitment bias
Regarding recruitment bias, we described whether participants were recruited before or after randomization of clusters. We regarded trials as having low risk of recruitment bias if participants were recruited before randomization of clusters; high risk of bias if they were recruited after randomization; and unclear risk of bias if information about the timing of recruitment was unclear.
Loss of clusters
We described the number of clusters lost, as well as the reasons for attrition.
Compatibility with RCTs randomized by individuals
We noted whether the intervention effects may be systematically different from individually RCTs — that is, whether it was likely that the effect size was over‐ or underestimated.
Measures of treatment effect
We compared intervention and control data using risk ratios. All results were presented with their associated 95% confidence intervals (95% CIs). Data regarding reduction in mosquito bites was compared using mean difference and standard deviation.
Unit of analysis issues
We combined results from cRCTs with individually RCTs if they had adjusted for clustering in their analysis and presented results using forest plots. If there was no adjustment for clustering in RCTs, we adjusted data before combining it with data from individually RCTs. We adjusted the data by multiplying standard errors by the square root of the design effect (Higgins 2011). If the trial did not report the ICC value, we estimated the ICC from a similar trial, or by searching external sources for example ICCs. Regarding studies which measured malaria transmission through active case detection and reported results from multiple cross‐sectional studies, only data from the last cross‐sectional study was included in the meta‐analysis.
Dealing with missing data
In case of missing data, we applied available‐case analysis, only including data on the known results. The denominator used was the total number of participants who had data recorded for the specific outcome. For outcomes with no missing data, we carried out analyses on an intention‐to‐treat (ITT) basis. We included all participants randomized to each group in the analyses and analysed participants in the group to which they were randomized.
Assessment of heterogeneity
We inspected forest plots for overlapping CIs and assessed statistical heterogeneity in each meta‐analysis using the I² and Chi² statistics. We regarded heterogeneity as moderate if I² values are between 30% to 60%; substantial if they are between 59% to 90%; and considerable if they are between 75% to 100%. We regarded a Chi² test statistic with a P value less than or equal to 0.10 as indicative of statistically significant heterogeneity. We explored clinical and methodological heterogeneity through consideration of the trial populations, methods and interventions, and by visualization of trial results.
Assessment of reporting biases
In cases where 10 or more trials were included in each meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually, and used formal tests for funnel plot asymmetry (Harbord 2006). We explored reasons for asymmetry.
Data synthesis
We grouped trials and analysed by these interventions:
topical repellents;
ITC;
spatial repellents.
Within each group, we stratified by whether LLINs were included in both intervention and control groups.
We analysed data using Review Manager 5 (RevMan 5) software (Review Manager 2014). We used fixed‐effect meta‐analysis to combine data when heterogeneity was absent. If considerable heterogeneity was present, we combined data using random‐effects meta‐analysis and reported an average treatment effect. We decided whether to use fixed‐effect or random‐effects meta‐analysis based on the consideration of clinical and methodological heterogeneity between trials, as described previously.
Certainty of the evidence
We rated the certainty of the evidence using the GRADE approach (Guyatt 2011). Each important outcome was rated as follows, as described by Balshem 2011:
high: we are very confident that the true effect lies close to that of the estimate of the effect;
moderate: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect;
low: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect;
very low: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
RCTs start as high certainty evidence but can be downgraded if there are valid reasons within the following five categories: risk of bias, imprecision, inconsistency, indirectness, and publication bias. Studies can also be upgraded if there is a large effect; a dose‐response effect; and if all plausible residual confounding would reduce a demonstrated effect or would suggest a spurious effect if no effect was observed (Balshem 2011). We summarized our findings in a 'Summary of findings' table.
Subgroup analysis and investigation of heterogeneity
We explored reasons for substantial heterogeneity using subgroup analysis. We subgrouped trial data on clinical malaria and malaria parasitaemia based on whether the study had investigated the repellent intervention in combination with insecticide‐treated bed nets. We assessed differences between subgroups using the Chi² test, with a P value less than or equal to 0.05 indicating statistically significant differences between subgroups.
Sensitivity analysis
We performed sensitivity analysis on the primary outcome to see the effect of exclusion of trials at high risk of bias (for improper randomization methods and allocation concealment) on overall results. The same analysis was done to investigate whether the exclusion of being placebo‐controlled had an effect. If the ICC value was estimated, we carried out sensitivity analyses to investigate the impact of varying the ICC on results from the meta‐analysis.
We conducted three sensitivity analyses to test the robustness of our results:
sensitivity analysis 1: excluded trials at high risk of bias for improper randomization and allocation concealment;
sensitivity analysis 2: excluded non‐placebo controlled trials;
sensitivity analysis 3: varied the estimated ICC for trials that did not report ICC.
Results
Description of studies
Results of the search
We searched the available literature up to 26 June 2017 and identified 440 citations from the electronic database searches and three from other sources. We identified two duplicates. We screened 441 articles by title and abstract. We selected abstracts that potentially matched our inclusion criteria, and also articles where it was unclear whether or not they fulfilled the inclusion criteria, for full‐text assessment. We excluded 425 articles and identified 16 full‐text articles for further assessment. After full‐text assessment of these articles, we excluded and listed six articles; and we gave reasons for exclusion in the Characteristics of excluded studies table. Ten articles met the inclusion criteria and were included in the qualitative and quantitative synthesis. We have illustrated the study selection process in Figure 1.
1.
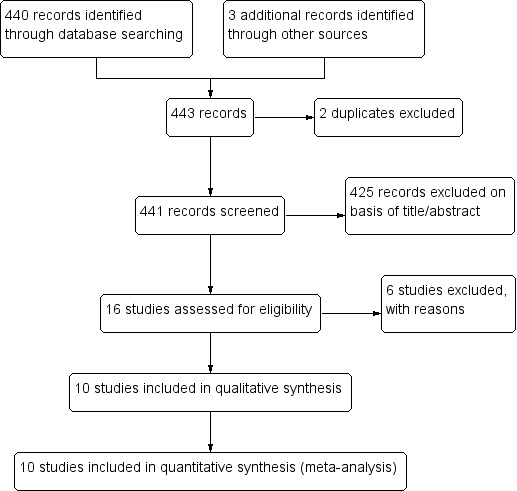
Study flow diagram.
Included studies
Two RCTs, McGready 2001 and Soto 1995, and eight cRCTs — Chen‐Hussey 2013, Hill 2007, Hill 2014, Rowland 1999, Rowland 2004, Sangoro 2014a, Sluydts 2016, and Syafruddin 2014 — met the inclusion criteria of this Cochrane Review. Data from McGready 2001 was obtained after we contacted the study author: the author provided the number of events (malaria cases) per treatment arm including the number of individuals who had more than one episode of P. falciparum or P. vivax. Only the first episode of P. vivax per participant was included in the analysis as individuals with multiple episodes of vivax malaria might suffer recurrent episodes of the same infection. We also contacted the authors of all the cRCTs that did not report ICC (Chen‐Hussey 2013; Hill 2007; Sangoro 2014a; Sluydts 2016): only one author provided the ICC used on their study (Sluydts 2016). The remaining studies, for which ICC was not available, were adjusted for clustering using an estimated ICC of 0.04 — obtained from Rowland 2004, a cRCT on topical repellents — as per protocol. Sensitivity analysis was done to evaluate if variation of the estimated ICC resulted in significant change to the main results and conclusions.
Six studies investigated the impact of topical repellent compared to placebo or no treatment (Chen‐Hussey 2013; Hill 2007; McGready 2001; Rowland 2004; Sangoro 2014a; Sluydts 2016). In total, 34,281 participants were included in the treatment arms and 33,016 in the control arms. The studies were conducted in a variety of countries: Laos (Chen‐Hussey 2013), Bolivia (Hill 2007), Thailand (McGready 2001), Pakistan (Rowland 2004), Tanzania (Sangoro 2014a), and Cambodia (Sluydts 2016). A variety of repellents and concentrations were used: 15% DEET (Chen‐Hussey 2013; Sangoro 2014a); 20% DEET (McGready 2001); 30% PMD (Hill 2007); 20% DEET and 0.5% permethrin (Rowland 2004); and picaridin (20% picaridin for adults and 10% picaridin for children) (Sluydts 2016). Three studies used LLINs as co‐interventions (Chen‐Hussey 2013; Hill 2007; Sangoro 2014a). Most studies included both children and adults in the population; however one study only included pregnant women (McGready 2001).
Two studies investigated the impact of ITC compared to placebo or no treatment (Rowland 2004; Soto 1995). In total, 524 individuals were in the treatment arms, and 473 individuals were in the control arms. One study was conducted with Afghan refugees in Pakistan (Rowland 1999); and the other with soldiers based in Colombia (Soto 1995). We extracted data from Rowland 1999 using inverse variance from adjusted odds ratio and confidence intervals reported in the article. The study follow‐up ranged from three to 16 weeks. Data from Soto 1995 on recorded adverse events included data from soldiers who were enrolled in the study and deployed in leishmania‐endemic regions (143 per arm). These individuals were not part of the component of the study investigating the effect of ITC on malaria incidence but because they also received the same treatments the results were included in the review regarding the outcome "recorded adverse events". No co‐interventions were used in either study.
Two studies investigated the impact of spatial repellents compared to placebo or no treatment (Hill 2014; Syafruddin 2014). One study was conducted in China with 1026 households in both the intervention and control arms. We extracted data from Syafruddin 2014 and Hill 2014 by using inverse variance from adjusted odds ratio and confidence intervals reported in the articles. The study conducted in China had a trial duration of six months and investigated 0.03% transfluthrin coils in combination with or without LLINs (Hill 2014). The other study, in Indonesia, was conducted for a period of 6 months and investigated 0.00975% metofluthrin coils. Both studies screened at start all participants enrolled for follow‐up and cleared pre‐existent malaria infections. Syafruddin 2014 was conducted in two villages with a total population of 2120 but only an active cohort of 170 participants was enrolled for follow‐up (87 in the control arm and 83 in the intervention arm). These individuals were screened and cleared at start but other villagers were not. The mosquito coils were rolled out to all village households according to treatment allocation (metofluthrin‐treated or placebo coils).
Excluded studies
Six studies were excluded: three studies only had two units of randomization (Abdulsalam 2014; Hamza 2016; Kimani 2006); one study did not specify in the published article the repellent compound that was used — we contacted the corresponding author but did not receive a response (Deressa 2014); and two were not RCTs or cRCTs (Dadzie 2013; Eamsila 1994).
Risk of bias in included studies
Overall the risk of bias in the included studies was high (see Figure 2).
2.
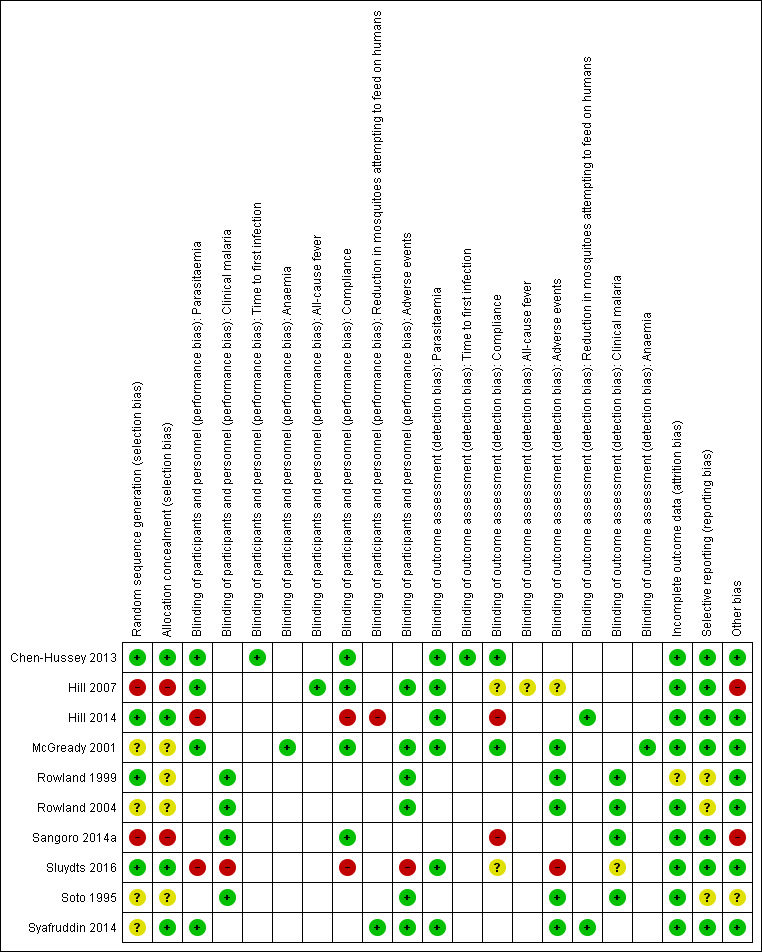
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Only three studies, Chen‐Hussey 2013, Hill 2014, and Sluydts 2016, described proper randomization and allocation concealment methods, and we therefore graded them as having low risk of selection bias. Rowland 1999 also used an adequate randomization method but did not clearly describe how allocation was performed. We considered two studies, Hill 2007 and Sangoro 2014a, to have high risk of selection bias because they used alternate allocation methods. All other studies — McGready 2001, Rowland 2004, Soto 1995, and Syafruddin 2014 — did not provide sufficient information to make a judgement about risk of bias and we judged them as having unclear risk of bias.
Blinding
Most trials were at low risk of bias and adequately blinded participants and personnel. We judged two trials, Hill 2014 and Sluydts 2016, to have high risk of performance bias as they were not placebo‐controlled. Sluydts 2016 did not provide sufficient information on how the clinical data regarding malaria cases were collected and was thus graded as having an unclear risk of bias.
Incomplete outcome data
All studies but one were graded as having low risk of attrition bias with comparable rates of loss to follow‐up between treatment arms. Rowland 1999 did not report on how many participants were lost to follow‐up from both intervention and control arm and was thus graded as having unclear risk of bias.
Selective reporting
Selective reporting bias was low for most studies. Three studies, Rowland 1999, Rowland 2004 and Soto 1995, did not have an available protocol and were therefore graded as having unclear risk of bias.
Other potential sources of bias
Two studies, Hill 2007 and Sangoro 2014a, described baseline imbalances which is considered as a potential source of bias. These two studies were assessed as having high risk of bias. One study, Soto 1995, described that soldiers were deployed to endemic areas for 3‐8 weeks but did not report deployment time per arm and so it was judged as having an unclear risk of baseline bias.
Effects of interventions
See: Table 1; Table 2; Table 3
The findings are presented by intervention type (topical repellents, ITC, and spatial repellents).
Comparison 1: topical repellents compared to placebo or no treatment for malaria prevention (see 'Summary of findings' table 1)
Clinical malaria caused by P. falciparum
Three studies investigated the impact on clinical malaria caused by P. falciparum (Rowland 2004; Sangoro 2014a; Sluydts 2016). Overall, topical repellents had no impact on clinical malaria (risk ratio (RR) 0.65, 95% confidence interval (CI) 0.40 to 1.07, 3 studies, 4447 participants, very low certainty evidence; Analysis 1.1) Figure 3. When sub‐grouped by inclusion of LLINs we found one study that, in the absence of LLINs, reported a significant reduction in clinical malaria (RR 0.40, 95% CI 0.23 to 0.71, 1 study, 869 participants). There was no significant impact on prevention of clinical malaria when LLINs were in place (RR 0.84, 95% CI 0.55 to 1.27, 2 studies, 3578 participants). Sensitivity analysis was done by excluding Sluydts 2016 which was not placebo controlled and Sangoro 2014a which had high risk of bias because of using an alternate allocation method. We also performed sensitivity analysis in regard to the estimated ICC of 0.04 (Sangoro 2014a), by varying this value between 0.03 and 0.05. The main results did not change and point estimates remained within the same values.
1.1. Analysis.
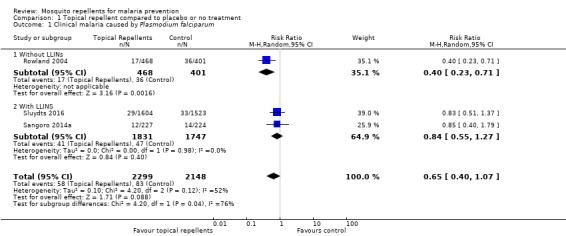
Comparison 1 Topical repellent compared to placebo or no treatment, Outcome 1 Clinical malaria caused by Plasmodium falciparum.
3.
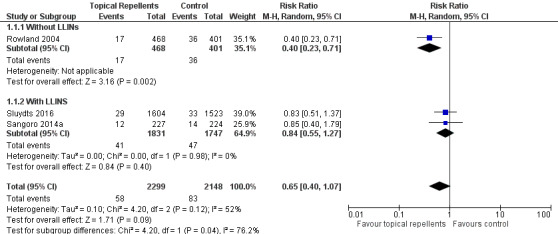
Forest plot of comparison: 1 Topical repellent compared to placebo or no treatment, outcome: 1.1 Clinical malaria caused by P. falciparum.
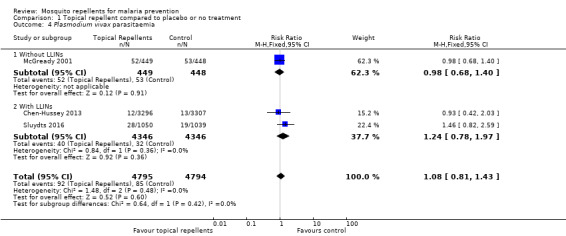
P. falciparum parasitaemia
Four studies investigated the impact on P. falciparum parasitaemia (Chen‐Hussey 2013; Hill 2007; McGready 2001; Sluydts 2016). Overall, topical repellents had no impact onP. falciparum parasitaemia (RR = 0.84, 95% CI 0.64 to 1.12, 4 studies, 13,310 participants, low‐certainty evidence; Analysis 1.2) Figure 4. There continued to be no impact on P. falciparum parasitaemia when used in conjunction with LLINs (RR 0.91, 95% CI 0.60 to 1.38, 3 studies, 12,413 participants) or without LLINs (RR 0.78, 95% CI 0.53 to 1.16, 1 study, 897 participants). We conducted a sensitivity analysis by excluding Sluydts 2016 as it was not placebo‐controlled: the point estimate remained the same. Point estimates were also narrowly affected by removing Hill 2007 from the analysis due to risk of bias for using alternate allocation. We also performed a sensitivity analysis by varying the ICC that was estimated for Chen‐Hussey 2013 and Hill 2007 of 0.04 between 0.03 and 0.05 and point estimates remained within the same values.
1.2. Analysis.

Comparison 1 Topical repellent compared to placebo or no treatment, Outcome 2 Plasmodium falciparum parasitaemia.
4.
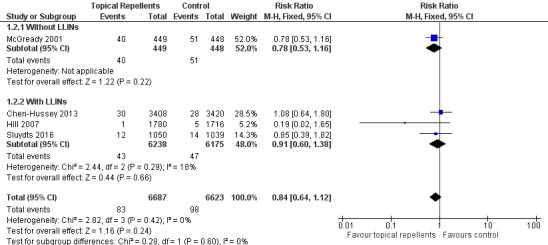
Forest plot of comparison: 1 Topical repellent compared to placebo or no treatment, outcome: 1.2 P. falciparum parasitaemia.
Clinical malaria caused by P. vivax
Two studies investigated the impact on clinical malaria for P. vivax (Rowland 2004; Sluydts 2016). Overall topical repellents had no impact on clinical malaria caused by P. vivax (RR 1.32, 95% CI 0.99 to 1.76, 2 studies, 3996 participants, low‐certainty evidence;Analysis 1.3) Figure 5. We conducted a sensitivity analysis by excluding Sluydts 2016, which was not placebo controlled. The point estimate remained close to 1 but shifted from favouring the control to favouring the intervention.
1.3. Analysis.
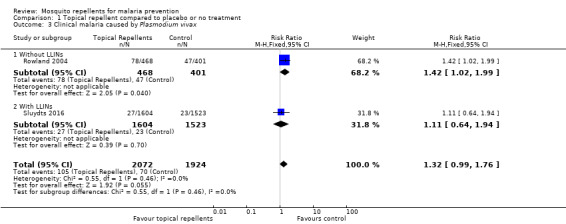
Comparison 1 Topical repellent compared to placebo or no treatment, Outcome 3 Clinical malaria caused by Plasmodium vivax.
5.
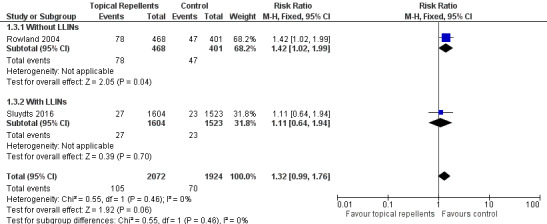
Forest plot of comparison: 1 Topical repellent compared to placebo or no treatment, outcome: 1.3 Clinical malaria caused by P. vivax.
P. vivax parasitaemia
Three studies investigated the impact on P. vivax parasitaemia (Chen‐Hussey 2013; McGready 2001; Sluydts 2016). Overall, topical repellents had no impact on P. vivax parasitaemia (RR 1.08, 95% CI 0.81 to 1.43, 3 studies, 9589 participants, low‐certainty evidence;Analysis 1.4) Figure 6. There continued to be no impact onP. vivax parasitaemia when used in conjunction with LLINs (RR 1.24, 95% CI 0.78 to 1.97, 2 studies, 8692 participants) or without LLINs (RR 0.98, 95% CI 0.68 to 1.40, 1 study, 897 participants). We conducted a sensitivity analysis by excluding Sluydts 2016, which was not placebo controlled. The main results did not change although point estimates shifted slightly in favour of the control. We also performed a sensitivity analysis by varying the ICC, estimated for Chen‐Hussey 2013 at 0.04, between 0.03 and 0.05: point estimates remained within the same values.
1.4. Analysis.
Comparison 1 Topical repellent compared to placebo or no treatment, Outcome 4 Plasmodium vivax parasitaemia.
6.
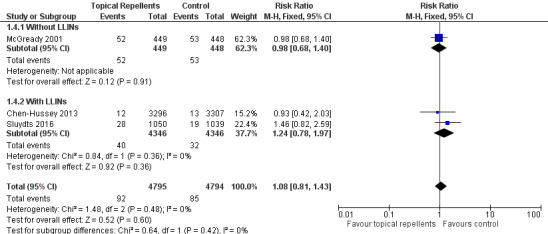
Forest plot of comparison: 1 Topical repellent compared to placebo or no treatment, outcome: 1.4 P. vivax parasitaemia.
Anaemia
One study investigated the impact on anaemia (McGready 2001). Topical repellents had no impact on anaemia (RR 1.06, 95% CI 0.91 to 1.23, 1 study, 587 participants; Analysis 1.5).
1.5. Analysis.
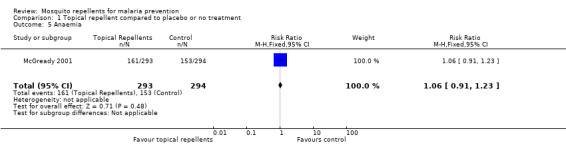
Comparison 1 Topical repellent compared to placebo or no treatment, Outcome 5 Anaemia.
All‐cause fever
One study investigated the impact on all‐cause fever (Hill 2007). Participants that used topical repellents were half as likely to develop a fever when compared to participant in the control arm (RR 0.44, 95% CI 0.35 to 0.55, 1 study, 3496 participants; Analysis 1.6).
1.6. Analysis.
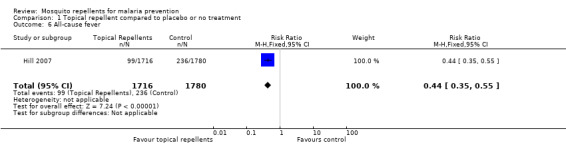
Comparison 1 Topical repellent compared to placebo or no treatment, Outcome 6 All‐cause fever.
Adherence to the intervention
Five studies reported adherence to the intervention (Chen‐Hussey 2013; Hill 2007; McGready 2001; Sangoro 2014a; Sluydts 2016). All five report self‐reported adherence, measured monthly or weekly or non‐periodically. Four studies report a variety of methods of objective monitoring of adherence: estimating weight of repellent bottles (Chen‐Hussey 2013; Hill 2007); random sniff checks or spot checks (Hill 2007; McGready 2001); number of bottles issued to households (Sangoro 2014a). Two studies reported the proportion of participants that have been adherent to the intervention. Chen‐Hussey 2013 reported 61.3% adherence in the intervention arm and 62.2% in the control arm. Hill 2007 reported 98.5% compliance in both arms (Analysis 1.7).
1.7. Analysis.
Comparison 1 Topical repellent compared to placebo or no treatment, Outcome 7 Adherence to the intervention.
| Adherence to the intervention | ||||
|---|---|---|---|---|
| Study | Follow up length | Method | Compliance repellent arm | Compliance treatment arm |
| Chen‐Hussey 2013 | Monthly | Self reporting Estimating weight of repellent bottles. |
61.3% | 62.2% |
| Hill 2007 | Monthly | Self reporting Random unanounced "sniff check" Estimating weight of repellent bottles. |
98.5% | 98.5% |
| McGready 2001 | Weekly | Self reporting Random spot checks |
Unclear | Unclear |
| Sangoro 2014a | Monthly | Self reporting Mean number of bottles issued to each household |
Unclear | Unclear |
| Sluydts 2016 | Non‐periodic | Self reporting Observational studies |
Unclear | Unclear |
Adverse events
Four studies reported adverse events (Hill 2007; McGready 2001; Rowland 2004; Sluydts 2016). Three studies used interviews to assess the occurrence of adverse events (Hill 2007; McGready 2001; Rowland 2004), of which one study also provided a questionnaire to a small sample of the study population (Rowland 2004). Sluydts 2016 did not describe the methods of measuring and recording adverse events. Very few adverse events were reported, and all related to skin irritation or warming sensation (Analysis 1.8). McGready 2001 reported the occurrence of adverse events to 6% of the participants but did not specify the nature of the adverse events or in which treatment arm they had occurred. We contacted the authors, and they informed us that all adverse events had been described as skin warming sensation and had been restricted to the study arm that had been allocated thanaka with 20% DEET. No serious adverse events were reported requiring intervention discontinuation.
1.8. Analysis.
Comparison 1 Topical repellent compared to placebo or no treatment, Outcome 8 Adverse events.
| Adverse events | ||||||
|---|---|---|---|---|---|---|
| Study | Follow up length | Method | Unit |
Description of adverse events |
Intervention arm | Control arm |
| Hill 2007 | Monthly surveys | Interview | Even per household | None reported | 0/424 | 0/436 |
| McGready 2001 | Weekly surveys | Interview | Unclear | 6% of the participants reported skin warming sensation. |
Unclear | Unclear |
| Rowland 2004 | End of trial | Interviews and questionnaires to 20 households from each treatment arm |
Event per household | Skin irritation | 1/20 | 0/20 |
| Sluydts 2016 | Unclear | Unclear | Unclear | Not described | 41/unclear | |
Other outcomes
No data were available for the comparisons of reduction in mosquito bites and time to first infection.
Comparison 2: ITC compared to placebo or no treatment for malaria prevention (see 'Summary of findings' table 2)
Clinical malaria caused by P. falciparum
Two studies investigated the impact on clinical malaria caused byP. falciparum (Rowland 1999; Soto 1995). Overall, ITC halved the incidence of clinical malaria (RR 0.49, 95% CI 0.29 to 0.83, 2 studies, 997 participants, low‐certainty evidence;Analysis 2.1; Figure 7). Results remained the same after we conducted a sensitivity analysis by excluding Rowland 1999 (cRCT).
2.1. Analysis.
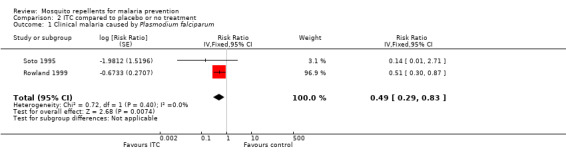
Comparison 2 ITC compared to placebo or no treatment, Outcome 1 Clinical malaria caused by Plasmodium falciparum.
7.
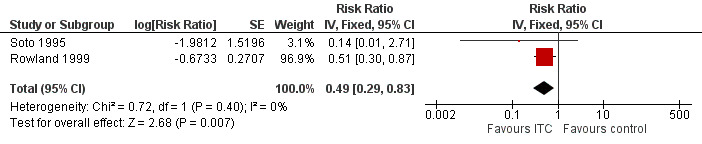
Forest plot of comparison: 2 ITC compared to placebo or no treatment, outcome: 2.1 Clinical malaria caused by P. falciparum.
Clinical malaria caused byP. vivax
Two studies investigated the impact on clinical malaria for P. vivax (Rowland 1999; Soto 1995). Overall, ITC reduced by 64% the risk of clinical malaria caused by P. vivax (RR 0.64, 95% CI 0.40 to 1.01, 2 studies, 997 participants, low‐certainty evidence;Analysis 2.2) Figure 8. After we carried out a sensitivity analysis by excluding Rowland 1999 (cRCT) results shifted in favour of the intervention but had wider confidence intervals, crossing the point estimate of no effect.
2.2. Analysis.
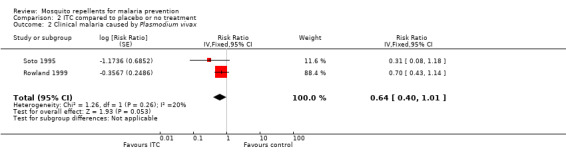
Comparison 2 ITC compared to placebo or no treatment, Outcome 2 Clinical malaria caused by Plasmodium vivax.
8.
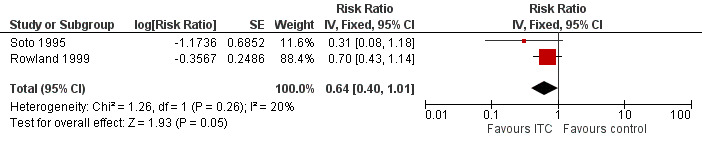
Forest plot of comparison: 2 ITC compared to placebo or no treatment, outcome: 2.2 Clinical malaria caused by P. vivax.
Adverse events
Two studies reported adverse events from interviews with participants (Rowland 1999; Soto 1995). Only two events of skin irritation were reported in the 997 participants across the two studies (Analysis 2.3). No serious adverse events requiring trial discontinuation were reported.
2.3. Analysis.
Comparison 2 ITC compared to placebo or no treatment, Outcome 3 Adverse events.
| Adverse events | ||||||
|---|---|---|---|---|---|---|
| Study | Follow up length | Method | Unit | Description of adverse events | Intervention arm | Control arm |
| Rowland 1999 | 16 weeks | Interview | Event per household | None reported | 0/438 | 0/387 |
| Soto 1995 | End of trial | Interview | Event per participant | Skin irritation | 2/229 | 0/229 |
Other outcomes
No data were available for the comparison of the following outcomes: P. falciparum parasitaemia, P. vivax parasitaemia, time to first infection, anaemia, all‐cause fever, adherence to the intervention, and reduction in mosquito bites.
Comparison 3: spatial repellents compared to placebo or no treatment for malaria prevention (see 'Summary of findings' table 3)
Plasmodium species' parasitaemia
Hill 2014 investigated the impact of spatial repellents on bothP. falciparum and P. vivax infections. Syafruddin 2014 did not differentiate between Plasmodium species and presented incidence numbers of malaria irrespective of causing agent. Both studies cleared P. vivax infections at start. In order to allow a meta‐analysis and compare data from both studies, we combined the data from Hill 2014 into total number of infections caused by Plasmodium species (13 cases in total: repellent arm reported 1 case of P. falciparum and the control arm reported 2 cases of P. falciparum and 10 of P. vivax). The papers reported results adjusted for clustering and we extracted these data and entered them in the analysis. Results from the meta‐analysis show that spatial repellents had no impact onPlasmodium species' parasitaemia (RR 0.24, 95% CI 0.03 to 1.72, 2 studies, 6683 participants, very low certainty evidence; Analysis 3.1) Figure 9.
3.1. Analysis.
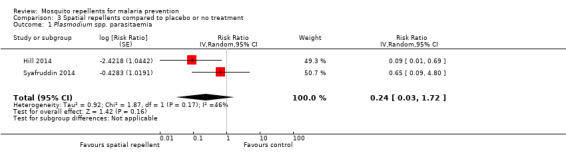
Comparison 3 Spatial repellents compared to placebo or no treatment, Outcome 1 Plasmodium spp. parasitaemia.
9.

Forest plot of comparison: 3 Spatial repellents compared to placebo or no treatment, outcome: 3.1 Plasmodium spp. parasitaemia.
Adherence to the intervention
One study — Hill 2014 — reported adherence to the intervention through self‐reporting and counting of empty coil boxes using a monthly survey. Hill 2014 reported compliance between 89.3% and 97.8% in the control arm and between 98.5% and 98.6% in the treatment arm (Analysis 3.2).
3.2. Analysis.
Comparison 3 Spatial repellents compared to placebo or no treatment, Outcome 2 Adherence to the intervention.
| Adherence to the intervention | ||||
|---|---|---|---|---|
| Study | Follow up length | Method | Compliance control arms | Compliance treatment arms |
| Hill 2014 | monthly survey | Self reporting Counting of empty coil boxes |
No treatment arm: 89.3% LLINs only arm: 97.8% |
Repellent coils arm: 98.6% Repellent coils + LLINs arm: 98.5% |
Reduction in mosquito bites
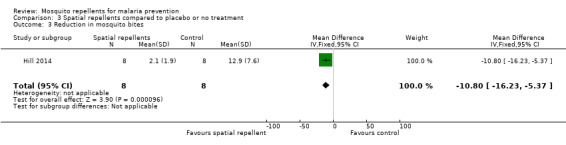
One study reported reduction in mosquito bites (Hill 2014). The mean number of bites was 2.1 in the spatial repellent arm (standard deviation (SD) 1.9) and 12.9 (SD 7.6) in the control arm (Analysis 3.3). Syafruddin 2014 also measured the reduction in mosquito bites caused by use of metofluthrin coils. Syafruddin 2014 reported a 32.9% reduction in mosquito landings in households using the metofluthrin coils, however the data presented in the article could not be extracted and added to the meta‐analysis.
3.3. Analysis.
Comparison 3 Spatial repellents compared to placebo or no treatment, Outcome 3 Reduction in mosquito bites.
Adverse events
One study investigated adverse events related to the use of mosquito coils (Syafruddin 2014). Participants were interviewed during random spot‐checks and asked if any adverse event had occurred. No adverse events were reported.
Other outcomes
No data were available for the comparison of the following outcomes: clinical malaria caused by P. falciparum or P. vivax; time to first infection; anaemia; and all‐cause fever.
Discussion
We have discussed the findings of the main outcomes by intervention type (topical repellents, ITC, and spatial repellents).
Summary of main results
Topical repellents
Results from the overall meta‐analysis indicate that the included trials did not demonstrate that topical repellents have a protective effect against clinical malaria caused by either P. falciparum or P. vivax (very low certainty evidence and low‐certainty evidence respectively). The same was observed in trials that used active case detection and measured the effect of topical repellents on malaria parasitaemia caused by P. falciparum or P. vivax (low‐certainty evidence). Regarding P. vivax infections, topical repellents may in fact increase the risk of infection. However, it is unclear if this result has a plausible biological explanation or if the finding was due to confounding factors. The most likely possible confounding factor is the recrudescent infections that may have been unbalanced between study arms because none of the studies investigating topical repellents cleared parasites at start. Subgroup analysis was undertaken to assess trials conducted with and without LLINs as co‐interventions. Only two studies, which were both conducted with displaced populations, did not include LLINs. Rowland 2004 reported fewer P. falciparum malaria cases in the intervention group given repellent soap (RR 0.40, 95% CI 0.23 to 0.71); however McGready 2001 measured no reduction in malaria infection incidence by either P. falciparum or P. vivax. Trials where LLINs were distributed to the participants and repellents were used as an additional protective generally reported no additional protection against malaria.
Compliance was an issue in the included studies (see Table 4). Most studies reported poor compliance or difficulty in reliably measuring compliance. Most studies used self‐reporting methods such as interviews and questionnaires or indirect methods such as weighing bottles or counting bottles of repellents. These methods are not reliable as participants may lie to please the investigating team (response bias), dispose of the repellent or share the repellent bottles with others. Sluydts 2016 conducted an observational study where compliance was evaluated in a pool of households from different clusters and observed compliance between 6% and 15%, as opposed to self‐reported compliance of 70%. Other studies — Chen‐Hussey 2013 and Sangoro 2014a — also reported difficulties measuring compliance. Self‐reported compliance was generally found to be high (> 80%); however the unreliable nature of the data might overestimate compliance. The issue of compliance may relate to product acceptability. Social studies showed that participants liked using topical repellents (Rowland 2004), but often forgot to use them or did not use them appropriately (Chen‐Hussey 2013). It is questionable if topical repellents can be used for malaria prevention in the general population as daily compliance and poor standardization (amount of repellent used, surface area applied, time of application, and period between repeated applications) are major limitations of this intervention. In addition, poor compliance leads to a decrease in study power and requires studies with very large numbers of participants which are also increasingly unfeasible as malaria prevalence drops across regions.
1. Assessment of compliance.
| Study | Intervention group | Design | Method of assessing compliance | Unit | Follow‐up time | Compliance level1 | |
| Intervention arm | Placebo arm | ||||||
| Chen‐Hussey 2013 | Topical repellent | cRCT | Self‐reported compliance. Self‐reported combined with an estimation of the proportion of lotion used by the participant by weighing the returned bottles. |
Percentage of self‐reported participants/night that adhered to the assigned treatment in a given month. Participants who reported to have used the repellent and confirmed by the weight of returned bottles. |
Monthly surveys | Moderate: 61.3% | Moderate: 62.2% |
| Hill 2007 | Topical repellent | cRCT | Self‐reported compliance through questionnaires combined with an estimation of the amount used by weighing the returned bottles, and verified by unannounced “sniff checks”. | Cumulative percentage of compliant households per month. A household was considered non‐compliant if they had reported to have not used the repellent 3 or more nights in a month or had more than 30 ml left in the bottle. |
Monthly surveys | High: 98.5% (119/8164) | High: 98.5% (110/7876) |
| Hill 2014 | Spatial repellent | cRCT | Daily recordings of compliance per household were reported by village leaders. Compliance was further confirmed by counting the number of empty mosquito coil boxes in each house. | Cumulative percentage of compliant households per month. A household was considered non‐compliant if it did not use the coils for 3 days or longer in one month. |
Monthly surveys | High No treatment arm: 89.3% LLIN arm: 97.8% |
High Repellent coils arm: 98.6% Repellent coils + LLINs: 98.5% |
| McGready 2001 | Topical repellent | RCT | Weekly self‐reporting and random spot checks. | Cumulative percentage of compliant participants per week. | Weekly surveys | Unclear Compliance was reported to be similar across treatment arms (P = 0.24) but was not reported for each arm. Self‐reported compliance: 90.5% (87,715/96,955) Compliance measured by spot checks: 84.6% (1918/2267) |
|
| Sangoro 2014a | Topical repellent | cRCT | Self‐reported compliance through questionnaires combined with an estimation of the amount used by counting the empty returned bottles. | Mean number of bottles of repellent issued to each household per month. | Monthly surveys | Unclear Authors stated that self‐reported data was unreliable so they used the data from the empty bottles to estimate compliance. Compliance was poorly reported. The authors reported mean number of bottles issued per household per month rather than estimating the compliance level for each treatment arm: Repellent arm: 6.73 bottles (95% CI 6.51 to 6.95) Placebo arm: 6.92 bottles (95% CI 6.68 to 7.16) |
|
| Sluydts 2016 | Topical repellent | cRCT | Self‐reported compliance was assessed using questionnaires during 3 surveys in October 2012, March 2013 and October 2013. The repellent consumption rate was measured per family every 2 weeks during the repellent distribution by visual inspection of the leftover repellent divided into categories (for example, empty, half full, full). A social science study was done to assess the acceptability and use of repellents in 10 selected clusters. |
Unit of measurement was not clearly defined. Self‐reported compliance is likely the percentage of compliant households during the survey period but was not defined in the article. The repellent consumption rate was not reported. Social study reported percentage of participants observed to comply with the application of the repellent from a small selection of 10 clusters in the intervention group. |
Non‐periodic surveys (in October 2012, March 2013 and October 2013) along the duration of the trial. | Self‐reported compliance was reported around 70%. However, observational studies reported compliance between 6% and 15% . |
No placebo |
1Levels of compliance: high: > 80%; moderate: 50% to 79%; low: < 50%.
The included studies were performed in diverse ecological and epidemiological settings (see Table 5), across hypo‐endemic regions (malaria prevalence < 5%) (Chen‐Hussey 2013; Hill 2007; Sluydts 2016), and meso‐endemic regions (malaria prevalence 5% to 15%) (McGready 2001; Rowland 2004; Sangoro 2014a), using both active and passive case detection and different diagnostic methods (see Table 6). We used malaria prevalence data from each study's control to calculate the necessary sample size and noted that except for Rowland 2004, which was only slightly underpowered, all other studies were severely underpowered. Even the very large trial that was conducted in Cambodia with over 48,000 participants was severely underpowered, effectively needing over half a million participants to reach its objectives (Sluydts 2016). Reasons included the very low prevalence of malaria in the study area (< 2%) as well as the large size of the clusters which reduced the effective sample size after adjusting for clustering. The sample size estimation that we calculated assumed 100% compliance, which is unrealistic, meaning that the sample sizes would need to be even larger. The sample size for a cRCT aiming to investigate the effect of topical repellents on malaria needs to be so large that its feasibility is questionable, making it arguable if RCTs and cRCTs are the best methodology. There is undeniable evidence from entomological studies that topical repellents can provide bite protection from mosquitoes and reduce vector‒human contact, making them a very efficient personal protection tool, but our review results conclude that, despite their high efficiency, topical repellents as an intervention might have very poor effectiveness with regard to malaria prevention.
2. Epidemiology of malaria and major vector of the study region.
| Study | Intervention | Design | Transmission intensity1,2 | Region | Main malaria vectors | Biting times | Efficacy of the intervention at repelling Anophelines tested at baseline? (V/N) |
| Chen‐Hussey 2013 | Topical repellent | cRCT | Hypoendemic 0.83% P. falciparum 0.4% P. vivax Measured through active case detection |
South East Asia ‐ Laos |
Anopheles dirus An. minimus An. maculatus |
From 18:00 to 2:00 with peak biting time from 21.00 to 02.00. | No |
| Hill 2007 | Topical repellent | cRCT | Hypoendemic 0.31% P. falciparum Measured through active case detection |
South America: Bolivian Amazon Region | An. darlingi | Peak biting activity between 8 p.m. and 10 p.m. | Yes Moore 2002 |
| Hill 2014 | Spatial repellent | cRCT | Hypoendemic 0.06% P. falciparum 0.28% P. vivax Measured through active case detection |
South East Asia: Yunnan Province of China |
An. sinensis An. minimus An. kochi An. splendidus An barbirostris An. vagus An. jeyporiensis An. annularis An. philippinsis An. tessallatus An. maculatus An. barbumbrosus An. dirus An culicifacies |
Given the diversity of vectors in the area the biting activity occurs from early evening extending to later in the night. | Yes |
| McGready 2001 | Topical repellent | RCT | Mesoendemic 11.4% P. falciparum 11.8% P. vivax Measured through active case detection |
South East Asia: Thailand | Not reported | Not reported | No |
| Rowland 1999 | Insecticide treated clothing | cRCT | Holoendemic 20.7% P. falciparum 17.6% P. vivax Measured through passive case detection |
North Western Pakistan |
An. nigerrimus An. subpictus An. stephensi |
Not reported | Yes |
| Rowland 2004 | Topical repellent | cRCT | Mesoendemic 8.9% P. falciparum 11.7% P. vivax Measured through passive case detection |
Asia: Pakistan |
An. culicifacies An. stephensi An. nigerrimus An. pulcherrimus |
Mosquito biting starts after dusk, peaks around 9 p.m. to 11 p.m. then declines gradually through the night. | Yes |
| Sangoro 2014a | Topical repellent | cRCT | Mesoendemic 6.22% P. falciparum Measured through passive case detection |
East Africa: Tanzania |
An gambiae s.s. An arabiensis |
Biting activity starts early evening and continues into the later hours of the night. | Yes Sangoro 2014c |
| Sluydts 2016 | Topical repellent | cRCT | Hypoendemic 1.33% P. falciparum 1.85% P. vivax Measured through active case detection |
Southeast Asia: Cambodia |
An. dirus s.s. An. maculatus An barbirostris An. minimus s.s. An. sawadwongporni An aconitus |
Early evening biting was common. | Yes Van Roey 2014 |
| Soto 1995 | Insecticide‐treated clothing | RCT | Mesoendemic for P.vivax and Hypoenemic for P. falciparum 3.4% P. falciparum 10.4% P. vivax Measured through passive case detection |
South America: Colombia | Unclear | Not reported | No |
| Syafruddin 2014 | Spatial repellent | cRCT | Holoendemic for Plasmodium spp. 70.1% Plasmodium spp. Measured through passive case detection |
Asia: Indonesia |
An. sundaicus An. subpictus s.l. An. indefinitus An. vagus An. barbirostris An. annularis An. maculatus An. aconitus An. kochi An. tessellatus |
Early evening biting was common with peaks between 18:00 and 20:00 continuing throughout the night. The high diversity of vectors also reflected diverse biting patterns. | Yes Barbara 2011 |
1Transmission intensity: holo‐endemic: malaria prevalence > 15%; meso‐endemic: malaria prevalence 5% to 15%; and hypo‐endemic: malaria prevalence < 5%. 2Calculated from prevalence in the control group.
3. Malaria diagnostic methods.
| Study | Intervention | Design | Diagnostic method | Validated | Plasmodium species in the region | Participants screened and cleared for vivax (Y/N) |
| Chen‐Hussey 2013 | Topical repellent | cRCT | mRDT | Yes, by PCR | 80% P. falciparum 20% P. vivax |
No |
| Hill 2007 | Topical repellent | cRCT | mRDT | No |
P. falciparum P. vivax |
No1 |
| Hill 2014 | Spatial repellent | cRCT | mRDTs | Yes, positive RDTs were validated through thick blood slide. | 32% P. falciparum 58% P. vivax |
Yes |
| McGready 2001 | Topical repellent | RCT | Blood smear | No |
P. falciparum P. vivax |
No |
| Rowland 1999 | Insecticide‐treated clothing | cRCT | Blood smear | No |
P. falciparum P. vivax |
No |
| Rowland 2004 | Topical repellent | cRCT | Blood smear | No |
P. falciparum P. vivax |
No |
| Sangoro 2014a | Topical repellent | cRCT | mRDT | Unclear if microscopy was used for validation of positive mRDTs | Mainly P. falciparum | No1 |
| Sluydts 2016 | Topical repellent | cRCT | PCR | No |
P. falciparum P. vivax |
No |
| Soto 1995 | Insecticide‐treated clothing | RCT | Blood smear | No |
P. falciparum P. vivax |
No |
| Syafruddin 2014 | Spatial repellent | cRCT | Blood smear | No |
P. falciparum P. vivax |
Yes |
1mRDT was only specific for P. falciparum.
ITC
Results from the meta‐analysis trials indicate that ITC may protect against clinical malaria caused by either P. falciparum or P. vivax (low‐certainty evidence). The studies were conducted with soldiers and refugees who did not have access to LLINs or other personal protection tools. Compliance with the intervention was not measured in either trial but it is highly likely to have been high, given the limited options of soldiers and refugees with regard to clothing. Also, studies reported that participants perceived additional protection from other insects, such as fleas and bedbugs, suggesting a high product acceptability.
Spatial repellents
A meta‐analysis of the outcome Plasmodium species' parasitaemia was performed. Both studies cleared P.vivax infections at start (Hill 2014; Syafruddin 2014). Results from the meta‐analysis did not demonstrate a protective effect of spatial repellents against acquiring Plasmodium species' parasitaemia (very low certainty evidence). There was a considerable heterogeneity between the studies and very large confidence intervals around the point estimates. The studies used two different volatile pyrethroids: Hill 2014 used transfluthrin 0.03% and Syafruddin 2014 used metofluthrin 0.00925%. However this difference between the studies is an unlikely explanation for the observed heterogeneity because both compounds were tested before trial start and reported to reduce vector biting rates (Barbara 2011; Hill 2014). Hill 2014 was severely underpowered and reported very few events — the study took place in an area with very low malaria transmission (see Table 5). Syafruddin 2014 was done in an area with higher malaria prevalence (see Table 5); however it only followed up 170 individuals (83 in the intervention arm and 87 in the control arm) and was also underpowered.
Overall completeness and applicability of evidence
Studies on topical repellents were undertaken in various malaria‐endemic countries (Bolivia, Cambodia, Laos, Pakistan, Tanzania, and Thailand) with malaria prevalence ranging between 0.31% and 11.4% forP. falciparum and 0.4% and 11.8% for P. vivax (see Table 5). Most studies were conducted with entire resident communities, involving adults and children of all ages. One study focused strictly on displaced pregnant women of Karen ethnicity in Thailand;and one study was conducted amongst Afghan refugees in a refugee camp in Pakistan. Some of the studies investigated topical repellents as complementary tool to LLINs. Given that LLINs are highly effective against malaria and are the backbone of all national malaria control programs, studies that did not include them may not be providing useful information to policy makers. However the included studies that did not use LLINs were conducted with vulnerable displaced populations and therefore the results may still be applicable to disaster situations or other situations where LLIN use may be compromised. Compliance may have affected the results of some of the included studies; however low compliance reflects what is likely to happen in the 'real world' and suggests that topical repellents may not be an option for malaria control programmes.
With regard to ITC, no study has been done in the general population. Both studies involved vulnerable populations: soldiers deployed in malaria‐endemic regions (Soto 1995); and refugees (Rowland 1999). These populations are exposed to a higher risk of malaria, potentially have lower immunity than resident populations of that endemic area, live in harsher conditions and potentially wash their clothing less frequently and differently compared to the general population. This might have implications on the efficacy and effectiveness of the intervention. It is arguable if the results from our meta‐analysis are applicable to the general population; further studies on civilian and undisplaced populations would be of interest to policy makers as ITC may to some extent reduce the risk of malaria. It is also important to evaluate the benefit of using ITC in combination with LLINs, as studies available so far did not include LLINs in their design and may not provide adequate information on the additional protection it may provide to populations who already use LLINs.
Two studies investigating spatial repellents met the inclusion criteria for this review. The studies were both conducted in Asia (China and Indonesia) amongst the general population. It is unclear if the studies could be representative of other regions.
This review focused on malaria; however, mosquito repellents may have a broader applicability in regard to protection from other vector‐borne diseases particularly transmitted by Aedes mosquitoes, such as dengue, chikungunya, and Zika viruses. An additional systematic review addressing this limitation would summarize the available evidence of the effect of this intervention on Aedes‐borne diseases.
Quality of the evidence
The results of the main outcomes were graded as either very low or low‐certainty evidence. We downgraded mainly due to risk of bias generated by improper methodologies for random sequence generation and allocation concealment; and by imprecision, as most studies were severely underpowered, estimates had wide confidence intervals, there were very few events, and the point estimate included the point of no effect (RR = 1). In the case of spatial repellents we also downgraded for inconsistency, as trials reported very different results, leading to a high degree of unexplainable heterogeneity.
Potential biases in the review process
We attempted to minimize bias in the review process by conducting a comprehensive search of published and unpublished literature, without language restrictions. Two review authors, who had no involvement in the included study, independently screened abstracts, extracted data and assessed risk of bias. We resolved any discrepancies by involving a third review author. We were unable to create funnel plots to assess reporting biases, since fewer than 10 RCTs/cRCT per intervention (topical repellents, ITC and spatial repellents) met the inclusion criteria.
Agreements and disagreements with other studies or reviews
A systematic review done by Wilson 2014 which included randomized and non‐RCTs on topical repellents concluded that these are unlikely to provide effective protection against malaria and called for further well‐designed trials. Our findings are in accordance with Wilson 2014, as we also conclude that there is insufficient evidence to make recommendations regarding topical repellents for malaria prevention. We did not find any other systematic review which aimed to investigate the effect of spatial repellents or ITC on malaria prevention.
Authors' conclusions
Implications for practice.
We are unable to make well‐informed recommendations with regard to including or not including topical repellents, ITC, or spatial repellents in malaria control programmes as the available evidence is low to very low certainty. The use of ITC in refugee camps or disaster situations may be useful as ITC provided some malaria prevention; however further research needs to be done in order to generate stronger evidence to support this.
Implications for research.
We conclude that there are insufficient well‐designed trials on topical repellents to draw evidence‐based conclusions and make well‐informed recommendations to policy makers regarding tropical repellents as a malaria prevention tool. However, there is lean evidence that the use of ITC may be useful in refugee camps or other disaster settings as they provided some malaria prevention in the absence of LLINs; further research needs to be done in order to generate stronger evidence to support this. There is a need to consider methodologies other than RCTs and cRCTs for the evaluation of malaria prevention methods such as topical repellents, ITC and spatial repellents at community level. Low compliance alongside decreasing malaria prevalence levels in potential study sites are major limitations for the design of future RCT or cRCTs because an unfeasible number of participants would need to be followed up to reach sufficient statistical power. All of the trials considered in this review were considered to be underpowered, including Sluydts 2016 which recruited over 20,000 participants per arm. Further studies on ITC involving general populations are needed to broaden the applicability of the results and to increase the certainty of the evidence. We also conclude that there are insufficient studies on spatial repellents to generate evidence‐based conclusions regarding spatial repellents for malaria prevention.
Acknowledgements
We are indebted to the mentorship of Prof. Paul Garner of the Liverpool School of Tropical Medicine.
This work was partly supported through a grant from the Global Malaria Programme, World Health Organization.
MM was supported by a fellowship offered by the Effective Health Care Research Consortium, supported by Cochrane South Africa and the South African Medical Research Council, to attend a five‐day workshop in Cape Town to work on this review. This Consortium and the editorial base for the Cochrane Infectious Diseases Group is funded by UKaid from the UK government for the benefit of low‐ and middle‐income countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
Appendices
Appendix 1. Search strategies
MEDLINE (PubMed)
| Search | Query |
| #21 | Search (#20) AND #17 |
| #20 | Search (#19) OR #18 |
| #19 | Search "Randomised Controlled Trial" [Publication Type] OR "Controlled Clinical Trial" [Publication Type] |
| #18 | Search randomised OR placebo OR randomly OR groups OR trial Field: Title/Abstract |
| #17 | Search (#16) AND #8 |
| #16 | Search ((((((#9) OR #10) OR #11) OR #12) OR #13) OR #14) OR #15) |
| #15 | Search “vaporizer mat*” Field: Title/Abstract |
| #14 | Search “personal protection*” Field: Title/Abstract |
| #13 | Search "impregnated cloth*" Field: Title/Abstract |
| #12 | Search “electric emanator*” Field: Title/Abstract |
| #11 | Search Spray OR sprays OR lotion* OR gel OR gels OR roll‐on* OR wipe* Field: Title/Abstract |
| #10 | Search "insecticide treated clothing" OR ITC Field: Title/Abstract |
| #9 | Search repellen* Field: Title/Abstract |
| #8 | Search ((#5) OR #6) OR #7 |
| #7 | Search "Anopheles"[Mesh] |
| #6 | Search "Mosquito Control"[Mesh] |
| #5 | Search (#4) AND #1 |
| #4 | Search (#2) OR #3 |
| #3 | Search ("Insect Vectors"[Mesh]) |
| #2 | Search vector* OR mosquito* Field: Title/Abstract |
| #1 | Search malaria Field: Title/Abstract |
Cochrane Library
#1 "malaria":ti,ab,kw (Word variations have been searched)
#2 vector* or mosquito*:ti,ab,kw (Word variations have been searched)
#3 MeSH descriptor: [Insect Vectors] explode all trees
#4 MeSH descriptor: [Anopheles] explode all trees
#5 #2 or #3 or #4
#6 #1 and #5
#7 MeSH descriptor: [Mosquito Control] explode all trees
#8 #6 or #7
#9 Spray or sprays or lotion* or gel or gels or roll‐on* or wipe* or repellen* or coil*:ti,ab,kw
#10 "insecticide treated clothing" or ITC:ti,ab,kw
#11 "passive emanator*" ti,ab,kw
#12 "electric emanator*" ti,ab,kw
#13 "vaporizer mat*" ti,ab,kw
#14 "personal protection" ti,ab,kw
#15 #9 or #10 or #11 or #12 or #13 or or #14
#16 #8 and #15
Embase (OVID)
| Results | Search Type |
| 1 | malaria.mp. or malaria/ |
| 2 | insect vectors.mp. or disease carrier/ |
| 3 | mosquito/ or mosquito.mp. |
| 4 | Anopheles/ |
| 5 | 2 or 3 or 4 |
| 6 | 1 and 5 |
| 7 | mosquito control.mp. |
| 8 | 6 or 7 |
| 9 | insect repellent/ or insecticide treated clothing.mp. |
| 10 | (coil* or spray or sprays or lotion* or gel or gels or roll‐on* or wipe*).ab. or (coil* or spray or sprays or lotion* or gel or gels or roll‐on* or wipe*).ti. |
| 11 | passive emanator*.ab. or passive emanator*.ti. |
| 12 | electric emanator*.ab. or electric emanator*.ti. |
| 13 | vaporizer mat*.ab. or vaporizer mat*.ti. |
| 14 | personal protection.ab. or personal protection.ti. |
| 15 | 9 or 10 or 11 or 12 or 13 or 14 |
| 16 | 8 and 15 |
| 17 | clinical trial/ |
| 18 | randomised controlled trial/ |
| 19 | 17 or 18 |
| 20 | randomisation/ |
| 21 | (single blind* or double blind*).mp. |
| 22 | random allocation.mp. |
| 23 | randomly allocated.mp. |
| 24 | cluster randomised.mp. |
| 25 | 17 or 18 or 20 or 21 or 22 or 23 or 24 |
| 26 | 16 and 25 |
CABI: CAB Abstracts®
| # 5 | #4 AND #3 Timespan=All years Search language=Auto |
| # 4 |
TOPIC: (randomised OR double‐blind* or single‐blind*OR placebo OR randomly) Timespan=All years Search language=Auto |
| # 3 | #2 AND #1 Timespan=All years Search language=Auto |
| # 2 |
TOPIC: (Spray or sprays or lotion* or gel or gels or roll‐on* or wipe* O repellen* or coil*) ORTOPIC: (insecticide treated clothing) ORTOPIC: (vaporizer mat*) ORTOPIC: (personal protection) Timespan=All years Search language=Auto |
| # 1 |
TOPIC: (malaria) ANDTOPIC: (vector* OR mosquito* OR anopheles) Timespan=All years Search language=Auto |
LILACS
| Database : | LILACS |
| Search on : | malaria and (mosquito$ or vector$) [Words] and repellent$ or spray$ or coils or emanator$ or vaporizer$ or clothing [Words] and randomised or trial or controlled or placebo [Words] |
Data and analyses
Comparison 1. Topical repellent compared to placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical malaria caused by Plasmodium falciparum | 3 | 4447 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.40, 1.07] |
| 1.1 Without LLINs | 1 | 869 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.23, 0.71] |
| 1.2 With LLINS | 2 | 3578 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.55, 1.27] |
| 2 Plasmodium falciparum parasitaemia | 4 | 13310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.64, 1.12] |
| 2.1 Without LLINs | 1 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.16] |
| 2.2 With LLINs | 3 | 12413 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.60, 1.38] |
| 3 Clinical malaria caused by Plasmodium vivax | 2 | 3996 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.99, 1.76] |
| 3.1 Without LLINs | 1 | 869 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.02, 1.99] |
| 3.2 With LLINs | 1 | 3127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.64, 1.94] |
| 4 Plasmodium vivax parasitaemia | 3 | 9589 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.81, 1.43] |
| 4.1 Without LLINs | 1 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.68, 1.40] |
| 4.2 With LLINs | 2 | 8692 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.78, 1.97] |
| 5 Anaemia | 1 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.91, 1.23] |
| 6 All‐cause fever | 1 | 3496 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.35, 0.55] |
| 7 Adherence to the intervention | Other data | No numeric data | ||
| 8 Adverse events | Other data | No numeric data |
Comparison 2. ITC compared to placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical malaria caused by Plasmodium falciparum | 2 | Risk Ratio (Fixed, 95% CI) | 0.49 [0.29, 0.83] | |
| 2 Clinical malaria caused by Plasmodium vivax | 2 | Risk Ratio (Fixed, 95% CI) | 0.64 [0.40, 1.01] | |
| 3 Adverse events | Other data | No numeric data |
Comparison 3. Spatial repellents compared to placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Plasmodium spp. parasitaemia | 2 | Risk Ratio (Random, 95% CI) | 0.24 [0.03, 1.72] | |
| 2 Adherence to the intervention | Other data | No numeric data | ||
| 3 Reduction in mosquito bites | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐10.8 [‐16.23, ‐5.37] |
| 4 Adverse events | Other data | No numeric data |
3.4. Analysis.
Comparison 3 Spatial repellents compared to placebo or no treatment, Outcome 4 Adverse events.
| Adverse events | ||||||
|---|---|---|---|---|---|---|
| Study | Follow‐up length | Method | Unit | Description of adverse events | Intervention arm | Control arm |
| Syafruddin 2014 | 6 months | Interviews | Random spot‐checks | None described | None reported | None reported |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen‐Hussey 2013.
| Methods | Cluster randomized controlled trial (RCT) Unit of randomization was household. Intra‐cluster correlation coefficient (ICC) was not reported. Trial duration: up to 8 months' follow‐up in 2009 and 2010 |
|
| Participants | Adults or children living in endemic regions of Laos in Attapeu Sekong Provinces. Participants were not screened at start for P. vivax. |
|
| Interventions | Topical repellent: 15% DEET and placebo Co‐interventions: LLINs Treatment arms: ‐ Repellent arm: 795 households; 3972 participants; and ‐ Placebo arm: 802 households; 4008 participants. |
|
| Outcomes | ‐ Participants with malaria parasitaemia confirmed through mRDTs (P. falciparum or P. vivax); ‐ Time to first infection (mean time in person/months to first malaria infection); and ‐ Self‐reported adherence to regular usage of the intervention. |
|
| Notes | Conducted in Laos. Trial registration number: NCT00938379 Funded by Population Services International. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Equal group allocation, stratified by village. Heads of households picked treatment codes through lottery system. |
| Allocation concealment (selection bias) | Low risk | Heads of households picked treatment codes out of a bowl. |
| Blinding of participants and personnel (performance bias) Parasitaemia | Low risk | The treatment allocation was blinded to both participants and field staff. |
| Blinding of participants and personnel (performance bias) Time to first infection | Low risk | The treatment allocation was blinded to both participants and field staff. |
| Blinding of participants and personnel (performance bias) Compliance | Low risk | The treatment allocation was blinded to both participants and field staff. |
| Blinding of outcome assessment (detection bias) Parasitaemia | Low risk | Assessment of parasitaemia or time to first infection are objective outcomes. “Field staff carrying out randomisation and follow‐up surveys and trial staff performing data entry and analysis were blinded for the length of the trial." |
| Blinding of outcome assessment (detection bias) Time to first infection | Low risk | Assessment of parasitaemia or time to first infection are not biased because these are objective outcomes. |
| Blinding of outcome assessment (detection bias) Compliance | Low risk | The treatment allocation was blinded to both participants and field staff. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attrition between 2 groups: 11.7% in intervention and 13.2% in control groups were lost to follow‐up/excluded/withdrew. |
| Selective reporting (reporting bias) | Low risk | Primary outcome was reported as per protocol. Secondary outcomes included all‐cause fever, but this was not reported; however it is non‐essential information for this study. The data presented on compliance was self‐reported, there was no reporting of compliance measured through "sniff‐checks" although it was described in the Methods section. |
| Other bias | Low risk | Baseline imbalance Study arms had similar baseline characteristics. |
Hill 2007.
| Methods | Cluster‐RCT Unit of randomization: household ICC was not reported. Trial duration: 6 months from March to September 2003. |
|
| Participants | Adults or children living in malaria‐endemic area | |
| Interventions | Topical repellent lotion containing 30% PMD versus placebo lotion. Co‐interventions: LLINs Treatment arms: ‐ Repellent arm (30% PMD) + LLINs: 424 households (1967 individuals) ‐ Placebo arm + LLINs: 436 households (2041 individuals) |
|
| Outcomes | ‐ Participants with malaria parasitaemia confirmed through mRDTs (specific to P. falciparum); ‐ All‐cause fever; ‐ Self‐reported adherence to regular usage of the intervention; and ‐ Recorded adverse events. |
|
| Notes | Conducted in the Bolivian Amazon, Vaca Diez and Pando Provinces Trial registration number: NCT00144716 Funded by Gates Malaria Partnership grant from London School of Hygiene and Tropical Medicine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generation was not random. “Field staff followed the strict inclusion criteria to randomise participants at the household level following a basic sequential alternate A/B/A/B regimen. Field staff and study participants were blind to the group allocation.” |
| Allocation concealment (selection bias) | High risk | Sequence generation was alternated. Personnel knew which treatment was given next. “Field staff followed the strict inclusion criteria to randomise participants at the household level following a basic sequential alternate A/B/A/B regimen. Field staff and study participants were blind to the group allocation.” |
| Blinding of participants and personnel (performance bias) Parasitaemia | Low risk | Field staff and participants were blinded to the treatment allocation. |
| Blinding of participants and personnel (performance bias) All‐cause fever | Low risk | Field staff and participants were blinded to the treatment allocation. |
| Blinding of participants and personnel (performance bias) Compliance | Low risk | Field staff and participants were blinded to the treatment allocation. |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk | Field staff and participants were blinded to the treatment allocation. |
| Blinding of outcome assessment (detection bias) Parasitaemia | Low risk | Primary outcome is objective (mRDT result), so although it is not described if the outcome assessor is blinded, lack of blinding was unlikely to bias the results. |
| Blinding of outcome assessment (detection bias) Compliance | Unclear risk | Blinding of outcome assessment for adherence to intervention is unclear. |
| Blinding of outcome assessment (detection bias) All‐cause fever | Unclear risk | Blinding of outcome assessment for all‐cause fever is unclear. |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Blinding of outcome assessment for adverse events is unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants lost to follow‐up was similar between treatment arms. |
| Selective reporting (reporting bias) | Low risk | All the outcomes set to be measured were reported. |
| Other bias | High risk | Baseline imbalance “There were no significant differences in most household characteristics (number of household members, roof material, water source, heating source, or possession of electricity, fridge, and radio) between the two groups (data not shown), but households allocated to the repellent group were slightly more likely to own a television than those allocated to the placebo group (P=0.056) (table 1). There were also no significant differences in age or sex between the groups but at baseline more participants in the repellent group were positive for P. falciparum (P=0.065) (table 1).” |
Hill 2014.
| Methods | Cluster‐RCT Unit of randomization: household ICC is not reported. Trial duration: 1 month baseline and 6 months' intervention from April to October 2007. |
|
| Participants | Adults or children living in an endemic region Participants were screened forP. vivax and parasites were cleared at start. |
|
| Interventions | Mosquito coils (0.03% transfluthrin) and no treatment. Co‐interventions: LLINs Treatment arms: ‐ Control (no treatments) arm ‒ 513 households ‐ 0.03% transfluthrin coils arm ‒ 512 households ‐ LLINs arm ‒ 513 households ‐ LLINs + 0.03% transfluthrin coils arm ‒ 514 households |
|
| Outcomes | ‐ Participants with malaria parasitaemia confirmed through mRDTs (P. falciparum or P. vivax) and verified by external microscopist through thick film; ‐ Adherence to regular usage of the intervention measured through village leaders' reports and self‐reporting; and ‐ Reduction in indoor density of mosquitoes measured through collections using CDC light traps indoor households from the four treatment arms (monthly arithmetic mean of mosquito densities). |
|
| Notes | Conducted in rural areas of China in the Ruili County, Yunnan Province, close to the Myanmar border. Trial registration number: NCT00442442 Funded by SC Johnson |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was done using lottery system: “Households enrolled at baseline were randomly allocated by the lottery method to one of the four intervention arms (i) nothing, (ii) coils alone, (iii) LLINs alone or (iv) coils and LLINs.” |
| Allocation concealment (selection bias) | Low risk | Allocation was done using lottery system so allocation was concealed: “Households enrolled at baseline were randomly allocated by the lottery method to one of the four intervention arms (i) nothing, (ii) coils alone, (iii) LLINs alone or (iv) coils and LLINs.” |
| Blinding of participants and personnel (performance bias) Parasitaemia | High risk | Participants and field staff were not blinded. Participants may have changed their behaviour if they knew to which treatment they had been allocated. “Field workers and participants were not blinded to treatment allocation, as this was impossible in practice. However, the field staff collecting monthly RDT data were not aware of the intervention which individuals had been using thus achieving single blinding (investigator) of the study.” |
| Blinding of participants and personnel (performance bias) Compliance | High risk | Participants and field staff were not blinded. Participants may have changed their behaviour by knowing the treatment they had been allocated to. "Field workers and participants were not blinded to treatment allocation, as this was impossible in practice. However, the field staff collecting monthly RDT data were not aware of the intervention which individuals had been using thus achieving single blinding (investigator) of the study.” ” …the untreated control group continued to use their own personal protection methods. It would be unethical to ask anyone not to do this but a record was kept of such ad‐hoc coil use in the negative control group and those reporting the use of one box or more (10 coils/5 nights) were excluded from the analysis for that round.(...) Conversely, those in the control arm were less likely to follow the request of the study directors to not use any intervention, with 13‐19% using local coils for 3 or more days in the month prior to the survey.” |
| Blinding of participants and personnel (performance bias) Reduction in mosquitoes attempting to feed on humans | High risk | The team collecting the mosquitoes could have been biased if they knew which houses belonged to each treatment. |
| Blinding of outcome assessment (detection bias) Parasitaemia | Low risk | Staff assessing parasitaemia were blinded. "However, the field staff collecting monthly RDT data were not aware of the intervention which individuals had been using thus achieving single blinding (investigator) of the study.Furthermore, microscopist’s at Yunnan Institute of Parasitic diseases that verified positive RDTs by microscopy and the statistician was blind to the allocation.” |
| Blinding of outcome assessment (detection bias) Compliance | High risk | Compliance was measured indirectly through counting of empty boxes of coils. |
| Blinding of outcome assessment (detection bias) Reduction in mosquitoes attempting to feed on humans | Low risk | Data is objective therefore the risk of detection bias is low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was less than 2% in all treatment arms. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | Baseline imbalance Study arms had similar baseline characteristics. |
McGready 2001.
| Methods | RCT Trial duration: 17 months between April 1995 and September 1996. |
|
| Participants | Participants were women who were 3 to 7 months' pregnant. Participants were not screened at start for P. vivax. |
|
| Interventions | 20% DEET added to Thanaka (popular local cosmetic) compared to Thanaka alone. Co‐intervention: none Treatment arms: ‐ Thanaka containing 20% DEET arm ‒ 449; and ‐ Thanaka arm ‒ 448. |
|
| Outcomes | ‐ Participants with malaria parasitaemia confirmed through blood smears (P. falciparum andP. vivax); ‐ Adherence to regular usage of the intervention measured through self‐reporting; ‐ Anaemia; and ‐ Recorded adverse events. |
|
| Notes | The study was carried out in camps for displaced people of the Karen ethnic minority in endemic regions of Thailand. The project was funded by the Danish Bilharziasis Laboratory and was part of the Wellcome‒Mahidol University of Oxford Tropical Medicine Research Programme funded by the Wellcome Trust. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Parasitaemia | Low risk | Double‐blinded RCT, both personnel and participants were blinded to the intervention. |
| Blinding of participants and personnel (performance bias) Anaemia | Low risk | Double‐blinded RCT, both personnel and participants were blinded to the intervention. |
| Blinding of participants and personnel (performance bias) Compliance | Low risk | Double‐blinded RCT, both personnel and participants were blinded to the intervention. |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk | Double‐blinded RCT, both personnel and participants were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Parasitaemia | Low risk | Double‐blinded RCT, both personnel and participants were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Compliance | Low risk | Double blinded RCT, both personnel and participants were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Adverse events | Low risk | Double‐blinded RCT, both personnel and participants were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Anaemia | Low risk | This is an objective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition between arms was similar, data was not reported in the published but retrieved through communication with the author. |
| Selective reporting (reporting bias) | Low risk | Reporting was not clear in the published article but data of events between treatment arms was sent to us after communicating with the author. |
| Other bias | Low risk | Baseline imbalance "Between April 1995 and September 1996, 897 pregnant women were enrolled in the study, 449 into the DET and thanaka group and 448 into the thanaka alone group with no difference in baseline characteristics" |
Rowland 1999.
| Methods | Cluster‐RCT Unit of randomization: household ICC was not reported. Trial duration: 16 weeks from July to November 1996 |
|
| Participants | Adults or children living in malaria‐endemic regions Participants were not screened at start for P. vivax. |
|
| Interventions | Treated clothing in the form of chaddars (permethrin 0.1 mg/cm²) versus placebo Co‐interventions: none Treatment arms: ‐ Treated chaddar arm: 51 households (438 individuals) ‐ Placebo arm: 51 households (387 individuals) |
|
| Outcomes | ‐ Participants with clinical malaria confirmed through blood smears or rapid diagnostic tests (P. falciparum or P. vivax); and ‐ Recorded adverse events. |
|
| Notes | Trial was conducted with Afghan refugees in Adizai settlement in north‐western Pakistan. Funded by HealthNet International’s Malaria and Leishmaniasis control and research programme. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator used against list of health centre family registration cards. “To achieve this sample size, 20% of refugee households were selected using a random number generator against the list of health centre family registration cards.” |
| Allocation concealment (selection bias) | Unclear risk | Not described. “Selected households were randomly divided into intervention and placebo groups, and if more than one family lived in a single house all families therein were allocated to the same treatment group.” |
| Blinding of participants and personnel (performance bias) Clinical malaria | Low risk | Participants and staff were blinded. “Field workers were under the assumption that both placebo and permethrin were effective. Health centre staff did not know which families were in which group.” |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk | Participants and staff were blinded. “Field workers were under the assumption that both placebo and permethrin were effective. Health centre staff did not know which families were in which group.” |
| Blinding of outcome assessment (detection bias) Adverse events | Low risk | “Health centre staff did not know which families were in which group" |
| Blinding of outcome assessment (detection bias) Clinical malaria | Low risk | “Health centre staff did not know which families were in which group" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated how many people were lost to follow‐up, or how/if this was measured. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available and author failed to communicate with the review team. |
| Other bias | Low risk | Baseline imbalance Study arms had similar baseline characteristics. |
Rowland 2004.
| Methods | Cluster‐RCT Unit of randomizations: household Intra‐cluster correlation coefficient factor of 0.04. Trial duration: 7 months between August 1999 and February 2000. |
|
| Participants | Adults and children living in malaria‐endemic regions Participants were not screened at start for P. vivax. |
|
| Interventions | Topical repellent ‐ Mosbar soap (20% DEET + 0.5% permethrin) versus placebo lotion Co‐interventions: none Treatment arms: ‐ Mosbar soap (20% DEET + 0.5% permethrin) arm: 67 households (618 participants) ‐ Placebo arm: 60 households (530 participants) |
|
| Outcomes | ‐ Participants with clinical malaria confirmed through blood smears or rapid diagnostic tests (P. falciparum or P. vivax); and ‐ Recorded adverse events. |
|
| Notes | Trial was conducted with Afghan refugees in malaria‐endemic region of Pakistan. Funded by HealthNet International’s Malaria and Leishmaniasis control and research programme. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described "By applying simple randomisation 13% (67 of 510) of households were allocated to the repellent soap group and a similar proportion (12%, 60 of 510) to the placebo control." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Clinical malaria | Low risk | Participants were blinded: although they had been given two different products, a soap or a lotion, they were not aware which one had repellent properties. |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk | Participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) Adverse events | Low risk | Participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) Clinical malaria | Low risk | Microscopists were blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available and author failed to communicate with the review team. |
| Other bias | Low risk | Baseline imbalance Study arms had similar baseline characteristics. |
Sangoro 2014a.
| Methods | Cluster‐RCT Unit of randomization: cluster of houses ICC is not reported. Trial duration: 14 months from July 2009 to August 2010 |
|
| Participants | Adults or children living in endemic areas. | |
| Interventions | 15% DEET lotion versus placebo lotion Co‐interventions: LLINs Treatment arms: ‐ DEET 15% + LLINs arm ‒ 10 clusters, 468 households and 2224 participants ‐ Placebo + LLINs arm ‒ 10 clusters, 469 households and 2202 participants |
|
| Outcomes | ‐ Participants with clinical malaria confirmed through blood smears or rapid diagnostic tests (P. falciparum); and ‐ Adherence to regular usage of the intervention. |
|
| Notes | Trial was conducted in rural communities of the Ulanga district, Kilombero Valley, Tanzania. Trial registration number: ISRCTN92202008 Funded by Population Services International. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generation was done using lottery system. |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed. The method described was basic sequential alternate A/B/A/B. |
| Blinding of participants and personnel (performance bias) Clinical malaria | Low risk | The treatment allocation was blinded to both participants and field staff. |
| Blinding of participants and personnel (performance bias) Compliance | Low risk | The treatment allocation was blinded to both participants and field staff. |
| Blinding of outcome assessment (detection bias) Compliance | High risk | Compliance was indirectly reported by measuring the amount of lotion remaining in the bottle. |
| Blinding of outcome assessment (detection bias) Clinical malaria | Low risk | Clinical malaria was diagnosed by mRDT which is an objective method. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up and withdrawals were identical between treatment groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | High risk | Baseline imbalance: “Bias was introduced into the study by an imbalance in socio‐economic status between the two study groups. The control group demonstrated a higher socio‐economic status than the control arm.” |
Sluydts 2016.
| Methods | Cluster‐RCT Unit of randomization: cluster of houses ICC was calculated per survey; survey 4 ICC was 0.0294. Trial duration: approximately 20 months from April 2012 until November 2013 inclusive. |
|
| Participants | Adults and children living in malaria‐endemic regions. Participants were not screened at start for P. vivax. |
|
| Interventions | Picaridin KBR3023 (topical repellent) versus no treatment Picaridin 10% for children < 10 years and Picaridin 20% in individuals < 10 years Co‐interventions: LLINs Treatment arms: ‐ Picaridin KBR3023 arm ‒ 49 clusters from 57 villages (5642 households, 25,051 individuals) ‐ No treatment arm ‒ 49 clusters from 56 villages (5287 households, 23,787 individuals) |
|
| Outcomes | ‐ Participants with clinical malaria confirmed through blood smears or rapid diagnostic tests (P. falciparum or P. vivax); ‐ Participants with malaria parasitaemia confirmed through thick or thin blood smears, mRDTs or PCR (P. falciparum or P.vivax); ‐ Adherence to regular usage of the intervention through self‐reporting and observational studies; and ‐ Recorded adverse events. |
|
| Notes | Trial was conducted in Ratanakiri province, Cambodia. Trial registration number: NCT01663831 Funded by the Bill and Melinda Gates Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence, calculation of restriction factor, and validity matrix was carried out in R using “onemillion_random.RData”. |
| Allocation concealment (selection bias) | Low risk | All clusters were allocated a treatment at start using a computer generated random sequence. |
| Blinding of participants and personnel (performance bias) Parasitaemia | High risk | There was no placebo given to control group. |
| Blinding of participants and personnel (performance bias) Clinical malaria | High risk | There was no placebo given to control group. |
| Blinding of participants and personnel (performance bias) Compliance | High risk | There was no placebo given to control group so it is unclear how compliance might have been affected. Control group was given LLIN and intervention group was given a topical repellent in addition to the LLIN. It is possible that participants felt they would be protected by the repellent and so would choose not to use their bed net. |
| Blinding of participants and personnel (performance bias) Adverse events | High risk | There was no placebo given to control so those given repellent lotions might have felt more likely to suffer adverse effects. |
| Blinding of outcome assessment (detection bias) Parasitaemia | Low risk | Parasitaemia was measured by PCR which is an objective test. |
| Blinding of outcome assessment (detection bias) Compliance | Unclear risk | Compliance was only measured in the treatment arm because there was no placebo. |
| Blinding of outcome assessment (detection bias) Adverse events | High risk | Adverse effects were self‐reported and could have been influenced by the participant knowing that he/she had been given a fully effective mosquito repellent. |
| Blinding of outcome assessment (detection bias) Clinical malaria | Unclear risk | The trial was not placebo‐controlled: individuals that received the repellent could have mentioned this to medical staff and influenced their diagnosis of clinical malaria. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was similar between groups. |
| Selective reporting (reporting bias) | Low risk | Reporting was done according to protocol. |
| Other bias | Low risk | Baseline imbalance. Restrained randomization controlled for baseline imbalances |
Soto 1995.
| Methods | RCT Duration of the trial: 3 to 5 weeks followed by 4 weeks' follow‐up |
|
| Participants | Colombian Army members stationed in endemic areas | |
| Interventions | Insecticide treated clothing versus placebo Treatment arms: ‐ ITC ‒ 86 individuals; ‐ Placebo ‒ 86 individuals. |
|
| Outcomes | ‐ Participants with clinical malaria confirmed through blood smears or rapid diagnostic tests (P. falciparum or P. vivax); and ‐ Recorded adverse events. |
|
| Notes | Trial was conducted in the Colombian Amazon. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The randomization process is not described. Quote “troops were randomly assigned to receive either permethrin‐impregnated or non‐impregnated uniforms” |
| Allocation concealment (selection bias) | Unclear risk | Poorly described. |
| Blinding of participants and personnel (performance bias) Clinical malaria | Low risk | Medical attendants and soldiers were blinded to the intervention. |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk | Medical attendants and soldiers were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Adverse events | Low risk | Medical attendants and soldiers were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) Clinical malaria | Low risk | Medical attendants and soldiers were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up, no treatment withdrawals, no trial group changes and no major adverse events. Adherence to instructions (wearing clothes) was not monitored so not possible to assess whether soldiers were compliant. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available and the corresponding author failed to communicate. |
| Other bias | Unclear risk | Baseline imbalance Both study arms recruited similar number of soldiers and deployed them to the same endemic area. However, the number of weeks soldiers in each study arm were deployed in the field was not reported per arm. "Each soldier was in the area of endemicity for 3‐8 weeks." |
Syafruddin 2014.
| Methods | Matched pair cluster‐RCT, with the matching done according to village. Unit of randomization: cluster ICC not reported. Trial duration: 6 months |
|
| Participants | Male adults between 18 and 60 years old, residents of malaria‐endemic regions. Participants were screened at start and parasites were cleared. |
|
| Interventions | Mosquito coils (0.00975% metofluthrin) versus Placebo coils No co‐interventions Treatment arms: ‐ Metofluthrin treated coils: 2 clusters with total of 216 households, population of 1001 individuals and 83 participants (males 18 to 60 years old) enrolled for follow‐up; ‐ Placebo coils: 2 clusters with total of 229 households, population of 1119 and 87 participants (males 18 to 60 years old) enrolled for follow‐up. |
|
| Outcomes | ‐ Participants with Plasmodium spp. parasitaemia confirmed through blood smear. ‐ Reduction in mosquito landings measured through human landing catch. ‐ Adverse events. |
|
| Notes | Trial was conducted in Umbugendo and Wainyapu in Southwest Sumba District, East Nusa Tenggara Province, Indonesia. Funded by Bill and Melinda Gates Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method was not described. |
| Allocation concealment (selection bias) | Low risk | The trial as a matched pair cRCT with matching done according to village level. There were only two clusters in each village: therefore after treatment was allocated to one cluster, it was obvious which treatment would be allocated to the next cluster. |
| Blinding of participants and personnel (performance bias) Parasitaemia | Low risk | Blinding of both participants and personnel was in place. “The study administrator obtained a list of lot manufacturing codes from the coil manufacturer (S.C. Johnson Co., Ho Chi Minh, Vietnam) that identified coils as either active or placebo. The administrator then assigned a code specific to each home and labelled packages of coils corresponding to cluster assignment to active or placebo coil treatment. These assignments were kept in a sealed envelope in a secure location within the managing centre of the research program (Jakarta). Thus, the investigators, research team, study subjects, and residents were blinded as to which cluster received active versus placebo coils until after completion of the study.” |
| Blinding of participants and personnel (performance bias) Reduction in mosquitoes attempting to feed on humans | Low risk | Technicians collecting the mosquitoes were blinded to the interventions. |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk | Blinding of both participants and personnel was in place. |
| Blinding of outcome assessment (detection bias) Parasitaemia | Low risk | Blinding of both participants and personnel was in place. Diagnosis was done through microscopy of blood smear. The method was not validated. |
| Blinding of outcome assessment (detection bias) Adverse events | Low risk | Blinding of both participants and personnel was in place. |
| Blinding of outcome assessment (detection bias) Reduction in mosquitoes attempting to feed on humans | Low risk | Blinding of both participants and personnel was in place. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were reported. |
| Selective reporting (reporting bias) | Low risk | The primary outcomes set out by the author in the registered protocol match those reported in the paper. |
| Other bias | Low risk | Baseline imbalance Study arms had similar baseline characteristics. |
Abbreviations: RCT: randomized controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdulsalam 2014 | The study only had two units of randomization. |
| Dadzie 2013 | The study was not a randomized controlled trial (RCT). |
| Deressa 2014 | The study did not specify the repellent compound tested. |
| Eamsila 1994 | The study was not a RCT. |
| Hamza 2016 | The study only had two units of randomization. |
| Kimani 2006 | The study only had two units of randomization. |
Abbreviations: RCT: randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616001434482.
| Trial name or title | Effectiveness of mosquito repellent delivered through village health volunteers on malaria incidence in artemisinin resistance containment programs in South‐East Myanmar |
| Methods | Open stepped‐wedge cluster‐randomized controlled trial (RCT) |
| Participants | Men and women of all ages residing in the study area. High‐risk populations (mobile and migrant people and residents who are also forest dwellers) will be targeted to receive the repellent. |
| Interventions | 12% DEET cream versus no treatment |
| Outcomes | The primary epidemiological outcomes will be incidence of Plasmodium spp. infection (diagnosed by an mRDT) and incidence of malaria illness. |
| Starting date | 01‐04‐2015 |
| Contact information | Freya Fowkes (freya.fowkes@burnet.edu.au) |
| Notes | www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616001434482 |
NCT02294188.
| Trial name or title | Spatial Repellent Products for Control of Vector Borne Diseases ‐ Malaria ‐ Indonesia |
| Methods | Cluster‐RCT |
| Participants | Residents of malaria‐endemic regions of Indonesia |
| Interventions | Spatial repellent passive emanators versus placebo |
| Outcomes | The primary epidemiological endpoint will be the incidence density of first time malaria infections among human cohorts during the follow‐up period as detected by polymerase chain reaction assay (PCR). |
| Starting date | May 2015 |
| Contact information | Neil Lobo (nlobo@nd.edu) |
| Notes | clinicaltrials.gov/ct2/show/NCT02294188 |
NCT02653898.
| Trial name or title | Malaria Elimination Pilot Study in Military Forces in Cambodia |
| Methods | Cluster‐RCT |
| Participants | Residents of military encampments on the Thai‐Cambodian border |
| Interventions | Focused screening and treatment, malaria prophylaxis and insecticide‐treated uniforms versus untreated uniforms |
| Outcomes | The primary epidemiological outcome will be the absolute risk reduction based on the proportion of subjects remaining malaria‐free at the end of 6 months between the study arms as diagnosed by PCR‐corrected malaria microscopy |
| Starting date | January 2016 |
| Contact information | Chanthap Lon (chantapl@afrims.org) |
| Notes | clinicaltrials.gov/ct2/show/record/NCT02653898 |
NCT02938975.
| Trial name or title | Field Efficacy Of Insecticide Treated Uniforms And Skin Repellents for Malaria Prevention (URCT) |
| Methods | Cluster‐RCT using a 4‐arm non‐inferiority design with 12 months of follow‐up |
| Participants | Healthy recruits of the Tanzanian National Service Program JKT Mgambo Camp. |
| Interventions | Ultra 30 insect repellent lotion (30% Lipo DEET) in combination or not with permethrin factory‐treated army combat uniforms. |
| Outcomes | The primary epidemiological outcome will be the incidence of P. falciparum malaria through monthly measurement of malaria positivity by direct polymerase chain reaction (PCR) to detect parasite DNA. |
| Starting date | November 2017 |
| Contact information | Sarah Moore (smoore@ihi.or.tz) |
| Notes | clinicaltrials.gov/ct2/show/record/NCT02938975 |
Abbreviations: RCT: randomized controlled trial.
Differences between protocol and review
With regard to P. vivax infections, we had originally described in the protocol that data onP. vivax from studies that had not cleared parasites at start would be excluded. However only two of the included studies, which both focused on spatial repellents, cleared parasites at start. If a study undertook a proper randomization, recrudescent infections would be similar between treatment arms. For this reason we decided to include data on P. vivax regardless of whether infections had been cleared at start or not.
We also decided to subgroup by use or not of LLINs as a co‐intervention rather than by endemicity level, compliance and diagnostic methods, as described in the protocol. This was done because we believe there was heterogeneity between studies that included and did not include LLINs as co‐interventions. Also, given that current malaria control programmes all incorporate LLINs, we believe policy makers are mostly interested in the combined effect of LLINs with topical repellents rather than these on their own.
Contributions of authors
MM, MV, and SJM developed the protocol with statistical input from MR. MM and MV screened search outputs, selected trials for inclusion, extracted the data, assessed risk of bias, analysed the data and prepared the draft manuscript. MR provided statistical support. CL and SJM critically engaged with the manuscript and provided comments.
SJM is an investigator on two of the studies included in this review; however the author was not involved in decisions regarding inclusion, assessment of risk of bias, data extraction or interpretation of the results of this trial. MM and MK evaluated the trials and conducted all tasks in regard to these studies with no input from SJM.
All review authors have seen and approved the final manuscript.
Sources of support
Internal sources
Cochrane Infectious Diseases Group, UK.
Liverpool School of Tropical Medicine, UK.
External sources
-
University of Notre Dame, USA.
Award number 261655. Salary of MM and SM.
-
Department of International Development (DFID), UK.
Grant: 5242
Declarations of interest
MM, MK, MR, and CL have no conflicts of interest in any way related to the content of the review. They have no financial interests in relation to repellents for mosquito avoidance, either in the form of participations or incomes from commercial activities, patents, or from any form of sponsorship.
SJM has received salary coverage from BMGF research grant and PSI for two studies included in this review. However she did not have any role in deciding inclusion, assessment of risk of bias, data extraction, or interpretation of the results of those trials. In addition, SJM currently holds a research grant funded by the Deployed War Fighters Protection Fund evaluating permethrin‐treated clothing, which has been included in the review as an on‐going study. SJM declares no further known conflicts of interest.
Unchanged
References
References to studies included in this review
Chen‐Hussey 2013 {published data only}
- Chen‐Hussey V, Carneiro I, Keomanila H, Gray R, Bannavong S, Phanalasy S, et al. Can topical insect repellents reduce malaria? A cluster‐randomised controlled trial of the insect repellent N,N‐diethyl‐m‐toluamide (DEET) in Lao PDR. PLoS ONE 2013;8(8):e70664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hill 2007 {published data only}
- Hill N, Lenglet A, Arnéz AM, Carneiro I. Plant based insect repellent and insecticide treated bed nets to protect against malaria in areas of early evening biting vectors: double blind randomised placebo controlled clinical trial in the Bolivian Amazon. BMJ 2007;335(7628):1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hill 2014 {published data only}
- Hill N, Zhou HN, Wang P, Guo X, Carneiro I, Moore SJ. A household randomized, controlled trial of the efficacy of 0.03% transfluthrin coils alone and in combination with long‐lasting insecticidal nets on the incidence of Plasmodium falciparum and Plasmodium vivax malaria in Western Yunnan Province, China. Malaria Journal 2014;13:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGready 2001 {published data only}
- McGready R, Hamilton KA, Simpson J A, Cho T, Luxemburger C, Edwards R, et al. Safety of the insect repellent N,N‐diethyl‐M‐toluamide (DEET) in pregnancy. American Journal of Tropical Medicine and Hygiene 2001;65(4):285‐9. [DOI] [PubMed] [Google Scholar]
Rowland 1999 {published data only}
- Rowland M, Durrani N, Hewitt S, Mohammed N, Bouma M, Carneiro I, et al. Permethrin‐treated chaddars and top‐sheets: appropriate technology for protection against malaria in Afghanistan and other complex emergencies. Transactions of the Royal Society of Tropical Medicine and Hygiene 1999;93(5):465‐72. [DOI] [PubMed] [Google Scholar]
Rowland 2004 {published data only}
- Rowland M, Downey G, Rab A, Freeman T, Mohammad N, Rehman H, et al. DEET mosquito repellent provides personal protection against malaria: a household randomized trial in an Afghan refugee camp in Pakistan. Tropical Medicine & International Health 2004;9(3):335‐42. [DOI] [PubMed] [Google Scholar]
Sangoro 2014a {published data only}
- Miller JE. Low cost repellents for malaria prevention in rural Africa: the jury is still out. ASTMH 60th Annual Meeting, 2011 Dec 4‐8; Philadelphia. American Journal of Tropical Medicine and Hygiene 2011;6(Suppl. 1):369‐70. [Google Scholar]
- Sangoro O, Turner E, Simfukwe E, Miller JE, Moore SJ. A cluster‐randomized controlled trial to assess the effectiveness of using 15% DEET topical repellent with long‐lasting insecticidal nets (LLINs) compared to a placebo lotion on malaria transmission. Malaria Journal 2014;13:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangoro P, Simfukwe E, Moore SJ. Cluster randomized controlled trial to determine the additional benefits of topical repellents to long lasting insecticide nets (LLINs) on malaria incidence. ASTMH 60th Annual Meeting, 2011 Dec 4‐8; Philadelphia. American Journal of Tropical Medicine and Hygiene 2011;6(Suppl. 1):229. [Google Scholar]
Sluydts 2016 {published data only}
- Sluydts V, Durnez L, Heng S, Gryseels C, Canier L, Kim S, et al. Efficacy of topical mosquito repellent (picaridin) plus long‐lasting insecticidal nets versus long‐lasting insecticidal nets alone for control of malaria: a cluster randomised controlled trial. Lancet Infectious Diseases 2016;16(10):1169‐77. [DOI] [PubMed] [Google Scholar]
Soto 1995 {published data only}
- Soto J, Medina F, Dember N, Berman J. Efficacy of permethrin‐impregnated uniforms in the prevention of malaria and leishmaniasis in Colombian soldiers. Clinical Infectious Diseases 1995;21(3):599‐602. [DOI] [PubMed] [Google Scholar]
Syafruddin 2014 {published data only}
- Syafruddin D, Bangs MJ, Sidik D, Elyazar I, Asih PB, Chan K, et al. Impact of a spatial repellent on malaria incidence in two villages in Sumba, Indonesia. American Journal of Tropical Medicine and Hygiene 2014;91(6):1079‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdulsalam 2014 {published data only}
- Abdulsalam YM, Muhammad H, Abduljalal A, Iliyasu Z, Muhammad B, Bello MM, et al. Effectiveness of transfluthrin‐coated inflammable‐fumes insecticide‐paper (Rambo) in the prevention of malaria in Kano, Nigeria. International Journal of Infectious Diseases 2014;21(1):154. [Google Scholar]
Dadzie 2013 {published data only}
- Dadzie S, Boakye D, Asoala V, Koram K, Kiszewski A, Appawu M. A community‐wide study of malaria reduction: evaluating efficacy and user‐acceptance of a low‐cost repellent in northern Ghana. American Journal of Tropical Medicine and Hygiene 2013;88(2):309‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deressa 2014 {published data only}
- Deressa W, Yihdego Y, Kebede Z, Batisso E, Tekalegne A, Dagne G. Effect of combining mosquito repellent and insecticide treated net on malaria prevalence in Southern Ethiopia: cluster randomised trial. Parasite & Vectors 2014;7:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eamsila 1994 {published data only}
- Eamsila C, Frances S P, Strickman D. Evaluation of permethrin‐treated military uniforms for personal protection against malaria in northeastern Thailand. Journal of the American Mosquito Control Association 1994;10(4):515‐21. [PubMed] [Google Scholar]
Hamza 2016 {published data only}
- Hamza M, Bello M, Ma'aruf M, Manu A, Ado A, Dalhatu Y, et al. Effectiveness of transfluthrin‐coated inflammable‐fumes insecticide‐paper (Rambo) in the prevention of malaria in Kano, Nigeria. Sub‐Saharan African Journal of Medicine 2016;3(2):111. [Google Scholar]
Kimani 2006 {published data only}
- Kimani EW, Vulule JM, Kuria IW, Mugisha F. Use of insecticide‐treated clothes for personal protection against malaria: a community trial. Malaria Journal 2006;5:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12616001434482 {published data only}
- ACTRN12616001434482. Effectiveness of mosquito repellent delivered through village health volunteers on malaria incidence in artemisinin resistance containment programs in South‐East Myanmar. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616001434482 14‐10‐2016.
NCT02294188 {published data only}
- NCT02294188. Spatial Repellent Products for Control of Vector Borne Diseases ‐ Malaria ‐ Indonesia (SR‐M‐IDR). clinicaltrials.gov/ct2/show/NCT02294188 17‐11‐2014.
NCT02653898 {published data only}
- NCT02653839. Malaria elimination pilot study in military forces in Cambodia. clinicaltrials.gov/ct2/show/NCT02653839 08‐01‐2016.
NCT02938975 {published data only}
- NCT02938975. Field efficacy of insecticide treated uniforms and skin repellents for malaria prevention. clinicaltrials.gov/ct2/show/NCT02938975.
Additional references
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Barbara 2011
- Barbara KA, Sukowati S, Rusmiarto S, Susapto D, Bangs MJ, Kinzer MH. Survey of Anopheles mosquitoes (Diptera:Culicidae) in West Sumba District, Indonesia. Southeast Asian Journal of Tropical Medicine and Public Health 2011;42(1):71‐82. [PubMed] [Google Scholar]
CDC 2014
- Centers for Disease Control and Prevention. In: Brunette GW editor(s). CDC Health Information for International Travel. New York: Oxford University Press, 2014. [Google Scholar]
Durnez 2013
- Durnez L, Coosemans M. Chapter 21: Residual transmission of malaria: an old issue for new approaches. In: Manguin S editor(s). Anopheles mosquitoes ‐ new insights into malaria vectors. Rijeka, Croatia: Intech, 2013. [Google Scholar]
Garrett‐Jones 1964
- Garrett‐Jones C. Prognosis for interruption of malaria transmission through assessment of the mosquito's vectorial capacity. Nature 1964;204:1173‐5. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Herodotus 1996
- Herodotus. Herodotus: The Histories. London: Penguin, 1996. [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed.) 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kitchen 2009
- Kitchen LW, Lawrence KL, Coleman RE. The role of the United States military in the development of vector control products, including insect repellents, insecticides, and bed nets. Journal of Vector Ecology: Journal of the Society for Vector Ecology 2009;34(1):50‐61. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
malERA 2011
- malERA Consultative Group on Vector Control. A research agenda for malaria eradication: vector control. PLoS Medicine 2011;8(1):e1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2002
- Moore SJ, Lenglet A, Hill N. Field evaluation of three plant‐based insect repellents against malaria vectors in Vaca Diez Province, the Bolivian Amazon. Journal of the American Mosquito Control Association 2002;18(2):107‐10. [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sangoro 2014b
- Sangoro PO, Moore SJ. Evaluation of Repellent Efficacy in Reducing Disease Incidencee. In: Debboun M, Frances SP, Strickman D editor(s). Repellents: principles, methods and uses. 2nd Edition. Boca Raton, Florida: CRC Press, 2014. [Google Scholar]
Sangoro 2014c
- Sangoro O, Lweitojera D, Simfukwe E, Ngonyani H, Mbeyela E, Lugiko D, et al. Use of a semi‐field system to evaluate the efficacy of topical repellents under user conditions provides a disease exposure free technique comparable with field data. Malaria Journal 2014;13:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinka 2010
- Sinka ME, Rubio‐Palis Y, Manguin S, Patil AP, Temperley WH, Gething PW, et al. The dominant Anopheles vectors of human malaria in the Americas: occurrence data, distribution maps and bionomic précis. Parasites & Vectors 2010;3:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinka 2011
- Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, et al. The dominant Anopheles vectors of human malaria in the Asia‐Pacific region: occurrence data, distribution maps and bionomic précis. Parasites & Vectors 2011;4:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Snow 1998
- Snow RW, Peshu N, Forster D, Bomu G, Mitsanze E, Ngumbao E, et al. Environmental and entomological risk factors for the development of clinical malaria among children on the Kenyan coast. Transactions of the Royal Society of Tropical Medicine and Hygiene 1998;92(4):381‐5. [DOI] [PubMed] [Google Scholar]
Sturrock 2013
- Sturrock HJ, Hsiang MS, Cohen JM, Smith DL, Greenhouse B, Bousema T, et al. Targeting asymptomatic malaria infections: active surveillance in control and elimination. PLoS Medicine 2013;10(6):e1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tatem 2010
- Tatem AJ, Smith DL. International population movements and regional Plasmodium falciparum malaria elimination strategies. Proceedings of the National Academy of Sciences of the United States of America 2010;107(27):12222‐7. [DOI: 10.1073/pnas.1002971107] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Roey 2014
- Roey K, Sokny M, Denis L, Broeck N, Heng S, Siv S, et al. Field evaluation of picaridin repellents reveals differences in repellent sensitivity between Southeast Asian vectors of malaria and arboviruses. PLoS Neglected Tropical Diseases 2014;8(12):e3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2014
- White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet 2014;383(9918):723‐35. [DOI] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. International Travel and Health. Geneva: World Health Organization, 2012. [Google Scholar]
WHO 2017
- World Health Organization. World Malaria Report 2017. http://apps.who.int/iris/bitstream/10665/259492/1/9789241565523‐eng.pdf?ua=1 (accessed 4 February 2018).
WHOPES 1998
- WHOPES. Report of First Meeting of the Global Collaboration for the Development of Pesticides for Public Health (GCDPP). 1998 14‐15 October; WHO/HQ Geneva. www.who.int/whopes/gcdpp/en/oct98_gcdpp_report.pdf (accessed 25 March 2014).
WHOPES 2006
- WHOPES. Pesticides and their application: For the control of vectors and pests of public health importance. 6th edition. whqlibdoc.who.int/hq/2006/WHO_CDS_NTD_WHOPES_GCDPP_2006.1_eng.pdf (accessed 25 March 2014).
Wilson 2014
- Wilson AL, Chen‐Hussey V, Logan JG, Lindsay SW. Are topical insect repellents effective against malaria in endemic populations? A systematic review and meta‐analysis. Malaria Journal 2014;13:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2010
- Zhang L, Jiang Z, Tong J, Wang Z, Han Z, Zhang J. Using charcoal as base material reduces mosquito coil emissions of toxins. Indoor Air 2010;20(2):176‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]


